Abstract
Recent studies in animal models of bronchopulmonary dysplasia (BPD) suggest that antioxidant treatments may be beneficial for the disease. However, the mechanisms by which these drugs improve the course of BPD are not completely known. Alpha1-antitrypsin (α1-AT) is one of the major serine protease inhibitors in human plasma that has anti-elastase and anti-apoptotic activities. Both activities of α1-AT are dependent on its reactive site loop (RSL), which is highly susceptible to oxidative inactivation. In this study, we investigated the elastase inhibitory activity of α1-AT in two different baboon models of BPD, the “new BPD” and the “severe BPD” models, and determined the effect of treatment with a catalytic antioxidant, Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin (MnTE-2-PyP), on the elastase inhibitory activity of α1-AT in the severe BPD model. Our results demonstrate the presence of sufficient elastase inhibitory activity of the airway α1-AT in the new, but not the severe BPD model. Treatment of severe BPD group baboons with the catalytic antioxidant MnTE-2-PyP resulted in augmentation of the elastase inhibitory activity of α1-AT. These findings suggest that prevention of the oxidative inactivation of α1-AT may be one of the mechanisms by which antioxidant therapy improves the pulmonary outcomes in animal models of severe BPD.
Introduction
Bronchopulmonary dysplasia (BPD) remains as the most common complication of very preterm birth (reviewed in (1–5)). Infants with BPD not only suffer from long-term pulmonary dysfunction, but are also at higher risk of having growth restriction and adverse neurodevelopmental outcomes compared with age-matched infants (6–11). The pathogenesis of BPD is multifactorial and complex. Barotrauma, volutrauma, oxygen toxicity, antenatal and postnatal inflammation, and patent ductus arteriosus have been implicated to play a role in the development of BPD (reviewed in (1, 5, 12)). An enhanced inflammatory reaction with persistent influx of neutrophils is observed in the airways of preterm infants, who subsequently develop BPD (13, 14). This inflammation is associated with an abundance of reactive oxygen species and proteases that may not be sufficiently regulated by antioxidants and antiproteases, respectively, of the preterm lung (15–17).
Several studies in animal models of BPD have demonstrated structural and functional improvements with antioxidant treatments. Transgenic newborn mice that overexpress human extracellular superoxide dismutase (SOD) demonstrated reduced inflammation, improved epithelial cell proliferation and preservation of alveolar surface and volume density when exposed to hyperoxia (18, 19). In hyperoxia-exposed baboons, intravenous treatment with a catalytic antioxidant, MnTE-2-PyP (Mn(III)meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin), resulted in improved alveolar surface area, decreased parenchymal mast cells, eosinophils, and neuroendocrine cells and urine bombesin-like-peptide levels (20). In a multicenter trial, treatment of premature infants with intratracheal recombinant human CuZn superoxide dismutase (r-CuZnSOD) failed to decrease the incidence of death or BPD, but resulted in a significant decrease in the number of patients who required asthma medications, had wheezing episodes, emergency room visits, or rehospitalizations at 1 year corrected gestational age compared with the controls (21). Thus although this study indicates that treatment with r-CuZnSOD may reduce lung injury, it is not clear why it did not have an effect on BPD incidence. Furthermore, the mechanisms by which antioxidant agents decrease inflammation and improve alveolarization in animal models are not completely understood.
Alpha1-antitrypsin (α1-AT) is one of the major serine protease inhibitors (serpin) in human plasma and has been a molecule of interest in BPD as one of the major inhibitors of neutrophil elastase (NE). In a study by Stiskal et al, i.v. administration of α1-AT to premature infants with respiratory distress syndrome decreased the incidence of pulmonary hemorrhage without having an effect on the incidence of BPD (22). In addition to its anti-elastase activity, recent studies have also identified a novel role for α1-AT in apoptosis as an inhibitor of caspase-3 (23–25). Similar to its anti-elastase activity, the anti-apoptotic activity of α1-AT is dependent on its reactive site loop (RSL), which is highly susceptible to oxidative inactivation (24). In this study, we investigated the elastase inhibitory activity of airway α1-AT in two different baboon models of BPD and determined the effect of the catalytic antioxidant, MnTE-2-PyP, on the elastase inhibitory activity of α1-AT recovered from the airways of baboons with hyperoxia-induced severe BPD.
Methods
Animal Model
Frozen baboon lung tissue and necropsy bronchoalveolar lavage fluid (BALF) samples were provided by the Southwest Foundation for Biomedical Research (San Antonio, TX). All animal procedures were reviewed and approved by the animal care committees of the Southwest Foundation for Biomedical Research and the University of Texas Health Science Center in San Antonio. In the new BPD model, baboons that were delivered by hysterotomy at 125 days were intubated, treated with exogenous surfactant (Survanta®; donated by Ross Laboratories, Columbus, OH) and maintained on pressure-limited, time-cycled infant ventilators (donated by InfantStar; Infrasonics, San Diego, CA) for 2 d, 6 d, or 14 d (new BPD group). The ventilator settings were adjusted to maintain the arterial carbon dioxide tension (PaCO2) between 45 and 55 mmHg and oxygen was provided on a pro re nata (PRN) basis to maintain the arterial oxygen tension (PaO2) between 55 and 70 mmHg. Animals that were sacrificed at 14 d had pathologic and biochemical findings that were characteristic of the new BPD seen in human infants as described previously (26). Baboons that were delivered at 125-d or 140-d and sacrificed immediately served as the gestational controls (125-d GC or 140-d GC groups). A third control group consisted of baboons that were born via natural delivery at full-term gestation (~185 d) and sacrificed 2–3 days later (full-term group). In the “severe BPD” model, baboons were delivered at 140 d gestation and were ventilated for a total of 10 d with 100% O2 (27). The catalytic antioxidant MnTE-2-PyP was administered to 7 baboons that were exposed to 100% O2 continuously. Since MnTE-2-PyP has a half-life of 0.5 to 1 hour in mice, it was administered by continuous intravenous infusion at a dose of 0.5 mg/kg/day using an infusion rate of 0.1 cc/hour as previously described (20). A preliminary 2-week toxicity study in mice using continuous infusion of the drug demonstrated no toxic effects at 5 mg/kg/day. Therefore a 10-fold lower dose was chosen for this initial study in immature baboons.
Isolation of total RNA and Reverse Transcription
Total RNA was isolated from fresh-frozen baboon lung (right middle lobe) or liver tissues using Trizol reagent (Invitrogen, Carlsbad, CA) and was treated with DNAse I (Invitrogen) following the manufacturer’s instructions. First-strand cDNA was synthesized from 0.5 µg of RNA using the Superscript First-Strand Synthesis System (Invitrogen) with 0.5 µg oligo-dT. The reaction mixture was incubated at 42°C for 50 min followed by incubation at 72°C for 15 min. cDNA was stored at −20°C until use.
Real-time PCR
Real-time PCR analysis was performed using the Mx4000 Multiplex Quantitative PCR System (Stratagene Inc, La Jolla, CA) and the brilliant SYBR Green QPCR Master Mix (Stratagene Inc). The sequences of PCR primers were designed using the IDT DNA web site (http://www.idtdna.com) and were as follows: alpha1-antitrypsin forward primer, 5’-AGGAGCTTGACAGAGACACAGT-3’, reverse primer, 5’-TCGGTGTCCTTGACTTCAAAGG-3’; cyclophilin A forward primer, 5’-TTCATCTGCACTGCCAAGACTG-3’, reverse primer, 5’-GGC CTC CAC AAT ATT CAT GCC T-3’. For PCR analysis, 2 µl cDNA was diluted 1:10, and the reactions were performed in 20 µl of reaction volume with the following conditions: initial denaturation at 95°C for 10 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 62°C for 60 s, and extension at 72°C for 30 s. All reactions were performed in triplicates and repeated at least 3 times. Cyclophilin A was used as an internal reference to normalize the target transcripts using the 2−ΔΔCT method (28, 29).
Baboon bronchoalveolar lavage fluid
BALF samples were obtained at the time of necropsy. Sterile 0.9% saline was instilled into the left lower lobe until the lobe was completely filled and drawn back a total of five times. BALF samples were centrifuged and supernatants were stored at −80°C until use. Total protein concentration was determined by Bradford’s Assay (Bio-Rad, Hercules, CA). The concentration of each sample was adjusted to 0.8 µg/µl either by adding PBS or by concentrating the sample using a Vivaspin Concentrator (ISC Bio Express, Kaysville, UT) according to the manufacturer’s protocol.
Determination of the elastase inhibitory activity of α1-AT in BALF by formation of NE/α1-AT complexes
Bronchoalveolar lavage fluids (10 µl, total protein concentration, 0.8 µg/µl) were incubated with two different concentrations of purified neutrophil elastase (2 µl of 16 ng/µl or 4 ng/µl, Athens Research, Athens, GA) or the same volume of PBS, pH 7.4, for 15 min at room temperature (RT). The samples were heated to 95°C in 2× Laemmli sample buffer for 5 min and subjected to immunoblotting as previously described (16). Briefly, the proteins were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. The membrane was blocked in a buffer containing PBS, pH 7.4, 0.1% Tween-20, and 5% dried milk for 1 h and incubated with a rabbit anti-human α1-AT polyclonal antibody at a dilution of 1 in 8000 (Sigma, St. Louis, MO) for 1 h at RT. Subsequently, the membrane was rinsed in wash buffer and incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit IgG (Jackson Immunoresearch Laboratories, Westgrove, PA). After three rinses in wash buffer, the protein bands were visualized by chemiluminescence (ECL Western Blotting Analysis System; Amersham Biosciences, Piscataway, NJ).
Statistical analysis
Mann-Whitney U test and Fisher’s exact test were used for analysis of non-parametric and categorical data, respectively. Non-categorical data are presented as mean ± SEM and p < 0.05 is considered to be significant.
Results
Effect of gestational age and BPD on airway α1-antitrypsin
We first determined the relative antigenic levels of α1-AT in necropsy BALF obtained from premature and full-term control baboons, and baboons with BPD by immunoblotting. Control premature baboons were delivered and sacrificed at 125 d or 140 d. Bronchopulmonary dysplasia groups included two different models. In the first model, animals were delivered at 125 d and treated with one dose of surfactant, mechanical ventilation and as needed (PRN) O2 for 14 d. This model induces lung injury that recapitulates the interrupted alveolarization and vascularization observed in human infants with “new BPD.” In the second model, baboons were delivered at 140-d gestation and exposed to 100% O2 for 10 days. This model results in lung injury that is consistent with hyperoxia-induced severe BPD. Seven baboons from this group were treated with the catalytic antioxidant MnTE-2-PyP as a continuous infusion for 10 days.
Alpha1-antitrypsin was detected primarily as a 52 kDa band in all control BALF samples (Fig.1, black arrow). All samples also contained lower molecular mass bands, likely due to proteolytic cleavage of α1-AT (Fig. 1, white arrow). In both BPD groups, there were increased levels of α1-AT compared with the control samples. In addition, BPD samples demonstrated more cleavage products compared with the control samples. There were no obvious differences in quantity or quality of α1-AT bands between the 125-d/PRN O2 and 140-d/100% O2 BPD models.
Figure 1. Alpha1-antitrypsin in baboon BALF samples.
BALF samples were collected at the time of necropsy from 125-d, 140-d, and full-term gestation baboons and baboons with new (125-d/14 d PRN O2) or severe (140-d/100% O2) BPD. Immunoblotting was performed for α1-AT using a polyclonal antibody. A representative immunoblot is shown. Black and white arrows indicate 52 kDa native α1-AT and cleaved α1-AT, respectively.
Synthesis of α1-antitrypsin in baboon lung and liver tissues
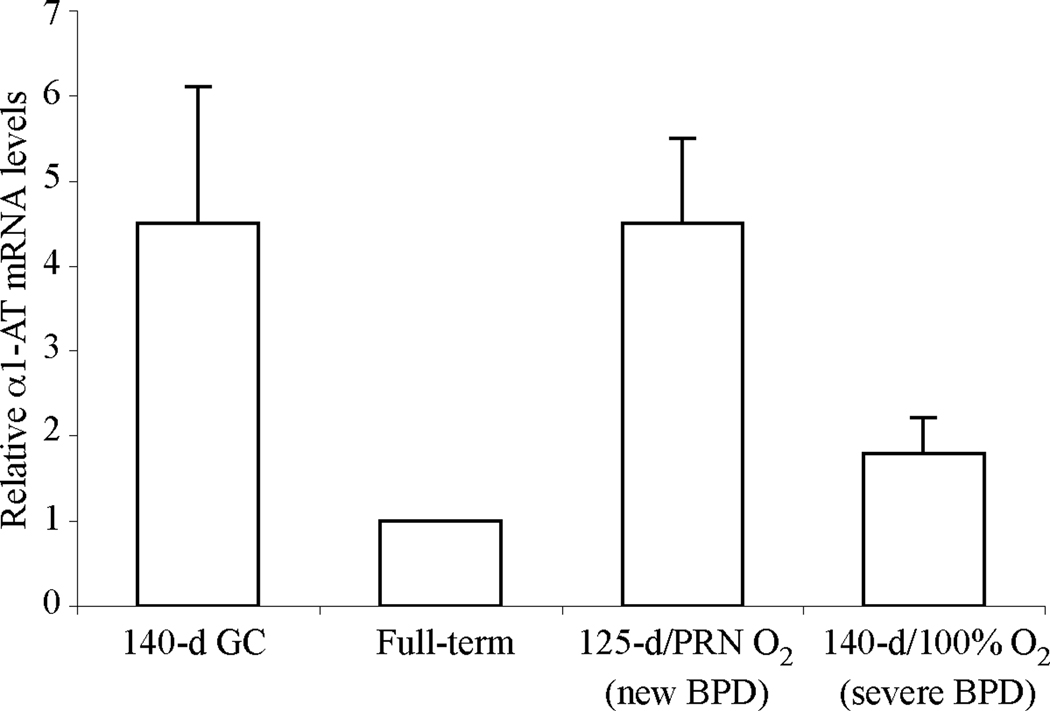
There are three major mechanisms that can lead to increased levels of α1-AT, a plasma serpin, in the airways of baboons with BPD. The first is the leakage of the protein into the airways as a result of impaired alveolar-capillary integrity, a well-known feature of several inflammatory lung diseases including BPD. Other possibilities include increased local production of α1-AT, for example by airway epithelial cells, and increased synthesis in the liver. To explore the latter two possibilities, we performed real-time PCR for α1-AT using baboon lung and liver cDNA samples. The relative steady-state mRNA levels of α1-AT in the lung were very low without any major differences among the groups (data not shown). In the liver, α1-AT mRNA levels were comparable in 140-d gestational control animals and BPD animals and higher than full-term animals, however this difference did not reach statistical significance (Fig. 2, p = 0.05). In the 140-d severe BPD group, α1-AT mRNA levels were lower than in the 125-d new BPD group, but this difference also did not reach statistical significance (p = 0.05). These data suggest that alterations in lung or liver mRNA synthesis of α1-AT are unlikely to account for the increased levels of α1-AT in the airways in BPD.
Figure 2. Steady-state relative mRNA levels of α1-AT in baboon liver samples.
Relative steady-state mRNA levels of α1-AT in liver tissues were determined by real-time reverse transcription-PCR using RNA isolated from 140-d gestational control, full-term (FT), new (125-d/14 d PRN O2) and severe (140-d/100% O2) BPD group baboons. N=4–5 animals/group. Data is expressed as mean ± SEM from two independent experiments performed in triplicates.
Functional activity of airway α1-antitrypsin in BPD
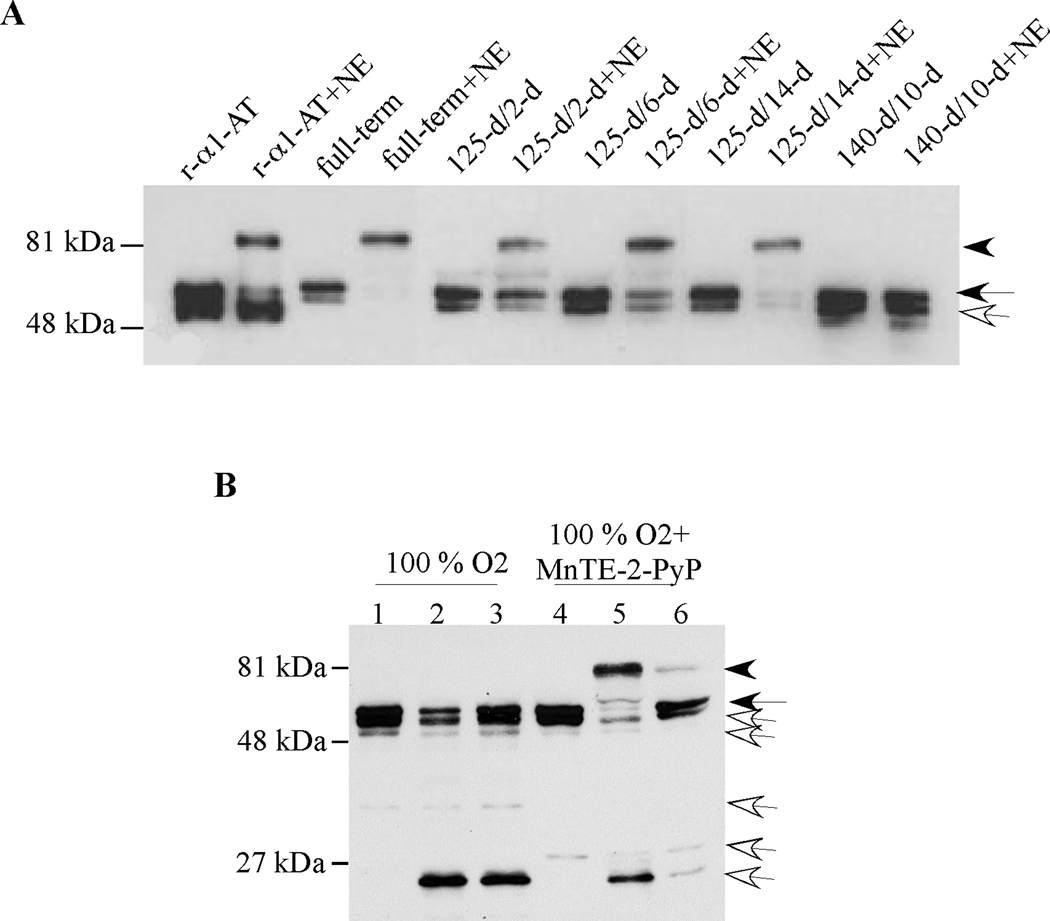
Proteolytic degradation and oxidative inactivation are two major mechanisms that regulate the activity of serpins. We detected a 52 kDa full-length α1-AT molecule in all BPD BALF samples along with some cleaved forms. The 52 kDa band could represent either functionally active native α1-AT or its inactive oxidized form. To determine the functional activity of α1-AT, we incubated BALF samples with exogenous NE and assessed whether α1-AT formed SDS-stable high molecular mass complexes with NE (Fig. 3A and Table 1). This method allowed us to specifically assess the NE-neutralizing activity of α1-AT rather than other potential inhibitors of NE in BALF, such as SerpinB1 (30). All full-term control BALF samples demonstrated the presence of functionally active α1-AT by formation of a high molecular mass complex upon incubation with NE (Fig. 3A, arrowhead). In the new BPD group, we tested the elastase inhibitory activity of BALF samples obtained following 2 d (n=3), 6 d (n=3), and 14 d (n=5) of ventilation and PRN O2 treatment. All 2-d and 6-d samples, and 80% of the 14-d BALF samples contained functionally active α1-AT as indicated by the detection of high molecular mass bands (Fig.3A, arrowhead). In contrast, high molecular mass complexes were detected in only 33% of the BALF samples from hyperoxia-induced severe BPD group animals (n=6). Remarkably, all BALF samples obtained from hyperoxia-exposed and MnTE-2-PyP-treated baboons demonstrated the presence of α1-AT with NE-inhibitory activity as evidenced by formation of high molecular mass complexes upon incubation with α1-AT (n=7, p < 0.05 vs 100% O2 treated group, Fisher’s exact test, Fig. 3B).
Figure 3. Assessment of anti-neutrophil elastase activity by formation of high molecular mass complexes between α1-AT and exogenous neutrophil elastase in baboon BALF samples.
(A) Recombinant α1-AT or baboon necropsy BALF samples were incubated with PBS or purified NE (32 ng) at RT for 15 min, then exposed to SDS-PAGE and immunoblotting using a polyclonal antibody for α1-AT under reducing conditions. A representative immunoblot is shown. Arrowhead, black arrow and white arrow indicate 81 kDa complexed α1-AT, 52 kDa native α1-AT, and cleaved α1-AT, respectively. (B) Representative immunoblot demonstrating α1-AT immunoreactivity in necropsy BALF samples of a baboon delivered at 140 d gestation and treated with mechanical ventilation and 100% O2 for 10 days and a baboon that received i.v. antioxidant MnTE-2-PyP for 10 days. Samples were incubated at RT for 15 min with PBS (lanes 1 and 4) or two different amounts of purified NE (32 ng, lanes 2 and 5, or 8 ng, lanes 3 and 6). Immunoblotting was performed under reducing conditions using a polyclonal antibody against α1-AT.
Table 1.
Summary of α1-antitrypsin activity in necropsy bronchoalveolar lavage samples of baboons with BPD.
| BALF sample |
125-d gestation/PRN O2 model (new BPD) |
140d/100%O2 model (Severe BPD) |
|||
|---|---|---|---|---|---|
| 2-d (n=3) |
6-d (n=3) |
14-d (n=5) |
Untreated (n=6) |
MnTE-2- PyP-treated (n=7) |
|
|
Activity of α1-AT* (%) |
100 | 100 | 80 | 33 | 100** |
Assessed by formation of high molecular mass complexes of α1-AT when treated with neutrophil elastase.
p < 0.05 versus untreated group (Fisher’s exact test)
Discussion
In this study, we determined the relative antigenic and functional levels of airway α1-AT in two different baboon models of BPD and evaluated the effect of a catalytic antioxidant, MnTE-2-PyP, on the activity of α1-AT in the “severe BPD” model. An imbalance between neutrophil elastase and its major endogenous inhibitor α1-AT was proposed to play a role in the development of BPD more than 25 years ago (31). Since then major advances in neonatology have led to a significant improvement in the clinical severity, but not the incidence of this disease. In the majority of surfactant-treated baboon and human infants with evolving BPD, NE is no longer the major offending protease (16, 30, 32, 33). Consistent with these observations, we found increased levels of functionally active α1-AT in the airways in the new BPD model, which indicates the presence of adequate elastase inhibitory activity. Our results also suggest that the increased airway levels of α1-AT in BPD are likely due to increased alveolar-capillary permeability rather than increased transcription of α1-AT in the lung or liver in this model although we can not rule out the possibility of increased mRNA or protein stability.
In baboon BALF samples, we detected only native and cleaved, but not complexed forms of α1-AT. The presence of cleaved forms of α1-AT can be explained by 3 potential mechanisms: cleavage of α1-AT by non-cognate proteases, such as cysteine proteases; cleavage of oxidized α1-AT by target proteases, such as NE; and cleavage of the 4 kDa COOH-terminal fragment as a result of complex formation between α1-AT and a target protease, thus yielding a ~ 48 kDa cleaved protein. In untreated BALF samples, some of the cleaved forms of α1-AT were the same size as the cleaved α1-AT (48 kDa) following in vitro complex formation between recombinant α1-AT and purified NE (Fig. 3). This suggests that these samples might have contained complexed α1-AT that was subsequently degraded during the retrieval process or storage of BALF.
Despite all advances in neonatology, a subgroup of extremely low birth weight infants continues to develop severe BPD following respiratory distress syndrome that may be complicated by sepsis, medical treatment-refractory patent ductus arteriosus, and/or pulmonary interstitial emphysema (34). The severe inflammatory response and treatment with high concentrations of oxygen in these fragile patients are inevitably associated with production of abundant reactive oxygen species and oxidant injury (15, 35). Our findings in the “severe BPD” model indicate that although the airway antigenic levels of α1-AT are similar to those in the new BPD model, the α1-AT recovered from the BALF of these animals does not have adequate elastase inhibitory activity as it fails to form SDS-stable complexes with exogenous NE. Since the majority of airway α1-AT in severe BPD was detected as a full-length 52 kDa band by immunoblotting, we reasoned that oxidative inactivation was a likely cause for failure of α1-AT to form inhibitory complexes with NE in these samples. Oxidation of methionine in P1 position of α1-AT converts this amino acid to Met-SO, thus resulting in a dramatic loss of functional activity (36). In a study that examined BAL protein oxidation in children with chronic lung disease, α1-AT was identified as one of the most sensitive proteins to oxidation in the airways (37). In another study, inactivation of α1-AT by smokers’ macrophages was prevented by addition of anti-oxidant enzymes such as superoxide dismutase and catalase (38). Consistent with our hypothesis and these previous studies, we detected active α1-AT in BALF of a significantly higher number of severe BPD-group baboons treated with the antioxidant MnTE-2-PyP compared with those who did not receive this treatment.
In summary, antioxidant treatment of baboons with severe BPD resulted in augmentation of the elastase inhibitory activity of airway α1-AT. Protection of the RSL of α1-AT against oxidative inactivation may be beneficial for anti-proteinase and anti-apoptotic effects of this serpin, both of which are relevant for the pathogenesis of BPD. Further studies are needed to determine whether preservation of the function of α1-AT is one of the potential mechanisms that contribute to the therapeutic effects of anti-oxidants in animal models of BPD and whether this approach could benefit human infants with evolving severe BPD.
Acknowledgements
We thank Dr. Richard Parad for his critical review of the manuscript. We also thank Vickie Winter and Dr. Jackie Coalson for provision of the baboon samples.
This study was supported by NIH HL075904 [to S.C.] and NIH HL63397 [to J.D.C.]
Abbreviations
- α1-AT
alpha-1 antitrypsin
- BALF
bronchoalveolar lavage fluid
- BPD
bronchopulmonary dysplasia
- GC
gestational control
- MnTE-2-PyP
Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin
- NE
neutrophil elastase
- PRN
pro re nata
- RSL
reactive site loop
- RT
room temperature
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Marter LJ. Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009;14:358–366. doi: 10.1016/j.siny.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 3.Bland RD. Neonatal chronic lung disease in the post-surfactant era. Biol Neonate. 2005;88:181–191. doi: 10.1159/000087581. [DOI] [PubMed] [Google Scholar]
- 4.Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008;358:1700–1711. doi: 10.1056/NEJMra0707601. [DOI] [PubMed] [Google Scholar]
- 5.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–172. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle LW. Respiratory function at age 8’9 years in extremely low birthweight/very preterm children born in Victoria in 1991–1992. Pediatr Pulmonol. 2006;41:570–576. doi: 10.1002/ppul.20412. [DOI] [PubMed] [Google Scholar]
- 7.Chye JK, Gray PH. Rehospitalization and growth of infants with bronchopulmonary dysplasia: a matched control study. J Paediatr Child Health. 1995;31:105–111. doi: 10.1111/j.1440-1754.1995.tb00756.x. [DOI] [PubMed] [Google Scholar]
- 8.Greenough A. Late respiratory outcomes after preterm birth. Early Hum Dev. 2007;83:785–788. doi: 10.1016/j.earlhumdev.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006;367:1421–1431. doi: 10.1016/S0140-6736(06)68615-7. [DOI] [PubMed] [Google Scholar]
- 10.Jeng SF, Hsu CH, Tsao PN, Chou HC, Lee WT, Kao HA, Hung HY, Chang JH, Chiu NC, Hsieh WS. Bronchopulmonary dysplasia predicts adverse developmental and clinical outcomes in very-low-birthweight infants. Dev Med Child Neurol. 2008;50:51–57. doi: 10.1111/j.1469-8749.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:227–232. doi: 10.1053/j.semperi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Speer CP. Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology. 2009;95:353–361. doi: 10.1159/000209301. [DOI] [PubMed] [Google Scholar]
- 13.Arnon S, Grigg J, Silverman M. Pulmonary inflammatory cells in ventilated preterm infants: effect of surfactant treatment. Arch Dis Child. 1993;69:44–48. doi: 10.1136/adc.69.1_spec_no.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groneck P, Gotze-Speer B, Oppermann M, Eiffert H, Speer CP. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics. 1994;93:712–718. [PubMed] [Google Scholar]
- 15.Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed. 2008;93:F455–F461. doi: 10.1136/adc.2007.121327. [DOI] [PubMed] [Google Scholar]
- 16.Altiok O, Yasumatsu R, Bingol-Karakoc G, Riese RJ, Stahlman MT, Dwyer W, Pierce RA, Bromme D, Weber E, Cataltepe S. Imbalance between cysteine proteases and inhibitors in a baboon model of bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2006;173:318–326. doi: 10.1164/rccm.200503-425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Didrik Saugstad O. Oxygen and oxidative stress in bronchopulmonary dysplasia. J Perinat Med. 2010;38:571–577. doi: 10.1515/jpm.2010.108. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed MN, Suliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, Auten RL. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med. 2003;167:400–405. doi: 10.1164/rccm.200202-108OC. [DOI] [PubMed] [Google Scholar]
- 19.Auten RL, O'Reilly MA, Oury TD, Nozik-Grayck E, Whorton MH. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;290:L32–L40. doi: 10.1152/ajplung.00133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang LY, Subramaniam M, Yoder BA, Day BJ, Ellison MC, Sunday ME, Crapo JD. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2003;167:57–64. doi: 10.1164/rccm.200203-232OC. [DOI] [PubMed] [Google Scholar]
- 21.Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics. 2003;111:469–476. doi: 10.1542/peds.111.3.469. [DOI] [PubMed] [Google Scholar]
- 22.Stiskal JA, Dunn MS, Shennan AT, O'Brien KK, Kelly EN, Koppel RI, Cox DW, Ito S, Chappel SL, Rabinovitch M. alpha1-Proteinase inhibitor therapy for the prevention of chronic lung disease of prematurity: a randomized, controlled trial. Pediatrics. 1998;101:89–94. doi: 10.1542/peds.101.1.89. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Lu Y, Campbell-Thompson M, Spencer T, Wasserfall C, Atkinson M, Song S. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes. 2007;56:1316–1323. doi: 10.2337/db06-1273. [DOI] [PubMed] [Google Scholar]
- 24.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, Petrache HI, Flotte TR, Tuder RM. alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrache I, Fijalkowska I, Zhen L, Medler TR, Brown E, Cruz P, Choe KH, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, Zhang B, Song S, Hicklin D, Voelkel NF, Flotte T, Tuder RM. A novel antiapoptotic role for alpha1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med. 2006;173:1222–1228. doi: 10.1164/rccm.200512-1842OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160:1333–1346. doi: 10.1164/ajrccm.160.4.9810071. [DOI] [PubMed] [Google Scholar]
- 27.Coalson JJ, Winter VT, Gerstmann DR, Idell S, King RJ, Delemos RA. Pathophysiologic, morphometric, and biochemical studies of the premature baboon with bronchopulmonary dysplasia. Am Rev Respir Dis. 1992;145:872–881. doi: 10.1164/ajrccm/145.4_Pt_1.872. [DOI] [PubMed] [Google Scholar]
- 28.VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619–626. doi: 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- 29.Ghelfi E, Karaaslan C, Berkelhamer S, Akar S, Kozakewich H, Cataltepe S. Fatty acid binding proteins and peribronchial angiogenesis in bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasumatsu R, Altiok O, Benarafa C, Yasumatsu C, Bingol-Karakoc G, Remold-O'Donnell E, Cataltepe S. SERPINB1 upregulation is associated with in vivo complex formation with neutrophil elastase and cathepsin G in a baboon model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L619–L627. doi: 10.1152/ajplung.00507.2005. [DOI] [PubMed] [Google Scholar]
- 31.Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, Edwards DD, Gluck L. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. J Clin Invest. 1983;72:656–666. doi: 10.1172/JCI111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sluis KB, Darlow BA, Vissers MC, Winterbourn CC. Proteinase-antiproteinase balance in tracheal aspirates from neonates. Eur Respir J. 1994;7:251–259. doi: 10.1183/09031936.94.07020251. [DOI] [PubMed] [Google Scholar]
- 33.Cederqvist K, Siren V, Petaja J, Vaheri A, Haglund C, Andersson S. High concentrations of plasminogen activator inhibitor-1 in lungs of preterm infants with respiratory distress syndrome. Pediatrics. 2006;117:1226–1234. doi: 10.1542/peds.2005-0870. [DOI] [PubMed] [Google Scholar]
- 34.Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, Richardson DK. Trends in severe bronchopulmonary dysplasia rates between 1994 and 2002. J Pediatr. 2005;146:469–473. doi: 10.1016/j.jpeds.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Schock BC, Sweet DG, Halliday HL, Young IS, Ennis M. Oxidative stress in lavage fluid of preterm infants at risk of chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1386–L1391. doi: 10.1152/ajplung.2001.281.6.L1386. [DOI] [PubMed] [Google Scholar]
- 36.Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- 37.Starosta V, Griese M. Protein oxidation by chronic pulmonary diseases in children. Pediatr Pulmonol. 2006;41:67–73. doi: 10.1002/ppul.20289. [DOI] [PubMed] [Google Scholar]
- 38.Hubbard RC, Ogushi F, Fells GA, Cantin AM, Jallat S, Courtney M, Crystal RG. Oxidants spontaneously released by alveolar macrophages of cigarette smokers can inactivate the active site of alpha 1-antitrypsin, rendering it ineffective as an inhibitor of neutrophil elastase. J Clin Invest. 1987;80:1289–1295. doi: 10.1172/JCI113204. [DOI] [PMC free article] [PubMed] [Google Scholar]