Abstract
Background
Pre- and early clinical studies on patients with autoimmune diseases suggested that induction of regulatory T(Treg) cells may contribute to the immunosuppressive effects of glucocorticoids(GCs).
Objective
We readdressed the influence of GC therapy on Treg cells in immunocompetent human subjects and naïve mice.
Methods
Mice were treated with increasing doses of intravenous dexamethasone followed by oral taper, and Treg cells in spleen and blood were analyzed by FACS. Sixteen patients with sudden hearing loss but without an inflammatory disease received high-dose intravenous prednisolone followed by stepwise dose reduction to low oral prednisolone. Peripheral blood Treg cells were analyzed prior and after a 14 day GC therapy based on different markers.
Results
Repeated GC administration to mice for three days dose-dependently decreased the absolute numbers of Treg cells in blood (100 mg dexamethasone/kg body weight: 2.8±1.8×104 cells/ml vs. 33±11×104 in control mice) and spleen (dexamethasone: 2.8±1.9×105/spleen vs. 95±22×105/spleen in control mice), which slowly recovered after 14 days taper in spleen but not in blood. The relative frequency of FOXP3+ Treg cells amongst the CD4+ T cells also decreased in a dose dependent manner with the effect being more pronounced in blood than in spleen. The suppressive capacity of Treg cells was unaltered by GC treatment in vitro. In immunocompetent humans, GCs induced mild T cell lymphocytosis. However, it did not change the relative frequency of circulating Treg cells in a relevant manner, although there was some variation depending on the definition of the Treg cells (FOXP3+: 4.0±1.5% vs 3.4±1.5%*; AITR+: 0.6±0.4 vs 0.5±0.3%, CD127low: 4.0±1.3 vs 5.0±3.0%* and CTLA4+: 13.8±11.5 vs 15.6±12.5%; * p<0.05).
Conclusion
Short-term GC therapy does not induce the hitherto supposed increase in circulating Treg cell frequency, neither in immunocompetent humans nor in mice. Thus, it is questionable that the clinical efficacy of GCs is achieved by modulating Treg cell numbers.
Introduction
Glucocorticoids (GCs) are a class of steroid hormones that bind to the glucocorticoid receptor, which is present in almost every vertebrate animal cell [1]. This explains the large variety of physiological roles played by GCs [2], [3]. They are crucial modifier of the metabolic [4] and immune system [5], [6], but are also important for development [7] as well as for arousal and cognition [8]. During an immune response, GCs contribute to the termination of inflammation by both suppressing and enhancing activities of the immune system [9]. At the level of the innate immune system GCs induce neutrophilia by increasing polymorphonuclear cell release from the bone marrow while inhibiting their transmigration to inflammatory sites [10], [11]. At the same time they induce apoptosis of basophils and eosinophils [12], [13]. In a similar way GCs control the movement of circulating monocytes while enhancing the phagocytotic ability and antigen uptake by the tissue macrophages and therefore speed up the clearance of foreign antigens and microorganisms [14]. At the level of leukocyte gene expression, a huge number of proinflammatory cytokines (IL1β, TNFα, IL-6, IL-8, IL-12, IL-18 etc) and chemokines (both CC and CXC) is strongly suppressed by GCs, while the anti-inflammatory cytokines IL-10 and TGFβ are upregulated [15]. On the other hand, GCs favor antibody production by promoting the generation of immunoglobulin secreting plasma cells [16]. Accordingly, GCs promote the polarization of naïve T-cells to a Th2 phenotype while at the same time promoting an immature dendritic cell phenotype [17]. Both effects may be in part responsible for a presumed upregulation of regulatory T (Treg) cells by GCs [18]. In the last decades, it became clear that Treg cells play an essential role in self-tolerance and for maintaining immune system homeostasis [19].
Failure of self-tolerance often leads to autoimmune diseases (AD), with an incidence of 5–10 % of the population [20]. Emergence of autoimmune diseases is not completely understood, it is however documented that T cells often play an essential role in their development (e.g. diabetes mellitus type I or type A gastritis [21]). Some of these autoimmune diseases are probably triggered by infections (e.g. Guillian Barré syndrome [22], [23]), whereas in others it has been shown that tolerance defects are due to a decrease in number or defective expression pattern in the Treg cell population [24], [25]. Since more than 60 years GCs are often the first line therapy in inflammatory and autoimmune responses [26] and around 30 years ago a connection between GCs and a “suppressor” T cell phenotype was initially shown in vitro [27], [28]. In the meantime, a number of different Treg cell populations have been described [25], [29], of which the most studied one expresses CD4 and high levels of the IL-2 receptor alpha-chain (CD25) [19], [30]. Several additional surface markers have been reported to discriminate naturally occurring Treg cells from other activated CD4+ Th2 cells such as cytotoxic T-lymphocyte antigen 4 (CTLA4) [31], [32], the nuclear transcription factor forkhead box P3 (FOXP3), the activation-inducible tumor necrosis factor receptor AITR [33] and low expression of the IL-7 receptor (CD127) [34], [35]. Although expression of FOXP3 is considered as the best tool to define Treg cells by many authors [36], [37], [38], even this marker (as all other markers) is not specific to human Treg cells and can be transiently induced on all effector T cell populations upon activation.
In 2004, Karagiannidis et al. showed for the first time that GC treatment in vivo (both systemic and inhaled) induces an increase in circulating Treg cells (as defined by the FOXP3 and IL-10 mRNA expression of CD4+ T cells) in patients with asthma bronchiale [39]. However, such a positive correlation between GC treatment and the number of Treg cells in the peripheral blood is still disputed. In the mouse, Chen et al. could demonstrate that the synthetic GC dexamethasone increased the proportion of Treg cells both in peripheral blood and secondary lymphoid organs [40], [41]. By contrast, Stock et al. showed the opposite in a mouse model of asthma [42] as did Wüst et al. in a mouse model of multiple sclerosis [43]. In humans, several small in vivo studies pointed towards a positive correlation between administration of GCs and the frequency of Treg cells in patients with different autoimmune diseases [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]. However, two recently performed larger studies, both including more than 50 patients with asthma bronchiale or autoimmune connective tissue diseases, respectively, arrived at exactly the opposite conclusion [55], [56]. The fact that these studies do not present a unified picture of the influence of GCs on Treg cells may be explained by two aspects. Firstly, there is a huge heterogeneity in the molecular characterization of Treg cells. Several studies just defined Treg cells as being CD4+CD25high. However, it is evident by now that many of these cells are not Treg cells but rather activated T cells [57]. Secondly, all but one study analyzed patients with an autoimmune background and it is likely that different autoimmune diseases come along with different levels of impairment of Treg cell frequency and/or function [47], [48], [51], [53]. The only study on healthy donors so far was performed ex vivo using mixed PBMC cultures in the presence of dexamethasone, epinephrine and IL-2 [58]. Nonetheless, it has been already shown in mice that IL-2 topple the balance in favor of Treg cells regardeless of GC treatment [40], [41].
Hence, the goal of this study was to determine the influence of short-term GC therapy, as frequently used in different clinical scenarios, on circulating Treg cells in immunologically uncompromised mice and humans in vivo.
Methods
Animal experiments
Mice
C57Bl/6 mice were bred in the animal facility at the University of Göttingen Medical School, kept in individually ventilated cages under specific pathogen free conditions and used at an age of 8–12 weeks. Mice of both sexes were included in the study. Blood samples were taken by bleedings from the tail, or the mice were sacrificed using CO2 to obtain the spleen or blood from the heart. All animal experiments were conducted according to ethical standards of humane animal care and approved by the authorities of Lower Saxonia (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit, LAVES; approval ID: 33.14.42502-04-107.08).
GC treatment was performed by intraperitoneal (IP) injection of 0.8 mg/kg, 4 mg/kg, 20 mg/kg or 100 mg dexamethasone dihydrogen phosphate (Dexa-ratio-pharm®, Ratiopharm, Ulm, Germany) / kg body weight on three consecutive days [43]. Mice treated IP with 100 mg/kg were treated for additional 11 days with oral glucocorticoids with decreasing dosages of water-soluble dexamethasone (Sigma, Taufkirchen, Germany) added to the drinking water (days 4 – 7: 10 mg/l, days 8 – 11: 5 mg/l, days 12 – 14: 1 mg/l). The supplemented drinking water was changed every second day.
Lymphocyte analysis
Lymphocytes were isolated from the spleen of the mice on days 0, 3 and 14 by passing the freshly isolated organs through a 40 µm Nylon mesh, washed in FACS-Buffer (PBS with 0.5% BSA and 0.05% NaN3), erythrolyzed and counted. Absolute cell numbers in blood were determined by flow cytometric analysis of a defined blood volume. Analysis of splenocytes and blood lymphocytes by six-color flow-cytometry was performed using a FACS Canto II device (BD Biosciences, Heidelberg, Germany) in combination with FlowJo software. Monoclonal antibodies directed against mouse leukocyte specific molecules were obtained from BD Biosciences unless otherwise indicated (clone name in brackets): CD3 ε (145-2C11), CD4 (RM4-5), CD8α (53–6.7), CD25 (PC61), TCRαβ (H57-597), GR-1 (RB6-8C5), B220 (RA3-6B2), FoxP3 (FJK-16S; eBiosciences, Frankfurt, Germany). The antibodies were directly labeled with FITC, PE, PerCP, PE-Cy7, Cy5, APC or APC-Cy7, respectively.
Analysis of the suppressive activity of Treg cells in vitro
CD4+CD25+ regulatory T (Treg) cells and conventional CD4+CD25− helper T (Th) cells serving as indicator cells were purified from spleens of C57BL/6 mice using the CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotech, Bergisch Gladbach, Germany). Th cells (5×104 cells / well) and different amounts of Treg cells were cocultured with γ-irradiated antigen-presenting cells (30 Gy, 105 cells / well) for 48 hrs in RPMI 1640 medium supplemented with 10 % FCS and 1% Pen/Strep in 96-well U-bottom plates. Polyclonal stimulation was achieved by adding Con A (2,5 µg/ml) into the cultures. Water-soluble dexamethasone (Sigma, Taufkirchen, Germany) was added to a final concentration of 5 nM where indicated. Unstimulated and stimulated Th cells and Treg cells alone served as controls. Proliferation was assessed by measuring 3H-TdR (Hartmann Analytics, Braunschweig, Germany) incorporation (37 kBq / well) during an additional culture period of 16 hours. The labeled DNA was harvested onto Filtermat A glassfibre filters and the radioactivity was quantified using a MicroBeta2 ß-scintillation counter (Perkin Elmer, Rodgau, Germany). In addition, supernatants were collected from cultures after 48 hrs and Interleukin-2 levels were measured using a commercially available ELISA kit (BioLegend, San Diego/CA, USA) according to the manufacturers' instructions.
Human experiments
Patients
16 patients (10 males and 6 females) with acute hearing loss were routinely treated in the Department of Ophthalmology and Otolaryngology of the University Hospital of Würzburg using a modified “Stennert scheme” (only GCs without pentoxifyllin as clinically indicated). All individuals were otherwise healthy and fulfilled the following inclusion criteria: age >18 years (mean patient age was 35.8±13 years), no GCs or other immunosuppressive therapy in the last 8 weeks, no suspected endogenous GC excess and no evidence for any malignant or autoimmune diseases or any acute or chronic infections. All patients gave written informed consent and the study was approved by the ethic committee of the University of Würzburg (Ethik-Kommission der Medizinischen Fakultät der Universität Würzburg, Amendment 1 zu Studien-Nr. 83/05). Prednisolone was administered according to the following regimen: day 1–3: 250 mg intra venous (IV), day 4: 150 mg IV, day 5–9: 100 mg per os (P.O.), day 10–11: 75 mg P.O., day 12: 50 mg P.O., day 13: 20 mg P.O. and day 14: 10 mg P.O.
Lymphocyte analysis
Before the first injection of prednisolone (day 0) and at the end of the 14 day treatment period 5 ml EDTA and 20 ml heparinized blood were taken from the patients. The EDTA blood was analyzed in the central laboratory of the University Hospital of Würzburg for a complete blood count with white blood cell differential. The heparinized blood was diluted with 20 ml phosphate buffer saline (PBS). Subsequently, the diluted blood was applied to a Ficoll hypaque® gradient and separated via centrifugation at 400 G for 30 minutes [59]. The peripheral blood mononuclear cells (PBMC) were pipetted from the interface and washed twice with PBS. For a detailed characterization of cellular changes induced by GCs, the PBMCs were stained according to manufacturers' protocols using six different sets of monoclonal antibodies directed against various surface molecules (clone numbers in brackets): Set 1: CD3 (UCHT1), CD14 (M5E2), CD19 (HIB19); Set 2: CD8 (SK-1), CD25 (M-A251), CTLA-4 (BNI3); Set 3: CD4 (RPA-T4), CD25 (M-A251), CD127 (hIL-7R-M21); Set 4: CD4 (RPA-T4), CD25 (M-A251), AITR (eBioAITR); Set 5: CD4/CD 25 (RPA-T4/BC96) and staining control Set 6: no antibodies. Subsequently, the cells which had been stained with the surface markers of sets 5 and 6 were fixed and permeabilized according to the manufacturer's instructions followed by intracellular staining with rat-anti-FOXP3 (PCH101) and the appropriate isotype control antibody (eBR2a). The antibodies used in the sets 1 to 4 were obtained from BD Biosciences (Heidelberg, Germany) and those in the sets 5 and 6 from eBiosciences (Frankfurt, Germany). All antibodies were directly labeled with FITC, PE and APC. Three-color flow cytometry measurements were performed using a FACS Calibur and data analysis was done by using CellQuest pro software (both BD Biosciences, Heidelberg, Germany).
Statistics
Results are depicted as mean ± standard deviation (SD). For statistical analysis a repeated measures Anova followed by Bonferroni comparison test was performed using Prism v.4.b. software for Macintosh (GraphPad Software Inc., La Jolla, CA, USA). A P value <0.05 was considered statistically significant.
Results
Systemic high-dose GC treatment of mice reduces Treg cells numbers in peripheral blood and spleen
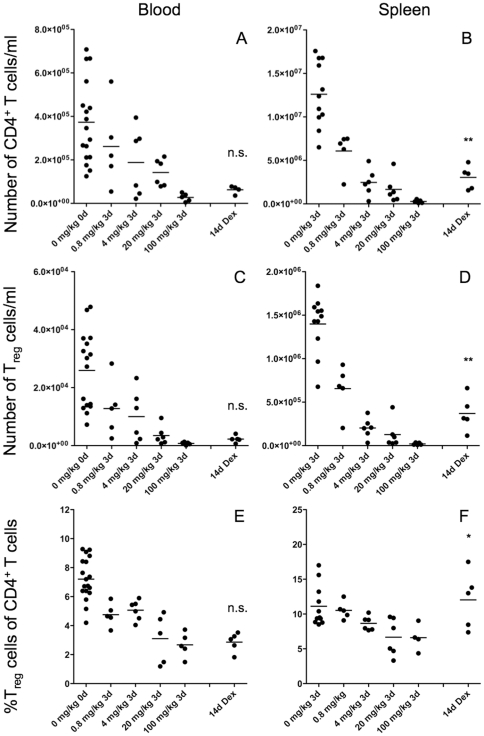
We have chosen a protocol for GC treatment of mice mimicking steroid regimens widely applied to human patients. Whilst therapy of acute relapses in multiple sclerosis patients for example consists of several repeated injection of high-dose GC followed by a taper [60], [61], lower concentrations of GC are used to treat rheumatoid arthritis or asthma patients. Our protocol has been further adapted to consider differences between mice and humans in terms of pharmacokinetics and handling by employing IP injections of dexamethasone [43], [62]. The number of CD4+ T cells in the blood of the mice decreased in a dose-dependent manner dropping to levels below 10% of control animals after 3 days of IP treatment with 100 mg/kg dexamethasone (Figure 1A). CD4+ T cell numbers remained low even after oral steroid taper (Table 1 and Figure 1A). The number of Treg cells defined as CD4+CD25highFOXP3+ T cells also decreased in a dose-dependent manner after the 3 day of GC therapy and did not recover after an additional 11 days of oral treatment (Figure 1C). Importantly, also the percentage of FOXP3+ Treg cells decreased amongst the CD4+ T cell population with the GC doses applied, and did not recover at day 14 (Figure 1E).
Figure 1. Modulation of CD4+ T cells and Treg cells by GCs in mice.
Peripheral blood (A, C, E) and spleen (B, D, E) cells from C57BL/6 mice were analyzed by flow cytometry before, 3 days after IP treatment with different dosages of dexamethasone and 14 days after IP treatment with 100 mg/kg dexamethasone followed by oral taper. The absolute numbers of CD4+ T cells (A, B) and CD4+CD25highFOXP3+ Treg cells (C, D) were assessed and the relative frequency of Treg cells amongst all CD4+ T cells was calculated (E, F); *p<0.05, **p<0.01.
Table 1. Lymphocyte counts and percentages in the blood and spleen of mice before and after GC therapy in vivo.
| cell numbers (cells / ml ± SD) | ||||||
| before therapy | 3d after IP therapy with dexamethason (mg/kg) | 14d after therapy | ||||
| Blood | 0.8 | 4 | 20 | 100 | ||
| CD4+ cells | 37.3×104±18.8×104 | 26.2×104±18.9×104 | 18.8×104±15.7×104 | 14.2×104±6.2×104 | 2.8×104±1.8×104 | 6.3×104±1.8×104 |
| FOXP3+ cells* | 2.6×104±1.3×104 | 1.3×104±1.0×104 | 1.0×104±0.9×104 | 0.3×104±0.3×104 | 0.07×104±0.05×104 | 0.22×104±0.12×104 |
| %FOXP3 in CD4+ cells | 7.2±1.4 | 4.8±0.8 | 5±0.7 | 3.1±1.7 | 2.7±0.8 | 2.9±0.7 |
| spleen | cell numbers (cells / spleen ± SD) | |||||
| CD4+ cells | 12.6×106±3.8×106 | 6.0×106±2.2×106 | 2.5×106±1.6×106 | 1.6×106±1.5×106 | 0.3×106±0.2×106 | 3.0×106±1.4×106 |
| FOXP3+ cells* | 1.4×106±0.3×106 | 0.7×106±0.3×106 | 0.2×106±0.1×106 | 0.1×106±0.1×106 | 0.02×106±0.01×106 | 0.4×106±0.2×106 |
| %FOXP3 in CD4+ cells | 11.1±2.9 | 10.53±1.3 | 8.7±1.0 | 6.7±2.7 | 6.6±1.9 | 12.0±.1 |
* = as subpopulation of CD4+CD25high cells.
‡ = % out of CD4+ cells ± SD.
In the spleen of the same mice the situation was slightly different. Although the absolute number of CD4+ T cells also strongly and dose-dependently decreased after the 3 days IP treatment (Table 1 and Figure 1B), the cell numbers partially recovered during the phase of oral GC taper (Figure 1B). The same situation applies to the FOXP3+ Treg cells, the number of which decreased after 3 days and slightly recovered after the 14 days of GC treatment (Figure 1D). Consequently, the relative proportion of Treg cells amongst the CD4+ T cell population significantly decreased (Figure 1F) after the high-dose systemic GC treatment. Interestingly, the percentage of Treg cells recovered after the additional 11 days of oral taper to values comparable to those before treatment (Figure 1F).
GC treatment does not impact the suppressive capacity of Treg cells in vitro
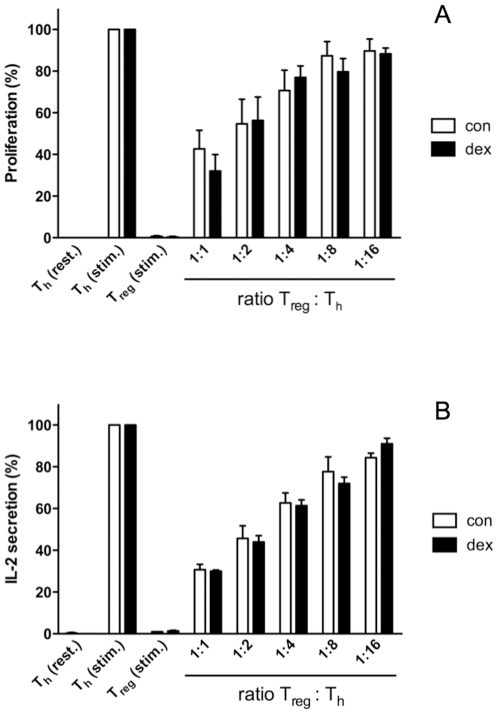
Besides altering the absolute or relative numbers of Treg cells in blood and peripheral lymphoid organs, it is also conceivable that GC impact the suppressive capacity of Treg cells. This functional characteristic is best studied in vitro. Furthermore, a concentration of 5 nM dexamethasone was chosen since pilot experiments had revealed that this already leads to a significant regulation of T cell function while at the same time proliferation is only slightly affected (data not shown). To assess whether GC alter the functional properties of Treg cells in vitro, we performed a standard suppression assay in the absence or presence of dexamethasone by adding decreasing amounts of Treg cells to ConA-stimulated Th cells serving as indicator cells. The activity of the Th cells was assessed by analyzing proliferation on the basis of 3H-TdR incorporation (Figure 2A) as well as by determining IL-2 production (Figure 2B). Importantly, addition of dexamethasone into the culture did not significantly alter the suppressive capacity of Treg cells, neither in terms of proliferation (42.7±15.5 % at 1∶1 Treg:Th ratio in control cells vs 32.0±13.7 % in cells treated with dexamethasone; n.s.) nor in terms of IL-2 secretion (30.7±4.5 % at 1∶1 Treg:Th ratio in control cells vs 30.0±1.0 % in cells treated with dexamethasone; n.s.) (Figure 2). This suggests that - at least in vitro - GCs do neither positively nor negatively influence the functional properties of Treg cells.
Figure 2. Effect of GC on the function of Treg cells in vitro.
Th cells (5×104 cells / well) were incubated with Treg cells at different ratios in the presence of irradiated APC (30 Gy, 105 cells / well) and Con A (2,5 µg / ml) for 48 hrs, either with dexamethasone (dex, 5 nM) or without it (con). Resting Th cells as well as Th and Treg cells stimulated with Con A served as controls. Proliferation was determined by 3H-TdR incorporation during an additional 16 hrs culture period (A), IL-2 levels were directly measured in the supernatants by ELISA (B). All values were normalized to stimulated Th cells treated with or without Dex, respectively. In both panels the combined data of three independent experiments are depicted.
GCs have little impact on the relative frequency of circulating Treg cells in immunologically uncompromised human subjects
The 14 days of prednisolone administration to human subjects, none of which suffered from immunological diseases, induced a doubling of circulating leukocytes (Table 2). As expected, this was mainly attributed to a significant increase in circulating neutrophils. In addition, the number of monocytes also doubled and the number of lymphocytes increased by 30%, while the numbers of basophils and eosinophils remained unaltered. Of note, the frequency of each cell type within the leukocyte population remained largely unchanged with the exception of the eosinophils that decreased slightly (Table 2).
Table 2. Blood lymphocyte counts in immunocompetent human subjects before and after GC therapy in vivo.
| cell numbers (cells/ml ± SD) | frequency (% ±SD) | |||||
| cell type | before therapy | after therapy | p value | before therapy | after therapy | p value |
| leukocytes | 7.3±3.0×106 | 13.0±4.2×106 | p<0.001 | - | - | - |
| neutrophils | 4.6±2.6×106 | 9.3±3.7×106 | p<0.001 | 60.4±11.6# | 68.0±10.3# | n.s. |
| basophils | 1.7±4.0×104 | 7.0±3.0×103 | n.s. | 0.2±0.5# | 0.1±0.3# | n.s. |
| eosinophils | 1.4±1.4×105 | 1.3±1.0×105 | n.s. | 2.5±2.6# | 0.9±0.7# | p<0.05 |
| monocytes | 5.0±1.5×105 | 8.5±2.6×105 | p<0.001 | 7.5±2.3# | 6.5±1.7# | n.s. |
| lymphocytes | 2.0±0.7×106 | 3.0±1.3×106 | p<0.01 | 28.7±8.5# | 24.0±9.6# | n.s. |
| CD3+ cells | 1.5±0.6×106 | 2.3±1.0×106 | p<0.001 | 58.0±11.0§ | 58.0±9.0§ | n.s. |
| CD8+ cells | 4.0±2.5×105 | 5.8±3.1×105 | p<0.001 | 24.5±9.0$ | 24.3±7.5$ | n.s. |
| CD4+ cells | 11.0±4.0×105 | 18.0±7.7×105 | p<0.001 | 75.5±9.0$ | 75.7±7.5$ | n.s. |
| FOXP3+ cells* | 6.2±3.5×104 | 8.2±4.7×104 | p<0.05 | 4.0±1.5‡ | 3.4±1.5‡ | p<0.05 |
| AITR+ cells* | 8.9±6.8×103 | 10.0±9.0×103 | n.s. | 0.6±0.4‡ | 0.5±0.3‡ | n.s. |
| CD127low cells* | 5.6±2.4×104 | 10.0±6.0×104 | p<0.001 | 4.0±1.3‡ | 5.0±3.0‡ | p<0.05 |
| CTLA4+ cells* | 1.6±0.7×105 | 3.0±2.0×105 | p<0.05 | 13.8±11.5‡ | 15.6±12.5‡ | n.s. |
* = as subpopulation of CD4+CD25high cells.
# = % out of leukocyte cells ± SD.
§ = % out of PBMC ± SD.
$ = % out of CD3+ cells ± SD.
‡ = % out of CD4+ cells ± SD.
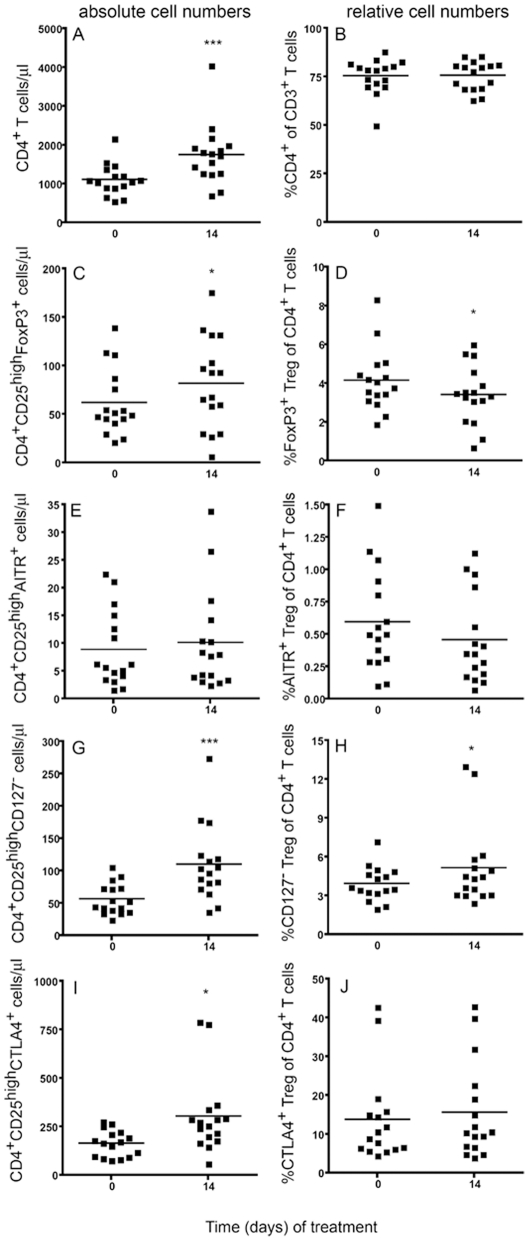
With regard to the T cell population, the 14 days of GC administration induced a 1.5-fold increase in the number of CD3+ T cells from 15±5.8×105 to 23±10×105 cells/ml (p<0.001; Table 2) due to an increase in CD8+ T cell numbers (4±2.5×105 to 5.8±3.1×105 cells/ml; p<0.001) as well as CD4+ cell numbers (11±4.0×105 to 18±7.7×105 cells/ml; p<0.001; Figure 3A). In contrast, the relative frequency of all T cell subpopulations remained unaffected (58±11 to 58±9.0% T-cells in PBMC; 24.5±9.0% to 24.3±7.5% CD8+ T cells and 75.5±9.0% to 75.7±7.5% CD4+ T cells within the T cell population; Figure 3B). The Treg cells, being a subpopulation of the CD4+ T cells, followed the positive trend in absolute cell numbers, however, depending on their molecular characterization, the amplitude differed (Figure 4). The number of Treg cells increased only slightly when defined as FOXP3+ (62±35×103 to 82±47×103 cells/ml; p<0.05; Figure 3C and 4A, B) or AITR+ (8.9±6.8×103 to 10±9.0×103 cells/ml; n.s.; Figure 3E and 4C, D), whereas the CD127low and the CTLA4 Treg cells doubled from 56±24×103 to 100±60×103 cells/ml (p<0.001; Figure 3G and 4E, F) and from 160±70×103 to 300±200×103 cells/ml (p<0.05; Figure 3I and 4G, H), respectively. The frequency of the Treg cells amongst the CD4+ T cells also varied depending on the molecular characterization. Whereas the frequency of FOXP3+ and AITR+ Treg cells within the CD4+ T cell population slightly decreased after GC treatment from 4.0±1.5 to 3.4±1.5% (p<0.05; Figure 3D) and 0.6±0.4% to 0.45±0.3% (n.s.; Figure 3F), respectively, their percentages did not increase in a relevant manner from 4.0±1.3% to 5±3.0% (p<0.05; Figure 3H) and from 13.8±11.5% to 15.6±12.5% (n.s.; Figure 3J) when the Treg cells were defined as CD127low and CTLA4+, respectively.
Figure 3. Modulation of CD4+ T cells and Treg cells by GCs in humans.
Peripheral blood cells from acute hearing loss patients before and 14 days after prednisolone treatment were analyzed by flow cytometry and the absolute numbers (A, C, E, G, I) and the frequency (B, D, F, H, J) of CD4+ T cells (A, B) and Treg cells (CD4+CD25high and FOXP3+ (C, D), AITR+ (E, F), CD127low (G, H) or CTLA4+ (I, J)) were assessed; *p<0.05, ***p<0.001.
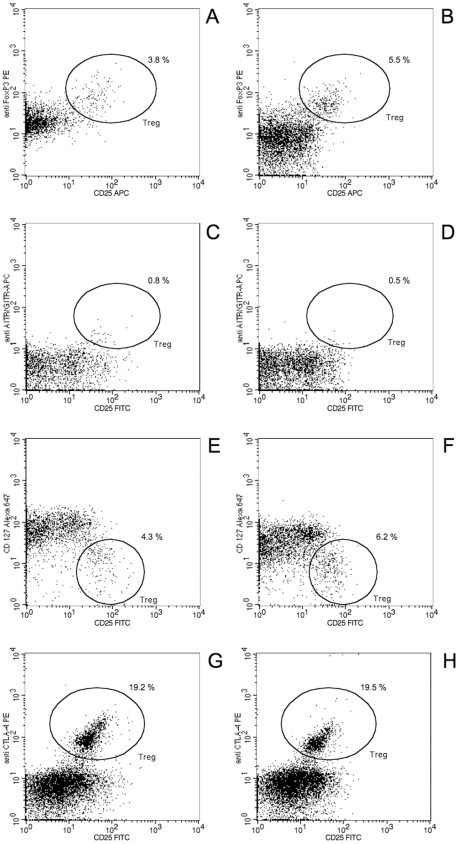
Figure 4. Flow cytometric analysis of Treg cells according to different markers.
Peripheral blood from one representative hearing-loss patient treated for 3 days (A, C, E, G) and 14 days (B, D, F, H) with glucocorticoid regimen was analyzed for the presence of regulatory T cells according to the following markers: CD4+ CD25high and FOXP3+ (A, B), AITR+ (C, D) CD127low (E, F) and CTLA4+ (G, H). Only CD4+ cells are depicted and the percentages indicate the relative frequency of Treg cells within this subpopulation.
Discussion
Treg cells exert potent suppressive effects on the immune system through a plethora of different mechanisms. It has been previously reported that the frequency of Treg cells is essential for the prevention of exaggerated inflammation and autoimmunity [24], [25], and some studies suggested that Treg cells are induced by GCs both in mice and humans [41], [50]. It was hypothesized that this is due to the differential responsiveness of this specialized T cell subpopulation to GCs [40]. However, the respective experiments were mostly performed not as a monotherapy but rather in combination with potent T-cell inducers like IL-2 or vitamin D3, or they were performed on vaccinated or immunodefficient mice [41], [63]. To reassess the impact of GC therapy on Treg cells in mice in the context of steroid monotherapy, we applied a protocol with increasing dosages of 0.8 to 100 mg/kg dexamethasone IP for 3 days. This therapy led to a dose dependant decrease of CD4+ T cells in peripheral blood and spleen. Surprisingly, the decrease in the number of Treg cells was even more pronounced than the number of total CD4+ T cells. This is in agreement with the data of Stock et al. [42] who show in a mouse asthma model that GCs induce Treg cell deficiency, which aggravates the long-term course of the allergic disease. It is also in line with the data of Wüst et al. [43] who reported a relative decrease of Treg cell numbers in a mouse model of multiple sclerosis following high-dose GC therapy. During the additional 11 days of oral taper (from 10 mg/l to 1 mg/l) the low T cell numbers and frequencies in the blood persisted whereas the number of CD4+ T cells in the spleen slightly recovered and the proportion of Treg cells even reached similar levels compared to before the therapy. The observed differences between peripheral blood and spleen might suggest that while under GC therapy no new Treg cells are being produced by the thymus, they are constantly generated de novo in the secondary lymphatic organs. This corroborates data showing that GCs induce an immature dendritic cell (DC) phenotype in the periphery capable of transforming naïve T cells into Treg cells [64]. We therefore postulate that high-dose CG therapy in the mouse induces massive apoptosis of CD4+ T cells, that Treg cells show an increased sensitivity to GCs, and that a prolonged lower dose of GCs favors their tolerogenic DC-induced regeneration in the periphery.
Our finding that the absolute and relative numbers of Treg cells in blood and spleen are decreased by GC treatment in vivo does not necessarily exclude that the functional properties of the Treg cells are altered. However, we found that the suppressive capacity of Treg cells was indistinguishable in the presence or absence of GC at least in vitro. Neither inhibition of Th cell proliferation nor repression of their IL-2 production by Treg cells was different when dexamethasone was added to the cultures. Although we cannot fully exclude that Treg cell function in vivo is affected by GC treatment, our in vitro results nevertheless strongly argue that GC do not impact the functional properties of Treg cells directly.
In the human subjects, 14 days of prednisolone administration in a dosage used for the treatment of many common diseases led to strong blood leukocytosis, mainly due to the already described increase in circulating neutrophils and monocytes but to a minor extent also in lymphocytes. Of note, T cells as well as their CD4+ and CD8+ subpopulations increased at the same rate. On average, the numbers of CD4+CD25high Treg cells increased independently of additional markers used for their identification. However, this change was only significant when using FOXP3+, CD127low and CTLA4+ as definition of Treg cells but not AITR+. Similarly, the frequency of FOXP3+ Treg cells within the CD4+ T cell population decreased slightly, whereas the relative number of CD127low Treg cells increased and the one of AITR+ and CTLA4+ Treg cells showed no relevant changes concerning the Treg/CD4+ T cell ratio. We therefore conclude that GCs have no relevant impact on the frequency of circulating Treg cells. However, our study also indicates that the outcome of the analysis of Treg cells in peripheral blood of human subjects depends - at least in part - on the markers used for their molecular characterization. This could explain - amongst others - the partially contradictory results published before. Considering FOXP3 expression, the most accepted marker of Treg cells, in our human cohort, the GC taper treatment seems to induce an increase in Treg cell numbers but at the same time an even stronger increase in CD4+ T cells. This even results in a significant reduction of the relative frequency of Treg cells as compared to the rest of the circulating CD4+ T cells and thereby confirms our results obtained in mice. Thus, our findings call the hypothesis that GCs exert some of their immunosuppressive capacity by inducing Treg cells into question. Therefore, it is tempting to speculate that GCs rather modify cell-cell interactions of Treg cells with other immune cells than increasing their frequency.
Our results are seemingly in contrast to several previously published in vivo studies that showed an increased number of Treg cells and, even more importantly, an elevated Treg/CD4+ T cell ratio in humans treated with GCs [44], [45], [50]. However, as mentioned before, most of these studies were performed in patients either suffering from hyperimmune or autoimmune diseases [51], [52], [53], [54]. Interestingly, the same studies show a deficiency of Treg cells in the same patients, probably due to thymic dysfunction, and thus the GC treatment seems to only restore their numbers to levels of healthy subjects. Based on our data we therefore hypothesize that GCs induce a tolerogenic environment in these patients leading to the generation of Treg cells in the secondary lymphatic organs which compensates for the thymic dyfunction by creating an apparent advantage of the Treg cells as compared to the rest of the T cells. However, the reconstitution of the Treg cells will last only until the therapy ends and the de novo generated Treg cells are recycled, which might explain the short-lived benefit and the high relapse rate described after GC therapy [65].
Our study has strengths and limitations. Firstly, the individuals of our study cohort consisting of patients with acute hear loss are most likely immunocompetent although the exact cause of the acute hear loss is unknown [66], [67]. Secondly, the dosage of GCs in our prospective study was chosen rather arbitrarily and might have impact on the results. Of note, 5 of 7 patients tested on day 14 showed impaired adrenocortical function as measured by ACTH stimulation test (data not shown). In the case of our mouse study, we administered a regimen consisting of increasing doses of dexamethasone [43], whereas in the human study we used the standard dosage for hear loss, which is also utilized for several autoimmune diseases such as lupus erythematosus [68] or idiopathic thrombocytopenic purpura [69]. Thirdly, we analyzed only selected time points and it thus remains open whether the effects that we see are only transient and would be different after shorter or longer time periods of GC treatment. Finally, we used several markers for Treg cell characterization, acknowledging the fact that there is still no consensus on the best definition of Treg cells in humans. However, despite the results being slightly different between each Treg cell characterization, the effect on the relative frequency of circulating Treg cells in general is weak.
In conclusion, short-term GC therapy did not induce the expected increase in the frequency of circulating Treg cells, neither in immunocompetent human subjects nor in mice. In the same time GC treatment in vitro did not have any direct effect on the functional ability of the Treg cells. Thus, it is doubtful whether GCs exerts their immunosuppressive effects via influencing the functionality and the (relative) number of Treg cells in blood and spleen.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the Deutsche Forschungsgemeinschaft (KFO124, TP6 to M.F. and H.M.R. (grant Fa466/3-1) and Re1631/8-1 to H.M.R.) and the Deutsche Krebshilfe (grant #107111 to M.F.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Norgaard P, Poulsen HS. Glucocorticoid receptors in human malignancies: a review. Ann Oncol. 1991;2:541–557. doi: 10.1093/oxfordjournals.annonc.a058018. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann N Y Acad Sci. 2009;1179:153–166. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanchi NE, Filho MA, Felitti V, Nicastro H, Lorenzeti FM, et al. Glucocorticoids: extensive physiological actions modulated through multiple mechanisms of gene regulation. J Cell Physiol. 2010;224:311–315. doi: 10.1002/jcp.22141. [DOI] [PubMed] [Google Scholar]
- 4.Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, et al. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 5.Gaillard RC. Interaction between the hypothalamo-pituitary-adrenal axis and the immunological system. Ann Endocrinol (Paris) 2001;62:155–163. [PubMed] [Google Scholar]
- 6.Da Silva JA. Sex hormones and glucocorticoids: interactions with the immune system. Ann N Y Acad Sci. 1999;876:102–117; discussion 117-108. doi: 10.1111/j.1749-6632.1999.tb07628.x. [DOI] [PubMed] [Google Scholar]
- 7.Giannopoulos G. Early events in the action of glucocorticoids in developing tissues. J Steroid Biochem. 1975;6:623–631. doi: 10.1016/0022-4731(75)90043-6. [DOI] [PubMed] [Google Scholar]
- 8.Martignoni E, Costa A, Sinforiani E, Liuzzi A, Chiodini P, et al. The brain as a target for adrenocortical steroids: cognitive implications. Psychoneuroendocrinology. 1992;17:343–354. doi: 10.1016/0306-4530(92)90040-e. [DOI] [PubMed] [Google Scholar]
- 9.Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- 10.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154:4719–4725. [PubMed] [Google Scholar]
- 11.Nakagawa M, Bondy GP, Waisman D, Minshall D, Hogg JC, et al. The effect of glucocorticoids on the expression of L-selectin on polymorphonuclear leukocyte. Blood. 1999;93:2730–2737. [PubMed] [Google Scholar]
- 12.Schleimer RP, Bochner BS. The effects of glucocorticoids on human eosinophils. J ALLERGY CLIN IMMUNOL. 1994;94:1202–1213. doi: 10.1016/0091-6749(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura C, Miyamasu M, Nagase H, Iikura M, Yamaguchi M, et al. Glucocorticoids induce basophil apoptosis. J ALLERGY CLIN IMMUNOL. 2001;108:215–220. doi: 10.1067/mai.2001.116575. [DOI] [PubMed] [Google Scholar]
- 14.van der Goes A, Hoekstra K, van den Berg TK, Dijkstra CD. Dexamethasone promotes phagocytosis and bacterial killing by human monocytes/macrophages in vitro. J Leukoc Biol. 2000;67:801–807. doi: 10.1002/jlb.67.6.801. [DOI] [PubMed] [Google Scholar]
- 15.Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. Faseb J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- 16.Cupps TR, Edgar LC, Thomas CA, Fauci AS. Multiple mechanisms of B cell immunoregulation in man after administration of in vivo corticosteroids. J Immunol. 1984;132:170–175. [PubMed] [Google Scholar]
- 17.Segerer SE, Muller N, van den Brandt J, Kapp M, Dietl J, et al. Impact of female sex hormones on the maturation and function of human dendritic cells. Am J Reprod Immunol. 2009;62:165–173. doi: 10.1111/j.1600-0897.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- 18.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 20.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 22.Hahn K, Husstedt IW. [HIV-associated neuropathies]. Nervenarzt. 2010;81:409–417. doi: 10.1007/s00115-010-2931-x. [DOI] [PubMed] [Google Scholar]
- 23.Vucic S, Kiernan MC, Cornblath DR. Guillain-Barre syndrome: an update. J Clin Neurosci. 2009;16:733–741. doi: 10.1016/j.jocn.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 24.Kondelkova K, Vokurkova D, Krejsek J, Borska L, Fiala Z, et al. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kralove) 2010;53:73–77. doi: 10.14712/18059694.2016.63. [DOI] [PubMed] [Google Scholar]
- 25.Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression - a diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hench P. Effects of cortisone in the rheumatic diseases. Lancet. 1950;2:483–484. [PubMed] [Google Scholar]
- 27.Hirschberg T, Randazzo B, Hirschberg H. Effects of methylprednisolone on the in vitro induction and function of suppressor cells in man. Scand J Immunol. 1980;12:33–39. doi: 10.1111/j.1365-3083.1980.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda T, Uchihara M, Daiguji Y, Hasumura Y, Takeuchi J. Immunological mechanisms of corticosteroid therapy in chronic active hepatitis: analysis of peripheral blood suppressor T-cell and interleukin 2 activities. Clin Immunol Immunopathol. 1988;48:371–379. doi: 10.1016/0090-1229(88)90031-1. [DOI] [PubMed] [Google Scholar]
- 29.Damoiseaux J. Regulatory T cells: back to the future. Neth J Med. 2006;64:4–9. [PubMed] [Google Scholar]
- 30.McNeill A, Spittle E, Backstrom BT. Partial depletion of CD69low-expressing natural regulatory T cells with the anti-CD25 monoclonal antibody PC61. Scand J Immunol. 2007;65:63–69. doi: 10.1111/j.1365-3083.2006.01870.x. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 34.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 37.Wieckiewicz J, Goto R, Wood KJ. T regulatory cells and the control of alloimmunity: from characterisation to clinical application. Curr Opin Immunol. 2010;22:662–668. doi: 10.1016/j.coi.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hori S. Developmental plasticity of Foxp3+ regulatory T cells. Curr Opin Immunol. 2010;22:575–582. doi: 10.1016/j.coi.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, et al. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004;114:1425–1433. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Murakami T, Oppenheim JJ, Howard OM. Differential response of murine CD4+CD25+ and CD4+CD25- T cells to dexamethasone-induced cell death. Eur J Immunol. 2004;34:859–869. doi: 10.1002/eji.200324506. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OM. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol. 2006;36:2139–2149. doi: 10.1002/eji.200635873. [DOI] [PubMed] [Google Scholar]
- 42.Stock P, Akbari O, DeKruyff RH, Umetsu DT. Respiratory tolerance is inhibited by the administration of corticosteroids. J Immunol. 2005;175:7380–7387. doi: 10.4049/jimmunol.175.11.7380. [DOI] [PubMed] [Google Scholar]
- 43.Wust S, van den Brandt J, Tischner D, Kleiman A, Tuckermann JP, et al. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J Immunol. 2008;180:8434–8443. doi: 10.4049/jimmunol.180.12.8434. [DOI] [PubMed] [Google Scholar]
- 44.Azab NA, Bassyouni IH, Emad Y, Abd El-Wahab GA, Hamdy G, et al. CD4+CD25+ regulatory T cells (TREG) in systemic lupus erythematosus (SLE) patients: the possible influence of treatment with corticosteroids. Clin Immunol. 2008;127:151–157. doi: 10.1016/j.clim.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Lee HY, Hong YK, Yun HJ, Kim YM, Kim JR, et al. Altered frequency and migration capacity of CD4+CD25+ regulatory T cells in systemic lupus erythematosus. Rheumatology (Oxford) 2008;47:789–794. doi: 10.1093/rheumatology/ken108. [DOI] [PubMed] [Google Scholar]
- 46.Suarez A, Lopez P, Gomez J, Gutierrez C. Enrichment of CD4+ CD25high T cell population in patients with systemic lupus erythematosus treated with glucocorticoids. Ann Rheum Dis. 2006;65:1512–1517. doi: 10.1136/ard.2005.049924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, et al. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009;64:1539–1546. doi: 10.1111/j.1398-9995.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- 48.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q, Qian FH, Liu H, Zhou LF, Huang M, et al. Expression of surface markers on peripheral CD4+CD25high T cells in patients with atopic asthma: role of inhaled corticosteroid. Chin Med J (Engl) 2008;121:205–212. [PubMed] [Google Scholar]
- 50.Braitch M, Harikrishnan S, Robins RA, Nichols C, Fahey AJ, et al. Glucocorticoids increase CD4CD25 cell percentage and Foxp3 expression in patients with multiple sclerosis. Acta Neurol Scand. 2009;119:239–245. doi: 10.1111/j.1600-0404.2008.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fattorossi A, Battaglia A, Buzzonetti A, Ciaraffa F, Scambia G, et al. Circulating and thymic CD4 CD25 T regulatory cells in myasthenia gravis: effect of immunosuppressive treatment. Immunology. 2005;116:134–141. doi: 10.1111/j.1365-2567.2005.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ling Y, Cao X, Yu Z, Ruan C. Circulating dendritic cells subsets and CD4+Foxp3+ regulatory T cells in adult patients with chronic ITP before and after treatment with high-dose dexamethasome. Eur J Haematol. 2007;79:310–316. doi: 10.1111/j.1600-0609.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 53.Xu L, Xu Z, Xu M. Glucocorticoid treatment restores the impaired suppressive function of regulatory T cells in patients with relapsing-remitting multiple sclerosis. Clin Exp Immunol. 2009;158:26–30. doi: 10.1111/j.1365-2249.2009.03987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu YF, Cai XH, Zhu JY, Jiang WP, Lan JH, et al. [Study of CD4+ CD25+ regulatory T cells and expression of Foxp3 mRNA in bronchiolitis and glucocorticoid regulation]. Zhonghua Yi Xue Za Zhi. 2009;89:1563–1566. [PubMed] [Google Scholar]
- 55.Banica L, Besliu A, Pistol G, Stavaru C, Ionescu R, et al. Quantification and molecular characterization of regulatory T cells in connective tissue diseases. Autoimmunity. 2009;42:41–49. doi: 10.1080/08916930802282651. [DOI] [PubMed] [Google Scholar]
- 56.Majak P, Rychlik B, Stelmach I. The effect of oral steroids with and without vitamin D3 on early efficacy of immunotherapy in asthmatic children. Clin Exp Allergy. 2009;39:1830–1841. doi: 10.1111/j.1365-2222.2009.03357.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhang B, Zhang X, Tang F, Zhu L, Liu Y. Reduction of forkhead box P3 levels in CD4+CD25high T cells in patients with new-onset systemic lupus erythematosus. Clin Exp Immunol. 2008;153:182–187. doi: 10.1111/j.1365-2249.2008.03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiang L, Marshall GD Immunomodulatory Effects of in vitroStress Hormones on FoxP3, Th1/Th2 Cytokine and Costimulatory Molecule mRNA Expression in Human Peripheral Blood Mononuclear Cells. Neuroimmunomodulation. 2010;18:1–10. doi: 10.1159/000311450. [DOI] [PubMed] [Google Scholar]
- 59.Fassnacht M, Lee J, Milazzo C, Boczkowski D, Su Z, et al. Induction of CD4(+) and CD8(+) T-cell responses to the human stromal antigen, fibroblast activation protein: implication for cancer immunotherapy. Clin Cancer Res. 2005;11:5566–5571. doi: 10.1158/1078-0432.CCR-05-0699. [DOI] [PubMed] [Google Scholar]
- 60.Milligan NM, Newcombe R, Compston DA. A double-blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1. Clinical effects. J Neurol Neurosurg Psychiatry. 1987;50:511–516. doi: 10.1136/jnnp.50.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noseworthy JH. Management of multiple sclerosis: current trials and future options. Curr Opin Neurol. 2003;16:289–297. doi: 10.1097/01.wco.0000073929.19076.cd. [DOI] [PubMed] [Google Scholar]
- 62.Wust S, Tischner D, John M, Tuckermann JP, Menzfeld C, et al. Therapeutic and adverse effects of a non-steroidal glucocorticoid receptor ligand in a mouse model of multiple sclerosis. PLoS One. 2009;4:e8202. doi: 10.1371/journal.pone.0008202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie Y, Wu M, Song R, Ma J, Shi Y, et al. A glucocorticoid amplifies IL-2-induced selective expansion of CD4(+)CD25(+)FOXP3(+) regulatory T cells in vivo and suppresses graft-versus-host disease after allogeneic lymphocyte transplantation. Acta Biochim Biophys Sin (Shanghai) 2009;41:781–791. doi: 10.1093/abbs/gmp067. [DOI] [PubMed] [Google Scholar]
- 64.Matyszak MK, Citterio S, Rescigno M, Ricciardi-Castagnoli P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur J Immunol. 2000;30:1233–1242. doi: 10.1002/(SICI)1521-4141(200004)30:4<1233::AID-IMMU1233>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 65.Crane J, Pearce N, Burgess C, Woodman K, Robson B, et al. Markers of risk of asthma death or readmission in the 12 months following a hospital admission for asthma. Int J Epidemiol. 1992;21:737–744. doi: 10.1093/ije/21.4.737. [DOI] [PubMed] [Google Scholar]
- 66.Wei BP, Mubiru S, O'Leary S. Cochrane Database Syst Rev; 2006. Steroids for idiopathic sudden sensorineural hearing loss.CD003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. A Meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582–586. doi: 10.1001/archotol.133.6.582. [DOI] [PubMed] [Google Scholar]
- 68.Viertel A, Weidmann E, Wigand R, Geiger H, Mondorf UF. Treatment of severe systemic lupus erythematosus with immunoadsorption and intravenous immunoglobulins. Intensive Care Med. 2000;26:823–824. doi: 10.1007/s001340051260. [DOI] [PubMed] [Google Scholar]
- 69.Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura (ITP). Blood. 2005;106:2244–2251. doi: 10.1182/blood-2004-12-4598. [DOI] [PubMed] [Google Scholar]