Abstract
It is becoming increasingly clear that signals generated in tumor microenvironments are crucial to tumor cell behavior, such as, survival progression, and metastasis. The establishment of these malignant behaviors requires that tumor cells acquire novel adhesion and migration properties to detach from their original sites for localizing into distant organs. CD44, an adhesion/homing molecule is a major receptor for the glycosaminoglycan hyaluronan, which is one of the major components of the tumor extracellular matrix (ECM). CD44, a multi structural and multifunctional molecule, detects changes in ECM components, and thus is well positioned to provide appropriate responses to changes in the microenvironment, i.e. engagement in cell-cell and cell-ECM interactions, cell traffic, lymph node homing, and presentation of growth factors/cytokines/chemokines to co-ordinate signaling events that enable the cell responses that change in the tissue environment. The potential involvement of CD44variants (CD44v), especially CD44v4-v7 and CD44v6-v9 in tumor progression was confirmed for many tumor types in numerous clinical studies. Down regulation of the standard CD44 isoform (CD44s) in colon cancer is postulated to result in increased tumorigenicity. CD44v-specific functions could be due to their higher binding affinity for hyaluronan than CD44s. Alternatively, CD44v-specific functions could be due to differences in associating molecules, which may bind selectively to the CD44v exon. This review summarizes how the interaction between hyaluronan and CD44v can serve as a potential target for cancer therapy, in particular how silencing the CD44v can target multiple metastatic tumors.
Introduction
Ten years ago, Hanahan and Weinberg proposed seven “Hallmarks of cancer” shared by most tumor cells, namely: self-sufficiency in growth signals, insensitivity to anti-growth signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, tissue invasion, and metastasis [1]. More recently, Kroemer and Pouyssegur further appended these essential hallmarks of cancer with the altered tumor cell-intrinsic metabolism [2], proposing as another hallmark of cancer avoidance of immunosurveillance due to metabolic reprogramming of tumor cells. In addition, it is now widely recognized that the tumor-associated stroma contributes to malignant tumor progression [1, 3]. The tumor micro-environment contains many distinct cell types, including vascular cells, fibroblasts, immune cells, and components of the ECM, i.e. growth factors and cytokines as well as structural molecules [4, 5]. Tumor cells sense paracrine signals from the local microenvironment and communicate these signals with their stromal cells. In this way they often alter the cellular and molecular composition of a particular tumor microenvironment to promote and maintain tumor progressions. Hence, the notion of the tumor microenvironment as an integrated and essential part of the metastatic phenotypes of carcinoma cells has been the subject of intense investigation. Disruption of ECM promotes abnormal inter- and/or intra- cellular signaling, leading to dysregulation of cell proliferation, growth and cytoskeleton reorganization [6, 7].
The glycosaminoglycan hyaluronan is a major component in the ECM of most mammalian tissues, that accumulates in sites of cell division and rapid matrix remodeling, which occurs during embryonic morphogenesis, inflammation and tumorigenesis [8–10]. Hyaluronan is found in pericellular matrices attached to hyaluronan synthesizing enzymes or its receptors, but is also found in intracellular compartments [11–14]. Regulation of transient interactions of hyaluronan with its hyaluronan binding proteins, hyaladherins (both extracellular and cell surface receptors), is crucial for fundamental physiological processes, e.g. embryonic development, but also during pathological conditions (Wang et al., FEBS J, submitted in this minireview series) where hyaluronan affects cell proliferation, migration and differentiation [10, 15, 16].
The adhesion/homing molecule CD44, which is implicated in cell-cell and cell-matrix adhesion, is the major cell-surface receptor for hyaluronan. CD44 proteins exist in three states with respect to hyaluronan binding: non-binding; non-binding unless activated by physiological stimuli; or constitutively binding [17–19]. Hyaluronan induces signaling when it binds to constitutively activated CD44variants [20, 21]. CD44 can also react with other molecules including collagen, fibronectin, osteopontin, growth factors and metalloproteinases (MMPs), but functional roles of such interactions are less well known [22]. CD44 is a transmembrane protein encoded by a single gene, but due to alternative splicing, multiple forms of CD44 are generated that further are modified by N- and O-linked glycosylations (Fig. 1). The smallest CD44 isoform that lacks variant exons, designated CD44s, is abundantly expressed by both normal and cancer cells, whereas the variant CD44 (CD44v) isoforms that contain a variable number of exon insertions (v1–v10) at the proximal plasma membrane external region, are expressed mostly by cancer cells. In addition, the CD44 ectodomain can be decorated with chondroitin sulphate and/or heparan sulphate enabling CD44 to bind growth factors, including fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF) or hepatocyte growth factor (HGF) [22, 23]. The rather short cytoplasmic tail of CD44 binds to ankyrin and ezrin-radixin-moesin (ERM) proteins providing a link to the cytoskeleton, as well as to merlin, which abrogates this binding. However, the multiple cellular functions of CD44 rely on its association with partner proteins that regulate cell migration, growth, survival and differentiation (Skandalis et al., FEBS J, submitted in this minireview series). CD44 is endogenously expressed in low levels on various cell types of normal tissues [24, 25] but requires activation before binding to hyaluronan [17, 18, 26–29]. The CD44 structure of normal cells is distinct from that of cancer cells because pathological conditions promote alternate splicing and post translational modifications to produce diversified CD44 molecules with enhanced HA binding that leads to increased tumorigenicity [30–36]. Glycosylation is required for spliced variant formation of CD44, which has high affinity to bind hyaluronan on certain cell types, while glycosylations rich in sialic acid decrease hyaluronan binding [37–39]. For example, circulating lymphocytes express CD44 but do not bind hyaluronan until CD44 is deglycosylated upon lymphocyte activation [37, 40, 41], and to internalize CD44 it must be acylated [42]. This diversification of CD44v functions allows the production of specific targeting agents that will be useful for both diagnosis and therapy. Systemic application of antibodies directed against the variant 6 epitope and the expression of antisense CD44v6 retard colon tumor growth and metastasis in vivo [43, 44]. Overexpression of variant, high molecular weight isoforms CD44v4-v7 and CD44v6-v9 in various cancers [45–52] as well as downregulation of the standard CD44 isoform (CD44s) in colon cancer are postulated to result in increased tumorigenicity [53], emphasizing the potential importance of CD44 splice variants in cancer.
FIG.1.
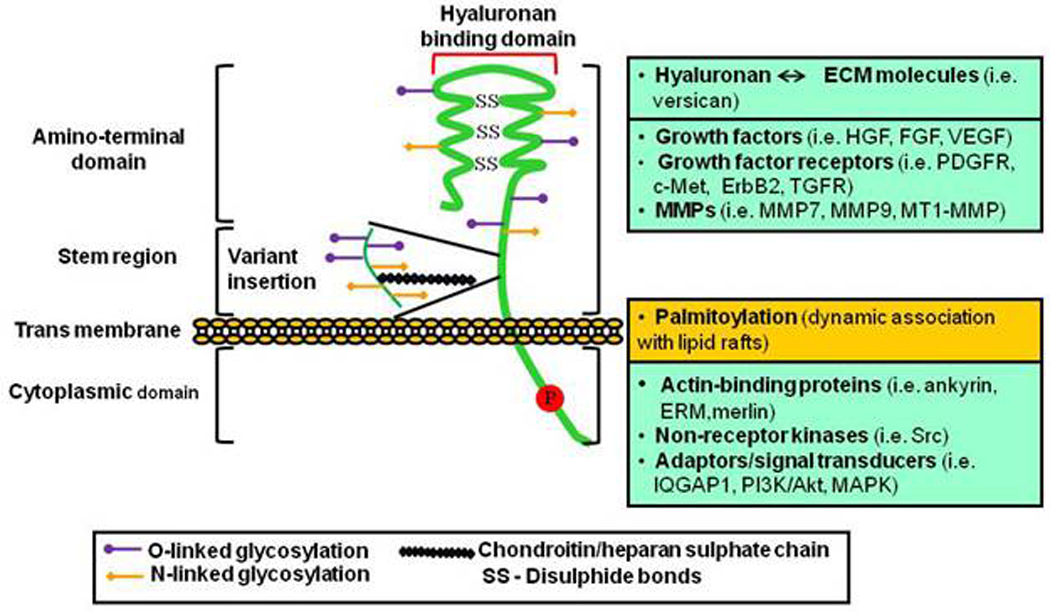
Structure, binding domains and interactions of CD44. The ectodomain of CD44 contains the hyaluronan-binding motifs and is decorated with chondroitin/heparan sulphate that both affect its hyaluronan binding capacity and enable its interactions with growth factors/growth factor receptors and MMPs. Transmembrane and cytoplasmic domains undergo multiple posttranslational modifications including palmitoylation and phosphorylation on cysteine and serine residues, respectively, promoting the binding of proteins with crucial functions in cytoskeletal organization and signaling.
Herein, we review the tumorigenic actions of hyaluronan and its receptor CD44 that occur extensively in several malignant conditions. We also discuss potential therapeutic interventions for the development of targeted therapies based on understanding the communication between hyaluronan and cell surface CD44. In particular, we highlight possible roles in hyaluronan - CD44v-induced tumor growth and invasion together with fresh insights into the enigmatic nature of CD44 splice variants, and how suppression of hyaluronan/CD44v interaction may be a therapeutic target.
Hyaluronan and CD44 in tumor initiation and progression
Influence of hyaluronan in tumorigenesis
Reactive stroma in cancer is often characterized by an increase in cancer-associated fibroblasts/myofibroblasts that produce an array of growth factors and chemokines, and amplify synthesis of hyaluronan (Tammi et al., FEBS J, submitted in this minireview series). The interaction of the anti-adhesion molecule versican with hyaluronan and CD44 promotes expansion of the pericellular matrix. These complexes increase the viscoelastic nature of the pericellular matrix, creating a highly malleable extracellular environment that supports a cell shape change necessary for cancer cell proliferation and migration. Furthermore, versican, via its chondroitin/dermatan sulfate side chains, is highly polyanionic, which amplifies the hydration of the environment caused by hyaluronan [54]. A large body of studies performed during the last three decades have demonstrated a close correlation between malignancy and hyaluronan-rich ECM as well as with CD44s and CD44v expressions. CD44 in cancer cells interacts with hyaluronan-rich microenvironments, which affects cell signaling pathways that trigger the ability of malignant cells to migrate, to invade basement membranes/ECM, and to lodge at distant sites of the tumor. [14, 22, 23, 55–58] (see also the interesting series of reviews on the Web “Science of Hyaluronan Today” at http://www.glycoforum.gr.jp). However, the underlying molecular mechanism whereby hyaluronan-CD44 cooperation influences the malignant phenotype and contributes to the tumor progression is not clear yet.
Divergent mechanisms control the expression of each hyaluronan synthase (HAS) gene in response to stimuli, and each HAS synthesizes hyaluronan molecules of different size and amount in a cell-type and context-specific manner. The study of HAS2-knockout mice [9, 59] clearly demonstrated that hyaluronan deposition in the ECM is required for embryonic heart valve morphogenesis. In HAS2 null embryos the endocardial cushion cells fail to undergo epithelial-to-mesenchymal transition (EMT) and do not migrate to the cardiac jelly. This is partly due to the lack of hyaluronan-CD44-induced Ras signaling. Importantly, this phenotype was seen only for the HAS2 isoform, indicating functional differences among the three HASs. The hyaluronan synthesizing capacity of HASs, and specifically HAS2, can be regulated by dimerization and ubiquitination [60]. In this study, mutation of HAS2 lysine residue 190, which is one major acceptor site for ubiquitin, led to total inactivation of its enzymatic activity. The different roles of the three HAS isoforms are likely to be related also to different expression patterns [61]. Studies of non-malignant cells over expressing different HASs revealed that the high levels of hyaluronan induced by HAS3 were inversely correlated to cell motility and CD44 expression [62]. Importantly, overproduction of hyaluronan in cancer cells such as fibrosarcomas, breast cancer, mesotheliomas and prostate cancer, transfected with HAS1, HAS2 and HAS3 genes, triggered intracellular signaling pathways that promoted anchorage-independent growth and invasiveness, which correlated with increased expression of CD44 [63–66]. HAS1 and its splice variants were detected in multiple myeloma patients but not in healthy donors, and were associated to poor survival of the patients [67]. Invasive and/or metastatic breast cancer cells deprived of HAS2 lost their aggressive phenotype [68]. These and many other studies, not referred to here because of space limitations, suggest that hyaluronan contributes in several ways to the hallmark properties of malignancy, especially anchorage-independent growth and invasiveness.
Although many studies have shown the importance of HASs in tumor growth and malignant progression, other studies have suggested a more complex role of hyaluronan. For example, HAS2 over expression was found to suppress the tumorigenesis of glioma cells lacking hyaluronidase (HYAL) activity [69], and HYAL1 expression promoted the HAS-mediated growth suppression and metastatic ability of prostate cancer cells [70]. Notably, over-expression of HAS2 in colon carcinomas that possessed HYAL1 activity promoted, whereas over-expression of HYAL1 suppressed tumorigenesis in an experimental model of colon carcinoma [71]. In addition the hyaluronan content in tissues was well correlated to tumor growth rate. Additional observations support the notion that HYAL1 can have both tumor promoting and tumor suppressing functions [72]. It is possible that excess hyaluronan synthesis and degradation in concert promote the metastatic phenotype of certain tumor types. However, the hyaluronan content in clinical samples is not always statistically correlated to tumor grade, suggesting that the transformation-induced hyaluronan overproduction may be a result of differential up-regulation of HAS isoforms and/or HYALs at different stages of malignant transformation. Recent work utilizing the mouse mammary tumor virus-Neu transgenic model conditionally expressing HAS2 highlighted the role of hyaluronan in the promotion of the malignant phenotype. The growth rate of mammary tumors increased, and a hyaluronan-rich intratumoral stroma was formed that most likely established interactions between tumor and stromal cells that promoted angiogenesis and lymphangiogenesis [73, 74]. This and other mouse models will be useful to further study the mechanisms regulating tumor-stroma interplay and stromal targeting therapy. It should also be mentioned that there is a connection between hyaluronan catabolism and energy generation most likely allowing hyaluronan to function as an alternate energy source to glucose for malignant cells [75]. Such metabolic reprogramming of tumor cells, could add a further dimension of the importance of hyaluronan in cancer progression.
Function of CD44 in tumor initiation and metastatic behavior
The increased deposition of hyaluronan in tumors is not a passive process during malignancy, rather, it triggers signaling events and promotes the association between CD44 and other cell surface receptors that become activated or inhibited either directly or indirectly through hyaluronan-activated CD44 [14, 16, 57, 76]. Early studies by us and other laboratories revealed that aggressive breast carcinomas expressed high levels of CD44s and CD44v as well as increased synthesis of hyaluronan [77, 78]. More recent studies have highlighted the importance of CD44 molecules in the onset of malignant transformation. There is now increasing evidence that a small population of tumor cells (less than 0.1%), referred as cancer stem-cells (CSCs) or cancer initiating cells, exhibit stem-cell properties, i.e. are responsible for maintaining the tumor and possibly formation of new tumors at metastatic loci. CD44 has been identified as an important marker of such a population of CSCs in breast, pancreas or colorectal cancers ([79–81]. Together, these findings suggest that CD44 plays an important role in the initiation and/or maintenance of CSCs in some tumors.
Specifically, CD44s interacts with growth factor receptors such as epidermal growth factor receptor-2 (ErbB2) and platelet-derived growth factor receptor (PDGFR). Most importantly, the binding of hyaluronan to CD44s either stimulates [82, 83] or inhibits [84] the tyrosine phosphorylation by the associated tyrosine kinase receptors. Most likely, the binding of hyaluronan to CD44 causes clustering, which triggers differential downstream events dependent on cell type and tissue context. Such a clustering appears to be important for the trapping of MMP9 and subsequent activation of transforming growth factor beta (TGFβ), which affects oncogenic functions including invasion and angiogenesis [85]. Moreover, clustering of CD44 also occurs upon extensive N- and O-glycosylations of the variant ectodomain of CD44 that can affect the binding of hyaluronan to CD44 [22, 23]. However, there are also indications of clustering-independent signaling via CD44. Thus, hyaluronan dodeccasacharides, which most likely are unable to induce CD44 clustering, induce chemokine CXCL1 secretion resulting in endothelial cell sprouting in a CD44 dependent manner [86]. During tumor progression, HAS and HYAL activities give rise to hyaluronan molecules of high or low molecular mass, with capacity to bind differentially to CD44 and thereby modulate its function. This complexity may explain why CD44 expression is not correlated to tumor aggressiveness in neuroblastomas and prostate cancer [23].
Since the detailed description of the expression of CD44v isoforms from less malignant to more advanced stages is beyond the scope of this review, we will highlight the relevance of CD44 variants in cancer, which seem to be suitable targets for anti-cancer therapy. In several primary and cancer cells CD44v6 forms a ternary complex with HGF and its receptor c-Met. Most likely, CD44v6 presents HGF to its receptor, triggering receptor kinase activity and signaling pathways involving ERM protein ezrin binding and thus actin cytoskeleton binding and Ras activation. HGF elicits metastatic behavior on various types of cells, mostly in a paracrine fashion. In a recent study, we found that insulin-like growth factor 1 (IGF1), TGFβ, prostaglandin E2 (PGE2) and tumor necrosis factor-alpha (TNF-α) secreted by prostate cancer cells stimulate synthesis of HGF by myofibroblasts. The HGF, in turn, stimulates production of splice variant 9 of CD44. The interaction of stromal-derived hyaluronan with the up-regulated CD44v9 initiates signaling pathways that stabilize androgen receptor (AR) functions and induce anti-apoptotic signaling [87]. Colon cancer cells have the same mechanism, but utilize CD44v6 (Misra et al., unpublished data). Silencing the appropriate CD44-variant inhibits tumor cell adhesion to tumor cell matrix and in vitro tumor cell invasion [87]. Cross-talk between the increased hyaluronan synthesized by the stromal cells that interacts with colon tumor cell CD44v6 sustains hyaluronan/CD44v6/phosphoinositide 3-kinase (PI3K) signaling through a positive feedback loop between CD44v6 and PI3K that induces invasiveness. In addition, we demonstrated that stromal-derived HGF stimulated synthesis of metalloproteinase (MT1MMP), which induces shedding of the CD44 variants, and promotes colon/prostate cancer cell invasiveness (Misra et al., unpublished data; depicted in the model Fig. 2). Thus, therapeutic approaches by targeting hyaluronan/CD44v interaction with CD44v-shRNA can target tumors at one or more of these levels: the micro-environment (stromal factors such as HGF and its inducers), receptor based signals (select CD44v, Met/RTK) and signal transducers such as PI3K/AKT, or mitogen-activated protein kinase (MAPK) (Fig. 2).
FIG.2.
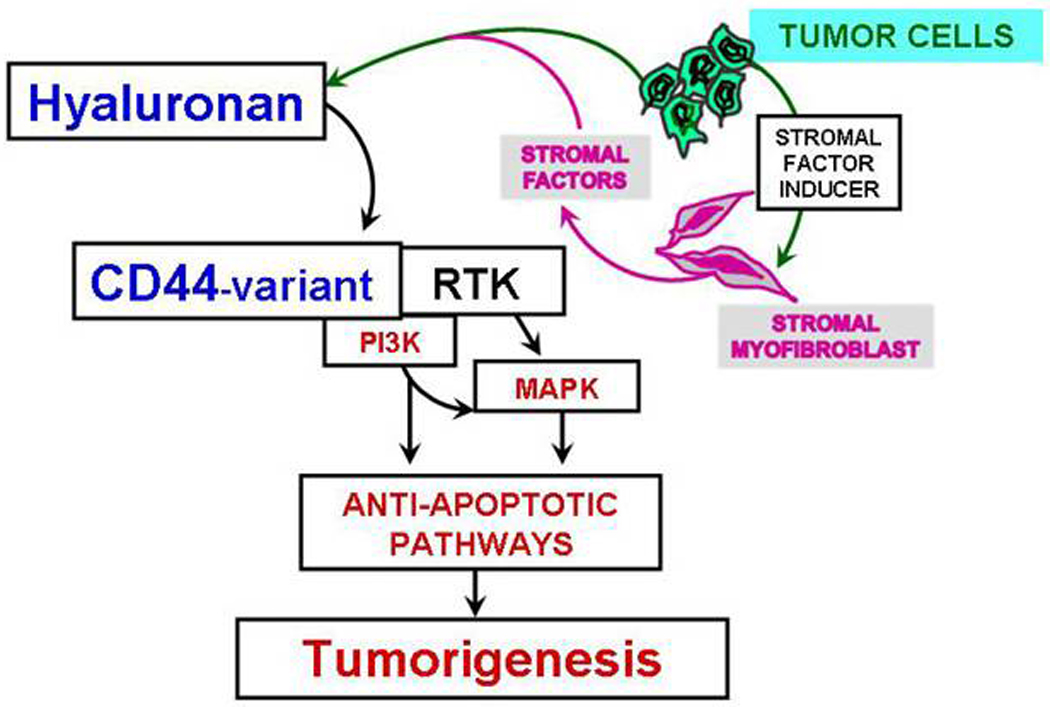
Proposed model for the cross-talk between tumor cells (epithelial cells) and tumor-associated stromal myofibroblasts. Cancer cells and stroma-derived fibroblasts influence each others’ development. The extracellular domain of CD44 variants that contain the sequence encoded for variants of CD44 and its interaction with HA are required for the stromal factor-dependent activation of receptor tyrosine kinases (RTK such as, HGF/Met) and its downstream anti-apoptotic signaling involving PI3K/AKT and MAPK pathways. Tumor associated srtromal myofibroblast derived hyaluronan synthesized in response to stromal factors (such as, HGF) and cancer cell-derived CD44variant, and RTK are involved in the tumorigenesis.
CD44 and hyaluronan in tumors, wounds that do not heal
The tumor microenvironment contains many distinct cell types, including endothelial cells and their precursors, pericytes, smooth muscle cells, fibroblasts, carcinoma-associated fibroblasts (CAFs/TAFs), myofibroblasts, neutrophils/eosinophils/basophils/mast cells, T/B lymphocytes, natural killer cells and antigen presenting cells (APC), such as macrophages and dendritic cells [4]. The micro-environment of a solid tumor closely resembles the environment of wound healing and tissue repair sites of an injured tissue. Upon tissue injury, platelets are activated. These activated platelets release vasoactive mediators for vascular permeability, serum fibrinogen for fibrin clot formation, growth factors/ cytokines/matricellular proteins to initiate granulation tissue formation, activation of fibroblasts, and induction and activation of matrix metalloproteinases necessary for ECM remodeling. Epithelial and stromal cell types engage in a reciprocal signaling cross-talk to assist healing. The reciprocal signaling collapses after the wound is healed. In case of tumorigenesis, the invasive inflammatory tumor cells produce an array of cytokines/chemokines that are mitogenic for granulocytes/monocytes/macrophages/fibroblasts/endothelial cells. These factors (cytokines/chemokines) potentiate tumor growth, stimulate angiogenesis, induce fibroblast migration, and enable metastatic spread. During this process, non-hematopoietic mesenchymal stem cells (MSCs) originating from bone marrow localize to the to sites of hematopoiesis, sites of inflammation, and sites of injury as well as to solid tumors [88–90]. Inactivated MSCs have been shown to inhibit tumor growth by inhibiting a PI3K/AKT pathway in an E-cadherin dependent way, prompting the use of these cells as tumor inhibitory cells in vivo [91], whereas activated MSCs within the solid tumors are the source of CAFs that contribute to tumor growth in several ways [92, 93]. Tissue injury and inflammation are accompanied by increased production of stromal hyaluronan, which in addition to cell–cell and cell–matrix adhesion [94, 95] and cell proliferation and survival [10, 83, 87, 96], helps to create highly hydrated ECM that may facilitate local cellular trafficking [97, 98]. In the bone marrow, hyaluronan is also abundantly produced by both stromal and hematopoietic cells. CD44 in addition to its function to regulate cell proliferation/differentiation/survival/migration into tissues is implicated in hematopoietic progenitor trafficking to the bone marrow and spleen [99–101]. The concept of use of MSCs as delivery vehicles came from the fact that the tumors, similar to the wounds, send out chemo-attractants such as the cytokines/chemokines (e.g. VEGF, TGF-β), to recruit MSCs to form the supporting stroma of the tumor, and also pericytes for angiogenesis. MSCs transfected with interferon-β gene can increase the production of interferon-β at the local site [102, 103]. Likewise, Herrera et al., [104] present a convincing case that interactions between CD44 and hyaluronan influence the homing of exogenous MSCs that localize to kidneys during acute renal failure, i.e., CD44 on exogenous cells is important in helping the MSCs to localize to the damaged renal tissue in vivo. However, this in vivo function of MSCs depends partly on signals from the target tissue microenvironment, i.e.; the endothelial progenitor cells were used as the gene delivery vehicles towards the site of angiogenesis rather than to the quiescent vasculature [105]. Based on these observations, it is possible to deliver immune activating cytokines/secreted proteins to the site of tumors through the MSCs [103]. Since hMSCs can be easily expanded in vitro and retain an extensive multipotent capacity for differentiation [106, 107], in a recent study we found that genetically engineered hMSCs that secrete soluble CD44 variants that act as antagonists to hyaluronan/CD44v signaling inhibit malignant properties in cancer cells (Misra et al., unpublished data). These studies and co-implantation models combining tumor cells and MSCs [102, 103, 108] hold great promise for therapeutic strategies [106], in which the interaction between tumor and stroma can be manipulated and studied (the concept of using MSCs for tumor therapy is depicted in the model Fig. 3).
FIG.3.
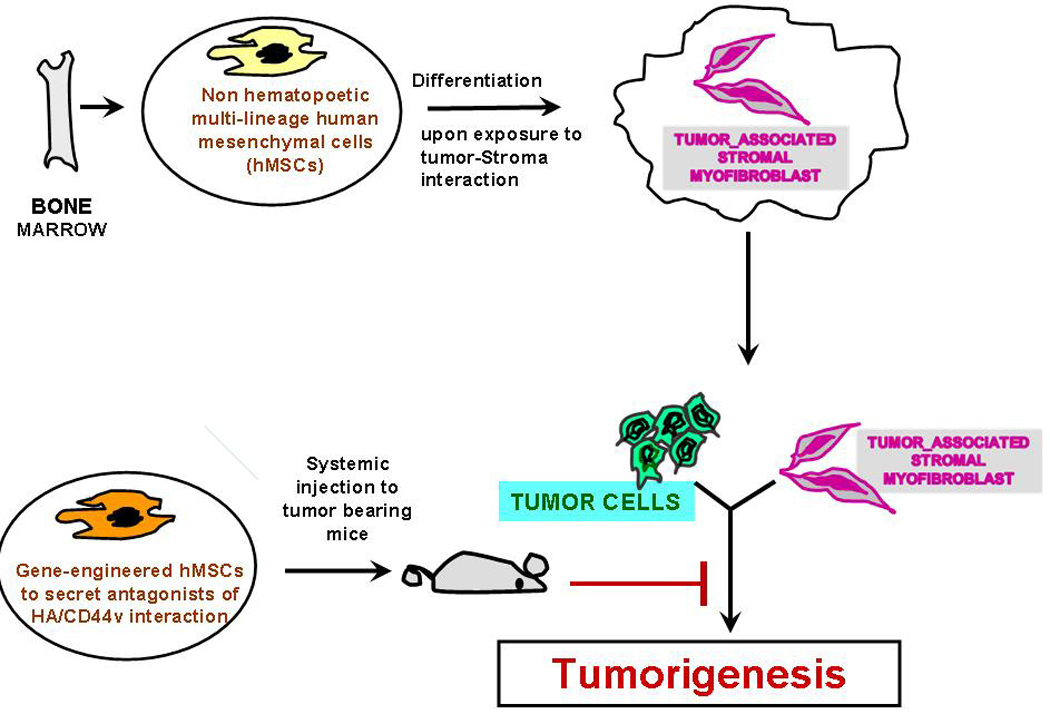
Bone marrow derived non hematopoetic hMSCs are pluripotent cells that are capable of differentiating into various tissue lineages, including osteoblasts, adipocytes, chondrocytes, myoblasts, hepatocytes and possibly even neural cells [107]. After systemic injection, hMSCs can selectively migrate to solid tumors, where they proliferate and become cancer associated stromal myofibroblasts [103]. Since hMSCs can be easily expanded in vitro and hold an extensive multipotent capacity for differentiation, hMSCs have been explored as vehicles for tissue repair and gene therapy [106], if they are appropriately engineered for therapy by circulating proteins for diseases. We established that tissue specific floxed plasmid/nanoparticle delivery is efficient to activate a gene of interest [120], and our pilot study (Misra et al., unpublished data) using genetically modified hMSCs in nanoparticles alters the tropism, because the secreted proteins from transduced hMSCs interact with stromal hyaluronan, and thus inhibit malignant properties of cancer cells by more than 20 fold by perturbing hyaluronan/CD44v interaction.
Therapeutic strategy involving perturbation of hyaluronan/CD44 interactions
Importance of targeting CD44variants in vivo
CD44 variant interaction with hyaluronan is known for its role in the metastatic cascade as this interaction regulates the ability of malignant cells to activate receptor tyrosine kinases, and to stimulate migration, invasion of basement membranes/ECM, and migration to distant sites [22, 57, 109–115]. Hyaluronan induces intracellular signaling when it binds to “constitutively activated” CD44 variants during cell dynamic processes, but does not do so under conditions of adult tissue homeostasis, which generally involve the standard form of CD44 (CD44s). The CD44 structure on normal cells is distinct from that on cancer cells because under various physiological and pathological conditions, the local environmental pressure (stromal factors) influences alternate splicing and post-translational modification to produce diversified CD44 molecules [35, 36]. This diversification allows the production of specific targeting agents that will be useful for both diagnosis and therapy. Pathological conditions that stimulate alternate splicing and post translational modifications produce diversified CD44 molecules with enhanced hyaluronan binding that leads to increased tumorigenicity [30–36]. Systemic application of antibodies directed against a CD44 variant epitope [43] reduced metastasizing activity of a pancreatic adenocarcinoma. Over expression of variant, high molecular weight isoforms CD44v4-v7 and CD44v6-v9 in various cancers, [45–52] as well as down regulation of the standard CD44s isoform in colon cancer, are postulated to result in increased tumorigenicity [53], emphasizing the potential importance of CD44 splice variants in cancer.
Inhibition of hyaluronan /CD44 interactions
To explore the mechanism of constitutive hyaluronan/CD44 interactions and the consequent outcomes in cancer cells, four different methods were used. The first method uses small hyaluronan oligosacharides (O-hyaluronan; ~2.5 kDa) that compete with the endogenous hyaluronan polymer [83, 96, 110, 116–118]. The second method over expresses soluble hyaluronan -binding proteins (e.g., soluble CD44) that act as competitive decoys for CD44 and thus bind to the endogenous hyaluronan [83, 96, 110, 116, 117]. The third method blocks hyaluronan/CD44 interaction specifically by treating the cells with a blocking antibody against the hyaluronan binding site of CD44 [96, 104, 117, 119]. The fourth inhibits post transcriptional expression of CD44 variants with CD44siRNAs [83, 87, 110, 112, 119, 120]. While these methods yielded valuable information on how epithelial cell-derived hyaluronan and its interaction with CD44 variants can influence malignant properties in vitro, they do not address the tumor cell responses to cell-specific perturbation of the hyaluronan/CD44v interaction at the genetic level in vitro and in vivo. In addition, by using the CD44siRNA to interrupt hyaluronan-CD44v6 signaling processes at a cellular level [110, 112], it has been observed that the phenotypic changes induced by siRNAs only persist one week due to lack of transfer of siRNA or to dilution of siRNA concentration after each cell division, or to lack of stability of siRNA, which limits their utility for use in inhibiting tumor progression in vivo. Moreover, the dose of siRNA remains undefined in that case, and the induction of innate immune responses will be another obstacle that will obscure the use of siRNA as therapeutics.
Srategies that target CD44 to perturb HA-CD44 interaction on tumors [121]
Hyaluronan Conjugated Drug
CD44 can internalize hyaluronan [122]. Thus hyaluronan carrying drug alone or encapsulated drug in liposomes has the potential to be used as a targeted drug as well as a drug transport vehicle. Chemical groups of hyaluronan such as carboxylate on the glucuronic acid, the N-acetylglucosamine hydroxyl, and the reducing end, can be potentially used to conjugate a drug [123]. Hyaluronan-drug conjugates are internalized via CD44, and the drug is released and activated mainly by intracellular enzymatic hydrolysis [124–126]. Activated CD44 is over expressed on solid tumors but not on their non-tumorigenic counterparts. Several preclinical studies have shown that hyaluronan chemically conjugated to cytotoxic agents improved anticancer properties of the agent in vitro [125, 127, 128]. Drugs with low solubility can be successfully applied when conjugated with hyaluronan. For instance, the antimitotic chemotherapeutic agent paclitaxel has low water solubility. Upon conjugation to hyaluronan, its solubility and CD44 dependent cellular uptake increased in vitro [126].
Hyaluronan conjugated nano-carrier
Hyaluronan when conjugated to a nanocarrier acts as a protective structural component and a targeting coating. Circulation time and biodistribution (pharmacokinetic properties) are influenced by incorporating the targeting and cell specific uptake properties of hyaluronan onto large carriers. Cargo liposomes or nanoparticles delivered to CD44 over-expressing cells include anti-cancer drugs: epirubicin [127], doxorubicin [129–139], paclitaxel [125, 126], and mitomycin c [127, 133], as well as siRNA [140, 141]. Results from the above studies using hyaluronan targeted nanocarriers do not differ from many of the studies performed with hyaluronan-drug conjugates.
Targeting with Anti-CD44 antibodies
Anti-CD44 antibodies against highly expressed variants can “actively” target drugs to CD44, inhibit and disrupt CD44 matrix interactions, occupy CD44 and induce CD44 signaling, which can cause apoptosis [142]. Anti-CD44 antibodies targeting ligands for either radio-labels or anti-cancer chemotherapeutics partially stabilize some patients [143, 144]. CD44v6 is expressed in – breast, cervical and colon cancers, and in squamous cell carcinomas. This variant of CD44 was chosen as a model for therapy. A Phase 1 clinical trial was done with an immunotoxin (humanized antibody coupled with a cytotoxic drug mertansine) against CD44v6 in thirty patients with incurable squamas cell carcinoma [76].Three patients showed partial response and thought the trial was successful. Unfortunately one of them died, and the trial was abruptly withdrawn.
Ttissue-specific deletion of CD44variant signalling
The technique of using shRNA in an expression vector is an alternative strategy to stably suppress selected gene expression, which suggests that the use of shRNA expression vectors holds potential promise for therapeutic approaches for silencing disease causing genes [145]. There are two ways to deliver shRNA in cancer cells – either using a viral vector or a non-viral vector. Viral vectors have been used to achieve this proof of principle in animal models and, in selected cases, in human clinical trials [146]. Systemic targeting by viral vectors towards the desired tissue is difficult because the host immune responses activate viral clearance. Systemic administration of a large amount of adenovirus (e.g. into the liver) can be a serious health hazard that even caused the death of one patient [146]. Nevertheless, there has been a considerable interest in developing non viral-vectors for gene therapy. In this regard, non viral vectors, such as positively charged polyethyleneimine (PEI)-complexes shielded with polyethylene glycol (PEG), can be used safely to avoid the non-specific interactions with non-target cells and blood components [147]. Non viral vectors were once limited for their low gene transfer efficiency. However, the incorporation of various ligands, such as peptides, growth factors and proteins, or antibodies for targets highly expressed on cancer cells, circumvented this obstacle [148]. Also, enhanced permeability due to aberrant vasculature in solid tumors and retention (known as EPR effect) of ligand coated vectors around the receptors of tumor cells can increase chances for high probability of interaction with the cells [120]. Thus, the non-viral vectors can acquire high gene transfer efficiency [120]. This concept has been tested by preparing non-viral vector nanoparticles with plasmids packed inside an outer PEG-PEI layer coated with transferrin (Tf), an iron transporting protein [120, 148] that binds with Tf-receptors (Tf-R) with high affinity. The Tf-R is present at much higher levels on the tumor cells [120] than on phenotypically normal epithelial cells. Association of transferrin with the nanoparticles significantly enhances transfection efficiency of shRNA generator-plasmids by promoting the internalization of nanoparticles in dividing and non-dividing cells through receptor-mediated endocytosis [148]. Finally, the uptake of nanoparticles carrying multiple functional domains (surface shielding particles Tf-PEG-PEI, shRNA generator plasmids, tissue specific promoter driven-Cre recombinase, and conditionally silenced plasmid) can overcome the intracellular barriers for successful delivery of the shRNA gene.
The newly developed cell-specific shRNA delivery approach by Misra et al [120] confirmed that targeting the hyaluronan/CD44v6-induced signaling pathway inhibited distant colon tumor growth in Apc Min/+ mice. The tissue specific shRNA delivery was made possible by the use of Cre-recombinase produced in response to a colon tissue specific promoter, which deletes the interruption between the U6 promoter and the CD44v6shRNA oligonucleotide. This approach, depicted in the model Fig. 4, has successfully demonstrated that the CD44v6shRNA is localized into the colon tumor cells by an end point assay of CD44v6 expression, and by perturbation of hyaluronan-CD44v6 interaction as reflected in the reduction in number of tumors [120]. In our recent in vivo studies with the C57Bl/6 mice, we are optimistic that systemic delivery of a mixture of the two plasmids in Tf/nanoparticles - (pARR2-Probasin-Cre/nanoparticles and the floxed pSico-CD44v9shRNA/nanoparticles) – can target both localized and metastatic prostate cancer cells (Ghatak et al., unpublished data). This novel approach opens up new ways to combat cancer, and to understand tumorigenesis in vivo for the following reasons: 1) cell specific release of shRNA by applying a tisuue specific promoter driven Cre-lox mechanism, 2) it silences the expression of the selected CD44 variant in the target tissue cancer cells, 3) it does not affect the normal target tissue cells, which do not express the targeted CD44variant, and which rely on the CD44s (standard) form, which is not affected by the plasmids, 4) the target CD44vshRNA is not expressed in other types of cells because the tissue specific promoter only unlocks the Cre recombinase in the targeted tissue cells thereby reducing potential side effects [120], 5) the nanoparticles that carry plasmids are biodegradable and cleared from the system, 6) it addresses the pathophysiological role of hyaluronan-CD44v interactions in cancer, 7) it can establish diagnostic markers for the targeted cancer including CD44variants, soluble CD44, and hyaluronan, and 8) it can establish CD44v/hyaluronan interactions as an innovative novel therapeutic target against cancer progression. Thus, the conditional suppression of gene expression by the use of an CD44vshRNA expressing plasmid holds potential promise for therapeutic approaches for silencing hyaluronan/CD44variant signaling and hence its downstream signaling that promotes disease causing genes [145] (Fig. 4).
FIG. 4.
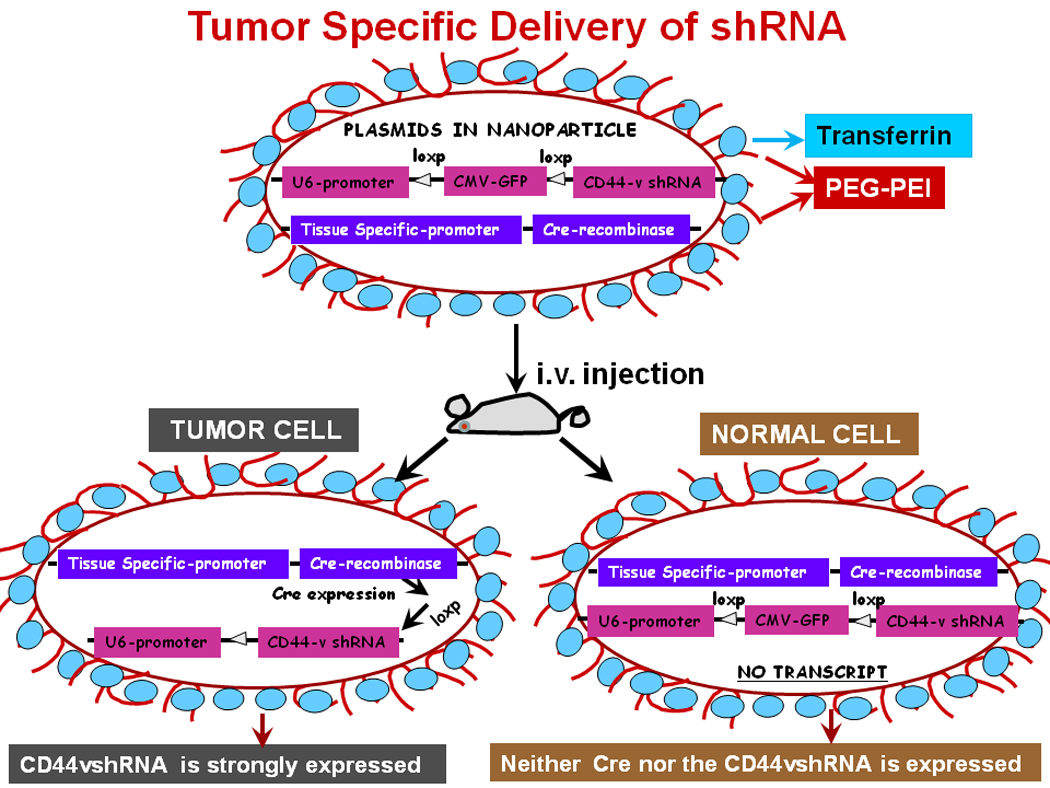
Model for delivery of shRNA. This illustration depicts cellular uptake of plasmid Tf-PEGPEI nanoparticles and the mechanism of action of shRNA. A: First, a pSico vector containing a U6 promoter-loxP-CMV-GFP-STOP signal-loxP-CD44vshRNA (gene of interest) is made. Second, an expression vector with the Cre recombinase gene controlled by the tissue specific promoter is created. Third, the two vectors are packaged in transferrin (Tf) coated-PEG-PEI nanoparticles that bind with Tf-receptors (Tf-R) present at high levels in the targeted tumor cells. Delivery of the vectors in normal and malignant cells from the targeted tissue results in deletion of the Stop signal and transcriprtion of Cre recombinase driven by the tissue specific promoter. The target gene (CD44vshRNA) is then unlocked and transcribed through the strong U6 promoter for high expression. The normal tissue cells are not affected because they do not make the targeted CD44 variant.
Advantages of tumor specific delivery of CD44variant-shRNA versus other therapeutic strategies
First, this technique avoids multiple chemical steps to prepare hyaluronan conjugated cytotoxic drugs and conjugation to nanocarriers. Second, it abolishes the CD44 variants in the cancer cells only. Third, a number of cell types in normal tissues that express CD44 will not be affected because these are not activated. Fourth, inflammation-associated cancers accumulate activated immune cells having upregulated transferrin receptors and CD44 variant. However, they may take up the nanoparticles but no deletion of CD44 variant will take place because the promoter is not lymphocyte specific (Misra et al., unpublished data). To target activated lymphocytes, specific promoter driven-cre plasmids should be used. Fifth, accumulation of antibody in non-tumor areas is a major limitation of anti-CD44 antibody therapy. Experiments so far do not produce any such effect in shRNA delivery.
Concluding remarks
Regardless of the increasing literature of studies conducted so far, the complete understanding of hyaluronan/CD44 induced signaling still remains elusive. However, both hyaluronan and CD44 appear to be vitally important from embryogenesis to morphogenesis, in inflammation, and in cancer, which accompanies over expression of CD44 and its splice variants and aberrant synthesis/turnover of the hyaluronan. Based on the above-mentioned functions of hyaluronan and its interaction with CD44, it seems likely that the impact of hyaluronan/CD44 and its variant-induced tumor growth is multi-factorial. Importantly CD44v-induced proteolysis [24, 149] of matrix facilitates detachment of malignant tumor cells from their confined tumor area and therefore promotes spread of the malignant tumor cells to distant sites. Moreover, partial degradation of hyaluronan molecules promotes angiogenesis, a vital requirement for tumor growth. Furthermore, by providing increased tissue hydration, hyaluronan molecules provide the suitable environment to support malignant cell migration similar to cardiac cushion cell movement [150–156]. In summary, CD44 and more specifically CD44variants are promising target molecules for therapy and diagnosis at least in some tumors.
Acknowledgements
This work was supported, in whole or in part, by National Institutes of Health Grants P20RR021949 (to S. G.) and P20RR016434 (to S. M., S. G., and R. R. M.), HL RO1 33756 and 1 P30AR050953 (to V. C. H.). This work was also supported by Mitral-07 CVD 04 (to R. R. M.), Medical University of South Carolina University Research Council Project 2204000-24330 (to S. M.) and 2204000-24329 (to S. G.).
Abbreviations
- CAFs
carcinoma-associated fibroblasts
- CD44s
standard CD44
- CD44v
variant CD44
- CSCs
cancer stem-cells
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal transition
- ERM
ezrin-radixin-moesin proteins
- FGF
fibroblast growth factor
- HAS
hyaluronan synthase
- HGF
hepatocyte growth factor
- HYAL
hyaluronidase
- MMP
matrix metalloproteinase
- MSCs
mesenchymal stem cells
- PDGFR
platelet-derived growth factor receptor
- PEG
polyethylene glycol
- PEI
polyethyleneimine
- PI3K
phosphoinositide 3-kinase
- Tf
transferin
- TGFβ
transforming growth factor beta
- VEGF
vascular endothelial growth factor
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, Turley EA, Bissell MJ. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammatory Cells and Cancer. Think different! J Exp Med. 2001;193:F23–F26. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. doi: 10.1111/j.1742-4658.2010.07800.x. [DOI] [PubMed] [Google Scholar]
- 7.Murphy G, Nagase H. Localising MMP activities in the pericellular environment. FEBS J. 2010 doi: 10.1111/j.1742-4658.2010.07918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 9.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 11.Evanko SP, Parks WT, Wight TN. Intracellular hyaluronan in arterial smooth muscle cells: association with microtubules, RHAMM, and the mitotic spindle. J Histochem Cytochem. 2004;52:1525–1535. doi: 10.1369/jhc.4A6356.2004. [DOI] [PubMed] [Google Scholar]
- 12.Hascall VC, Majors AK, De La Motte CA, Evanko SP, Wang A, Drazba JA, Strong SA, Wight TN. Intracellular hyaluronan: a new frontier for inflammation? Biochim Biophys Acta. 2004;1673:3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Heldin P, Pertoft H. Synthesis and assembly of the hyaluronan-containing coats around normal human mesothelial cells. Exp. Cell Res. 1993;208:422–429. doi: 10.1006/excr.1993.1264. [DOI] [PubMed] [Google Scholar]
- 14.Toole BP. Hyaluronan-CD44 Interactions in Cancer: Paradoxes and Possibilities. Clin Cancer Res. 2009;15:7462–7468. doi: 10.1158/1078-0432.CCR-09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurent TC, Fraser JRE. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 16.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 18.Lesley J, Hyman R. CD44 can be activated to function as an hyaluronic acid receptor in normal murine T cells. Eur J Immunol. 1992;22:2719–2723. doi: 10.1002/eji.1830221036. [DOI] [PubMed] [Google Scholar]
- 19.Lesley J, English N, Charles C, Hyman R. The role of the CD44 cytoplasmic and transmembrane domains in constitutive and inducible hyaluronan binding. Eur J Immunol. 2000;30:245–253. doi: 10.1002/1521-4141(200001)30:1<245::AID-IMMU245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.He Q, Lesley J, Hyman R, Ishihara K, Kincade PW. Molecular isoforms of murine CD44 and evidence that the membrane proximal domain is not critical for hyaluronate recognition. J Cell Biol. 1992;119:1711–1719. doi: 10.1083/jcb.119.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sleeman J, Rudy W, Hofmann M, Moll J, Herrlich P, Ponta H. Regulated clustering of variant CD44 proteins increases their hyaluronate binding capacity. J Cell Biol. 1996;135:1139–1150. doi: 10.1083/jcb.135.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 23.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271–1277. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Cichy J, Pure E. The liberation of CD44. J Cell Biol. 2003;161:839–843. doi: 10.1083/jcb.200302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackay CR, Terpe HJ, Stauder R, Marston WL, Stark H, Gunthert U. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 1994;124:71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sneath RJ, Mangham DC. The normal structure and function of CD44 and its role in neoplasia. Mol Pathol. 1998;51:191–200. doi: 10.1136/mp.51.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lesley J, He Q, Miyake K, Hamann A, Hyman R, Kincade PW. Requirements for hyaluronic acid binding by CD44: a role for the cytoplasmic domain and activation by antibody. J Exp Med. 1992;175:257–266. doi: 10.1084/jem.175.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesley J, Howes N, Perschl A, Hyman R. Hyaluronan binding function of CD44 is transiently activated on T cells during an in vivo immune response. J Exp Med. 1994;180:383–387. doi: 10.1084/jem.180.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesley J, Hyman R. CD44 structure and function. Front Biosci. 1998;3:D616–D630. doi: 10.2741/a306. [DOI] [PubMed] [Google Scholar]
- 30.Bourguignon LY, Zhu H, Shao L, Zhu D, Chen YW. Rho-kinase (ROK) promotes CD44v(3, 8–10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton. 1999;43:269–287. doi: 10.1002/(SICI)1097-0169(1999)43:4<269::AID-CM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Bourguignon LY, Zhu H, Shao L, Chen YW. CD44 interaction with tiam1 promotes Rac1 signaling and hyaluronic acid- mediated breast tumor cell migration. J Biol Chem. 2000;275:1829–1838. doi: 10.1074/jbc.275.3.1829. [DOI] [PubMed] [Google Scholar]
- 32.Bourguignon LY, Singleton PA, Zhu H, Zhou B. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- 33.Bourguignon LY, Zhu H, Shao L, Chen YW. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem. 2001;276:7327–7336. doi: 10.1074/jbc.M006498200. [DOI] [PubMed] [Google Scholar]
- 34.Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- 35.van Weering DH, Baas PD, Bos JL. A PCR-based method for the analysis of human CD44 splice products. PCR Methods Appl. 1993;3:100–106. doi: 10.1101/gr.3.2.100. [DOI] [PubMed] [Google Scholar]
- 36.Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- 37.Katoh S, Zheng Z, Oritani K, Shimozato T, Kincade PW. Glycosylation of CD44 negatively regulates its recognition of hyaluronan. J Exp Med. 1995;182:419–429. doi: 10.1084/jem.182.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skelton TP, Zeng C, Nocks A, Stamenkovic I. Glycosylation provides both stimulatory and inhibitory effects on cell surface and soluble CD44 binding to hyaluronan. J Cell Biol. 1998;140:431–446. doi: 10.1083/jcb.140.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rochman M, Moll J, Herrlich P, Wallach SB, Nedvetzki S, Sionov RV, Golan I, Ish-Shalom D, Naor D. The CD44 receptor of lymphoma cells: structure-function relationships and mechanism of activation. Cell Adhes Commun. 2000;7:331–347. doi: 10.3109/15419060009015004. [DOI] [PubMed] [Google Scholar]
- 40.Hathcock KS, Hirano H, Murakami S, Hodes RJ. CD44 expression on activated B cells. Differential capacity for CD44- dependent binding to hyaluronic acid. J Immunol. 1993;151:6712–6722. [PubMed] [Google Scholar]
- 41.Lesley J, English N, Perschl A, Gregoroff J, Hyman R. Variant cell lines selected for alterations in the function of the hyaluronan receptor CD44 show differences in glycosylation. J Exp Med. 1995;182:431–437. doi: 10.1084/jem.182.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thankamony SP, Knudson W. Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. J Biol Chem. 2006;281:34601–34609. doi: 10.1074/jbc.M601530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seiter S, Arch R, Reber S, Komitowski D, Hofmann M, Ponta H, Herrlich P, Matzku S, Zoller M. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993;177:443–455. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeder JA, Gotley DC, Walsh MD, Fawcett J, Antalis TM. Expression of antisense CD44 variant 6 inhibits colorectal tumor metastasis and tumor growth in a wound environment. Cancer Res. 1998;58:3719–3726. [PubMed] [Google Scholar]
- 45.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 46.Naor D, Sionov RV, Zahalka M, Rochman M, Holzmann B, Ish-Shalom D. Organ-specific requirements for cell adhesion molecules during lymphoma cell dissemination. Curr Top Microbiol Immunol. 1998;231:143–166. doi: 10.1007/978-3-642-71987-5_9. [DOI] [PubMed] [Google Scholar]
- 47.Ochiai S, Nakanishi Y, Mizuno K, Hashimoto S, Inutsuka S, Kawasaki M, Yatsunami J, Hara N. [Expression of CD44 standard and CD44 variant 6 in human lung cancer] Nihon Kyobu Shikkan Gakkai Zasshi. 1997;35:1179–1185. [PubMed] [Google Scholar]
- 48.Kurozumi K, Nishida T, Nakao K, Nakahara M, Tsujimoto M. Expression of CD44 variant 6 and lymphatic invasion: importance to lymph node metastasis in gastric cancer. World J Surg. 1998;22:853–857. doi: 10.1007/s002689900481. discussion 857–8. [DOI] [PubMed] [Google Scholar]
- 49.Foekens JA, Dall P, Klijn JG, Skroch-Angel P, Claassen CJ, Look MP, Ponta H, Van Putten WL, Herrlich P, Henzen-Logmans SC. Prognostic value of CD44 variant expression in primary breast cancer. Int J Cancer. 1999;84:209–215. doi: 10.1002/(sici)1097-0215(19990621)84:3<209::aid-ijc2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 50.Ayhan A, Tok EC, Bildirici I. Overexpression of CD44 variant 6 in human endometrial cancer and its prognostic significance. Gynecol Oncol. 2001;80:355–358. doi: 10.1006/gyno.2000.6014. [DOI] [PubMed] [Google Scholar]
- 51.Ishida T. Immunohistochemical expression of the CD44 variant 6 in colorectal adenocarcinoma. Surg Today. 2000;30:28–32. doi: 10.1007/PL00010042. [DOI] [PubMed] [Google Scholar]
- 52.Ishibashi M, Nishida T, Murakami H, Shiraishi M, Aritomi T, Yoshida M. [The role of interstitial hyaluronan in acute lung injury] Nihon Kyobu Shikkan Gakkai Zasshi. 1995;33 Suppl:225–230. [PubMed] [Google Scholar]
- 53.Choi SH, Takahashi K, Eto H, Yoon SS, Tanabe KK. CD44s expression in human colon carcinomas influences growth of liver metastases. Int J Cancer. 2000;85:523–526. doi: 10.1002/(sici)1097-0215(20000215)85:4<523::aid-ijc13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Skandalis SS, Kletsas D, Kyriakopoulou D, Stavropoulos M, Theocharis DA. The greatly increased amounts of accumulated versican and decorin with specific post-translational modifications may be closely associated with the malignant phenotype of pancreatic cancer. Biochim Biophys Acta. 2006;1760:1217–1225. doi: 10.1016/j.bbagen.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Dev. 2006;20:1715–1720. doi: 10.1101/gad.1430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anttila MA, Tammi RH, Tammi MI, Syrjanen KJ, Saarikoski SV, Kosma VM. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000;60:150–155. [PubMed] [Google Scholar]
- 57.Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–231. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 58.Theocharis AD, Vynios DH, Papageorgakopoulou N, Skandalis SS, Theocharis DA. Altered content composition and structure of glycosaminoglycans and proteoglycans in gastric carcinoma. Int J Biochem Cell Biol. 2003;35:376–390. doi: 10.1016/s1357-2725(02)00264-9. [DOI] [PubMed] [Google Scholar]
- 59.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2–ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 60.Karousou E, Kamiryo M, Skandalis SS, Ruusala A, Asteriou T, Passi A, Yamashita H, Hellman U, Heldin CH, Heldin P. The activity of hyaluronan synthase 2 is regulated by dimerization and ubiquitination. J Biol Chem. 2010;285:23647–23654. doi: 10.1074/jbc.M110.127050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacobson A, Brinck J, Briskin MJ, Spicer AP, Heldin P. Expression of human hyaluronan synthases in response to external stimuli. Biochem J. 2000;348(Pt 1):29–35. [PMC free article] [PubMed] [Google Scholar]
- 62.Brinck J, Heldin P. Expression of recombinant hyaluronan synthase (HAS) isoforms in CHO cells reduces cell migration and cell surface CD44. Exp. Cell Res. 1999;252:342–351. doi: 10.1006/excr.1999.4645. [DOI] [PubMed] [Google Scholar]
- 63.Kosaki R, Watanabe K, Yamaguchi Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res. 1999;59:1141–1145. [PubMed] [Google Scholar]
- 64.Itano N, Sawai T, Miyaishi O, Kimata K. Relationship between hyaluronan production and metastatic potential of mouse mammary carcinoma cells. Cancer Res. 1999;59:2499–2504. [PubMed] [Google Scholar]
- 65.Li Y, Heldin P. Hyaluronan production increases the malignant properties of mesothelioma cells. Br. J. Cancer. 2001;85:600–607. doi: 10.1054/bjoc.2001.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu N, Gao F, Han Z, Xu X, Underhill CB, Zhang L. Hyaluronan synthase 3 overexpression promotes the growth of TSU prostate cancer cells. Cancer Res. 2001;61:5207–5214. [PubMed] [Google Scholar]
- 67.Adamia S, Maxwell CA, Pilarski LM. Hyaluronan and hyaluronan synthases: potential therapeutic targets in cancer. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:3–14. doi: 10.2174/1568006053005056. [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Li L, Brown TJ, Heldin P. Silencing of hyaluronan synthase 2 suppresses the malignant phenotype of invasive breast cancer cells. Int J Cancer. 2007;120:2557–2567. doi: 10.1002/ijc.22550. [DOI] [PubMed] [Google Scholar]
- 69.Enegd B, King JA, Stylli S, Paradiso L, Kaye AH, Novak U. Overexpression of hyaluronan synthase-2 reduces the tumorigenic potential of glioma cells lacking hyaluronidase activity. Neurosurgery. 2002;50:1311–1318. doi: 10.1097/00006123-200206000-00023. [DOI] [PubMed] [Google Scholar]
- 70.Bharadwaj AG, Kovar JL, Loughman E, Elowsky C, Oakley GG, Simpson MA. Spontaneous metastasis of prostate cancer is promoted by excess hyaluronan synthesis and processing. Am J Pathol. 2009;174:1027–1036. doi: 10.2353/ajpath.2009.080501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobson A, Rahmanian M, Rubin K, Heldin P. Expression of hyaluronan synthase 2 or hyaluronidase 1 differentially affect the growth rate of transplantable colon carcinoma cell tumors. Int J Cancer. 2002;102:212–219. doi: 10.1002/ijc.10683. [DOI] [PubMed] [Google Scholar]
- 72.Lokeshwar VB, Cerwinka WH, Isoyama T, Lokeshwar BL. HYAL1 hyaluronidase in prostate cancer: a tumor promoter and suppressor. Cancer Res. 2005;65:7782–7789. doi: 10.1158/0008-5472.CAN-05-1022. [DOI] [PubMed] [Google Scholar]
- 73.Koyama H, Hibi T, Isogai Z, Yoneda M, Fujimori M, Amano J, Kawakubo M, Kannagi R, Kimata K, Taniguchi S, Itano N. Hyperproduction of hyaluronan in neu-induced mammary tumor accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG-M. Am J Pathol. 2007;170:1086–1099. doi: 10.2353/ajpath.2007.060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koyama H, Kobayashi N, Harada M, Takeoka M, Kawai Y, Sano K, Fujimori M, Amano J, Ohhashi T, Kannagi R, Kimata K, Taniguchi S, Itano N. Significance of tumor-associated stroma in promotion of intratumoral lymphangiogenesis: pivotal role of a hyaluronan-rich tumor microenvironment. Am J Pathol. 2008;172:179–193. doi: 10.2353/ajpath.2008.070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smedsrod B, Seljelid R. Fate of intravenously injected aminated beta(1----3) polyglucose derivatized with 125I-tyraminyl cellobiose. Immunopharmacology. 1991;21:149–158. doi: 10.1016/0162-3109(91)90020-y. [DOI] [PubMed] [Google Scholar]
- 76.Orian-Rousseau V, Ponta H. Adhesion proteins meet receptors: a common theme? Adv Cancer Res. 2008;101:63–92. doi: 10.1016/S0065-230X(08)00404-1. [DOI] [PubMed] [Google Scholar]
- 77.Heldin P, de la Torre M, Ytterberg D, Bergh J. Differential synthesis and binding of hyaluronan by human breast cancer cell lines: Relationship to hormone receptor status. Oncology Rep. 1996;3:1011–1016. doi: 10.3892/or.3.6.1011. [DOI] [PubMed] [Google Scholar]
- 78.Kaufmann M, Heider KH, Sinn HP, von Minckwitz G, Ponta H, Herrlich P. CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet. 1995;345:615–619. doi: 10.1016/s0140-6736(95)90521-9. [DOI] [PubMed] [Google Scholar]
- 79.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simeone DM. Pancreatic cancer stem cells: implications for the treatment of pancreatic cancer. Clin Cancer Res. 2008;14:5646–5648. doi: 10.1158/1078-0432.CCR-08-0584. [DOI] [PubMed] [Google Scholar]
- 81.Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y, Chen Q. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 82.Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC. Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biol Chem. 1997;272:27913–27918. doi: 10.1074/jbc.272.44.27913. [DOI] [PubMed] [Google Scholar]
- 83.Ghatak S, Misra S, Toole BP. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem. 2005;280:8875–8883. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
- 84.Li L, Heldin CH, Heldin P. Inhibition of platelet-derived growth factor-BB-induced receptor activation and fibroblast migration by hyaluronan activation of CD44. J Biol Chem. 2006;281:26512–26519. doi: 10.1074/jbc.M605607200. [DOI] [PubMed] [Google Scholar]
- 85.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takahashi Y, Li L, Kamiryo M, Asteriou T, Moustakas A, Yamashita H, Heldin P. Hyaluronan fragments induce endothelial cell differentiation in a CD44- and CXCL1/GRO1-dependent manner. J Biol Chem. 2005;280:24195–24204. doi: 10.1074/jbc.M411913200. [DOI] [PubMed] [Google Scholar]
- 87.Ghatak S, Hascall VC, Markwald RR, Misra S. Stromal hyaluronan interaction with epithelial CD44 variants promotes prostate cancer invasiveness by augmenting expression and function of hepatocyte growth factor and androgen receptor. J Biol Chem. 285:19821–19832. doi: 10.1074/jbc.M110.104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mendes SC, Robin C, Dzierzak E. Mesenchymal progenitor cells localize within hematopoietic sites throughout ontogeny. Development. 2005;132:1127–1136. doi: 10.1242/dev.01615. [DOI] [PubMed] [Google Scholar]
- 89.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 90.Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, Zerbini G, Allavena P, Bonifacio E, Piemonti L. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 91.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem. 2008;283:19864–19871. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- 93.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 94.Fraser JR, Cahill RN, Kimpton WG. Hyaluronic acid and cell adhesion molecules in haematology. Aust N Z J Med. 1994;24:71. doi: 10.1111/j.1445-5994.1994.tb04435.x. [DOI] [PubMed] [Google Scholar]
- 95.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 96.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–38020. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 97.Hallgren R, Gerdin B, Tufveson G. Hyaluronic acid accumulation and redistribution in rejecting rat kidney graft. Relationship to the transplantation edema. J Exp Med. 1990;171:2063–2076. doi: 10.1084/jem.171.6.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tufveson G, Gerdin B, Larsson E, Laurent T, Wallander J, Wells A, Hallgren R. Hyaluronic acid accumulation; the mechanism behind graft rejection edema. Transpl Int. 1992;5 Suppl 1:S688–S689. doi: 10.1007/978-3-642-77423-2_202. [DOI] [PubMed] [Google Scholar]
- 99.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 100.Khaldoyanidi S, Denzel A, Zoller M. Requirement for CD44 in proliferation and homing of hematopoietic precursor cells. J Leukoc Biol. 1996;60:579–592. doi: 10.1002/jlb.60.5.579. [DOI] [PubMed] [Google Scholar]
- 101.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 102.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 103.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 104.Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430–441. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 105.Anderson SA, Glod J, Arbab AS, Noel M, Ashari P, Fine HA, Frank JA. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood. 2005;105:420–425. doi: 10.1182/blood-2004-06-2222. [DOI] [PubMed] [Google Scholar]
- 106.Gafni Y, Turgeman G, Liebergal M, Pelled G, Gazit Z, Gazit D. Stem cells as vehicles for orthopedic gene therapy. Gene Ther. 2004;11:417–426. doi: 10.1038/sj.gt.3302197. [DOI] [PubMed] [Google Scholar]
- 107.Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. 2005;306:330–335. doi: 10.1016/j.yexcr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 108.Kanehira M, Xin H, Hoshino K, Maemondo M, Mizuguchi H, Hayakawa T, Matsumoto K, Nakamura T, Nukiwa T, Saijo Y. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007;14:894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 109.Marhaba R, Bourouba M, Zoller M. CD44v6 promotes proliferation by persisting activation of MAP kinases. Cell Signal. 2005;17:961–973. doi: 10.1016/j.cellsig.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 110.Misra S, Ghatak S, Toole BP. (JBC Paper of the Week)) Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J Biol Chem. 2005;280:20310–20315. doi: 10.1074/jbc.M500737200. [DOI] [PubMed] [Google Scholar]
- 111.Misra S, Obeid LM, Hannun YA, Minamisawa S, Berger FG, Markwald RR, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of COX-2-mediated cell survival activity in intestinal epithelial and colon carcinoma cells. J Biol Chem. 2008;283:14335–14344. doi: 10.1074/jbc.M703811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. 2006;281:34936–34941. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- 113.Heldin P, Karousou E, Bernert B, Porsch H, Nishitsuka K, Skandalis SS. Importance of hyaluronan-CD44 interactions in inflammation and tumorigenesis. Connect Tissue Res. 2008;49:215–218. doi: 10.1080/03008200802143323. [DOI] [PubMed] [Google Scholar]
- 114.Herold-Mende C, Seiter S, Born AI, Patzelt E, Schupp M, Zoller J, Bosch FX, Zoller M. Expression of CD44 splice variants in squamous epithelia and squamous cell carcinomas of the head and neck. J Pathol. 1996;179:66–73. doi: 10.1002/(SICI)1096-9896(199605)179:1<66::AID-PATH544>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 115.Regidor PA, Callies R, Regidor M, Gunthert U, Zoller M, Schindler AE. Expression of the CD44 variant isoforms 6 and 4/5 in breast cancer. Correlation with established prognostic parameters. Arch Gynecol Obstet. 1996;258:125–135. doi: 10.1007/s004040050113. [DOI] [PubMed] [Google Scholar]
- 116.Misra S, Ghatak S, Zoltan-Jones A, Toole BP. Regulation of multidrug resistance in cancer cells by hyaluronan. J Biol Chem. 2003;278:25285–25288. doi: 10.1074/jbc.C300173200. [DOI] [PubMed] [Google Scholar]
- 117.Zoltan-Jones A, Huang L, Ghatak S, Toole BP. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem. 2003;278:45801–45810. doi: 10.1074/jbc.M308168200. [DOI] [PubMed] [Google Scholar]
- 118.Marieb EA, Zoltan-Jones A, Li R, Misra S, Ghatak S, Cao J, Zucker S, Toole BP. Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res. 2004;64:1229–1232. doi: 10.1158/0008-5472.can-03-2832. [DOI] [PubMed] [Google Scholar]
- 119.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 120.Misra S, Hascall VC, De Giovanni C, Markwald RR, Ghatak S. Delivery of CD44 shRNA/nanoparticles within cancer cells: perturbation of hyaluronan/CD44v6 interactions and reduction in adenoma growth in Apc Min/+ MICE. J Biol Chem. 2009;284:12432–12446. doi: 10.1074/jbc.M806772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Platt VM, Szoka FC., Jr Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm. 2008;5:474–486. doi: 10.1021/mp800024g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Culty M, Nguyen HA, Underhill CB. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992;116:1055–1062. doi: 10.1083/jcb.116.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pouyani T, Prestwich GD. Functionalized derivatives of hyaluronic acid oligosaccharides: drug carriers and novel biomaterials. Bioconjug Chem. 1994;5:339–347. doi: 10.1021/bc00028a010. [DOI] [PubMed] [Google Scholar]
- 124.Luo Y, Kirker KR, Prestwich GD. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release. 2000;69:169–184. doi: 10.1016/s0168-3659(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 125.Luo Y, Prestwich GD. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjug Chem. 1999;10:755–763. doi: 10.1021/bc9900338. [DOI] [PubMed] [Google Scholar]
- 126.Luo Y, Ziebell MR, Prestwich GD. A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules. 2000;1:208–218. doi: 10.1021/bm000283n. [DOI] [PubMed] [Google Scholar]
- 127.Akima K, Ito H, Iwata Y, Matsuo K, Watari N, Yanagi M, Hagi H, Oshima K, Yagita A, Atomi Y, Tatekawa I. Evaluation of antitumor activities of hyaluronate binding antitumor drugs: synthesis, characterization and antitumor activity. J Drug Target. 1996;4:1–8. doi: 10.3109/10611869609046255. [DOI] [PubMed] [Google Scholar]
- 128.Coradini D, Pellizzaro C, Miglierini G, Daidone MG, Perbellini A. Hyaluronic acid as drug delivery for sodium butyrate: improvement of the anti-proliferative activity on a breast-cancer cell line. Int J Cancer. 1999;81:411–416. doi: 10.1002/(sici)1097-0215(19990505)81:3<411::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 129.Eliaz RE, Szoka FC., Jr Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001;61:2592–2601. [PubMed] [Google Scholar]
- 130.Eliaz RE, Nir S, Marty C, Szoka FC., Jr Determination and modeling of kinetics of cancer cell killing by doxorubicin and doxorubicin encapsulated in targeted liposomes. Cancer Res. 2004;64:711–718. doi: 10.1158/0008-5472.can-03-0654. [DOI] [PubMed] [Google Scholar]
- 131.Eliaz RE, Nir S, Szoka FC., Jr Interactions of hyaluronan-targeted liposomes with cultured cells: modeling of binding and endocytosis. Methods Enzymol. 2004;387:16–33. doi: 10.1016/S0076-6879(04)87002-2. [DOI] [PubMed] [Google Scholar]
- 132.Peer D, Dekel Y, Melikhov D, Margalit R. Fluoxetine inhibits multidrug resistance extrusion pumps and enhances responses to chemotherapy in syngeneic and in human xenograft mouse tumor models. Cancer Res. 2004;64:7562–7569. doi: 10.1158/0008-5472.CAN-03-4046. [DOI] [PubMed] [Google Scholar]
- 133.Peer D, Margalit R. Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int J Cancer. 2004;108:780–789. doi: 10.1002/ijc.11615. [DOI] [PubMed] [Google Scholar]
- 134.Peer D, Margalit R. Physicochemical evaluation of a stability-driven approach to drug entrapment in regular and in surface-modified liposomes. Arch Biochem Biophys. 2000;383:185–190. doi: 10.1006/abbi.2000.2046. [DOI] [PubMed] [Google Scholar]
- 135.Peer D, Margalit R. Tumor-targeted hyaluronan nanoliposomes increase the antitumor activity of liposomal Doxorubicin in syngeneic and human xenograft mouse tumor models. Neoplasia. 2004;6:343–353. doi: 10.1593/neo.03460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yadav AK, Agarwal A, Rai G, Mishra P, Jain S, Mishra AK, Agrawal H, Agrawal GP. Development and characterization of hyaluronic acid decorated PLGA nanoparticles for delivery of 5-fluorouracil. Drug Deliv. 17:561–572. doi: 10.3109/10717544.2010.500635. [DOI] [PubMed] [Google Scholar]
- 137.Yadav AK, Mishra P, Agrawal GP. An insight on hyaluronic acid in drug targeting and drug delivery. J Drug Target. 2008;16:91–107. doi: 10.1080/10611860802095494 . [DOI] [PubMed] [Google Scholar]
- 138.Yadav AK, Mishra P, Jain S, Mishra P, Mishra AK, Agrawal GP. Preparation and characterization of HA-PEG-PCL intelligent core-corona nanoparticles for delivery of doxorubicin. J Drug Target. 2008;16:464–478. doi: 10.1080/10611860802095494 . [DOI] [PubMed] [Google Scholar]
- 139.Yadav AK, Mishra P, Mishra AK, Mishra P, Jain S, Agrawal GP. Development and characterization of hyaluronic acid-anchored PLGA nanoparticulate carriers of doxorubicin. Nanomedicine. 2007;3:246–257. doi: 10.1016/j.nano.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 140.Lee H, Mok H, Lee S, Oh YK, Park TG. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J Control Release. 2007;119:245–252. doi: 10.1016/j.jconrel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 141.Yun YH, Goetz DJ, Yellen P, Chen W. Hyaluronan microspheres for sustained gene delivery and site-specific targeting. Biomaterials. 2004;25:147–157. doi: 10.1016/s0142-9612(03)00467-8. [DOI] [PubMed] [Google Scholar]
- 142.Song G, Liao X, Zhou L, Wu L, Feng Y, Han ZC. HI44a, an anti-CD44 monoclonal antibody, induces differentiation and apoptosis of human acute myeloid leukemia cells. Leuk Res. 2004;28:1089–1096. doi: 10.1016/j.leukres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 143.Tijink BM, Buter J, de Bree R, Giaccone G, Lang MS, Staab A, Leemans CR, van Dongen GA. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res. 2006;12:6064–6072. doi: 10.1158/1078-0432.CCR-06-0910. [DOI] [PubMed] [Google Scholar]
- 144.Sauter A, Kloft C, Gronau S, Bogeschdorfer F, Erhardt T, Golze W, Schroen C, Staab A, Riechelmann H, Hoermann K. Pharmacokinetics, immunogenicity and safety of bivatuzumab mertansine, a novel CD44v6-targeting immunoconjugate, in patients with squamous cell carcinoma of the head and neck. Int J Oncol. 2007;30:927–935. [PubMed] [Google Scholar]
- 145.Paul CP, Good PD, Winer I, Engelke DR. Effective expression of small interfering RNA in human cells. Nat Biotechnol. 2002;20:505–508. doi: 10.1038/nbt0502-505. [DOI] [PubMed] [Google Scholar]
- 146.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 147.Kursa M, Walker GF, Roessler V, Ogris M, Roedl W, Kircheis R, Wagner E. Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjug Chem. 2003;14:222–231. doi: 10.1021/bc0256087. [DOI] [PubMed] [Google Scholar]
- 148.Bellocq NC, Pun SH, Jensen GS, Davis ME. Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug Chem. 2003;14:1122–1132. doi: 10.1021/bc034125f. [DOI] [PubMed] [Google Scholar]
- 149.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 150.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 151.West DC, Kumar S. Hyaluronan and angiogenesis. Ciba Found Symp. 1989;143:187–201. doi: 10.1002/9780470513774.ch12. [DOI] [PubMed] [Google Scholar]
- 152.West DC, Kumar S. Tumour-associated hyaluronan: a potential regulator of tumour angiogenesis. Int J Radiat Biol. 1991;60:55–60. doi: 10.1080/09553009114551541. [DOI] [PubMed] [Google Scholar]
- 153.Camenisch TD, McDonald JA. Hyaluronan: is bigger better? Am J Respir Cell Mol Biol. 2000;23:431–433. doi: 10.1165/ajrcmb.23.4.f201. [DOI] [PubMed] [Google Scholar]
- 154.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 155.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Raija TaM T. Hyaluronan in the epidermis, Glycoforum. http://www.glycoforum.gr.jp/science/hyaluronan/HA04/HA04E.html. [Google Scholar]


