Abstract
Many cytokines transmit signals to the cell interior through activation of receptor-associated, Janus family protein tyrosine kinases (Jak PTKs). The interleukin-2 receptor (IL-2R) is associated with the Jak1 and Jak3 PTKs, and ligand-induced activation of these PTKs is essential for lymphocyte proliferation. Here, the nonreceptor PTK, Pyk2, was found to be activated following IL-2 stimulation in a Jak-dependent manner. Furthermore, physical association was detected between endogenous Pyk2 and Jak3, and a dominant interfering mutant of Pyk2 inhibited IL-2-induced cell proliferation without affecting Stat5 activation. Collectively, these results suggest that Pyk2 is a newly identified component of the Jak-mediated IL-2 signaling pathway.
Keywords: IL-2, IL-2R, Jak PTK, Pyk2, Stat5
Interleukin-2 (IL-2), a cytokine critical for lymphocyte proliferation, transmits the signals to cell interior through binding to IL-2R (receptor), a complex composed of the IL-2Rα, βc, and γc chains (for review, see Taniguchi and Minami 1993; Leonard et al. 1994; Sugamura et al. 1996). It has been shown that the IL-2Rβc and γc chains are associated with two Janus protein tyrosine kinases (Jak1 and Jak3 PTKs), respectively, and that IL-2-induced activation of these PTKs is crucial for invoking cell proliferation (Johnston et al. 1994; Miyazaki et al. 1994; Russell et al. 1994; Witthuhn et al. 1994; Kawahara et al. 1995). Few of the downstream targets of Jak PTKs are known, except for the Stat (signal transducer and activator of transcription) factors (Darnell et al. 1994; Stahl et al. 1995; Ihle 1996). However, IL-2-induced cell proliferation occurs without Stat5 activation in some cells (Fujii et al. 1995), indicating the presence of an additional, unknown target(s) of the Jaks. Pyk2 (CAKβ/RAFTK), a member of focal adhesion kinase (FAK) family PTKs (Avraham et al. 1995; Lev et al. 1995; Sasaki et al. 1995), has been characterized as a mediator of G-protein-coupled receptors (Lev et al. 1995) and stress signals, leading to activation of the MAP kinase (Dikic et al. 1996) and Jun N (amino)-terminal kinase (JNK) signaling pathways (Tokiwa et al. 1996), respectively. In addition, Pyk2 was shown to be activated by a variety of extracellular stimuli that elevate intracellular Ca2+ concentration (Lev et al. 1995). Notably, Pyk2 is highly expressed in the cells of the immune system (Avraham et al. 1995; Tokiwa et al. 1996; Qian et al. 1997) in addition to the neuronal system (Lev et al. 1995), and it was shown to be involved in signaling triggered by T cell antigen receptor (TCR) stimulation (Qian et al. 1997). However, the role of Pyk2 in cytokine signaling has been unknown. In the present study, we report on the IL-2-induced activation of Pyk2 and provide evidence demonstrating the role of Pyk2 as a mediator of the IL-2R–Jak signaling pathway.
Results and Discussion
Pyk2 activation following IL-2R stimulation
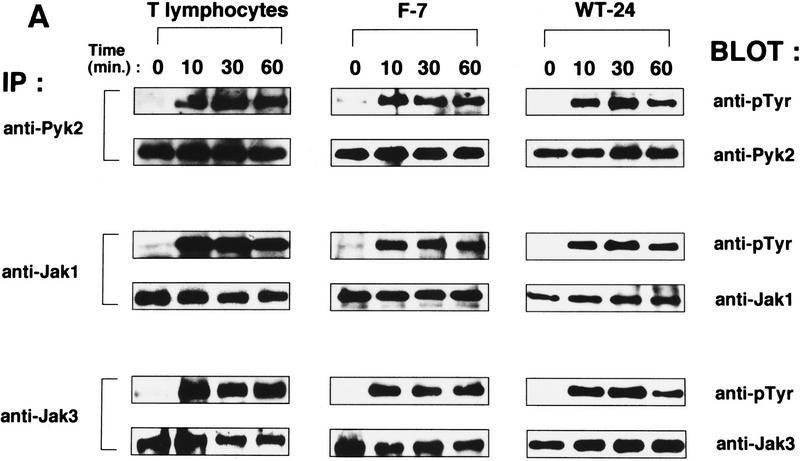
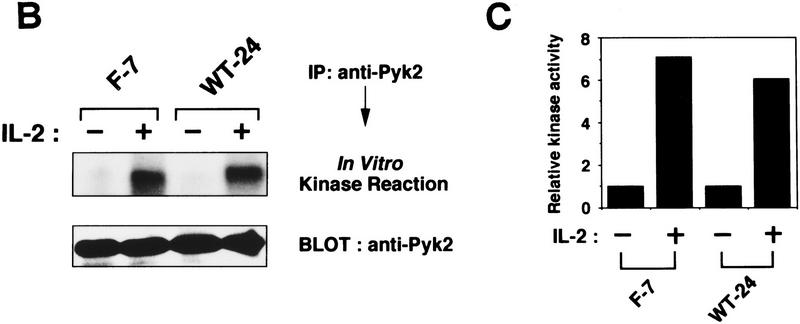
Activation of Pyk2 by IL-2 was first examined in human peripheral T lymphocytes. Induction of Pyk2 tyrosine phosphorylation was detectable within 10 min after IL-2 stimulation and persisted for at least 60 min (Fig. 1A, left). Notably, the kinetics of IL-2-induced Pyk2 phosphorylation are similar to those observed for Jak1 and Jak3 (Fig. 1A), suggesting a potential link among these PTKs in IL-2 signaling (see below). Similar results were obtained with the IL-2-responsive cell clone F-7 (Hatakeyama et al. 1989), which was established from the IL-2Rα+, βc−, γc+, murine hematopoietic cell line BAF-B03 by stable expression of the wild-type IL-2Rβc cDNA (Fig. 1A, middle). Furthermore, IL-2-induced Pyk2 phosphorylation was also confirmed with a human T cell clone WT-24, which was established from the IL-2Rα+, βc+, γc−, ED40515 (−) cell line (Arima et al. 1992) by stable expression of the wild-type IL-2Rγc cDNA (Fig. 1A, right). IL-2-induced activation of Pyk2 PTK was demonstrated in both F-7 and WT-24 cells by autophosphorylation and by in vitro phosphorylation of an exogenous substrate (Fig. 1B,C).
Figure 1.
Activation of Pyk2 following IL-2R stimulation. (A) Time course of IL-2-induced tyrosine phosphorylation of Pyk2, Jak1, and Jak3 in human peripheral T lymphocytes, F-7, and WT-24 cells. Quiescent T lymphocytes and F-7 cells or WT-24 cells were stimulated with IL-2 for the indicated intervals (see Materials and Methods). Cell lysates from these cells were subjected to immunoprecipitation (IP) and analyzed by immunoblotting (BLOT). (B) Autophosphorylation of Pyk2 following IL-2 stimulation. Factor-starved F-7 or WT-24 cells were stimulated (+) or not (−) with IL-2 for 30 min. Pyk2 was immunoprecipitated from these cells, and the immunoprecipitates were subjected to an in vitro kinase assay or immunoblotting with anti-Pyk2 antibody. The phosphorylated products were analyzed by autoradiography. (C) Tyrosine phosphorylation of exogenous substrate by Pyk2. Immunoprecipitated Pyk2 from F-7 or WT-24 cells, stimulated (+) or not (−) with IL-2 as described in B, were subjected to an in vitro kinase assay in the presence of the exogenous substrate, poly(Glu–Tyr) (4:1), as reported by Lev et al. (1995). The phosphorylated products were analyzed by autoradiography, and quantified by the incorporated radioactivity of the phosphorylated poly(Glu–Tyr). The data are presented as kinase activity relative to unstimulated cells.
Identification of the critical regions of the IL-2Rβc and γc chains for Pyk2 activation
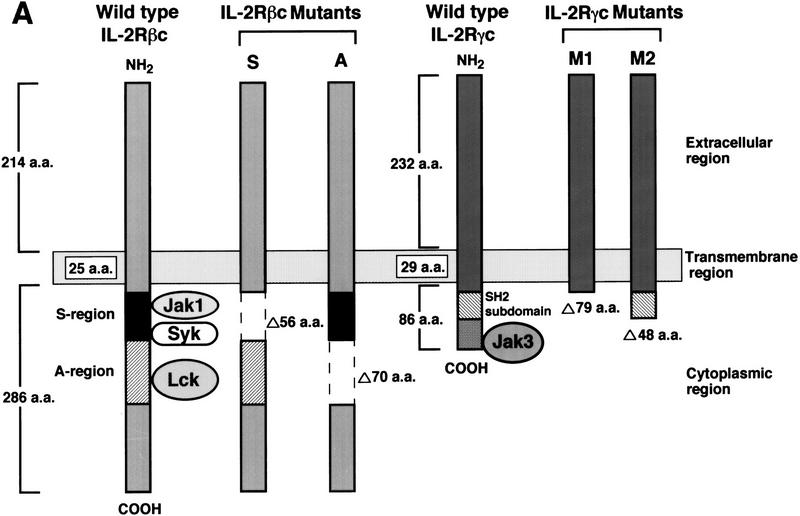
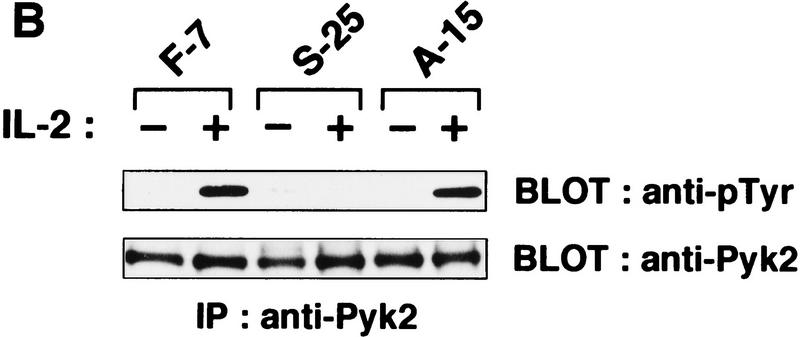
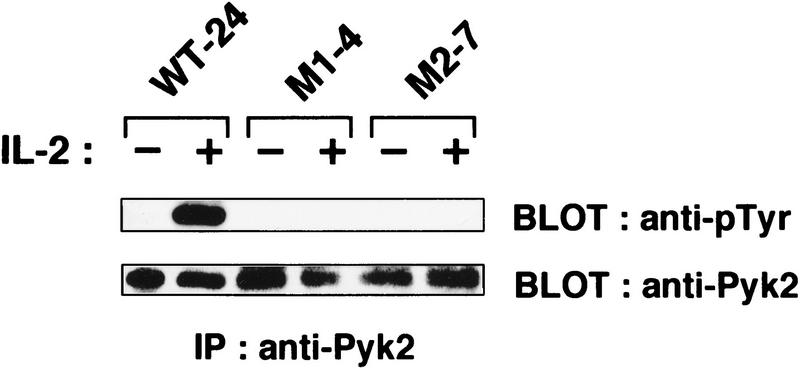
The membrane–proximal S-region of IL-2Rβc (Hatakeyama et al. 1989) and the 48-amino-acid carboxy-terminal region of IL-2Rγc are essential for IL-2 signaling (Asao et al. 1993; Ishii et al. 1994) (Fig. 2A). In fact, these two regions are critical for the interaction with Jak1 and Jak3, respectively, and for the activation of both Jak kinases (Ishii et al. 1994; Johnston et al. 1994; Miyazaki et al. 1994; Russell et al. 1994; Witthuhn et al. 1994; Nelson et al. 1996). We thus examined whether these regions are also critical for Pyk2 activation. As shown in Figure 2B (top), IL-2-induced tyrosine phosphorylation of Pyk2 does not occur in BAF-B03-derived S-25 cells, which express a mutant IL-2Rβc lacking the S region (S mutant). On the other hand, Pyk2 phosphorylation is still seen in A-15 cells, which express a mutant IL-2Rβc lacking the A region (A mutant) (Fig. 2A). The A region is essential for the binding and activation of src family members, for example, Lck, but not for Jak1 (Taniguchi 1995; Miyazaki and Taniguchi 1996), indicating that the former PTKs are not involved in the IL-2-induced Pyk2 activation. As for IL-2Rγc, tyrosine phosphorylation of Pyk2 was no longer observed in M2-7 cells, which express a mutant IL-2Rγc lacking its carboxy-terminal 48 amino acids (Fig. 2, A and B, bottom), a region that is critical for Jak3 binding (Miyazaki et al. 1994; Russell et al. 1994). These results suggest that IL-2-induced Pyk2 phosphorylation/activation may be triggered by the Jak1/Jak3 signaling pathway.
Figure 2.
Critical regions of the IL-2Rβc and γc chains for the IL-2-induced Pyk2 activation. (A) A schematic representation of the wild-type and mutant forms of IL-2Rβc and γc (see text and Materials and Methods for details). (B) Identification of the critical regions of the IL-2Rβc and γc chains for the activation of Pyk2. Factor-starved F-7, S-25 or A-15 cells were cultured in the presence (+) or absence (−) of IL-2 for 30 min, and cell lysates were prepared. The lysates were subjected to immunoprecipitation (IP) with anti-Pyk2 antibody and subsequent immunoblotting (BLOT) with anti-pTyr or anti-Pyk2 antibody. The expression levels of the wild-type and mutant IL-2Rβc in these cells were comparable (Hatakeyama et al. 1989). Clones M1-4 and M2-7 express the M1 and M2 mutants, respectively (see Materials and Methods). The expression levels of the wild-type and mutant IL-2Rγc were similar, as revealed by FACS analysis (data not shown). Pyk2 activation by IL-2 was analyzed by the same procedures described above.
Requirement of the Jak signaling pathway for Pyk2 activation
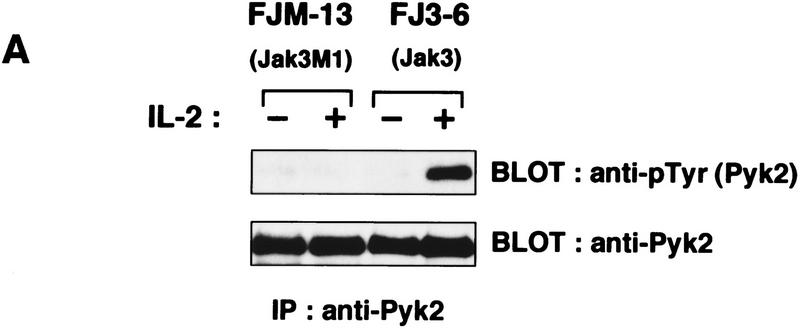
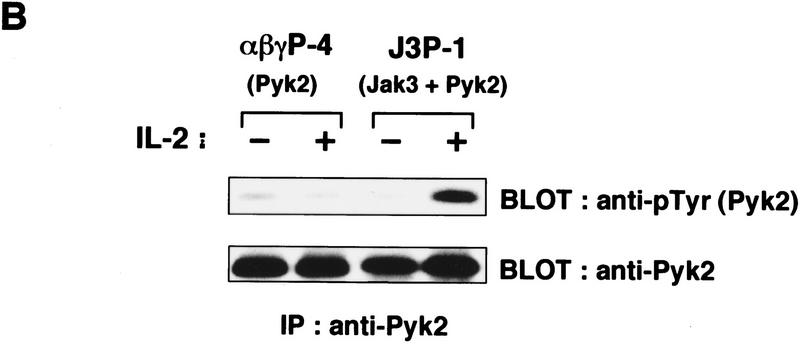
To study further the functional relationship between Pyk2 and the Jaks, Pyk2 activation was compared in the F-7-derived clones FJM-13 and FJ3-6, which overexpress a dominant interfering mutant and the wild-type Jak3 molecule, respectively (Kawahara et al. 1995). It was found that in FJM-13 cells, in which IL-2-induced activation of Jak1 and Jak3 is inhibited (Kawahara et al. 1995), Pyk2 phosphorylation was also inhibited (Fig. 3A). To examine further the role of the Jaks, IL-2-induced Pyk2 phosphorylation was examined in NIH-3T3-derived cell lines expressing IL-2R (Miyazaki et al. 1994). The cell line 3T3αβγ, which expresses all three IL-2R chains from transfected cDNAs, was established (Minami et al. 1994). 3T3αβγ expresses endogenous Jak1 but not Jak3. Another cell line, J3, was established from 3T3αβγ by expressing the cDNA encoding Jak3, which became responsive to IL-2 for cell proliferation in the absence of serum (Miyazaki et al. 1994). As shown in Figure 3B, expression of the cDNA-driven human Pyk2 was detected similarly between the established clones αβγP-4 and J3P-1; however, induction of Pyk2 phosphorylation by IL-2 was observed only in the latter cells expressing Jak3. Furthermore, tyrosine phosphorylation of endogenous Pyk2 was induced in J3 but not in 3T3αβγ (data not shown). These results indicate that the Jak signaling pathway may participate in Pyk2 phosphorylation/activation.
Figure 3.
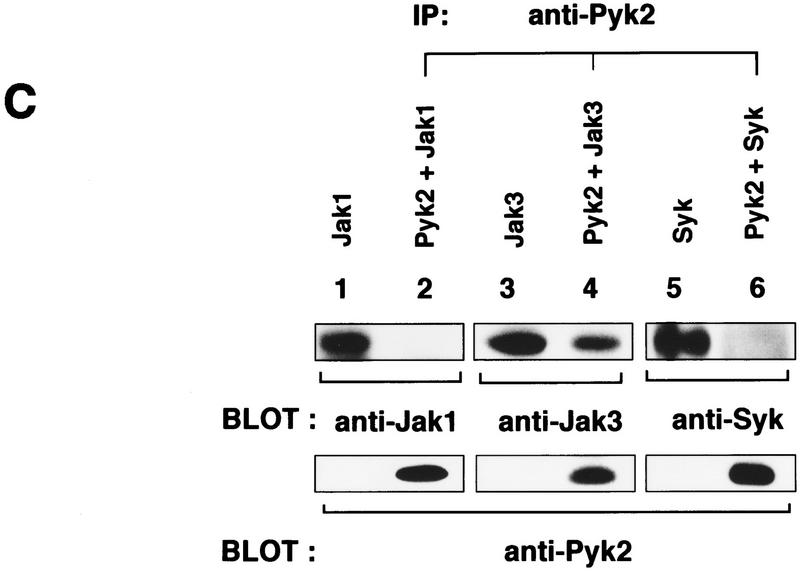
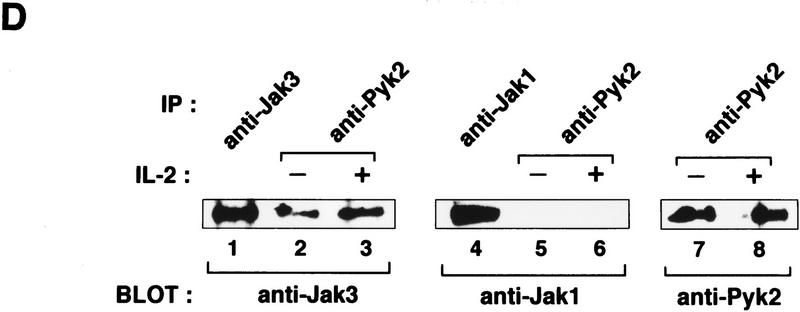
Requirement of the Jak signaling pathway for Pyk2 activation. (A) Inhibition of IL-2-induced Pyk2 activation by Jak3M1, a dominant-interfering mutant of Jak3. Factor-starved FJM-13 and FJ3-6 cells were cultured in the presence (+) or absence (−) of IL-2 for 30 min, and the cell lysates were immunoprecipitated (IP) with anti-Pyk2 antiody and immunoblotted (BLOT) with anti-pTyr or anti-Pyk2 antibody. These cells express similar levels of the cDNA-directed Jak molecules. (B) Requirement of Jak3 in Pyk2 activation by IL-2. αβγP-4 and J3P-1 cells (1 × 107) were serum-starved and stimulated (+) or not (−) with IL-2 (5 nm) for 30 min and analyzed as in A. αβγP-4 expresses Pyk2 cDNA, and J3P-1 expresses Jak3 and Pyk2 cDNA. (C) The selective association of Pyk2 with Jak3. After COS cells were transfected with cDNA expression vector, pEF-Pyk2, together with pEF–Jak1, pEF–Jak3 (Miyazaki et al. 1994), or pEF–Syk (Minami et al. 1995), cell lysates were immunoprecipitated (IP) with anti-Pyk2 antibody (lanes 2,4,6) and immunoblotted with antibodies as indicated. As controls, cell lysates of COS cells transfected with the expression vector, pEF–Jak1, pEF–Jak3, or pEF–Syk were subjected to immunoblotting with antibodies against the respective molecules (lanes 1,3,5). Under the same conditions, Pyk2 association with the cytoplasmic regions of the IL-2Rβc and γc chains (Miyazaki et al. 1994) was not detected (data not shown). (D) Constitutive association of Pyk2 with Jak3. Cell lysates of factor-starved F-7 cells (2 × 108) were prepared before (−) or after (+) IL-2 stimulation for 60 min; immunoprecipitated with anti-Pyk2 antibody (lanes 2,3,5–8); and immunoblotted with anti-Jak3 (lanes 2,3), anti-Jak1 (lanes 5,6), or anti-Pyk2 (lanes 7,8) antibody. As controls, the F-7 cell lysates were immunoprecipitated and immunoblotted for the expression of Jak3 (lane 1) and Jak1 (lane 4). This association was also observed in human peripheral T lymphocytes and J3P-1 cells (data not shown).
The above observations prompted us to examine whether Pyk2 can associate with Jaks or Syk, another tyrosine kinase that associates with the S region of IL-2Rβ (Minami et al. 1995). COS cells were transfected with an expression vector for Pyk2, together with either of the PTK expression vectors. Then, cell lysates were subjected to immunoprecipitation with anti-Pyk2 antibody, which was followed by immunoblotting with respective PTK antibodies. As shown in Figure 3C (top, lanes 2, 4, and 6), Jak3 was coimmunoprecipitated with Pyk2, whereas Jak1 and Syk were not. Furthermore, the in vitro kinase assay results (data not shown) are consistent with the notion that Jak3 can phosphorylate Pyk2 directly. Perhaps more importantly, association between endogenous Pyk2 and Jak3 was observed in F-7 cells (Fig. 3D) before and after IL-2 stimulation.
The functional role of Pyk2 on IL-2-induced growth and Stat5 activation
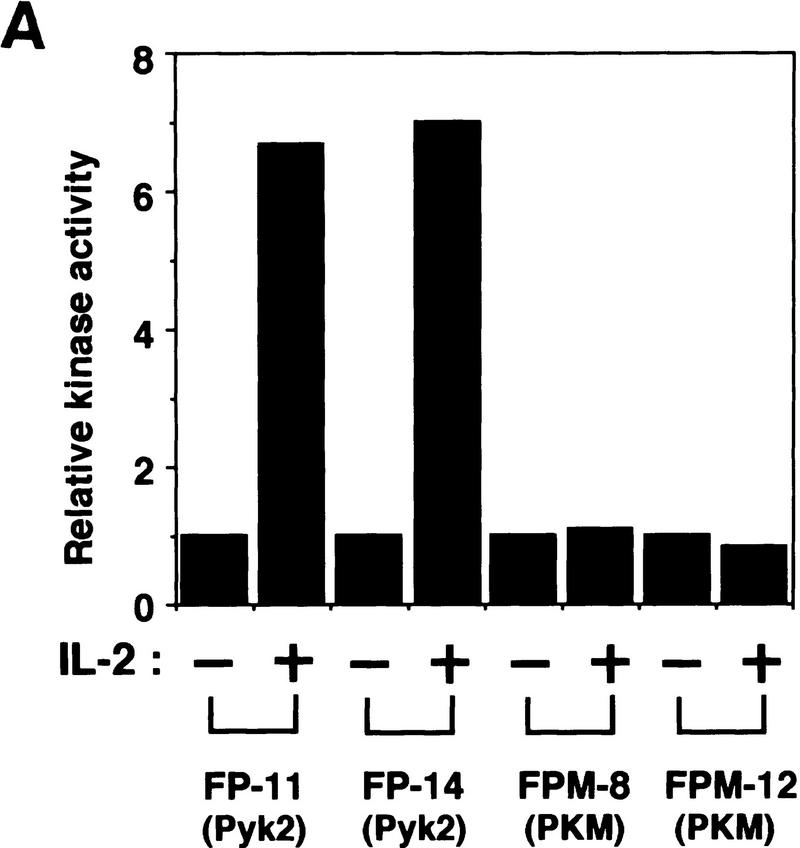
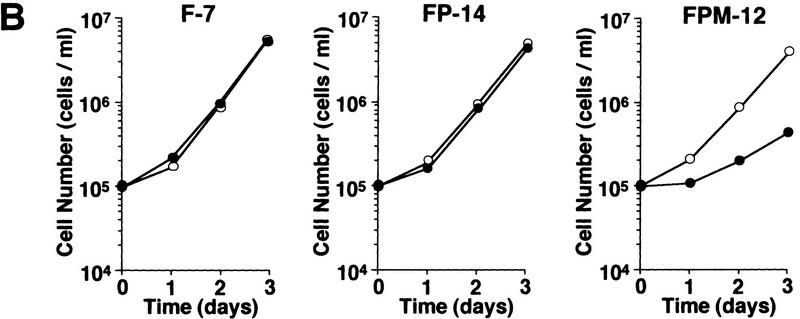
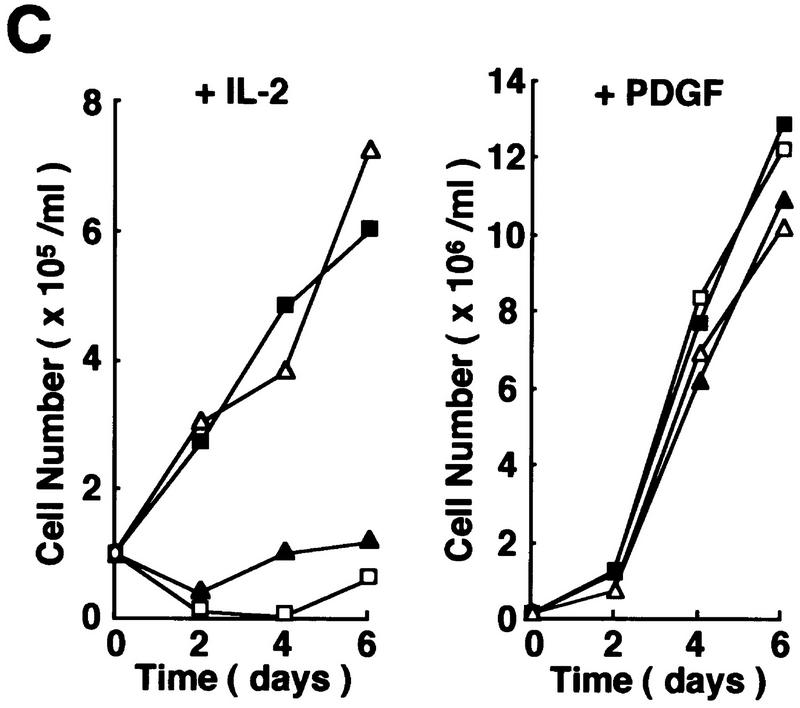
It was shown previously that a kinase-negative mutant of Pyk2 (PKM) acts as an inhibitor of wild-type Pyk2-mediated activation of MAP kinase induced by lysophosphatidic acid (LPA) or bradykinin (Dikic et al. 1996). To assess the functional role of Pyk2 in the proliferative signal transmission elicited by IL-2, we examined the effect of PKM on the growth of F-7 cells. Because PKM may affect IL-3-induced proliferation of F-7 during cDNA transfection and clonal selection, a tetracycline-inducible promoter-based expression system (Gossen et al. 1995) was employed for the expression of the PKM cDNA (see Materials and Methods). Two independent F-7-derived transformants, FPM-8 and FPM-12, were obtained that expressed high levels of PKM on addition of a tetracycline derivative, doxycycline (Dox) (at least 10-fold over endogenous Pyk2) (data not shown). Clones FP-11 and FP-14, which express wild-type Pyk2 at levels similar to that of PKM in the previous clones, were established by similar methods. As shown in Figure 4A, IL-2-induced activation of Pyk2 is strongly inhibited in Dox-treated FPM-8 and FPM-12 cells. Concomitantly, IL-2-induced thymidine uptake (data not shown) and cell proliferation (Fig. 4B) were also inhibited in these cells. We also tested the effect of overexpression of PKM on the NIH-3T3-derived cell line J3, which expresses low levels of endogenous Pyk2 (data not shown) and proliferates in response to IL-2 stimulation (Miyazaki et al. 1994). As shown in Figure 4C, expression of the PKM cDNA (J3PM-3 cells) also inhibited IL-2-induced cell proliferation. On the other hand, PKM expression showed no effect on platelet derived growth factor (PDGF)-induced cell proliferation (Fig. 4C), supporting the notion that PKM selectively inhibits the IL-2R–Jak pathway. In addition, we also examined the effect of antisense oligonucleotides against Pyk2 on IL-2 signaling and found that IL-2-induced thymidine uptake was inhibited by the Pyk2 antisense (5′-GGCTCGGACACCCCAGACAT-3′) but not sense (5′-ATGTCTGGGGTGTCCGAGCC-3′) oligonucleotides in both human IL-2-dependent T-cell lines, Kit225 (Arima et al. 1992) and ILT–Mat (Takeshita et al. 1989; data not shown).
Figure 4.
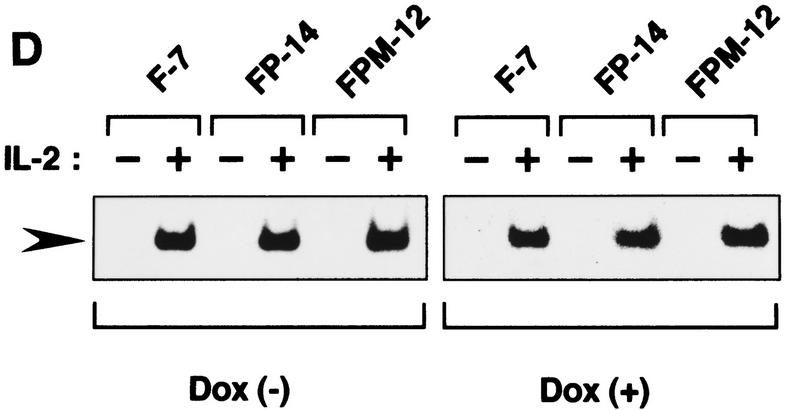
Effects of the overexpressed Pyk2 or PKM on IL-2-induced growth and Stat5 activation. (A) Effect of PKM on IL-2-induced Pyk2 activation. FP-11, FP-14, FPM-8, and FPM-12 cells were incubated in culture medium in the presence of 1 μg/ml Dox for 1 day and cultured in the presence (+) or absence (−) of IL-2 for 30 min. Pyk2 was immunoprecipitated from these cells, and subjected to an in vitro kinase assay as described in Fig. 1. (B) Effect of PKM on IL-2-induced proliferation of F-7-derived clones. F-7, FP-14, and FPM-12 cells were incubated in the presence (•) or absence (○) of Dox for 1 day. After factor starvation, cells were incubated with IL-2, and the viable cell numbers were counted. F-7, FP, and FPM cells were analyzed for their abilities to incorporate [3H]thymidine in response to IL-2 in the presence or absence of Dox. Incorporation of [3H]thymidine by IL-2 was inhibited selectively in the presence of Dox in FPM cells (data not shown). The expression of Jak3M1, a dominant-interfering mutant of Jak3, in F-7 cells results in growth inhibition of [3H]thymidine uptake (Kawahara et al. 1995) and cell proliferation (A. Kawahara, unpubl.) at levels similar to that caused by PKM. (C) Effect of PKM on IL-2-induced proliferation of J3-derived clones. 3T3αβγ (□), J3 (▪), and J3-derived clones, J3P-1 (▵) and J3PM-3 (▴), were cultured (see Materials and Methods). These cells were incubated with IL-2 or PDGF-AB after FCS starvation for 24 hr, and the viable cell numbers were counted by trypan blue staining. Essentially identical results were obtained in the three independent experiments. (D) Effect of PKM on IL-2-induced DNA-binding activity of Stat5. F-7, FP-14, and FPM-12 cells were incubated with (+) or without (−) Dox, and stimulated (+) or not (−) with IL-2 for 15 min. Cell extracts were prepared and subjected to EMSA, using a 32P-labeled probe DNA containing the IRF-1-derived GAS element (Fujii et al. 1995). Complexes were separated by 4% acrylamide gel and detected by autoradiography. The positions of IL-2-induced DNA-binding complex are shown (arrowhead). The IL-2-induced Stat activities in F-7 cells are exclusively Stat5 (Fujii et al. 1995; data not shown). Essentially identical results were obtained in other FP and FPM clones, FP-11 and RPM-8 in B and D.
It has been well-established that cytokine-induced activation of Jak PTKs results in tyrosine phosphorylation of Stat factors to induce their DNA-binding activities. We therefore examined whether or not the expression of PKM affects the IL-2-induced tyrosine phosphorylation and DNA-binding activity of Stat5. IL-2 stimulation of F-7 and derivative clones expressing either wild-type Pyk2 or PKM all showed similar tyrosine phosphorylation levels for Stat5 (data not shown) and similar induction of Stat5 DNA-binding activities (Fig. 4D; Fujii et al. 1995; data not shown). Furthermore, we found that PKM has no effect on the transcriptional activity of Stat5 induced by IL-2.
Our results indicate that Pyk2 is a critical mediator of IL-2 signaling. We infer that Pyk2 is also involved in other cytokine signaling, as PKM expression in FPM-8 and FPM-12 cells also inhibited IL-3-induced cell proliferation (data not shown), suggesting a role for Pyk2 in Jak2-mediated signaling (Ihle 1995; Taniguchi 1995; Miyazaki and Taniguchi 1996). Our results suggest the diversification of the Jak signaling pathway, wherein the Stat and Pyk2 pathways function independently of one another. Consistently, IL-2-induced activation of Jak3 remains unaffected by PKM (data not shown). In view of our findings that Pyk2 but not Stat5 is required for IL-2-induced cell proliferation in F-7 cells, the Jak–Pyk2 pathway must be linked to a downstream target(s) other than Stat5. How Pyk2 participates in the regulation of other Jak-associated molecules (Endo et al. 1997; Naka et al. 1997; Starr et al. 1997; Takeshita et al. 1997) remains to be clarified. The present work offers another example of a signaling cascade involving PTK and suggests a unique convergence of the cytokine receptor–Jak signaling pathway and mitogenic G-protein-coupled receptor signaling pathway through Pyk2.
Materials and methods
Cells and culture conditions
Human peripheral blood lymphocytes were isolated from normal adults by Ficoll–Hypaque centrifugation and cultured in RPMI1640 supplemented with 10% (vol/vol) FCS in the presence of Con A at 10 μg/ml for 3 days. Peripheral T lymphocytes were subsequently cultured and enriched in RPMI1640 supplemented with human recombinant IL-2 (Takeda Chemicals, Japan) for 7 days. BAF–B03-derived F-7, A-15, S-25, FJ3-6, and FJM-13 cells were cultured as described previously (Hatakeyama et al. 1989; Kawahara et al. 1995). ED40515 (−) was cultured in RPMI1640 supplemented with 10% FCS (vol/vol). COS, 3T3αβγ, and J3 cells were cultured as described previously (Miyazaki et al. 1994).
Cell lysis, stimulation, immunoprecipitation, immunoblotting, and in vitro kinase assay
Cell lysis, immunoprecipitation, immunoblotting, and in vitro kinase assays were carried out as described previously (Miyazaki et al. 1994; Lev et al. 1995; Dikic et al. 1996; Qian et al. 1997). Unless stated, the cell number used in these assays was 5 × 107, and human IL-2 was used at 2nm. Peripheral T lymphocytes or F-7 cells were washed with PBS three times, cells were cultured with RPMI1640/ 10% (vol/vol) FCS for 24 and 12 hr, respectively, and stimulated with IL-2. For immunoprecipitation and immunoblotting of Pyk2, rabbit antiserum against Pyk2 (no. 600) was used as described (Dikic et al. 1996). Antibodies against phosphotyrosine (4G10), Jak1, and Jak3 were obtained from Upstate Biotechnology for immunoprecipitation and immunoblotting analyses (Miyazaki et al. 1994). For immunoblotting of Syk, rabbit anti-Syk antibody was used as described (Minami et al. 1995).
Plasmid construction and DNA transfection
The cDNA encoding IL-2RγM2, which lacks the carboxy-terminal 48 amino acids of the human IL-2Rγc chain, was generated by PCR and cloned into the pEF expression vector (Kawahara et al. 1994; H. Fujii, S. Tsujino, and T. Miyazaki, in prep.). WT-24, M1-4, and M2-7 cells were established by transfecting the expression vector plasmids for the wild-type IL-2Rγc, the mutant IL-2RγM1 (Kawahara et al. 1994), and IL-2RγM2, respectively, with a hygromycin-resistance gene into ED40515(−) cells by electroporation (Miyazaki et al. 1991). Expression of wild-type or mutant IL-2Rγc was analyzed by a FACS Caliber Flow Cytometer (Beckton-Dickinson). The wild-type or mutant Pyk2 (PKM) cDNA was subcloned into pEF vector to generate the expression vectors pEF–Pyk2 or pEF–PKM, respectively. αβγP-4 and J3P-1 cells were obtained by cotransfection of pEF–Pyk2 plasmid with the blasticidin-resistance gene into 3T3αβγ or J3-13 cells, respectively, by the calcium phosphate method (Miyazaki et al. 1991). Similarly, J3PM-3 cells were obtained by transfection of pEF–PKM plasmid with the blasticidin-resistance gene into J3-13 cells. The wild-type or mutant Pyk2 (PKM) cDNA was subcloned into the plasmid pUHD10-3 to generate inducible expression vectors for wild-type Pyk2 or PKM, respectively. FP and FPM cells were generated by cotransfecting the plasmids encoding the reverse tetracycline (Tc)-controlled transactivator (rtTA), pUHD172-1 neo (Gossen et al. 1995), and pUHD10-3-derived expression vector for Pyk2 or PKM cDNA, along with the hygromycin-resistance gene into F-7 cells by electroporation. Selection was initiated 24 hr after transfection. Transfection of plasmids, pEF vector (control), pEF–Pyk2, pEF–Jak1, pEF–Jak3 (Miyazaki et al. 1994), or pEF–Syk (Minami et al. 1995) into COS cells was performed by the calcium phosphate method.
Cell growth assay
For cell growth analysis of F-7 or F-7-derived cells, cells were seeded into six-well plates at 1 × 105 cells/ml in RPMI1640 medium/10% FCS. After factor starvation, cells were stimulated by IL-2. For cell growth analysis in 3T3αβγ, J3, and J3-derived cells, cells were seeded into six-well plates at 1 × 105 cells/ml in Dulbecco’s modified Eagle medium (DMEM; GIBCO BRL)/10% FCS. After 24 hr, cells were starved of FCS and subsequently stimulated by IL-2 (5 nm) or 20 ng/ml PDGF-AB (supplemented with 10% FCS). The viable cell number was counted by trypan blue staining.
Acknowledgments
We thank J.N. Ihle for the Jak1 and Jak3 cDNA, H. Yamamura for the Syk cDNA and anti-Syk antibody, H. Bujard for the tetracycline-inducible expression system, T. Sasaki for the poly(Glu–Tyr) substrate, and M.S. Lamphier and E.L. Barsoumian for invaluable suggestions. This work was supported by the Research for the Future (RFTF) Program (96L00307) from the Japan Society for the Promotion of Science (JSPS) and by a special grant for Advanced Research on Cancer, a grant for Molecular Pathogenesis, Grant-in-Aid for Scientific Research on Priority Areas (09273105) and Intervention of Immune Disorders from the Ministry of Education, Science and Culture of Japan and a grant for the Human Frontier Science Program Organization (HFSP). A.T. is a JSPS Research Associate and H.F. is a JSPS Research Fellow. L.N. is a European Union Science and Technology Fellow.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL tada@m.u-tokyo.ac.jp; FAX 81-3-5689-7214.
References
- Arima N, Kamio M, Imada K, Hori T, Hattori T, Tsudo M, Okuma M, Uchiyama T. Pseudo-high affinity interleukin-2 (IL-2) receptor lacks the third component that is essential for functional IL-2 binding and signaling. J Exp Med. 1992;176:1265–1272. doi: 10.1084/jem.176.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asao H, Takeshita T, Ishii N, Kumaki S, Nakamura M, Sugamura K. Reconstitution of functional interleukin-2 receptor complexes on fibroblastoid cells: Involvement of the cytoplasmic domain of the γ chain in two distinct signaling pathways. Proc Natl Acad Sci. 1993;90:4127–4131. doi: 10.1073/pnas.90.9.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor LM, White RA, Groopman JE, Avraham H. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Nogueira L, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- Fujii H, Nakagawa Y, Schindler U, Kawahara A, Mori H, Gouilleux F, Groner B, Ihle JN, Minami Y, Miyazaki T, Taniguchi T. Activation of Stat5 by interleukin-2 requires a carboxyl-terminal region of the interleukin-2 receptor β chain but is not essential for the proliferative signal transmission. Proc Natl Acad Sci. 1995;92:5482–5486. doi: 10.1073/pnas.92.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M, Mori H, Doi T, Taniguchi T. A restricted cytoplasmic region of IL-2 receptor β chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989;59:837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Ihle JN. Cytokine receptor signaling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- ————— STATs: Signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- Ishii N, Asao H, Kimura Y, Takeshita T, Nakamura M, Tsuchiya S, Konno T, Maeda M, Uchiyama T, Sugamura K. Impairment of ligand binding and growth signaling of mutant IL-2 receptor γ-chains in patients with X-linked severe combined immunodeficiency. J Immunol. 1994;153:1310–1317. [PubMed] [Google Scholar]
- Johnston JA, Kawamura M, Kirken RA, Chen YQ, Blake TB, Shibuya K, Ortaldo JR, McVicar DW, O’Shea JJ. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Kawahara A, Minami Y, Taniguchi T. Evidence for a critical role for the cytoplasmic region of the interleukin-2 receptor γ chain in IL-2, IL-4, and IL-7 signaling. Mol Cell Biol. 1994;14:5433–5440. doi: 10.1128/mcb.14.8.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Minami Y, Miyazaki T, Ihle JN, Taniguchi T. Critical role of the interleukin-2 (IL-2) receptor γ-chain-associated Jak3 in the IL-2-induced c-fos and c-myc, but not bcl-2 gene induction. Proc Natl Acad Sci. 1995;92:8724–8728. doi: 10.1073/pnas.92.19.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WJ, Noguchi M, Russell SM, McBride OW. The molecular basis of X-linked severe combined immunodeficiency: The role of the interleukin-2 receptor γ chain as a common γ chain, γ c. Immunol Rev. 1994;138:61–86. doi: 10.1111/j.1600-065x.1994.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Minami Y, Oishi I, Liu ZJ, Nakagawa S, Miyazaki T, Taniguchi T. Signal transduction mediated by the reconstituted IL-2 receptor: Evidence for a cell type-specific function of IL-2 receptor β-chain. J Immunol. 1994;152:5680–5690. [PubMed] [Google Scholar]
- Minami Y, Nakagawa Y, Kawahara A, Miyazaki T, Sada K, Yamamura H, Taniguchi T. Protein tyrosine kinase Syk is associated with and activated by the IL-2 receptor: Possible link with the c-myc induction pathway. Immunity. 1995;2:89–100. doi: 10.1016/1074-7613(95)90081-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Taniguchi T. Coupling of the IL2 receptor complex with non-receptor protein tyrosine kinases. Cancer Surv. 1996;27:25–40. [PubMed] [Google Scholar]
- Miyazaki T, Maruyama M, Yamada G, Hatakeyama M, Taniguchi T. The integrity of the conserved “WS motif” common to IL-2 and other cytokine receptors is essential for ligand binding and signal transduction. EMBO J. 1991;10:3191–3197. doi: 10.1002/j.1460-2075.1991.tb04881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu ZJ, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, Taniguchi T. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- Nelson BH, Lord JD, Greenberg PD. A membrane-proximal region of the interleukin-2 receptor γc chain sufficient for Jak kinase activation and induction of proliferation in T cells. Mol Cell Biol. 1996;16:309–317. doi: 10.1128/mcb.16.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Lev S, van Oers NSC, Dikic I, Schlessinger J, Weiss A. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. J Exp Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, Goldman AS, Schmalstieg FC, Ihle JN, O’Shea JJ, Leonard WJ. Interaction of IL-2Rβ and γc chains with Jak1 and Jak3: Implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nagura K, Ishino M, Tobioka H, Kotani K, Sasaki T. Cloning and characterization of cell adhesion kinase β, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell Jr JE, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signaling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor γ chain: Its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- Takeshita T, Goto Y, Tada K, Nagata K, Asao H, Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med. 1989;169:1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T, Arita T, Higuchi M, Asao H, Endo K, Kuroda H, Tanaka N, Murata K, Ishii N, Sugamura K. STAM, signal transducing adaptor molecule, is associated with Janus kinases and involved in signaling for cell growth and c-myc induction. Immunity. 1997;6:449–457. doi: 10.1016/s1074-7613(00)80288-5. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995;268:251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: A current overview. Cell. 1993;73:5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- Tokiwa G, Dikic I, Lev S, Schlessinger J. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science. 1996;273:792–794. doi: 10.1126/science.273.5276.792. [DOI] [PubMed] [Google Scholar]
- Witthuhn BA, Silvennoinen O, Miura O, Lai KS, Cwik C, Liu ET, Ihle JN. Involvement of the Jak-3 Janus kinase in signaling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]