Abstract
Background
Visceral adipose tissue (VAT) is widely recognized as conveying the highest health risk in humans among the currently measurable adipose tissue compartments. A recent study indicated that the traditionally measured VAT area at L4–L5 is not the VAT area with the highest correlation with total VAT volume. At present, it is unknown whether the area with the highest correlation is also the most strongly associated with obesity-related health risk.
Objective
The study aim was to establish which VAT slice area(s) are most strongly associated with obesity-related health risk indicators.
Design
The subjects were a convenience sample of healthy adults who completed whole-body magnetic resonance imaging (MRI) scans. The correlations, with appropriate adjustments, were examined between individual MRI slice VAT areas and fasting serum/plasma triglycerides (TG), high-density lipoprotein cholesterol (HDL), glucose, insulin and blood pressure.
Results
The sample consisted of 283 healthy men (age (mean±s.d.) 41.9±15.8 years; BMI, 26.0±3.2 kg/m2; VAT, 2.7±1.8 L) and 411 women (age, 48.1±18.7 years; BMI 27.0±5.4 kg/m2; VAT, 1.7±1.2 L). After adjusting for age, race, menopause status, scan position and specific blood analysis laboratory, VAT area at L4–L5 had lower correlations with most metabolic risk factors including serum/plasma TG, HDL, glucose, insulin and blood pressure than VAT volume in both men and women. The VAT areas 10 and 15 cm above L4–L5 in men had higher or equal correlations with health risk measures than VAT volume. In women, the VAT area 5 cm above or below L4–L5 and total VAT volume had similar correlations with health risk measures.
Conclusions
An appropriately selected single slice VAT area is an equally reliable phenotypic marker of obesity-related health risk as total VAT volume. However, in both men and women the VAT slice area at the traditional L4–L5 level is not the best marker of obesity-related health risk.
Keywords: metabolic syndrome, magnetic resonance imaging, computed tomography, body composition, abdominal obesity
Introduction
Accumulating evidence suggests a close link between central adiposity and obesity-related morbidity and mortality.1–5 A majority of studies report that visceral adipose tissue (VAT) conveys the highest health risks among the currently measurable adipose tissue compartments in humans.1,2,6–10 Although there is still controversy surrounding the question of whether VAT is the cause of these health risks and whether VAT is a superior measure of obesity than subcutaneous adipose tissue or anthropometric measures,11–15 investigators generally agree that these questions can best be answered by optimizing VAT measurement methods.
Computerized axial tomography (CT) and magnetic resonance imaging (MRI) provide the important opportunity to quantify VAT in vivo. Because the application of multi-slice volume imaging is limited by radiation exposure with CT and by the relatively high cost of image analyses with MRI, most investigators use a single cross-sectional image to measure VAT area in their research as a compromise between accuracy and cost.16–21 The accuracy of a single image slice in predicting obesity-related health risks is of particular importance for both future studies applying a single image slice for phenotyping subjects and for evaluating the large number of published reports over the past two decades that rely on a single slice as an estimate of total VAT.
We recently systematically investigated the relationships between single VAT slice areas and total VAT volume in a diverse sample of healthy adult subjects who completed whole-body MRI scans.22 Although the L4–L5 level has been widely adopted for VAT measurement over the past two decades, we found that the correlation between VAT area at L4–L5 and total VAT is lower than for single slice VAT areas 5–10 cm above the L4–L5 level. This observation prompted us to investigate whether a single VAT slice area other than L4–L5 is maximally associated with weight-related health risks. Recently, Kuk et al.23 reported VAT measured at an upper abdominal level is more closely related to metabolic syndrome than VAT measured at the L4–L5 level in a sample of 85 Caucasian men. At present, there is no reported investigation to identify the anatomic location of a single slice whose area is most highly correlated with health risk indicators in a large ethnically diverse sample of adult males and females.
The aim of the present study was to investigate the relationships between single cross-sectional VAT image areas, total VAT volume and weight-related health risk factors in a large sample of adult subjects who completed whole-body MRI studies. Our focus was to identify the slice location with the strongest association to health risk factors with adjustment of age and ethnic groups. We also examined the associations between total VAT volume and health risk factors.
Methods
Protocol and design
The main study aim was to evaluate the associations between single cross-sectional image areas and total VAT volume with fasting serum insulin levels along with four metabolic syndrome clinical criteria components as defined by the Adult Treatment Panel III:24 serum glucose, triglycerides (TG), high-density lipoprotein cholesterol (HDL) and systolic and diastolic blood pressure (SBP, DBP).
Subjects were a convenience sample of healthy adults, over the age of 18 years, who completed a screening medical history, physical examination and blood studies. Race was established in each subject by self-report and included whites, blacks, Hispanics and Asians. Subjects who had a fasting serum glucose>140 mg/dl, serum TG>400 mg/dl, SBP>180 mm Hg, DBP>110 mm Hg or serum insulin >40 uIU/ml were excluded from participation. Specific lipid, insulin/glucose and blood pressure values were excluded from analysis in patients treated with lipid, glucose and blood pressure-lowering pharmacologic agents, respectively.
Data from three sites were combined to produce the final study database: Obesity Research Center, St Luke’s-Roosevelt Hospital in New York (NYORC), University of Alabama at Birmingham (UAB) and Kaiser Permanente Northern California (KPNC), Oakland, CA. The NYORC data included two chronologically separated studies, one carried out between 1990 and 1995 and the other between 1995 and 2003. The remaining data were from CARDIA study participants at the UAB and KPNC sites25 that were studied as part of the FRAM study.26
Anthropometric measurements
Body weight was measured to the nearest 0.1 kg and height to the nearest 0.1 cm using appropriately calibrated scales and stadiometers. Waist circumference was measured by trained observers between the lower rib margin and the iliac crest27 with subjects standing with their heels together. Subjects weighing more than 136.2 kg (300 pounds) were excluded from the study due to the weight limit of the MRI scanner platform. Weight, height and body composition were evaluated on the same day as the screening examination.
Magnetic resonance imaging
Whole-body MRI scans were performed and analyzed as previously reported by our group.28,29 T1-weighted MRI scans were acquired using 1.5T General Electric systems (6X Horizon, Milwaukee, WI, USA) with a matrix of 256 × 256 and a field of view of 48 cm. Spin echo sequence was used at NYORC site with repetition time/echo time (TR/TE) 300/15 ms and Gradient echo sequence was used at UAB and KPNC site with TR/TE 140/2.2 ms. The protocol involved acquisition of approximately 40 axial images of 10 mm thickness and at 40 mm intervals from fingers to toes with the subject in either a prone or supine position, using the L4–L5 intervertebral disc as the point of origin. Following acquisition, the VAT was segmented by trained and quality-controlled technicians using image analysis software (SliceOmatic, Tomovision Inc., Montreal, Canada). The intraclass correlation coefficient for volume rendering of VAT by different technicians at our center is 0.95. The VAT volume was calculated as:
where V is volume, Ai is each scan’s cross-sectional area, h is the between-slice interval, t is the thickness of each slice and N is the number of total slices. Abdominopelvic VAT volumes were calculated using all slices between the dome of the liver and the bottom of the pelvis (abdominopelvic region), whereas abdominal VAT was calculated using all slices between the dome of the liver to one slice below the L4–L5 level. Abdominal and abdominopelvic VAT were chosen in this study because they were the most frequently measured compartments in previous studies. Shen et al.30 provide an extended critical review of VAT definitions and the use of VAT estimations in clinical research.
Blood studies
Serum glucose, lipids (total cholesterol, TG and HDL) and insulin levels were evaluated at Center for Disease Control-certified chemistry laboratories (NYORC, Quest Diagnostics (Teterboro, NJ, USA), Hospital Laboratory and CARDIA, Covance Inc. (Indianapolis, IN, USA)).
Statistical methods
Group data are presented as the mean±s.d. (Table 1; includes median and quartiles). The adjusted correlation coefficients between the residuals of each regression equation and VAT volume and single slice VAT areas were calculated as follows. Regression equations were derived with each of the health risk measures (serum glucose, insulin, TG, HDL, SBP and DBP) as dependent variables and age, ethnicity, menopause status, prone or supine position and blood work laboratory as independent variables. These regression analyses were conducted within each sex group. We then calculated the correlation coefficients between the residuals of each regression equation and VAT volume and single slice VAT areas for each slice. The calculated correlations were used to identify the slice location with the highest correlation with health risks. Differences between correlated correlation coefficients were tested using the method of Steiger.31
Table 1.
Subject characteristics
| Men | Women | |
|---|---|---|
| Sample size | 283 | 411 |
| Ethnicity | ||
| Caucasian | 132 (46.6%) | 183 (44.5%) |
| African American | 110 (38.9%) | 184 (44.8%) |
| Hispanic | 20 (7.1%) | 23 (5.6%) |
| Asian† | 20 (7.1%) | 21 (5.1%) |
| Age (years) | 41.1±13.4 (39.0, 34.0, 44.0) | 44.1±15.6 (42.0, 34.0, 49.0) |
| BMI (kg/m2) | 26.6±4.2 (26.1, 24.0, 29.1) | 26.7±6.4 (25.5, 21.9, 31.5) |
| Waist circumference (cm) | 90.3±11.0 (89.8, 82.8, 95.7) | 82.5±14.7 (80.9, 71.0, 92.9) |
| Glucose (mg/dl) | 90.0±10.3 (90.0, 83.0, 97.0) | 88.0±9.0 (87.0, 83.0, 93.0) |
| TG (mg/dl) | 110.6±71.6 (97.0, 59.0, 134.0) | 83.2±46.1 (71.0, 51.0, 104.0) |
| HDL-cholesterol (mg/dl) | 48.3±14.1 (47.0, 39.0, 55.0) | 57.0±16.4 (55.0, 45.0, 65.0) |
| Insulin (uIU/ml)‡ | 10.4±5.8 (9.1, 6.2, 13.1) | 10.5±6.3 (9.4, 5.6, 14.1) |
| SBP (mm Hg)‡ | 117.8±10.7 (117.0, 111.0, 124.0) | 111.9±14.5 (110.0, 102.0, 120.0) |
| DBP (mm Hg)‡ | 78.2±9.1 (78.7, 72.0, 84.0) | 73.8±9.4 (74.0, 67.0, 80.7) |
| VAT (L) | 2.3±1.7 (3.0, 1.1, 3.1) | 1.5±1.2 (1.1, 0.6, 2.1) |
| L4–L5 VAT (cm2) | 72.9±54.4 (60.8, 34.5, 98.5) | 53.9±44.8 (40.2, 19.5, 74.0) |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; SBP, systolic blood pressure; TG, triglyceride; VAT, visceral adipose tissue. Data are presented as mean±s.d. (median, 25 percentile, 75 percentile), except that N (proportion of each sex group) is presented for each ethnic group;
the Asian sample is a multi-generation mixture of Chinese, Indian, Korean and Japanese;
data only available in 231 men and 243 women for blood pressure and in 175 men and 186 women for insulin.
Levene’s test was used to evaluate the equality of variance among groups and the Shapiro–Wilk test was applied to test the normality of the residual distributions. When necessary, health risk measures were mathematically transformed in regression models to normalize the residual distributions and to equalize the residual variance across centers or laboratories. Log transformations were applied initially and followed by Box–Cox transformations if necessary.32
When the Box–Cox transformation failed to normalize the residual distributions or to equalize the residual variance across centers, subjects with residuals >3 s.d.s from the group mean were excluded from the analyses. Correlation coefficients were calculated both before transformation with the outlying subjects included and after transformation without the outlying subjects.
A polynomial curve was fitted to describe the smoothed relation of correlation strength for slice area and VAT volume over the range of different anatomic locations for men and women separately. A similar polynomial was fitted to describe the relationship between slice area and health risk measures. The location of the peak of the polynomial curve was taken as an estimate of the anatomic level of the single slice which would have the highest correlation between VAT area and volume or health risk measures.
All statistical analyses were carried out using SPSS (SPSS for Windows, 11.5, SPSS Inc., Chicago, USA). Two-tailed (α= 0.05) tests of significance were used.
Results
VAT and health risk factor associations
The correlations between a single slice VAT area, total VAT volume, and fasting serum/plasma TG, HDL, glucose, insulin and blood pressure are presented in Table 2.
Table 2.
Adjusted correlation coefficients of VAT volume, single slice VAT areas and health risk factors
| Group | Glu† | Insulin†,‡ | TG† | HDL† | SBP†,‡ | DBP†,‡ |
|---|---|---|---|---|---|---|
| Men (n = 283) | ||||||
| Abdominal VAT | 0.211 | 0.505 | 0.361 | −0.242 | 0.312 | 0.318 |
| Abdominopelvic VAT | 0.217 | 0.522 | 0.356 | −0.255 | 0.309 | 0.316 |
| −5 cm | 0.186 | 0.389** | 0.272** | −0.194 | 0.244** | 0.264 |
| L4–L5 | 0.193 | 0.384** | 0.276** | −0.162** | 0.277 | 0.266** |
| +5 cm | 0.187 | 0.458** | 0.354 | −0.235 | 0.307 | 0.306 |
| +10 cm | 0.210 | 0.520 | 0.401* | −0.271* | 0.306 | 0.313 |
| +15 cm | 0.201 | 0.522 | 0.332 | −0.238 | 0.295 | 0.313 |
| Women (n = 411) | ||||||
| Abdominal VAT | 0.218 | 0.569 | 0.397 | −0.383 | −0.054 | 0.104 |
| Abdominopelvic VAT | 0.222 | 0.558 | 0.398 | −0.385 | −0.051 | 0.110 |
| −5 cm | 0.192 | 0.547 | 0.386 | −0.386 | −0.072 | 0.069 |
| L4–L5 | 0.161** | 0.569 | 0.369 | −0.350** | −0.054 | 0.139 |
| +5 cm | 0.219 | 0.562 | 0.392 | −0.392 | −0.060 | 0.076 |
| +10 cm | 0.230 | 0.514** | 0.385 | −0.348** | −0.062 | 0.083 |
| +15 cm | 0.220 | 0.467** | 0.305** | −0.299** | 0.013 | 0.118 |
Abbreviations: DBP, diastolic blood pressure; Glu, serum glucose; HDL, HDL-cholesterol; SBP, systolic blood pressure; TG, triglyceride; VAT, visceral adipose tissue; ‘+’, above L4–L5; ‘−’, below L4–L5.
Either log or Box–Cox transformed when needed to normalize the distribution of the residuals and to equalize the residual variance among groups; TG, and HDL in men and women, SBP in men were log transformed; DBP in men, SBP in women and insulin in men and woman were Box–Cox transformed (λ= 1.1, −0.4, 0.3, 0.3, respectively).
Data only available in 231 men and 243 women for blood pressure and in 175 men and 186 women for insulin.
Significantly higher (P<0.05) than abdominal VAT;
, significantly lower (P<0.05) than abdominal VAT.
In men, compared to abdominal VAT volume, the VAT area measurement located at L4–L5 had significantly lower (TG, P = 0.0002; HDL, P<0.0001; insulin, P = 0.0001; DBP, P = 0.0434) or equal (Glu, SBP, P>0.05) correlations with health risk measures. The VAT slice 5 cm above and 5 cm below L4–L5 also had significantly lower or equal correlations with health risk measures compared to abdominal VAT volume (Table 2). In contrast, the VAT measurement in men 10 cm above the L4–L5 level had equal (Glu, SBP, DBP, insulin, P>0.05) or significantly higher (TG, P = 0.0026; HDL, P = 0.0370) correlations with health risk measures than total abdominal VAT volume. The slice 15 cm above the L4–L5 level had equal correlations with health risk measures to total abdominal VAT volume. In women, compared to abdominal VAT volume, the VAT measurements located at L4–L5 had significantly lower (Glu, P = 0.0004; HDL, P = 0.0304) or equal (HDL, SBP, DBP, insulin, P>0.05) correlations with health risk measures. The slices 10 and 15 cm above L4–L5 also had significantly lower or equal correlations with health risk measures (Table 2). In contrast, the VAT measurement in women 5 cm above the L4–L5 level and 5 cm below the L4–L5 level had equal correlations with all health risk measures (Glu, TG, HDL, SBP, DBP, insulin, P>0.05) to abdominal VAT.
When the correlations between single slice VAT areas and health risk measures were compared with that of abdomi-nopelvic VAT instead of abdominal VAT, the correlation coefficient ranking of VAT areas at different anatomic levels did not change (data not shown). The significance levels of the correlation coefficient differences between VAT areas and volumes also did not change.
When the data analyses were run without excluding outliers and without normalizing transformations on the dependent variables, the correlation coefficient ranking of VAT areas at the different anatomic levels did not change (data not shown). The significance levels of the correlation coefficient differences between VAT areas and volumes also did not change.
Polynomial interpolation
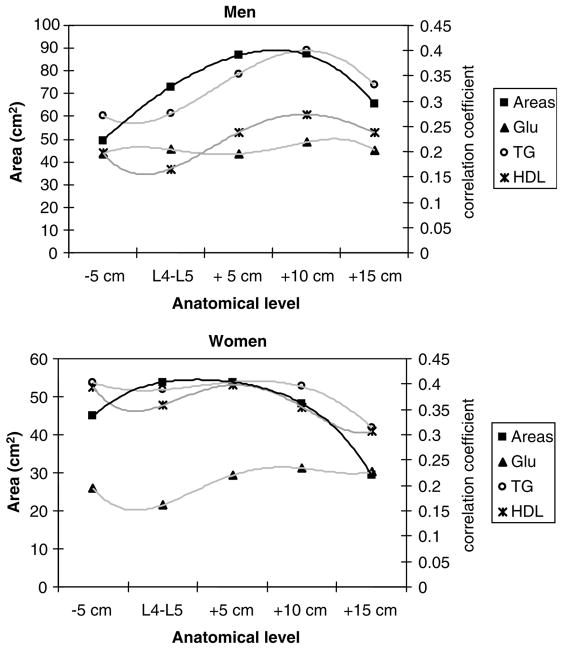
As contiguous slices were not available in this data set, we estimated the anatomic location of maximum correlation using polynomial interpolation. Fourth-order polynomials were fitted to the five correlation coefficients describing the relations between VAT area and health risk measures, and to single slice VAT areas and anatomic levels (Figure 1) (equations not shown). The maxima of the fitted curves suggest that the single slice with the strongest association between VAT area and health risk measures is the same slice as the highest slice area in men (i.e. 10 cm above L4–L5). However, in women, whereas the slice located at the L4–L5 level had an equal amount of VAT to the slice 5 cm above the L4–L5 level and had a higher amount of VAT than the slice 5 cm below the L4–L5 level, the L4–L5 slice had a lower correlation with health risk measures than either of the two single slices. The maxima of the polynomials relating correlation coefficients with anatomic location, and single slice area with anatomic location, occur at different anatomic locations suggesting that the slice with the largest area does not necessarily have the strongest association with health risks.
Figure 1.
Relationships between correlation coefficients of VAT slice area with glucose, TG, HDL and VAT slice areas (in cm2) with anatomic level. The data were fit with fourth-order polynomial equations in men (upper), and women (lower). L4–L5 is taken as the zero point and slices are identified as below (−) or above (+) this level.
Discussion
After adjusting for age, race, menopause status, scan position and blood work laboratory in this large healthy subject pool, we found that the highest correlation between VAT area and health risk factors is 5 cm above or below the L4–L5 level in women and 10 cm above the L4–L5 level in men. This finding is consistent with others and our previous reports that VAT areas measured 5 cm above L4–L5 in women and 10 cm above L4–L5 in men have the highest correlations with total VAT volume.22,33,34 The present study provided further evidence that a slice in the upper abdomen is a more appropriate anatomic location for single slice VAT studies than that at the traditional L4–L5 level, if VAT volume cannot be performed. It has been recently reported that VAT measured at T12–L1 and L1–L2 levels is more closely related to metabolic syndrome than VAT measured from contiguous CT images at the L4–L5 level in a sample of 85 Caucasian men.23 Our results in men generally agree with this study as our images 10 and 15 cm above the L4–L5 level would approximately cover the region between T12–L1 and L2.
A related finding in the present study is that there are sex differences in the relationship between VAT slice area and obesity-related health risk factors. VAT area measured by single slices tended to be larger higher in the abdomen in male subjects than in female subjects. In addition, the best single VAT slice area related to health risk factors is located higher in the abdomen in males than in females; the slice located at 15 cm above L4–L5 in men also had similar correlations to health risk factors as total VAT volume. In females, the VAT slice 5 cm below L4–L5 had similar correlations to health risk factors as total VAT volume. This observation may be explained by the large pelvis of females that can accommodate more excess omental or mesenteric adipose tissue. In our previous study, we found that the slice at L4–L5 in women had the largest amount of VAT, although this slice does not have the highest correlation with total VAT volume,22 a finding confirmed in the present study (Figure 1). VAT has two compartments, intra-peritoneal and extra-peritoneal adipose tissue (IPAT and EPAT).30 Whereas EPAT components serve primarily as mechanical cushions for organs such as kidneys, rectum, uterus and bladder, IPAT depots are of high metabolic activity and thus may account for a large proportion of the between-individual variation in observed VAT volume.22 Consequently, a slice that contains mostly IPAT may show the highest correlation between slice area and health risk factors even though the slice does not have the largest VAT area. IPAT is more mobile than EPAT and this may be why the large female pelvis can accommodate more IPAT (i.e., mesenteric AT) than the L4–L5 level.
Another observation of this study is that an appropriately selected single slice location may have equal or higher correlations with health risk factors than total VAT volume. As multi-slice MRI scans are unavailable to many investigators because of complexity and high cost of imaging, our findings point to the potential of achieving equivalent or higher study power as multi-slice MRIs by using single slice imaging. If our results can be confirmed for other health risk factors, morbidity and mortality studies, the research field could benefit by increased study power and decreased cost.
In the last two decades, the L4–L5 VAT area has been used as a measure of VAT in almost all studies, including both biological studies 16–21 and method validations or optimizations.35–37 Previous investigations favored the L4–L5 level or its approximation, the umbilical level, for a variety of reasons: ease in detecting the landmarks including L4–L5 intervertebral disc, umbilicus or iliac crest;20,38,39 same level as WC measurement site;40 maximum adipose to total tissue area ratio38 and percentage of total tissue as VAT found at the umbilical level cross-section is closer to the mean value for all slices combined than for any other single slice.38 Subsequent papers cited these earlier investigations 19,40 or did not give a reason for choosing L4–L5 as a measure of VAT volume.37,41–44 Whereas the choice of a selected slice for VAT measurement may have merit depending on the study aim, the level where the slice area has the highest correlation with health risk indicators is usually of the most interest to investigators.
There are several limitations of the present study. We do not have continuous scans and the exact location of the slice with the highest correlation with health risk indicators cannot be identified precisely. We also cannot study land-marks such as L2–L3 or L3–L4. The degree of correspondence between a single slice at L2–L3 (usually located 7–8 cm above the L4–L5 level) versus a slice 5 or 10 cm above the L4–L5 level cannot be evaluated in the current study. In addition, our currently available data cannot be used to differentiate between IPAT and EPAT, and we cannot therefore identify the slice having the highest correlation with the proposed highly active IPAT compartment. As we did not adjust our analyses for multiple comparisons, there is a chance of a type I error occurring in some of the comparisons. However, as our study focus was on overall trends rather than on individual comparisons, our conclusion would not be influenced by the presence of type I error in specific cases. Whereas the present study only focused on clinical components of metabolic syndrome in a cross-sectional convenience sample, metabolic syndrome includes other components such as athero-genic dyslipidemia, prothrombotic and pro-inflammatory status.45 Furthermore, although practical metabolic syndrome clinical criteria have been widely adopted in recent years, there is still controversy on whether metabolic syndrome adds measurably to patient evaluation beyond that of the individual risk factors.45 Our results should be cautiously applied until future studies confirm our findings in longitudinal data sets and population-based samples as well as for other obesity-related health risks or in patients with health-related disorders. Another limitation is that we have relatively small numbers of Hispanics and Asians and there may be metabolic difference within Asian populations. Our findings therefore should be applied cautiously in racial groups other than Caucasians and African Americans. We also note that the present study was based on correlations and we cannot conclude whether or not the relationships between VAT and health risks are causal. Similarly, whether or not VAT is the fat component that conveys the highest health risk is also beyond the scope of the present study. Future studies would need to examine the mechanisms linking adipose tissue distribution and health risks along with other potential influencing factors.
Conclusions
Single slice VAT areas 5–10 cm above the L4–L5 level have a higher correlation with health risk factors than the VAT area at the traditional L4–L5 level adjusted for age, race, menopause status, scan position and measurement laboratory. The present study results suggest that an appropriately selected single slice may have equal or higher power in detecting health risks than total VAT volume. Future studies need to validate our findings with health risk indicators other than those of the metabolic syndrome as well as morbidities and mortalities.
Acknowledgments
This work was supported by National Institutes of Health Grants NIDDK R21 DK66360-01, R01 DK40414, R01 DK42618, R01 DK57508, 1 and P30 DK26687; NHLBI RO1 53359 and RO1 74814; R29-AG14715, F32-AG05679, M01 RR00645, NO1HC48047-UAB and N01-HC-48050; and grants to General Clinical Research Centers (M01-RR00036, M01-RR00051, M01-RR00052, M01-RR00054, M01-RR00636 and M01-RR00865).
References
- 1.Faria AN, Ribeiro Filho FF, Gouveia Ferreira SR, Zanella MT. Impact of visceral fat on blood pressure and insulin sensitivity in hypertensive obese women. Obes Res. 2002;10:1203–1206. doi: 10.1038/oby.2002.164. [DOI] [PubMed] [Google Scholar]
- 2.Phillips GB, Jing T, Heymsfield SB. Relationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarction. Metabolism. 2003;52:784–790. doi: 10.1016/s0026-0495(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76:743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 4.Oppert JM, Charles MA, Thibult N, Guy-Grand B, Eschwege E, Ducimetiere P. Anthropometric estimates of muscle and fat mass in relation to cardiac and cancer mortality in men: the Paris Prospective Study. Am J Clin Nutr. 2002;75:1107–1113. doi: 10.1093/ajcn/75.6.1107. [DOI] [PubMed] [Google Scholar]
- 5.Nicklas BJ, Penninx BW, Cesari M, Kritchevsky SB, Newman AB, Kanaya AM, et al. Health, Aging and Body Composition Study. Related articles, links. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol. 2004;160:741–749. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 6.Rissanen J, Hudson R, Ross R. Visceral adiposity, androgens, and plasma lipids in obese men. Metabolism. 1994;43:1318–1323. doi: 10.1016/0026-0495(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 7.Björntorp P. ‘Portal’ adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–496. [PubMed] [Google Scholar]
- 8.Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, et al. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–433. doi: 10.1161/01.hyp.38.3.429. [DOI] [PubMed] [Google Scholar]
- 9.Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord. 2002;26:193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- 10.Rendell M, Hulthen UL, Tornquist C, Groop L, Mattiasson I. Relationship between abdominal fat compartments and glucose and lipid metabolism in early postmenopausal women. J Clin Endocrinol Metab. 2001;86:744–749. doi: 10.1210/jcem.86.2.7260. [DOI] [PubMed] [Google Scholar]
- 11.Tulloch-Reid MK, Hanson RL, Sebring NG, Reynolds JC, Premkumar A, Genovese DJ, et al. Both subcutaneous and visceral adipose tissue correlate highly with insulin resistance in African Americans. Obes Res. 2004;12:1352–1359. doi: 10.1038/oby.2004.170. [DOI] [PubMed] [Google Scholar]
- 12.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Adams-Huet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes. 1996;45:1684–1693. doi: 10.2337/diab.45.12.1684. [DOI] [PubMed] [Google Scholar]
- 13.Lovejoy JC, Smith SR, Rood JC. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: the Healthy Transitions Study. Obes Res. 2001;9:10–16. doi: 10.1038/oby.2001.2. [DOI] [PubMed] [Google Scholar]
- 14.Seidell JC, Bouchard C. Visceral fat in relation to health: is it a major culprit or simply an innocent bystander? Int J Obes Relat Metab Disord. 1997;21:626–631. doi: 10.1038/sj.ijo.0800467. [DOI] [PubMed] [Google Scholar]
- 15.Frayn KN. Visceral fat and insulin resistance – causative or correlative? Br J Nutr. 2000;83:S71–S77. doi: 10.1017/s0007114500000982. [DOI] [PubMed] [Google Scholar]
- 16.Gray DS, Fujioka K, Colletti PM, Kim H, Devine W, Cuyegkeng T, et al. Magnetic-resonance imaging used for determining fat distribution in obesity and diabetes. Am J Clin Nutr. 1991;54:623–627. doi: 10.1093/ajcn/54.4.623. [DOI] [PubMed] [Google Scholar]
- 17.Leenen R, Kooy Kvd, Seidell JC, Deurenberg P. Visceral fat accumulation measured by magnetic resonance imaging in relation to serum lipids in obese men and women. Atherosclerosis. 1992;94:171–181. doi: 10.1016/0021-9150(92)90242-9. [DOI] [PubMed] [Google Scholar]
- 18.Despres JP, Moorjani S, Ferland M, Tremblay A, Lupien PJ, Nadeau A, et al. Adipose tissue distribution and plasma lipoprotein levels in obese women. Importance of intra-abdominal fat. Arteriosclerosis. 1989;9:203–210. doi: 10.1161/01.atv.9.2.203. [DOI] [PubMed] [Google Scholar]
- 19.Stallone DD, Stunkard AJ, Wadden TA, Foster GD, Boorstein J, Arger P. Weight loss and body fat distribution: a feasibility study using computed tomography. Int J Obes Relat Metab Disord. 1991;15:775–780. [PubMed] [Google Scholar]
- 20.Hendler RG, Welle SL, Statt MC, Barnard R, Amatruda JM. The effects of weight reduction to ideal body weight on body fat distribution. Metabolism. 1995;44:1413–1416. doi: 10.1016/0026-0495(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 22.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–278. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuk JL, Church TS, Blair SN, Ross R. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome? Diabetes Care. 2006;29:679–684. doi: 10.2337/diacare.29.03.06.dc05-1500. [DOI] [PubMed] [Google Scholar]
- 24.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 25.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DRJ, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 26.Tien P, Benson C, Zolopa A, Sidney S, Osmond D Investigators. CGftFS. The Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Thornton JC, Bari S, Williamson B, Gallagher D, Heymsfield SB, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr. 2003;77:379–384. doi: 10.1093/ajcn/77.2.379. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi- Sunyer FX, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–58. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 29.Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282:E132–E138. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- 30.Shen W, Wang ZM, Punyanita M, Lei J, Sinav A, Kral JG, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res. 2003;11:5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–261. [Google Scholar]
- 32.NIST/SEMATECH e-Handbook of Statistical Methods. 2004 http://www.itl.nist.gov/div898/handbook/eda/section3/eda336.htm.
- 33.Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr. 1997;65:403–408. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- 34.Han TS, Kelly IE, Walsh K, Greene RM, Lean ME. Relationship between volumes and areas from single transverse scans of intraabdominal fat measured by magnetic resonance imaging. Int J Obes Relat Metab Disord. 1997;21:1161–1166. doi: 10.1038/sj.ijo.0800530. [DOI] [PubMed] [Google Scholar]
- 35.Clasey JL, Bouchard C, Wideman L. The influence of anatomical boundaries, age, and sex on the assessment of abdominal visceral fat. Obes Res. 1997;5:395–401. doi: 10.1002/j.1550-8528.1997.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 36.Terry JG, Hinson WH, Evans GW, Schreiner PJ, Hagaman AP, Crouse JRr. Evaluation of magnetic resonance imaging for quantification of intraabdominal fat in human beings by spinecho and inversion-recovery protocols. Am J Clin Nutr. 1995;62:297–301. doi: 10.1093/ajcn/62.2.297. [DOI] [PubMed] [Google Scholar]
- 37.Elbers JM, Haumann G, Asscheman H, Seidell JC, Gooren LJ. Reproducibility of fat area measurements in young, non-obese subjects by computerized analysis of magnetic resonance images. Int J Obes Relat Metab Disord. 1997;21:1121–1129. doi: 10.1038/sj.ijo.0800525. [DOI] [PubMed] [Google Scholar]
- 38.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 39.Snel YE, Brummer RJ, Doerga ME, Zelissen PM, Bakker CJ, Hendriks MJ, et al. Adipose tissue assessed by magnetic resonance imaging in growth hormone-deficient adults: the effect of growth hormone replacement and a comparison with control subjects. Am J Clin Nutr. 1995;61:1290–1294. doi: 10.1093/ajcn/61.6.1290. [DOI] [PubMed] [Google Scholar]
- 40.Seidell JC, Oosterlee A, Thijssen MA, Burema J, Deurenberg P, Hautvast JG, et al. Assessment of intra-abdominal and subcutaneous abdominal fat: relation between anthropometry and computed tomography. Am J Clin Nutr. 1987;45:7–13. doi: 10.1093/ajcn/45.1.7. [DOI] [PubMed] [Google Scholar]
- 41.Seidell JC, Bakker CJ, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution – a comparison between computed tomography and 1. 5-T magnetic resonance. Am J Clin Nutr. 1990;51:953–957. doi: 10.1093/ajcn/51.6.953. [DOI] [PubMed] [Google Scholar]
- 42.Dixon AK. Abdominal fat assessed by computed tomography: sex difference in distribution. Clin Radiol. 1983;34:189–191. doi: 10.1016/s0009-9260(83)80303-1. [DOI] [PubMed] [Google Scholar]
- 43.Weits T, van der Beek EJ, Wedel M, Ter Haar Romeny BM. Computed tomography measurement of abdominal fat deposition in relation to anthropometry. Int J Obes Relat Metab Disord. 1988;12:217–225. [PubMed] [Google Scholar]
- 44.Ashwell M, Cole TJ, Dixon AK. Obesity: new insight into the anthropometric classification of fat distribution shown by computed tomography. BMJ (Clin Res Ed) 1985;290:1692–1694. doi: 10.1136/bmj.290.6483.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reaven GM. The metabolic syndrome: is this diagnosis necessary? Am J Clin Nutr. 2006;83:1237–1247. doi: 10.1093/ajcn/83.6.1237. [DOI] [PubMed] [Google Scholar]