Abstract
Parkinson disease (PD) is the second most common neurodegenerative disorder1,2. Growing evidence suggests a causative role of misfolded forms of the protein, α-synuclein (αSyn), in the pathogenesis of PD3,4. Intraneuronal aggregates of αSyn occur in Lewy bodies and Lewy neurites5, the cytopathological hallmarks of PD and the related disorders called synucleinopathies. αSyn has long been defined as a “natively unfolded” monomer of ∼14 kDa6 that is believed to acquire α-helical secondary structure only upon binding to lipid vesicles7. This concept derives from the widespread use of recombinant bacterial expression protocols for in vitro studies, and of overexpression, sample heating and/or denaturing gels for cell culture and tissue studies. In contrast, we report that endogenous αSyn isolated and analyzed under non-denaturing conditions from neuronal and non-neuronal cell lines, brain tissue and living human cells occurs in large part as a folded tetramer of ∼58 kDa. Multiple methods, including analytical ultracentrifugation, scanning transmission electron microscopy and in vivo cell crosslinking, confirmed the occurrence of the tetramer. Native, cell-derived αSyn showed α-helical structure without lipid addition and had much greater lipid binding capacity than the recombinant αSyn studied heretofore. Whereas recombinantly expressed monomers readily aggregated into amyloid-like fibrils in vitro, native human tetramers underwent little or no amyloid-like aggregation. Based on these findings, we propose that destabilization of the helically folded tetramer precedes αSyn misfolding and aggregation in PD and other human synucleinopathies and that small molecules which stabilize the physiological tetramer could reduce αSyn pathogenicity.
To identify the native state of αSyn in cells while avoiding the potential breakdown of physiological assemblies by detergents, we initially used native gel electrophoresis. αSyn is expressed endogenously in many cell types, so we chose to analyze the dopaminergic human neuroblastoma line, M17D8 and the commonly used cell lines HEK293, HeLa, and COS-7. Each of these cell lines predominantly contained a non-denatured αSyn-immunoreactive species migrating in Blue Native PAGE (BN-PAGE) at ∼45-50 kDa (Fig. 1A, lanes 1-4). Because these initial results suggested an apparently stable oligomeric form under native conditions, we next probed the endogenous state of αSyn in normal brain. The frontal cortex of wild-type mice also revealed a ∼45-50 kDa form of endogenous αSyn as the main species in the buffer-soluble fraction (Fig. 1A, lane 6).
Figure 1.

Western blot analysis of lysates of M17D, HeLa, HEK293 and COS-7 cells, mouse cortex and human RBCs probed for endogenous αSyn. A: Blue Native PAGE. B: Clear Native PAGE. The band just below the main ∼55-60 kDa RBC species (B, lane 6) may represent an alternatively spliced form of αSyn. . Arrow marks a possible dimeric species. C: Left: SDS-PAGE/Western blot (antibody C20) analysis of cell lysates without crosslinking. Right: Proteins were crosslinked in intact living cells with membrane permeable DSS (M17D, HeLa, HEK 293, COS-7) or in RBC lysate with water soluble BS3 and then run on SDS-PAGE.
To assess the state of endogenous αSyn in living human cells, we examined freshly collected red blood cells (RBC), which were recently found to have high αSyn expression9. Human RBC contained a ∼45-50 kDa αSyn immunoreactive band on BN-PAGE (Fig 1A, lane 5). As a second non-denaturing gel system that precludes effects of the Coomassie dye used in BN-PAGE, we performed Clear Native PAGE (CN-PAGE)10. The main αSyn species in all samples migrated at ∼55-60 kDa, suggesting a tetramer (theoretical mass of monomer = 14,460 Daltons) (Fig. 1B, lanes 1-6). The better resolution of CN-PAGE without Coomassie dye also revealed small amounts of apparent monomers running below the 14 kDa MW marker (Fig. 1B, lanes 1-4, 6) and distinguished the small differences in amino acid length of the human and mouse αSyn monomers and putative tetramers (Fig. 1B, lane 6). The endogenous ∼55-60 kDa species was detected by monoclonal αSyn antibodies syn1, 211 and LB509 and polyclonal antibody C20 in both native gel systems.
Because the migration of a protein on BN- or CN-PAGE does not depend solely on its mass but also on its conformation and charge, we used in vivo cross-linking to preserve the assembled state of the putative αSyn oligomer, followed by denaturing SDS-PAGE. We observed SDS-stable αSyn bands migrating at the expected positions of a tetramer (∼55 kDa) and non-crosslinked monomer in all cells, plus some putative dimer in the HeLa, HEK and red blood cells (Fig 1C). This in vivo crosslinking supports the existence of native tetramers in cells. We performed 2D gel analysis after the in vivo crosslinking, i.e., isoelectric focusing (IEF) to separate proteins by charge in a pH gradient followed by denaturing SDS-PAGE. The higher-migrating αSyn species in the cross-linked RBC lysates had the same pKa as monomers, within the limits of IEF resolution (Fig S1), consistent with their being homo-oligomers.
Next, we developed a non-denaturing method to purify native αSyn from soluble RBC lysates [see Methods and Protocol Exchange (http://www.nature.com/protocolexchange/)]. This allowed us to estimate the mass of native αSyn based on distinct measurement principles that are not affected by protein conformation, unlike gel electrophoresis. Scanning transmission electron microscopy (STEM) is useful for measuring the masses of purified, non-covalently bonded complexes that may not resist ionization during mass spectrometry11,12. STEM images of αSyn purified under non-denaturing conditions from human RBC (Fig. S2) yielded a homogenous distribution of roughly spherical particles measuring ∼3.0-3.5 nm diameter (Fig. 2A). Unbiased automatic sampling by the STEM of 1,000 particles gave a size distribution pattern with a peak at ∼55 kDa (Fig 2B). Importantly, we next applied sedimentation equilibrium analytical ultracentrifugation (SE-AUC), a technique commonly used to establish the oligomeric state of native proteins independent of their conformation. SE-AUC analysis of purified, native RBC αSyn performed at three different concentrations and at different rotor speeds yielded an average molecular weight of 57.8 kDa (4.78 Svedbergs), strongly supporting a tetrameric assembly state (Fig 2C).
Figure 2.
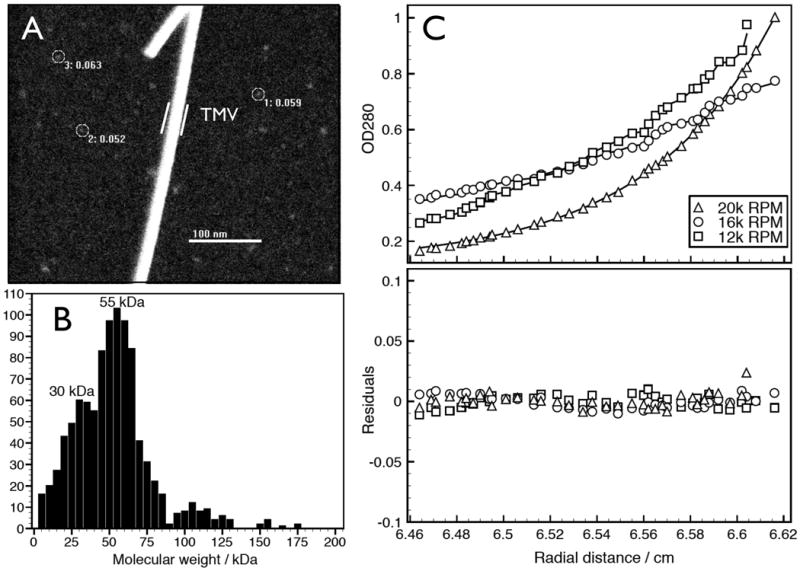
Sizing analyses of αSyn from human RBCs. A: Representative large-angle dark-field STEM image of purified αSyn from human RBC. A few representative particles are circled. As an internal size standard, tobacco mosaic virus (TMV) helical rod was included during EM specimen preparation. B: Mass histogram (bin size = 5 kDa) of 1,000 automatically selected αSyn particles. C: Sedimentation equilibrium AUC of purified, native RBC αSyn. Upper panel shows the individual experimental analyses fitting an ideal single-species model to the equilibrium data obtained at 12k, 16k, and 20k RPM for 1.1 mg/ml αSyn solution. The fitting yielded a molecular weight of 57,753 Da (SD: +/- 655.199) with a root mean square deviation of 0.004533. Lower panel shows an overlay of the residuals of data and theoretical fit for the three different speeds.
Numerous studies have reported conformational changes in αSyn, with a focus on the natively unfolded recombinant monomer undergoing a random coil to α-helix transition upon in vitro interaction with small lipid vesicles7. This change is believed to be relevant to the poorly defined physiological function of αSyn in cells and could potentially decrease the likelihood of its aggregation into β-sheet-rich neurotoxic assemblies13. Unexpectedly, we found that circular dichroism (CD) spectra of the human RBC tetramer purified under non-denaturing conditions showed two minima of mean residue ellipticity at 222 and 208 nm (Fig. 3A), characteristic of an α-helically folded protein14. This result is inconsistent with the common assumption that αSyn is natively unfolded. Addition of negatively charged, small unilamellar lipid vesicles did not induce a significant conformational change in the native tetramer by CD (Fig. 3A), but a random coil to α-helical conversion did occur (as reported) with recombinant monomer that had been expressed in bacteria (Fig. 3B). Incubation of the purified RBC αSyn tetramer with Lipidex 1000, a reagent used to strip proteins of bound lipids and fatty acids15, did not change the conformation of the α-helical αSyn tetramer (Fig S3), suggesting that significant lipid association is not required to maintain the folded structure of cellular αSyn. To support this possibility, we conducted a quantitative elemental phosphate analysis16 on the purified native αSyn to search for phospholipid. We obtained an average value of 0.25 mol phosphate per mol αSyn, making a significant presence of phospholipids on the α-helical αSyn purified from normal cells unlikely. Because post-translational modifications also could have an impact on the conformational differences between the native human RBC tetramer and the bacterially expressed, recombinant human monomer, we performed mass spectrometry. The recombinant protein showed a mass peak at 14,462 kDa, very close to the theoretical predicted mass of 14,460 kDa, whereas the purified erythrocyte αSyn showed a peak at 14,505 kDa, indicative of only an N-α-acetylation commonly present on human proteins (theoretical predicted mass = 14,502 kDa) (Supp. Fig. S4).
Figure 3.

A: CD-spectra of native tetrameric αSyn (isolated under non-denaturing conditions from human RBC) before vs. after addition of PC/PS SUV (protein/lipid 1:500). B: CD spectra of recombinant αSyn monomer purified from E. coli alone and with addition of PC/PS SUV (protein/lipid 1:500). C: SPR sensorgram of equal protein concentrations of αSyn recombinant monomer vs. endogenous tetramer injected on a L1 chip covered with a PC/PS membrane. D: Amyloid-type aggregation kinetics of recombinant αSyn monomer vs. native RBC tetramer monitored by ThT fluorescence; average values from 3 independent experiments (error bars = SD; some SD for RBC-derived αSyn are smaller than the symbol size). AU, arbitrary units.
To validate the above results obtained on RBC αSyn using a different human cell type and a different non-denaturing purification method, we isolated αSyn from a M17D human neuroblastoma cell line stably overexpressing wt human αSyn (3D5 cells17). from untransfected M17D cell lysates migrated above bacterially expressed αSyn of confirmed random coil structure on CN-PAGE (Fig. S5A). This was also true of native (α-helical) but not denatured (random coil) purified RBC αSyn (Fig. S5B). After αSyn was purified from the stably transfected 3D5 cell line or from RBC, the two differentially purified and α-helically folded (by CD) cellular proteins co-migrated at ∼55-60 kDa on CN-PAGE, as expected (Fig. S6). Unbiased, automated STEM measurements of 3,000 particles revealed that the 3D5 neuroblastoma cells contained αSyn tetramers of closely similar estimated MW (peak mass ∼55 kDa) to those of the RBC αSyn (Fig S7; compare to Fig. 2B). CD spectroscopy revealed the purified 3D5 cell αSyn to have two minima of mean residue ellipticity at 222 and 208 nm (Fig S8). To further exclude artifacts arising during purification of cellular αSyn such as adventitious association of biomolecules (e.g., cellular lipids not removed by Lipidex 1000) that artificially fold the protein, we repeated our experiments with the 3D5 parental line M17D, which has only low levels of endogenous αSyn. We added (“spiked”) bacterially expressed recombinant human monomer onto the M17D cells before performing lysis and the full purification, and then assayed its structural properties. This exposure to cell lysates and the purification procedure lead to no induction of helical folding in the recombinant human αSyn (Fig. S9), whereas simultaneously purified 3D5 cell human αSyn did show this conformation, supporting our conclusion that α-helically folded αSyn does not arise due to artificial manipulation of the protein.
Membrane association has been viewed as a principal functional property of αSyn in vitro7 and in living cells18. We searched for differential binding of recombinant monomeric human αSyn vs. RBC tetrameric human αSyn to a lipid membrane using surface plasmon resonance (SPR). Because recombinant αSyn is reported to have preferential affinity for negatively charged lipids, especially phosphatidyl serine7, we chose a mixed phosphatidyl serine and phosphatidyl choline (PS/PC) membrane as a model membrane. Exposure of a PS/PC membrane to cell-derived, purified native αSyn in a Biacore instrument produced a markedly increased resonance angle shift compared to conventional recombinant monomers at identical concentrations in solution (Fig. 3C), indicating dramatically increased lipid binding. Fitting a dilution series of αSyn tetramer injections to a two-state binding model (Fig. S10) gave an apparent dissociation constant of Kapp = 56 ± 61 nM, which is several orders of magnitude lower than values obtained for recombinant monomer in analogous SPR studies19. We next tested the amyloid aggregation propensity of the distinct species in a Thioflavin T fluorescence assay. Monomeric and tetrameric αSyn displayed very different characteristics, with samples of purified cellular αSyn incubated under identical conditions showing no evidence of fibril formation in a time (10 days) more than sufficient to form mature, Thioflavin-bound fibrils from equivalent amounts of unfolded recombinant αSyn (Fig. 3D). Analysis of protein concentration in the solution after the 10-day incubation showed that the RBC αSyn was still present and soluble, ruling against non-amyloid (i.e., Thioflavin-negative) aggregation of the tetramers. Interestingly, melting curves of purified tetrameric αSyn showed that heat denaturation (at 95°C) appeared irreversible under our conditions (Fig. S11).
Our experiments provide multiple, independent lines of evidence that endogenous cellular αSyn exists in large part as an α-helically folded, ∼58 kDa tetramer under native conditions. This finding is in contrast to many biophysical and biochemical studies describing αSyn as a natively unfolded ∼14 kDa monomer. An early study of bacterially expressed recombinant protein purified under non-denaturing conditions or with heat treatment observed no conformational differences, concluding that αSyn is a natively unfolded monomer6. This suggests problems in generating properly folded protein in E. coli, although a modified bacterial expression protocol avoiding heating and denaturants has recently been found to yield a helical αSyn tetramer closely resembling the species found by us in native human samples20. The reasons for the conformational differences observed in these two bacterial studies are unknown. Using gel filtration on unfolded recombinant αSyn also showed an apparent molecular weight of ∼60 kDa in earlier studies; the data were interpreted as a decrease in mobility of the extended state of an unfolded protein in the tested matrices6. This suggests the possibility of a similar hydrodynamic radius for the unfolded monomer and the more compact, helically folded tetramer, making gel filtration an unreliable indicator. Our evidence for a tetrameric molecular mass of endogenous αSyn was particularly supported by the analytical ultracentrifugation and the unbiased STEM analysis, both of which sizing methods are not based on conformation. The STEM sizing was performed on intrinsic αSyn isolated from two cell types and using two distinct non-denaturing procedures.
Our apparent disagreement with most published findings on the monomeric state of αSyn in cells and brain tissue, usually as judged by SDS-PAGE and Western blotting, can be explained by the common use of denaturing detergents. Our tetramer aggregation data (Fig. 3D) are consistent with a recent report describing non-neurotoxic, aggregation-resistant αSyn oligomers in vivo21. Moreover, an oligomeric species of αSyn (size undefined) was observed by in vivo fluorescence lifetime imaging in an intact cell culture model22. Given the close match between our observed molecular weights using SE-AUC (Fig. 2C) and STEM (Fig. 2B and Supp Fig. S7) and the theoretical weight of a tetramer, the detection of a tetrameric band on denaturing gels after in vivo crosslinking (Fig. 1C), and the IEF evidence post-crosslinking that the endogenous tetramer and dimer bands have pKa's similar to that of a monomer (Fig. S1), we conclude that the predominant physiological species of αSyn in cells and brain is a helically folded tetramer, although minor and variable amounts of monomers, dimers and trimers were detected in some cell types. The closely similar properties of αSyn observed to date in neural cells and fresh human RBC recommends the latter as an abundant, available source for future studies of physiological αSyn.
The higher lipid-binding capacity of native αSyn leads us to speculate that the monomer represents a not fully functional and less abundant species in normal cells. Given the much lower propensity of the native tetramer to aggregate into fibrils (Fig. 3D), it is likely that tetramers undergo destabilization prior to αSyn aggregation into abnormal oligomeric and fibrillar assemblies that can confer cytotoxicity in PD and other α-synucleinopathies. Hypothetically, such a mechanism could be analogous in part to transthyretin amyloidosis, in which a native metastable tetramer circulates in human plasma but can become destabilized (e.g. by pathogenic missense mutations) to allow monomers to aggregate aberrantly in tissue23. Our identification of helically folded αSyn tetramers encourages the design of compounds that, like those for transthyretin24, could kinetically stabilize native tetramers and prevent pathogenic αSyn aggregation as a novel treatment approach for PD, dementia with Lewy bodies and other synucleinopathies25.
Methods summary
Native gel electrophoresis was conducted as described10. For crosslinking, 1-5 mM DSS was added to living cells. RBC lysates were treated analogously but utilizing 1 mM BS3. To purify αSyn from fresh or packed frozen RBC, an initial 25% (NH4)2SO4 cut followed by a 50% (NH4)2SO4 precipitation substantially enriched αSyn. The resolubilized 50% pellet was injected onto a hydrophobic interaction column (HiTrap Phenyl HP, GE Healthcare) and eluted in a 1M to 0M (NH4)2SO4, pH 7. Alternatively, αSyn-overexpressing 3D5 neuroblastoma cell lysate after (NH4)2SO4 was injected onto a 5 ml HiTrap Q HP column. A 25 to 500 mM NaCl (pH 8.0) gradient eluted αSyn at ∼300 mM NaCl. αSyn from both cell sources underwent a final purification step on a Superdex 75 SEC column. STEM analysis was conducted at the Brookhaven National Laboratory STEM user facility. Sedimentation equilibrium data were acquired on a Beckman XL-I analytical ultracentrifuge at speeds of 12k, 16k, and 20k RPM (AN-60 Ti rotor) and protein concentrations of 0.6, 1.1 and 1.6 mg/ml. CD spectroscopy for lipid-induced αSyn folding was conducted in the presence of 4 mM POPC/POPS (4:1) SUVs. SPR spectroscopy was conducted as described19. To quantify amyloid fibril growth, aliquots (10 μL) of purified αSyn were added to a 10 μM Thioflavin T (ThT) solution in 10 mM glycine buffer, pH 9. ThT fluorescence was measured by exciting at 444 nm and scanning the emission wavelengths from 460 to 550 nm.
Supplementary Material
Acknowledgments
Mass measurements were carried out at the Brookhaven National Laboratory STEM facility, a user facility supported by the US Department of Energy. We are grateful to Donald Walker and John Anderson (Elan Pharmaceuticals, San Francisco, CA) for conducting mass spectrometry of our purified αSyn samples and for helpful comments. We thank Xu Simon and Iva Perovic (Brandeis University, Waltham, MA) for their assistance with the AUC and phosphate analyses. Supported by NIH grants NS051318 and NS038375 (DJS). We thank our colleagues at the Center for Neurologic Diseases for many helpful discussions.
Footnotes
Author Contributions: All experiments were planned by T.B. and D.J.S. and conducted by T.B. and J.G.C. The manuscript was prepared by T.B. and D.J.S.
References
- 1.Obeso JA, et al. Missing pieces in the Parkinson's disease puzzle. Nat Med. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A, Dawson VL, Dawson TM. What causes cell death in Parkinson's disease? Annals of Neurology. 2008;64:S3–S15. doi: 10.1002/ana.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. The EMBO Journal. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong J, et al. Brain {alpha}-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain. 2010;133:172–188. doi: 10.1093/brain/awp282. [DOI] [PubMed] [Google Scholar]
- 5.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 6.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PTJ. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 7.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. The Journal of biological chemistry. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 8.DeTure M, et al. Missense tau mutations identified in FTDP-17 have a small effect on tau-microtubule interactions. Brain Res. 2000;853:5–14. doi: 10.1016/s0006-8993(99)02124-1. [DOI] [PubMed] [Google Scholar]
- 9.Scherzer CR, et al. GATA transcription factors directly regulate the Parkinson's disease-linked gene alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10907–10912. doi: 10.1073/pnas.0802437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittig I, Schagger H. Advantages and limitations of clear-native PAGE. PROTEOMICS. 2005;5:4338–4346. doi: 10.1002/pmic.200500081. [DOI] [PubMed] [Google Scholar]
- 11.Osenkowski P, et al. Cryoelectron microscopy structure of purified gamma-secretase at 12 A resolution. Journal of molecular biology. 2009;385:642–652. doi: 10.1016/j.jmb.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wall JS, Simon MN, Lin BY, Vinogradov SN. Mass mapping of large globin complexes by scanning transmission electron microscopy. Methods Enzymol. 2008;436:487–501. doi: 10.1016/S0076-6879(08)36027-3. [DOI] [PubMed] [Google Scholar]
- 13.Beyer K. Mechanistic aspects of Parkinson's disease: alpha-synuclein and the biomembrane. Cell Biochem Biophys. 2007;47:285–299. doi: 10.1007/s12013-007-0014-9. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Yang JT, Martinez HM. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 2002;11:4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- 15.Sharon R, et al. alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9110–9115. doi: 10.1073/pnas.171300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen PS, Toribara TY, Warner H. Microdetermination of Phosphorus. Analytical Chemistry. 1956;28:1756–1758. [Google Scholar]
- 17.Ko LW, Ko HH, Lin WL, Kulathingal JG, Yen SH. Aggregates assembled from overexpression of wild-type alpha-synuclein are not toxic to human neuronal cells. J Neuropathol Exp Neurol. 2008;67:1084–1096. doi: 10.1097/NEN.0b013e31818c3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean PJ, Kawamata H, Ribich S, Hyman BT. Membrane association and protein conformation of alpha-synuclein in intact neurons. Effect of Parkinson's disease-linked mutations. The Journal of biological chemistry. 2000;275:8812–8816. doi: 10.1074/jbc.275.12.8812. [DOI] [PubMed] [Google Scholar]
- 19.Smith DP, et al. Formation of a High Affinity Lipid-Binding Intermediate during the Early Aggregation Phase of alpha-Synuclein. Biochemistry. 2008;47:1425–1434. doi: 10.1021/bi701522m. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, et al. The Parkinson disease-associated protein alpha-synuclein is folded in solution. Proceedings of the National Academy of Sciences of the United States of America [Google Scholar]
- 21.Tsika E, et al. Distinct Region-Specific {alpha}-Synuclein Oligomers in A53T Transgenic Mice: Implications for Neurodegeneration. Journal of Neuroscience. 2010;30:3409–3418. doi: 10.1523/JNEUROSCI.4977-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klucken J, Outeiro TF, Nguyen P, McLean PJ, Hyman BT. Detection of novel intracellular alpha-synuclein oligomeric species by fluorescence lifetime imaging. FASEB J. 2006;20:2050–2057. doi: 10.1096/fj.05-5422com. [DOI] [PubMed] [Google Scholar]
- 23.Quintas A, Saraiva MJM, Brito RMM. The Tetrameric Protein Transthyretin Dissociates to a Non-native Monomer in Solution. The Journal of biological chemistry. 1999;274:32943–32949. doi: 10.1074/jbc.274.46.32943. [DOI] [PubMed] [Google Scholar]
- 24.Connelly S, Choi S, Johnson SM, Kelly JW, Wilson IA. Structure-based design of kinetic stabilizers that ameliorate the transthyretin amyloidoses. Current Opinion in Structural Biology. 2010;20:54–62. doi: 10.1016/j.sbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


