Abstract
Permanent closure of the newborn ductus arteriosus requires the development of neointimal mounds to completely occlude its lumen. Vascular endothelial growth factor is required for neointimal mound formation. The size of the neointimal mounds (composed of proliferating endothelial and migrating smooth muscle cells) is directly related to the number of VLA4+ mononuclear cells that adhere to the ductus lumen after birth. We hypothesized that VEGF plays a crucial role in attracting CD14+/CD163+ mononuclear cells (expressing VLA4+) to the ductus lumen and that CD14+/CD163+ cell adhesion to the ductus lumen is important for neointimal growth. We used neutralizing antibodies against VEGF and VLA-4+ to determine their respective roles in remodeling the ductus of premature newborn baboons. Anti-VEGF treatment blocked CD14+/CD163+ cell adhesion to the ductus lumen and prevented neointimal growth. Anti-VLA-4 treatment blocked CD14+/CD163+ cell adhesion to the ductus lumen, decreased the expression of PDGF-B (which promotes smooth muscle migration), and blocked smooth muscle influx into the neointimal subendothelial space (despite the presence of increased VEGF in the ductus wall). We conclude that VEGF is necessary for CD14+/CD163+ cell adhesion to the ductus lumen and CD14+/CD163+ cell adhesion is essential for VEGF-induced expansion of the neointimal subendothelial zone.
Introduction
Closure of the full-term ductus arteriosus after birth occurs in two phases. First, smooth muscle constriction narrows the ductus’ lumen. Then, anatomic remodeling permanently occludes the lumen. In contrast with the full term ductus, the preterm ductus frequently fails to narrow its lumen or undergo anatomic remodeling. Remodeling of the preterm ductus will occur if it can be made to constrict tightly and obstruct luminal blood flow (1).
Several of the steps involved with ductus remodeling have been elucidated. The initial constriction creates a zone of ischemic hypoxia within the ductus’ muscle media that appears to be the required stimulus for the following anatomic changes: 1) formation of neointimal mounds (composed of proliferating luminal endothelial cells and migrating medial smooth muscle cells expanding the intima’s subendothelial space), 2) vasa vasorum proliferation within the muscle media, and 3) smooth muscle cell death (2). While the cell death and penetration of vasa vasorum into the ductus wall appear to be due to profound ATP depletion (3, 4) and vascular endothelial growth factor induction (5), respectively, the mechanism(s) responsible for the neointimal changes are still unknown.
In mice, platelets and platelet thrombi appear to be essential for permanent closure of the ductus lumen (6). In contrast, in human and non-human primates, recent studies suggest that platelets are not required for permanent ductus closure (7).
Inflammatory processes play a crucial role in the pathogenesis of several vascular disorders (8, 9). During atherogenesis, direct interactions between circulating monocytes and luminal endothelial cells increase the synthesis of factors like platelet derived growth factor (PDGF) and matrix metalloproteinase (MMP)-9, that facilitate the migration of smooth muscle cells into the expanding neointima (10, 11). We previously hypothesized that the attachment of circulating mononuclear cells to the ductus’ luminal endothelium may also be essential for ductus neointima formation (12). This hypothesis is supported by several observations: following ductus constriction, endothelial cells lining the lumen, increase their expression of vascular cell adhesion molecule-1 (VCAM-1), an important ligand for the mononuclear cell adhesion receptor, VLA-4 (also known as integrin α4β1 or CD49d) (12, 13); paralleling the increase in VCAM-1, VLA-4+ mononuclear cells increase their adherence to the ductus lumen (12); and, finally, the degree of VLA-4+ mononuclear cell adherence to the ductus lumen is associated with the extent of neointimal remodeling of the ductus (12).
The factors responsible for attracting circulating mononuclear cells to the ductus wall are still unknown. The hypoxia-inducible growth factor, VEGF appears to play an important role in ductus neointimal mound formation and vasa vasorum ingrowth (1, 5, 12). VEGF promotes the proliferation and migration of endothelial cells (14, 15). VEGF is also a direct chemoattractant for monocytes (16, 17)).
In the experiments described below, we used neutralizing antibodies against VEGF and VLA-4+ to determine their roles in mononuclear cell adhesion and ductus neointima formation in preterm baboons.
Methods
VLA-4 inhibition – in vivo
Animal experiments were approved (by the Institutional Animal Care and Use Committee) and performed at the Southwest National Primate Research Center in San Antonio, TX. Complete descriptions of the animal care and surgical procedures have been published elsewhere (5, 12). Briefly, timed pregnant baboon (Papio papio) dams were delivered by C-section at 125 + 1 days gestation (full term = 185 days); their fetuses were either (a) euthanized before breathing (Fetal Controls) or (b) mechanically ventilated for 6 days (Newborn groups) at which point the animals were euthanized. Preterm newborn baboons usually fail to constrict and obstruct their ductus luminal blood flow unless they are treated with inhibitors of both prostaglandin and nitric oxide production (1). Therefore, all preterm newborn baboons were treated with a combination of indomethacin (Indocin ®, 0.1 mg/kg/dose, given intravenously at 24, 48, 72, 84, 96, 108, 120 and 132 h after delivery) and N-nitro-L-arginine (a nitric oxide synthase inhibitor: 6 mg/kg/h as a continuous infusion, starting at 50 h after delivery and continuing until necropsy). Preterm newborn baboons were randomly assigned to one of three treatment groups: 1) Controls (no antibody treatment), 2) anti-VLA-4 treated newborns, and 3) anti-VEGF treated newborns. Because of the expense and need to restrict the use of this precious animal model, we were limited in the number of animals we could study. As a result, we were not able to have a Control group that received an isotype control antibody. The anti-VLA-4 newborns (group 2) received two intravenous doses (5 mg/kg) of HP1/2 (a humanized anti-VLA-4 monoclonal antibody (MAb) (Biogen Idec, Cambridge, MA) (18)) at 24 and 72 hr after delivery. Similar doses of HP1/2 have been shown to reduce mononuclear cell adhesion to arterial walls in mice (19), rabbits (20), pigs (21), and baboons (18). The anti-VEGF newborns (group 3) received a single intravenous dose (10 mg/kg) of a neutralizing monoclonal antibody made against VEGF (MAb A.4.6.1, Genentech, S. San Francisco, CA) (22, 23)) 24 hr after delivery (5). The animals in the anti-VEGF group (group 3) have been previously reported (5); however, the findings presented below were not included in the original report.
A complete echocardiographic examination including assessment of ductal patency was performed daily (1). At necropsy, the ductus was dissected in 4°C phosphate buffered saline solution and (a) embedded and frozen in Tissuetek (American Master Tech, Merced, CA) for immunohistochemistry (newborn baboon groups 1, 2, and 3), and/or (b) frozen in liquid nitrogen for RNA analysis (newborn baboon groups 1 and 2). Tissue was not available for RNA analysis from the group 3 animals.
Flow cytometry
To identify the cell populations most likely to be affected by the anti-VLA-4 antibody, HP1/2, we used multicolor direct immunofluorescence, as previously described (24, 25). The antibodies used were anti-human CD3 (clone SP34-2, BD Biosciences), CD8 (clone SK1, BD Biosciences), CD14 (clone 322A-1, Beckman-Coulter), CD31 (clone WM59, Biolegend), CD45 (clone DO58–1283, Biolegend), and CD49d (clone AF10, Biolegend).
Quantitative PCR (polymerase chain reaction)
Total RNA was isolated from each individual ductus (26). Gene expression was quantified using TaqMan Universal PCR master mix, Taqman probes, and ABI PRISM 7500 Sequence detection system as previously described (3, 26). The degree of expression of the gene of interest was determined using the relative gene expression method. Malate dehydrogenase (MDH) was used as an internal control to normalize the data (12, 27). ΔCT represents the difference in cycle threshold between the expression of the housekeeping gene, MDH, and the gene of interest. Each unit of ΔCT represents a 2-fold change in a gene’s mRNA. The more negative the ΔCT, the fewer the number of starting copies of a gene (mRNA).
We have previously shown that the expression of HIF1α mRNA in the ductus arteriosus correlates with the degree of smooth muscle hypoxia (3). In our real-time PCR experiments, we used HIF1α as a surrogate marker for hypoxia.
Immunohistochemistry
Immunohistochemistry protocols were similar to those reported previously (1, 12, 28). Endothelial cells were detected with anti-eNOS ({Clone 3} BD Biosciences, San Jose, CA) and anti-VE cadherin ({F-8} Santa Cruz Biotechnology, Santa Cruz, CA). Both antibodies showed identical findings. Monocytes/macrophages were detected with anti-CD163 ({GHI/61} Santa Cruz Biotechnology) or anti-CD14 ({TUKU} Dako, Carpenteria, CA). Both antibodies showed identical findings. Antibodies against the following antigens were used to identify T-lymphocytes (CD3 {rabbit polyclonal} Dako), T cells/NK cells (CD8 {UCH-T4} Santa Cruz Biotechnology), dendritic cells (CD83 {HB15a} Santa Cruz Biotechnology), and granulocytes (neutrophil elastase {NP57} Dako). Antibodies against GP1bα ({AN51} Dako) were used to identify platelets. Antibodies against VCAM-1 ({1G11} Santa Cruz Biotechnology) were used to identify adhesion molecules expressed by activated endothelial cells. The number of positively stained cells lining the ductus lumen was expressed per 100 luminal-lining cells.
Histologic measurements in the newborn ductus were made at the level of minimal luminal area, which was determined from 6µm serial cross-sections. The neointimal zone was defined as the region between the lumen and the internal elastic lamina (identified by phase contrast microscopy). The endothelial zone of the neointima was the luminal cell layer(s) that stained positive for eNOS (endothelial nitric oxide synthase) and VE-cadherin (vascular endothelial cell cadherin). The subendothelial zone was defined as the region between the luminal endothelial cells and the internal elastic lamina. Neointimal dimensions were determined by averaging measurements made from eight predetermined regions of the section, using an overlay template and NIH ImageJ software.
Mononuclear cells and vasa vasorum invade the ductus muscle media from the surrounding adventitia (see below). To determine the extent of muscle media invasion by mononuclear cells and vasa vasorum, we used an overlay template, containing a grid, and NIH ImageJ software to divide the muscle media into eight clock-hours sections. In each section of the wall, we measured the maximal distance that the cells migrated from the adventitia into the media (expressed as a percent of the distance from the adventitia to the internal elastic lamina in that section of the wall). The maximal distances from each of the eight sections of the ductus wall were averaged and reported as “% muscle media thickness” for that vessel.
Statistics
Results are presented as means ± standard deviations (SD) and percentages. Intergroup differences were evaluated with either a Chi-Square analysis, or unpaired t-test. When more than one comparison was made, Bonferroni’s correction was used.
Results
Combined treatment with indomethacin and N-nitro-L-arginine produced constriction of the ductus arteriosus and complete loss of Doppler-demonstrable luminal blood flow in all of the preterm newborn baboons. Neither the anti-VLA-4 MAb, nor the anti-VEGF MAb, affected the rate of ductus constriction measured by daily pulsed-Doppler exams (age of Doppler-demonstrable ductus closure: group 1 (Control newborns, n=18) = 79±31 hours; group 2 (anti-VLA-4, n=8) = 91±28 hours; group 3 (anti-VEGF, n=6) = 88±24 hours).
We used HIF1α mRNA expression as a surrogate marker for ductus hypoxia. There was a significant increase in HIF1α expression between fetus and newborns following postnatal closure (Table 1) (3), however, there was no difference in HIF1α expression between newborns in the Control group (group 1) and those treated with anti-VLA-4 MAb (group 2) (Table 1). Nor were there differences in the degree of hypoxia between the Control (group 1) and the anti-VEGF treated newborns (group 3) (previously reported in (5).
Table 1. Effects of ductus treatment on Real Time polymerase chain reaction (PCR) measurements of molecules involved with ductus arteriosus closure.
Fetal ductus obtained from 125 days gestation baboons (n=10 separate animals), were compared with ductus from Control premature newborns (n=10 separate animals) and from preterm newborns treated with HP1/2 (anti-VLA-4 MAb) (n=6 separate animals). ΔCT represents the difference in cycle threshold (CT) between the expression of the housekeeping gene Malate dehydrogenase (MDH) and the gene of interest. Each unit of ΔCT represents a 2-fold change in a gene’s mRNA. The more negative the ΔCT, the fewer the number of starting copies of a gene (mRNA).
| Fetus | Newborn Control |
Newborn Anti-VLA-4 |
||||
|---|---|---|---|---|---|---|
| ΔCT (MDH – gene) |
ΔCT (MDH – gene) |
ΔCT (MDH – gene) |
||||
| mean | SD | mean | SD | mean | SD | |
| CD14 | −5.01 | 0.37 | −3.20 * | 0.33 | −4.28 *† | 0.42 |
| PTGS1/COX-1 | −5.72 | 0.51 | −4.31 * | 0.64 | −5.41 † | 0.60 |
| PTGS2COX-2 | −6.96 | 0.88 | −5.26 * | 0.82 | −5.43 * | 0.85 |
| NOS3/eNOS | −6.07 | 0.59 | −4.35 * | 1.13 | −4.84 * | 0.25 |
| FLT1/FLT-1 | −2.99 | 0.33 | −2.28 * | 0.44 | −2.19 * | 0.40 |
| HIF1a/HIF1α | −3.72 | 0.23 | −1.46 * | 0.28 | −1.69 * | 0.39 |
| IFNG/IFNα | −6.21 | 1.10 | −2.37 * | 0.53 | −3.29 *† | 0.81 |
| IL6/IL-6 | −5.49 | 1.07 | −2.71 * | 0.88 | −2.97 * | 0.84 |
| IL8/IL-8 | −6.03 | 0.92 | −2.56 * | 0.51 | −2.93 * | 0.91 |
| CSF1/MCSF | −3.60 | 0.81 | −1.27 * | 0.74 | −1.43 * | 0.94 |
| MMP9/MMP-9 | −3.47 | 0.69 | −1.09 * | 0.50 | −2.05 *† | 0.53 |
| PDGF-B | −4.69 | 0.60 | −2.25 * | 0.35 | −2.96 *† | 0.50 |
| PTGIS | 1.45 | 0.79 | 0.39 * | 0.82 | 0.08 * | 0.97 |
| TGFB1/TGFβ1 | −5.74 | 0.43 | −3.87 * | 0.56 | −4.93 *† | 0.64 |
| TNFA/TNFα | −9.98 | 0.49 | −8.72 * | 0.71 | −9.22 | 1.18 |
| VCAM-1 | −4.93 | 0.64 | −2.48 * | 0.29 | −2.42 * | 0.34 |
| CDH5/VE-cadherin | −2.35 | 0.40 | −1.12 * | 0.12 | −1.57 * | 0.39 |
| VEGFA | −3.24 | 0.28 | −0.41 * | 0.78 | −0.84 * | 0.88 |
FLT, fms-related tyrosine kinase; IL, interleukin; MCSF, colony stimulating factor 1 (macrophage); PTGIS, prostacyclin synthase
p < 0.05 vs fetal ductus;
p < 0.05 vs newborn control ductus.
Distribution of VLA-4 (CD49d) on baboon leukocytes
To identify the cell populations most likely to be affected by the anti-VLA-4 antibody, HP1/2, we used flow cytometry to determine the distribution of expression of VLA-4 on umbilical cord blood leukocytes obtained from 4 fetal baboons. VLA-4 was expressed on the surface of 64±17% (mean ±SD) of baboon monocytes (defined as CD45+ CD14+ or CD45+ CD163+ cells), and 0±0% of baboon granulocytes (large cells that were CD45+ and CD14−CD163−). This distribution is similar to what we observed in human cord blood monocytes (80±18% expressed VLA-4) and granulocytes (0±0%). On the other hand, the percentage of baboon T-cells that expressed VLA-4 was significantly less than the percentage of human T-cells that expressed VLA-4: CD4+ T-cells (defined as CD45+ CD3+ CD14− CD8−) cells: baboon = 8±2%, human = 84±7% expressed VLA-4; CD8+ T-cells (defined as CD45+ CD3+ CD14− CD8+) cells: baboon =18±5%, human = 97±3% expressed VLA-4. Therefore, we anticipated that, in the baboon, the anti-VLA-4 MAb, HP1/2, would primarily affect CD14+/CD163+ cells (monocytes) from adhering to the luminal endothelium.
Anti-VLA-4 experiments
Following delivery of the Control newborns, there was an increase in expression of VCAM-1 (the ligand for the monocyte VLA-4 receptor (13)) on ductus luminal endothelial cells (Figure 1; Tables 1, 2), as well as an increase in the number of CD14+ and CD163+ cells (monocytes) (Figure 2; Table 2), CD3+ cells (data not shown), and CD8+ T-cells (Figure 2; Table 2) adhering to the ductus luminal endothelium. We found no evidence of platelets (GP1bα+ cells), neutrophils (neutrophil elastase+ cells), or dendritic (CD83+) cells adhering to the fetal or neonatal ductus lumen (data not shown).
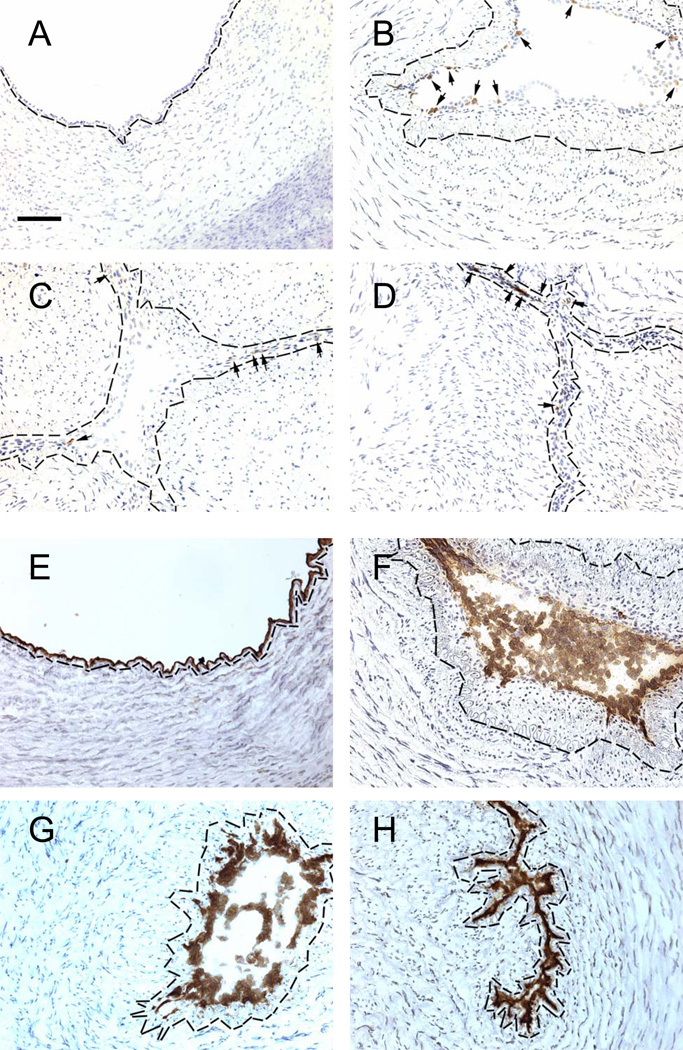
Figure 1.
VCAM-1 (A–D) and eNOS (E–H) expression in cells lining the ductus lumen. Endothelial cells were detected by eNOS expression. Ductus come from fetuses (A,E) and neonates exposed to Control conditions (B,F), anti-VLA-4 (C,G) or anti-VEGF (D,H) antibodies. Small arrows (A–D) indicate brown immunostained cells. Nuclei counterstained with hematoxylin (blue). Dashed black line = internal elastic lamina (identified by phase-contrast microscopy). Horizontal bar = 50 µm.
Table 2. Effect of ductus treatment on VCAM-1 expression and mononuclear cell adhesion to the ductus lumen (immunohistochemistry).
The number of endothelial cells lining the ductus lumen that are positive for VCAM-1, and the number of VLA-4, CD163, CD14, or CD8 positive mononuclear cells that adhere to the ductus luminal wall, are expressed per 100 lumen-lining cells.
| Positive expressing cells/100 luminal cells | ||||
|---|---|---|---|---|
| Fetus | Newborn Control |
Newborn Anti-VLA-4 |
Newborn Anti-VEGF |
|
| (N=7) | (N=14) | (N=7) | (N=6) | |
| VCAM-1 | 0±0 | 4±4* | 3±4* | 4±4* |
| VLA-4 | 0±0 | 4±2* | NA | 0±0† |
| CD163 | 0±0 | 7±4* | 2±2† | 0±1† |
| CD14 | 0±0 | 6±4* | 2±2† | 1±1† |
| CD8 | 0±0 | 7±5* | 4±3* | 4±3* |
p < 0.05 vs fetal ductus;
p < 0.05 vs newborn control ductus.
N, number of ductus examined. NA, tissue could not be examined because the monocyte VLA-4 receptors were already saturated with the HP1/2 anti-VLA-4 MAb.
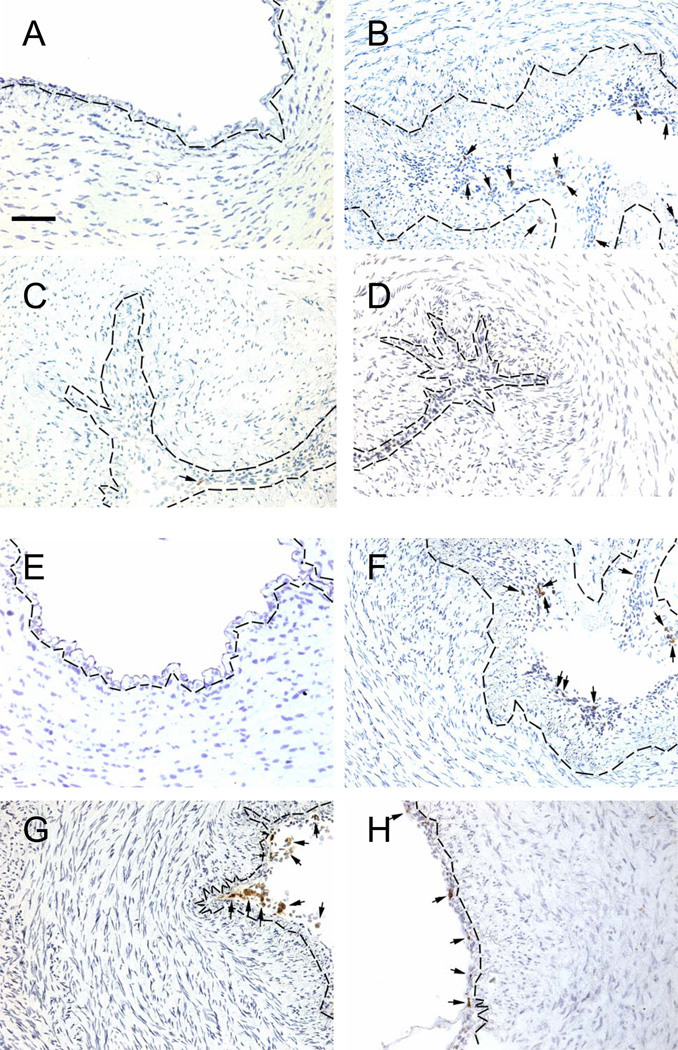
Figure 2.
Adherence of CD14+ (A–D) and CD8+ (E–H) cells to the ductus luminal surface. Small arrows indicate brown immunostained cells. See legend Figure 1.
Treatment with the anti-VLA-4 MAb did not alter the expression of VCAM-1 on the luminal endothelium (Figure 1; Tables 1, 2), but inhibited CD14+ cells and CD163+ cells (monocytes) (Figure 2; Tables 1, 2) from adhering to the ductus lumen. CD8+ T-cells did not appear to be affected by the anti-VLA-4 MAb treatment (Figure 2; Table 2).
Many of the genes that are essential for vascular remodeling are expressed by the ductus after postnatal constriction (Table 1) (12). Treatment with the anti-VLA-4 MAb decreased the expression of cyclooxygenase-1, IFNγ, MMP-9, PDGF-B chain, and TGFβ1 in the ductus wall (Table 1), which suggests that VLA4+/CD14+/CD163+ cell adhesion to the ductus lumen may be responsible, in part, for their expression.
The ductus of the Control newborn animals (group 1) developed the same anatomic changes that have previously been observed when postnatal constriction produces profound ductus wall hypoxia (1, 2, 5, 12). The Control newborn ductus developed a significantly thicker neointima than the fetal ductus (Figure 1, Table 3). The neointima was composed of an endothelial zone (filled with multiple layers of endothelial cells) and an expanding subendothelial zone (Figure 1, Table 3) (filled with non-endothelial cells that expressed α-smooth muscle actin (data not shown)).
Table 3. Effect of anti-VLA-4 and anti-VEGF treatment on ductus neointima and vasa vasorum remodeling (see Methods for definitions).
A) Luminal endothelial thickness: number of ductus in each group that have either a single or multiple (2, 3 or more) layers of endothelial cells that completely surround the ductus lumen (Chi-Square analysis). B) Neointimal (endothelial, subendothelial and total) thickness: Total thickness is the distance between the lumen and the internal elastic lamina. C) Subendothelial zone thickness > 20 µm: this represents the percentage of the luminal circumference that is surrounded by a thickened (> 20 µm) subendothelial zone. D) Vasa vasorum ingrowth: the distance that vasa vasorum migrate from the adventitia into the muscle media is expressed as the percentage of muscle media thickness.
| Fetus | Newborn Control |
Newborn Anti-VLA-4 |
Newborn Anti-VEGF |
|
|---|---|---|---|---|
| (N=7) | (N=14) | (N=7) | (N=6) | |
| A) Luminal endothelial thickness | * | * | * † | |
| 1 layer – n (%) | 7 (100) | 0 (0) | 0 (0) | 1 (17) |
| 2 layers – n (%) | 0 (0) | 2 (14) | 2 (30) | 4 (67) |
| ≥ 3 layers – n (%) | 0 (0) | 12 (86) | 5 (70) | 1 (17) |
| B) Neointimal thickness | ||||
| Endothelial zone - µm | 8±1 | 62±38 * | 53±25 * | 24±10 * † |
| Subendothelial zone - µm | 20±14 | 66±29 * | 27±12 † | 19±10 † |
| Total thickness - µm | 29±14 | 128±47 * | 81±30 † | 44±10 * † |
| C) Subendothelial zone > 20 µm | ||||
| % circumference of lumen | 18±7 | 60±23 * | 20±10 † | 15±7 † |
| D) Vasa vasorum ingrowth | ||||
| % muscle media thickness | 8±4 | 14±6 * | 14±6 * | 5±3 † |
p < 0.05 vs fetal ductus;
p < 0.05 vs newborn control ductus.
N, number of ductus examined.
Treatment with the anti-VLA-4 MAb inhibited neointimal expansion (Figure 1, Table 3). The anti-VLA-4 MAb had no effect on the postnatal increase in the number of endothelial cell layers but blocked the influx of non-endothelial cells into the subendothelial zone (Figure 1, Table 3).
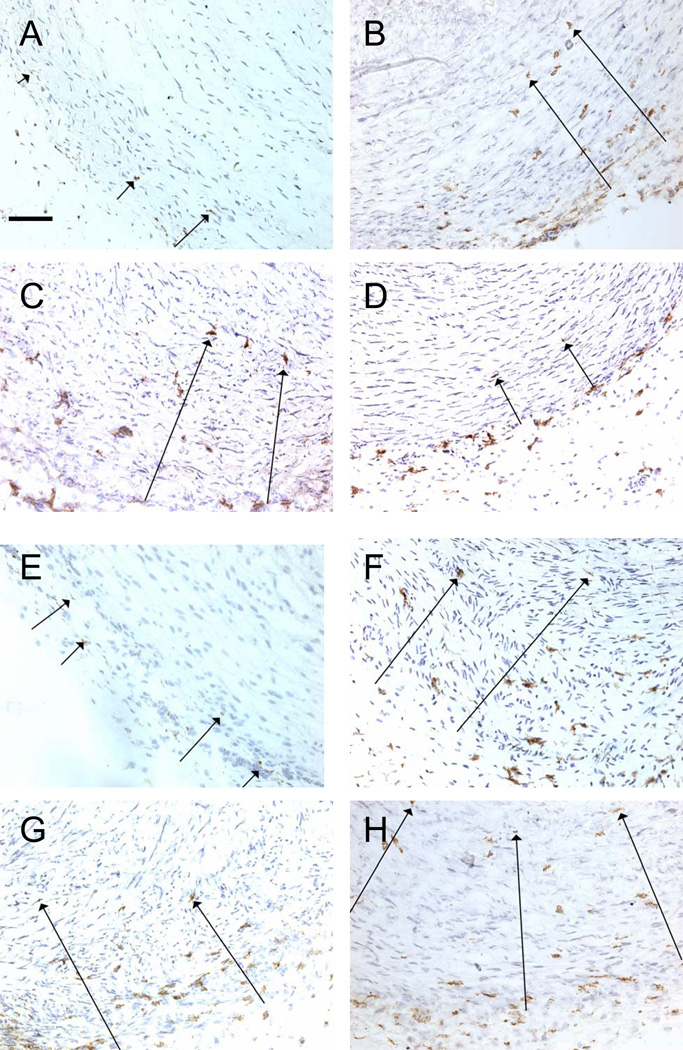
After birth, vasa vasorum invade the muscle media from the adventitia of the preterm ductus (1, 5, 12). Prior studies have shown that resident CD14+ and CD68+ mononuclear cells are present in the adventitia and outer muscle media of the fetal ductus (12). These mononuclear cells invade the muscle media after birth and the extent of their invasion correlates with the extent of vasa vasorum penetration into the muscle media (12). We found that treatment with the anti-VLA-4 MAb had no effect on either the postnatal invasion of CD14+, CD163+, or CD8+ cells (Figure 3, Table 4) or the penetration of vasa vasorum into the muscle media (Table 3).
Figure 3.
Invasion of CD14+ (A–D) and CD8+ (E–H) cells into the ductus muscle media. Long arrows indicate the furthest distance brown immunostained cells have migrated from the adventitia in this particular visual field. Horizontal bar = 50 µm.
Table 4.
Effect of ductus treatment on invasion of ductus muscle media by VLA-4+, CD163+, CD14+ and CD8+ cells (expressed as percentage of muscle media thickness containing positive-expressing cells (see Methods)).
| % muscle media thickness | ||||
|---|---|---|---|---|
| Fetus | Newborn Control |
Newborn Anti-VLA-4 |
Newborn Anti-VEGF |
|
| (N=7) | (N=14) | (N=7) | (N=6) | |
| VLA-4 | 1±3 | 15±6* | NA | 3±5† |
| CD163 | 2±3 | 18±6* | 20±7* | 3±6† |
| CD14 | 1±3 | 16±6* | 18±6* | 2±5† |
| CD8 | 8±5 | 23±11* | 18±9* | 19±11* |
p < 0.05 vs fetal ductus;
p < 0.05 vs newborn control ductus.
N, number of ductus examined. NA, tissue could not be examined because the monocyte/macrophage VLA-4 receptors were already saturated with the HP1/2 anti-VLA-4 MAb.
Anti-VEGF experiments
Treatment with the neutralizing anti-VEGF MAb did not alter the expression of VCAM-1 on the luminal endothelium (Figure 1, Table 2), but inhibited CD14+ and CD163+ cells (Figure 2; Table 2) from adhering to the ductus lumen. CD8+ T-cells did not appear to be affected by the anti-VEGF MAb treatment (Figure 2; Table 2).
Treatment with the anti-VEGF MAb decreased the postnatal neointimal expansion by reducing the number of endothelial cell layers and by completely blocking the influx of non-endothelial cells into the subendothelial zone of the neointima (Figure 1, Table 3).
The anti-VEGF MAb also blocked VLA-4+, CD14+ and CD163+ mononuclear cells from migrating into the outer muscle media (Figure 3, Table 4) and inhibited vasa vasorum penetration into the muscle media (Table 3) (5). The anti-VEGF MAb did not appear to affect migration of CD8+ cells into the outer muscle media (Figure 3, Table 4).
Discussion
The mechanisms responsible for leukocyte attachment, during ductus arteriosus remodeling, differ significantly from those involved in other “injury-induced” models of vascular remodeling. In most vascular injury models, leukocyte recruitment depends on the induced expression on the endothelial cell surface of P-selectin, E-selectin, ICAM-1, and VCAM-1 (29–31). Leukocyte interactions with both P-selectin and ICAM-1 appear to be necessary for recruitment to occur when physiologic shear forces are present (30, 32). Interactions between VCAM-1 and VLA-4 (the integrin receptor for VCAM-1) are capable of mediating leukocyte rolling and adhesion, without the need for selectins or β2 integrin/ICAM-1 interactions, but only when very low flow conditions exist (33–35).
During ductus closure, the ductus luminal endothelial cells have a very limited pattern of adhesion molecule expression (E-selectin and VCAM-1 are the only ones expressed) (12). As a result, leukocytes fail to attach in the presence of physiologic shear forces (12). VLA-4+ leukocytes can adhere to the ductus wall, but only after tight constriction and loss of luminal flow have occurred (12).
We found that VLA-4 was most frequently expressed by baboon CD14+ and CD163+ cells (monocytes), less frequently by T-lymphocytes, and not at all by neutrophils. These findings help to explain why neutrophils, which normally contribute to most inflammatory conditions, are notably absent during ductus closure (see Results and (12)). They also explain why the anti-VLA-4 MAb primarily blocked CD14+ and CD163+ mononuclear cells from attaching to the ductus luminal endothelium (Figure 2, Tables 1, 2). The VLA-4 MAb also appeared to have little effect on the postnatal migration of CD14+, CD163+, or CD8+ cells from the adventitia into the muscle media (Figure 3, Table 4).
The present experiments demonstrate that after birth, there is increased synthesis of PDGFB chain, MMP-9, COX-1, TGFβ, and IFNγ (Table 1) as well as smooth muscle migration into the neointima of the tightly constricted ductus arteriosus (Figure 1, Table 3). The anti–VLA-4 MAb inhibited these events from occurring (Figure 1, Tables 1,3). VLA4+/CD14+/CD163+ monocytes are the likely candidates for promoting smooth muscle cell migration into the neointima. They have the capacity to secrete proteolytic enzymes (e.g., MMP-9 (Table 1)), which are capable of degrading the extracellular matrix surrounding smooth muscle cells (9), and chemotactic substances for smooth muscle cell migration (e.g., PDGF (Table 1) (36)). We hypothesize that during ductus closure, monocyte-stimulated PDGF-B production may be one of the mediators of smooth muscle cell migration, as it is in other experimental models of neointimal thickening (37).
It is possible that the anti–VLA-4 MAb could have effects on cells other than monocytes that would affect ductus arteriosus cells and gene expression; however, it is unlikely that the anti–VLA-4 MAb exerted a direct suppressive effect on smooth muscle cell migration because VLA-4 is not detected on ductus smooth muscle cells (12).
Although the expansion of the subendothelial zone of the neointima appears to depend on VLA4+/CD14+/CD163+ monocytes adhering to the ductus lumen, the proliferation and layering of endothelial cells within the neointima appear to be independent of VLA4+/CD14+/CD163+ cell attachment (Figure 1, Table 3). Other mechanisms (e.g., VEGF) must be responsible for regulating ductus endothelial proliferation. Although VEGF is essential for luminal endothelial cell layering (Figure 1, Table 3) (5), additional factors must contribute to the luminal endothelial cell proliferation since ductus treated with the anti-VEGF MAb still had a thicker luminal endothelial zone than fetal ductus (Table 3).
In addition to its effects on luminal endothelial cell proliferation, VEGF plays an essential role in smooth muscle cell expansion of the ductus neointima and vasa vasorum ingrowth (5). Our current studies demonstrate that VEGF is necessary for the adherence of VLA4+/CD14+/CD163+ cells (monocytes) to the ductus lumen (Figure 2, Table 2). The VLA-4 dependent, CD14+/CD163+ cell adhesion to the ductus lumen appears to be essential for the VEGF-induced subendothelial expansion of the neointima; when VLA4+/CD14+/CD163+ cells are prevented from adhering to the ductus lumen by the MAb against VEGF, smooth muscle cell expansion of the neointima fails to occur (Figure 1, Table 3) despite the presence of increased VEGF in the ductus wall (Table 1).
It is also intriguing to speculate that CD14+/CD163+ cell migration may play a significant role in the VEGF-induced vasa vasorum ingrowth, since vasa vasorum ingrowth was only observed in association with CD14+ and CD163+ cell (macrophage) invasion (Tables 3 and 4).
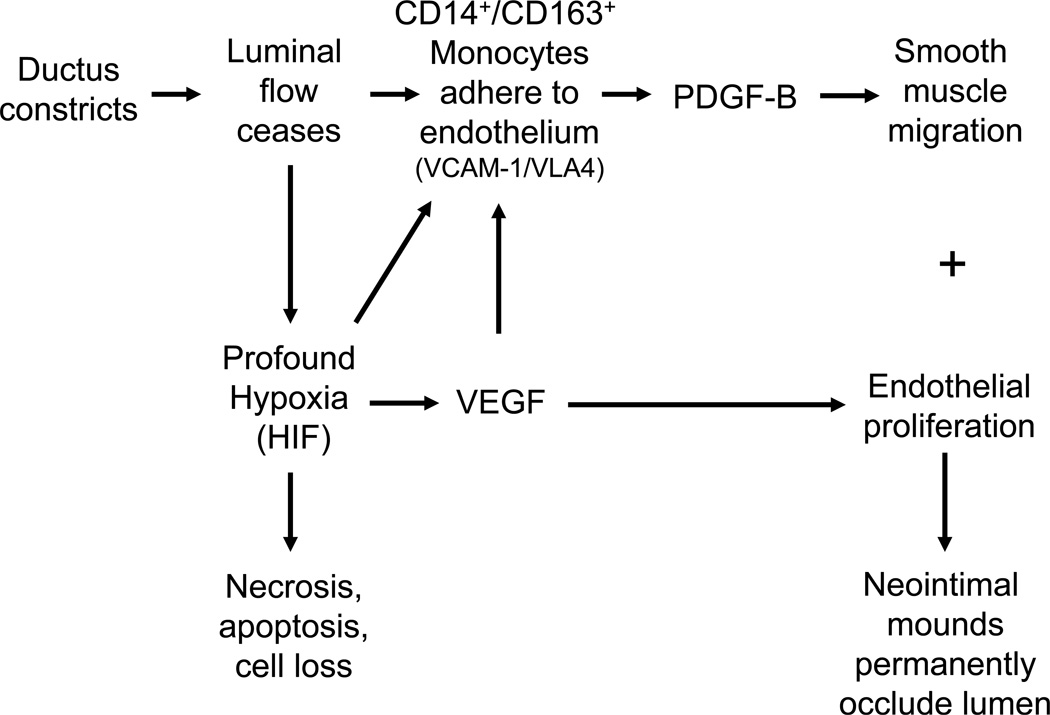
Our current findings are consistent with the hypothetical model depicted in Figure 4. If VEGF and VLA4+/CD14+/CD163+ monocyte recruitment are central to ductus remodeling, then the current studies help to explain why tight ductus constriction and loss of luminal flow are essential for remodeling and permanent closure after birth (1, 2). Decreased luminal flow has a direct effect on VEGF and VCAM-1 expression (38). Decreased flow also enables the VLA4+/CD14+/CD163+ monocytes to adhere to the ductus wall through VCAM-1/VLA-4 interactions. Once the VLA4+/CD14+/CD163+ monocytes adhere to the ductus wall, the subsequent neointimal expansion helps to permanently plug the residual lumen.
Figure 4.
Hypothetical model of critical events in ductus arteriosus anatomic remodeling.
Acknowledgments
We would like to thank Dr. Napoleone Ferrara (Genentech) for providing us with MAb A.4.6.1. We also thank Ms. Vickie Winter, Dr. Jackie Coalson and all the personnel at the BPD Resource Centre and the University of Texas Health Science Center San Antonio pathology division.
Financial Support: This work was supported by grants from U.S. Public Health Service (NIH grants HL46691, HL56061, HL52636 BPD Resource Center and P51RR13986 (NCRR) and by gift from the Jamie and Bobby Gates Foundation. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant number C06 RR12087 (NCRR).
Abbreviations
- COX
cyclooxygenase
- ΔCT
the difference in cycle threshold between the expression of the housekeeping gene MDH and the gene of interest
- eNOS
endothelial nitric oxide synthase
- HP1/2
monoclonal antibody against VLA-4
- MDH
Malate dehydrogenase
- MMP-9
matrix metalloproteinase-9
- VCAM-1
vascular cell adhesion molecule-1
- VE
vascular endothelial cell
- VLA-4 or CD49d
α4β1 integrin mononuclear cell adhesion receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Seidner SR, Chen Y-Q, Oprysko PR, Mauray F, Tse MM, Lin E, Koch C, Clyman RI. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res. 2001;50:365–373. doi: 10.1203/00006450-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Clyman RI, Chan CY, Mauray F, Chen YQ, Cox W, Seidner SR, Lord EM, Weiss H, Wale N, Evan SM, Koch CJ. Permanent anatomic closure of the ductus arteriosus in newborn baboons: the roles of postnatal constriction, hypoxia, and gestation. Pediatr Res. 1999;45:19–29. doi: 10.1203/00006450-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Levin M, McCurnin D, Seidner SR, Yoder B, Waleh N, Goldbarg S, Roman C, Liu BM, Boren J, Clyman RI. Postnatal constriction, ATP depletion, and cell death in the mature and immature ductus arteriosus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R359–R364. doi: 10.1152/ajpregu.00629.2005. [DOI] [PubMed] [Google Scholar]
- 4.Goldbarg S, Quinn T, Waleh N, Roman C, Liu BM, Mauray F, Clyman RI. Effects of hypoxia, hypoglycemia, and muscle shortening on cell death in the sheep ductus arteriosus. Pediatr Res. 2003;54:204–211. doi: 10.1203/01.PDR.0000072519.61060.E5. [DOI] [PubMed] [Google Scholar]
- 5.Clyman RI, Seidner SR, Kajino H, Roman C, Koch CJ, Ferrara N, Waleh N, Mauray F, Chen YQ, Perkett EA, Quinn T. VEGF regulates remodeling during permanent anatomic closure of the ductus arteriosus. Am J Physiol Regul Integr Comp Physiol. 2002;282:R199–R206. doi: 10.1152/ajpregu.00298.2001. [DOI] [PubMed] [Google Scholar]
- 6.Echtler K, Stark K, Lorenz M, Kerstan S, Walch A, Jennen L, Rudelius M, Seidl S, Kremmer E, Emambokus NR, von Bruehl ML, Frampton J, Isermann B, Genzel-Boroviczeny O, Schreiber C, Mehilli J, Kastrati A, Schwaiger M, Shivdasani RA, Massberg S. Platelets contribute to postnatal occlusion of the ductus arteriosus. Nat Med. 2010;16:75–82. doi: 10.1038/nm.2060. [DOI] [PubMed] [Google Scholar]
- 7.Shah NA, Hills NK, Waleh N, McCurnin D, Seidner S, Chemtob S, Clyman R. Relationship between circulating platelet counts and ductus arteriosus patency after indomethacin treatment. J Pediatr. 2011;158:919.e2–923.e2. doi: 10.1016/j.jpeds.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kevil CG, Bullard DC. Roles of leukocyte/endothelial cell adhesion molecules in the pathogenesis of vasculitis. Am J Med. 1999;106:677–687. doi: 10.1016/s0002-9343(99)00132-1. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 10.Funayama H, Ikeda U, Takahashi M, Sakata Y, Kitagawa S, Takahashi Y, Masuyama J, Furukawa Y, Miura Y, Kano S, Matsuda M, Shimada K. Human monocyte-endothelial cell interaction induces platelet-derived growth factor expression. Cardiovasc Res. 1998;37:216–224. doi: 10.1016/s0008-6363(97)00224-1. [DOI] [PubMed] [Google Scholar]
- 11.Abedi H, Zachary I. Signalling mechanisms in the regulation of vascular cell migration. Cardiovasc Res. 1995;30:544–556. [PubMed] [Google Scholar]
- 12.Waleh N, Seidner S, McCurnin D, Yoder B, Liu BM, Roman C, Mauray F, Clyman RI. The role of monocyte-derived cells and inflammation in baboon ductus arteriosus remodeling. Pediatr Res. 2005;57:254–262. doi: 10.1203/01.PDR.0000148278.64777.EF. [DOI] [PubMed] [Google Scholar]
- 13.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 14.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 15.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 16.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 17.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lumsden AB, Chen C, Hughes JD, Kelly AB, Hanson SR, Harker LA. Anti-VLA-4 antibody reduces intimal hyperplasia in the endarterectomized carotid artery in nonhuman primates. J Vasc Surg. 1997;26:87–93. doi: 10.1016/s0741-5214(97)70151-4. [DOI] [PubMed] [Google Scholar]
- 19.Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- 20.Kling D, Fingerle J, Harlan JM, Lobb RR, Lang F. Mononuclear leukocytes invade rabbit arterial intima during thickening formation via CD18-and VLA-4-dependent mechanisms and stimulate smooth muscle migration. Circ Res. 1995;77:1121–1128. doi: 10.1161/01.res.77.6.1121. [DOI] [PubMed] [Google Scholar]
- 21.Labinaz M, Hoffert C, Pels K, Aggarwal S, Pepinsky RB, Leone D, Koteliansky V, Lobb RR, O'Brien ER. Infusion of an antialpha4 integrin antibody is associated with less neoadventitial formation after balloon injury of porcine coronary arteries. Can J Cardiol. 2000;16:187–196. [PubMed] [Google Scholar]
- 22.Borgström P, Bourdon MA, Hillan KJ, Sriramarao P, Ferrara N. Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumors in vivo. Prostate. 1998;35:1–10. doi: 10.1002/(sici)1097-0045(19980401)35:1<1::aid-pros1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 24.Hodara VL, Velasquillo MC, Parodi LM, Giavedoni LD. Expression of CD154 by a simian immunodeficiency virus vector induces only transitory changes in rhesus macaques. J Virol. 2005;79:4679–4690. doi: 10.1128/JVI.79.8.4679-4690.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giavedoni LD, Schlabritz-Loutsevitch N, Hodara VL, Parodi LM, Hubbard GB, Dudley DJ, McDonald TJ, Nathanielsz PW. Phenotypic changes associated with advancing gestation in maternal and fetal baboon lymphocytes. J Reprod Immunol. 2004;64:121–132. doi: 10.1016/j.jri.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Bouayad A, Kajino H, Waleh N, Fouron JC, Andelfinger G, Varma DR, Skoll A, Vazquez A, Gobeil FJ, Clyman RI, Chemtob S. Characterization of PGE2 receptors in fetal and newborn lamb ductus arteriosus. Am J Physiol Heart Circ Physiol. 2001;280:H2342–H2349. doi: 10.1152/ajpheart.2001.280.5.H2342. [DOI] [PubMed] [Google Scholar]
- 27.Waleh N, Kajino H, Marrache AM, Ginzinger D, Roman C, Seidner SR, Moss TJ, Fouron JC, Vazquez-Tello A, Chemtob S, Clyman RI. Prostaglandin E2--mediated relaxation of the ductus arteriosus: effects of gestational age on g protein-coupled receptor expression, signaling, and vasomotor control. Circulation. 2004;110:2326–2332. doi: 10.1161/01.CIR.0000145159.16637.5D. [DOI] [PubMed] [Google Scholar]
- 28.Clyman RI, Goetzman BW, Chen YQ, Mauray F, Kramer RH, Pytela R, Schnapp LM. Changes in endothelial cell and smooth muscle cell integrin expression during closure of the ductus arteriosus: an immunohistochemical comparison of the fetal, preterm newborn, and full-term newborn rhesus monkey ductus. Pediatr Res. 1996;40:198–208. doi: 10.1203/00006450-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171:223–229. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 30.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 33.Johnston B, Issekutz TB, Kubes P. The alpha 4-integrin supports leukocyte rolling and adhesion in chronically inflamed postcapillary venules in vivo. J Exp Med. 1996;183:1995–2006. doi: 10.1084/jem.183.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaboury JP, Kubes P. Reductions in physiologic shear rates lead to CD11/CD18-dependent, selectin-independent leukocyte rolling in vivo. Blood. 1994;83:345–350. [PubMed] [Google Scholar]
- 35.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinet Y, Bitterman PB, Mornex JF, Grotendorst GR, Martin GR, Crystal RG. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986;319:158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- 37.Jackson CL, Raines EW, Ross R, Reidy MA. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993;13:1218–1226. doi: 10.1161/01.atv.13.8.1218. [DOI] [PubMed] [Google Scholar]
- 38.Walpola PL, Gotlieb AI, Cybulsky MI, Langille BL. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol. 1995;15:2–10. doi: 10.1161/01.atv.15.1.2. [DOI] [PubMed] [Google Scholar]