Abstract
Memory phases, dependent on different neural and molecular mechanisms, strongly influence memory performance. Our understanding, however, of how memory phases interact is far from complete. In Drosophila, aversive olfactory learning is thought to progress from short-term through long-term memory phases. Another memory phase termed anesthesia resistant memory, dependent on the radish gene, influences memory hours after aversive olfactory learning. How does the radish-dependent phase influence memory performance in different tasks? It is found that the radish memory component does not scale with the stability of several memory traces, indicating a specific recruitment of this component to influence different memories, even within minutes of learning.
Introduction
Memory phases strongly influence memory performance over time. These phases can be influenced by different neural structures and molecular mechanisms. In the honeybee, for example, the role of the cAMP/PKA cascade is required in a short time window after training to induce a long-term memory phase [1]. And in vertebrate animals, it is widely held that the vertebrate hippocampus is needed in a transient way for some new memories to be formed [2]. Our understanding, however, of how memory phases ultimately influence conditioned behavior is far from complete.
Current models of how memory phases influence memory performance in Drosophila largely depends on results from aversive olfactory learning. In this type of learning flies are conditioned by associating electric shock with an odorant. A memory test allows flies to choose between the shock-associated odorant and a second odorant not previously associated with shock [3]. Memory performance in this paradigm is thought to mature through short-term memory (STM), middle-term memory (MTM), and long-term memory (LTM) phases [4], [5]. Memory in the minutes range after training is influenced by the cAMP / PKA signaling cascade, among other genes [3], [6]–[9]. An intriguing memory phase termed anesthesia resistant memory (ARM) has also been identified, which develops within hours of learning, and is operationally defined as the memory component that is resistant to the effects of cold-shock induced anesthesia [4]. The radish (rsh) gene (formerly CG15720, now referred to as CG42628) provides the main molecular insight into the mechanisms of ARM [4], [10]–[13]. While mutation of the rsh gene leads to a minor reduction in memory performance shortly after aversive olfactory training [13], this gene plays an increasingly important role in memory performance as ARM provides a more significant component of the overall memory [4], [13]. Thus, the currently accepted model is that memory consolidation in the range of hours after training is critically regulated by a rsh-dependent ARM.
We asked whether the rsh gene influence on memory formation is restricted to an hours-long memory phase following other forms of learning. Appetitive olfactory learning and operant place conditioning induce memories that decay at different rates compared to aversive classical olfactory learning. Aversive olfactory memory after one training session decays to near-zero levels within 24 hrs [3], [14]. In contrast, appetitive olfactory learning that associates sugar with an odorant in a single training session leads to memory that is stable for at least 24 hrs [15]–[17]. In operant place learning, where individual flies are conditioned to avoid part of a long narrow chamber using high temperature as a negative reinforcer, short training leads to a memory that decays within minutes [18], [19]. With prolonged intermittent-training memory decays to negligible levels within 2 hrs [20]. We asked how the rsh-dependent memory is established under these learning conditions.
Results
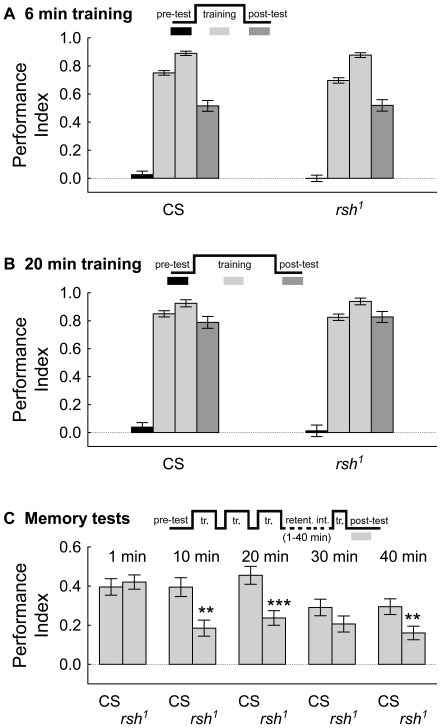
The role of the rsh gene in place learning and memory in the heat-box was examined. Both wild-type CS and rsh1 mutant flies were trained for either 6 or 20 min and place memory was tested directly afterward. The CS flies were used for comparison as this strain best represents the genetic background of the rsh1flies [12]. The rsh1 allele is either a strong hypomorphic or null allele as there is a stop codon toward the end of the coding region which reduces Rsh protein levels to below detection limits [12]. Only subtle differences were identified between flies of these genotypes in their avoidance behavior during training and in the memory post-test (Fig. 1A and B). Thus, consistent with previous tests of rsh1 flies in aversive olfactory memory and visual pattern learning [13], [21], up to this point the rsh gene has a minor role in early stages of memory formation.
Figure 1. Mutation of the rsh gene does not influence conditioning or place memory tested directly after training.
Following a 30 s pre-test period (black bars), wild-type CS and rsh1 mutant flies were trained in two equal length periods for a total of either 6 or 20 min with 41°C (light gray bars). A 3 min memory was tested directly following in the post-test period (dark gray bars). The training, retention intervals, and testing patterns (both pre and post) are diagrammed for each panel, the time axis is not to scale. (A), Conditioning and memory tests were similar between the genotypes with 6 min of training (N = 331; pre-test: U = 12753.5, z = 1.07, P = 0.28; 1st training period: U = 11877.0, z = 2.08, P = 0.04; 2nd training period: U = 12888.5, z = 0.92, P = 0.36; post-test: U = 13237.0, z = 0.51, P = 0.61). (B) Conditioning and memory tests were also similar between the genotypes with 20 min of training (N = 232; pre-test: U = 6106.5, z = 1.22, P = 0.22; 1st training period: U = 5740.5, z = 1.93, P = 0.06; 2nd training period: U = 5802.0, z = −1.81, = 0.07; post-test: U = 6463.0, z = −0.52, P = 0.60). (C) The rsh gene is necessary for normal short-term place memory. Flies were trained with intermittent training and then held for varying times (1 – 40 min) before being tested for memory with a short reminder training. The rsh1 flies had memory performance similar to wild-type CS levels with a 1 min delay between training and the memory test (N = 447, U = 24641.5, z = 0.24, P = 0.8). Significant differences were found at several time points following training (10 min: N = 295, U = 8637.0, z = .02, ** = P<0.01; 20 min: N = 330, U = 10074.5, z = 3.95, *** = P<0.001; 30 min: N = 311, U = 10926.0, z = 1.45, P = 0.1; 40 min: N = 351, U = 12941.5, z = 2.48, ** = P<0.01). The values are means and error bars represent s.e.m.
Because the memory trace rapidly decays in place learning, we thought that the rsh gene might be important in place memory consolidation shortly after training. Thus, wild-type CS and rsh1 flies were conditioned with intermittent training, removed from the chambers for 1 to 40 min in the retention interval, and tested for memory after a short 1 min reminder training. As in the memory test directly after training, a test of memory after a 1 min delay did not reveal a difference between flies of these genotypes (Fig. 1C). Memory with a short retention interval and reminder training, however, does have the expected lower memory performance levels in wild-type CS flies compared to memory tested directly after training (Fig. 1B and C) [20], [22]. From 10 to 40 min after conditioning the memory levels are lower in rsh1 flies compared to CS, although not significantly so at 30 min post-training. This latter case might indicate an interesting dynamic in memory processes in this time range. Nevertheless, these results show that the rsh gene is necessary to partially consolidate place memory several minutes after training.
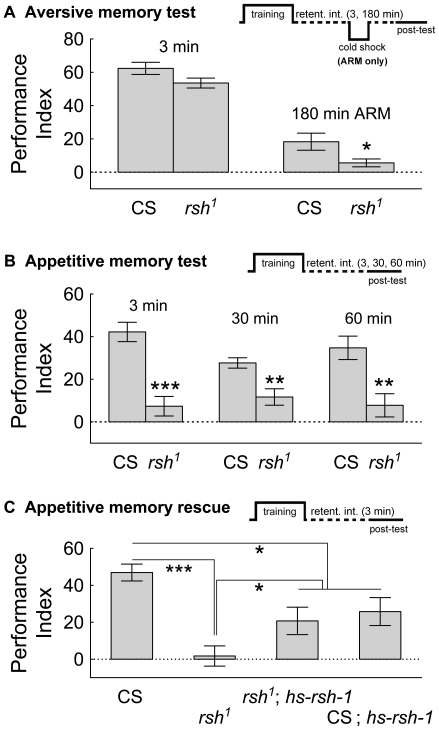
We next examined the role of rsh1 mutation in aversive olfactory memory as a way to affirm that we are indeed measuring rsh-dependent processes; a minor role in 3 min memory but a strong defect in ARM is a defining feature of the rsh1 mutation [4], [13]. As found previously [12], rsh1 mutant flies have a minor deficit in 3 min memory, but a significant ARM defect measured 3 hrs post-training (Fig. 2A).
Figure 2. Mutation of the rsh gene reveals a major role in aversive olfactory memory (ARM) and is necessary for appetitive olfactory memory shortly after conditioning.
Flies were either trained with odorants paired with electric shock or sugar reward. The training, cold-shock, retention intervals, and testing patterns (both pre and post) are diagrammed for each panel, the time axis is not to scale. (A) Olfactory memory tested three min after training is reduced in rsh1 flies compared to CS flies, although levels do not reach statistical significance (F(1,12) = 3.5, P = 0.09). To reveal the rsh function in aversive olfactory memory, wild-type CS and rsh1 flies were trained with odorant / shock pairings, then after 2 hrs were given a cold-shock, memory was tested 1 hr later. Memory performance of rsh1 flies was significantly lower than wild-type CS flies with this procedure (F(1,10) = 5.0, * = P = 0.04). (B) Appetitive olfactory short-term memory was tested at 3, 30, and 60 min after the odorant / sucrose training session. A rsh1 phenotype was evident at all tested time points after training (3 min: F(1,16) = 29.2, *** = P<0.001; 30 min: F(1,14) = 12.3, ** = P<0.01; 60 min: F(1,14) = 12.1, ** = P<0.01). (C) The rsh1 appetitive short term olfactory memory phenotype is rescued with a transgenic copy of the wild-type version of the rsh gene (F(3,32) = 13.0, P<0.0001; post-hoc tests: CS vs rsh1 *** = P<0.001, rsh1 vs. rsh1; hs-rsh-1 * = P<0.05, CS vs. rsh1; hs-rsh-1, * = P<0.05; rsh1 vs. CS; hs-rsh-1 * = P<0.05; CS vs. CS; hs-rsh-1 * = P<0.05). The values are means and error bars represent s.e.m.
That the rsh gene provides a memory consolidation function in a short time window for a memory which rapidly decays in the heat-box prompted examination of the rsh gene in appetitive olfactory memory performance. After training a memory was tested at several retention intervals. Surprisingly, we found that rsh1 mutant flies had a severe deficit in appetitive memory as soon as we could measure the flies post-training (3 min) (Fig. 2B), and this deficit was still evident 60 min afterwards (a 180 min deficit of rsh1 mutant flies was previously described) [16]. Partial rescue of the rsh1 phenotype with expression of a wild-type version of the rsh gene (hs-rsh-1) indicates that the phenotypes we measured are a consequence of mutation of the rsh gene (Fig. 2C). In this case, no heat-shock was necessary to increase the memory performance levels of rsh1 mutant flies to levels higher than rsh1 mutant flies, and toward wild-type CS levels. Furthermore, addition of the hs-rsh1 transgene does not improve wild-type CS flies memory levels which might have been the case if more rsh expression simply gave rise to higher memory performance. Indeed, slightly lower memory levels were found in CS, hs-rsh1 flies compared to CS levels, suggesting there are optima in expression levels / domains for rsh function and olfactory memory formation. It is also possible that the partial rescue indicates that the phenotypes measured are partially dependent on mutation of the rsh gene. Nevertheless, that addition of the hs-rsh1 transgene increases rsh1 mutant flies appetitive olfactory memory levels provides strong evidence that rsh-dependent functions were measured.
Finally, we examined control behaviors of rsh1 mutant and wild-type CS flies. The ability to avoid the odors used in conditioning of rsh1 and rescued flies after starvation were similar to wild-type CS and other genetic control flies (Table 1). The olfactory tests used the same odorant concentrations and time allowed in the T-maze choice point as for conditioning. Furthermore, the ‘attractiveness’ of the sucrose used in the conditioning experiments was tested. The attractiveness of sucrose was tested in vials with a dried stripe of sucrose (the same concentration used in the conditioning experiment) [23]. The proportion of flies on the stripe over time was measured, and the average over two minutes was used as a sucrose responsivity measure. Differences in sucrose responsiveness in CS, rsh1, and other genetic control flies were not detected (Table 1), in contrast to previous findings in which a dilution of the sucrose reward was used to test for sucrose responsivity [17]. As a control for place learning we measured activity levels. The measure of activity is the average probability of moving in the pre-test period [24]. The activity levels were similar between wild-type CS and rsh1 mutant flies (Table 1). Most importantly, normal conditioning and early place memory in rsh1 flies suggests that they can sense and avoid the high temperatures used in the conditioning experiments (Fig. 1A). Thus, rsh–dependent changes in memory performance levels are independent of changes in olfactory, sugar-related, and temperature sensory defects, and locomotor activity differences.
Table 1. Control behaviors of wild-type CS and rsh1 mutant flies.
| Genotype | MCH avoidance (PI) N = 36 | Oct avoidance (PI) N = 24 | Sugar attractiveness N = 48 | Activity (rel. units) N = 563 |
| CS | 20.6±5.2 | 12.7±5.2 | 0.61±0.04 | 0.73±0.01 |
| rsh1 | 19.2±8.9 | 22.8±10.8 | 0.53±0.05 | 0.70±0.01 |
| rsh1; hs-rsh-1 | 8.2±5.9 | 31.5±5.5 | 0.52±0.05 | ND |
| CS; hs-rsh-1 | 25.8±7.9 | 33.1±9.7 | 0.55±0.04 | ND |
MCH avoidance: ANOVA F(3,32) = 1.07, P = 0.4; Oct avoidance: F(3,20) = 1.3, P = 0.3; Sugar attractiveness: ANOVA F(3,44) = 0.75, P = 0.53; Activity: F(1,561) = 3.3, P = 0.07.
Discussion
Our results challenge the traditional view that the rsh gene provides a consolidation function for ARM in the range of hours after learning [4], [5]. The results described here reveal that the timing of rsh function depends on the learning context. In aversive place learning, with a memory trace that decays within hours, the rsh function is already evident within 10 min of conditioning. This is the first mutation that reduces place memory levels without also altering conditioned behavior during training. Furthermore, that rsh1 flies have a phenotype in place memory indicates that rsh has a more general function in memory formation, which is not always the case [7], [25]–[28]. In appetitive olfactory learning, the rsh function is also critical for a memory tested within minutes of training. Remarkably, the appetitive memory is much more stable than either the place memory or the aversive olfactory memory [15], [16], [20]. Thus, one cannot simply scale the role of rsh with the stability of a memory trace, but the timing of the role of rsh in memory formation depends on the learning task.
The balance of memory phases or components that supports memory performance depends on conditioning parameters. One gains access to two primary memory components after aversive olfactory learning by training flies with either massed or spaced protocols. That is, with massed training of multiple training sessions, flies form a memory that is predominantly resistant to the effects of anesthesia (ARM) and sensitive to mutation of the rsh gene [4]. With spaced training, the same amount of training as in massed training but interspersed with some periods of rest, both ARM and an LTM component are induced [4]. ARM and LTM are thought to exist in parallel, or the LTM is antagonistic to ARM several hours after training [4], [5]. Indeed, it has been proposed that rsh might be important for a pathway parallel to the cAMP / PKA pathway in memory formation [5]. In appetitive olfactory memory with a single training session, inducing maximal memory levels, and place memory with an extended intermittent training session, we may be inducing a memory that is strongly influenced by the so-called ARM component. This interpretation depends on the thus far perfect correlation between the effects of anesthesia on aversive olfactory memory and mutation of the rsh gene. The partial effects of rsh1 on place memory and appetitive olfactory memory suggests a second component is also important, which could correspond to a rutabaga (rut) adenylyl cyclase function [23], [29]–[31]. Double mutant tests with these genes would address this possibility.
Why does aversive olfactory memory largely require the rsh component hours after conditioning but appetitive olfactory memory (more stable) and place memory (less stable) require rsh within minutes of training? Three possible explanations for the timing difference of the rsh memory component are explored. The first possible explanation is the complexity of the memories that are induced. The electric-shock reinforced olfactory memory is complex in the sense that it induces both an odorant approach and avoidance memory; the net odorant avoidance behavior of flies after this type of training is a combined effect of these memories. This complexity is revealed in altering the timing of shock / odor presentation and by genetic mutation [31]–[33]. There is no evidence for this sort of complexity in rewarded olfactory memory or place memory. A second possibility is the degree to which operant conditioning contributes to a memory. In aversive olfactory conditioning there should be very little if any operant learning as flies are presented with both odorants and electric shocks on a fixed timing schedule. In contrast, place memory should have a strong operant component since a fly learns about the space / temperature contingency by walking back and forth inside the heat-box chamber. Similarly, in rewarded olfactory learning flies presumably actively taste / ingest the sugar that is paired with the odorants during training (although the odorants are still surrounding the flies when they are not feeding too, so an operant component in this paradigm would require an emphasis on the pairing of feeding and perception of odorant). Third, an attention deficit with visual cues has been identified in rsh1 mutant flies [34], which might influence the interpretation of the memory deficits of rsh1 mutant flies described here. As much as one can extrapolate results from one test of attention onto different learning paradigms, it is possible that as the memories that are formed depend more on operant learning, an interaction of attention deficits and memory might give rise to a more severe memory deficit. Two sets of results argue against a major influence of rsh-dependent attention on memories tested here. Flies mutant for the rsh gene perform well in control experiments, which of course also require operant behaviors and, therefore, also likely require attention. Moreover, rsh mutant flies have largely normal learning in place memory and aversive olfactory memory (tests of appetitive olfactory memory at 3 min after training cannot directly address a learning deficit), suggesting that the attention in early phases of the experiments is sufficient for conditioning behavior. Altogether, it might be that the systems recruited in the more straight-forward memory forming conditions (i.e., one that does not elicit a mixture of approach and avoidance behavior to a stimulus that predicts the reinforcer) or with a significant operant component, establish the rsh-dependent phase earlier. In aversive olfactory memory, a delayed system recruits the rsh-dependent memory component.
The rsh memory component receives input from multiple sensory modalities and signal cascades. Aversive olfactory conditioning requires the dopaminergic system, and activation of these neurons paired with an odorant can be used to induce an aversive memory [23], [35]–[37]. In appetitive olfactory learning, the octopamine system is both necessary and sufficient for reinforcing this memory [23], [37]. Finally, in place learning, serotonin, but not dopamine or octopamine, are critical for memory formation [38], [39]. Each of these aminergic systems provide critical input to an associative process that in turn acts on a rsh-dependent consolidation [4], [13], [15], [16]. The different G-protein coupled receptor cascades that transduce these aminergic signals should eventually feed into the rsh pathway. Furthermore, since the neural structures for olfactory and place memory are different [7], [14], [30], [40], if the input to the rsh pathway is direct, rsh should be functioning in multiple parts of the fly brain. Alternatively, if rsh acts in an indirect fashion, there might be a single neural structure that requires rsh across different types of learning. Localized gene expression and behavioral rescue experiments will address these latter possibilities.
In conclusion, the rsh gene identifies a memory component that can be induced with different training regimens. When this component critically influences behavior depends on the learned task. It can influence memory performance from minutes to hours after training. Importantly, the rsh memory component does not scale with the stability of a memory trace. This suggests that what one ultimately measures as a change in behavior with training is the combined influence of multiple memory components, each of which has its own temporal property.
Materials and Methods
Flies and rearing conditions
Wild-type Canton S (CS), radish1 (rsh1), and rsh1; hs- rsh1flies [12] were reared under standard conditions [31]. Flies with the rsh1 allele had a white+ X-chromosome. Flies used for attempted appetitive olfactory memory rescue experiments were the male progeny of CS or rsh1 female flies crossed with w1118; hs-rsh1 male flies. Because heat-shocks of temperatures from 37 to 41°C for 15 to 40 min durations after starvation were deleterious to appetitive olfactory memory (not shown), no heat-shock was given prior to the behavioral experiments shown in Fig. 2C. Flies were between 2 and 7 days old for behavioral experiments.
Behavioral experiments
Place learning used the heat-box [41]. Flies were trained as described in the results section or with intermittent training (three 6 min sessions with 3 min intervals) using 24/41°C temperatures [18], [19], [42]. Memory was tested for 3 min, either tested directly after conditioning or after an interval in which flies were held in fly food vials [20]. A 1 min reminder training was used to test memory after intermittent training. The measure of activity is the average probability of moving in the pre-test period [24].
Olfactory learning. Undiluted 4-methylcyclohexanol (MCH) and 3-octanol (OCT) were used as odorants with protocols previously described [16], [30]. To test for a potential rsh aversive olfactory memory deficit, memory was tested 3 min after training; for the 3 hr memory a 2 min cold-shock was presented 2 hrs after conditioning [4]. Flies were held in fly food vials in the longer retention intervals. Flies were trained by pairing either MCH or OCT with 12, 100 V electric shocks [30]. For appetitive memories, flies were tested at several time points after conditioning. Conditioning of flies was done after 16 to 20 hrs with access to only water by pairing MCH or OCT with 1 M sucrose dried on filter paper for 2 min (similar to) [16], the other odorant was paired with filter paper that was water treated and then dried. Flies were held in empty fly food vials in the retention intervals. Flies were given 1 min to choose between converging odorant streams in both the aversive and appetitive olfactory memory tests. Control experiments tested the ability of flies to sense and avoid the odorants used in the conditioning experiments against air, the testing period was again for 1 min [25], [30]. The ability of flies to sense sucrose was tested in vials with a stripe of sucrose (similar to) [23]. The proportion of flies on the stripe every 10 seconds was determined over 2 min, and the average over this period was used as a sucrose responsivity measure.
Indices of behavior
For place learning, a Performance Index (PI) is used to calculate altered place preference [29]. Statistical tests use non-parametric Kruskal Wallis tests with Multiple Comparisons [28]. For olfactory learning, flies avoiding the odorant associated with shock (or approaching the odorant associated with sucrose) are used to generate an olfactory memory PI [3]. Olfactory memory PIs from flies of different genotypes are compared with an ANOVA and Newman-Keuls post-hoc tests [30].
Acknowledgments
We offer thanks to Divya Sitaraman for providing critical comments on an early version of this manuscript. Flies were generously provided by Chip Quinn (MIT).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grants from the National Science Foundation (IOS 0613708) and the University of Missouri (MU) Research Board. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Muller U. Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron. 2000;27:159–168. doi: 10.1016/s0896-6273(00)00017-9. [DOI] [PubMed] [Google Scholar]
- 2.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 3.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 4.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 5.Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- 6.Zars T. Visualizing PKA activity in a learning center. Neuron. 2010;65:442–444. doi: 10.1016/j.neuron.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Zars T. Short-term memories in Drosophila are governed by general and specific genetic systems Learn Mem. 2010;17:246–251. doi: 10.1101/lm.1706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gervasi N, Tchenio P, Preat T. PKA dynamics in a Drosophila learning center: coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. Neuron. 2010;65:516–529. doi: 10.1016/j.neuron.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Tomchik SM, Davis RL. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron. 2009;64:510–521. doi: 10.1016/j.neuron.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knapek S, Sigrist S, Tanimoto H. Bruchpilot, a synaptic active zone protein for anesthesia-resistant memory. J Neurosci. 2011;31:3453–3458. doi: 10.1523/JNEUROSCI.2585-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horiuchi J, Yamazaki D, Naganos S, Aigaki T, Saitoe M. Protein kinase A inhibits a consolidated form of memory in Drosophila. Proc Natl Acad Sci U S A. 2008;105:20976–20981. doi: 10.1073/pnas.0810119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkers E, Waddell S, Quinn WG. The Drosophila radish gene encodes a protein required for anesthesia-resistant memory. Proc Natl Acad Sci U S A. 2006;103:17496–17500. doi: 10.1073/pnas.0608377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folkers E, Drain P, Quinn WG. radish, a Drosophila mutant deficient in consolidated memory. Proc Natl Acad Sci U S A. 1993;90:8123–8127. doi: 10.1073/pnas.90.17.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colomb J, Kaiser L, Chabaud MA, Preat T. Parametric and genetic analysis of Drosophila appetitive long-term memory and sugar motivation. Genes Brain Behav. 2009;8:407–415. doi: 10.1111/j.1601-183X.2009.00482.x. [DOI] [PubMed] [Google Scholar]
- 18.Zars T. Spatial orientation in Drosophila. J Neurogenet. 2009;23:104–110. doi: 10.1080/01677060802441364. [DOI] [PubMed] [Google Scholar]
- 19.Zars M, Zars T. High and low temperatures have unequal reinforcing properties in Drosophila spatial learning. J Comp Physiol A. 2006;192:727–735. doi: 10.1007/s00359-006-0109-6. [DOI] [PubMed] [Google Scholar]
- 20.Putz G, Heisenberg M. Memories in Drosophila heat-box learning. Learn Mem. 2002;9:349–359. doi: 10.1101/lm.50402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong Z, Xia S, Liu L, Feng C, Guo A. Operant visual learning and memory in Drosophila mutants dunce, amnesiac and radish. J Insect Physiol. 1998;44:1149–1158. doi: 10.1016/s0022-1910(98)00076-6. [DOI] [PubMed] [Google Scholar]
- 22.Zars M, Zars T. Rapid matching in Drosophila place learning. Naturwissenschaften. 2009;96:927–931. doi: 10.1007/s00114-009-0550-9. [DOI] [PubMed] [Google Scholar]
- 23.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gioia A, Zars T. Thermotolerance and place memory in adult Drosophila are independent of natural variation at the foraging locus. J Comp Physiol A. 2009;195:777–782. doi: 10.1007/s00359-009-0455-2. [DOI] [PubMed] [Google Scholar]
- 25.Laferriere H, Ostrowski D, Guarnieri DJ, Zars T. The arouser EPS8L3 gene is critical for normal memory in Drosophila. PLoS ONE. 2011;6:e22867. doi: 10.1371/journal.pone.0022867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahsai L, Zars T. International Review of Neurobiology; Learning and memory in Drosophila: behavior, genetics, and neural systems. in press. [DOI] [PubMed] [Google Scholar]
- 27.LaFerriere H, Guarnieri DJ, Sitaraman D, Diegelmann S, Heberlein U, et al. Genetic dissociation of ethanol sensitivity and memory formation in Drosophila melanogaster. Genetics. 2008;178:1895–1902. doi: 10.1534/genetics.107.084582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Putz G, Bertolucci F, Raabe T, Zars T, Heisenberg M. The S6KII (rsk) gene of Drosophila melanogaster differentially affects an operant and a classical learning task. J Neurosci. 2004;24:9745–9751. doi: 10.1523/JNEUROSCI.3211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zars T, Wolf R, Davis R, Heisenberg M. Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: In search of the engram. Learn Mem. 2000;7:18–31. doi: 10.1101/lm.7.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- 31.Diegelmann S, Zars M, Zars T. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learn Mem. 2006;13:72–83. doi: 10.1101/lm.45506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanimoto H, Heisenberg M, Gerber B. Experimental psychology: event timing turns punishment to reward. Nature. 2004;430:983. doi: 10.1038/430983a. [DOI] [PubMed] [Google Scholar]
- 33.Yarali A, Krischke M, Michels B, Saumweber T, Mueller MJ, et al. Genetic distortion of the balance between punishment and relief learning in Drosophila. J Neurogenet. 2009;23:235–247. doi: 10.1080/01677060802441372. [DOI] [PubMed] [Google Scholar]
- 34.van Swinderen B, Brembs B. Attention-like deficit and hyperactivity in a Drosophila memory mutant. J Neurosci. 2010;30:1003–1014. doi: 10.1523/JNEUROSCI.4516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selcho M, Pauls D, Han KA, Stocker RF, Thum AS. The role of dopamine in Drosophila larval classical olfactory conditioning. PLoS ONE. 2009;4:e5897. doi: 10.1371/journal.pone.0005897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 38.Sitaraman D, Zars M, Zars T. Place memory formation in Drosophila is independent of proper octopamine signaling. J Comp Physiol A. 2010;196:299–305. doi: 10.1007/s00359-010-0517-5. [DOI] [PubMed] [Google Scholar]
- 39.Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, et al. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwaerzel M, Heisenberg M, Zars T. Extinction antagonizes olfactory memory at the sub-cellular level. Neuron. 2002;35:951–960. doi: 10.1016/s0896-6273(02)00832-2. [DOI] [PubMed] [Google Scholar]
- 41.Wustmann G, Rein K, Wolf R, Heisenberg M. A new paradigm for operant conditioning of Drosophila melanogaster. J Comp Physiol A. 1996;179:429–436. doi: 10.1007/BF00194996. [DOI] [PubMed] [Google Scholar]
- 42.Sitaraman D, Zars M, Zars T. Reinforcement pre-exposure enhances spatial memory formation in Drosophila. J Comp Physiol A. 2007;193:903–908. doi: 10.1007/s00359-007-0243-9. [DOI] [PubMed] [Google Scholar]