Abstract
Poor prosthetic fit is often the result of heterotopic ossification (HO), a frequent problem following blast injuries for returning service members. Osseointegration technology offers an advantage for individuals with significant HO and poor socket tolerance by using direct skeletal attachment of a prosthesis to the distal residual limb, but remains limited due to prolonged post-operative rehabilitation regimens. Therefore, electrical stimulation has been proposed as a catalyst for expediting skeletal attachment and the bioelectric effects of HO were evaluated using finite element analysis in 11 servicemen with transfemoral amputations. Retrospective computed tomography (CT) scans provided accurate reconstructions, and volume conductor models demonstrated the variability in residual limb anatomy and necessity for patient-specific modeling to characterize electrical field variance if patients were to undergo a theoretical osseointegration of a prosthesis. In this investigation, the volume of HO was statistically significant when selecting the optimal potential difference for enhanced skeletal fixation, since higher HO volumes required increased voltages at the periprosthetic bone (p = 0.024, r = 0.670). Results from Spearman’s rho correlations also indicated that the age of the subject and volume of HO were statistically significant and inversely proportional, in which younger service members had a higher frequency of HO (p = 0.041, r = −0.622). This study demonstrates that the volume of HO and age may affect the voltage threshold necessary to improve current osseointegration procedures.
Keywords: Electrical stimulation, Finite element analysis, Heterotopic ossification, Osseointegration, Residual limb, Service members
INTRODUCTION
Heterotopic ossification (HO) has been reported as “bizarre overgrowth” of mature bone in neighboring soft tissue.46 This lamellar bone formation44 is metabolically active,1 variable in nature1 and has been described as “serpentine-like.”45 Reports of HO are replete in the orthopedic literature and occur predominately in periarticular regions following: total hip arthoplasty,1,13 head injury,18 spinal cord injury,16,21 rotator cuff surgery33 and burns.20 While the etiology of HO is not fully understood,13 there is a general agreement in the literature that HO is associated with damage to soft tissue and inflammation6,13 and is the most pronounced in combat-related trauma to servicemen and women following blast injuries.47
The frequency of HO from improvised explosive devices (IEDs) and rocket-propelled grenades (RPGs) in Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) have been reported as high as 63% in the wounded service members.46 Because HO manifests months after a blast injury and has a maturation rate upward of 18 months,18,56 problems may arise with poor prosthetic fit and discomfort for those requiring assistive ambulatory devices.12 An improper interface between the residual limb and prosthetic socket may lead to skin breakdown51 and significantly limit the mobility of individuals with limb loss,5,47 particularly in the case of injured service members who wish to return to active duty or an energetic lifestyle.43
In order to help alleviate the frequent problems associated with traditional socket prostheses42 and assist service members with limited residual limb lengths (which may preclude the use of these devices), scientists are developing percutaneous osseointegrated implants as an alternative. Osseointegration is the direct skeletal attachment between an implant and host tissue3 and alleviates the need for a socket prosthesis. The advantage of this docking system is the improved loading at the bone–implant interface which is more physiologic and avoids problems with stress shielding and osteopenia. Most importantly, common problems with HO may be offset in the future by osseointegration technology since ambulation will not require compressing a soft tissue socket against sharp osseous formations in the adjacent musculature.
While osseointegration procedures have been reported with relatively high success rates in Europe,23,29 one primary limitation preventing wide-spread acceptance of this technology is the required lengthy rehabilitation regimen following surgery (12– 18 months).55 The slow progression from post-operative to full weight-bearing is designed to prevent overloading at the bone–implant construct; however, this protracted restriction of activities may negatively impact a patient’s quality of life.
In order to alleviate these rehabilitation limitations, our team has developed an external electrical stimulation device which utilizes the exposed exoprosthetic attachment as a functional cathode.28 While controlled electrical stimulation has proven effective in fracture healing8 and non-traumatized bone models,15 this idea has not been expanded as a method for expediting skeletal fixation of percutaneous osseointegrated implants. The use of controlled electrical stimulation is believed to assist with calcium deposition, slight alterations in oxygen content and pH, recruitment of growth factors, assisting with osteoblast migration and secretion of additional extracellular matrix.28 Therefore, by limiting the current density and electric field between the osseointegrated implant and external electrodes, the team aims to stimulate positive bone remodeling at the periprosthetic interface using an osseoinductive pathway.4
Improving skeletal fixation of osseointegrated implants with controlled electrical stimulation may drastically reduce rehabilitation protocols for individuals with limb deficiencies. However, before employing this technique in human subjects, extensive scientific experimentation must be conducted to better understand the bioelectric properties and pathways through human residual limbs. For example, case reports of patient discomfort from excessive current densities in fracture healing applications are common31,39 and have caused unnecessary pain to participants. While meta-analyses have confirmed the overall utility of electrical stimulation,2 variations in experimental design, treatment time, dosage, and overall poor scientific understanding have significantly limited the expansion of this technology.22
Prior to implementing electrical stimulation as a rehabilitation tool for service members with limb loss using osseointegrated implants, safety and efficacy must be evaluated for a broad patient population; especially given the variations that exist in residual limb volume, geometry, and factors such as HO. Finite element analysis (FEA) offers a plausible experimental model for better understanding the bioelectric effects in the distal residual limb for an amputee seeking osseointegration technology. In addition, the variability of HO formation in subjects with limb deficiencies, provides robust simulations which may alter the electric field and current density distributions at the bone–implant construct. Therefore, the goals of this study were (1) to demonstrate that safe and effective electric stimulation of osseointegrated implants is possible even in patients with significant HO, (2) to show that patient-specific modeling and simulation is necessary to determine the relevant metrics for such stimulation, (3) to develop a quantitative method for determining the volume of HO, and (4) to characterize the prevalence, the extent, and the structure of HO in returning service members.
MATERIALS AND METHODS
Study Population
In order to account for variations in patient anatomy and HO in service member residual limbs, computed tomography (CT) scans were obtained retrospectively from Walter Reed Army Medical Center in accordance with Institutional Review Board approvals. CT scans were selected as the preferred imaging modality and femoral slice thicknesses ranged from 1.0 to 2.5 mm for all subjects included in the study. Inclusion criteria required the absence of metallic implants in the residual limb to prevent image artifacts during three dimensional reconstructions.
Eleven male service members satisfied the above criteria and were included in the study. Subjects were on average 28.3 ± 5.0 years at the time of injury, and 84.5 ± 11.3 kg and 181.2 ± 4.4 cm prior to injury. While age was routinely recorded for each subject, weight and height were reported in only 10/11 and 6/11 of the patient’s medical records respectively. The study population consisted of transfemoral amputations with an average limb length of 26.7 ± 6.1 cm, as measured from the apex of the greater trochantor to most distal bone in the residual limb using CT scans and computer software (OsiriX 3D, version 3.1). Ten (91%) subjects included in this study sustained a limb amputation as a result of a combat-related injury (9/11 OIF, 1/11 OEF), while one subject sustained a limb loss from a non-military conflict. The injury mechanism most frequently reported was IEDs which resulted in 82% of traumatic amputations (9/11), while 9% were the direct result of an RPG (1/11) and motor vehicle accident (1/11), respectively (Table 1).
TABLE 1.
Demographical information of the service member sample group.
| Patient | Age (yrs) | Injury Mechanism | Event | Admission height (cm) | Admission weight (kg) | Residual limb bone length (cm) | Volume of HO (cm3) |
|---|---|---|---|---|---|---|---|
| 1 | 27 | IED | OIF | 175.26 | 90.56 | 28.40 | 47.88 |
| 2 | 24 | IED | OIF | 177.80 | 90.72 | 30.00 | 74.25 |
| 3 | 22 | IED | OIF | 187.96 | 80.00 | 35.10 | 115.96 |
| 4 | 32 | IED | OIF | – | 90.00 | 25.90 | 0.00 |
| 5 | 30 | IED | OIF | – | – | 23.30 | 26.53 |
| 6 | 39 | RPG | OEF | 180.34 | 67.92 | 13.00 | 0.00 |
| 7 | 24 | IED | OIF | – | 106.50 | 31.50 | 12.75 |
| 8 | 28 | IED | OEF | 182.88 | 90.00 | 24.40 | 47.78 |
| 9 | 23 | IED | OIF | – | 74.00 | 21.20 | 77.43 |
| 10 | 31 | IED | OIF | – | 73.30 | 29.60 | 0.00 |
| 11 | 31 | MVA | NBI | 182.88 | 81.80 | 30.70 | 261.89 |
IED = improvised explosive device; RPG = rocket propelled grenade; NBI = non-battle injury; MVA = motor vehicle accident.
Image Reconstructions
The CT files collected during the study were imported into Seg3D (version 1.11.0, Scientific Computing and Imaging Institute, Salt Lake City, UT), a volume segmentation and processing tool, used to create anatomically accurate patient-specific models. The tissue boundaries of the internal organs, bone, bone marrow, and adipose tissue were generated by thresholding the CT files interactively using fixed thresholded values for all of the patients’ CTs.28 Musculature was obtained by manually setting seed points inside the tissue and using a confidence connected filter to find all the tissue connected to the seed points since the complex geometry required additional image processing.28 Because the skin was impossible to discern reliably from CT images, an estimate of the skin layer was generated by dilating the outermost visible tissue to produce a 2-mm layer of homogeneous thickness (the average thickness of human skin)54 to surround the full model. Skin was modeled with 0.26 S/m, a conductivity representative of hydrated skin, since the moisture content on the surface of the tissue will significantly alter electric fields and the related current densities at the bone–implant construct. Segmentations were lastly manually inspected, corrected to ensure accuracy, and combined in a hierarchy into a single label map required to generate meshes for FEA (Fig. 1).28
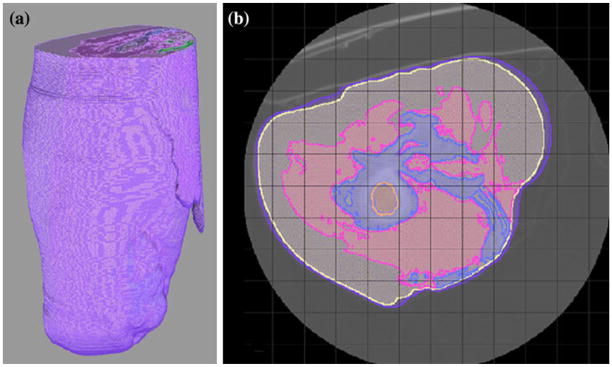
FIGURE 1.
A unilateral hierarchical model was assembled as a representative image consisting of skin (purple) adipose tissue (yellow), musculature (pink), bone (blue), bone marrow (orange), and internal organs (green) (a). Each tissue type was assigned a specific conductivity using SCIRun. A large serpentine-like mass of HO was identified in the medial aspect of the residual limb, and was demonstrated in more detail in an axial cross section of the affected limb (b).
The bioelectric effect of HO was evaluated by computing the volume of ectopic osseous overgrowth throughout the residual limb. Axial cross sections of CT scans were manually inspected and all “small islands”1 and “serpentine”45 HO formations were included. The entire volumetric data were collected using customized software that multiplied voxel height and width by the slice thickness to determine the volume of HO (Analyze 9.0, Mayo Clinic, OH). Three-dimensional reconstructions were created in OsiriX 3D to visualize the HO and were necessary to fully understand the intricate morphology of heterotopic bone45 (Figs. 2–5).
FIGURE 2.
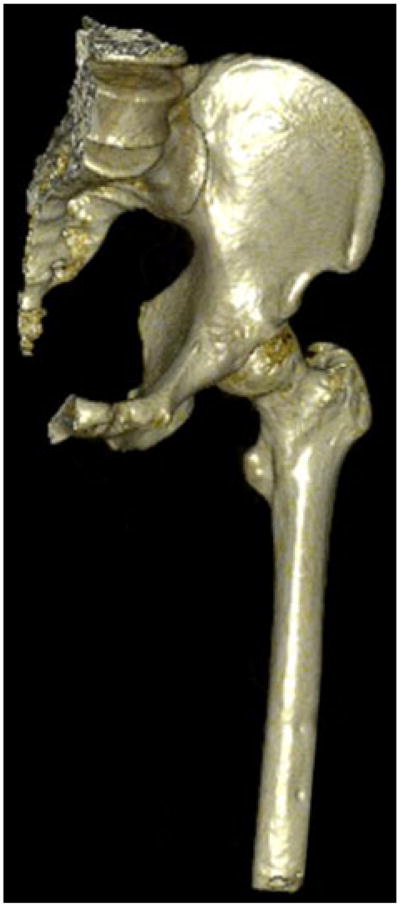
Three dimensional reconstruction of a service member with no visible signs of HO.
FIGURE 5.
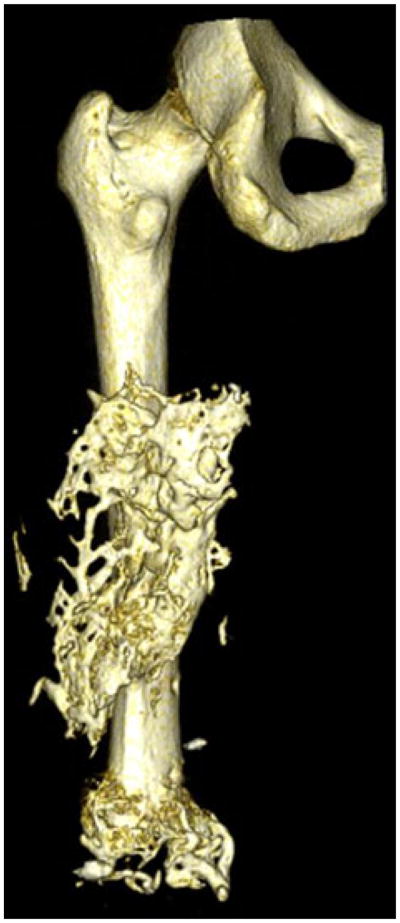
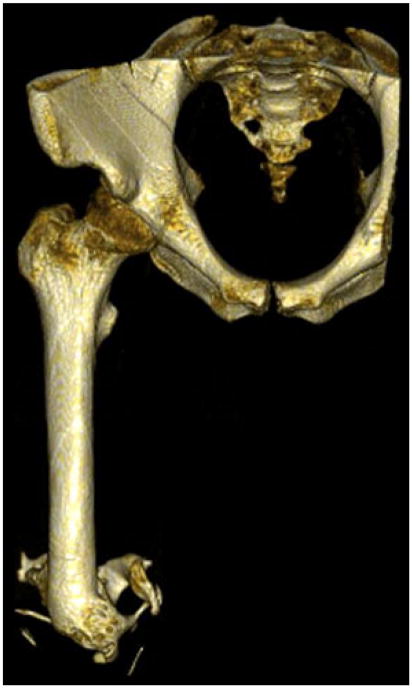
Three dimensional reconstruction of a service member with 115.96 cm3 of HO.
Finite Element Analysis
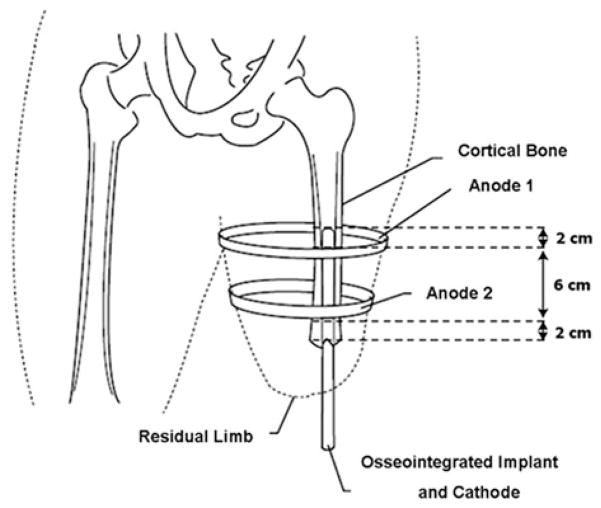
SCIRun, a problem-solving environment which included modules to carry out FEA for bioelectric field problems, provided support for electrode design and bioelectric evaluation (version 4.0, Scientific Computing and Imaging Institute, Salt Lake City, UT). The interactive platform allowed for real-time electrode manipulation and helped to assure reproducibility in the model. To make certain that electrodes were placed at the same location on each amputee, a 10-cm intermedullary device was simulated as the osseointegrated implant and set to the endosteal wall, since gaps in excess of 50 lm may lead to fibrous encapsulation without bone ingrowth.26 External electrodes, which consisted of two continuous bands, 1.6 cm in thickness, were placed equidistant from the implant site, and were designed to match the size of similar commercially available capacitive stimulation devices.7 The electrode bands were positioned 2 cm from the most proximal and distal ends of the osseointegrated implant on the residual limb because this experimental setup has previously demonstrated homogenous fields at the bone–implant construct (Fig. 6).28
FIGURE 6.
External electrode placement was standardized by placing 2 electrodes bands 2 cm from the most distal and proximal ends of the osseointegrated implant to create a homogenous electric field at the bone–implant construct.
The models generated from retrospective CTs consisted of a hexahedral mesh with approximately 1.8 million elements; a quantity determined to be sufficient following a mesh sensitivity study for subject 1’s residual limb which verified a less than 5% relative difference in voltage gradients between mesh densities. Elements in the model were treated as piecewise homogenous, ohmic, and isotropic, and were assigned conductivities using measurements reported from physiologic tissues28 and included the following: internal organs 0.22 S/m, skin 0.26 S/m, adipose tissue 0.09 S/m, muscle 0.25 S/m, cortical bone 0.02 S/m, and bone marrow 0.07 S/m. FEA was conducted using a quasi-static approach and by solving Laplace’s equation for each tissue type.28
In this model, the boundary conditions were formed by the electrodes that injected electrical current and the homogenous Neumann boundary condition on the limb’s surface forcing the electric current to remain in the limb, as electrical conductivity of the surrounding air is zero. Since the electrodes and the implant had a much larger conductivity than the surrounding tissues, it was assumed that the osseointegrated implant (cathode) was at a constant potential; similarly, the surface electrodes (anodes) were modeled with a constant potential difference from the percutaneous implant. Electrodes incorporated in the finite element meshing were assigned a constant potential difference from 1.0 to 2.0 V, in ¼ volt increments, a predetermined range selected to assure tissue integrity based on the expected tissue conductivities.
Outcome Criteria
The overall outcome measure of “optimal potential difference” was satisfied when current density and electric fields were at their highest attainable value within predetermined measures. Electric fields were restricted between 1 and 10 V/cm to prevent joule heating effects,53 while current densities were limited to 1.8 mA/cm2 to prevent localized tissue necrosis. The current density threshold was preset to 1.8 mA/cm2 to adhere to International Electrotechnical Commissions regulations that 2 mA/cm2 should not be exceeded in electrical devices designed for the general population.35 Maintaining a value below the standard of practice was also important in providing a factor of safety since fluctuations may occur in vivo due to variations in ion concentrations, temperature and hydration; variables which were not accounted for with this finite element model.
Statistical Evaluation
The volume of HO in each service members’ residual limb was compared to the optimal potential difference to determine whether ectopic bone growth correlated with the electric field and current density at the bone–implant interface. HO formation was also independently assessed to determine whether demographical information (age, height, weight, residual limb length) correlated with the volume of HO since inconsistencies have been presented in the orthopedic literature.6,21 All the statistical evaluations were performed by computing Spearman’s rho correlation coefficients and nonparametric statistical evaluations, given the limited sample size. In addition, in order to accurately associate the predictor and outcome measures, without introducing overfitting or having confounding variables, each factor was correlated independently. All the statistical comparisons were conducted with commercially available software and α = 0.05 (SPSS, Inc., Chicago, IL, USA).
RESULTS
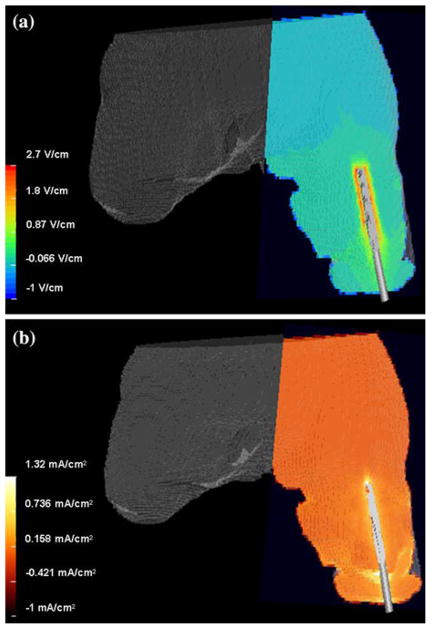
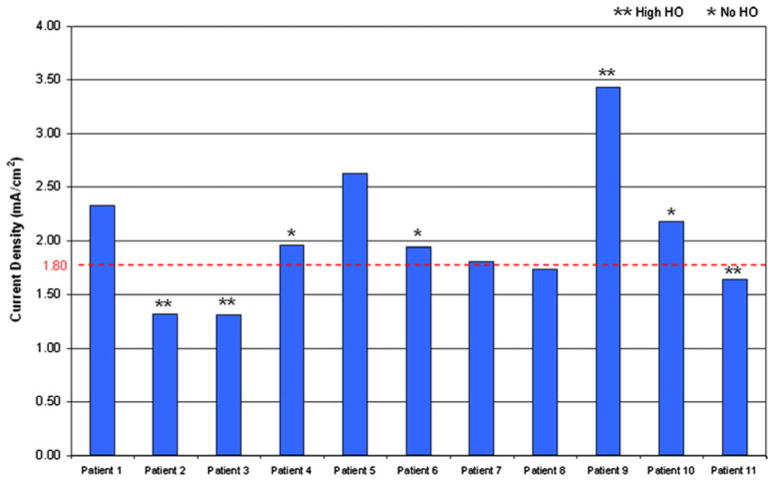
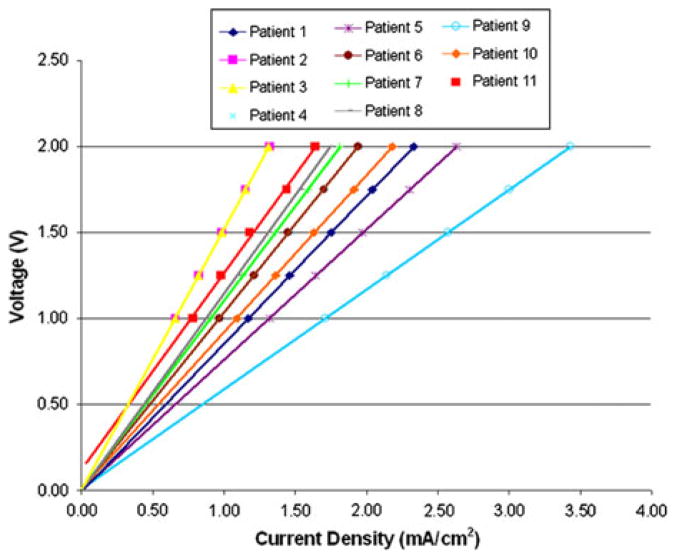
For all the reported cases, voltage gradients at the bone–implant interface were within the required range, and, therefore, the limiting factor for selecting the optimal potential difference for each service member was based on current density magnitudes (Fig. 7). Electric fields fluctuated from 1.30 to 3.10 V/cm for all the patients, a value which should theoretically induce osteoblast migration14 (Table 2). However, current densities ranged from 0.66 to 2.63 mA/cm2 for the potential differences selected and would require individual adjustments if this technology were to be implemented clinically (Fig. 8 and Table 3).
FIGURE 7.
Electric field (a) and current density (b) distributions for service member 2 using a potential difference of 2 volts.
TABLE 2.
Voltage gradients at the bone implant-interface given in units of V/cm.
| Patient | Potential difference
|
||||
|---|---|---|---|---|---|
| 1.00 V | 1.25 V | 1.50 V | 1.75 V | 2.00 V | |
| 1 | 1.3 | 1.6 | 2.0 | 2.3 | 2.6 |
| 2 | 1.4 | 1.7 | 2.1 | 2.4 | 2.7 |
| 3 | 1.4 | 1.7 | 2.1 | 2.4 | 2.8 |
| 4 | 1.5 | 1.9 | 2.3 | 2.6 | 3.0 |
| 5 | 1.5 | 1.9 | 2.3 | 2.7 | 3.1 |
| 6 | 1.3 | 1.6 | 1.9 | 2.2 | 2.5 |
| 7 | 1.4 | 1.7 | 2.1 | 2.4 | 2.8 |
| 8 | 1.4 | 1.8 | 2.1 | 2.5 | 2.8 |
| 9 | 1.4 | 1.7 | 2.1 | 2.4 | 2.8 |
| 10 | 1.5 | 1.9 | 2.3 | 2.7 | 3.1 |
| 11 | 1.4 | 1.7 | 2.1 | 2.4 | 2.7 |
FIGURE 8.
Current densities in the distal residual limb for the 2 volt potential difference are shown for each patient in the study. The critical threshold for current density (1.8 mA/cm2) is indicated by the horizontal dashed line.
TABLE 3.
Current densities at the bone-implant interface given in units of mA/cm2.
| Patient | Potential difference
|
||||
|---|---|---|---|---|---|
| 1.00 V | 1.25 V | 1.50 V | 1.75 V | 2.00 V | |
| 1 | 1.17 | 1.46 | 1.75a | 2.04 | 2.33 |
| 2 | 0.658 | 0.822 | 0.986 | 1.15 | 1.32a |
| 3 | 0.656 | 0.82 | 0.984 | 1.15 | 1.31a |
| 4 | 0.98 | 1.22 | 1.47 | 1.71a | 1.96 |
| 5 | 1.32 | 1.64a | 1.97 | 2.30 | 2.63 |
| 6 | 0.970 | 1.21 | 1.45 | 1.70a | 1.94 |
| 7 | 0.907 | 1.13 | 1.36 | 1.59a | 1.81 |
| 8 | 1.01 | 1.26 | 1.52 | 1.77a | 2.02 |
| 9 | 0.872 | 1.09 | 1.31 | 1.53 | 1.74a |
| 10 | 1.09 | 1.36 | 1.63a | 1.91 | 2.18 |
| 11 | 0.784 | 0.980 | 1.18 | 1.44 | 1.64a |
Signifies the recommended threshold for current density.
Investigation of the current densities at the periprosthetic interface demonstrated lower current density magnitudes when the volume of HO increased (Fig. 8). For each potential selected in Subjects 2, 3, and 11, current densities remained below the 1.8 mA/cm2 threshold. In each of these cases, a dense aggregation of HO was located at the anode site and resulted in more resistive medium at the point of current injection. This trend was consistent throughout the study and results of a Spearman’s rho correlation coefficient, assessing the relationship between the volume of HO and optimal potential difference, were statistically significant (p = 0.024, r = 0.670).
The volume of HO was also compared to demographic information provided in the subjects’ medical records to determine whether correlations existed between patient history and HO. While literature has speculated that the frequency of HO is dependent on genetic predispositions6 and body type, there is little evidence to directly support these claims. Our results indicated that only age was statistically significant (p = 0.041, r = −0.622) and that the volume of HO decreased with increasing age.
DISCUSSION
Ectopic bone formation presents a difficult challenge for rehabilitation and post-amputation quality of life. While HO can be detected early as indicated by redness, swelling in the periarticular regions, and increased alkaline phosphotase levels,27 few treatments are available to quell the metabolically active osseous growth.6 Non-steroidal anti-inflammatory drugs and irradiation treatments are available but are limited33 and present additional health risks to the patient. Compounding this problem is that the presence of significant HO within a residual limb may prohibit the use of a prosthesis. Therefore, electrically induced osseointegration offers a potential alternative to traditional prosthetic socket fitting, may promote bone remodeling, and avoid frequent complications with HO. It should be noted that it is unlikely that electrically induced osseointegration would increase HO formation in the residual limb, since HO develops from inflammation and trauma,6,13 and is not solely dependent on electrical signals. While electrical stimulation has demonstrated to be effective in augmenting nonunions, 8 the co-authors are unaware of case studies in the peer-reviewed literature that note the manifestation of HO from electrically induced fracture healing with direct current, inductive coupling, or capacitive-coupled devices.
In this experiment, FEA demonstrated that HO will significantly affect the bioelectricity in the residual limb, since larger volumes of HO required a higher potential difference to satisfy the electric field and current density criterion needed to accelerate bone healing using simulations. Therefore, effective use of electrical stimulation in this patient population would require altering the voltage in the system or modifying the band placements slightly to avoid resistive HO medium on a personal basis. The only exception noted in this trend was in service member 9 who had a smaller residual limb size, and a reduction in soft tissue may have offset the resistive effect of HO. While the admission height for this patient was not available for retrospective review, serviceman 9 was 11.5 kg below the average weight of the other subjects in the study and, therefore, may have also had associated decreased muscle mass or adipose tissue.
When assessing the correlation between age and HO frequency, our results indicate that higher volumes of HO were most prevalent in younger subjects. The inconsistent reporting of age-related ectopic bone formation has been subject to debate in the orthopedic literature,21,50 and a discrepancy exists since HO studies are often small, unrandomized, and lack control groups.34 A contributing factor in age-related HO may be the result of the decreasing proliferation of stem cells and progenitor cells, which occurs naturally with age48 since “skeletal tissue is a complex, multicellular, multifunctional system mutually interactive with and dependent on all other organ systems.”11 Evidence of the decline in stem cell regeneration is apparent in older individuals in which the cavities of long bones become vacant and bone marrow resides only in the pelvis, sternum, and vertebrae.41 The decreased bone-forming capacity of aged osteoblasts and reduced cell population with age11 may limit the likelihood of developing HO, but was not likely the case in our study. Because the patient population in this study consisted of a small sample of relatively young service members (28 ± 5.0 years), age was not likely to be a causative factor for HO formation. The statistical trend reported in the study was most likely the result of comorbidities, which are highly prevalent with blast injuries40 and may have contributed to the HO formation. In fact, previous peer-reviewed publications clearly demonstrate that the likelihood of developing HO significantly increases with head and spinal cord injuries, variables which were not assessed in the study. In order to confirm the hypothesis of age-related HO, a study must be organized with a more uniform patient population and a wider age range distribution to prevent confounding variables.
Innovation
In this sample population of injured service members, the frequency of HO occurred in 73%of the cases (8/11) and was variable in severity and location. The formation of HO resulted in “serpentine”45 structures which connected to the skeleton and “small islands”1 in the neighboring soft tissue. In order to help categorize the HO in a non-subjective manner, thresholding software provided volumetric measurements which assisted in determining the severity of HO in each residual limb. To the authors’ knowledge, this is the first grading criteria to directly quantify the volume of HO in patient extremities. Traditional methods for determining the magnitude of HO include: measuring the length of HO on anteroposterior radiographs,9 developing grading scales to group HO severity based on a percentage of occupied space around the affected area,13 or designing studies which subjectively include patients only displaying signs of decreased motion or pain.17 These techniques are very limited because only three dimensional reconstructions or direct volume measurements have the ability to completely demonstrate the intricate morphology of HO.45 In addition, grading criteria’s which group HO by percentages and classifications of mild, moderate, and severe19 tend to mislead the scientific community since readers assume that a higher value of HO (severe vs. mild) results in more pain or impaired movement for a patient, but this is not necessarily the case. A reduced activity level is often the result of the location of HO in relation to the periarticular region, neurovascular damage and individual patient pain tolerances.
Computational Modeling Limitations
Because our model used a quasi-static approach, the current density and electric fields in this experiment scaled linearly. Therefore, the direct relationship depicted in Fig. 9 demonstrated that the optimal potential difference may be determined for each patient by evaluating individual trend lines derived from the FEA. The ability to use a simple algorithm is important for improving periprosthetic attachment, however, fluctuations in temperature, hydration, and ion concentration will undoubtedly affect recordings in vivo. While computational modeling has a broad utility, FEA in this study only served to provide initial proof of concept for future device development.
FIGURE 9.
General trend lines for each service member selected in the study demonstrate that optimal potential difference may be selected using an algorithm on a patient-specific basis.
Study Limitations
While osseointegration is currently being practiced in Europe and Australia, this technology has yet to be expanded in the United States and will not be available until Food and Drug Administration’s (FDA) approval. Therefore, since our model cannot be validated until osseointegration technology is accepted as a standard of practice, further investigation will require a larger sample population to better understand the effect of HO in residual limbs using these extrapolations.
Prior to use clinically, electrically induced osseointegration will require further FEA refinements. For example, the tissue conductivities used in this experiment were fixed and treated as homogenous, ohmic, and isotropic and did not vary based on anatomical location, temperature, or hydration. While these basic model simplifications were effective for the proposed hypotheses and served as initial proof of concept for further investigation, it seems reasonable from a model perspective that interpersonal differences may have significantly affected the electric metrics at the bone–implant construct. Individual variances in tissues may arise particularly in returning service members because IED injuries generate a high quantity of scar tissue formation, and peer-reviewed literature has indicated that the hydration of scar tissue varies from that of normal physiologic tissue and would therefore, have a different localized conductivity.32
Direct comparisons which have resulted from this investigation may have also been influenced by slight variations in patient anatomy. Because the service members used in this study varied in residual limb size, the percutaneous osseointegrated implant was set to the endosteal wall to ensure uniform skeletal attachment prior to FEA. While this simplification ensured reproducibility with the host bone–implant contact, it did create another variable in itself, given the fluctuations in medullary canal diameters. Because our service members varied in height and weight, there is reason to believe that custom-fitting the prosthetic device to the residual limb may have slightly altered the diameter of the metal cathode. While there is reason to believe this did not introduce large variations in the data, given the much greater conductivity of the cathode than that of human tissue, a study by Mahaisavariya et al. did show that medullary canal diameters ranged from 10.3 mm at the greater trochantor to 11.8 mm in the metaphysis of 98 human cadaveric femurs (range 22–83 years).37
One limitation of the FEA research conducted in this study was the assumption that tissue was homogenous, ohmic, and isotropic. While it is well known that bone is a highly organized, anisotropic composite structure,49 this tissue type like the other five reconstructed in this experiment (bone marrow, adipose tissue, musculature, internal organs, and skin) were modeled with constant tissue properties to reduce experimental variations. Confounding errors are possible when assuming tissue fiber directions, since the dielectric constant and conductivity of tissue has not been well characterized, and it is very difficult to measure field strengths inside living organisms in vivo.38 While experimental calculations provide a range of expected field strengths, current densities can only be “crudely estimated” without the use of FEA.36 It is also well known that tissue conductivity may fluctuate in the same tissue or organ due to changes in fiber orientation,10 and bone, for example, has been reported as 100% more resistive in the circumferential direction compared to longitudinal direction.52
A final limitation of our study design resulted from using a sample population that consisted of all servicemen. Because the team was unable to obtain retrospective CTs of female subjects which satisfied the preexisting criterion, this investigation was unable to evaluate the effect of gender as a causation for HO development; an area of frequent debate in the literature. 1,21,50 While the authors are unable to comment on their own data at this point, it is very unlikely that HO formation is gender specific and more likely attributed to the size of the residual limbs. In general, males tend to have an increased volume of muscle and since osteoblast progenitor cells reside in the neighboring soft tissue,6,30 a greater volume of muscle mass may increase the likelihood of HO formation. Previous studies have indicated that HO formation generally occurs in tissues with high aggregations of fibroblasts1 and between 3 weeks to 6 months after injury.24,27 This commonality may be the result of overexpression of bone morphogenetic proteins30 which may directly increase alkaline phosphotase levels25 and contribute to HO development.
CONCLUSION
Osseointegration may offer significant improvements in prosthetic management of individuals with limb loss, especially those with complex residual limbs with HO and subsequent difficultly with socket fit and comfort. Controlled electrical stimulation may accelerate rehabilitation programs for osseointegrated implants once safety and efficacy are verified, but research has presently shown that current density must be controlled in the distal residual limb on a patientspecific basis using FEA. Statistical evaluations in this model demonstrated that the volume of HO compared to age and volume of HO compared to the optimal potential difference were significant. Therefore, if electrical stimulation is to be used in the future for individuals with percutaneous osseointegrated implants, careful electrode placement must be based on the volume of HO, age of the patient population, and comorbidities from blast injuries.
FIGURE 3.
Three dimensional reconstruction of a service member with 26.53 cm3 of HO.
FIGURE 4.
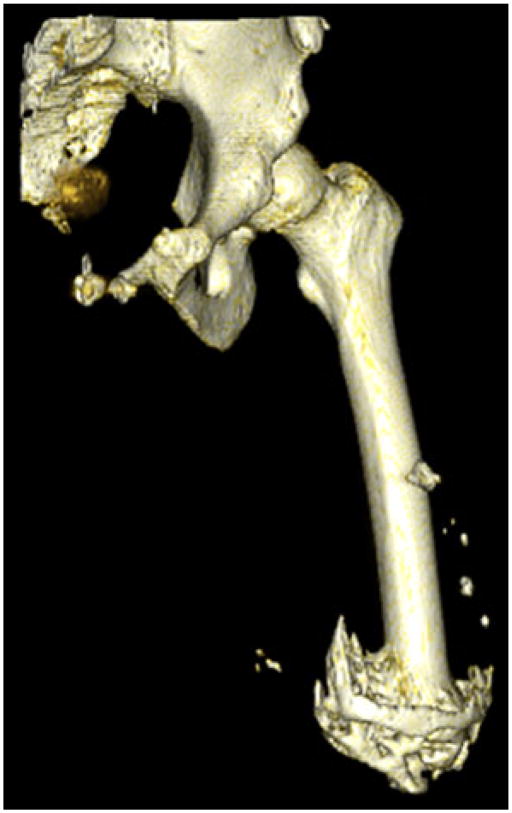
Three dimensional reconstruction of a service member with 74.25 cm3 of HO.
Acknowledgments
Gratitude is expressed to Gregory Stoddard for assistance and interpretation of statistical analysis, Darrell Swenson for support with image visualizations, and Gwenevere Shaw and Sharon Weeks for support with manuscript preparation. Grant & Financial Support: This material is based on study supported by the Veterans Affairs Office of Research and Development, Rehabilitation R&D Service, DVA SLC Health Care System, Salt Lake City, UT; the Albert & Margaret Hofmann Chair and the Department of Orthopaedics, University of Utah School ofMedicine, Salt Lake City, UT; the Technology Commercialization Office, University of Utah, Salt Lake City, UT. Technical support for the simulations was provided by the Center for Integrative Biomedical Computing of Scientific Computing and Imaging Institute, and was made possible in part by software from the NIH/NCRR Center for Integrative Biomedical Computing, P41-RR12553-07.
Footnotes
DISCLAIMER
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army, the Department of Defense, or the United States government.
References
- 1.Ahrengart L. Periarticular heterotopic ossification after total hip arthroplasty. Risk factors and consequences. Clin Orthop Relat Res. 1991;263:49–58. [PubMed] [Google Scholar]
- 2.Akai M, Hayashi K. Effect of electrical stimulation on musculoskeletal systems; a meta-analysis of controlled clinical trials. Bioelectromagnetics. 2002;23:132–143. doi: 10.1002/bem.106. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsson T, Albrektsson B. Osseointegration of bone implants. A review of an alternative mode of fixation. Acta Orthop Scand. 1987;58:567–577. doi: 10.3109/17453678709146401. [DOI] [PubMed] [Google Scholar]
- 4.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10(Suppl 2):S96–S101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen R, Davis S, Scoville C. Rehabilitation of military amputees: from injury to independence. Orthopedics. 2008;31:1000–1002. doi: 10.3928/01477447-20110525-05. [DOI] [PubMed] [Google Scholar]
- 6.Bayley SJ. Funnybones: a review of the problem of heterotopic bone formation. Orthop Rev. 1979;8:113–120. [Google Scholar]
- 7.Black J. Methods for Stimulating Tissues. Electrical Stimulation: Its Role in Growth, Repair and Remodeling of the Musculoskeletal System. Westport: Greenwood Press; 1986. pp. 92–97. [Google Scholar]
- 8.Brighton CT, Friedenberg ZB, Zemsky LM, Pollis PR. Direct-current stimulation of non-union and congenital pseudarthrosis. Exploration of its clinical application. J Bone Joint Surg Am. 1975;57:368–377. [PubMed] [Google Scholar]
- 9.Brumback RJ, Wells JD, Lakatos R, Poka A, Bathon GH, Burgess AR. Heterotopic ossification about the hip after intramedullary nailing for fractures of the femur. J Bone Joint Surg Am. 1990;72:1067–1073. [PubMed] [Google Scholar]
- 10.Crile GW, Hosmer HR, Rowland AF. The electrical conductivity of animal tissues under normal and pathological conditions. Am J Physiol. 1921;60:59–106. [Google Scholar]
- 11.Doll BA, Tegtmeier F, Koch H, Acarturk O, Hollinger JO. Evidence for a cellular and molecular decline in bone healing with age. Oper Tech Orthop. 2002;12:72–77. [Google Scholar]
- 12.Dudek NL, DeHaan MN, Marks MB. Bone overgrowth in the adult traumatic amputee. Am J Phys Med Rehabil. 2003;82:897–900. doi: 10.1097/01.PHM.0000087459.94599.2D. [DOI] [PubMed] [Google Scholar]
- 13.Errico TJ, Fetto JF, Waugh TR. Heterotopic ossification. Incidence and relation to trochanteric osteotomy in 100 total hip arthroplasties. Clin Orthop Relat Res. 1984;190:138–141. [PubMed] [Google Scholar]
- 14.Ferrier J, Ross SM, Kanehisa J, Aubin JE. Osteoclasts and osteoblasts migrate in opposite directions in response to a constant electrical field. J Cell Physiol. 1986;129:283–288. doi: 10.1002/jcp.1041290303. [DOI] [PubMed] [Google Scholar]
- 15.Friedenberg ZB, Zemsky LM, Pollis RP, Brighton CT. The response of non-traumatized bone to direct current. J Bone Joint Surg Am. 1974;56:1023–1030. [PubMed] [Google Scholar]
- 16.Furman R, Nicholas JJ, Jivoff L. Elevation of the serum alkaline phosphatase coincident with ectopic-bone formation in paraplegic patients. J Bone Joint Surg Am. 1970;52:1131–1137. [PubMed] [Google Scholar]
- 17.Garland DE, Blum CE, Waters RL. Periarticular heterotopic ossification in head-injured adults. Incidence and location. J Bone Joint Surg Am. 1980;62:1143–1146. [PubMed] [Google Scholar]
- 18.Garland DE, Hanscom DA, Keenan MA, Smith C, Moore T. Resection of heterotopic ossification in the adult with head trauma. J Bone Joint Surg Am. 1985;67:1261–1269. [PubMed] [Google Scholar]
- 19.Garland DE, Miller G. Fractures and dislocations about the hip in head-injured adults. Clin Orthop Relat Res. 1984;186:154–158. [PubMed] [Google Scholar]
- 20.Gaur A, Sinclair M, Caruso E, Peretti G, Zaleske D. Heterotopic ossification around the elbow following burns in children: results after excision. J Bone Joint Surg Am. 2003;85-A:1538–1543. doi: 10.2106/00004623-200308000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Goldman J. Heterotopic ossification in spinal cord injuries. Physiotherapy. 1980;66:219–220. [PubMed] [Google Scholar]
- 22.Haddad JB, Obolensky AG, Shinnick P. The biologic effects and the therapeutic mechanism of action of electric and electromagnetic field stimulation on bone and cartilage: new findings and a review of earlier work. J Altern Complement Med. 2007;13:485–490. doi: 10.1089/acm.2007.5270. [DOI] [PubMed] [Google Scholar]
- 23.Hagberg K, Branemark R, Gunterberg B, Rydevik B. Osseointegrated trans-femoral amputation prostheses: prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthet Orthot Int. 2008;32:29–41. doi: 10.1080/03093640701553922. [DOI] [PubMed] [Google Scholar]
- 24.Hierton C, Blomgren G, Lindgren U. Factors associated with heterotopic bone formation in cemented total hip prostheses. Acta Orthop Scand. 1983;54:698–702. doi: 10.3109/17453678308996614. [DOI] [PubMed] [Google Scholar]
- 25.Hirata K, Mizuno A, Yamaguchi A. Transplantation of skin fibroblasts expressing BMP-2 contributes to the healing of critical-sized bone defects. J Bone Miner Metab. 2007;25:6–11. doi: 10.1007/s00774-006-0721-0. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann AA, Bachus KN, Bloebaum RD. Comparative study of human cancellous bone remodeling to titanium and hydroxyapatite coated implants. J Arthroplasty. 1993;8:157–166. doi: 10.1016/s0883-5403(06)80056-2. [DOI] [PubMed] [Google Scholar]
- 27.Hsu JD, Sakimura I, Stauffer ES. Heterotopic ossification around the hip joint in spinal cord injured patients. Clin Orthop Relat Res. 1975;112:165–169. [PubMed] [Google Scholar]
- 28.Isaacson BM, Stinstra JG, MacLeod RS, Webster JB, Beck JP, Bloebaum RD. Bioelectric analyses of an osseointegrated intelligent implant design system for amputees. J Vis Exp. 2009;29:1–6. doi: 10.3791/1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isaacson BM, Vance RE, Rosenbaum Chou TG, Bloebaum RD, Bachus KN, Webster JB. The effectiveness of resonance frequency in predicting orthopedic implant strength and stability in an in vitro osseointegration model. J Rehabil Res Dev. 2009;46:1109–1120. doi: 10.1682/jrrd.2009.06.0080. [DOI] [PubMed] [Google Scholar]
- 30.Jackson WM, Aragon AB, Bulken-Hoover JD, Nesti LJ, Tuan RS. Putative heterotopic ossification progenitor cells derived from traumatized muscle. J Orthop Res. 2009;27:1–7. doi: 10.1002/jor.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorgensen TE. Electrical stimulation of human fracture healing by means of a slow pulsating, asymmetrical direct current. Clin Orthop Relat Res. 1977;124:124–127. [PubMed] [Google Scholar]
- 32.Karp FB, Bernotski NA, Valdes TI, Böhringer KF, Ratner BD. Foreign body response investigated with an implanted biosensor by in situ electrical impedance spectroscopy. IEEE Sens. 2008;8:104–112. [Google Scholar]
- 33.Kircher J, Martinek V, Mittelmeier W. Heterotopic ossification after minimally invasive rotator cuff repair. Arthroscopy. 2007;23:1359, e1351–1353. doi: 10.1016/j.arthro.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Kolbl O, Knelles D, Barthel T, Kraus U, Flentje M, Eulert J. Randomized trial comparing early postoperative irradiation vs. the use of nonsteroidal antiinflammatory drugs for prevention of heterotopic ossification following prosthetic total hip replacement. Int J Radiat Oncol Biol Phys. 1997;39:961–966. doi: 10.1016/s0360-3016(97)00496-3. [DOI] [PubMed] [Google Scholar]
- 35.Leitgeb N, Cech R, Schrottner J. Electromagnetic field spectral evaluation problems in exposure assessment. Radiat Prot Dosimetry. 2007;124:124–129. doi: 10.1093/rpd/ncm174. [DOI] [PubMed] [Google Scholar]
- 36.Liboff AR, Rinaldi RA, Lavine LS, Shamos MH. On electrical condution in living bone. Clin Orthop Relat Res. 1975;106:330–335. doi: 10.1097/00003086-197501000-00045. [DOI] [PubMed] [Google Scholar]
- 37.Mahaisavariya B, Sitthiseripratip K, Oris P, Chaichanasiri E, Suwanprateeb J. Fit-and-fill analysis of trochanteric gamma nail for the Thai proximal femur: a virtual simulation study. J Med Assoc Thai. 2004;87:1315– 1320. [PubMed] [Google Scholar]
- 38.Marino AA, Cullen JM, Reichmanis M, Becker RO, Hart FX. Sensitivity to change in electrical environment: a new bioelectric effect. Am J Physiol. 1980;239:R424–R427. doi: 10.1152/ajpregu.1980.239.5.R424. [DOI] [PubMed] [Google Scholar]
- 39.Minkin C, Poulton BR, Hoover WH. The effect of direct current on bone. Clin Orthop Relat Res. 1968;57:303– 309. [PubMed] [Google Scholar]
- 40.Pasquina PF. Optimizing care for combat amputees: experiences at Walter Reed Army Medical Center. J Rehabil Res Dev. 2004;41:vii–xii. doi: 10.1682/jrrd.2004.05.0051. [DOI] [PubMed] [Google Scholar]
- 41.Pearce D, Bonnet D. Ageing within the hematopoietic stem cell compartment. Mech Ageing Dev. 2009;130:54–57. doi: 10.1016/j.mad.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Pendegrass CJ, Goodship AE, Price JS, Blunn GW. Nature’s answer to breaching the skin barrier: an innovative development for amputees. J Anat. 2006;209:59–67. doi: 10.1111/j.1469-7580.2006.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierce RO, Jr, Kernek CB, Ambrose TA., II The plight of the traumatic amputee. Orthopedics. 1993;16:793–797. doi: 10.3928/0147-7447-19930701-08. [DOI] [PubMed] [Google Scholar]
- 44.Poolman RW. Adjunctive non-invasive ways of healing bone fractures. BMJ. 2009;338:b11. doi: 10.1136/bmj.b11. [DOI] [PubMed] [Google Scholar]
- 45.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski D. Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J Am Acad Orthop Surg. 2006;14:S191–S197. doi: 10.5435/00124635-200600001-00042. [DOI] [PubMed] [Google Scholar]
- 46.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–486. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- 47.Potter BK, Scoville CR. Amputation is not isolated: an overview of the US Army Amputee Patient Care Program and associated amputee injuries. J Am Acad Orthop Surg. 2006;14:S188–S190. doi: 10.5435/00124635-200600001-00041. [DOI] [PubMed] [Google Scholar]
- 48.Quarto R, Thomas D, Liang CT. Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Int. 1995;56:123–129. doi: 10.1007/BF00296343. [DOI] [PubMed] [Google Scholar]
- 49.Reilly DT, Burstein AH. The mechanical properties of cortical bone. J Bone Joint Surg [Am] 1974;56-A:1001–1022. [PubMed] [Google Scholar]
- 50.Riegler HF, Harris CM. Heterotopic bone formation after total hip arthroplasty. Clin Orthop Relat Res. 1976;117:209–216. [PubMed] [Google Scholar]
- 51.Salawu A, Middleton C, Gilbertson A, Kodavali K, Neumann V. Stump ulcers and continued prosthetic limb use. Prosthet Orthot Int. 2006;30:279–285. doi: 10.1080/03093640600836139. [DOI] [PubMed] [Google Scholar]
- 52.Singh S, Saha S. Electrical properties of bone. A review. Clin Orthop Relat Res. 1984;186:249–271. [PubMed] [Google Scholar]
- 53.Soong HK, Parkinson WC, Bafna S, Sulik GL, Huang SC. Movements of cultured corneal epithelial cells and stromal fibroblasts in electric fields. Invest Ophthalmol Vis Sci. 1990;31:2278–2282. [PubMed] [Google Scholar]
- 54.Tortora GJ, Nielsen MT. Structure of the skin. In: Roesch B, Trost K, Wojcik L, Muriello L, Raccuia L, editors. Principles of Human Anatomy. Hoboken, NJ: John Wiley & Sons, Inc; 2009. p. 117. [Google Scholar]
- 55.Ward DA, Robinson KP. Osseointegration for the skeletal fixation of limb prostheses in amputations at the trans-femoral level. In: Brånemark PI, Chien S, Gröndahl HG, Robinson K, editors. The Osseointegration Book from Calvarium to Calcaneus. Berlin, Germany: Quintessenz Verlags-GmbH; 2005. pp. 463–476. [Google Scholar]
- 56.Wharton GW. Heterotopic ossification. Clin Orthop Relat Res. 1975;112:142–149. [PubMed] [Google Scholar]