Abstract
The prognosis of advanced melanoma remains poor in spite of treatment advances, emphasizing the importance of additional preventive measures. Flavonoids, natural components of our diet are being investigated for their chemopreventive/therapeutic properties. Microphthalmia-associated transcription factor (Mitf), downstream of Wnt/β-catenin pathway has become an important prognostic marker of melanoma. Here, we show that treatment of 451Lu melanoma cells with the dietary flavonoid fisetin resulted in decreased cell viability with G1-phase arrest and disruption of Wnt/β-catenin signaling. This was accompanied with a decrease in expression of Wnt protein and its co-receptors and a parallel increase in the expression of endogenous Wnt inhibitors. Fisetin-treated cells showed increased cytosolic levels of Axin and β-TrCP and decreased phosphorylation of GSK3-β assocaited with decreased β-catenin stabilization. Fisetin-mediated interference with the functional cooperation between β-catenin and LEF/TCF-2 resulted in downregulation of positively regulated TCF targets such as c-myc, Brn-2 and Mitf. Flowcytometric analysis of Mitf overexpressing cells showed that fisetin repressed Mitf-induced cell proliferation. Finally, administration of fisetin to 451Lu xenografted nude mice resulted in inhibition of tumor development and decreased Mitf expression. Our data suggest that fisetin can be developed as an effective agent against melanoma due to its potential inhibitory effect on β-catenin/Mitf signaling.
INTRODUCTION
Constitutive activation of Wnt signaling pathway is a feature of a number of cancers including malignant melanoma with aberrant nuclear accumulation and subsequent up-regulation of β-catenin transcription response (Larue and Delmas, 2006). Binding of Wnt to the transmembrane Frizzled (FZD) receptor prompts Dishevelled (DVL) to prevent proteolytic destruction of β-catenin. Stabilized β-catenin then transits to the nucleus, where it converts transcriptional repressors called the T cell factors (TCF) into activators and regulates cell fate through gene expression (Bowerman, 2008). Microphthalmia-associated transcription factor (Mitf) has been shown to reside downstream of the canonical Wnt pathway during melanocyte differentiation from pluripotent neural crest cells. Although expression of many melanocytic/pigmentation markers is lost in human melanoma, Mitf expression remains intact, even in non-pigmented tumors, suggesting a role for Mitf beyond its role in differentiation (Widlund et al., 2002). It has been suggested that Mitf can redirect β-catenin transcriptional activity away from canonical Wnt signaling-regulated genes toward Mitf-specific target promoters thereby enhancing the repertoire of genes regulated by β-catenin (Schepsky et al., 2006).
Epidemiological evidence suggests that a plant-based diet rich in flavonoids is effective against cancer (Khan et al., 2008; Kundu and Surh, 2005). Thus, flavonoids have become not only important potential chemopreventive, but also therapeutic natural agents. Fisetin (3,7,3′,4′-tetrahydroxyflavone) belongs to the flavonol subgroup of flavonoids together with quercetin, myricetin, and kaempferol, and is found in many fruits and vegetables such as strawberries, apple, persimmon, kiwi, onion and cucumber. Fisetin has been shown to facilitate long-term memory potentiation and enhance object recognition in mice, and reduced behavioral deficits following stroke in other animal models (Maher et al., 2007). Emerging data from our laboratory and others suggest that fisetin in addition to its neuroprotective effects possesses anti-cancer properties (Syed et al., 2008). The aim of the present study was to investigate whether fisetin can inhibit the growth of highly aggressive human melanoma cells and to define the molecular basis of this effect. Here, we determined that fisetin exerts its anti-proliferative actions in human melanoma cells by interference with the key elements of the Wnt pathway. In addition, we investigated the effect of fisetin on cellular localization of β-catenin and studied the impact of fisetin on the TCF-2/LEF-1-regulated proteins with emphasis on Mitf as a direct target of Wnt/β-catenin signaling.
RESULTS
Fisetin decreases the viability of 451Lu human melanoma cells
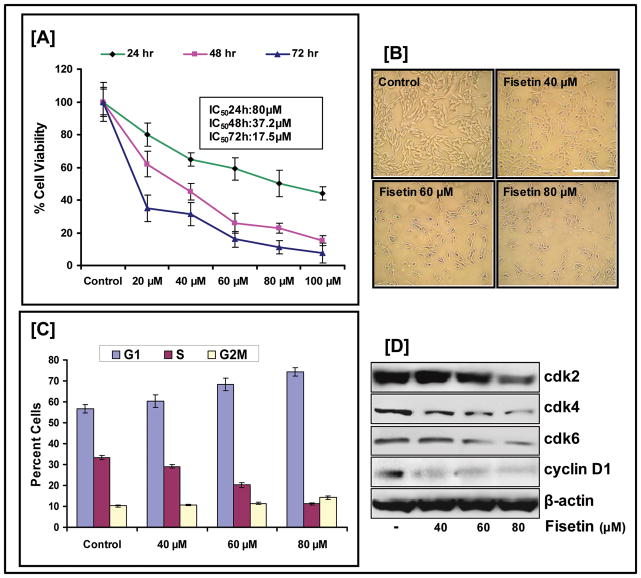
We evaluated the effect of fisetin on the growth of metastatic 451Lu human melanoma cells which exhibit constitutive Wnt signaling in addition to harboring a mutation in the B-Raf gene (Maddodi et al.; Tarapore et al.). Fisetin treatment of 451Lu cells at various time points displayed a dose dependent decrease in cell viability as analyzed by MTT assay (Fig. 1A). The IC50 was estimated to be 80 μM, 37.2 μM, and 17.5 μM at 24, 48 and 72 h respectively. These observations led to the selection of doses for further mechanistic studies that ranged from 40 to 80 μM over 24 h. The dose dependent effect of fisetin on cellular morphology after 24 h treatment as observed by phase contrast microscopy can be visualized in Fig. 1B. Relative to 451Lu cells, normal human melanocytes were resistant to fisetin-mediated growth repression (data not shown).
Figure 1. Effect of fisetin on melanoma cell.
(A) 451Lu cells were treated with fisetin for 24, 48 and 72 h, and MTT assay was performed. The data expressed as the percentage of cell viability represent the mean±standard errors of three experiments (B) Phase contrast microscopy: Representative pictures of 451Lu cells treated with fisetin for 24 h (bar=50μm). (C) Cells treated with fisetin were collected and stained with PI using the Apo-Direct Kit. Following FACS analysis, cellular DNA histograms were analyzed by ModiFitLT V3.0. The data are representative of duplicate experiments. (D) Whole cell lysates were analyzed by immunoblot analysis. Equal loading was confirmed by reprobing for β-actin. Data shown are representative of three independent experiments.
Fisetin induces G1-phase arrest in 451Lu human melanoma cells
To examine the effect of fisetin on 451Lu cell cycle, flowcytometric analysis was performed, and expression of cell cycle regulatory molecules was evaluated by western blot analysis. Fig. 1C shows that fisetin treatment arrested the cells in the G1 phase with an increase in the mean percentage (68.2% and 74.5% at 60 and 80 μM respectively) of cells in G1 as compared to 56.6% of cells in untreated control samples. Furthermore, inhibition of cell-cycle progression by fisetin correlated with a reduction in the protein levels of cyclin-dependent-kinases (cdk) −2,−4 and −6, key cell cycle regulatory proteins involved in the G1 phase progression (Fig. 1D).
Downregulation of Wnt protein and its co-receptors by fisetin
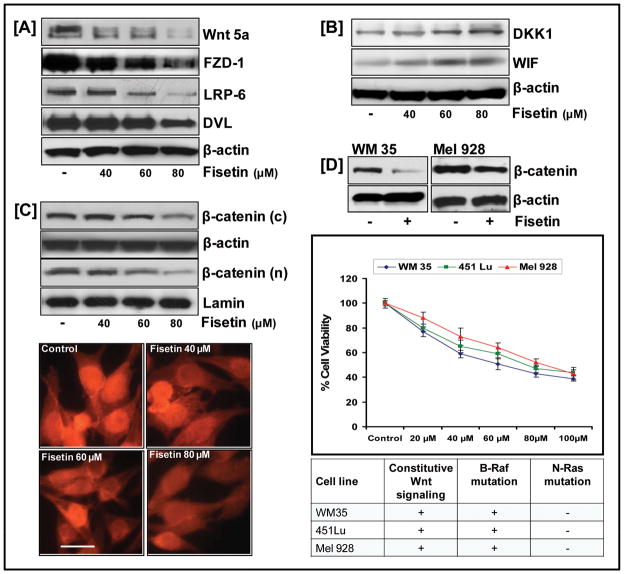
To assess the functional relevance of Wnt/β-catenin signaling in the context of cell growth inhibition, we analyzed the effect of fisetin on Wnt protein and its co-receptors. Fig. 2A demonstrates that cells treated with increasing doses of fisetin showed a dose-dependent decrease in the expression of Wnt growth factor protein, Wnt5a. Interestingly, downregulation of co-receptor (FZD/LRP-6) expressions along with a decrease in DVL expression coincided with an increase in endogenous Wnt inhibitors (DKK1 and WIF) in fisetin-treated cells (Fig. 2A&B).
Figure 2. Effect of fisetin on Wnt signaling pathway.
(A,B) Whole cell (C, top) cytosolic and nuclear lysates were analyzed by western blotting and equal loading confirmed by β-actin and lamin; (bottom) 451Lu cells seeded on tissue culture slides and treated with/without fisetin, fixed in 2% paraformaldehyde, and incubated with β-catenin anti-antibody were observed under a Zeiss Axiophot microscope (bar=6μm). (D, top) Whole cell lysates of WM35 and Mel928 melanoma cells were analyzed by western blot analysis and equal loading confirmed by β-actin; (middle) MTT assay performed on WM35 and Mel928 melanoma cells treated with fisetin for 24 h. Data shown are representative of three independent experiments; (bottom) Table showing mutations in cell lines used.
Decrease in nuclear β-catenin levels by fisetin
Next, we examined the effect of fisetin on cellular localization of β-catenin. Western blot analysis indicated that β-catenin was mainly localized to the cytosolic compartment of the untreated cells, where as increasing doses of fisetin was associated with a decrease in the cytosolic β-catenin with a concomitant decrease in the nuclear β-catenin (Fig. 2C, top). Immunofluorescence studies further elucidated this effect. β-catenin staining was notably reduced in the nuclei of fisetin-treated melanoma cells indicating that a significant amount of β-catenin was phosphorylated and degraded resulting in decreased nuclear accumulation (Fig. 2C, bottom).
We investigated if the inhibitory effect of fisetin on β-catenin expression extended to other melanoma cell lines. For this, we selected two cell lines WM35 and Mel928 with increased β-catenin levels and performed MTT assay and western blot analysis after exposing them to increasing doses of fisetin. As shown in Fig. 2D, fisetin treatment effectively decreased β-catenin protein expression in both cell lines accompanied with dose-dependent inhibition in growth and viability.
Stimulation of cytosolic degradation of β-catenin by fisetin through modulation of destruction complex
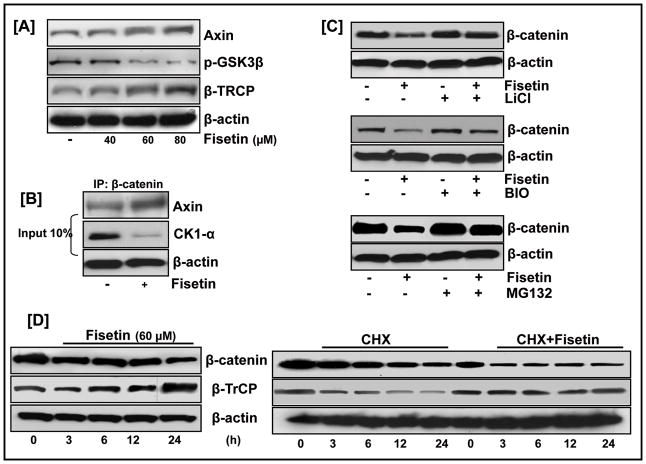
From a mechanistic perspective, the effect of fisetin on suppressing β-catenin expression might be facilitated by two distinct mechanisms:augmenting the destruction complex and increase in proteasomal degradation. Fig. 3A shows an increase in the expression levels of Axin and β-TrCP proteins in the cytosol of fisetin-treated cells indicating a stimulatory effect of fisetin on the destruction complex proteins. To substantiate the involvement of Axin in fisetin-mediated decrease in β-catenin levels, we used an in vitro pull-down assay to assess the binding of Axin with endogenous β-catenin in the lysates of fisetin-treated cells (Fig. 3B). 451Lu cells were treated with fisetin for 24 h, and the cell lysates were incubated with agarose beads coated with β-catenin antibody and subjected to western blotting. As shown in Fig. 3B, treatment of 451Lu cells with fisetin significantly increased the amount of Axin associated with the pull-down.
Figure 3. Fisetin regulates cellular β-catenin levels through modulation of the destruction complex.
(A) Cytosolic fraction of fisetin treated cells were analyzed by western blotting and equal loading confirmed by β-actin. (B) Equal amounts of cell lysates treated with/without fisetin (60 μM) were immunoprecipitated with anti-β-catenin antibody followed by western blot analysis with anti-Axin antibody or immunoblotted with anti-CKIα and β-actin antibodies (input, bottom). (C) 451Lu cells co-treated with fisetin and GSK3β inhibitors LiCl (20 mM) or BIO (10 nM) or MG132 (1 μM) for 24 h followed by Western blot analysis. (D, left) 451Lu cells treated with/without 60 μM fisetin harvested at different time points were analyzed by western blotting; (right) 451Lu cells treated with/without 60 μM fisetin and 50 μg/ml CHX, for different time intervals, followed by western blotting were analyzed for β-TrCP protein stability. Equal loading was confirmed by β-actin. Data shown is representative of three independent experiments.
The phosphorylation of β-catenin by GSK3β targets it for proteasomal degradation. GSK3β activity, in turn is regulated by its phosphorylation status, with phosphorylation at Ser9 rendering the protein functionally inactive. Fig. 3A shows that fisetin treatment resulted in decreased phosphorylation of GSK3β in a dose-dependent manner. To establish if fisetin-induced suppression of β-catenin is mediated through a GSK3β-dependent mechanism, we assessed the effect of fisetin on β-catenin expression in combination with the known GSK3β inhibitor, LiCl. Immunoblot analysis showed that LiCl could protect 451Lu cells from fisetin-mediated suppression of β-catenin expression (Fig. 3C, top). Since several structurally diverse inhibitors of GSK3 must affect the given biological process similarly before concluding that the fisetin is acting via inhibition of GSK3, we co-treated 451Lu cells with fisetin and the highly selective GSK3β inhibitor, BIO and analyzed the lysates for β-catenin protein levels. As before, fisetin was unable to counteract BIO-facilitated activation of β-catenin, indicating that GSK3β played a key role in fisetin-induced β-catenin degradation (Fig. 3C, middle).
β-TrCP is involved in fisetin-mediated β-catenin proteolysis
β-TrCP, the F-box protein in the E3 ubiquitin ligase complex, binds and tags β-catenin for proteasomal degradation (Liu et al., 2004). Fisetin treatment increased the expression of β-TrCP in a dose-dependent manner parallel to that of β-catenin repression, indicating a mechanistic link between two diverse responses (Fig. 3A). To further examine this, we used MG-132 to block proteasome-mediated protein degradation. Treatment with fisetin consistently induced a decrease in β-catenin levels in 451Lu melanoma cells. However, the effect of fisetin on the reduction of β-catenin was abrogated by the addition of MG-132 indicating that fisetin induces the degradation of β-catenin in a proteasome-dependent manner (Fig. 3C, bottom). Time-dependent studies further verified the role of β-TrCP in the suppression of β-catenin expression by fisetin (Fig. 3D). Next, we investigated whether fisetin promoted β-catenin degradation by regulating β-TrCP gene expression or by increasing the stability of the protein. RT-PCR analysis indicated that exposure of451Lu cells to different doses of fisetin did not affect the mRNA level of β-TrCP (data not shown) despite a robust increase in its protein level (Fig. 3A&D). This finding suggested that the up-regulation of β-TrCP by fisetin was mediated by prolonging its protein stability. To verify this, we assessed the effect of fisetin on the half-life of β-TrCP in 451Lu cells co-treated with fisetin and the protein synthesis inhibitor cycloheximide (CHX). In untreated cells, β-TrCP had a half-life of approximately 6 h whereas in fisetin-treated cells, the protein level of β-TrCP remained unaltered throughout the course of CHX treatment (Fig. 3D).
Pleiotropic effects of fisetin on multiple β-catenin signaling targets
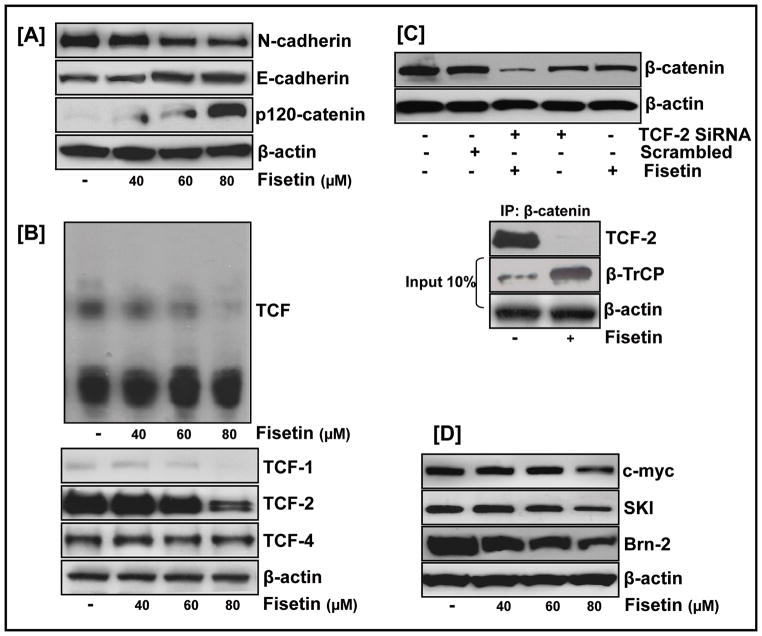
We next examined the effect of fisetin on the cadherin signaling network. As shown in Fig. 4A, fisetin caused differential repression of the cadherin family of proteins with a decrease in N-cadherin and an increase in β-catenin’s binding partner E-cadherin. Also noteworthy was the up-regulation of cadherin-associated p120 catenin protein expression in fisetin treated cells reported to be significantly reduced in cancer tissues (Perez-Moreno and Fuchs, 2006).
Figure 4. Fisetin downregulates β-catenin target proteins.
(A) Differential repression of the cadherin family of proteins by fisetin as analyzed by western blot analysis of 451Lu whole cell lysates treated with fisetin; equal loading confirmed by β-actin. (B, top) Nuclear extracts of fisetin-treated 451Lu cells assayed for TCF transcriptional activity by EMSA; (bottom) Western blot analysis of fisetin treated whole cell lysates showed a dose-dependent inhibitory effect of fisetin on TCF-2 protein (C, top) Cells electroporated with 75 nm of TCF-2 Si RNA or scrambled RNA, treated with fisetin (60 μM) for 24 h and whole cell lysates analyzed by western blot; (bottom) Cells treated with/without fisetin for 24 h, incubated with β-catenin immobilized onto agarose beads and subjected to western blot analysis with anti-TCF-2 antibody. One tenth of cell lysates was collected as input and β-TrCP expression examined (D) Western blot analysis of fisetin-treated cells showed decrease in the protein expression of known β-catenin targets. Equal loading confirmed by β-actin; data representative of at least two independent experiments.
Fisetin interferes with the functional cooperation between TCF-2 and β-catenin
Because β-catenin transactivates gene expression in a complex with TCF proteins, we studied the effect of fisetin on the TCF complex employing mobility shift assay. Fig. 4B (top) shows a dose-dependent decrease of the TCF complex in fisetin-treated 451Lu cells. We next examined the effect of fisetin on different proteins of the TCF family. Fisetin caused differential repression of these proteins in the order of TCF-2>TCF-1 with minimal change in TCF-4 (Fig. 4B, bottom). We wanted to know if fisetin-regulated β-catenin-dependent control is mediated through its effect on TCF-2/β-catenin interaction. Treatment of cells with TCF-2-siRNA, but not scrambled RNA resulted in a significant decrease in the protein expression of β-catenin (Fig. 4C, top). This decrease in β-catenin expression was comparable to the decrease observed in fisetin-treated cells and was more pronounced when cells were co-treated with TCF-2-siRNA and fisetin. A pull-down assay with β-catenin further identified TCF-2 as the key regulator of β-catenin mediated effects in fisetin treated cells (Fig. 4C, bottom). We next studied the effect of fisetin on the protein expression of known TCF-regulated genes. Fig. 4D shows that fisetin treatment resulted in downregulated protein levels of positively regulated β-catenin/TCF targets such as c-myc, Brn2, and Mitf.
Suppression of proliferation by fisetin involves Mitf
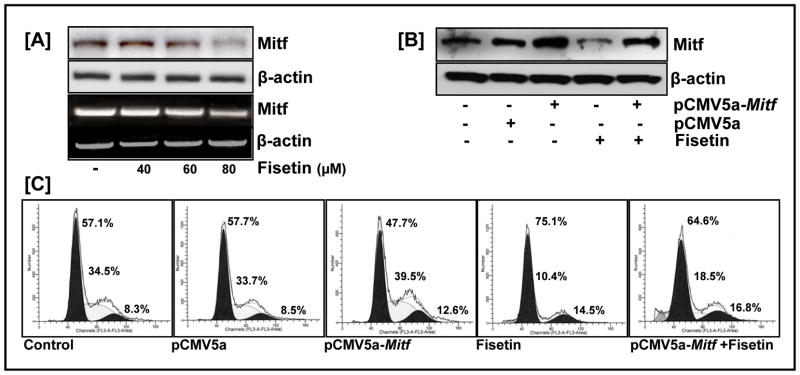
We then examined the effect of fisetin on Mitf, critical in melanoma cell proliferation. Reduced levels of Mitf protein and mRNA in fisetin treated cells suggested that fisetin-induced Mitf repression was mediated at the transcriptional level (Fig. 5A). Furthermore, downregulated Mitf expression was accompanied by a decrease in the anti-apoptotic Bcl-2 protein expression (data not shown). To examine the involvement of Mitf in β-catenin’s mitogenic effect, we over-expressed Mitf in 451Lu melanoma cells. Flowcytometric analysis revealed that ectopic expression of Mitf resulted in an increase of the S-phase fraction of the cell cycle as compared to the untreated control (39.5% versus 34.5%), whereas fisetin treated cells showed a significant decrease in S-phase content (10% versus 34.5%) respectively. Moreover, fisetin was able to relieve cells from Mitf-induced cell proliferation (18% versus 39.5%) (Fig. 5B&C).
Figure 5. Suppression of proliferation by fisetin involves Mitf.
(A) Western blot and RT-PCR analysis of fisetin treated cells showed a dose-dependent inhibitory effect of fisetin on Mitf protein and mRNA levels. Equal loading was confirmed by β-actin. Data shown are representative of at least two independent experiments with similar results (B) Western blot analysis of 451Lu cell transfected with the Mitf expression plasmid pCMV5a-Mitf (1μg) and treated without/with fisetin (60 μM) for 24 h. Equal loading was confirmed by β-actin (C) Flowcytometric analysis of 451Lu cell transfected with the Mitf expression plasmid pCMV5a-Mitf (1μg) and treated without/with fisetin (60 μM) for 24 h. Following FACS analysis, cellular DNA histograms were analyzed by ModiFitLT V3.0. The data are representative of duplicate experiments.
Fisetin inhibits the growth of 451Lu human melanoma cells and decreases Mitf levels in athymic mice
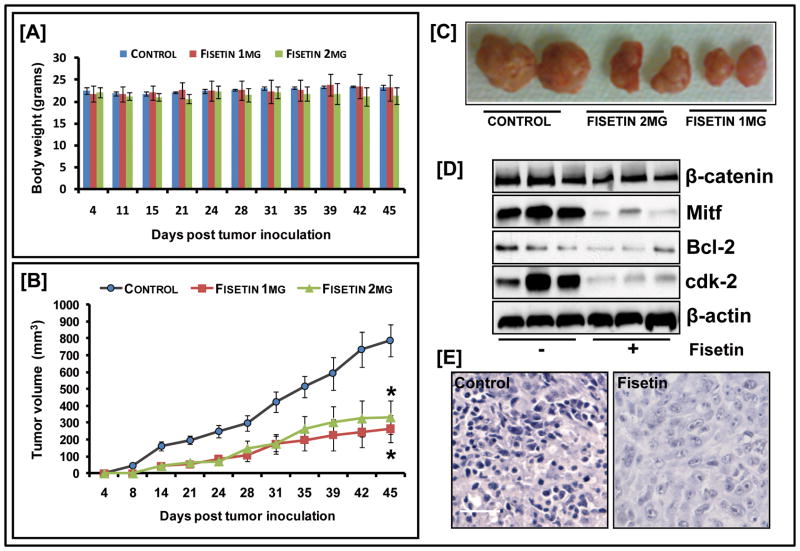
Although our in vitro data unambiguously demonstrated that fisetin had potent growth inhibitory activity, questions remained regarding its in vivo efficacy. Athymic nude mice were implanted with 451Lu melanoma cells and divided into three cohorts, each with 6 animals that were administered fisetin/vehicle intra-peritoneally. The first group received the vehicle (DMSO) only, whereas the second and the third group received fisetin 1mg and 2mg/animal (45 and 90mg/kg body weight) respectively. Fisetin was administered twice weekly and appeared tolerable as depicted by body weight measurements (Fig. 6A). On day 7, the appearance of small tumors was observed in the control cohorts followed by tumors in the fisetin-treated groups by day 14. A smaller average tumor volume was consistently observed in mice treated with fisetin. This was more marked in animals receiving 1mg of fisetin as compared to animals receiving the 2mg dose, indicating a non-linear dose response (Fig. 6B&C). In the control group, the average tumor volume of 788.5 mm3 was reached at day 45, while mice receiving 1mg of fisetin had an average tumor volume of 263.8 mm3 representing a significant suppression in tumor growth by 66.6% (p=0.0012). The 2 mg group, at the same time point had an average tumor volume of 331 mm3 and tumor suppression by about 58.1% (p=0.0007). A significant decrease in the protein expression of Mitf as well as downstream target Bcl-2 and cdk-2 was observed in the treated mice indicating that the growth inhibitory effect of fisetin extended to the in vivo situation (Fig. 6D&E).
Figure 6. Effect of fisetin on 451Lu tumor growth in athymic nude mice.
(A) Average weight of control and fisetin-treated mice plotted over days after tumor cell inoculation: points; mean of 12 tumors in six animals; bars represent SD, p<0.05, versus the control group (B) Average tumor volume of control and fisetin-treated mice plotted over days after tumor cell inoculation. Points, mean of 12 tumors in six animals; bars, SEM*, p<0.001, versus the control group (C) Photographs of excised tumors from each group (D) Whole cell lysates of tumor tissues analyzed by western blot; equal loading confirmed by β-actin (E) Mitf staining in tumor sections of fisetin-treated/untreated mice (bar=25μm). Data shown is representative of samples from each group repeated thrice with similar results.
DISCUSSION
Malignant melanoma is a deadly human cancer with no effective cure for metastatic disease. The aim of this study was to identify natural compounds that may be used against melanoma for chemopreventive/therapeutic purposes. Not only does their use circumvent the need to introduce foreign compounds into human body, but also natural agents are expected, in general, to be less toxic, more accessible and less expensive than synthetic agents (Syed et al., 2008). Strategies are being developed to limit constitutive Wnt signaling involved in the genesis of a number of malignancies including melanoma (Takahashi-Yanaga and Sasaguri, 2007). Fisetin-mediated decrease in the expression of transmembrane receptors, FZD and LRP6 as well as upregulation of the Axin protein indicates that fisetin interferes with the interaction ofLRP6, Axin, and β-catenin (Fig. 2&3). In addition, an increase in the endogenous Wnt inhibitors DKK and WIF-1 may also contribute to suppress the canonical Wnt pathway in fisetin treated cells.
Activated Wnt signaling positively feeds into the PI3K/Akt pathway through inhibition of GSK3, a key component shared by both pathways (Fang et al., 2007). Fisetin treatment resulted in dephosphorylating activation of GSK3β (Fig. 2). Moreover, fisetin suppressed Akt activation in 451Lu melanoma cells through decreased phosphorylation at both Ser473 and Thr308 residues (data not shown). This is important as Akt is thought to act as a molecular switch that increases angiogenesis and generation of superoxide, fostering more aggressive behavior in melanoma tumors (Govindarajan et al., 2007).
Decreased HOS/β-TrCP-2 levels hinder the degradation of β-catenin (Spiegelman et al., 2002). Fisetin and other dietary flavonoids have been shown to enhance proteasome activity and promote nerve cell survival (Maher, 2008). This is consistent with our data that indicate that fisetin increased β-TrCP expression levels through protein stabilization thereby facilitating ubiquitin-dependent proteasomal degradation of β-catenin (Fig. 3). The ability of fisetin to target β-TRCP further provides a molecular basis to account for its reported effect on modulating the expression of cell-cycle regulatory proteins (Lu et al., 2005).
The transcriptional activity of β-catenin through its interaction with the TCF family is recognized as the major effector of the Wnt signaling pathway and its activation has been associated with malignancy (Larue and Delmas, 2006). Our data show that fisetin interferes with the β-catenin/TCF axis through targeting TCF-2/LEF-1 protein in 451Lu melanoma cells (Fig. 4). This effect of fisetin was specific for melanoma cells when compared to other cancer cell lines where fisetin exerts its effect through targeting TCF-4 (Syed et al., 2008). Analysis of protein expression of fisetin-treated melanoma cells showed a dose-dependent reduction in the levels of TCF targets involved in the β-catenin signaling network, including ubiquitous genes such as c-myc; cell lineage-restricted genes such as Brn2 and melanocyte-specific genes such as Mitf (Fig. 4&5). The retention of Mitf expression in the vast majority of human melanomas has led to its use as a diagnostic tool in this malignancy. Suppression of melanoma clonogenic growth by disruption of β-catenin-TCF-2 could be rescued by constitutive MITF (Widlund et al., 2002). In addition, reduction of Mitf activity sensitizes melanoma cells to chemotherapeutic agents. It is suggested that targeting Mitf in combination with B-RAF or cdk inhibitors may offer a rational therapeutic avenue into melanoma, a highly chemotherapy-resistant neoplasm (Garraway et al., 2005). Our data showed that fisetin, a known cdk inhibitor was able to override the proliferative effect of Mitf and induced growth repression in human melanoma cells. The inhibitory effect of fisetin was evident in the tumor xenografted mice as well. Treatment with fisetin at two different doses significantly slowed the progression of 451Lu tumor growth in nude mice as shown in Fig. 6. This inhibition of tumor growth was coupled with a decrease in Mitf levels. Thus it is conceivable that fisetin can inhibit melanoma cell proliferation via targeting the Wnt/β-catenin pathway and its downstream targets in conjunction with its direct effect on the cdk-cyclin network. It will be of interest to determine whether other Mitf-regulated genes can be targeted by fisetin to modulate the mitogenic response. Using dose scaling as advised in FDA guidance the human equivalent dose of fisetin in our in vivo study translated to approximately 97mg of fisetin in a 60kg adult and represents a reasonable starting point for future clinical investigations.
In summary, our goal in this study was to gain a mechanistic understanding of the mode of action of fisetin in melanoma cells. Our in vitro studies showed that decrease in β-catenin levels, induction of β-TrCP and a reduction of Mitf mRNA and protein levels are possible criteria for fisetin-mediated suppression of Wnt signaling in melanoma cells (Fig. 6). Importantly, this was reflected in our in vivo studies that showed significant growth inhibition in mice implanted with melanoma xenografts and decreased Mitf levels upon treatment with fisetin with two different doses. In contemplating future applications, there is strong evidence that this compound may be a strong candidate in novel cancer prevention and therapeutic strategies.
MATERIALS AND METHODS
Materials
Fisetin, LiCl, C2211 (MG132), and CHX were purchased from Sigma Chemical Co. (St. Louis, MO). BIO was obtained from Calbiochem (Gibbstown, NJ).
Antibodies
were obtained from Cell Signaling Technology (Beverly, MA). FZD, WIF1, β-TRCP and Mitf antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell culture/treatment
Mel 928 (ATCC), WM35 and 451Lu human melanoma cell lines kindly provided by Dr. Meenhard Herlyn (Wistar Institute, PA) were cultured in MEM from Gibco (Carlsbad, CA), with 10% FBS and 1% penicillin-streptomycin, at 37°C with 5% CO2 in a humid environment. For dose/time-dependent studies, cells (70% confluent) were treated with fisetin dissolved in DMSO (20–80 μM) for 24 h at 37°C in media and harvested for further studies. For time-dependent studies, cells treated with 60 μM fisetin were harvested at the specified time points.
Cell Viability
was determined by 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described (Johnson et al., 2008).
Cell cycle analysis and Apoptosis
451Lu treated with/without fisetin for 24 h were processed for labeling with fluorescein-tagged dUTP nucleotide and propidium iodide using the APO-DIRECT kit (Phoenix Flow Systems, CA) and analyzed using Modfit software.
Preparation of cell lysate
After fisetin treatment, whole, cytosolic and nuclear lysates were prepared, and western blot analysis performed as described (Syed et al., 2007).
Immunochemistry
Immunocytochemical analysis of 451Lu cells, seeded in tissue culture slides treated with/without fisetin was done as described earlier (Syed et al., 2007). Tumor tissues collected from mice fixed in 10% formalin were blocked (5% goat serum/PBS) for 30 minutes after deparaffinization and rehydration in xylene and graded series of ethanol. After incubation with MITF and HRP--conjugated secondary antibodies, slides were counterstained with haemotoxylin and immunoreactive complexes detected using 3,3′-diaminobenzidene (Dako Corp., CA). Sections were visualized on a Zeiss-Axiophot DMHT microscope and images captured with a camera attached to computer. Figures were composed using ADOBE PHOTOSHOP 7.0(Adobe Systems, CA).
Transient transfection and RNA interference
Transfection of cells was performed by electroporation using an Amaxa Nucleofector according to manufacturer’s protocol (Amaxa Biosystems, Germany)with pCMV5a-Mitf plasmid and a corresponding vector control pCMV5a plasmid/siRNA against TCF-2with scrambled siRNA as control (SantaCruz, CA). Transfected cells were harvested 24 h after fisetin treatment for immunoblot/flowcytometric analysis.
Electrophoretic mobility shift assay (EMSA)
EMSA for TCF was performed using lightshift™ chemiluminiscent EMSA kit (Pierce, Rockford, IL) as per manufacturer’s protocol. Briefly, double-stranded TCF oligonucleotide 5′-AGT TGA GGG GAC TTT CCC AGG C-3′; 3′-TCA ACT CCC CTG AAA GGG TCC G-5′ was labeled using the Biotin 3′ end labeling kit as described (Syed et al., 2007).
Xenograft studies
Athymic (nu/nu) female nude mice (NxGen Biosciences) were housed under pathogen-free conditions with a 12-h light/12-h dark schedule and fed with an autoclaved diet ad libitum. 1×106 451Lu cells suspended in matrigel were injected s.c. into each flank of the eighteen mice randomly divided into 3 groups. The first and the second group received i.p. injection of fisetin (1 and 2 mg/animal respectively) dissolved in DMSO, biweekly, whereas the third group (DMSO only) served as the control group. Mice weight and tumor volumes were recorded. After 45 days, mice were sacrificed and tumor tissues harvested.
Statistical analysis
Results were analyzed using a two-tailed Student’s paired t test, using GraphPad QuickCals software; p<0.05 was considered statistically significant.
Acknowledgments
This work was supported by grants T32 ES007015 (Syed DN) and 1KL2RR025012-01(Johnson JJ) from the NIH.
Footnotes
CONFLICT OF INTEREST: The authors state no conflict of interest.
References
- Bowerman B. Cell signaling. Wnt moves beyond the canon. Science. 2008;320:327–328. doi: 10.1126/science.1157590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Sligh JE, Vincent BJ, Li M, Canter JA, Nickoloff BJ, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JJ, Syed DN, Heren CR, Suh Y, Adhami VM, Mukhtar H. Carnosol, a dietary diterpene, displays growth inhibitory effects in human prostate cancer PC3 cells leading to G2-phase cell cycle arrest and targets the 5′-AMP-activated protein kinase (AMPK) pathway. Pharm Res. 2008;25:2125–2134. doi: 10.1007/s11095-008-9552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- Kundu JK, Surh YJ. Breaking the relay in deregulated cellular signal transduction as a rationale for chemoprevention with anti-inflammatory phytochemicals. Mutat Res. 2005;591:123–146. doi: 10.1016/j.mrfmmm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Front Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- Liu J, Stevens J, Matsunami N, White RL. Targeted degradation of beta-catenin by chimeric F-box fusion proteins. Biochem Biophys Res Commun. 2004;313:1023–1029. doi: 10.1016/j.bbrc.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Lu X, Jung J, Cho HJ, Lim DY, Lee HS, Chun HS, et al. Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J Nutr. 2005;135:2884–2890. doi: 10.1093/jn/135.12.2884. [DOI] [PubMed] [Google Scholar]
- Maddodi N, Huang W, Havighurst T, Kim K, Longley BJ, Setaluri V. Induction of autophagy and inhibition of melanoma growth in vitro and in vivo by hyperactivation of oncogenic BRAF. J Invest Dermatol. 130:1657–1667. doi: 10.1038/jid.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch Biochem Biophys. 2008;476:139–144. doi: 10.1016/j.abb.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepsky A, Bruser K, Gunnarsson GJ, Goodall J, Hallsson JH, Goding CR, et al. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol Cell Biol. 2006;26:8914–8927. doi: 10.1128/MCB.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman VS, Tang W, Katoh M, Slaga TJ, Fuchs SY. Inhibition of HOS expression and activities by Wnt pathway. Oncogene. 2002;21:856–860. doi: 10.1038/sj.onc.1205132. [DOI] [PubMed] [Google Scholar]
- Syed DN, Afaq F, Kweon MH, Hadi N, Bhatia N, Spiegelman VS, et al. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-kappaB activation in normal human bronchial epithelial cells. Oncogene. 2007;26:673–682. doi: 10.1038/sj.onc.1209829. [DOI] [PubMed] [Google Scholar]
- Syed DN, Suh Y, Afaq F, Mukhtar H. Dietary agents for chemoprevention of prostate cancer. Cancer Lett. 2008;265:167–176. doi: 10.1016/j.canlet.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi-Yanaga F, Sasaguri T. The Wnt/beta-catenin signaling pathway as a target in drug discovery. J Pharmacol Sci. 2007;104:293–302. doi: 10.1254/jphs.cr0070024. [DOI] [PubMed] [Google Scholar]
- Tarapore RS, Siddiqui IA, Saleem M, Adhami VM, Spiegelman VS, Mukhtar H. Specific targeting of Wnt/beta-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis. 31:1844–1853. doi: 10.1093/carcin/bgq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlund HR, Horstmann MA, Price ER, Cui J, Lessnick SL, Wu M, et al. Beta-catenin-induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J Cell Biol. 2002;158:1079–1087. doi: 10.1083/jcb.200202049. [DOI] [PMC free article] [PubMed] [Google Scholar]