Abstract
Summary
The risk of hip and other fractures was examined among a population-based group of older women with breast cancer. Women using aromatase inhibitors (AIs) were found to be over three times more likely to have a hip fracture over approximately 3 years’ follow-up. Other fracture risk factors were also identified.
Introduction
Aromatase inhibitors have been shown in randomized trials to increase total fracture risk compared with tamoxifen, but the fracture risks in the trials were relatively low, and no difference in hip fracture has been demonstrated.
Methods
A population-based cohort of 2003 breast cancer survivors ≥65 were followed prospectively for a median of 36 months. Patient survey information regarding adjuvant breast cancer therapies, prescription osteoporosis treatments, and other factors potentially associated with fracture was supplemented with cancer registry information. Hip and total nonvertebral fractures were determined using a validated Medicare algorithm, and the association of these fractures with adjuvant hormonal therapies was examined using Cox models.
Results
The cohort of 2,748 women with a mean age of 72.8 (SD 5.4) included 28.2% who took an aromatase inhibitor and 27.8% tamoxifen. There were 41 hip fractures (1.5%) and 218 nonvertebral fractures (7.9%) among the cohort. Subjects using AIs (adjusted hazard ratio 3.24 (1.05, 9.98)) and subjects not using hormone therapy (3.32 (1.14, 9.65)) were more likely than users of tamoxifen to have a hip fracture. Bisphosphonate use was more common among AI users but did not explain these results. Users of AIs were more likely to have nonvertebral fractures, but this result did not reach statistical significance (adjusted hazard 1.34 (0.92, 1.94)).
Conclusions
Hip and other fractures were common in an older population-based cohort of breast cancer survivors, and aromatase inhibitor use was associated with an increase in the short-term risk of hip fractures not detected in randomized controlled trials
Keywords: Breast cancer, Hip fracture, Medication-associated osteoporosis
Introduction
Adjuvant aromatase inhibitor (AI) therapy has been shown in several randomized trials to reduce breast cancer recurrence by about 50% when compared to tamoxifen therapy [1–5] and is now recommended for virtually all postmenopausal women with hormone-sensitive breast cancer. Unfortunately, the same trials showing benefit from aromatase inhibitors have also shown increases in the total number of bony fractures with their use [1, 3–5]. The trials have reported modest absolute risks of fractures in both placebo and control groups, however, and none have yet detected a difference in hip fracture between AI and tamoxifen users. Several questions about AIs and their effects on fractures thus remain unanswered by clinical trials.
First of these questions is whether the fracture risk with aromatase inhibitors is larger for community-based patients than for clinical trial enrollees. In the USA, only about 5% of breast cancer patients enroll in clinical trials, and these enrollees represent a selected group who are younger [6], have fewer comorbidities and higher functional status [7], and are more likely to be White [6]. The large aromatase inhibitor trials varied somewhat in their enrollment criteria, but most excluded patients with major comorbid cardiopulmonary diseases or poor performance status [4, 8–10]. The median cohort age in all of the large AI trials was less than 65, and even the largest study had less than 300 participants over age 75 [11]. Because of concerns about bony risks, one large trial [10] specifically excluded patients with prior “severe” osteoporosis diagnoses. It is likely that AIs are being used in broader groups of patients outside clinical trial settings, so examination of an older and unselected group of patients is essential.
The difference in risk of hip fracture between community-based and randomized trial subjects may be particularly large. Hip fracture risk increases more than tenfold between the sixth and ninth decades [12, 13], a substantially greater increase than for other fractures. Hip fractures also occur much more among frail patients [14], so younger patients at high hip fracture risk because of comorbidity would also be underrepresented in trials. It is important that hip fracture risk be well-studied; less than half of patients resume baseline levels of functioning, and nearly one in five requires long-term nursing home care after hip fracture [12, 14].
Second, randomized trials will not be able to address a continuing question regarding whether AIs cause the entire excess of fractures observed or whether tamoxifen provides some protection. Tamoxifen has been shown since the 1990s to increase bone mineral density (BMD) by −1% to 2% yearly when compared with placebo [15–17], but little has been published regarding its effect upon fractures. The only large trial of an aromatase inhibitor which included a placebo arm [1] enrolled patients who already had taken tamoxifen for 5 years and found no significant increase in total or hip fracture. One small study of stage I breast cancer patients [18] reported a reduction in BMD over 2 years with exemestane compared with placebo but was too small to examine fractures. The fracture risk of AIs compared with placebo could not ethically be examined among a larger group because of the well-established efficacy of adjuvant tamoxifen for breast cancer [19].
To further address the question of the risk of fracture among a community-based, higher-risk group, we measured fractures and factors associated with them among a population-based cohort of postmenopausal breast cancer patients with few exclusions. We hypothesized that the fracture risk in the cohort would be higher than in the randomized controlled trials and that risk factors for fracture could be identified. We further hypothesized that the risk of hip and total nonvertebral fractures would be higher with aromatase inhibitors than with tamoxifen, with an intermediate fracture risk for those not taking any hormonal therapy. This cohort included only women aged 65 and over, included information regarding fracture-specific risk factors, and enrolled participants at a time when a substantial number of patients were taking tamoxifen.
Materials and methods
Data sources and study cohort
The primary data source for the study was a population-based survey of community-dwelling elderly women with incident breast cancer in 2003 being followed prospectively in a National Cancer Institute-sponsored study of breast cancer outcomes [20, 21]. Potential subjects for the ongoing study were identified with a published algorithm [22] utilizing Medicare administrative data and included women aged 65–89 who underwent surgery for an incident breast cancer March 1–September 30, 2003 in Illinois, New York, Florida, or California. Subjects were required to be enrolled in Medicare parts A and B and not be in a Medicare health maintenance organization for calendar year 2003. The study protocol was approved by the Medical College of Wisconsin Institutional Review Board and the Centers for Medicare and Medicaid Services (CMS) Privacy Board.
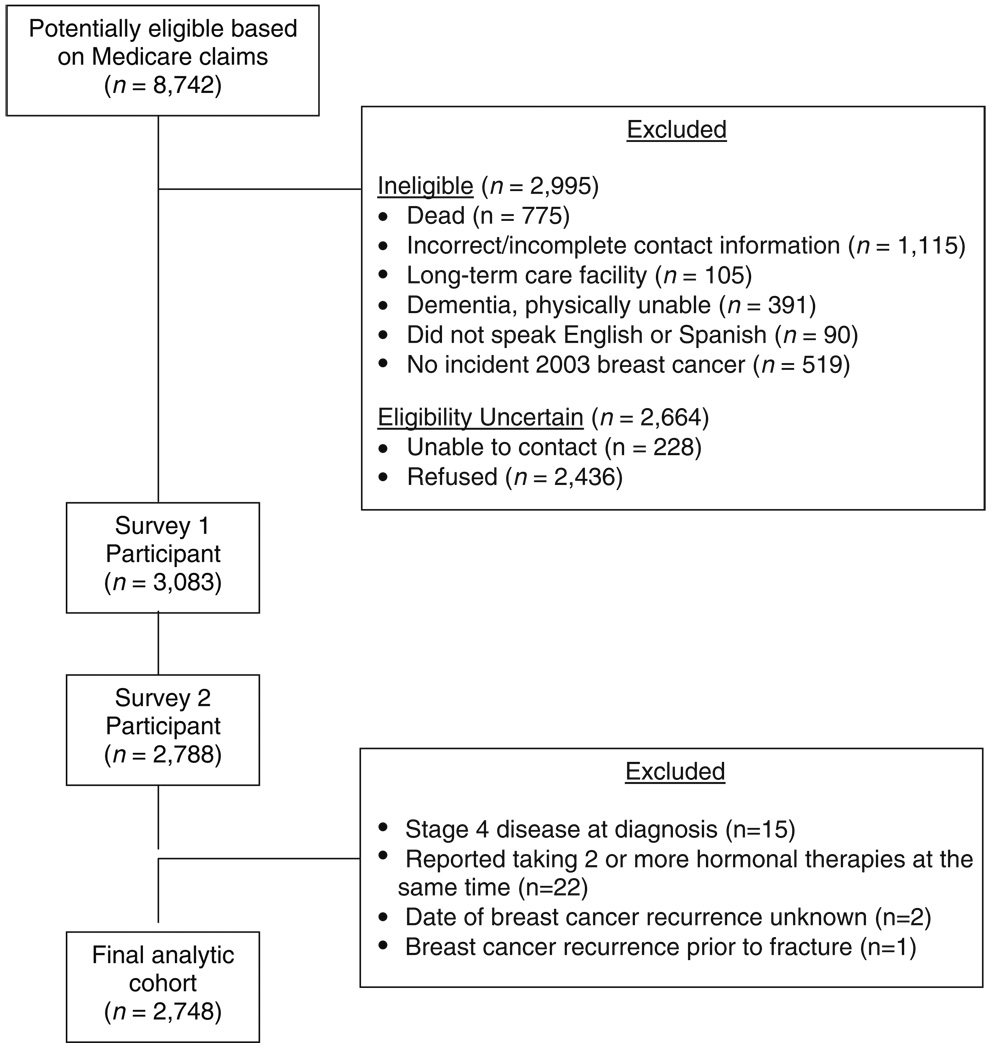
Women were invited to participate in the study through a letter which included opt-out information on Center for Medicare and Medicaid Services’ letterhead. The flow of potential participants in the study has been previously described [23]. In brief, potentially eligible subjects were excluded if by the time of recruitment they were deceased or had incorrect contact information, had Medicare claims for dementia or a long-term care facility stay of ≥100 days, did not speak English or Spanish or were physically unable to answer the survey, or did not confirm a 2003 incident breast cancer. All enrolled women provided informed consent by telephone.
Of the 8,742 subjects initially mailed a letter by the CMS contractor, 2,995 subjects were determined to be ineligible, 228 had contact information identified but were never reached by telephone, and 2,436 declined participation (Fig. 1) for a total cohort for the first survey numbering 3,083 women (response rate 70%). As previously reported [23], participants were no different from nonparticipants in race/ethnicity, income or comorbidity, or initial treatment, although they were slightly younger.
Fig. 1.
Participant flow and reasons for exclusion
Subjects initially enrolled in the study in 2005 provided demographic, socioeconomic, treatment, and cancer-related outcome factors including recurrence. These subjects were offered participation in follow-up telephone surveys focused on continuing ascertainment of treatments and outcomes at approximately yearly intervals. In addition, subjects gave informed consent for use of their Medicare administrative data and their tumor registry information.
Subjects for this analysis were selected from respondents to the second follow-up survey, conducted between June 2006 and February 2007, when information regarding fracture risk factors was elicited. The participation rate for survey 2 was 90.4%. Fifteen women with distant (stage 4) disease at the time of breast cancer diagnosis based on tumor registry were excluded from this analysis because of the greater risk that any fractures would be related to bony metastases for a final study cohort of 2,748 patients.
Fracture outcomes
We measured hip and total nonvertebral fractures from Medicare administrative data using an adaptation [23, 24] of an algorithm that had been validated with chart and radiologic review [25] among Medicare patients. Medicare data examined for evidence of fractures included the Inpatient Standard Analytical Files (SAF) of inpatient hospital claims, the Outpatient SAF of Medicare claims for outpatient facilities, the 100% Carrier SAF of physician services, and the denominator file, which contains information on beneficiary enrollment and zip code of residence. The algorithm first identified possible fractures using International Statistical Classification of Diseases and Related Health Problems, 9th Revision (ICD-9) diagnostic and procedure or Common Procedural Terminology procedure codes, including some nonspecific codes for confirmation of fracture site (e.g., casting codes). The algorithm then excluded subjects if further claims suggested that a new fracture did not occur, for example, a surgical procedure code with the only ICD-9 diagnosis codes indicating arthritis, old fracture, or other bone disease or a fracture diagnosis code seen only on hospital admission without operative procedure or discharge diagnosis codes. The algorithm does not attempt to identify vertebral fractures. A recent study showed that incident vertebral fractures continue to be difficult to identify using claims [26], so we did not attempt to identify them. The positive predictive value of the published algorithm is 98% for hip fracture and 94% overall [25] which compares favorably to published results for fracture self-report [27].
Hormonal therapy
In each study wave, subjects were asked to give detailed information about all breast cancer medications they had received, including dates they stopped and started the treatments. Each of the hormonal therapy medications (letrozole, exemestane, anastrozole, and tamoxifen) was asked about by brand and generic name. Hormonal therapy for breast cancer was defined as the medication (aromatase inhibitor, tamoxifen, or none) the subject was using 6 months after breast cancer surgery.
Other covariates
Race/ethnicity was determined by self-report. Self-reported height and weight were used to calculate body mass index (BMI), which was categorized in cohort quartiles as in published osteoporosis cohorts [28]. A family fracture history was defined as a history of a hip, pelvis, wrist, or vertebral fracture reported for a first-degree relative. Oral bisphosphonate start and stop dates were ascertained from survey as for hormonal therapy and intravenous bisphosphonates from Medicare administrative files from 2003 to 2006. Variables obtained from Medicare administrative files from 2003 included age at time of breast cancer surgery, nonvertebral fractures over the 12 months before AI initiation, and comorbidity using the methodology described by Klabunde et al. [29].
Initial breast cancer stage was obtained and categorized from routinely collected state tumor registry information (in situ, local, regional, or unknown). Recurrences and their dates were based on patient’s report of a physician-diagnosed breast cancer recurrence, with supplementary information from state tumor registries used to confirm that recurrences were not incident cancers of another primary type.
Analysis
The analysis was focused on the development of fractures and their association with medication use prior to breast cancer recurrence, as in several AI trials [3, 11, 30]. There were 247 members of our cohort who reported switching from tamoxifen to an aromatase inhibitor during the course of follow-up and 27 who switched from an aromatase inhibitor to tamoxifen. These patients were analyzed based on their original medication because based on the AI trials, this would bias our results toward a null finding for the most clinically relevant comparison of tamoxifen vs aromatase inhibitors. Little evidence exists to suspect that different AI agents would have different risks of fracture, so all AI users (87% of users of aromatase inhibitors took anastrozole) were grouped together. Non-Hispanic Whites made up 92% of the cohort and were compared with women of other races/ethnicities. We excluded 22 cohort patients from analyses: 19 who reported taking two different hormone therapy agents at the same time, two who could not recall the date of their breast cancer recurrences, and one who had a recurrence prior to fracture.
The unadjusted association between hormone therapy type and other potential risk factors [31, 32] and (1) hip and (2) total nonvertebral fractures occurring between 6 months after breast cancer surgery and December 31, 2006 were examined using the log-rank test. Primary adjusted analyses used time-to-event methods based on Cox proportional hazard models to examine the hazard for fracture by hormone therapy category adjusted for other fracture risk factors. Potential confounders including breast cancer stage and bisphosphonate use were examined by entering each individually into the models. We also examined any possible differences in the association of adjuvant hormonal therapies by patient age using interaction terms.
Results
There were 2,748 women in the final cohort of older postmenopausal breast cancer survivors from four large states. Of these, 28.2% of the women were taking an aromatase inhibitor and 27.8% tamoxifen 6 months after surgery. The mean age of cohort members was 72.8 (S.D. 5.4), and 12.6% of women were aged 80 and over.
The characteristics of the study sample by hormonal therapy category are shown in Table 1. Most fracture risk factors including age, history of fracture, and family history of fracture were evenly distributed between those taking an AI, tamoxifen, or neither. Body mass index was slightly higher and bisphosphonate use greater among women taking AIs. As expected among women with in situ tumors, only 9.5% took AIs and 31.5% took tamoxifen.
Table 1.
Characteristics of 2003 breast cancer cohort by adjuvant hormonal therapy type
| Aromatase inhibitor. (n = 775) | Tamoxifen (n = 764) | Neither (n = 1,209) | p value for difference | |
|---|---|---|---|---|
| Mean age (SD) | 72.5 (5.3) | 72.8 (5.3) | 72.9 (5.6) | 0.386 |
| Race/ethnicity (%) | ||||
| Non-Hispanic White | 91.0 | 91.6 | 92.2 | 0.610 |
| Other | 9.0 | 8.4 | 7.8 | |
| Breast cancer stage (%) | ||||
| In situ | 5.2 | 17.3 | 20.4 | <.001 |
| Local | 64.9 | 57.3 | 48.1 | |
| Regional | 17.0 | 11.8 | 18.2 | |
| Unknown | 12.9 | 13.6 | 13.3 | |
| Mean body mass index (SD)a | 27.6 (5.6) | 26.8 (4.9) | 26.8 (5.3) | 0.003 |
| History of nonvertebral fracture in prior year (%)a | 1.7 | 2.1 | 2.1 | 0.790 |
| Family history of fracture (%)a | 25.3 | 24.8 | 23.9 | 0.761 |
| No comorbidity (%)a | 62.3 | 66.9 | 62.2 | 0.141 |
| Cytotoxic chemotherapy use (%) | 14.1 | 9.4 | 29.8 | <.001 |
| Oral bisphosphonate use (%)a | 35.2 | 27.8 | 25.7 | <.001 |
Among female breast cancer patients without known metastatic disease at diagnosis (see text). Cohort n for BMI = 2,667, prior fractures n = 2,720, family history n = 2,669, osteoporosis medication use n = 2,738; for remainder of variables n = 2,748
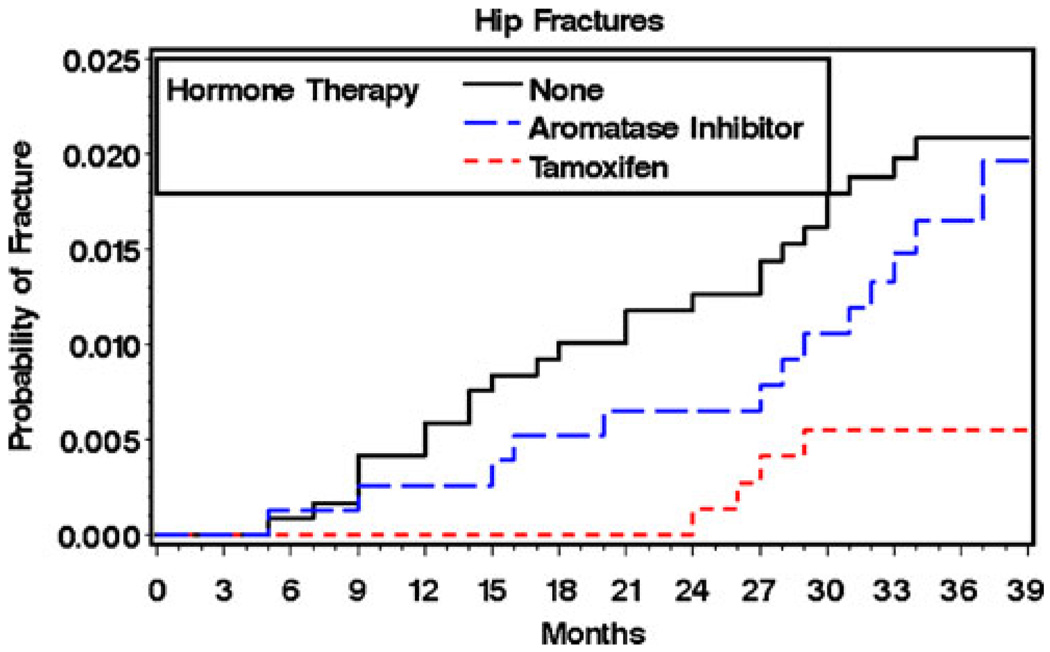
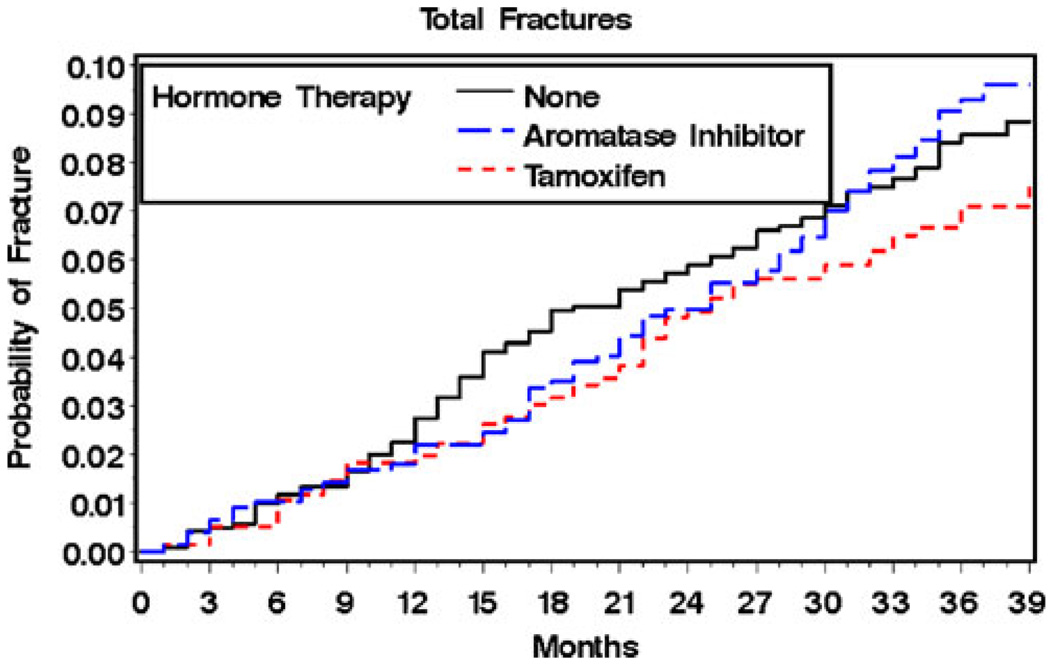
During a median of 36 months of follow-up, 1.5% of the total cohort subjects (n = 41) had a hip fracture and 7.9% (n = 216) a nonvertebral fracture. Hip fractures occurred among 1.7% of subjects taking AIs, 0.5% of subjects taking tamoxifen, and 2.0% of subjects not using hormone therapy (p = 0.028). Nonvertebral fractures occurred among 8.8% of users of aromatase inhibitors, 6.8% of users of tamoxifen, and 8.1% of subjects not using hormone therapy (p = 0.346). The unadjusted Kaplan–Meyer curves by category of hormonal therapy use are shown in Figs. 2 and 3. For hip fractures (Fig. 2), the curve for tamoxifen use stayed consistently below those for the other treatments throughout the study period. For total fracture, the tamoxifen curve appeared to separate from the other treatment curves at 30 months (Fig. 3), although the differences were not statistically significant.
Fig. 2.
Probability of hip fracture by type of adjuvant hormonal therapy unadjusted Kaplan–Meier estimates by type of adjuvant hormone therapy use (see text)
Fig. 3.
Probability of total nonvertebral fracture by type of adjuvant hormonal therapy unadjusted Kaplan–Meier estimates by type of adjuvant hormone therapy use (see text)
The associations of other potential fracture risk factors with hip and total fractures are shown in Table 2. Age was strongly associated with hip and total fractures in unadjusted analyses. BMI in the lowest quartile was associated with hip fracture, and the characteristics of fractures in the prior year and comorbidity were each associated with total nonvertebral fracture. Race/ethnicity was not associated with fractures, but only 14 nonwhite patients had nonvertebral fractures. Breast cancer stage was not associated with fracture (p = 0.873).
Table 2.
Risk of hip and total nonvertebral fracture among older breast cancer survivors
| Hip fracture (%) |
p value | Total fracture (%) |
p value | |
|---|---|---|---|---|
| Age | 0.006 | <.001 | ||
| 65–69 | 0.7 | 4.9 | ||
| 70–74 | 1.2 | 7.8 | ||
| 75–79 | 2.1 | 10.2 | ||
| 80+ | 3.2 | 11.8 | ||
| Race/ethnicity | 0.424 | 0.296 | ||
| Non-Hispanic | 1.5 | 8.1 | ||
| White | ||||
| Other | 0.9 | 6.1 | ||
| Body mass index | 0.002 | 0.16 | ||
| <23.2 | 2.8 | 9.4 | ||
| ≥23.2 | 1.1 | 7.5 | ||
| Prior fracture | 0.169 | <.001 | ||
| Yes | 3.7 | 24.1 | ||
| No | 1.4 | 7.6 | ||
| Family history of fracture | 0.363 | 0.43 | ||
| Yes | 1.8 | 8.5 | ||
| No | 1.3 | 7.6 | ||
| Cytotoxic chemotherapy use | 0.83 | 0.025 | ||
| Yes | 1.6 | 10.4 | ||
| No | 1.5 | 7.4 |
n = 2,748 except as noted in Table 2 footnote above
Adjusted results
In models of the risk of hip fracture adjusted for age, comorbidity, and BMI (Table 3), the hazard for hip fracture among AI users compared with tamoxifen users was 3.24 (95% confidence interval (CI) 1.05, 9.98). The hazard was similar for subjects not taking any hormone therapy. Age and BMI continued to be strongly associated with hip fracture. Race/ethnicity, prior fracture, comorbidity, and breast cancer stage were not important confounders.
Table 3.
Adjusted association of hip and total nonvertebral fracture with (a) adjuvant hormonal therapy type and patient characteristics and (b) adjuvant hormonal therapy type, patient characteristics, and bisphosphonate use
| Hip fracture adjusted hazard ratio (95% CI) | Total fracture adjusted hazard ratio (95% CI) | |||
|---|---|---|---|---|
| Hormonal therapy type | ||||
| Tamoxifen | – | – | – | |
| Aromatase inhibitor | 3.24 (1.05, 9.98) | 3.19 (1.03, 9.83) | 1.34 (0.92, 1.94) | 1.32 (0.91, 1.91) |
| None | 3.32 (1.14, 9.65) | 3.39 (1.16, 9.85) | 1.07 (0.75, 1.54) | 1.07 (0.75, 1.53) |
| Age (per 10-year interval) | 2.63 (1.53, 4.53) | 2.68 (1.55, 4.64) | 1.83 (1.43, 2.36) | 1.87 (1.45, 2.41) |
| BMI (lowest quartile) | 2.16 (1.14, 4.10) | 2.08 (1.09, 3.97) | 1.14 (0.84, 1.56) | 1.12 (0.82, 1.53) |
| Prior fracture | –a | 2.68 (1.55, 4.64) | 3.59 (2.04, 6.31) | 3.49 (1.98, 6.15) |
| Chemotherapy use | –a | –a | 1.65 (1.17, 2.33) | 1.64 (1.16, 2.32) |
| Bisphosphonate use | – | 1.27 (0.67, 2.41) | – | 1.36 (1.02, 1.81) |
Among 2,526 subjects in model also adjusted for comorbidity
Prior fractures and chemotherapy were not associated with subsequent hip fracture and were not included in the hip fracture models
In models of the risk of total nonvertebral fracture adjusted for age, comorbidity, BMI, chemotherapy use, and prior fracture (Table 3), there were no statistically significant differences in the hazard for total fracture for AI users or subjects not taking any hormone therapy compared with tamoxifen users (p = 0.11). Race, prior fracture, comorbidity, and breast cancer stage were not important confounders of this association.
Although users of AIs were more likely to be taking bisphosphonates, the addition of bisphosphonate use to all models had minimal effects on the results for hormonal therapies (Table 3). The results were unchanged in an analysis restricted to subjects who remained fully adherent to medications (84.2% of AI users and 81.8% of tamoxifen users). There was no interaction between age and the effect of aromatase inhibitors on hip or total fractures.
Discussion
In this population-based study of 2,748 women 65 and over with breast cancer in 2003, the hazard for hip fracture for users of an aromatase inhibitors compared with users of tamoxifen was 3.24 (95% CI 1.05, 9.98), with an absolute increase in hip fracture of 1.1% over 36 months. Hip fracture risk among women not taking any hormone therapy was also elevated compared to users of tamoxifen. There were no statistically significant differences between these three groups in risk of total nonvertebral fractures. Although AI users were more likely than other women to be taking a bisphosphonate, this did not alter any of these findings. Major fracture risk factors in non-cancer populations including age, low body mass index, and prior fractures were strongly associated with fractures.
This study offers support for the possibility that some of the differences in fracture risk between tamoxifen and AIs occur through a protective effect of tamoxifen. Placebo-controlled studies from the 1990s reported that tamoxifen increased BMD in postmenopausal women by 1% to 2% yearly compared with placebo-controlled studies, but evidence regarding fractures has been mixed [15, 17]. The NSABP Breast Cancer Prevention Study P-1 reported a substantial but not statistically significant reduction in the risk of hip (relative risk (RR) 0.55 (0.25–1.15)) and total (RR 0.81 (0.63–1.05)) fractures in its primary report on 5 years of tamoxifen chemoprevention [33]. The effect of tamoxifen vs placebo on total fractures, however, became statistically significant with two additional years of follow-up [34]. A smaller Danish randomized trial of tamoxifen compared with placebo among breast cancer patients reported no difference in fractures [35]. Our study is thus consistent with existing knowledge from the largest prior tamoxifen study, but to our knowledge is the first to show that adjuvant tamoxifen is associated with a lower risk of hip fracture than no hormonal therapy.
This study also provides some of the first direct evidence of the fracture risk of aromatase inhibitors when compared with no adjuvant hormonal therapy. The findings of no difference in fractures between AI users and those who did not take hormonal therapy may at first appear contradictory to the limited bone density literature to date. Adjuvant exemestane for 2 years worsened hip BMD loss compared with placebo in one study of 147 women (2.72% vs 1.42%, p = 0.024) [18], and another study of 2 years of letrozole after 5 years of tamoxifen [36] found greater spine and hip BMD reductions for letrozole users than placebo [36]. However, no patients with normal baseline BMD in either study became osteoporotic, and no differences were demonstrated in progression from osteopenia to osteoporosis. Given the potential for a delay in bone density impacts upon fracture risk, our study may thus be consistent with these previous findings. It is also possible that a smaller AI effect was not detectable, while the tamoxifen effect of 1% to 2% yearly difference between tamoxifen and placebo could be observed. Our large study of patients at high baseline fracture risk also adds to these previous reports by showing that even in a real-world setting, BMD effects of AIs did not translate into substantial short-term increases in fracture risk compared with no hormonal treatment.
Our lack of ability to identify total nonvertebral fractures between AI and tamoxifen users is somewhat surprising given the difference we found for hip fractures by AI and tamoxifen use. Given the confidence intervals in our study, it is possible that the risk conferred by aromatase inhibitors is actually similar for total and hip fractures. If there are differences, our study cannot directly address the possible reasons. However, our patients are likely to have been more frail than those in the randomized trials. They were older, with a median age near 73, and over one third had a major comorbidity. The incidence of hip fracture in our study was three times higher than corresponding early results from ATAC and was consistent with a subgroup analysis of 295 women ≥75 from the BIG 1–98 trial, in which there were four hip fractures with letrozole and one with tamoxifen [11]. It is also possible that women who switched from tamoxifen to AIs led us to underestimate the differences between these two groups in our total nonvertebral fracture results. However, the annualized absolute risk of total fracture in the tamoxifen group in our study was higher than in the ATAC anastrozole trial [5], and the hazard for the comparison of AIs with tamoxifen was only slightly smaller.
Our results offer important information to supplement the clinical trials regarding the absolute risk of fracture among breast cancer patients, both treated and untreated with hormonal therapy. The study cohort’s higher risk compared with AI trial enrollees is probably conferred by age, comorbidity, and the higher number of nonwhite patients in our study [11]. Several large trials were performed primarily in Western Europe and the USA and had few nonwhite participants. Since personal or family history of fractures were not systematically measured in the randomized trials, comparison with our cohort is difficult, although it is possible that these risk factors were more prevalent in our cohort as well. The fracture risks in the breast cancer cohort are comparable to non-cancer US estimates based on a Rochester Minnesota cohort [37]
Our study has limitations, several of which are common to observational research. Subjects were not randomly assigned to therapies, and it is possible that providers took fracture risk into account in treatment decisions. Our results are consistent with the possibility that physicians used lower BMI in decisions to prescribe tamoxifen. We were able, however, to adjust for this and multiple other fracture risk factors that clinicians might consider, and this adjustment did not appreciably change our findings. Our survey study could not obtain reliable information regarding bone density test results, although we did have information about bisphosphonate use. Our study was limited by the cohort’s relatively small number of fractures and short duration, although it included more older patients than any of the large AI trials. We could not exclude from our fracture outcomes any high-trauma fractures, but we would not expect these to differ by hormonal therapy use. As discussed above, women who switched therapies may have affected our results. Given that most switched from tamoxifen to AIs, however, they are not likely to explain the higher rate of hip fractures among AI vs tamoxifen users.
Our study results should also be considered in the context of recent studies of bone loss prevention in breast cancer patients. Bisphosphonates have been shown to maintain BMD in postmenopausal patients taking AIs [38, 39], although none of these studies was powered to examine fractures. Enthusiasm for using bisphosphonates may be further bolstered by early results of studies showing an antitumor effect of adjuvant bisphosphonates in postmenopausal women [40]. However, bisphosphonates can cause osteonecrosis of the jaw, a severe and potentially disfiguring condition [41], and recent warnings of possible increased risks of atrial fibrillation [42, 43] and esophageal cancer [44] might also raise concerns. Furthermore, adjustment for bisphosphonate use in our study did not substantially change our results. Perhaps this occurred because of insufficient study treatment time and/or the lack of ability to adjust for baseline bone density. It is also possible, however, that the preservation of bone density shown in trials of bisphosphonates for AI-related BMD losses does not translate into measurable fracture effects. Better understandings of the comparative bony risks of hormonal therapies are thus crucial for decision making about both hormonal and bisphosphonate treatments.
In conclusion, in a population-based cohort of older female breast cancer patients, users of aromatase inhibitors had a substantially higher risk of hip fracture, though not total nonvertebral fracture, than users of tamoxifen. Furthermore, the absolute hip fracture risk difference of just over 1% over 3 years that we identified is unlikely to outweigh the benefits of aromatase inhibitors over tamoxifen in improved breast cancer survival and reduced thromboembolism and endometrial cancer [45, 46]. This information, including the higher fracture risks measured among our cohort than reported in randomized trials, can, however, be useful prognostically to clinicians and patients considering bony protective treatment options. Trials of AI use beyond 5 years are currently enrolling, and continuing evaluation of AIs’ long-term effects on bone will be needed.
Acknowledgments
This study was supported by R21 CA131643-01A1, K07 CA125586-01A1, and PHS Grant R01 CA81379 from the National Institutes of Health.
Footnotes
Conflicts of interest None.
Contributor Information
J. M. Neuner, Email: jneuner@mcw.edu, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, USA; Center for Patient Care and Outcomes Research, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA.
T. W. Yen, Department of Surgery, Medical College of Wisconsin, Milwaukee, WI, USA Center for Patient Care and Outcomes Research, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA.
R. A. Sparapani, Center for Patient Care and Outcomes Research, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA
P. W. Laud, Center for Patient Care and Outcomes Research, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA Division of Biostatistics, Medical College of Wisconsin, Milwaukee, WI, USA.
A. B. Nattinger, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, USA Center for Patient Care and Outcomes Research, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA.
References
- 1.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. New England Journal of Medicine. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [see comment] [DOI] [PubMed] [Google Scholar]
- 2.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. Journal of Clinical Oncology. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [see comment] [DOI] [PubMed] [Google Scholar]
- 3.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. The Lancet. 2007;369:559–570. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 4.Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. The Lancet. 2005;366:455. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 5.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [see comment] [DOI] [PubMed] [Google Scholar]
- 6.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Boccardo F, Rubagotti A, Puntoni M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian tamoxifen anastrozole trial. J Clin Oncol. 2005;23:5138–5147. doi: 10.1200/JCO.2005.04.120. [DOI] [PubMed] [Google Scholar]
- 9.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [see comment][erratum appears in Lancet 2002 Nov 9;360(9344):1520] [DOI] [PubMed] [Google Scholar]
- 10.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 11.Crivellari D, Sun Z, Coates AS, et al. Letrozole compared with tamoxifen for elderly patients with endocrine-responsive early breast cancer: the big 1–98 trial. Journal of Clinical Oncology. 2008;26:1972–1979. doi: 10.1200/JCO.2007.14.0459. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services. Bone health and osteoporosis: a report of the surgeon general. Office of the Surgeon General. Rockville: U.S. Department of Health and Human Services; 2004
- 13.Cooper C, Melton LJ. Epidemiology of osteoporosis. Trends Endocrinol Metab. 1992;3:224–229. doi: 10.1016/1043-2760(92)90032-v. [DOI] [PubMed] [Google Scholar]
- 14.Leibson CLTA, Gabriel SE, Ransom JE, Melton LJ. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644–1650. doi: 10.1046/j.1532-5415.2002.50455.x. [DOI] [PubMed] [Google Scholar]
- 15.Powles T, Hickish T, Kanis J, et al. Effect of tamoxifen on bone mineral density measured by dual-energy X-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 16.Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. New England Journal of Medicine. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 17.Kristensen B, Ejlertsen B, Dalgaard P, et al. Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: a randomized study. Journal of Clinical Oncology. 1994;12:992–997. doi: 10.1200/JCO.1994.12.5.992. [DOI] [PubMed] [Google Scholar]
- 18.Lonning PE, Geisler J, Krag LE, et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol. 2005;23:5126–5137. doi: 10.1200/JCO.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 19.Early Breast Cancer Trialists’ Collaborate Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 20.Pezzin LE, O’Niel M, Nattinger AB. The economic consequences of breast cancer adjuvant hormonal treatments. J Gen Intern Med. 2009;24 Suppl 2:446–450. doi: 10.1007/s11606-009-1079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yen T, Fan X, Sparapani R, Laud P, Walker A, Nattinger A. A contemporary, population-based study of lymphedema risk factors in older women with breast cancer. Ann Surg Oncol. 2009;16:979–988. doi: 10.1245/s10434-009-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nattinger AB, Laud PW, Bajorunaite R, Sparapani RA, Freeman JL. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Services Research. 2004;39:1733–1749. doi: 10.1111/j.1475-6773.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nattinger AP, Pezzin LE, Sparapani RA, Neuner JM, King TK, Laud PW. Heightened attention to medical privacy: challenges for unbiased sample recruitment, and one solution. Am J Epidemiol. 2010;172:637–644. doi: 10.1093/aje/kwq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuner JM, Zhang X, Sparapani R, Laud PW, Nattinger AB. Racial and socioeconomic disparities in bone density testing before and after hip fracture. J Gen Intern Med. 2007;22:123–145. doi: 10.1007/s11606-007-0217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 26.Curtis JR, Mudano AS, Solomon DH, Xi J, Melton ME, Saag KG. Identification and validation of vertebral compression fractures using administrative claims data. Medical Care. 2009;47:69–72. doi: 10.1097/MLR.0b013e3181808c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11:264–274. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 28.Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the national osteoporosis risk assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 29.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group. Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 31.Cummings SR. Risk factors for hip fracture in white women. N Engl J Med. 1995;322:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 32.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. Journal of Bone & Mineral Research. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 33.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. Journal of the National Cancer Institute. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [see comment] [DOI] [PubMed] [Google Scholar]
- 34.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the national surgical adjuvant breast and bowel project P-1 study. Journal of the National Cancer Institute. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 35.Kristensen B, Ejlertsen B, Mouridsen HT, Andersen KW, Lauritzen JB. Femoral fractures in postmenopausal breast cancer patients treated with adjuvant tamoxifen. Breast Cancer Res Treat. 1996;39:321–326. doi: 10.1007/BF01806160. [DOI] [PubMed] [Google Scholar]
- 36.Perez EA, Josse RG, Pritchard KI, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol. 2006;24:3629–3635. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 37.Melton Iii LJ, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporosis International. 1999;9:29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 38.Brufsky A, Bundred N, Coleman R, et al. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13:503–514. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 39.Greenspan SL, Brufsky A, Lembersky BC, et al. Risedronate prevents bone loss in breast cancer survivors: a 2-year, randomized, double-blind, placebo-controlled clinical trial. J Clin Oncol. 2008;26:2644–2652. doi: 10.1200/JCO.2007.15.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. Journal of Clinical Oncology. 2007;25:829–836. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- 41.Woo S-B, Hellstein JW, Kalmar JR. Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 42.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. New Engl J Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Archives of Internal Medicine. 2008;168:826–831. doi: 10.1001/archinte.168.8.826. [DOI] [PubMed] [Google Scholar]
- 44.Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. New Engl J Med. 2009;360:89–90. doi: 10.1056/NEJMc0808738. [DOI] [PubMed] [Google Scholar]
- 45.Hillner BE. Benefit and projected cost-effectiveness of anastrozole versus tamoxifen as initial adjuvant therapy for patients with early-stage estrogen receptor-positive breast cancer. Cancer. 2004;101:1311–1322. doi: 10.1002/cncr.20492. [DOI] [PubMed] [Google Scholar]
- 46.Locker GY, Mansel R, Cella D, et al. Cost-effectiveness analysis of anastrozole versus tamoxifen as primary adjuvant therapy for postmenopausal women with early breast cancer: a US healthcare system perspective. The 5-year completed treatment analysis of the ATAC (‘Arimidex’, Tamoxifen Alone or in Combination) trial. Breast Cancer Res Treat. 2007;106:229–238. doi: 10.1007/s10549-006-9483-6. [DOI] [PubMed] [Google Scholar]