Abstract
SynGAP is a Ras GTPase activating protein present at the postsynaptic density (PSD) in quantities matching those of the core scaffold protein PSD-95. SynGAP is reported to inhibit synaptic accumulation of AMPA receptors. Here, we characterize by immunogold electron microscopy the distribution of SynGAP at the PSD under basal and depolarizing conditions in rat hippocampal neuronal cultures. The PSD core, extending up to 40 nm from the postsynaptic membrane, typically shows label for SynGAP, while half of the synapses exhibit additional labeling in a zone 40–120 nm from the postsynaptic membrane. Upon depolarization with high K+, labeling for SynGAP significantly decreases at the core of the PSD and concomitantly increases at the 40–120 nm zone. Under the same depolarization conditions, label for PSD-95, the presumed binding partner of SynGAP, does not change its localization at the PSD. Depolarization-induced redistribution of SynGAP is reversible and also occurs upon application of NMDA. Activity-induced movement of SynGAP could vacate sites in the PSD core allowing other elements to bind to these sites, such as transmembrane AMPA receptor regulatory proteins, and simultaneously facilitate access of SynGAP to CaMKII and Ras, elements of a regulatory cascade.
Keywords: SynGAP, PSD-95, AMPA receptor, postsynaptic density, electron microscopy, immunogold
The postsynaptic density (PSD) is a disc-shaped structure lining the cytoplasmic side of the postsynaptic membrane at glutamatergic excitatory synapses in the mammalian central nervous system. The PSD is a complex of regulatory proteins, membrane receptors and cell-adhesion molecules associated with a central scaffold (reviews: Sheng and Hoogenraad 2007, Feng and Zhang 2009). PSDs undergo changes in structure and composition upon stimulation (Geinisman et al., 1991, Hu et al., 1998, Dosemeci et al., 2001, Ehlers 2003), and these changes may impact synaptic strength.
SynGAP, a Ras GTPase activating protein, is highly enriched in the PSD (Kim et al., 1998, Chen et al., 1998). Analysis of PSD fractions purified from adult rat forebrain shows that SynGAP is as abundant as PSD-95, and that the two are the most abundant proteins in the PSD fraction after CaMKII (Cheng et al., 2006). Yeast two-hybrid studies show that the α-isoform of SynGAP interacts with the PDZ domains of PSD-95 and SAP102, a similar MAGUK prominent in immature brain, via its C-terminal PDZ-binding domain (Kim et al., 1998). SynGAP and PSD-95 co-immunoprecipitate from solubilized PSD fractions (Chen et al., 1998). Knockout and overexpression implicate SynGAP in the inhibition of accumulation of AMPA receptors at the synapse and spine formation (Kim et al., 2003, Vazquez et al., 2004, Rumbaugh et al., 2006,). Heterozygous null mutation of SynGAP (SynGAP−/+) results in reduced induction of LTP in the CA1 region of the hippocampus (Komiyama et al., 2002). SynGAP−/+ and PSD-95−/− double mutants show enhanced induction of LTP, which is also observed in PSD-95−/− single mutants, suggesting that the role of SynGAP in the regulation of LTP is dependent on the presence of PSD-95 (Komiyama et al., 2002).
The interaction of SynGAP with the PDZ domains of PSD-95 is well-characterized (Kim et al., 1998, Chen et al., 1998, Li et al., 2001). The PDZ-binding property of SynGAP, as well as its GTPase-activating property, has been shown to be necessary for the inhibitory action of SynGAP on accumulation of AMPA receptors at the synapse (Rumbaugh et al., 2006). The requirement for the PDZ-binding property of SynGAP suggests that direct association between SynGAP and PSD-95 is necessary for this inhibition. As the PDZ domains of PSD-95 lie within the PSD, close to the postsynaptic membrane (Chen et al., 2008), the association between SynGAP and PSD-95 would organize a laminar localization of SynGAP within the PSD. In the present study, we examine the distribution of SynGAP within the PSD under basal and excitatory conditions. The results add details to the picture of a dynamic PSD which reorganizes in response to synaptic activity.
EXPERIMENTAL PROCEDURES
1.1 Materials
A rabbit monoclonal antibody (clone EPR2883), which specifically recognizes isoforms with a PDZ-binding motif (QTRV) at the C-terminus of SynGAP (SynGAP Ab1), is from Millipore (Billerica, MA, USA; 1:2500 for Western blots, 1:500 for microscopy). Unless otherwise indicated, data presented are from samples labeled with SynGAP Ab1. A rabbit polyclonal antibody to residues 947–1167 of SynGAP, conserved in all isoforms (SynGAP Ab2), is from Abcam (Cambridge, MA, USA; 1:1000 for Western, 1:500 for microscopy). Polyclonal antibodies raised to residues 290–307 [PRRYSPVAKDLLGEEDIC] of PSD-95 (1:1000 for Western, 1:500 for microscopy) were custom made by New England Peptide (Gardener, MA, USA). A mouse monoclonal antibody (clone 9A11.2) recognizing all three primary isoforms of ras (H-, N- and K-ras) is from Millipore (1:1000 for Western). A mouse monoclonal antibody (clone 6G9) to the α-subunit of CaMKII is from Millipore (1:100 for microscopy). Rabbit anti-synaptophysin (1:2000 for Western) is from Dako (Carpinteria, CA, USA). NMDA and APV, an NMDA antagonist, are from Tocris (Ellisville, MO, USA).
1.2 Subcellular Fractionation and Western immunoblotting
Homogenate, synaptosome and PSD fractions from adult rat brains, collected and frozen in liquid nitrogen within two minutes of decapitation by Pel-Freeze Biologicals (Rogers, AR, USA), were prepared as described previously by Dosemeci et al. (2000). Fractions were separated by SDS-PAGE, transferred to nitrocellulose membrane and visualized by either chemiluminescence (Thermo Scientific, Waltham, MA, USA) or colorimetric methods (BCIP/NBT Phosphatase System, KPL, Gaithersburg, MD, USA).
1.3 Preparation and treatment of dissociated hippocampal neuronal cultures
The animal protocol was approved by NIH Animal Use and Care Committee and conformed to NIH guidelines. Hippocampal cells from 21-day embryonic Sprague Dawley rats were dissociated and grown on a glial cell layer as described previously by Lu et al. (1998) for 19–21 days.
Control incubation medium (124 mM NaCl, 2mM KCl, 1.24 mM KH2PO4, 1.3 mM MgCl2, 2.5 mM CaCl2, 30 mM glucose in 25 mM Hepes at pH 7.4) was prepared and, where indicated, was modified to include 90 mM KCl (compensated by reducing concentration of NaCl), 30 µM NMDA, 30 µM NMDA with 50 µM APV, or 50 µM APV. Cell cultures were washed with control incubation medium and treated with control incubation medium, high K+, NMDA or NMDA + APV medium for 2–3 minutes. For recovery experiments, cultures were treated with high K+ medium for 2 minutes, washed with control incubation medium (4 times within 2 minutes) then left in control incubation medium for a total of 30 minutes. Treatment of samples was performed with dishes floating on a platform in a water bath at 37°C.
1.4 Preparation and treatment of hippocampal slice cultures
The animal protocol was approved by NIH Animal Use and Care Committee and conformed to NIH guidelines. The hippocampus was removed from 6–10 day old Sprague Dawley rat pups and sections 250 µm thick cut with a McIlwain tissue chopper (Nickle Laboratory Engineering, Surrey, UK). The slices were placed in cell culture inserts and kept at the air-media interface in media containing HMEM with 12.5 mM Hepes, Hanks’ salts, 25% horse serum, 1 mM glutamine, 27 mM glucose, 1 mM pyruvate, 0.5 mM ascorbate and N3 (a growth factor cocktail) in a 5% carbon dioxide incubator at 35°C. The medium was changed to one containing 5% horse serum on the second day and changed every second day until use at in vitro days 12–14 (cf Tao-Cheng et al., 2009). Thus, the effective age of the slices used in experiments is approximately three weeks, the same as the dissociated hippocampal neurons. By P21, SynGAP expression in the hippocampus of mice stabilizes and remains largely unchanged until adulthood (Porter et al., 2005).
For experiments, slice culture inserts were placed in six-well dishes on a floating water bath at 37°C. Medium changes were made by first removing the old medium, transferring the insert into a new well with 1 ml of fresh medium and adding 1 ml of fresh medium on top to submerge the slice culture. Slice cultures were washed with control incubation medium before the addition of control or high K+ medium with the same protocol used for dissociated hippocampal neuronal cultures.
1.5 Pre-embedding immunogold-labeling
After treatment, both the dissociated and slice cultures were fixed in 4% paraformaldehyde (EMS, Hatfield, PA, USA) in PBS for 35–45 minutes at room temperature, then washed and stored in PBS. Slice cultures were lifted off the insert filter with a brush and trimmed for sampling from the zonula radiatum of the CA1 region as described in Tao-Cheng et al. (2009).
Immunolabeling procedures were carried out at room temperature. Samples were permeabilized and blocked in 0.1% saponin and 5% normal goat serum for 40–60 minutes, incubated with primary antibodies for 1–1.5 hours, then washed and incubated with secondary antibodies (Nanogold, Nanoprobes, Yaphank, NY, USA). Samples were then washed and fixed with 2% gluteraldehyde in PBS for 1 hour, silver enhanced (HQ kit, Nanoprobes), treated in 0.2% Os2O4 for 30 minutes then 0.5% uranyl acetate for one hour, dehydrated in graded ethanols and embedded in epoxy resin. Because the overall labeling sensitivity may differ between experiments, only parallel samples from the same experiment were directly compared. Illustrations within a figure are from parallel samples from the same experiment.
1.6 Morphometry
Every synaptic profile with a well-delineated postsynaptic membrane was photographed at 40,000X with a CCD digital camera system (XR-100 from AMT, Danvers, MA) until at least 30 profiles were sampled. Two separate measurements were carried out to characterize the activity-induced redistribution of SynGAP label. (1) Distance of SynGAP label from the postsynaptic membrane was measured from the center of each label to the cleft edge of the postsynaptic membrane using Image J (National Institutes of Health, Bethesda, MD, USA). Distance measurements were plotted as histograms to illustrate the distribution profiles under different experimental conditions for different antibodies. (2) Labels within the two sub-compartments of the PSD complex (as described below and defined in Fig. 1) were counted at a final magnification of 150,000X. These numbers were then normalized to the area of the sampled zone (cf. Fig. 1) and expressed as number of label/µm length in each zone for each synaptic profile.
Fig. 1.
Zones for measurement at the PSD. Synapse labeled with SynGAP (A) with superimposed mask (B) to illustrate zones for measuring amount of label in the PSD complex. The PSD core is defined as the area less than 40 nm from the postsynaptic membrane (between the postsynaptic membrane and upper dashed lines) and the adjacent area 40–120 nm from the postsynaptic membrane is defined as the contiguous network (between the upper and lower dashed lines). Each particle is assigned to the zone containing its center. Scale bar = 100nm.
The border of the PSD core is marked by a change in density approximately 30 nm from the cleft edge of the postsynaptic membrane. However, a web of filaments, indistinct by routine EM preparation, but visualized dramatically by special staining methods, constitutes a lower stratum of the PSD, a subsynaptic web ~100 nm wide (Valtschanoff and Weinberg, 2001). The subsynaptic web is not visualized directly by the fixation methods used for immunolabeling, though its presence can be inferred by the distribution of certain components, implying the presence of a network of proteins contiguous to the PSD (Tao-Cheng et al., 2010). The edge of the contiguous network for purposes of measurement was set at 120 nm from the postsynaptic membrane, based on the pattern of labeling for Shank, a major PSD protein that specifically occupies the contiguous network (Tao-Cheng et al., 2010). Because the position of a label might not reflect the exact position of its epitope due to the presence of primary and secondary antibodies, the line separating the core and contiguous network compartments of the PSD was extended to 40 nm in order that true numbers of SynGAP in the core not be undercounted. The entire zone less than 120 nm from the postsynaptic membrane will be referred to as the PSD complex, which is further divided into the PSD core (less than 40 nm from the postsynaptic membrane) and the PSD contiguous network (40–120 nm from the postsynaptic membrane). The area beyond 120 nm from the postsynaptic membrane is considered as the adjacent cytoplasmic compartment outside of the PSD complex.
Statistical analysis was carried out by Student’s t-test (unmatched sets, unequal variances; or paired t-test when appropriate; KaleidaGraph, Synergy Software, Reading, PA, USA) or ANOVA for comparing means, and by the Wilcoxin-Mann-Whitney or the Kruskal-Wallis rank sum test (KaleidaGraph) for comparing patterns of distribution.
RESULTS
2.1 SynGAP is enriched in the PSD fraction
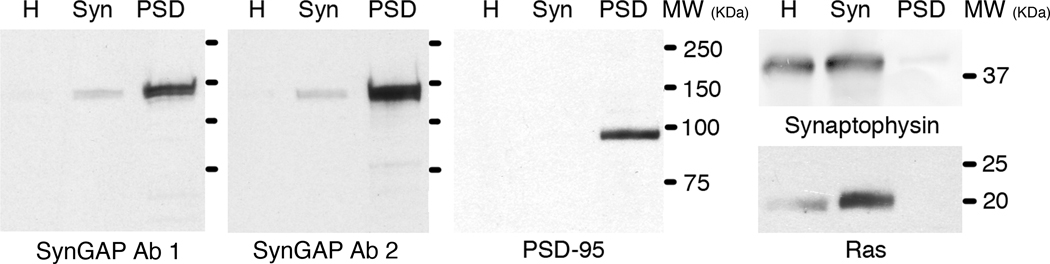
Western immunoblots (Fig. 2) comparing band intensities in homogenate, synaptosome and PSD fractions using two different antibodies to SynGAP showed that SynGAP is greatly enriched in the PSD fraction as previously reported (Chen et al., 1998). Ras, a soluble SynGAP substrate, is enriched in the synaptosome fraction, but not in the PSD fraction. All antibodies recognized a single strong band at the expected molecular weight range for their specific proteins, confirming their suitability for immunoEM studies.
Fig. 2.
SynGAP is enriched in the PSD fraction. Western immunoblots of homogenate (H), synaptosome (Syn) and postsynaptic density (PSD) fractions. Equal amounts of protein were loaded into each lane. Comparison of the subcellular fractions indicated enrichment of SynGAP in the PSD by two different antibodies, but not of Ras, a small GTPase regulated by SynGAP. As expected, PSD-95 was enriched in the PSD fraction while synaptophysin, an integral membrane protein of synaptic vesicles, was barely detectable.
2.2 Distribution of SynGAP at the PSD complex under basal conditions
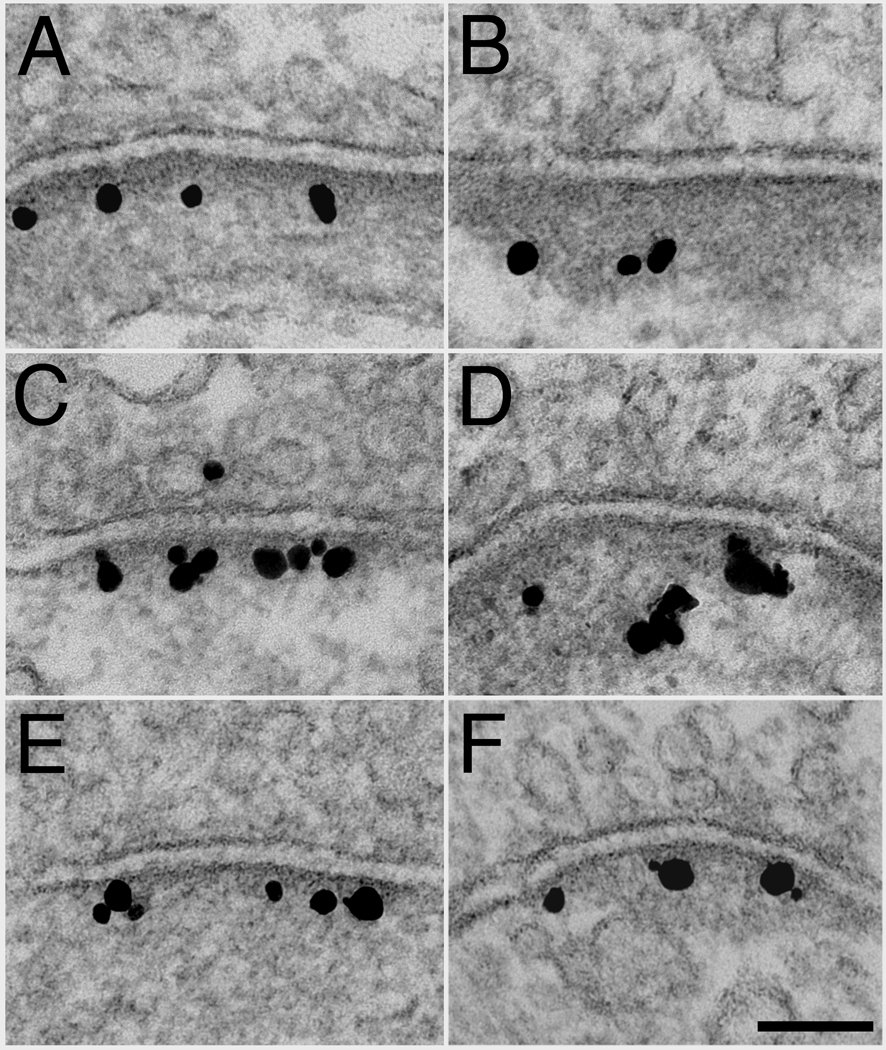
The great majority (> 90%) of synaptic profiles labeled for SynGAP in the PSD complex in dissociated hippocampal cultures under basal conditions. However, labeling for SynGAP varied in intensity and distribution from synapse to synapse (Fig. 3). Analysis of 181 synapses from two sets of cultures showed that approximately 40% of synaptic profiles labeled only in the PSD core, less than 40 nm from the postsynaptic membrane (Fig. 3A, B), while approximately 50% labeled in both the PSD core and the contiguous network, an adjacent area lying 40–120 nm from the postsynaptic membrane (Fig. 3C, D). The amount of label within the contiguous network was highly variable, ranging from low (Fig. 3C) to high (Fig. 3D). In addition, approximately 25% of synaptic profiles also had variable amounts of SynGAP label in the adjacent cytoplasmic compartment outside of the PSD complex (Fig. 3D).
Fig. 3.
Postsynaptic distribution of SynGAP under basal conditions. Immunolabeling with SynGAP antibody in dissociated hippocampal cultures shows variations in labeling patterns. Some synapses show labeling only at the PSD core (A, B) while others also show light (C) or heavy (D) labeling in the contiguous network, sometimes extending into the cytoplasm beyond the PSD complex (D). Scale bar = 100 nm.
2.3 Depolarization induces redistribution of SynGAP within the PSD complex
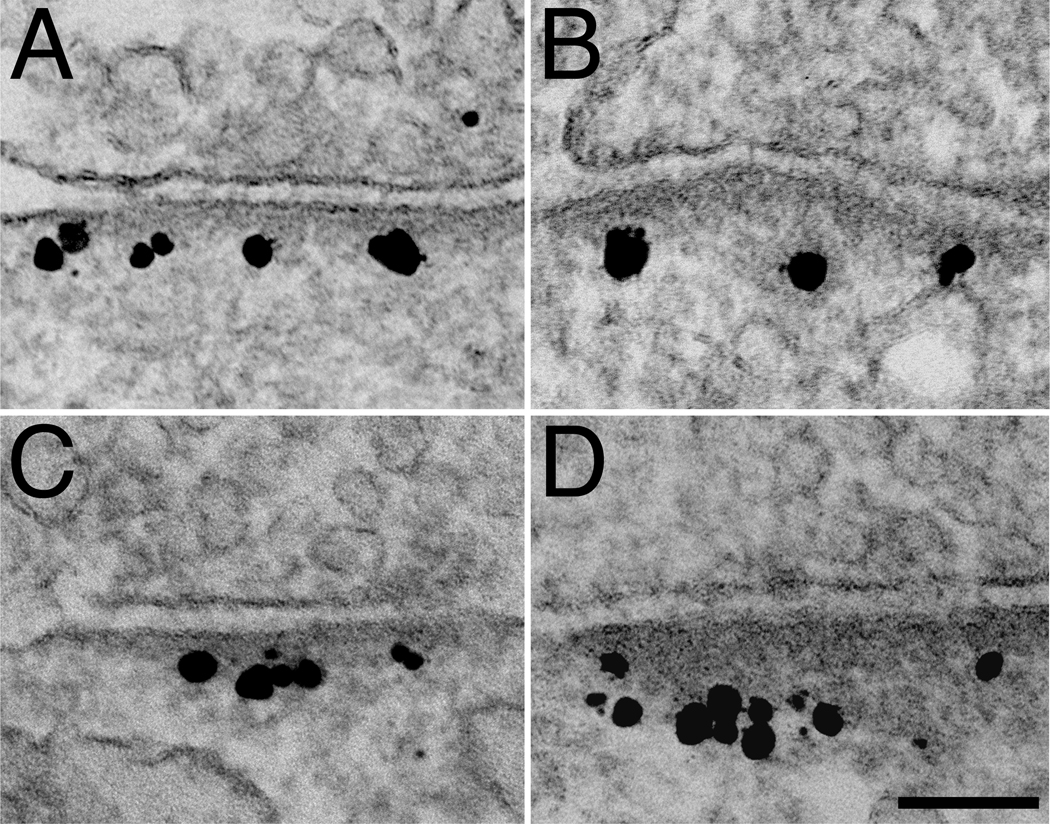
Hippocampal neurons were depolarized by two minutes of exposure to high extracellular K+ to determine how activity affects the distribution of SynGAP and PSD-95. As previously reported (Dosemeci et al., 2001), PSDs appeared thickened upon depolarization (Fig. 4, right column) with a concomitant accumulation of CaMKII on the cytoplasmic side of the PSD (data from current parallel samples not shown). These observations confirmed the effectiveness of the stimulation.
Fig. 4.
Effect of depolarization on the distribution of SynGAP and PSD-95. Immunogold label for SynGAP Ab1 (A, B), SynGAP Ab2 (C, D), and PSD-95 (E, F) under basal conditions (left column) and after two minutes of treatment with high K+ (right column). Upon depolarization, the distribution of label for SynGAP moves away from the PSD core and into the contiguous network, while label for PSD-95 remains unchanged. Scale bar = 100 nm.
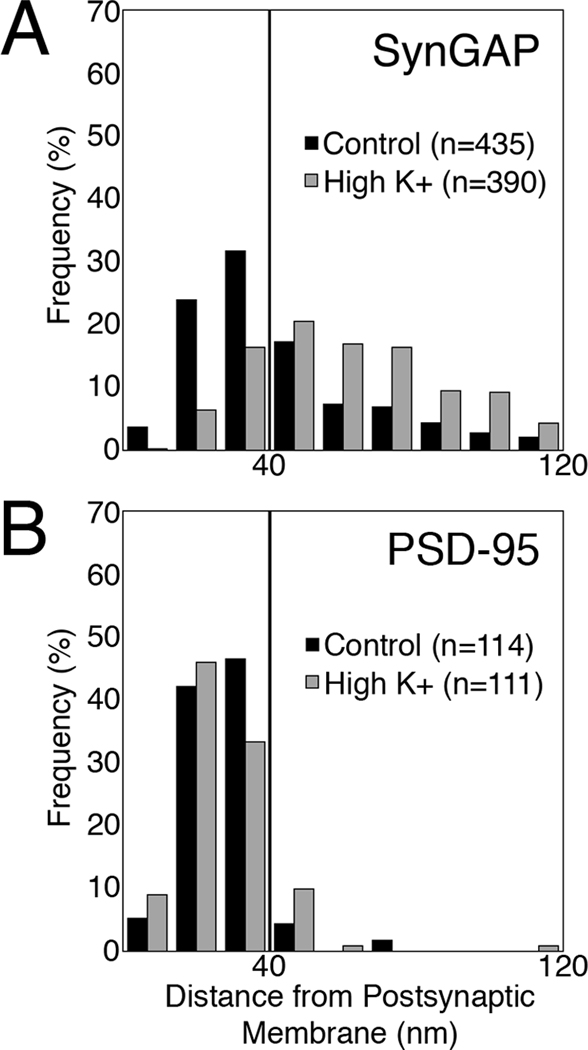
Depolarization also induced redistribution of SynGAP label away from the postsynaptic membrane (Fig. 4, A vs B; C vs D). The distribution of SynGAP label was skewed under basal conditions (Fig. 5A, black bars) with the majority of the label located within the PSD core. Upon depolarization, label for SynGAP became distributed further away from the postsynaptic membrane (Fig. 5A, gray bars). This depolarization–induced redistribution of label for SynGAP was consistent in four separate experiments with an average shift of 25 nm in the median distance from the postsynaptic membrane (Table 1). Redistribution was also seen in samples labeled with a different antibody to SynGAP (SynGAP Ab2; Fig. 4C vs. D). In contrast, the distribution of label for PSD-95 in the PSD complex remained unchanged after depolarization (Fig. 4E vs. F and Fig. 5B, Table 1).
Fig. 5.
SynGAP, but not PSD-95, moves out of the PSD core upon depolarization. Histograms from representative experiments showing the distribution of label for SynGAP (A) and PSD-95 (B) under control (black bars) and depolarizing conditions (gray bars). The vertical line denotes the boundary between the PSD core and the contiguous network. Upon depolarization, distribution of SynGAP label shifts to the contiguous network (P < 0.0001, Wilcoxin-Mann-Whitney rank sum test), while the distribution of PSD-95 label remains unchanged.
Table 1.
Effect of stimulation on the distance (nm) between label for SynGAP or PSD-95 and the postsynaptic membrane.
| Antibody | Control | High K+ | High K+/30' Recovery | NMDA | ||||
|---|---|---|---|---|---|---|---|---|
| Median(n) | Mean±SEM | Median(n) | Mean±SEM | Median(n) | Mean±SEM | Median(n) | Mean±SEM | |
| SynGAP | 33.3 (171) | 36.8 ± 1.4 | 60.0 (257) | 62.3 ± 1.7** | - | - | - | - |
| 38.5 (144) | 45.5 ± 2.1 | 58.0 (114) | 59.8 ± 2.3** | 35.4 (162) | 43.9 ± 2.0 | - | - | |
| 30.2 (332) | 33.2 ± 0.9 | 62.4 (79) | 62.6 ± 2.9** | 33.8 (138) | 35.3 ± 1.2 | 54.9 (201) | 59.6 ± 1.8** | |
| 35.8 (435) | 42.1 ± 1.1 | 57.6 (390) | 61.0 ± 1.3** | 37.7 (258) | 40.1 ± 1.2 | 56.1 (280) | 58.6 ± 1.5** | |
| 32.0 (185) | 36.7 ± 1.3 | - | - | - | - | 57.5 (240) | 61.6 ± 1.8** | |
| Average | 34.0 | 38.9 | 59.5 | 61.4 | 35.6 | 39.8 | 56.2 | 59.9 |
| PSD-95 | 27.1 (114) | 27.2 ± 1.0 | 25.8 (111) | 26.8 ± 1.2 | - | - | - | - |
| 26.5 (234) | 28.2 ± 0.9 | 25.0 (78) | 27.0 ± 1.5 | - | - | - | - | |
| 26.8 (36) | 28.3 ± 1.9 | 24.9 (58) | 27.1 ± 1.3 | - | - | - | - | |
| Average | 26.8 | 27.9 | 25.2 | 27.0 | ||||
Values are from individual experiments. n = # of labels measured.
P < 0.0001 against control, Student’s t test. Average values from experiments in bold.
The amount of label for SynGAP in the two sub-compartments of the PSD complex, the PSD core and the contiguous network (Fig. 1), were evaluated in a separate analysis. The amount of label for SynGAP in the PSD core significantly decreased in all four experiments upon depolarization (Table 2). Labeling for SynGAP in the contiguous network upon depolarization showed a concomitant increase, which reached statistical significance in three out of four experiments (Table 2). Because the overall labeling sensitivity varied from experiment to experiment, the ratio of labeling in the PSD core to the labeling in the contiguous network was evaluated in each of the four experiments. In control cultures, the mean ratio of labeling in the PSD core to the labeling in the contiguous network was 2.55 ± 0.31, while the mean ratio after high K+ stimulation was 0.39 ± 0.02 (P < 0.01, paired t-test), confirming that upon stimulation there is a significant redistribution of label for SynGAP out of the core of the PSD. Thus, a population of SynGAP leaving the PSD core appears to accumulate in the contiguous network.
Table 2.
Amount of SynGAP label by sub-compartments of the PSD complex (number of labels/µm length of measurement area) from four experiments.
| PSD Core | Contiguous Network | ||
|---|---|---|---|
| Control (n) | High K+ (n) | Control | High K+ |
| 15.5 ± 1.3 (67) | 6.7 ± 0.9 (66)** | 7.6 ± 0.9 | 18.2 ± 1.7 ** |
| 17.8 ± 1.4 (32) | 4.9 ± 0.9 (33)** | 8.5 ± 1.7 | 11.2 ± 1.3 |
| 14.2 ± 1.4 (41) | 6.3 ± 0.9 (45)** | 5.3 ± 0.9 | 16.5 ± 2.2 ** |
| 20.0 ± 1.5 (43) | 4.3 ± 1.1 (30)** | 5.9 ± 0.9 | 11.2 ± 2.3* |
Values given as mean ± SEM. n = number of synaptic profiles sampled,
P < 0.05 and
P < 0.0001, Student’s t-test.
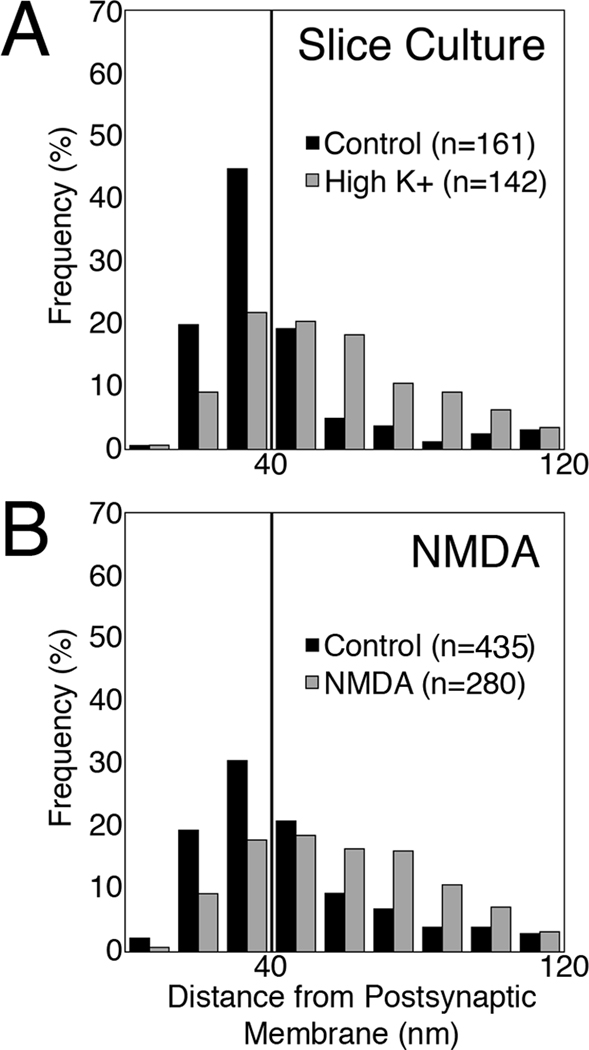
The same depolarization protocol carried out in hippocampal slice cultures yielded changes in the distribution of SynGAP similar to those in dissociated cultures (Fig. 6A vs B; Fig. 7A). Furthermore, a second stimulation protocol consisting of application of NMDA to dissociated hippocampal cultures resulted in a similar redistribution of label for SynGAP (Fig. 6C vs. D; Fig. 7B; Table 1). The effects of treatment with NMDA were blocked upon inclusion of 50 µM APV in all incubation media.
Fig. 6.
Immunogold label for SynGAP in hippocampal slice cultures is concentrated in the PSD core at rest (A) and shifts to the contiguous network after two minutes of high K+ (B). NMDA treatment induces a similar redistribution of SynGAP labels in dissociated hippocampal cultures (C at rest and D after NMDA treatment). Scale bar = 100 nm.
Fig. 7.
Histograms showing the distribution of label for SynGAP in hippocampal slice cultures (A) under control (black bars) and depolarizing conditions (gray bars), and in dissociated hippocampal cultures (B) under control conditions (black bars) and after two minutes of treatment with 30 µM NMDA (gray bars). The vertical line denotes the boundary between the PSD core and the contiguous network. Both histograms showed significant shifts after treatment (P < 0.0001, Wilcoxin-Mann-Whitney rank sum test).
2.4 Depolarization-induced redistribution of SynGAP is reversible
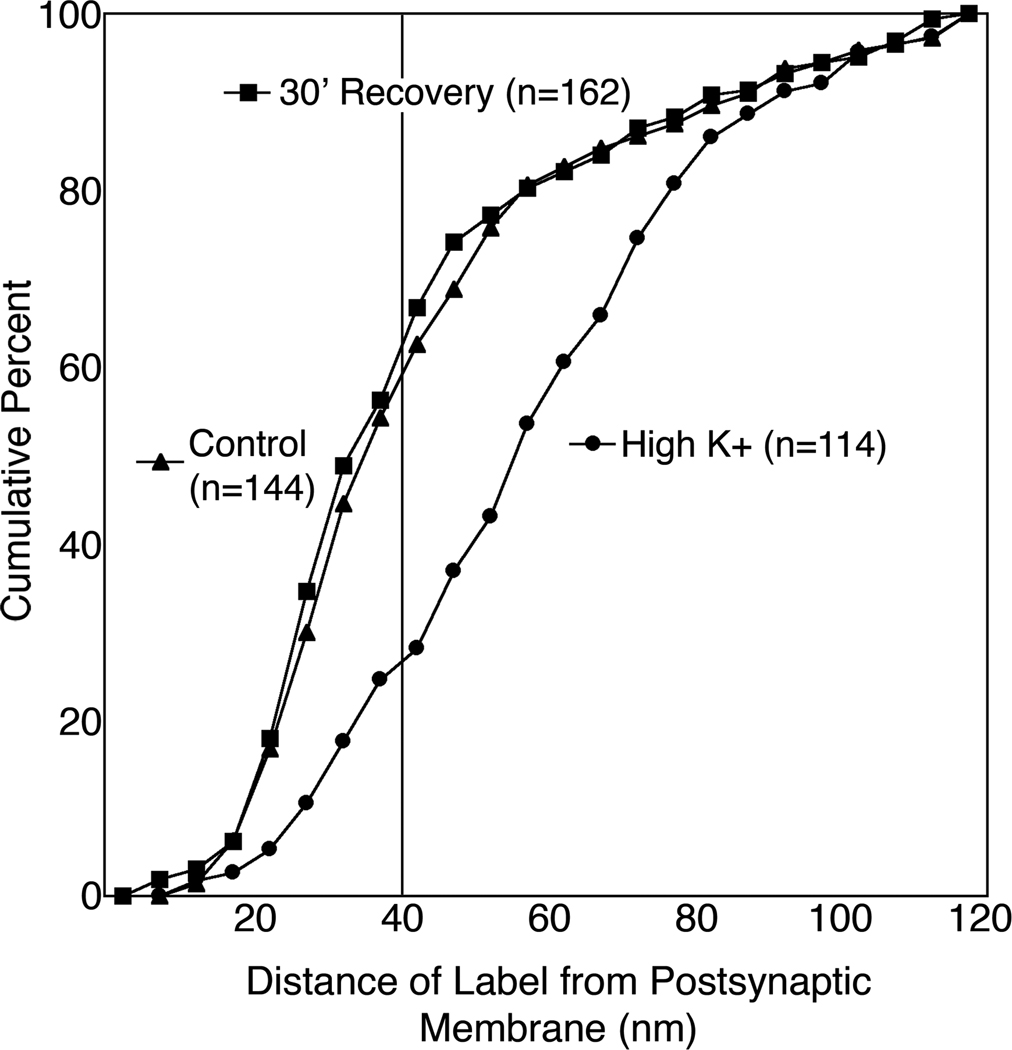
Depolarization-induced redistribution of SynGAP label became reversed after a thirty-minute period of recovery (Fig. 8). Three separate recovery experiments yielded similar results with the average of the median distances of label from the postsynaptic membrane increasing from 34.8 ± 2.5 nm in matched controls to 59.3 ± 1.5 nm after depolarization and returning to 35.6 ± 1.1 nm after recovery (P < 0.001, ANOVA). A cumulative frequency plot from a representative experiment (Fig. 9) shows that the distribution of SynGAP label is virtually identical under control and recovery conditions, with ~60% of the label residing in the PSD core. In contrast, only 26% of the label remains in the PSD core upon high K+ depolarization.
Fig. 8.
Redistribution of SynGAP label is reversible. SynGAP labels are in the PSD core close to the postsynaptic membrane at rest (A), move further away upon depolarization with high K+ (B), and return to the PSD core after 30 min of recovery from high K+ (C). Scale bar = 100 nm.
Fig. 9.
Cumulative frequency graph showing distance between label for SynGAP and the postsynaptic membrane at rest, after stimulation, and upon recovery. The vertical line at 40 nm denotes the boundary between the PSD core and the contiguous network (P < 0.0001 Kruskal-Wallis rank sum test).
DISCUSSION
In agreement with previous studies (Petralia et al., 2005), both the core of the PSD and its contiguous network label for SynGAP. Pre-embedding immunogold electron microscopy shows that label for SynGAP is concentrated in the PSD core under basal conditions. Depolarization with high K+ induces a reversible movement of SynGAP away from the PSD core into the contiguous network. High K+ also induces a similar redistribution of label for SynGAP in hippocampal slice cultures, showing that activity-induced redistribution is not limited to dissociated hippocampal neuronal cultures.
Depolarization with high K+ is a relatively strong stimulation protocol and was chosen as a reliable first strategy to uncover activity-induced protein traffic at the PSD based on previous studies for CaMKII (Dosemeci et al., 2001), Shanks (Tao-Cheng et al., 2010) and AMPA receptors (Tao-Cheng et al., 2011). Next we showed that application of NMDA is sufficient to induce a pattern of redistribution of SynGAP similar to that after application of high K+. Further studies should clarify whether the redistribution of SynGAP, like that of CaMKII (Otmakhov et al., 2004) is persistent under certain stimulation protocols.
In contrast to SynGAP, label for PSD-95 does not change position within the PSD complex upon depolarization, indicating that the decrease of SynGAP label at the PSD core is not due to failure of antibody to penetrate the denser PSD complex after stimulation. Although the mean distance of PSD-95 label from the postsynaptic membrane is unchanged after stimulation, our observations, which measure label only at the PSD complex, do not exclude the possibility that some PSD-95 leaves the spine during activity (Steiner et al., 2008).
The α-isoform of SynGAP can bind to the PDZ domains of PSD-95 through its PDZ-binding domain (Kim et al., 1998). The PDZ domains of PSD-95 appear to lie within 30 nm from the postsynaptic membrane (Chen et al., 2008). Allowing 10 nm for the length of the antibodies, the labels for the PDZ domain of PSD-95 and the PDZ-binding domain of SynGAP (used in this study) would lie within 40 nm of the postsynaptic membrane when the two domains are directly associated. Indeed, under basal conditions, both PSD-95 and SynGAP labels are concentrated within the PSD core, less than 40 nm from the postsynaptic membrane. Upon depolarization, label for PSD-95 remains at the PSD core while the majority of label for SynGAP moves into the contiguous network. The magnitude of the difference in the distributions of the two labels is such that the two interacting domains cannot remain associated upon depolarization.
There may be several functional consequences of activity-dependent transient movement of SynGAP away from the PSD core to the contiguous network where it could interact with new partners that modify its activity. Under experimental conditions identical to those used in this study, CaMKII accumulates at the cytoplasmic side of the PSD and in the contiguous network (Dosemeci et al., 2001), generally within the same zone where SynGAP becomes concentrated. Activity-induced colocalization of SynGAP and CaMKII could promote phosphorylation of SynGAP by CaMKII. Phosphorylation of SynGAP at multiple residues by CaMKII (Oh et al., 2004, Dosemeci and Jaffe, 2010) results in increased GAP activity (Oh et al., 2004), which down-regulates Ras. Also, SynGAP movement away from the PSD core may allow better access to its soluble substrate, Ras. CaMKII, SynGAP and Ras have been proposed to be elements of a cascade involved in the reorganization of the actin cytoskeleton (Carlisle et al., 2008).
SynGAP is as abundant as PSD-95 in the PSD (Cheng et al., 2006) and binds to the PDZ domains of PSD-95 (Kim et al., 1998). The PDZ domains of PSD-95 are also binding sites of multiple PSD components including NMDA receptors, transmembrane AMPA receptor regulatory proteins (TARPs), neuroligin and n-nitric oxide synthase (review: Kim and Sheng 2004). Activity-induced movement of SynGAP away from the PSD core would free the PDZ domains of PSD-95, allowing their interaction with other proteins. The removal of SynGAP, a large protein with a molecular weight of 135 KDa, could even allow more space and flexibility within the matrix of the PSD for addition of new molecules.
Of particular interest is the regulatory role of SynGAP in AMPA receptor trafficking at the synapse. Overexpression of SynGAP decreases the number of AMPA receptors at the synapse, while SynGAP knockouts show an increase in mEPSCs mediated by AMPA receptors (Rumbaugh et al., 2006) and an increase in number of clusters of AMPA receptors at synapses (Kim et al., 2003). AMPA receptors interact indirectly with the PDZ domains of PSD-95 through TARPs. Translocation of SynGAP away from the PSD core could vacate the binding sites for TARPs, which would allow addition of AMPA receptors at the synapse. Indeed, the number of AMPA receptors at the PSD increases under the depolarizing conditions identical to those used in the current study (Tao-Cheng et al., 2011). Thus, movement of SynGAP away from the PSD core upon activity could provide a window for synaptic plasticity.
SynGAP differs from other proteins at and near the PSD in that it moves away from the PSD core upon stimulation. This movement implies that it vacates its binding sites on PSD-95, allowing the core scaffold of the PSD to interact with and stabilize other components of the PSD. Movement of SynGAP would also promote its co-localization with CaMKII and thus facilitate phosphorylation of SynGAP and regulation of Ras activity. The structural observations of the current study explain how a protein with multiple functions, such as SynGAP, can segregate these functions in time and space at the synapse.
Highlights.
Characterize activity-induced changes in distribution of SynGAP by immunoEM
Depolarization induces movement of SynGAP away from the PSD core
PSD95, its binding partner, doesn’t move away from the PSD core upon depolarization
SynGAP may block integration of AMPARs into the PSD at rest
Shows how a multi-functional protein segregates its functions in time and space
ACKNOWLEDGEMENTS
We thank Christine A. Winters for hippocampal neuronal cultures and hippocampal slice cultures, Virginia Crocker and Rita Azzam for EM technical support. This research was supported by the Intramural Research Program of the NIH, NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Carlisle HJ, Manzerra P, Marcora E, Kennedy MB. SynGAP regulates steady-state and activity-dependent phosphorylation of cofilin. J Neurosci. 2008;50:13673–13683. doi: 10.1523/JNEUROSCI.4695-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, Reese TS. Organization of the core structure of the postsynaptic density. PNAS. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, Sheng M, Peng J. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Dosemeci A, Reese TS, Petersen J, Tao-Cheng JH. A novel particulate form of Ca(2+)/calmodulin-dependent [correction of Ca(2+)/CaMKII-dependent] protein kinase II in neurons. J Neurosci. 2000;20:3076–3084. doi: 10.1523/JNEUROSCI.20-09-03076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Tao-Cheng JH, Vinade L, Winters CA, Pozzo-Miller L, Reese TS. Glutamate-induced transient modification of the postsynaptic density. Proc Natl Acad Sci U S A. 2001;98:10428–10432. doi: 10.1073/pnas.181336998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Jaffe H. Regulation of phosphorylation at the postsynaptic density during different activity states of Ca2+/calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 2010;391:78–84. doi: 10.1016/j.bbrc.2009.10.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res. 1991;566:77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- Hu BR, Park M, Martone ME, Fischer WH, Ellisman MH, Zivin JA. Assembly of proteins to postsynaptic densities after transient cerebral ischemia. J Neurosci. 1998;18:625–633. doi: 10.1523/JNEUROSCI.18-02-00625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Liao D, Lau LF, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee HK, Takamiya K, Huganir RL. The role of synaptic GTPase-activating protein in neuronal development and synaptic plasticity. J Neurosci. 2003;23:1119–1124. doi: 10.1523/JNEUROSCI.23-04-01119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Komiyama NH, Watabe AM, Carlisle HJ, Porter K, Charlesworth P, Monti J, Strathdee DJ, O’Carroll CM, Martin SJ, Morris RG, O’Dell TJ, Grant SG. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci. 2002;22:9721–9732. doi: 10.1523/JNEUROSCI.22-22-09721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Okano A, Tian QB, Nakayama K, Furihata T, Nawa H, Suzuki T. Characterization of a novel SynGAP isoform, SynGAP-beta. J Biol Chem. 2001;276:21417–21424. doi: 10.1074/jbc.M010744200. [DOI] [PubMed] [Google Scholar]
- Lu Z, McLaren RS, Winters CA, Ralston E. Ribosome association contributes to restricting mRNAs to the cell body of hippocampal neurons. Mol Cell Neurosci. 1998;12:363–375. doi: 10.1006/mcne.1998.0723. [DOI] [PubMed] [Google Scholar]
- Oh JS, Manzerra P, Kennedy MB. Regulation of the neuron-specific Ras GTPase activating protein, synGAP, by Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:17980–17988. doi: 10.1074/jbc.M314109200. [DOI] [PubMed] [Google Scholar]
- Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–452. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Komiyama NH, Vitalis T, Kind PC, Grant SG. Differential expression of two NMDA receptor interacting proteins, PSD-95 and SynGAP during mouse development. Eur J Neurosc. 2005;21:351–362. doi: 10.1111/j.1460-9568.2005.03874.x. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Adams JP, Kim JH, Huganir RL. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci U S A. 2006;103:4344–4351. doi: 10.1073/pnas.0600084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008;60:788–802. doi: 10.1016/j.neuron.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Dosemeci A, Gallant PE, Miller S, Galbraith JA, Winters CA, Azzam R, Reese TS. Rapid turnover of spinules at synaptic terminals. Neuroscience. 2009;160:42–50. doi: 10.1016/j.neuroscience.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Dosemeci A, Gallant PE, Smith C, Reese T. Activity induced changes in the distribution of Shanks at hippocampal synapses. Neuroscience. 2010;168:11–17. doi: 10.1016/j.neuroscience.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Crocker VT, Winters CA, Azzam R, Chludzinski J, Reese TS. Trafficking of AMPA receptors at plasma membranes of hippocampal neurons. J Neurosci. 2011;31:4834–4843. doi: 10.1523/JNEUROSCI.4745-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez LE, Chen HJ, Sokolova I, Knuesel I, Kennedy MB. SynGAP regulates spine formation. J Neurosci. 2004;24:8862–8872. doi: 10.1523/JNEUROSCI.3213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]