Abstract
Background
Therapy with HIV protease inhibitors (PI) has been shown to worsen glucose and lipid metabolism, but whether these changes are caused by direct drug effects, changes in disease status, or body composition is unclear. Therefore, we tested the effects of the PI combination lopinavir and ritonavir on glucose and lipid metabolism in HIV-negative subjects.
Methods
A dose of 400 mg lopinavir/100 mg ritonavir was given twice a day to 10 HIV-negative men. Fasting glucose and insulin, lipid and lipoprotein profiles, oral glucose tolerance, insulin sensitivity by euglycemic hyperinsulinemic clamp, and body composition were determined before and after lopinavir/ritonavir treatment for 4 weeks.
Results
On lopinavir/ritonavir, there was an increase in fasting triglyceride (0.89 ± 0.15 versus 1.63 ± 0.36 mmol/l; P =0.007), free fatty acid (FFA; 0.33 ± 0.04 versus 0.43 ± 0.06 mmol/l; P =0.001), and VLDL cholesterol (15.1 ± 2.6 versus 20 ± 3.3 mg/dl; P =0.05) levels. There were no changes in fasting LDL, HDL, IDL, lipoprotein (a), or total cholesterol levels. Fasting glucose, insulin, and insulin-mediated glucose disposal were unchanged, but on a 2 h oral glucose tolerance test glucose and insulin increased. There were no changes in weight, body fat, or abdominal adipose tissue by computed tomography.
Conclusion
Treatment with 4 weeks of lopinavir/ritonavir in HIV-negative men causes an increase in triglyceride levels, VLDL cholesterol, and FFA levels. Lopinavir/ritonavir leads to a deterioration in glucose tolerance at 2 h, but there is no significant change in insulin-mediated glucose disposal rate by euglycemic hyperinsulinemic clamp.
Keywords: HIV protease inhibitors, ritonavir, insulin resistance, body composition, cholesterol, triglycerides, lipodystrophy, HIV
Introduction
With the advent of highly active antiretroviral therapy (HAART), the treatment of HIV has been associated with alterations in lipid metabolism, insulin resistance, and hyperglycemia [1–3]. HIV-infected patients treated with some protease inhibitors (PI) develop increased levels of triglycerides, VLDL and LDL cholesterol, but usually no change in HDL cholesterol levels [3–5]. PI treatment has also been associated with elevations in fasting glucose, insulin resistance, and impaired glucose tolerance [6–9].
It is unclear to what extent these metabolic changes are directly caused by protease inhibitor therapy, the underlying HIV disease state, immune reconstitution, or associated body composition changes [10–13]. PI induce some changes in metabolism in HIV-positive patients before the onset of body composition changes. To address these issues, our laboratory and others have studied the effects of PI on glucose and lipid metabolism in HIV-negative, healthy volunteers. We have previously found that 4 weeks of treatment with indinavir caused insulin resistance, but had no effect on lipoproteins [7]. The insulin resistance occurred in the absence of any changes in visceral or subcutaneous fat measured by computed tomography (CT) scan. In contrast, treatment of HIV-negative subjects with ritonavir for 2 weeks induced increases in triglyceride levels, VLDL, IDL, apolipoprotein B and lipoprotein (a), with a small decrease in HDL [14,15]. There was also no increase in LDL. Amprenavir, on the other hand, had no effect on lipoproteins in HIV-negative subjects [14]. However, the effects of other PI on glucose metabolism have not been studied in HIV-negative subjects.
On the basis of these observations, it appears that the effects of PI on lipid metabolism are drug specific, but there are inadequate data on the effects of PI on human glucose metabolism in the absence of the confounding effects of HIV. In-vitro data suggest that several PI block glucose uptake into cells by inhibiting GLUT4 [16,17]. To date, only indinavir has been shown to induce insulin resistance in humans. Therefore, in this study, we examined the effects of a second PI, lopinavir/ritonavir (Kaletra) on glucose and lipid metabolism in HIV-seronegative men. We found that 4 weeks of treatment with lopinavir/ritonavir increased triglyceride levels, free fatty acids (FFA), and VLDL with no effect on LDL, lipoprotein (a), or HDL. There was a small effect on glucose tolerance on the oral glucose tolerance test (OGTT), but no effect on fasting glucose and insulin or insulin resistance as measured by euglycemic hyperinsulinemic clamp.
Methods
Ten healthy men were recruited from the community or staff at the University of California, San Francisco (UCSF). The study protocol was approved by the Committee on Human Research at UCSF and informed consent was obtained. The subjects had no history of medical illness and had normal screening physical examinations. Hematology and chemistry results were within normal limits. All subjects had a negative HIV-1 antibody test before the study.
Exclusion criteria included a body mass index (BMI) greater than 27 kg/m2, serum total cholesterol greater than 6.2 mmol/l, triglyceride level greater than 3.8 mmol/l, fasting glucose greater than 7.0 mmol/l, serum aspartate or alanine aminotransferases greater than 50 U/l and creatinine greater than 124 μM.
Study design
Subjects were admitted to the General Clinical Research Center (GCRC) at San Francisco General Hospital (SFGH) for 5 days and were placed on a constant calorie diet with fixed proportions of carbohydrate, fat, and protein designed to maintain body weight and minimize dietary influences on metabolism [18]. After baseline studies, subjects were discharged, given 400 mg lopinavir/100 mg ritonavir (Kaletra; Abbott Laboratories, Abbott Park, IL, USA) to take twice a day with food, and were seen weekly for safety and adherence monitoring. They were instructed to resume their usual diet and physical activity. After 4 weeks on treatment, subjects were readmitted to the GCRC for a second 5-day period for repeat studies. To achieve adequate drug levels, lopinavir/ritonavir was given at the start of the OGTT and 40 min before the euglycemic hyperinsulinemic clamp on an empty stomach.
Euglycemic hyperinsulinemic clamp
The clamp was performed as described by DeFronzo et al. [19]. An antecubital vein cannulae was inserted for infusion, and a vein in the dorsum of the contralateral hand was cannulated and heated to 50–55°C for arterialized venous blood sampling. Subjects fasted overnight before the procedure. At t =0 min, insulin (Humulin R; Eli Lilly, Indianapolis, IN, USA) was administered as a primed continuous intravenous infusion for 10 min, followed by a constant infusion at the rate of 40 mU/m2 per min until t =180 min. The whole blood glucose concentration was measured every 5 min. Dextrose (20%) was infused to maintain the plasma glucose concentration at 4.5 mmol/l with a coefficient of variation of less than 5% based on the negative feedback principle. Blood samples were also collected for post hoc determination of serum insulin concentrations.
Resting energy expenditure
Oxygen consumption and carbon dioxide production were measured by indirect calorimetry (DeltaTrac metabolic monitor; Yorba Linda, CA, USA). The non-protein respiratory quotient and substrate oxidation rates were calculated after correction for protein oxidation, as estimated by urea nitrogen excretion measured in the 24 h urine collection [7]. The rate of non-oxidative glucose metabolism was calculated by subtracting the rate of carbohydrate oxidation from the rate of dextrose infusion during the clamp. At the insulin levels achieved during this procedure, hepatic glucose production is completely suppressed in healthy individuals.
Fat clearance
At 08:00 hours, after a 10 h overnight fast, an intravenous fat tolerance test was performed as described previously [7]. Intralipid (Liposyn II 20%; Abbott Laboratories, Chicago, IL, USA) was infused at 0.1 g/kg body weight over 2 min, and blood samples were collected at 0, 5, 10, 15, 20, 30, 40, and 50 min for nephelometry.
Oral glucose tolerance test
At 08:00 hours, after a 10 h overnight fast, subjects received 75 g glucose orally. Blood samples were collected at 0, 30, 60, 90, 120, and 180 min and assayed from plasma glucose (sodium fluoride-containing tubes) and serum insulin. The area under the curve (AUC) for glucose and insulin were calculated by the trapezoid method. The following glucose tolerance criteria from the World Health Organization were used for clinical characterization: normal glucose tolerance (2 h glucose < 7.8 mmol/l); impaired glucose tolerance (2 h glucose 7.8–11.0 mmol/l); and overt glucose intolerance or diabetes (2 h glucose > 11.1 mmol/l) [20]. Fasting plasma glucose and fasting serum insulin levels were measured on 2 days, and the average data were used to calculate the insulin resistance index by the homeostasis model assessment (HOMA) [21].
Measurements
Fasting lipids and FFA were measured using enzymatic colorimetric methods (Sigma Diagnostics, St Louis, MO, USA and Wako Chemicals, Richmond, VA, USA). Lipoprotein and cholesterol measurements were measured by ultracentrifugation (Atherotec, Birmingham, AL, USA). Whole blood and plasma glucose and lactate were measured using a glucose analyser (YSI 2300 STAT-Plus Glucose and Lactate Analyzer; YSI Inc., Yellow Springs, OH, USA). Serum insulin levels were determined by Coat-A-Count radioimmunoassay (Diagnostic Products Corp., Los Angeles, CA, USA) with an intra-assay coefficient of variation of 7.3%, a lower detection limit of 9.3 pmol/l and 20% cross reactivity with proinsulin.
Lopinavir levels were measured by liquid chromatography, tandem mass spectrometry at the Drug Research Unit, SFGH. The method has a lower detection limit of 105 ng/ml, inter and intra-assay coefficient of variations ranging from 6.0 to 9.9 and 3.3 to 6.4%, respectively.
Studies of body composition
BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). CT was performed on a helical HiSpeed CTI Scanner (General Electric Medical Systems, Milwaukee, WI, USA) as previously described [7,22]. A single 7 mm slice obtained at the level of the L4–L5 intervertebral disc space was used for the quantification of visceral and subcutaneous fat. All images were analysed in a matrix of 512 × 512 pixels by one investigator, with a coefficient of variation less than 1% on repeat analysis.
Total and regional body composition was measured by dual-energy X-ray absorptiometry (DEXA; Lunar model DPX, Madison, WI, USA, software version 3.65). An analysis of scans was performed as previously described [7,22,23], with a coefficient of variation for repeated regional analysis of trunk, arm, and leg fat at 1.0, 2.7, and 1.4%.
Bioelectric impedance analysis was performed using a tetra polar BIA101 Quantum (RJL Systems Inc., Clinton Township, MI, USA). Body cell mass was calculated using software version 3.1b.
Adherence
Subjects were instructed to take 400 mg lopinavir/100 mg ritonavir twice a day with water. Adherence was monitored at each weekly visit by three methods: (i) self report/direct questioning; (ii) electronic pill counts using a medication event monitoring system (Aprex Corp., Union City, CA, USA) for quantification of adherence rate and dosing intervals; and (iii) measurement of plasma lopinavir trough levels.
Statistical analysis
Data were analysed using Sigma Stat version 2.03 (SPSS, Inc., San Rafael, CA, USA). Paired t-tests were used for normally distributed data. Data are presented as mean ± SEM. P values are two-tailed.
Results
Ten male subjects ranged in age from 29 to 66 years (mean 43.8 ± 4.5); one subject was Hispanic whereas the other nine were Caucasian. The average therapeutic coverage measured by the medication event monitoring system was 94.4 ± 1% (range 87–99). All subjects had detectable lopinavir levels that were within two standard deviations of the expected level. Average weekly lopinavir levels were 13.4 ± 7.3 μmol/l. Before the euglycemic hyperinsulinemic clamp, lopinavir levels were 10.5 ± 1.2 μmol/l at t0 min and increased during the study to 12.5 ± 1.5 μmol/l at t180 min. Peak therapeutic lopinavir levels have been shown to average 8.49 ± 5.4 μmol/l in HIV-infected patients [24]. The most common reported adverse effects were loose stools (four subjects) and diarrhea (two subjects); one subject had a single episode of nausea and vomiting. No one complained of dry skin or mouth. There were no abnormalities in electrolytes, white blood cell counts, or liver function tests. All 10 subjects completed the study.
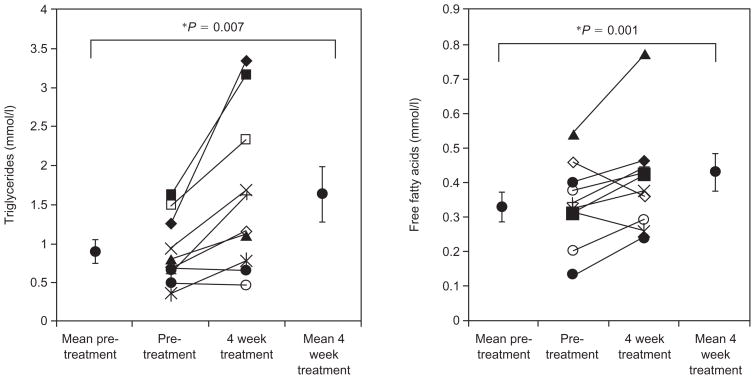
After 4 weeks of lopinavir/ritonavir treatment, fasting triglyceride levels increased nearly twofold, whereas VLDL cholesterol levels increased by 33% (Table 1). Eight out of 10 subjects showed an increase in triglyceride levels (Fig. 1). There were no changes noted in fasting LDL, HDL, IDL, lipoprotein (a), total cholesterol, or HDL. Among the VLDL subclasses, VLDL2 levels significantly increased and there was a smaller trend in VLDL3 levels. A small decrease in LDL subspecies cannot be ruled out. FFA increased by 30% after lopinavir/ritonavir treatment. Eight out of 10 subjects showed an increase in FFA levels (Fig. 1). The clearance time of triglyceride levels as measured by an intravenous fat tolerance test showed a trend towards a small 14% delay in fat clearance (Table 1).
Table 1.
Lipoproteins and lipids at baseline and after 4 weeks of treatment with lopinavir/ritonavir.
| Baseline | 4 weeks’ treatment | P value | |
|---|---|---|---|
| Total cholesterol (mmol/l) | 4.5 ± 0.3 | 4.4 ± 0.4 | 0.74 |
| Triglycerides (mmol/l) | 0.89 ± 0.15 | 1.63 ± 0.36 | 0.007 |
| Free fatty acids (mmol/l) | 0.33 ± 0.04 | 0.43 ± 0.06 | 0.001 |
| LDL cholesterol (mmol/l) | 3.0 ± 0.2 | 2.8 ± 0.2 | 0.31 |
| LDL-A (mmol/l) | 1.1 ± 0.2 | 1.0 ± 0.1 | 0.05 |
| LDL-B (mmol/l) | 1.3 ± 0.3 | 1.2 ± 0.2 | 0.59 |
| HDL cholesterol (mmol/l) | 1.13 ± 0.1 | 1.12 ± 0.1 | 0.85 |
| HDL2 cholesterol (mmol/l) | 0.21 ± 0.03 | 0.22 ± 0.03 | 0.49 |
| HDL3 cholesterol (mmol/l) | 0.92 ± 0.08 | 0.90 ± 0.07 | 0.63 |
| VLDL cholesterol (mmol/l) | 0.39 ± 0.07 | 0.52 ± 0.09 | 0.05 |
| VLDL2 cholesterol (mmol/l) | 0.19 ± 0.04 | 0.27 ± 0.05 | 0.03 |
| VLDL3 cholesterol (mmol/l) | 0.2 ± 0.04 | 0.26 ± 0.03 | 0.11 |
| IDL cholesterol (mmol/l) | 0.35 ± 0.04 | 0.39 ± 0.04 | 0.39 |
| Lipoprotein (a) (mmol/l) | 0.20 ± 0.04 | 0.19 ± 0.03 | 0.60 |
| Half-life fat clearance (min) | 21.3 ± 3.6 | 24.2 ± 3.5 | 0.07 |
Fig. 1.
Fasting triglyceride and free fatty acid levels at baseline and after 4 weeks of treatment with lopinavir/ritonavir.
In contrast to what we found with 4-week treatment with indinavir, fasting glucose, insulin, and insulin resistance index by HOMA did not change after 4 weeks of treatment with lopinavir/ritonavir (Table 2). Likewise, insulin resistance as measured by euglycemic hyperinsulinemic clamp was not significantly altered. During the euglycemic hyperinsulinemic clamp, a steady state insulin level of approximately 360 pmol/l was achieved after 30 min and maintained until 180 min. Steady-state glucose levels of approximately 4.5 mmol/l were achieved at 60 min and were maintained to 180 min. The insulin-mediated glucose disposal rate per unit of insulin (M/I) from 120 to 180 min remained unchanged with lopinavir/ritonavir treatment (15.9 ± 2.1 mg/kg per min per μU/ml at baseline versus 16.2 ± 2.3 at 4 weeks; P =0.81).
Table 2.
Glucose metabolism at baseline and after 4 weeks of treatment with lopinavir/ritonavir.
| Baseline | 4 weeks’ treatment | P value | |
|---|---|---|---|
| Fasting plasma glucose (mmol/l) | 4.8 ± 0.1 | 4.8 ± 0.1 | 0.91 |
| Fasting serum insulin (pmol/l) | 40.9 ± 8.3 | 36.4 ± 5.3 | 0.51 |
| Fasting serum insulin/fasting plasma glucose (pmol/mmol) | 8.5 ± 1.7 | 7.5 ± 1.1 | 0.51 |
| Homeostasis model assessment insulin resistance | 1.2 ± 0.3 | 1.1 ± 0.2 | 0.52 |
| Lactate (mmol/l) | 0.77 ± 0.08 | 0.78 ± 0.07 | 0.85 |
| Insulin-mediated glucose disposal (mg/kg/min) | 7.4 ± 0.6 | 7.1 ± 0.7 | 0.27 |
| Insulin-mediated glucose disposal (mg/kg/min per μU/ml insulin) | 15.9 ± 2.1 | 16.2 ± 2.3 | 0.81 |
| Oxidative component of total glucose disposal (mg/kg/min) | 1.8 ± 0.2 | 1.6 ± 0.2 | 0.18 |
| Non-oxidative component of total glucose disposal (mg/kg/min) | 5.6 ± 0.6 | 5.2 ± 0.5 | 0.27 |
| Glucose 2 h (OGTT) (mmol/l) | 4.6 ± 0.3 | 5.9 ± 0.6 | 0.05 |
| Insulin 2 h (OGTT) (pmol/l) | 99.6 ± 21.9 | 187.6 ± 48.4 | 0.04 |
| AUC glucose (OGTT) (mmol/h/l) | 16.9 ± 0.8 | 18.6 ± 1.4 | 0.07 |
| AUC insulin (OGTT) (pmol/h/l) | 523 ± 12.8 | 552 ± 73.6 | 0.63 |
| Free fatty acids 2h (OGTT) (mmol/l) | 0.13 ± 0.03 | 0.1 ± 0.02 | 0.18 |
AUC, Area under the curve; OGTT, oral glucose tolerance test.
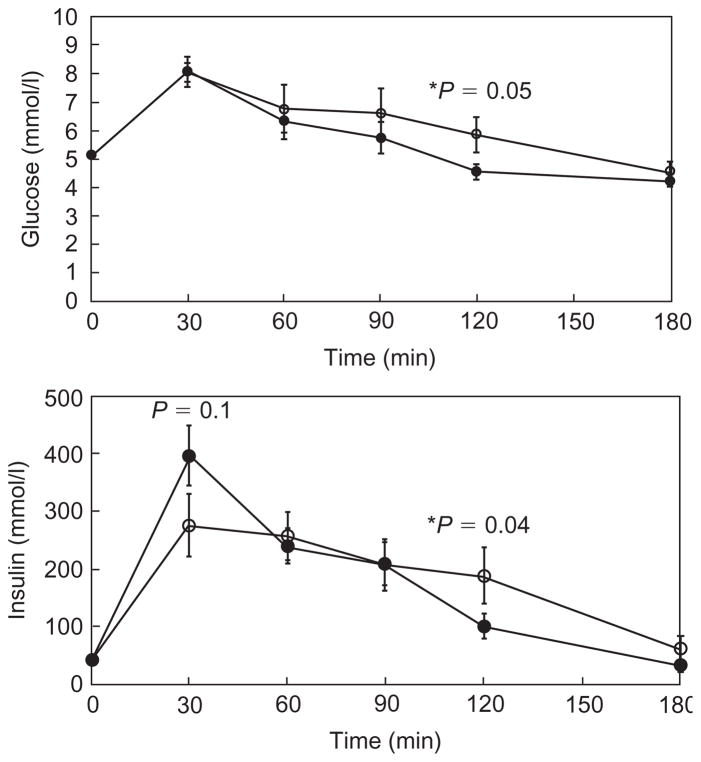
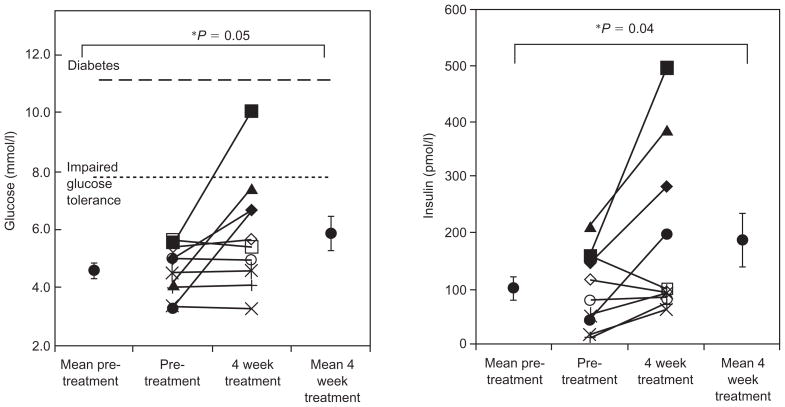
During the OGTT, glucose levels were increased at 2 h after 4 weeks of treatment (Fig. 2 and Table 2). Eight out of 10 subjects showed an increase in 2-h glucose levels and one subject developed impaired glucose tolerance (2 h glucose of 10.1 mmol/l) (Fig. 3). The AUC for glucose showed a trend towards an increase (Table 2). There was a trend towards a decrease in insulin levels early in the OGTT (30 min), and insulin levels also increased slightly by 2 h (Fig. 2). FFA were suppressed to the same level both before and during treatment with lopinavir/ritonavir (Table 2).
Fig. 2. Glucose and insulin levels during oral glucose tolerance test at baseline and after 4 weeks of treatment with lopinavir/ritonavir.
—●— Pre-treatment; —○— 4 weeks of treatment.
Fig. 3.
Glucose and insulin levels at 120 min during oral glucose tolerance test before and after 4 weeks of treatment with lopinavir/ritonavir.
After 4 weeks treatment with lopinavir/ritonavir, there were no significant changes in body composition, including weight, BMI, body cell mass by bioelectric impedence analysis, lean body mass, bone mineral content, or fat mass by DEXA, or visceral or subcutaneous fat by CT (Table 3).
Table 3.
Body composition and energy expenditure at baseline and after 4 weeks of treatment with lopinavir/ritonavir.
| Baseline | 4 weeks’ treatment | P value | |
|---|---|---|---|
| Weight (kg) | 78.6 ± 3.8 | 78.2 ± 3.8 | 0.21 |
| Body mass index (kg/m2) | 24.6 ± 0.7 | 24.4 ± 0.6 | 0.13 |
| Abdominal adipose tissue by CT scan | |||
| Subcutaneous (mm2) | 17 629 ± 2025 | 16 922 ± 2371 | 0.29 |
| Visceral (mm2) | 8950 ± 1933 | 8560 ± 2094 | 0.11 |
| Visceral + subcutaneous (mm2) | 26 579 ± 3177 | 25 482 ± 3638 | 0.16 |
| DEXA scan | |||
| Total fat tissue (kg) | 16.8 ± 1.9 | 16.6 ± 1.2 | 0.31 |
| Appendicular fat (kg) | 8.5 ± 0.7 | 8.4 ± 0.7 | 0.60 |
| Trunk fat (kg) | 7.6 ± 1.3 | 7.4 ± 1.4 | 0.38 |
| Total lean tissue (kg) | 57.3 ± 3.3 | 57.0 ± 3.4 | 0.23 |
| Appendicular lean (kg) | 27.9 ± 2.2 | 27.9 ± 2.2 | 0.83 |
| Trunk lean (kg) | 26.1 ± 1.3 | 25.9 ± 1.2 | 0.47 |
| Bone mineral content | 3.1 ± 0.2 | 3.1 ± 0.3 | 0.14 |
| Body cell mass (kg) | 30.5 ± 0.2 | 30.4 ± 0.2 | 0.77 |
| Energy expenditure during clamp (kcal/day) | 2095 ± 106 | 2055 ± 92 | 0.41 |
| Non-protein respiratory quotient | |||
| Fasting | 0.85 ± 0.03 | 0.82 ± 0.02 | 0.16 |
| Clamp | 0.91 ± 0.01 | 0.88 ± 0.02 | 0.15 |
CT, computed tomography; DEXA, dual-energy X-ray absorptiometry.
Discussion
The most notable effect of 4 weeks of lopinavir/ritonavir treatment on HIV seronegative men was the 83% increase in triglyceride levels with the greatest increase seen in VLDL particles. These results are similar to but quantitatively less than the threefold increase in triglyceride levels seen in HIV-negative subjects treated with ritonavir for 2 weeks [15]. Ritonavir is the likely agent causing the increase in triglyceride levels in the lopinavir/ritonavir combination. The effect of lopinavir cannot be studied in isolation, as ritonavir is needed as a pharmocokinetic booster by blocking cytochrome CYP 3A4; therefore, one cannot determine whether lopinavir alone contributes to the elevation in triglyceride levels seen in this study.
One postulated mechanism for the elevation in triglyceride levels is an increase in VLDL production. Our results support this hypothesis over a delay in lipoprotein clearance for several reasons. First, the small 14% decrease in triglyceride level clearance seen during the intravenous fat tolerance test cannot explain the nearly twofold elevation in triglyceride levels. Second, we did not see the appearance of increased IDL cholesterol levels or remnants. Finally, others have shown that lipoprotein lipase and hepatic lipase levels remained unchanged after ritonavir treatment, thus excluding the hypothesis of the decreased lipolysis of lipoproteins as a cause of triglyceride elevation [15]. In-vitro studies have suggested several cellular mechanisms for the increase in VLDL production. Ritonavir has been shown to inhibit apolipoprotein B degradation, resulting in an increased secretion of apolipoprotein B from cultured hepatoma cells [25]. Others have suggested the involvement of activated sterol regulatory element binding proteins (SREBP) in the liver [26]. Further work is needed on the mechanism of increased VLDL cholesterol production induced by ritonavir and lopinavir/ritonavir.
In contrast to the effects on VLDL and triglyceride levels, lopinavir/ritonavir did not increase LDL cholesterol levels. Increases in LDL have been observed in HIV-positive patients on HAART with several PI [15,27,28]. However, HAART with a non-nucleoside reverse transcriptase inhibitor (NNRTI), such as nevirapine, raises LDL cholesterol to similar levels as indinavir, ritonavir, nelfinavir and saquinavir [3,5,29]. In contrast, the treatment of HIV-negative individuals with four different PI: ritonavir [15], indinavir [7], amprenavir [14], and lopinavir/ritonavir (this study), did not lead to an increase in LDL levels. These data suggest that PI therapy does not directly cause alterations in LDL metabolism. Whether these changes in LDL levels seen in HIV-positive patients on PI and NNRTI represent a restoration to health, or an interaction between HAART and HIV remains to be determined. Preliminary data indicate that atazanavir does not increase LDL in HIV-positive patients [30]. Therefore, multiple mechanisms for alterations in LDL levels may be involved.
Although lopinavir/ritonavir had marked effects on triglyceride, FFA and VLDL cholesterol metabolism, less dramatic effects were seen on glucose metabolism and insulin resistance. Unlike indinavir, lopinavir/ritonavir had no effect on fasting glucose, fasting insulin, or HOMA, an assessment of fasting insulin resistance. No difference in insulin sensitivity or the induction of insulin resistance was seen in 10 patients using the euglycemic hyperinsulinemic clamp. However, there was a small, but significant, decrease in glucose tolerance at 120 min of OGTT. A similar impairment in glucose tolerance during OGTT, but with little insulin resistance by the minimal model, was found with amprenavir treatment of HIV-positive patients [31]. We also previously found an increase in insulin and glucose at 120 min with indinavir 4-week treatment of healthy normal volunteers [7]. There are several potential explanations for the different effects of lopinavir/ritonavir on glucose metabolism compared with amprenavir and indinavir. First, the changes seen during the OGTT may be independent of GLUT4 blockade, and may be caused by impaired first and second-phase insulin secretion. Woerle et al. [32] showed in 13 HIV-infected patients starting various PI including lopinavir/ritonavir and indinavir that first-phase insulin secretion was decreased by 25% during the hyperglycemic clamp. Second-phase insulin secretion did not decrease, but was inappropriately reduced in the setting of peripheral insulin resistance, as reflected in a decreased disposition index. Lopinavir/ritonavir may thus impair both first and second-phase insulin secretion more than peripheral resistance, resulting in impaired glucose tolerance on OGTT, with little evidence of impaired insulin-mediated glucose disposal on euglycemic hyperinsulinemic clamp. The decreased insulin levels at 30 min during the OGTT are consistent with impaired first-phase insulin secretion. An effect on insulin clearance is less likely, as levels during the clamp were not affected. Alternatively, the 10% decrease induced by lopinavir/ritonavir in glucose tolerance by AUC during the OGTT is small, and it is possible that a small change may have been missed on euglycemic hyperinsulinemic clamp or fasting glucose and insulin levels. The finding that 2 h insulin levels on OGTT increased in those with the largest increase in glucose supports this hypothesis. Although insulin levels at 120 min are elevated, second-phase insulin secretion may not be adequately increased in the setting of insulin resistance. A combination of mild insulin resistance coupled with impaired secretion preventing an adequate compensatory increase in insulin is therefore likely. Although we have not performed a randomized trial of lopinavir/ritonavir versus indinavir, it is of note that on lopinavir/ritonavir one patient developed impaired glucose tolerance, whereas on indinavir, one developed diabetes and two developed impaired glucose tolerance. The clinical significance of the small impairment of glucose tolerance on lopinavir/ritonavir is uncertain.
The lack of significant insulin resistance seen with lopinavir/ritonavir treatment during the clamp differs from the current in-vitro data on PI. Indinavir, amprenavir, and ritonavir have been shown to inhibit glucose uptake in 3T3-L1 adipocytes, and indinavir has been shown to inhibit glucose uptake by the acute blockade of GLUT4 transporters in a Xenopus laevis oocyte GLUT4 expression system. Of note, the effect of lopinavir on GLUT4 in vitro remains to be studied. One consideration in comparing the in-vivo effects of indinavir with other PI is the lower protein binding of indinavir. Whereas indinavir is only 60–65% protein bound, ritonavir and lopinavir are over 98% protein bound. The in-vitro studies of PI did not use normal concentrations of serum proteins, and therefore may have had higher free drug levels that do not account for the possible in-vivo effects of protein binding seen with lopinavir and ritonavir. Indinavir may thus achieve higher serum unbound drug concentrations in vivo than other PI.
Another difference between indinavir and lopinavir/ritonavir treatment was their effects on the FFA level. Four-week treatment with indinavir did not increase FFA, whereas lopinavir/ritonavir raised FFA by 30%. Although the stimulation of lipolysis with elevated FFA could be considered a mechanism for the insulin resistance of PI, this is not likely to be the case for several reasons. First, treatment with indinavir induced more insulin resistance than lopinavir/ritonavir by euglycemic hyperinsulinemic clamp, and yet did not increase fasting FFA levels, but tended to decrease them. Second, both indinavir and lopinavir/ritonavir showed FFA suppression during the OGTT. Third, indinavir acutely induced insulin resistance during a euglycemic hyperinsulinemic clamp with normal suppression of FFA. Finally, lopinavir/ritonavir induced a robust increase in fasting FFA without inducing insulin resistance. Although some have speculated that insulin resistance in HIV infection occurs secondary to the increased release of FFA, such studies demonstrate an uncoupling of FFA release and an induction of insulin resistance.
Likewise, it might be postulated that PI-induced insulin resistance might be the cause of the observed hypertriglyceridemia after lopinavir/ritonavir treatment. However, indinavir induced insulin resistance without increasing triglyceride levels, and lopinavir/ritonavir increased triglyceride levels without inducing significant insulin resistance. These data demonstrate that the effects of the drugs on each metabolic pathway are independent. They also emphasize the need to study other PI drugs on multiple metabolic pathways.
There are several potential limitations to the current study. There were no significant changes in body composition after 4 weeks of treatment with lopinavir/ritonavir. There was a trend towards a small decrease (4%) in visceral fat by CT scanning, but no decrease in subcutaneous fat, or total fat by DEXA. We cannot rule out the possibility that this small decrease in visceral adipose tissue may have dampened the induction of insulin resistance, but it might also have dampened the increase in FFA and triglyceride levels. The lack of increase in visceral adipose tissue suggests that changes in lipid and glucose metabolism seen with lopinavir/ritonavir are independent of increased central fat accumulation.
The drug combination of lopinavir/ritonavir limits the ability to identify which of the individual drugs or whether the combination of both are the causative agents of the effects on glucose and lipid metabolism. However, the combination is currently used to treat HIV patients and may be more relevant clinically [33]. Four-week treatment with lopinavir/ritonavir may not have been taken long enough to induce significant changes in body composition. Only men were enrolled in the current study, and pre and postmenopausal women may have different metabolic outcomes.
In summary, 4-week treatment with lopinavir/ritonavir increased plasma triglyceride levels and worsened glucose tolerance during OGTT; however, insulin sensitivity as measured by the euglycemic hyperinsulemic clamp was not impaired. These results contrast with those seen with indinavir, which had little effect on lipid metabolism but significantly increased insulin resistance. The metabolic effects of PI thus appear to be drug specific and not class specific. Individual PI need to be studied with respect to their effects on lipid and glucose metabolism in vivo and in the presence and absence of HIV. As of yet, no PI has been shown to increase LDL significantly in HIV-negative individuals. Further exploration of the mechanism of altered lipid metabolism with PI may lead to a better understanding of lipoprotein production, and long-term studies may offer a better insight into the risk of coronary artery disease in patients with HIV infection.
Acknowledgments
The authors would like to thank M. Pang, B. Chang, J. Hirai, J. Shigenaga, and the GCRC nursing staff for technical assistance.
Sponsorship: Funding was provided by the University-wide Aids Research Program ID01-SF-014, Merck, NIH DK54615, DK66999, RR00083 (UCSF-SFGH General Clinical Research Center), and P30 MH59037 (UCSF-SFGH Drug Research Unit). This study was also partly funded by Merck, Inc., who make a competing compound as previously noted. Merck played no role in accruing or analysing the data or in writing the paper.
References
- 1.Sullivan AK, Feher MD, Nelson MR, Gazzard BG. Marked hypertriglyceridaemia associated with ritonavir therapy [Letter] AIDS. 1998;12:1393–1394. doi: 10.1097/00002030-199811000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Dube MP, Johnson DL, Currier JS, Leedom JM. Protease inhibitor-associated hyperglycaemia. Lancet. 1997;350:713–714. doi: 10.1016/S0140-6736(05)63513-1. [DOI] [PubMed] [Google Scholar]
- 3.Mulligan K, Grunfeld C, Tai VW, Algren H, Pang M, Chernoff DN. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquired Immune Defic Syndr. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Echevarria KL, Hardin TC, Smith JA. Hyperlipidemia associated with protease inhibitor therapy. Ann Pharmacother. 1999;33:859–863. doi: 10.1345/aph.18174. [DOI] [PubMed] [Google Scholar]
- 5.Periard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 6.Roge BT, Katzenstein TL, Gerstoft J. Comparison of P-triglyceride levels among patients with human immunodeficiency virus on randomized treatment with ritonavir, indinavir or ritonavir/saquinavir. Scand J Infect Dis. 2001;33:306–311. doi: 10.1080/003655401300077388. [DOI] [PubMed] [Google Scholar]
- 7.Noor MA, Lo JC, Mulligan K, Schwarz JM, Halvorsen RA, Schambelan M, Grunfeld C. Metabolic effects of indinavir in healthy HIV-seronegative men. AIDS. 2001;15:F11–F18. doi: 10.1097/00002030-200105040-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarasheski KE, Tebas P, Sigmund C, Dagogo-Jack S, Bohrer A, Turk J, et al. Insulin resistance in HIV protease inhibitor-associated diabetes. J Acquired Immune Defic Syndr. 1999;21:209–216. doi: 10.1097/00126334-199907010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walli R, Herfort O, Michl GM, Demant T, Jèager H, Dieterle C, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–F173. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Saint-Marc T, Partisani M, Poizot-Martin I, Rouviere O, Bruno F, Avellaneda R, et al. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: preliminary results of the LIPOCO study. AIDS. 2000;14:37–49. doi: 10.1097/00002030-200001070-00005. [DOI] [PubMed] [Google Scholar]
- 11.Saves M, Raffi F, Capeau J, Rozenbaum W, Ragnaud JM, Perronne C, et al. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2002;34:1396–1405. doi: 10.1086/339866. [DOI] [PubMed] [Google Scholar]
- 12.Shumak SL. Increased insulin sensitivity and increased rates of insulin clearance in men with human immunodeficiency virus (HIV) infection. Metabolism. 1992;41:682. doi: 10.1016/0026-0495(92)90064-h. [DOI] [PubMed] [Google Scholar]
- 13.Hommes MJ, Romijn JA, Endert E, Eeftinck Schattenkerk JK, Sauerwein HP. Insulin sensitivity and insulin clearance in human immunodeficiency virus-infected men. Metabolism: Clin Exp. 1991;40:651–656. doi: 10.1016/0026-0495(91)90059-6. [DOI] [PubMed] [Google Scholar]
- 14.Sadler BM, Piliero PJ, Preston SL, Lloyd PP, Lou Y, Stein DS. Pharmacokinetics and safety of amprenavir and ritonavir following multiple-dose, co-administration to healthy volunteers. AIDS. 2001;15:1009–1018. doi: 10.1097/00002030-200105250-00009. [DOI] [PubMed] [Google Scholar]
- 15.Purnell JQ, Zambon A, Knopp RH, Pizzuti DJ, Achari R, Leonard JM, et al. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS. 2000;14:51–57. doi: 10.1097/00002030-200001070-00006. [DOI] [PubMed] [Google Scholar]
- 16.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–20254. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 17.Hruz PW, Murata H, Qiu H, Mueckler M. Indinavir induces acute and reversible peripheral insulin resistance in rats. Diabetes. 2002;51:937–942. doi: 10.2337/diabetes.51.4.937. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96:2735–2743. doi: 10.1172/JCI118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Technical Report Series. World Health Organization; 1985. Diabetes mellitus: report of a WHO study group. [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Lo JC, Mulligan K, Noor MA, Schwarz JM, Halvorsen RA, Grunfeld C, Schambelan M. The effects of recombinant human growth hormone on body composition and glucose metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2001;86:3480–3487. doi: 10.1210/jcem.86.8.7785. [DOI] [PubMed] [Google Scholar]
- 23.Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. ’Buffalo hump’ in men with HIV-1 infection. Lancet. 1998;351:867–870. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- 24.Corbett AH, Lim ML, Kashuba AD. Kaletra (lopinavir/ritonavir) Ann Pharmacother. 2002;36:1193–1203. doi: 10.1345/aph.1A363. [DOI] [PubMed] [Google Scholar]
- 25.Liang JS, Distler O, Cooper DA, Jamil H, Deckelbaum RJ, Ginsberg HN, Sturley SL. HIV protease inhibitors protect apolipoprotein B from degradation by the proteasome: a potential mechanism for protease inhibitor-induced hyperlipidemia. Nat Med. 2001;7:1327–1331. doi: 10.1038/nm1201-1327. [DOI] [PubMed] [Google Scholar]
- 26.Riddle TM, Kuhel DG, Woollett LA, Fichtenbaum CJ, Hui DY. HIV protease inhibitor induces fatty acid and sterol biosynthesis in liver and adipose tissues due to the accumulation of activated sterol regulatory element-binding proteins in the nucleus. J Biol Chem. 2001;276:37514–37519. doi: 10.1074/jbc.M104557200. [DOI] [PubMed] [Google Scholar]
- 27.Segerer S, Bogner JR, Walli R, Loch O, Goebel FD. Hyperlipidemia under treatment with proteinase inhibitors. Infection. 1999;27:77–81. doi: 10.1007/BF02560501. [DOI] [PubMed] [Google Scholar]
- 28.Berthold HK, Parhofer KHG, Ritter MM, Addo M, Wasmuth JC, Schliefer K, et al. Influence of protease inhibitor therapy on lipoprotein metabolism. J Intern Med. 1999;246:567–575. doi: 10.1046/j.1365-2796.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 29.van der Valk M, Kastelein JJ, Murphy RL, van Leth F, Katlama C, Horban A, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15:2407–2414. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 30.Sanne I, Piliero P, Squires K, Thiry A, Schnittman S. Results of a phase 2 clinical trial at 48 weeks (AI424-007): a dose-ranging, safety, and efficacy comparative trial of atazanavir at three doses in combination with didanosine and stavudine in antiretroviral-naive subjects. J Acquired Immune Defic Syndr. 2003;32:18–29. doi: 10.1097/00126334-200301010-00004. [DOI] [PubMed] [Google Scholar]
- 31.Dube MP, Qian D, Edmondson-Melancon H, Sattler FR, Goodwin D, Martinez C, et al. Prospective, intensive study of metabolic changes associated with 48 weeks of amprenavir-based antiretroviral therapy. Clin Infect Dis. 2002;35:475–481. doi: 10.1086/341489. [DOI] [PubMed] [Google Scholar]
- 32.Woerle H, Mariuz R, Meyer C, Reichman R, Popa E, Dostou J, et al. Mechanisms for the deterioration in glucose tolerance associated with HIV protease inhibitor regimens. Diabetes. 2003;52:918–925. doi: 10.2337/diabetes.52.4.918. [DOI] [PubMed] [Google Scholar]
- 33.Walmsley S, Bernstein B, King M, Arribas J, Beall G, Ruane P, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]