Abstract
Pulsed CO2 lasers show great promise for the rapid and efficient ablation of dental hard tissues. Our objective was to demonstrate that CO2 lasers operated at high repetition rates can be used for the rapid removal of dentin without excessive thermal damage and without compromising adhesion to restorative materials. Human dentin samples (3×3mm2) were rapidly ablated with a pulsed CO2 laser operating at a wavelength of 9.3-μm, pulse repetition rate of 300-Hz and an irradiation intensity of 18-J/cm2. The bond strength to composite was determined by the modified single plane shear test. There were 8 test groups each containing 10 blocks: negative control (non-irradiated non-etched), positive control (non-irradiated acid-etched), and six laser treated groups (three etched and three non-etched sets). The first and second etched and non-etched sets were ablated at a speed of 25 mm/sec and 50 mm/sec with water, respectively. The third set was also ablated at 50 mm/sec without application of water during laser irradiation. Minimal thermal damage was observed on the dentin surfaces for which water cooling was applied. Bond strengths exceeded 20 MPa for laser treated surfaces that were acid-etched after ablation (25-mm/sec: 29.9-MPa, 50-mm/sec: 21.3-MPa). The water-cooled etched laser groups all produced significantly stronger bonds than the negative control (p<0.001) and a lower bond strength than the positive control (p<0.05). These measurements demonstrate that dentin surfaces can be rapidly ablated by a CO2 lasers with minimal peripheral thermal damage. Additional studies are needed to determine if a lower bond strength than the acid-etched control samples is clinically significant where durability of these bonded restoration supersedes high bond strength.
Keywords: dentin, CO2 laser, adhesion, composite
1. INTRODUCTION
Several studies have demonstrated that carbon dioxide lasers operating at wavelengths of λ = 9.3 and 9.6-μm that are highly absorbed by dental hard tissues are ideally suited for the efficient ablation of dental caries and for surface treatments to increase the resistance to acid dissolution.1–5 If pulse durations in the range of 5–20-μs are used efficient ablation occurs with minimal thermal peripheral damage 3,5,6. Transverse excited atmospheric pressure (TEA) CO2 lasers operate through a simple high voltage discharge that excites the gas matrix and can potentially be manufactured at relatively low cost. Another advantage of this laser system is that it is capable of operating efficiently at high pulse repetition rates and the system can be combined with a laser beam scanning system for high speed precision removal of dental caries. One concern at higher repetition rates is the increased potential for peripheral thermal damage due to heat accumulation from multiple laser pulses delivered in rapid succession. This heat accumulation can be offset by the rapid scanning of the laser beam. Dentin is particularly susceptible to thermal damage that may compromise the adhesion of restorative materials because of the high percentage of collagen 6,7,8.
The results of adhesion studies involving laser treated dentin have been mixed and groups report that laser treated surfaces yield similar adhesion characteristics to conventional etching procedures while other groups report that treatment by conventional free-running Er:YAG or Er:YSGG laser yield lower bond strengths even if the surface is acid etched after laser treatment 9–15. Composite bonding to dentin surfaces poses a greater challenge than bonding to enamel due to the added complexity of the collagen matrix and since collagen comprises almost 50% of dentin, a principal concern during laser heating is thermal damage to the underlying collagen matrix. Conventional dentin bonding schemes depend on an acid etchant to remove the smear layer and widen the tubule lumen to increase the penetration of the resin to form resin tags and demineralize the intertubular dentin to form a collagen/resin hybrid layer 16. Such a hybrid zone results in a higher bond strength and tighter seal to reduce microleakage. Several studies carried out on laser treated dentin have resulted in a wide range of success. Studies have also showed mixed results with microleakage 17–21. The morphological and chemical changes induced in dentin as a result of laser irradiation have also been examined 22–28.
We found that the Er:YAG and Er:YSGG pulses of 150–250-μs duration which are used clinically can result in thermal modification of the collagen matrix reducing the bond strength. When the laser pulse duration was reduced to 35-μs, bond strengths approaching 30 MPa were attainable that were similar to the phosphoric acid etch control samples even when the surfaces were not etched after laser treatment 29. This clearly demonstrates that thermal modification of the dentin can compromise adhesion. Measurements of the thermal emission from tooth surfaces during ablation with Er:YAG, Er:YSGG and CO2 lasers indicates that the surface temperature at the time of ablation depends on the wavelength and pulse duration. The surface temperature is higher for CO2 lasers than for the erbium lasers 30. This is obviously an advantage for caries inhibition around caries preparations for enamel but the higher temperatures may result in greater peripheral thermal damage to dentin. Last year we demonstrated that an inexpensive rapidly scanned CO2 laser could be used to remove enamel and dentin 31. That laser operated with longer pulse durations 35, 50 and 75-μs and the shear bond strength to dentin was not evaluated.
The purpose of this study was to determine if a CO2 laser scanning system operating with laser pulses of 10–15-μs and pulse repetition rates of 300 Hz operating at various beam scanning rates with and without a water spray will produce peripheral thermal damage in dentin. Another aim was to compare the shear bond strength of dentin to composite after laser treatment, with and without post-ablation acid etching to conventionally prepared dentin surfaces.
2. MATERIALS AND METHODS
Dentin blocks (n=80), 3 × 3 mm2 with a thickness of 2 mm were prepared from non-carious human molars. There were a total of 8 groups each containing 10 blocks for repeated measurements. The blocks were cut using Isomet 2000 Buehler (Lake Bluff, IL) precision saw and kept well hydrated before ablation. Blocks were polished using 360 carbide grit, and the debris produced was removed by sonication.
2.1 Test Groups
Eight test groups were used. These consisted of one non-irradiated non-etched group and one non-irradiated acid etched group, the negative and positive control groups, respectively. There were six laser treated groups, three etched and three non-etched sets, with the same laser parameters. The groups and the laser beam scanning rates used are listed in Table I.
Table I.
The test groups and associated parameters.
| Sets | Groups | Laser beam scanning rate (mm/sec) | Water Spray |
|---|---|---|---|
| A | 1. Acid etched | 25 | Yes |
| 2. Not acid etched | |||
| B | 3. Acid etched | 50 | Yes |
| 4. Not acid etched | |||
| C | 5. Acid etched | 50 | No |
| 6. Not acid etched | |||
| D | 7. Acid etched | Positive control | NA |
| 8. Not acid etched | Negative control |
Joulemeter ED-200 from Gentec (Quebec, Canada). The laser beam was focused to a spot size of ~300-μm using a planoconvex ZnSe lens of 125-mm focal length. The laser energy was 13 mJ per pulse for a fluence of 18 J/cm2. A razor blade was scanned across the beam to determine the diameter (1/e2) of the laser beam. Computer-controlled XY galvanometers 6200HM series with MicroMax Series 671 from Cambridge Technology, Inc. (Cambridge) were used to scan the laser beam over sample surfaces. Figure 2 shows the basic setup for the laser apparatus. Following ablation, the blocks were embedded onto epoxy resin blocks and tested using the shear-bond test.
Fig 2.
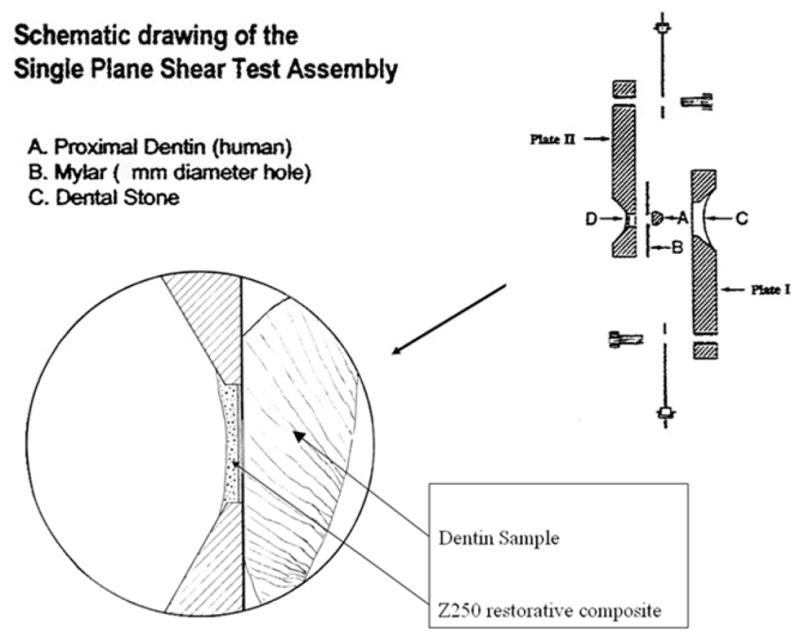
Setup for modified single plane shear test assembly.
2.2 Tissue Irradiation and Laser Parameters
An industrial marking laser, Impact 2500 from GSI Lumonics (Rugby, United Kingdom) operating at a wavelength of 9.3 μm was used. The laser was custom modified to produce a Gaussian output beam (single spatial mode) and a pulse duration of between 10–15-μs. This laser is capable of high repetition rates up to 500 Hz, and a fixed repetition rate of 300 Hz was used for fthese experiments. The laser energy output was monitored using a power meter EPM 1000, Coherent-Molectron (Santa Clara, CA), and the
2.3 Shear-Bond Test
The adhesive strength of the bonding agent to dentin was determined by the shear-bond test. The bonding material was Single Bond along with the Z-250 composite, 3M (Minneapolis, MN). The negative control group was neither irradiated nor etched and was just rinsed with water and gently dried, leaving a moderately moist surface. The positive control group was only etched with 35% phosphoric acid, rinsed with water, and gently dried. One group of laser treated blocks was etched with 35% phosphoric acid, rinsed with water and gently dried. The other group of laser treated blocks was not etched. Subsequently, the bonding resin was applied to all the blocks in two coats, dried, and cured for 10 seconds prior to bonding with composite.
The modified single plane shear test assembly (SPSTA) followed the procedure used by Sheth et al. and Watanabe et al. 32,33. Figure 3 shows the shear-bond test setup for the SPSTA method. Two aligning plates were used to connect the SPSTA to an Instron testing machine, that recorded measurements in kilograms with the crosshead speed set to 5 mm/min. When the two plates separated, the force level was recorded. The force-failure data (in kilograms) was divided by the surface area of the region and a conversion factor was used to calculate the force in Mega-Pascals (MPa).
Fig 3.
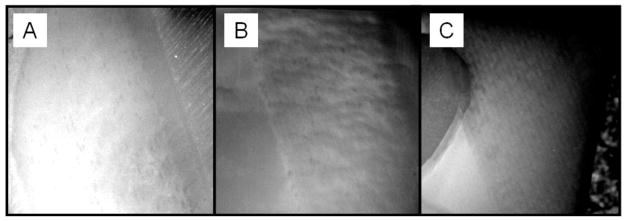
Surfaces treated with laser. CO2 laser at 300 Hz, with a speed of A) 25-mm/s with water, B) 50-mm/s with water, and C) 50-mm/sec without water applied during irradiation.
3. RESULTS
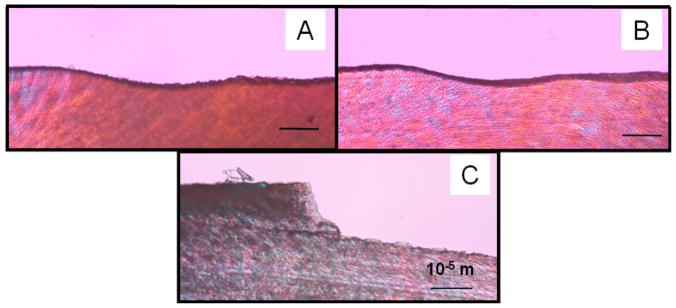
Minimal thermal damage was observed on the dentin surfaces for which water cooling was applied. Figure 3 shows the surfaces of ablated surfaces at 25 mm/sec with water, 50 mm/sec with water, and 50 mm/sec without water. As can be observed, the only speed that produced thermal damage was the 50 mm/sec in the absence of water during irradiation. Otherwise, no thermal damage was observed. Figure 4 shows the image of the cross section of the ablated surfaces, which demonstrates minimal thermal damage on the surface of the ablated surface. The highest thermal damage is observed when water cooling is not done during laser irradiation.
Fig 4.
Cross-section of surfaces treated with laser. CO2 laser at 300 Hz, with a speed of A) 50-mm/sec without water, B) 50-mm/sec with water, and C) 25-mm/sec with water applied during irradiation.
Bond strengths exceeded 20 MPa for laser treated surfaces that were acid-etched after ablation. Table 2 lists the mean bond strengths for each test group.
Table II.
The test groups and associated parameters. nw: no water, ne: non-etched.
| Groups | Treatment parameters | n | Mean (Mpa) | s.d | Statistical Groups* |
|---|---|---|---|---|---|
| 1 | 25-mm/sec | 10 | 29.9 | 6.4 | 3 |
| 2 | 25-mm/sec ne | 10 | 14.1 | 7.2 | 8,3–6 |
| 3 | 50 mm/sec | 10 | 21.3 | 5.5 | 1,2,4,5 |
| 4 | 50 mm/sec ne | 10 | 17.3 | 3 | 2,3,5,6 |
| 5 | 50 mm/sec nw | 10 | 14.1 | 5.3 | 8,2–4,6 |
| 6 | 50 mm/sec ne, nw | 10 | 11.3 | 5.8 | 8,2,4,5 |
| 7 | Positive control | 10 | 39.0 | 5.4 | |
| 8 | Negative control | 10 | 5.1 | 8 | 2,5,6 |
Groups with similar # are statistically similar (p>0.05).
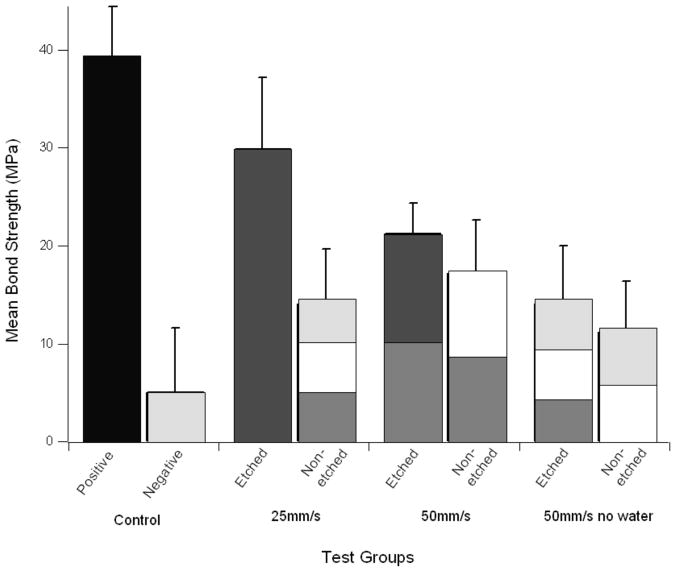
Figure 5 compares the bond strengths to the positive and negative control. As can be seen the water cooled etched laser groups all produced significantly stronger bonds than the negative control (p<0.001) and a lower bond strength than the positive control (p<0.05).
Fig 5.
Mean bond strengths for the test groups. The groups are all significantly lower than the positive control (p<0.05). The water-cooled etched laser groups are both significantly higher than the negative control (p<0.001). Bars with the same shading are statistically similar (p>0.05).
4. DISCUSSION
This study demonstrated that dentin could be rapidly removed by a CO2 laser operating at 9.3-μm with a pulse duration of 10–15-μs with minimal peripheral thermal damage. Peripheral thermal damage was not evident under examination using polarized light microscopy indicating that the zone of thermal damage is minimal and under 10-μm. We have previously demonstrated that CO2 laser pulses delivered under similar ablative conditions thermally modifies a thin layer of enamel around the incision converting it to a more acid resistance mineral phase that increases the resistance to acid dissolution, even when water-cooling is used 34. Since the enamel is modified under similar irradiation conditions then we must assume that there is also some peripheral thermal modification of dentin even though the layer is too thin to be observed using PLM. The width of thermal damage/modification must be very small, i.e., < 10-μm and is less than what we have observed in previous studies using longer laser pulses 6. This indicates that the heat accumulation due to the high pulse repetition rate, 300-Hz in this case, can be successfully offset by rapidly scanning the laser beam. Scanning rates of 25 mm/sec and 50 mm/sec were both successful in minimizing thermal damage, we did not explore lower scanning speeds. Such scanning rates are not excessive and can easily be employed in vivo.
The shear bond strength for the CO2 laser treated samples with water spray and acid etching exceeded 20 MPa. Twenty MPa is an important milestone since it represents the threshold for a clinically useful bond-strength 35,36. However, it was necessary to acid etch after laser treatment to remove the thin layer that was modified by the laser.
Recent measurements have indicated that thermal modification of dentin by CO2 laser irradiation does not increase the acid resistance of dentin in a similar to enamel37. This means that it is not necessary to avoid acid etching dentin since the layer of thermally modified dentin provides no benefit. One potential concern is that the bond strength was lower than the bond strength of the positive control which was the conventional preparation with 35% phosphoric acid etch. The mean shear bond strength was 39.0 MPa for the positive control which is extremely high. In our last adhesion study using exactly the same adhesion model, the mean shear bond strength of the positive control was only 30.7 MPa and the mean shear bond strength in that previous study for surfaces treated by Er:YAG lasers with pulse durations of 0.5 and 20–30-μs were 26.3 and 28.8 respectively with water-cooling 29.
The respective bond strengths of dentin to composite for the etched and water-cooled irradiated groups were significantly higher than the negative control which indicates that etching following ablation is a benefit. In addition, the bond strengths were all significantly lower than the positive control. This can indicate that laser ablation decreases the bonding strengths possibly due to the minimal thermal damage induced in the dentinal surfaces. It is not clear that there is a clinical significance to the lower bond strength of the CO2 laser treated surfaces vs. the positive control or if there is any clinical significance of having a bond strength far in excess of the cohesive strength of dentin. Other more sophisticated studies involving restoration longevity and microleakage are needed to answer these questions. For example, most adhesion studies including this one, report bond-strengths after only 24-hours and the bond strength deteriorates with time and it is not clear whether the longevity of bonds to laser-treated surfaces would be similar to non-treated surfaces. In summary, CO2 laser pulses of 10–15-μs duration delivered at a repetition rate of 300-Hz produced minimal peripheral thermal damage during laser ablation and the laser treated surfaces yield high bond strengths to composite after post-ablation acid etching.
Fig 1.
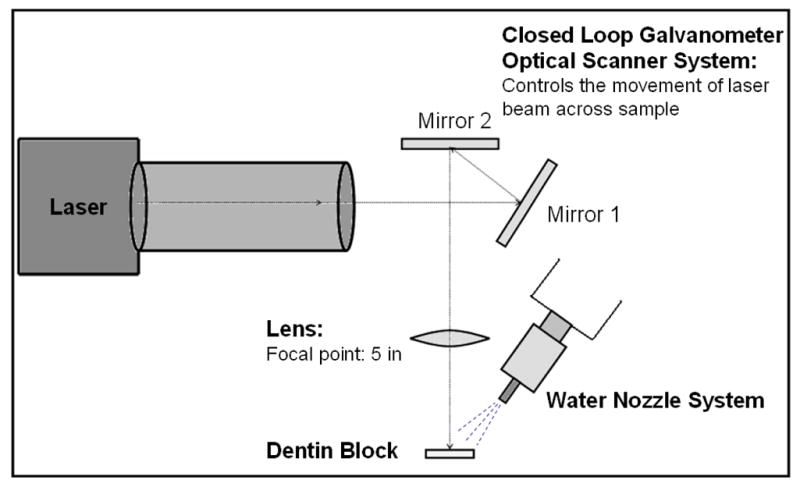
Setup for irradiating dentin blocks.
Acknowledgments
This study was supported by NIH grants T32 DE007306 and RO1 DE14554.
References
- 1.Featherstone JDB, Barrett-Vespone NA, Fried D, Kantorowitz Z, Lofthouse J, Seka W. Lasers in Dentistry. Vol. 2394. San Jose, CA: 1995. Rational choice of CO2 laser conditions for inhibition of caries progression; pp. 57–67. [Google Scholar]
- 2.Featherstone JDB, Barrett-Vespone NA, Fried D, Kantorowitz Z, Lofthouse J. CO2 laser inhibition of artificial caries-like lesion progression in dental enamel. J Dent Res. 1998;77:1397–1403. doi: 10.1177/00220345980770060401. [DOI] [PubMed] [Google Scholar]
- 3.Fan K, Bell P, Fried D. The Rapid and Conservative Ablation and Modification of Enamel, Dentin and Alveolar Bone using a High Repetition Rate TEA CO2 Laser Operating at λ=9.3 μm. J Biomed Opt. 2006;11:064008, 064001-064011 . doi: 10.1117/1.2401151. [DOI] [PubMed] [Google Scholar]
- 4.Fried D, Glena RE, Featherstone JDB, Seka W. Lasers in Dentistry. Vol. 2394. San Jose, CA: 1995. Multiple pulse irradiation of dental hard tissues at CO2 wavelengths; pp. 41–50. [Google Scholar]
- 5.Fried D, Murray MW, Featherstone JDB, Akrivou M, Dickenson KM, Duhn C. Dental hard tissue modification and removal using sealed TEA lasers operating at λ=9.6 μm. J Biomedical Opt. 2001;6:231–238. doi: 10.1117/1.1344192. [DOI] [PubMed] [Google Scholar]
- 6.Dela Rosa AA, Sarma AV, Le CQ, Jones RS, Fried D. Peripheral thermal and mechanical damage to dentin with microsecond and sub-microsecond 9.6 μm, 2.79 μm, and 0.355 μm laser pulses. Lasers in Surgery and Medicine. 2004;35:214–228. doi: 10.1002/lsm.20090. [DOI] [PubMed] [Google Scholar]
- 7.Sheth KK, Staninec M, Sarma AV, Fried D. Selective targeting of protein, water and mineral in dentin using UV and IR pulsed lasers: The effect on the bond strength to composite restorative materials. Lasers in Surgery and Medicine. 2004;35:245–253. doi: 10.1002/lsm.20102. [DOI] [PubMed] [Google Scholar]
- 8.Lee C, Ragadio J, Fried D. Lasers in Dentistry VI. Vol. 3910. San Jose: 2000. Influence of wavelength and pulse duration on peripheral thermal and mechanical damage to dentin and alveolar bone; pp. 193–203. [Google Scholar]
- 9.Keller U, Hibst R. Lasers in orthopedic, Dental, and Veterinary Medicine II. Vol. 1880. Los Angeles, CA: 1993. Effects of Er:YAG laser on enamel bonding of composite materials; pp. 163–165. [Google Scholar]
- 10.Shahabi S, Brockhurst PJ, Walsh LJ. Effect of tooth-related factors on the shear bond strengths obtained with CO2 laser conditioning of enamel. Aust Dent J. 1997;42:81–84. doi: 10.1111/j.1834-7819.1997.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 11.Altshuler GB, Belikov AV, Vlasova SN, Erofeev AV. Medical Applications of Lasers II. Vol. 2327. Lille, France: 1994. The research of seal materials adhesion to walls of cavity in enamel and dentine formation by Er-laser radiation; pp. 101–112. [Google Scholar]
- 12.Cooper LF, Myers ML, Nelson DGA, Mowery AS. Shear strength of composite resin bonded to laser pretreated dentin. J Prosth Dent. 1988;60:45–49. doi: 10.1016/0022-3913(88)90348-4. [DOI] [PubMed] [Google Scholar]
- 13.Visuri SR, Gilbert JL, Walsh JT. Lasers in Dentistry. Vol. 2394. San Jose: 1995. Shear test of composite bonded to dentin: Er:YAG laser vs. dental handpiece preparations; pp. 223–227. [Google Scholar]
- 14.Martinez-Insua A, Da Silva Dominguez L, Rivera FG, Santana-Penin UA. Differences in bonding to acid-etched or Er:YAG-laser-treated enamel and dentin surfaces. J Prosthet Dent. 2000;84:280–288. doi: 10.1067/mpr.2000.108600. [DOI] [PubMed] [Google Scholar]
- 15.Ceballos L, Toledano M, Osorio R, Tay F, Marshall GW. Bonding of Er:YAG laser treated Dentin. J Dent Res. 2002;81:119–122. [PubMed] [Google Scholar]
- 16.Marshall GW, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25:441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 17.Hossain M, Nakamura Y, Yamada Y, Murakami Y, Matsumoto K. Microleakage of composite resin restoration in cavities prepared by Er, Cr: YSGG laser irradiation and etched bur cavities in primary teeth. J Clin Pediatr Dent. 2002;26:263–268. doi: 10.17796/jcpd.26.3.q8747j711g425582. [DOI] [PubMed] [Google Scholar]
- 18.Ceballos L, Osorio R, Toledano M, Marshall GW. Microleakage of composite restorations after acid or Er-YAG laser cavity treatments. Dent Mater. 2001;17:340–346. doi: 10.1016/s0109-5641(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 19.Yazici AR, Frentzen M, Dayangac B. In vitro analysis of the effects of acid or laser etching on microleakage around composite resin restorations. J Dent. 2001;29:355–361. doi: 10.1016/s0300-5712(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 20.Roebuck EM, Saunders WP, Whitters CJ. Influence of Erbium:YAG laser energies on the microleakage of Class V resin-based composite restorations. Am J Dent. 2000;13:280–284. [PubMed] [Google Scholar]
- 21.Palma Dibb RG, Milori Corona SA, Borsatto MC, Ferreira KC, Pereira Ramos R, Djalma Pecora J. Assessing Microleakage on Class V Composite Resin Restorations after Er:YAG Laser Preparation Varying the Adhesive Systems. J Clin Laser Med Surg. 2002;20:129–133. doi: 10.1089/104454702760090209. [DOI] [PubMed] [Google Scholar]
- 22.Dostalova T, Jelinkova H, Kresja O, Hamal K. Evaluation of the surface changes in enamel and dentin due to possiblity of thermal overheating induced by Er:YAG laser radiation. Scanning Microscopy. 1996;10:285–291. [PubMed] [Google Scholar]
- 23.Ishizaka Y, Eguro T, Maeda T, Tanaka K. Effects of Er:YAG laser irradiation on human dentin:Polarizing microscopic, light microscopic and microradiographic observations and FTIR analysis. Lasers in Surgery and Medicine. 2002;31:171–176. doi: 10.1002/lsm.10061. [DOI] [PubMed] [Google Scholar]
- 24.Schein MT, Bocangel JS, Nogueira GEC, Schein PAL. SEM evaluation of the interaction pattern between dentin and resin after cavity preparation using the Er:YAG laser. J Dent. 2003;31:127–135. doi: 10.1016/s0300-5712(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 25.Keller U, Hibst R. Experimental studies of the application of the Er:YAG laser on dental hard substances: II. Light microscopic and SEM investigations. Lasers Surg Med. 1989;9:345–351. doi: 10.1002/lsm.1900090406. [DOI] [PubMed] [Google Scholar]
- 26.Freiberg RJ, Cozean CD. Lasers in Dentistry VIII. Vol. 4610. San Jose: 2002. Pulsed erbium laser ablation of hard dental tissue: the effects of atomized water spray vs water surface film; pp. 74–84. [Google Scholar]
- 27.Fried D, Ashouri N, Breunig TM, Shori RK. Mechanism of Water Augmentation during IR Laser Irradiation of Dental Enamel. Lasers Surg Med. 2002;31:186–193. doi: 10.1002/lsm.10085. [DOI] [PubMed] [Google Scholar]
- 28.Fried D, Zuerlein MJ, Le CQ, Featherstone J. Thermal and chemical modification of dentin by 9–11 μm CO2 laser pulses of 5–100-μs duration. Lasers Surg Med. 2002;3:275–282. doi: 10.1002/lsm.10100. [DOI] [PubMed] [Google Scholar]
- 29.Le CQ, Staninec M, Fried D. Lasers in Dentistry XI. Vol. 5687. San, Jose, CA: 2005. The influence of pulse duration on the bond strength of dentin to composite after Er:YAG laser irradiation; pp. 151–156. [Google Scholar]
- 30.Fried D, Visuri SR, Featherstone JDB, Seka W, Glena RE, Walsh JT, McCormack SM, Wigdor HA. Infrared radiometry of dental enamel during Er:YAG and Er:YSGG laser irradiation. J Biomedical Optics. 1996;1:455–465. doi: 10.1117/12.250668. [DOI] [PubMed] [Google Scholar]
- 31.Assa S, Meyer S, Fried D. Lasers in Dentistry XIV. Vol. 6843. San Jose, CA, USA: 2008. Ablation of dental hard tissues with a microsecond pulsed carbon dioxide laser operating at 9.3-μm with an integrated scanner; pp. 68430E68431–68437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe LG, Marshall GW, Marshall SJ. Dentin shear Strength: Effect of Tubule Orientation and Intratooth Location. Dent Mater. 1996;12:109–115. doi: 10.1016/S0109-5641(96)80077-7. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe LG, Marshall GW, Marshall SJ. Variables Influence on shear bond strength testing to dentin. Advanced Adhesive Dentistry, 3rd International Kuraray Symposium, Vol. Granada International Symposium; 1999. pp. 75–90. [Google Scholar]
- 34.Can AM, Darling CL, Ho CM, Fried D. Non-destructive Assessment of Inhibition of Demineralization in Dental Enamel Irradiated by a λ=9.3-μm CO2 Laser at Ablative Irradiation Intensities with PS-OCT. Lasers in Surgery and Medicine. 2008;40:342–349. doi: 10.1002/lsm.20633. [DOI] [PubMed] [Google Scholar]
- 35.Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005;21:864–881. doi: 10.1016/j.dental.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 36.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118–132. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 37.Le CQ, Fried D, Featherstone JDB. Lasers in Dentistry XIV. Vol. 6843. San Jose, CA, USA: 2008. Lack of dentin acid resistance following 9.3 um CO2 laser irradiation; pp. 68430J–68435. [Google Scholar]