Abstract
Sensory dendrites fall into many different morphological and functional classes. Polymodal nociceptors are one subclass of sensory neurons, which are of particular note due to their elaborate dendritic arbors. Complex developmental programs are required to form these arbors, and there is striking conservation of morphology, function, and molecular determinants between vertebrate and invertebrate polymodal nociceptors. Based on these studies, we argue that arbor morphology plays an important role in the function of polymodal nociceptors. Similar associations between form and function may explain the plethora of dendrite morphologies seen among all sensory neurons.
Introduction
Pioneering work by Ramón y Cajal revealed that neurons adopt many diverse morphological forms [1]. This diversity is clearly demonstrated by the diversity of dendritic arbors (Glossary) found in neurons of the central nervous system (CNS), and by the many forms of peripheral sensory dendritic arbors. In both cases, these dendritic arbors receive multiple inputs, and in the case of peripheral sensory neurons, their arbors physically define their sensory fields.
The range of signals perceived by a dendritic arbor is principally determined by the channels and receptors that it expresses. But if input collection is the sole function of these arbors, then an arbor’s function will depend only on the gross size of the area covered by it and on the density and type of receptors expressed on it. In contrast, we argue that the precise morphological form of sensory arbors also contributes to the ability of sensory neurons to perceive external stimuli. Indeed, it was previously suggested that the exact form of the dendritic arbor can affect summation and propagation of synaptic stimuli perceived by distal dendrites at the site of action potential initiation, and therefore can control the transmission efficacy of each stimulus [2]. However, the relationship between dendritic-arbor-form and signal propagation is still unclear. On the one hand, voltage-gated ion channels in CNS dendrites can function to dampen propagation effects [3]. On the other hand, recent evidence suggests a role for dendritic arbors in computing synaptic signals (reviewed in [4]). In any case, the sheer diversity and complexity of sensory dendritic arbors and the tight regulation of their development (reviewed in [5]) suggest that we should look for a link between form and function of these arbors.
Indeed, detailed analysis of GFP reporters for the DES-2 protein or for the F49H12.4 promoter expressed in PVD demonstrated a complex, but well-ordered, array of dendrites that formed repeating “menorah-like” structures covering the animal’s body [7,9] (Figs 1 and 2). In addition, electron microscopic analyses have shown that the terminal (quaternary) branches are as thin as 30–60nm, less than half the diameter of any other known neurite [10]. These fine branches are exceptionally wispy, lying within a very thin hypodermal sheet and showing no prominent cytoskeletal elements inside, or extracellular matrix (ECM)-derived structures outside, as well as no periodic attachments to surrounding tissues [10] (Figure 2). By comparison, the dendrites of the well characterized touch receptor neurons (TRN) (Table 2) of C. elegans contain prominent microtubule bundles and are deeply invested into a thick epidermal layer [22,23]. Furthermore, the TRN neurons display a thickened basement membrane (mantle) and periodic electron-dense attachments to anchor themselves to the outer cuticle (Table 2) [23].
Figure 1. Organization and development of PVD and FLP arbors in C.elegans.
Schematic diagrams of PVD (blue) and FLP (red) processes as they are seen at 5 different stages of development [late larval stage 2 (late L2) to late L4/ young adult]. Dashed lines (in black) indicate processes that appeared earlier in development but have retracted by this stage. All views are from the left aspect showing only the left side cells of each cell pair; anterior is to the left. For convenience, all stages are shown at constant size. A single axon emerges ventralward from the cell body (colored ball) before going rostrally within the ventral cord. The process(es) emerging laterally from the cell body elaborate into highly branched dendrites. Note that knowledge of FLP development is meager compared to that of PVD, but available data suggest that FLP dendrites undergo the same sequence of events, and are likely obey the same rules.
Figure 2. Proposed mechanism linking organization of PVD branches to mechanosensation in C.elegans.
A. Schematic of an adult C. elegans midbody showing three ventral and three dorsal menorah-like structures growing out of the PVD primary dendrite (dark blue). Movement of a single menorah upon pressure application by a sharp needle is indicated (white lines indicate position of the central dorsal menorah before pressure application). Transverse cross-section on the left edge of the diagram indicates positions of the four muscle quadrants (green) and hypodermis (beige) in the bodywall. The cuticle and thin layer of hypodermis are peeled back in part of the diagram to reveal the PVD quaternary dendrites (but quaternary dendrites are really within the hypodermis). One dorsal menorah on the left has been numbered, to indicate the temporal stage of branch development [ie. primary branches appeared first (1), followed by secondary (2) etc.]. On the right of the diagram, the overlying structures are shown: the outer cuticle layer (grey) and cuticle annuli (grey lines).
B. PVD quaternary dendrite enmeshed within the thin hypodermal layer (beige) in a transmission electron microscopic (TEM) image [colors have been superimposed on the image for clarity: PVD, blue; M (bodywall muscle), green; H (hypodermis), yellow]. Adapted, with permission, from [10].
C. Enlarged schematic showing how stretching of affected processes may lead to local DEG/ENaC channel opening. Both the position and likely activation state of these channels are indicated (filled circle indicates fully open, filled ellipse partly open, and thin line is closed).
Table 2.
Mechanosensory neurons in the C. elegans hermaphrodite.
| Cell name | Morphological features | Molecular sensor | Refs |
|---|---|---|---|
| ADEa | Cilium | [94] | |
| ALM, AVM, PLM, PVM (TRNs) | Large diameter microtubules, ECM derived mantle, and periodic dense plaques. | MEC-4, MEC-10, and DEGT-1 (DEG/ENaC) | [8,23,58,69], |
| ASH | Cilium | OSM-9 and OCR-2 (TRP)b | [17,38,39] |
| CEP | Cilium | TRP | [66,94] |
| DVA | Unspecialized neurite | TRP | [95] |
| FLP | Cilium and multiple branched dendrites | MEC-10 (DEG/ENaC) | [12,17] |
| IL1 | Cilium | [17] | |
| OLQ | Cillum | TRPA-1 and OSM-9 (TRP) | [12,17,96] |
| PDE | Cilium | [94] | |
| PVD | Multiple branched dendrites | MEC-10 and DEGT-1 (DEG/ENaC) | [8,10] |
C. elegans neurons have three letter names indicating characteristics of their soma and/or processes. ADE- Anterior DEirid, AFD- Amphid Filamentous D, ALM- Anterior Lateral Microtubule-filled, AVM- Anterior Ventral Microtubule-filled, PLM- Posterior Lateral Microtubule-filled, PVM- Posterior Ventral Microtubule-filled, IL1- Inner Labial 1, OLQ- Outer Labial Quadrant, PDE-Posterior DEirid.
In ASH, the TRP subunits [OSM-9 (OSMotic avoidance abnormal) and OCR-2 (Osm-9 and Capcaisin receptor-Related)] subunits are needed for all sensory modalities, and thus, may function downstream of stimulus reception.
Drosophila larvae, like C. elegans, provide an experimentally accessible system for the analysis of neuronal form, function, and development. Sensory dendrites innervating the larval surface are conveniently organized in two dimensions beneath a transparent skin [24]. Anatomical studies have classified these neurons according to whether their dendrites have, or lack, encapsulation. Free endings, lacking encapsulation, have been further classified according to their degree of arborization, leading to four classes of dendritic arborization (DA) neurons [25]. Functional analysis of these DA neuron classes shows that class IV (DA-IV) neurons, the most highly branched class, are polymodal nociceptors, while class I neurons, the least branched class, function as proprioceptors [26,27] (Table 1 and Fig 3a). Specifically, DA-IV neurons are responsible for the sensation of noxious heat, high-threshold mechanical stimuli, parasitoid wasps, and noxious light [13,14,27,28]. Moreover, similar to mammalian polymodal nociceptors, their sensitivity to heat can be modulated by signals caused by tissue damage [15].
Table 1.
Function and morphology of nociceptors discussed in this review
| Organism | Cell type | Modalities | Multi-dendritic | Tilingc |
|---|---|---|---|---|
| C. elegans | PVD | Mechanical, thermal | Yes | Yes |
| FLP | Mechanical, thermal | Yes | Yes | |
| ASH | Mechanical, chemical | No | NA | |
| Drosophila | DA-IV | Mechanical, thermal, light, chemical (cytotkines) | Yes | Yes |
| Mammalsa | C-fibers | Mechanical, thermal, chemical | Yes | No |
| Aδ-fibers | Mechanical, thermal | Yes | ? | |
| Hirudo medicinalis | N- Cells | Mechanical, thermal, chemical | Yes b | No |
| P-Cells | Mechanical (pressure) | Yes | No |
Mammalian nociceptors are heterogeneous. Both C-fibers and Aδ-fibers include multiple types of neurons having different functions, properties, and molecular markers
N-cells appear to be multi-dendritic, however, detailed analysis of their morphology is currently lacking
Abbreviations: NA, data not available.
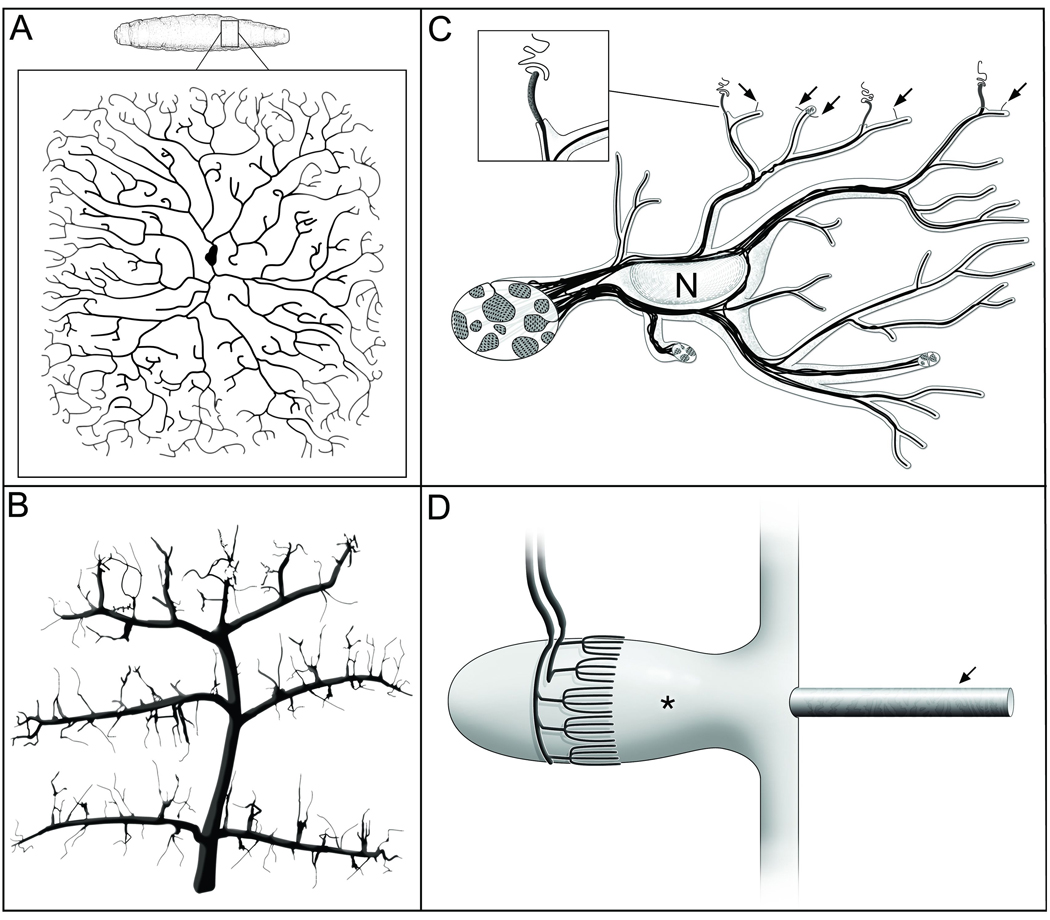
Figure 3. Multi-dendritic polymodal nociceptors and mechanosensors from different organisms.
A. Drosophila DA-IV neurons. The skin of the fly third instar larva is tiled with dendritic arbors that cover large receptive fields; the many free endings of each cell show self-avoidance. Redrawn with permission, based on immunohistochemical images from [25].
B. Leech P-cell. Pressure receptors in leech skin have distinctive multi-branched arbors with very fine endings that show self-avoidance (adapted, with permission, based on dye-filling images in [37]). Their receptive fields cover large portions of the leech skin and are known to substantially overlap circumferentially (i.e. no tiling).
C. Penicillate neuron of human skin. Nociceptors form highly branched dendrites ensheathed by a Schwann cell and its processes, except for short free endings at their termini (gray process shown in inset) that insert themselves at the borders between epidermal cells (wiggly line extending from the ending) or by invagination into a subepidermal cell. Short terminal branches of the neuron run within peripheral branches of the Schwann cell, and anchor to the epidermal basal lamina (arrows). These neurons are unmeylinated and belong to the C-fiber neuron class. N, Schwann cell nucleus. Based on schematic diagram in [30].
D. Palisade neuron of mammalian fur. Mechanosensitive dendrites end in candelabra-like arbors. These arbors show self-avoidance, and perhaps tiling, and wrap around the base of a vibrissal hair follicle (indicated by *). A single protruding hair is indicated by an arrow. Adapted, with permission, from [60].
In mammals, somatosensory neurons innervating the skin have sensory endings that are either encapsulated (known as Ruffini’s end organ, Meissner’s corpuscle or Merkel disk) or free [1]. Neurons having free sensory endings are a heterogeneous group whose morphology is difficult to characterize. Currently, sensory neurons innervating the skin are classified using several features: morphology, conductance, sensory modalities, and molecular markers. Encapsulated sensory endings are associated with fast conducting fibers (ie. Aβ) and are low threshold mechanoreceptors. In contrast, sensory endings lacking encapsulation (ie. free endings) are associated with slow conducting fibers that are poorly myelinated (Aδ-fibers) or unmyelinated (C-fibers) (Table 1 and Fig 3). These free endings mostly sense noxious signals and function as polymodal nociceptors. For example, many Aδ neurons sense sharp pressure and cold, while many C-fibers respond to heat, high threshold mechanical stimuli, and chemical stimuli (reviewed in [6,29]). Interestingly, neurons having “free” endings are mostly multi-dendritic [30–34], although it should be noted that naming sensory arbors of these neurons “multi-dendritic” may be confusing since the peripheral (sensory) branch of these neurons conducts action potentials, and therefore is sometimes considered an axon rather than a dendrite.
Taken together, morphological analysis of polymodal nociceptors in vertebrates and invertebrates highlights two shared morphological features: free endings lacking encapsulation and a multi-dendritic arbor. Further support for conservation of these features in evolution comes from another model organism, the leech Hirudo medicinalis, whose polymodal nociceptors (N-cells) also have a multi-dendritic arbor lacking encapsulation (Table 1) [35,36]. Unfortunately, details on the morphology of N-cells are poorly defined, possibly due to the small caliber of their terminal dendrites [35]. Detailed morphological data is, however, available for leech P-cells (Table 1). These neurons are responsible for sensation of pressure and have well ordered multi-dendritic arbors (Fig 3b) [37]. It is important to point-out one well-characterized exception to this conservation, the C. elegans ASH (Amphid Single-cillium H) neurons. These neurons are polymodal, sensing noxious mechanical and chemical stimuli applied to the nose, but unlike other polymodal nociceptors are not multi-dendritic [17,38–40] (Tables 1 and 2).
Development of multi-dendritic sensory arbors in C. elegans and Drosophila
PVD development in C. elegans involves the progressive addition of more branches, starting at the second larval stage and ending late in the fourth larval stage, the last stage of larval development [7,9,11] (Fig 1). Development of PVD branches is temporally ordered: primary branches appear first, secondaries and tertiaries later, and quaternaries appear last (see Fig 2 for branch numberings). Branch formation is dynamic and shows self-avoidance: both secondary and quaternary branches grow and retract to finally form a regularly spaced array of parallel branches, and growing tertiary branches retract their ends to avoid contact with tertiary branches from neighboring menorahs [9]. Thus PVD development is a dynamic process that is likely to depend on mechanisms of self-avoidance and competition to form a well-ordered and regularly spaced array of non-overlapping branches [9] (Fig 1).
PVD development is likely to depend on both extrinsic and intrinsic factors. External guideposts for PVD development may include processes of adjacent neurons, the overlying seam cells, the borders of muscle quadrants, and/or ECM components [9,10]. Important internal factors may include multiple transcription factors, signaling molecules, cytoskeletal proteins, and regulators of cytoskeleton dynamics [7,9,11]. Three molecules have been studied in detail. MEC-3 (MEChanosensory abnormality) is a LIM homeodomain transcription factor required for proper development of several mechanosensory neurons and is needed for appearance of PVD side branches [9,16,21,41]. Loss of the EFF-1 (Epithelial Fusion Failure) fusogen leads to ectopic branching of PVD processes and to considerable disorganization these processes [7], while BICD-1 (BICaudal D), a dynein accessory factor, is known to regulate the number and position of PVD branches [11].
Detailed analyses of the development of Drosophila DA neurons have led to the identification and characterization of many more regulators of dendritic arbor morphology. These include transcription factors [42–44], molecules involved in vesicle trafficking [45–47], components of signaling pathways [48–50], cell adhesion proteins [51–53], regulators of the actin cytoskeleton, and regulators of RNA trafficking and translation [54]. This large array of molecules and diverse cellular pathways regulates many different aspects of dendritic development: the number of dendrites, their position, their morphology, their length, self-avoidance between dendrites of the same neuron, tiling of neighboring arbors belonging to neurons of the same class, and continued maintenance of dendrites.
Overall, recent studies demonstrate that both Drosophila and C. elegans have well-ordered and molecularly complex developmental programs responsible for formation of the multi-dendritic sensory arbors of polymodal nociceptors. This tight regulation of arbor morphology suggests that the exact shape of the sensory arbor is important for functional reasons. Indeed, differences in arborization patterns of different DA neuron classes are also tightly regulated at the molecular level [42–44]. Specifically, the transcription factors Knot/Collier and Cut control dendrite numbers and dendrite length to regulate class specific arborization patterns in Drosophila DA neurons [42–44]. Therefore, we suggest that the morphology of sensory arbors is tightly regulated in order to enable their function. Although much less is known about the molecular details governing the development of sensory neurons in leech, evidence for well-ordered sensory arbors and for active self-avoidance has been reported in the leech Haementeria ghilianii [55,56].
Molecules and morphological features enabling mechanosensation
Early analysis of PVD function showed that, together with TRNs, they mediate the response to high-threshold prodding to the midbody [16]. Further analysis showed increased Ca2+ levels inside PVD neurons following strong mechanical stimuli [8]. MEC-10 and DEGT-1 (DEG/ENaC involved in Touch), both DEG/ENaC subunits, were identified as likely subunits of the PVD mechanosensory channel, and were found to colocalize to puncta on the primary and tertiary branches of PVD [8] (Fig 2).
The Drosophila DA-IV neurons also respond to high threshold mechanical stimuli [13], and like PVD neurons, depend on a DEG/ENaC subunit, Pickpocket, for this function [14]. Similar to MEC-10 and DEGT-1, Pickpocket was demonstrated to be localized to puncta on the dendritic arbor of sensory neurons [57]. It is important to note that heterologous expression of DEG/ENaC subunits, which have been suggested to function as mechanosensors, has not yet demonstrated their gating by mechanical stimuli [57,58]. Thus, gating of these channels by mechanical stimuli is likely to require accessory structures and proteins that are absent in heterologous expression systems.
Involvement of DEG/ENaC channels in mechanosensation has also been suggested for mammalian low-threshold and high-threshold mechanoreceptors [59,60]. However, genetic knockout studies are inconsistent with a central role for this family of receptors in mammalian mechanosensation, possibly due to molecular redundancy [61,62]. Indeed, a recent study showed that the Piezo-2 protein is responsible for a subset of mechanically activated currents in mammalian mechanosensors [63]. Piezo-2, a novel multipass membrane protein, is expressed in mammalian mechanosensory neurons, including some polymodal nociceptors [63]. Members of the Piezo family are found across the animal kingdom, including C. elegans and Drosophila [63], however their function in invertebrates is yet unknown.
Mechanosensory neurons are mostly associated with specialized ECM and/or cytoskeletal structures, which have been suggested to enable their function (reviewed in [64,65]). Specifically, C. elegans has multiple mechanosensory neurons; these neurons, with the exception of DVA (DorsoVentral A) and PVD, contain cilia or specialized large diameter microtubule bundles (Table 2) [40]. Deflection of the single cilium in the CEP (CEPhalic cilium) neuron by pressure application was observed to produce ion currents in this neuron, consistent with cilium’s suggested role in enabling mechanosensation [66]. Similarly, MEC-7, which encodes a microtubule subunit specific to TRNs, has been suggested to be involved in mechanosensation. Mutations in mec-7 resulted in the elimination of TRN-specific 15-protofilament microtubules [67]. These microtubules are attached to the membrane of TRNs at multiple sites and were suggested to gate the mechanosensory channel [23,68]. However, the membrane-attached ends of these microtubules do not co-localize with the mechanosensory channels, as measured by coimmunofluorescent and immuno-electron microscopy (EM) studies, nor does elimination of MEC-7 abolish all mechanically activated currents [68,69]. Such findings suggest that specialized microtubules are unlikely to directly gate mechanosensory channels, nor are they absolutely necessary for activation of these channels. Nevertheless, elimination of MEC-7 leads to greatly diminished responses to mechanical stimuli, and to a significantly higher threshold for activation of these responses, demonstrating the importance of MEC-7 for mechanosensation [69]. MEC-5, which encodes a collagen needed for mechanosensation [70], has also been found to not directly co-localize with the mechanosensory channel [68]. Thus, direct gating via microtubules or the ECM appears unlikely. Instead, it has been suggested that mechanical force applied to TRNs is transmitted via the microtubule bundles onto the membrane leading to local membrane stretch at the site of microtubule attachment sites, and thus, to stretch-activated channel opening [68].
PVD neurons, unlike other mechanosensitive neurons, do not contain cilia or other specialized microtubules, and are not encapsulated. Instead, we suggest that the morphology of PVD neurons and the organization of their processes serve as an alternative means for directing stretch forces onto the mechanosensory channels. Specifically, quaternary branches are embedded in a thin hypodermal layer between muscles and the animal’s outer envelope [10] (Fig 2). This position serves to anchor the quaternary dendrites between two relatively strong rigid structures, the muscle sarcomeres and the cuticle. In light of this, large displacement of the animal’s outer envelope by strong mechanical stimuli is likely to displace one quaternary branch relative to its neighbor, thereby stretching the tertiary processes linking one quaternary to its neighbor (Figure 2). By extension, it is also possible that such a displacement displaces one menorah relative to its neighbor, thereby stretching the primary branches linking one menorah to its neighbor. Importantly, the two subunits of the PVD mechanosensory channel are not found on its terminal (ie. quaternary) branches. Therefore, these branches are unlikely to function as mechanosensors themselves. Indeed, our proposed model suggests that these dendrites only serve as anchors whose displacement by local pressure transmits pulling forces onto tertiary and primary branches. Moreover, subunits of the mechanosensory channel are found in puncta on the primary and tertiary branches [8], and are thus in the right position to sense stretching of these processes (Fig 2). Additional support for our model comes from the analysis of worms lacking eff-1, a protein essential for cell fusion. Such mutants were observed to have highly disorganized PVD branches, and were also defective for mechanosensation [7]. Further support for this model requires similar analyses of the many other mutations known to affect PVD morphology, such as BICD-1 [11] and others [9].
Interestingly, studies of mammalian high-threshold mechanoreceptors are consistent with a mechanism whereby forces applied to external rigid structures result in one branch of a high threshold mechanoreceptor being pulled relative to other branches. Specifically, high threshold mechanoreceptors in joints are embedded within dense collagenous tissues, such as fibrous capsules or ligaments, and in the skin they are tethered to the basal lamina of the epidermis via collagen fibers [30,32](Fig 3c). By analogy, we propose that (low or high threshold) mechanosensation in other multi-dendritic neurons can also be achieved by local forces causing independent motions of branching dendrites spanning a large area, where these dendrites are independently embedded within, or tethered to, rigid structures. For instance, leech Hirudo medicinalis P-cells have many evenly spaced terminal dendrites that are embedded inbetween muscle layers [37]. Instances of low threshold mechanosensors that are multi-dendritic include T-cells in leech, DA class I neurons in Drosophila, and Palisade neurons in mammals (Fig 3d)[26,27,71,72].
Multi-dendritic neurons and temperature sensation
PVD neurons, like multi-dendritic sensory neurons in Drosophila, leech, and mammals, respond to both high-threshold mechanical stimuli and to noxious temperatures [8,13,36,73]. Cold sensation in PVD neurons has been determined to be mediated by TRPA-1 (Transient Receptor Potential Ankyrin), a member of the TRP (Transient Receptor Potential) channel family previously suggested to function in cold sensation in other species [8,74]. Mutation of TRPA-1 does not affect the responses of PVD neurons to mechanical stimuli [8],and expression of this channel in other neurons (ie. FLP and ALM), or in mammalian cells, also enables a response to cold [8].
The role of TRP channels in thermosensation, like the role of DEG/ENaC channels in mechanosensation, has been evolutionarily conserved. Specifically, thermoTRPs belonging to the TRPA, TRPM, and TRPV branches of the TRP ion-channel family have been shown to function in temperature sensation in Drosophila (Painless is a TRPA homolog) [13] and in mammalian polymodal nociceptors [75–79]. However, the precise role of thermo-TRPs in mammalian nociception, and particularly of TRPA1 in the sensation of noxious cold, is under debate [80–82]. One perplexing issue is that gating of thermoTRPs by temperature seems to be different in heterologous systems compared to the in vivo situation. Heterologous expression studies show that thermoTRPs respond to temperature, suggesting that they are directly gated by temperature [8,74–76] In vivo, chemical signals produced by inflammation or tissue damage facilitate temperature-dependent activation of thermoTRPs, and may lower the threshold for their activation by hot or cold. Indeed, knockout of TRPV1 in mice has been shown to eliminate inflammatory heat hyperalgesia, but does not eliminate nociceptor heat sensation; defects in the response to heat are only seen at temperatures far exceeding the threshold for activation of this receptor [75,83]. Multiple lines of evidence suggest roles for chemical signals in temperature sensation by TRP channels. Firstly, TRPA1 has been shown to function downstream of a G-protein coupled receptor (GPCR) and phospholipase C (PLC) cascade in Drosophila temperature discrimination [84]. Secondly, heat-dependent release of lipid metabolites from skin has been shown to be sufficient for TRPV activation [85]. Thirdly, agonist application is needed for significant cold-activated responses of TRPA [86], and lastly, the neuroactive steroid pregnenolone sulfate synergizes with heat to activate TRPM3 [79].
The role of PVD’s complex arbor in its ability to sense and respond to temperature remains unclear. Two facts argue against a role of PVD’s form in cold sensation. First, TRPA-1 also functions as a cold-sensor when expressed in a heterologous system [ie. human embryonic kidney (HEK) cells] [8] and in ALM neurons [8], which have simple unbranched sensory processes. Second, TRPA-1 is apparently localized to the cell body and not to sensory dendrites [8]. However, it is possible that the inability to detect GFP-tagged TRPA-1 in sensory dendrites of PVD was due to low expression levels, as other TRPA-1 homologs have been detected in sensory dendrites [13,87]. It is also notable that other known thermosensors are either multi-dendritic or have numerous villi (eg. AFD in C. elegans) [13,32,88]. Thus, one possibility is that a high surface-to-volume ratio may enhance the ability of a cell to sense temperature. Another possibility is that the placement of multiple terminal branches along the outer surface of the animal may enhance their sensitivity to temperature changes in the outside environment of the animal, rather than monitoring internal temperature. Other explanations are also possible, but further studies are needed to evaluate the role of PVD morphology in thermosensation.
Chemosensitivity of polymodal nociceptors
In mammals, polymodal nociceptors associated with C-fibers often function as chemosensors sensitive to ATP, protons, and other molecules that are released or secreted by nearby cells upon tissue damage or inflammation [73,89–91]. Chemicals acting on polymodal nociceptors either activate them directly or sensitize them to thermal or mechanical stimuli [92,93]. The heat response of Drosophila DA-IV neurons, like responses of mammalian C-fibers, is sensitized by tissue damage. Specifically, ultraviolet light-mediated damage to the overlying epidermis leads to release of cytokines that regulate the heat response of these neurons [15]. Leech nociceptors have also been demonstrated to be sensitive to chemicals (i.e protons) [36]. Protons are likely to be released from injured cells, and thus, may function as signals of tissue damage. Studies have yet to demonstrate that C. elegans PVD and FLP neurons are responsive to chemicals, however, the possibility cannot be ruled out entirely. To detect chemical signals, sensory dendrites of polymodal nociceptors need to be exposed to the external milieu, and thus need to be free of a protective capsule. It is important to note that naming the sensory endings of mammalian nociceptors as “free” or “bare” is misleading, as their terminals are known to be ensheathed by Schwann cells [30]. However, short segments lacking this sheath, and thus, accessible to the chemical milieu surrounding these endings, are found at their distal ends, which are likely to be sensory specializations [30,32](Fig 3c). Signals of tissue damage and inflammation may not diffuse widely due to localized release or secretion, combined with limited diffusion capabilities of signaling molecules that are easily buffered (eg. protons), quickly degraded (eg. ATP), or large in size (eg. cytokines). This may explain the need for multiple closely spaced sensory dendrites. Therefore, we suggest that chemical sensitivity of polymodal nociceptors in Drosophila and mammals requires both free dendrites and closely spaced dendrites.
Conclusions
Polymodal nociceptors detect noxious signals associated with tissue damage and elicit protective responses. As such, these neurons are likely to be of great importance to the animal’s survival. Indeed, many features of polymodal nociceptors are found in multiple organisms, suggesting evolutionary conservation. Specifically, a morphological feature found in polymodal nociceptors of vertebrates and invertebrates is a multi-dendritic arbor whose terminal dendrites are free of glial ensheathment. Recent findings from Drosophila and C. elegans demonstrate that generation of this multi-dendritic morphology depends on a complex developmental program. Furthermore, polymodal nociceptors throughout evolution depend on the same families of sensory channels, TRP and DEG/ENaC, for their function. The conserved association between specific functions and molecular sensors with specific morphology of dendrites and dendritic arbors suggests that the form of the dendritic arbor may contribute to the function of these neurons and molecular sensors. However, many questions need to be answered in order to validate this hypothesis (Box 1).
Outstanding questions.
Which particular features of the multi-dendritic arbor of polymodal nociceptors are important for which specific functions? One way to examine this question is to analyze sensory functions of mutants defective for specific morphological features.
Are terminal dendrites of all multi-dendritic mechanosensors either embedded in or tethered to external rigid structures?
Does activation of DEG/ENaC channels by mechanical stimuli require structural elements such as the large diameter microtubules found in C. elegans touch receptor neurons, a cilium, or alternatively, a multi-dendritic morphology?
Are C. elegans polymodal nociceptors sensitive to chemical signals? If so, which signals, and what functional roles do such signals confer?
Is a dense dendritic arbor required for efficient activation/sensitization by chemicals serving as signals of tissue damage?
Some multi-dendritic arbors involve exceptionally fine processes (eg. <100 nm diameter in C. elegans PVD neurons [10]). Do the terminal branches of other nociceptors also contain such fine branches? To examine this question at a detailed level, better intracellular tracers, and/or high-resolution microscopy are needed.
Similar contributions of form to function may explain the morphologies of diverse sensory neurons. Progress in imaging the morphology of individual cells within living tissues, as well as in advancements in functional imaging techniques and in the genetic labeling of specific neuronal classes should facilitate more detailed investigations of the underlying correlations between form and function in the various different subclasses of sensory neurons.
Acknowledgements
We thank Drs. Avi Priel, Bill Kristan and the anonymous reviewers for helpful comments. Chris Crocker supplied new artwork. We thank John White and the LMB/MRC for donation of their TEM archive of C. elegans to the Hall lab. This work was funded in part by National Institutes of Health grants (RR 12596 to DHH and a BSF 2005036 grant to MT).
Glossary
- Cilium
(plural cilia) A thin microtubule-based organelle. In invertebrates, sensory cilia often lie within a protected tube formed by glial cells. In C. elegans non-motile cilia sense the chemical or physical environment, via thin tube-like connections linking the glial channel to the exterior of the animal. Chemical and/or thermal signals can traverse these connections to bathe the cilia inside the channel.
- DEG/ENaC channels
The DEGenerin/Epithelial Na+ Channel gene family encodes for proteins with two transmembrane domain subunits that assemble as multimers (trimers) to form cation permeable ion channels. These ion channels have many functional roles, including in the regulation of cellular homeostasis and the sensation of mechanical stimuli or protons.
- Dendritic arbor
a tree like organization of branched neuronal-processes responsible for receiving synaptic or sensory inputs; also called a “sensory arbor” in sensory neurons.
- Encapsulated ending
a sensory ending (ie. a dendrite) that is fully wrapped by specialized protective glia
- Free ending
a sensory ending (ie. a dendrite or part of a dendrite) that has no accompanying glial wrapping, also called a “bare” ending or a “naked” ending.
- Hyperalgesia
(increased sensitivity to pain): sometimes a result of activation of nociceptor TRP channels, due to chemical signals resulting from inflammation and tissue damage.
- Hypodermis
a single layer syncytium covering the nematode’s body and lying underneath the non-living cuticle. The hypodermal layer is much thinner where it extends between cuticle and bodywall muscles. The function of the hypodermis is analogous to that of the living skin or epidermis.
- Nociceptor
a neuron that senses high threshold, noxious signals.
- Polymodal nociceptor
a nociceptor that is sensitive to two or more sensory modalities, usually mechanical and thermal, and sometimes chemical.
- Self-avoidance
a property of highly branched dendrites in certain neurons to develop evenly spaced arbors that never touch or cross neighboring dendrites from the same neuron
- Tiling
a property of branched dendrites of some neuron classes to never touch or cross dendrites from neighboring neurons of the same modality, but instead cover the body with non- overlapping receptive fields.
- TRP channels
Transient Receptor Potential genes encode for a diverse family of subunits, each comprised of six transmembrane domain subunits that form cation permeable ion channels. TRP channels have many roles in cellular homeostasis and in sensory transduction. Several subgroups (eg. TRPV, TRPA, and TRPM) can be activated by temperature changes and are thus called thermoTRPs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramon y, Cajal S. Histology of the Nervous System of Man and Vertebrate. Oxford University Press: 1995. [Google Scholar]
- 2.Rall W, Rinzel J. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophys. J. 1973;13:648–687. doi: 10.1016/S0006-3495(73)86014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cash S, Yuste R. Linear summation of excitatory inputs by CA1 pyramidal neurons. Neuron. 1999;22:383–394. doi: 10.1016/s0896-6273(00)81098-3. [DOI] [PubMed] [Google Scholar]
- 4.Branco T, Hausser M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr. Opin. Neurobiol. 2010;20:494–502. doi: 10.1016/j.conb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Jan Y-N, Jan LY. Branching out: mechanisms of dendritic arborization. Nat. Rev. Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith ES, Lewin GR. Nociceptors: a phylogenetic view. J. Comp. Physiol. A. 2009;195:1089–1106. doi: 10.1007/s00359-009-0482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oren-Suissa M, et al. The fusogen EFF-1 controls sculpting of mechanosensory dendrites. Science. 2010;328:1285–1288. doi: 10.1126/science.1189095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatzigeorgiou M, et al. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat. Neurosci. 2010;13:861–868. doi: 10.1038/nn.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith CJ, et al. Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Dev. Biol. 2010;345:18–33. doi: 10.1016/j.ydbio.2010.05.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albeg A, et al. C. elegans multi-dendritic sensory neurons: morphology and function. Mol. Cell. Neurosci. 2010;46:308–317. doi: 10.1016/j.mcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguirre-Chen C, et al. C. elegans bicd-1 homolog of the Drosophila dynein accessory factor, Bicaudal D, regulates the branching of PVD mechanosensory neuron dendrites. Development. 2011;138:507–518. doi: 10.1242/dev.060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatzigeorgiou M, Schafer WR. Lateral facilitation between primary mechanosensory neurons controls nose touch perception in C. elegans. Neuron. 2011;70:299–309. doi: 10.1016/j.neuron.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tracey WD, et al. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhong L, et al. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr. Biol. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babcock DT, et al. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr. Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Way JC, Chalfie M. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 1989;3:1823–1833. doi: 10.1101/gad.3.12a.1823. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan JM, Horvitz HR. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1993;90:2227–2231. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White JG, et al. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 19.Halevi S, et al. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002;21:1012–1020. doi: 10.1093/emboj/21.5.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yassin L, et al. Mutations in the extracellular domain and in the membrane-spanning domains interfere with nicotinic acetylcholine receptor maturation. Biochemistry. 2002;41:12329–12335. doi: 10.1021/bi020193y. [DOI] [PubMed] [Google Scholar]
- 21.Tsalik EL, et al. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev. Biol. 2003;263:81–102. doi: 10.1016/s0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalfie M, Thomson JN. Organization of neuronal microtubules in the nematode Caenorhabditis elegans. J. Cell Biol. 1979;82:278–289. doi: 10.1083/jcb.82.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalfie M, Sulston J. Developmental Genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- 24.Gao F-B, et al. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1999;13:2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grueber WB, et al. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 26.Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol. Cell. Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang RY, et al. Nociceptive Neurons Protect Drosophila Larvae from Parasitoid Wasps. Curr. Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang Y, et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 30.Cauna N. The free penicillate nerve endings of the human hairy skin. J. Anat. 1973;115:277–288. [PMC free article] [PubMed] [Google Scholar]
- 31.Kruger L, et al. Fine Structure of myelinated mechanical nociceptor endings in cat hairy skin. J. Comp. Neurol. 1981;198:137–154. doi: 10.1002/cne.901980112. [DOI] [PubMed] [Google Scholar]
- 32.Messlinger K. Functional morphology of nociceptive and other fine sensory endings (free nerve endings) in different tissues. In: Kumazawa T, et al., editors. Prog. Brain Res. Vol. 113. Elsevier; 1996. pp. 273–298. [DOI] [PubMed] [Google Scholar]
- 33.Peng YB, et al. Electrophysiological assessment of the cutaneous arborization of Adelta-fiber nociceptors. J. Neurophysiol. 1999;82:1164–1177. doi: 10.1152/jn.1999.82.3.1164. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt R, et al. Innervation territories of mechano-insensitive C nociceptors in human skin. J Neurophysiol. 2002;88:1859–1866. doi: 10.1152/jn.2002.88.4.1859. [DOI] [PubMed] [Google Scholar]
- 35.Blackshaw SE, et al. Physiological responses, receptive fields and terminal arborizations of nociceptive cells in the leech. J. Physiol. 1982;326:251–260. doi: 10.1113/jphysiol.1982.sp014189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastor J, et al. Properties of the nociceptive neurons of the leech segmental ganglion. J. Neurophysiol. 1996;75:2268–2279. doi: 10.1152/jn.1996.75.6.2268. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Macagno ER. The establishment of peripheral sensory arbors in the leech: in vivo time-lapse studies reveal a highly dynamic process. J. Neurosci. 1997;17:2408–2419. doi: 10.1523/JNEUROSCI.17-07-02408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobin D, et al. Combinatorial Expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 39.Colbert HA, et al. OSM-9, a novel protein with structural similarity to channels is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall DH, Altun ZF. C. elegans Atlas. Cold Spring Harbor Press; 2008. Nervous system; pp. 57–130. [Google Scholar]
- 41.Way JC, Chalfie M. mec-3, a homeobox-containing gene that specifies differentiation of the touch receptor neurons in C. elegans. Cell. 1988;54:5–16. doi: 10.1016/0092-8674(88)90174-2. [DOI] [PubMed] [Google Scholar]
- 42.Grueber WB, et al. Different levels of the homeodomain protein Cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell. 2003;112:805–818. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- 43.Hattori Y, et al. Selective expression of Knot/Collier, a transcriptional regulator of the EBF/Olf-1 family, endows the Drosophila sensory system with neuronal class-specific elaborated dendritic patterns. Genes Cells. 2007;12:1011–1022. doi: 10.1111/j.1365-2443.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 44.Jinushi-Nakao S, et al. Knot/Collier and Cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron. 2007;56:963–978. doi: 10.1016/j.neuron.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 45.Satoh D, et al. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat. Cell Biol. 2008;10:1164–1171. doi: 10.1038/ncb1776. [DOI] [PubMed] [Google Scholar]
- 46.Ye B, et al. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweeney NT, et al. The coiled-coil protein shrub controls neuronal morphogenesis in Drosophila. Curr. Biol. 2006;16:1006–1011. doi: 10.1016/j.cub.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 48.Emoto K, et al. Control of dendritic branching and tiling by the Tricornered-Kinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 2004;119:245–256. doi: 10.1016/j.cell.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 49.Emoto K, et al. The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature. 2006;443:210–213. doi: 10.1038/nature05090. [DOI] [PubMed] [Google Scholar]
- 50.Dimitrova S, et al. Slit and Robo regulate dendrite branching and elongation of space-filling neurons in Drosophila. Dev. Biol. 2008;324:18–30. doi: 10.1016/j.ydbio.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 51.Hughes ME, et al. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matthews BJ, et al. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Soba P, et al. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–416. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee A, et al. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development. 2003;130:5543–5552. doi: 10.1242/dev.00792. [DOI] [PubMed] [Google Scholar]
- 55.Kramer AP, et al. Developmental arborization of sensory neurons in the leech Haementeria ghilianii. I. Origin of natural variations in the branching pattern. J. Neurosci. 1985;5:759–767. doi: 10.1523/JNEUROSCI.05-03-00759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kramer AP, Stent GS. Developmental arborization of sensory neurons in the leech Haementeria ghilianii. II. Experimentally induced variations in the branching pattern. J. Neurosci. 1985;5:768–775. doi: 10.1523/JNEUROSCI.05-03-00768.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams CM, et al. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J. Cell Biol. 1998;140:143–152. doi: 10.1083/jcb.140.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodman MB, et al. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- 59.Price MP, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 60.García-Añoveros J, et al. Transport and localization of the DEG/ENaC ion channel BNaC1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. J. Neurosci. 2001;21:2678–2686. doi: 10.1523/JNEUROSCI.21-08-02678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roza C, et al. Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J. Physiol. 2004;558:659–669. doi: 10.1113/jphysiol.2004.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drew LJ, et al. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J. Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coste B, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lumpkin EA, et al. The cell biology of touch. J. Cell Biol. 2010;191:237–248. doi: 10.1083/jcb.201006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson RI, Corey DP. The force be with you: a mechanoreceptor channel in proprioception and touch. Neuron. 2010;67:349–351. doi: 10.1016/j.neuron.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 66.Kang L, et al. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Savage C, et al. mec-7 is a β-tubulin required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes & Development. 1989;3:870–881. doi: 10.1101/gad.3.6.870. [DOI] [PubMed] [Google Scholar]
- 68.Cueva JG, et al. Nanoscale Organization of the MEC-4 DEG/ENaC Sensory Mechanotransduction Channel in Caenorhabditis elegans Touch Receptor Neurons. J. Neurosci. 2007;27:14089–14098. doi: 10.1523/JNEUROSCI.4179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Hagan R, et al. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 70.Du H, et al. Extracellular proteins needed for C. elegans mechanosensation. Neuron. 1996;16:183–194. doi: 10.1016/s0896-6273(00)80035-5. [DOI] [PubMed] [Google Scholar]
- 71.Blackshaw SE. Morphology and distribution of touch cell terminals in the skin of the leech. J. Physiol. 1981;320:219–228. doi: 10.1113/jphysiol.1981.sp013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Millard CL, Woolf CJ. Sensory innervation of the hairs of the rat hindlimb: a light microscopic analysis. J. Comp. Neurol. 1988;277:183–194. doi: 10.1002/cne.902770203. [DOI] [PubMed] [Google Scholar]
- 73.Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J. Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- 74.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 75.Caterina MJ, et al. Impaired nociceptrion and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 76.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 77.McKemy DD, et al. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 78.Bautista DM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 79.Vriens J, et al. TRPM3 Is a Nociceptor Channel Involved in the Detection of Noxious Heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 80.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 81.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 82.Knowlton WM, et al. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Woodbury CJ, et al. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J. Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwon Y, et al. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 2008;8:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- 85.Patwardhan AM, et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1and produce pain in rodents. J. Clin. Invest. 2010;120:1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.del Camino D, et al. TRPA1 contributes to cold hypersensitivity. J. Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagata K, et al. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perkins LA, et al. Mutant sensory cillia in Caenorhabditis elegans. Dev. Biol. 1985;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 89.Beck PW, Handwerker HO. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974;347:209–222. doi: 10.1007/BF00592598. [DOI] [PubMed] [Google Scholar]
- 90.Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- 91.Mandadi S, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perl ER. Cutaneous polymodal receptors: characteristics and plasticity. In: Kumazawa T, et al., editors. Prog. Brain Res. Vol. 113. Elsevier; 1996. pp. 21–37. [DOI] [PubMed] [Google Scholar]
- 93.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010;16:1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sawin ER, et al. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 95.Li W, et al. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kindt KS, et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat. Neurosci. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]