Abstract
Experimentally naïve rats show variance in their locomotor reactivity to novelty, some displaying higher (HR) while others displaying lower (LR) reactivity, associated with vulnerability to stress. We employed a chronic variable physical stress regimen incorporating intermittent and random exposures of physical stressors or control handling during the peripubertal-juvenile period to assess interactions between stress and the LRHR phenotype in depressive- and anxiety-like behaviors on the forced swim and social interaction tests respectively. A decrease in immobility in the forced swim test along with a decrease in social contact in the social interaction test were observed in the juvenile HRs, coupled with increases in brain-derived neurotrophic factor (BDNF) mRNA in the hippocampus and in the basolateral amygdala with chronic variable physical stress. In contrast, an increase in immobility in the forced swim test and a decrease in social contact was observed in the LR counterparts coupled with an increase in the BDNF mRNA in the basolateral amygdala following chronic variable physical stress. Furthermore, chronic physical stress led to increased H3 and H4 acetylation at the P2 and P4 promoters of the hippocampal BDNF gene in the HR rats that is associated with increased suprapyramidal mossy fibre (SP-MF) terminal field volume. In contrast, chronic variable physical stress led to decreased H4 acetylation at the P4 promoter, associated with decreased SP-MF volume in the LR rats. These findings show dissociation in depressive- and anxiety-like behaviors following chronic variable physical stress in the juvenile HR animals that may be mediated by increased levels of BDNF in the hippocampus and in the amygdala respectively. Moreover, chronic variable physical stress during the peripubertal-juvenile period results in opposite effects in depressive-like behavior in the LRHR rats by way of inducing differential epigenetic regulation of the hippocampal BDNF gene that, in turn, may mediate mossy fibre sprouting.
Keywords: forced swim test, social interaction, chromatin plasticity, acetylated histone 3, acetylated histone 4, suprapyramidal mossy fibres
To study normally-occurring individual differences in responsiveness to stress, an outbred rat model of novelty-seeking phenotype was introduced (Piazza et al., 1989), where experimentally naïve rats were screened based on degrees of locomotor reactivity in a novel environment. In such settings, some rats display high rates of locomotor reactivity to novelty (high responders; HR), and others display low rates (low responders; LR). HR rats repeatedly choose novel environments over familiar ones (Piazza et al., 1989), appear less anxious in the light–dark box and the elevated plus maze (Kabbaj et al., 2000), and demonstrate prolonged stress-induced secretion of corticosterone when compared to LR rats (Piazza and LeMoal, 1996), suggesting a hyperactive hypothalamic pituitary adrenal axis (HPA). In further support of this, HR rats express lower levels of basal corticotropin releasing hormone (CRH) mRNA in the central nucleus of the amygdala and show higher levels of CRH mRNA in the paraventricular nucleus of the hypothalamus compared to LR counterparts (Kabbaj et al., 2000). In the hippocampus, HR rats also show glucocorticoid receptor (GR) deficit compared to LRs, and a phenotypic switch is induced by hippocampal microinjection of a GR antagonist (Kabbaj et al., 2000). These findings collectively provide a compelling evidence for the validity of the LRHR phenotype in studying molecular and neural mechanisms underlying the development of stress responsiveness, and to identify critical developmental periods where phenotype interactions with stressful environmental events may induce or mask behavioral vulnerability.
We have previously described a chronic variable physical stress regimen when applied during the peripubertal-juvenile period (postnatal days 28–56), led to alterations in the physiological response to stress, hippocampal GR expression and morphology of the hippocampus (Isgor et al., 2004a). We showed that hippocampal CA1, CA3 field and the dentate gyrus (DG) volumes continued to increase as a factor of normal maturation from the peripubertal-juvenile period into young adulthood. However chronic variable physical stress exposure led to inhibition of volume growth in all subfields of the hippocampus where the growth came to a complete arrest in the CA3 field. Moreover, with transition into young adulthood (3 wks of recovery from stress), persistent effects were observed as impairments in the Morris water-maze navigation, downregulation in the hippocampal GR expression, and deficits in the shutdown of acute stress-induced corticosterone secretion following the peripubertal-juvenile chronic variable physical stress exposure. These findings implicated this developmental period as a critical period for hippocampal maturation, where a nonhabituating and variable chronic physical stress regimen could alter various hippocampal functions including learning and memory and the regulation of the stress response. Moreover, a previous report showed that adolescent rats displayed higher magnitude of novelty-seeking behavior compared to young adults in the form of moving faster and traveling farther in a novel environment, with approximately 16–18% of adolescents exhibiting activity above the highest activity manifested by young adults (Philpot and Wecker, 2008). Interestingly these authors accounted the difference in activity levels between adolescent and young adult rats to a greater percentage of the HR phenotype within the adolescent population, which validates studying the novelty-seeking phenotype within this critical period.
In the present study, we will behaviorally characterize the LRHR phenotype following the peripubertal-juvenile exposure of the chronic physical stress or control handling in depressive-like behavior measured on the forced swim test and in social anxiety-like behavior measured on the social interaction test. Following this, we will study interactions between the LRHR phenotype and stress condition in hippocampal BDNF gene regulation, and subsequent morphological changes in the mossy fibre terminal fields that may be associated with the depressive-like behavior. Furthermore, we will evaluate BDNF mRNA levels in the basolateral amygdala (BLA) as a substrate for anxiety-like behavior. Traditionally, chronic stress in adult animals is reported to decrease the expression of BDNF in the hippocampus (Smith et al., 1995), and to increase the expression of BDNF in the amygdala in a sustained manner (Fanous et al., 2010). BDNF is critical for providing neurotrophic support for plasticity in the mossy fibre-CA3 synapse (Gómez-Palacio-Schjetnan and Escobar, 2008); hence stress-induced changes in the hippocampal BDNF levels may be critical for hippocampal synaptic reorganization. Particularly, we will investigate whether chronic physical stress during the peripubertal-juvenile period will induce differential chromatin plasticity at the hippocampal BDNF gene in the LRHR rats. Namely, differential acetylation of histones at the promoter regions of the hippocampal BDNF gene will be assessed following the stress exposure or control handling in the LRHR rats, and such effects will be followed by morphological assessments of the mossy fibre terminal fields.
EXPERIMENTAL PROCEDURES
LRHR phenotype screening
Animals were treated in accordance with the National Institutes of Health guidelines on laboratory animal use and care. Male Sprague-Dawley rats (N=36, Charles River, Wilmington, MA) arrived at weaning (postnatal day, PN 22), were housed in 43 × 21.5 × 25 cm clear acrylic cages, and were allowed ad libitum access to food and water. Animals were kept on a 12 hr light/dark cycle (lights on at 7:00 A.M.). Animals were allowed to habituate to the housing conditions and were handled daily for 2 days. On PN 25, animals underwent locomotor screening for 60 min in commercially-available locomotion chambers (San Diego Instruments, San Diego, CA). Locomotor reactivity to novelty was tested in 43 × 43 × 24.5 cm clear Plexiglas cages with stainless steel grid flooring. Activity was monitored by means of two banks of photocells (total of photocells X=16; Y=16) connected to a microprocessor. Two frames of photocells were used; the lower frame was located 2.5 cm above the grid floor and the upper frame was located 12.5 cm above the floor of the box. Horizontal locomotion was monitored by the lower bank of photocells. Each horizontal activity count recorded a minimum 14.3 cm traversing of the cage. Rearing was monitored by activity on the upper bank of photocells. At the end of a 60 min screening session, total locomotor activity (i.e., X, Y and Z locomotion) were pooled and the rats were ranked as HRs (i.e., rats that exhibited locomotor scores in the highest third of the sample, n=12) or LRs (i.e., rats that exhibited locomotor scores in the lowest third of the sample, n=12). Intermediary responders (middle 1/3rd, n=12) are eliminated from further testing.
Chronic variable physical stress
Table 1 summarizes the order and time of stressors employed in the chronic variable physical stress paradigm. Three stressors that are widely used in the literature and were previously utilized in published work (Isgor et al., 2004a; Kabbaj and Isgor, 2007) were selected. Stressors were applied in a systematic random order and at varying times of day for a total of 14 stress exposures to avoid habituation. Control animals were handled daily. Chronic stress or control handling occurred between PN 28–41. 1) Restraint: Animals were wrapped individually in flexible Teflon, which was secured with Velcro closures to limit movement for 2-hr. 2) Cold exposure: Animals were transferred to a cold box at 4°C for 2-hr in their home cages. 3) Ether exposure: Animals were transferred to an ether chamber located outside of the home colony in groups of four, for a maximum of 30 s. After complete recovery from ether, animals were returned to the home colony. Twenty-four hours after the last stress exposure or control handling (PN 42), all animals were tested on the social interaction and forced swim tests in a counterbalanced fashion.
Table 1.
Stressors were applied at systematic and random fashion for 14 days during the CVP regimen (PN 28–41). Control animals recieved handling daily during this time.
| Stressor | Day | Time |
|---|---|---|
| Cold exposure | 1 | 11:00am-1:00pm |
| Ether exposure | 2 | 12:30pm-2:30pm |
| Restraint | 3 | 3:00pm-5:00pm |
| Ether exposure | 4 | 12:00pm-2:00pm |
| Cold exposure | 5 | 8:00am-10:00am |
| Ether exposure | 6 | 2:00pm-4:00pm |
| Restraint | 7 | 1:00pm-3:00pm |
| Ether exposure | 8 | 10:00am-12:00pm |
| Cold exposure | 9 | 2:30pm-4:30pm |
| Restraint | 10 | 10:30am-12:30pm |
| Cold exposure | 11 | 9:00am-11:00am |
| Restraint | 12 | 12:00pm-2:00pm |
| Ether exposure | 13 | 11:30am-1:30pm |
| Cold exposure | 14 | 8:00am-10:00am |
Social interaction
Rats were placed individually in a 23 × 15 × 13 cm rubber rectangular box and allowed to habituate for 8 min. After the initial 8 min, an age- and weight-matched conspecific was placed in the box and both rats were allowed to interact for 5 min. Conspecifics were used only once and, between each trial, the box was wiped thoroughly with ethyl alcohol. Social behaviors elicited by the experimental rat towards the conspecific were measured (i.e., sniffing, following, grooming and crawling over or under) as described in File (1980) and published by our lab (Aydin et al., 2010, 2011). No aggressive behaviors were observed. Total amount of social interaction per animal was measured by adding the duration of all of the social interaction behaviors demonstrated by the experimental animal, and percent time spent in social interaction was calculated.
Forced swim
This test was performed according to a published protocol (Porsolt et al., 1977) with some modifications. The pretest (15 min) was conducted on the afternoon of the last day of stress exposure. The following morning, animals were tested on the forced swim test for 5 min. Briefly, rats were placed in a 30 cm diameter cylinder filled with water (25°C). Each rat was tested individually and the cylinder was cleaned and filled with fresh water following each animal. Behavior constituting as immobile (lack of movement except necessary movements to keep the head above water) was scored. Total amount of immobilization per animal was measured by adding the duration of immobility demonstrated by the experimental animal in a session and percent time spent in immobility was calculated.
At the completion of behavioral testing, animals were sacrificed by rapid decapitation. One hemisphere of each brain was harvested and snap frozen for in situ hybridization histochemistry, and Timm’s method for silver sulfide staining (n=6 per experimental group). The dorsal hippocampus from the remaining hemisphere was hand dissected and used for the chromatin immunoprecipitation assay (n=6 per experimental group).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed following a published protocol (Tsankova et al., 2004). Briefly, tissue punches from dorsal hippocampi were pooled and fixed to cross-link DNA with associated proteins. Fixed hippocampal tissue was sonicated twice for 10 sec at low setting and then sonicated three more times for 15 sec at maximum power to shear the chromatin to 400–500 bp using a cell lysis buffer (1% SDS (10%), 1 mM Tris-HCl (pH 8.0) and 100 mM 0.5 M EDTA). After determination of the optical densities, equal amounts of chromatin lysate (60 μg) were diluted with ChIP dilution buffer to a final volume of 1 ml. Samples were then immunoprecipitated overnight at 4°C with the following antibodies: 5 μg of anti-acetyl H3 antibody (acetylated on Lys9 and Lys14 Millipore, CA), 5 μg of anti-acetyl H4 antibody (acetylated at Lys5, Lys8, Lys12, and Lys16 Millipore, CA) and 5 μg nonimmune rabbit IgG antibody (Millipore, CA) used as control. The immunoprecipitant was collected using Protein A beads from Thermo Scientific (Rockford, IL). The beads were washed once with low salt, high salt, and LiCl and washed twice with TE buffers. After washing, the DNA–histone complexes were eluted from the beads and then reverse cross-linked using proteinase K. DNA was extracted, purified and quantified using quantitative real time-PCR.
Quantitative real time-PCR (qPCR)
qPCR was performed to determine the levels of acetylated H3 (acH3) and H4 (acH4) at the BDNF gene promoters P2 and P4 as described in previously published work (Tsankova et al., 2004). Specifically, experimental primers were custom designed as the following: BDNF P2: 5′-TGAGGATAGGGGTGGAGTTG-3′, 5′-GCAGCAGGAGGAAAAGGTTA-3′; for BDNF P4: 5′-TGCAGGGGAATTAGGGATAC-3′, 5′-TCTTCGGTTGAGCTTCGATT-3′ and endogenous control (GAPDH, Applied Biosystems, CA) was used for normalization. qPCR was performed on MxPro-Mx3005P QPCR System (Agilent Technologies, CA) and the reaction mixture consisted of 12.5 μl of Stratagene Brilliant II SYBR® Green Low ROX QPCR Master Mix (Agilent Technologies, CA), 2.5 μl of forward and backward primers and 4 μl of DNA in a total volume of 25 μl. Cycling conditions comprised of 10 min polymerase activation at 95°C, an amplification step consisting of 40 cycles at 95°C for 30 sec each and 60°C for 1 min and followed by 1 cycle each of 95°C for 1 min, 55°C for 30 sec and 95°C for 30 sec to obtain the disassociation curve. Immunoprecipitated DNA was PCR amplified in duplicate, and Ct values from each sample were obtained using the MxPro software. Ct values obtained from age-matched intermediary responders (i.e., rats that exhibited locomotor scores in the middle third of the same sample) that were handled as control animals were used as phenotype control to calculate the logarithmic changes in Ct values of LRHR rats exposed to chronic variable physical stress or handled as controls.
In situ hybridization histochemistry
Coronal brain sections were collected at 20 μm thickness throughout the dorsal hippocampus. On the day of hybridization, sections were fixed in 4% paraformaldehyde at room temperature for 1 hr, followed by three washes in 2X SSC (1X SSC is 150 mM sodium chloride, 15 mM sodium citrate). Sections were placed in a solution containing acetic anhydride (0.25%) in triethanolamine (0.1 M, pH 8) for 10 min, rinsed in distilled water, dehydrated through graded alcohols (50%, 75%, 85%, 95% and 100%) and air dried. Antisense 35S-labeled cRNA probes for rat BDNF (585 bp complementary to the mature rat sequence), was labeled in reaction mixture consisting of 1 ml of linearized plasmid, 1X transcription buffer, 125 mCi [35S]UTP, 125 mCi [35S]CTP, 150 mM each of ATP, and GTP, 12.5 mM dithiothreitol, 20 U RNAase inhibitor, and 6 U polymerase. In this manner, BDNF riboprobe (kindly donated by Dr. Stanley J. Watson, University of Michigan) was produced using T3 RNA polymerase as the transcription enzyme after the plasmid was antisense linearized with XhoI. Reactions were incubated for 90 min at 37°C, and separated from unincorporated nucleotides over Biorad columns (Biorad Laboratories, CA). Probe was diluted in hybridization buffer (50% formamide, 10% dextran sulfate, 2X SSC, 50 mM sodium phosphate buffer, pH 7.4, 1X Denhardt’s solution, 0.1 mg/ml yeast tRNA and 10 mM dithiothreitol) to yield 106 dpm/70 μl. Sections were hybridized with probe mixture inside a humidified box over night at 55°C. Next day, sections were washed in 3X SSC for 5 min each, then incubated for 1 hr in RNAase (20 mg/ml in Tris buffer containing 0.5 M NaCl, pH 8) at 37°C. Sections were washed with 2X, 1X and 0.5X SSC, and incubated for 1 hr in 0.1X SSC at 65°C. After rinsing in distilled water, sections were dehydrated, air dried and exposed to a Kodak XAR film (Eastman Kodak, NY). Section images were captured digitally from x-ray films with a CCD camera, and relative optical densities were determined using the Scion image software. Only pixels with gray values exceeding 3.5X above background were considered signal and included in the analyses. BDNF mRNA expression is quantified in the hippocampal CA1 and CA3 fields and the DG, and in the BLA.
Timm’s method for silver sulfide staining
Coronal brain sections were collected at 160μm intervals throughout the hippocampus, and were kept at −80°C until staining. On the day of staining, sections were air-dried and immersed in a phosphate-buffered (pH 7.4) 0.5% sodium sulfide solution for 2 min, briefly rinsed in two changes of phosphate buffer, fixed in 96% ethanol and rehydrated. Subsequently, sections were stained as described previously (Danscher, 1981; Geneser et al., 1993) by immersion in a citrate-buffered hydrochinone – silver lactate developer containing gum arabic as a protective colloid. Sections were rinsed vigorously with tap water following development and counterstained with cresyl violet stain and coverslipped. This protocol was successfully used on fresh frozen tissue in a previously published article (Isgor et al., 2004b).
Stereological estimation of mossy fiber terminal field volumes
The volumes of the two major components of the mossy fiber system were estimated using the Cavalieri estimator (Stereoinvestigator, Micro-BrightField; Colchester, VT). The tissue was viewed under brightfield illumination on a Zeiss Axiophot microscope interfaced with a CCD color video camera, and displayed on a high-resolution video monitor at a final magnification of 250X. Rostral and caudal extents of the hippocampus were determined following the convention of Paxinos and Watson (1982). A systematic, random sampling scheme was utilized such that estimates are based on every 10th section throughout the rostra-caudal extent of the hippocampus, yielding an average of 20 analyzed sections. The cross-sectional areas of the intra/infra pyramidal mossy fibre (IIP-MF) and SP-MF terminal fields were estimated by an automated point-counting technique using a grid of test points displayed on the video monitor superimposed upon the structure of interest. Volumes of different mossy fiber terminal fields were estimated from the total number of points that fell within the respective field, the sampling interval and the nominal section thickness. Values were plotted for unilateral terminal field volume estimations for SP-MF, IIP-MF, and total (SP + IIP) mossy fiber systems.
Statistical analyses
Two-way ANOVAs were conducted for (1) percent time spent in social interaction (2) percent time spent in immobility on the forced swim test (3) amounts of acH3 and acH4 in the P2 and P4 promoters of the BDNF gene in the hippocampus, (4) levels of BDNF mRNA expression in the hippocampus and the amygdala following in situ hybridization histochemistry, and (5) the SP-MF, IIP-MF, and total MF terminal field volumes between phenotypes (LR, HR) and stress condition (PHY, CONT). Furthermore, significant interactions and main effects of ANOVAs were followed by post-hoc comparisons. All significance levels were set at p = 0.05.
RESULTS
Performance on the social interaction and forced swim tests
Body weights at the start of the CVP regimen or control handling (on PN 28) were: LR CONT: 79 ± 9 gm, HR CONT: 85 ± 10 gm, LR CVP: 82 ± 5 gm, HR CVP: 77 ± 8gm. Body weights at the completion of CVP regimen or control handling (on PN 41) were: LR CONT: 185 ± 13 gm, HR CONT: 189 ± 7 gm, LR CVP: 148 ± 8 gm, HR CVP: 155 ± 5 gm.
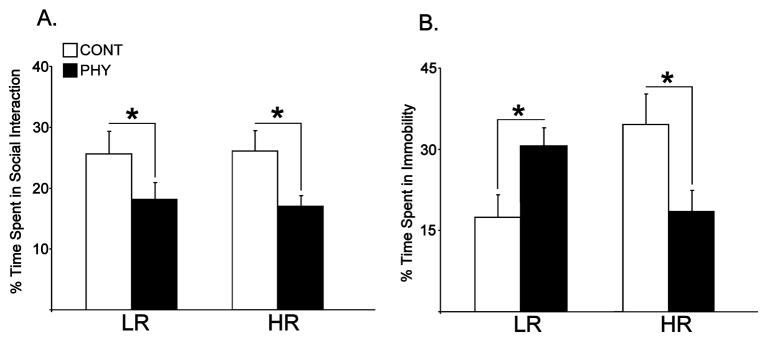
Figure 1A shows the percent time engaged in social interaction in the LRHR rats. A two-way ANOVA revealed a significant main effect of Stress (PHY, CONT) on social interaction between groups [F(1,20) = 24.64, p = 0.006]. Subsequent posthoc comparisons showed a significant decrease in percent time spent in social interaction in both phenotypes exposed to chronic physical stress compared to control animals [ps ≤ 0.022].
Figure 1.
Percent time spent in social interaction (A) and in immobility in forced swim test (B) in LRHR rats exposed to chronic variable physical stress (PHY) or handled as controls (CONT, n=6 per experimental group). Means are plotted in bar graphs ± SEMs. *: p ≤ 0.05.
Figure 1B shows the percent time spent in immobility in LRHR rats on forced swim test. A two-way ANOVA showed a significant interaction between Phenotype (LR, HR) and Stress (PHY, CONT) in immobility behavior [F(1,20) = 9.095, p = 0.005]. Subsequent posthoc comparisons showed a significant increase in percent time spent in immobility in LRs exposed to chronic physical stress compared to handled controls [p = 0.031], and a significant decrease in percent time spent in immobility in HRs exposed to chronic physical stress compared to handled controls [p = 0.002].
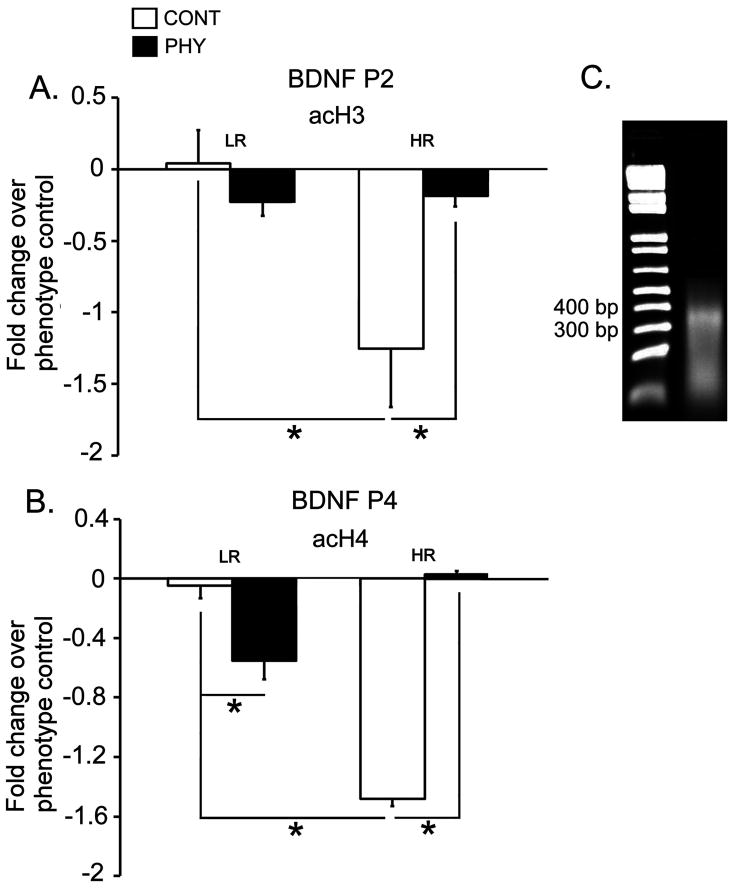
Levels of acetylated histones at the BDNF promoters in the hippocampus
Figures 2A and 2B show levels of acH3 at the P2 promoter of the BDNF gene and levels of acH4 at the P4 promoter of the BDNF gene respectively in the dorsal hippocampus of the LRHR rats exposed to chronic variable physical stress or control handling. Figure 2C shows representative gel picture of fragmented chromatin (roughly 400 bp) after the sonication. A two-way ANOVA revealed a significant interaction between Phenotype (LR, HR) and Stress (PHY, CONT) in the acH3 levels at the BDNF P2 promoter [F(1,20) = 7.431, p = 0.018]. Posthoc comparisons showed that control HRs had lower levels of acH3 at the BDNF P2 promoter compared to control LRs [p = 0.001]. Furthermore, acH3 levels at the P2 promoter were increased with physical stress in HRs compared to control levels [p = 0.027]. No significant effects were detected in the acH3 levels at the P2 promoter of the BDNF gene in the dorsal hippocampus of the LR rats, as well as acH3 levels at the P4 promoter of the BDNF gene between phenotypes and stress conditions (data not shown). Moreover, a two-way ANOVA showed a significant interaction between Phenotype (LR, HR) and Stress (PHY, CONT), a significant main effect of Stress and Phenotype in the acH4 levels at the BDNF P4 promoter [Fs(1,20) ≥ 24.650, ps ≤ 0.0004]. Posthoc comparisons showed that control HRs had lower levels of acH4 at the BDNF P4 promoter compared to control LRs [p = 0.001]. Furthermore, physical stress exposure resulted in decreased levels of acH4 at the BDNF P4 promoter in LRs, while same stress condition caused an increase in acH4 at the P4 promoter in HRs compared to handled controls [ps ≤ 0.015]. No significant effects were detected in acH4 levels at the P2 promoter of the BDNF gene between phenotypes and stress conditions (data not shown).
Figure 2.
Levels of acH3 at the BDNF P2 promoter (A), and acH4 at the BDNF P4 promoter (B) in the LRHR rats exposed to chronic variable physical stress (PHY) or handled as controls (CONT, n=6 per experimental group). Fold changes in Ct values of immunoprecipitated samples obtained from LRHR rats exposed to physical stress or handled controls were calculated with the Ct values obtained from intermediary responders (phenotype control), and quantified using real-time PCR. Data are expressed as means ± SEMs. *: p ≤ 0.05. Sonicated chromatin was run on a 2% agarose gel to confirm proper fragmentation (roughly 400 bp from a representative gel in C).
BDNF mRNA in the hippocampus and the basolateral amygdala
Figure 3 shows expression of the BDNF mRNA in the dorsal hippocampus of the LRHR rats. Two-way ANOVAs showed significant interactions between Phenotype (LR, HR) and Stress (PHY, CONT) in BDNF mRNA levels in the DG and the CA3 field of the hippocampus [Fs(1,20) ≥ 3.529, ps ≤ 0.035]. Specific post-hoc comparisons showed that BDNF mRNA was upregulated in the hippocampal DG and CA3 in HR rats exposed to chronic physical stress compared to handled controls [ps ≤ 0.007].
Figure 3.
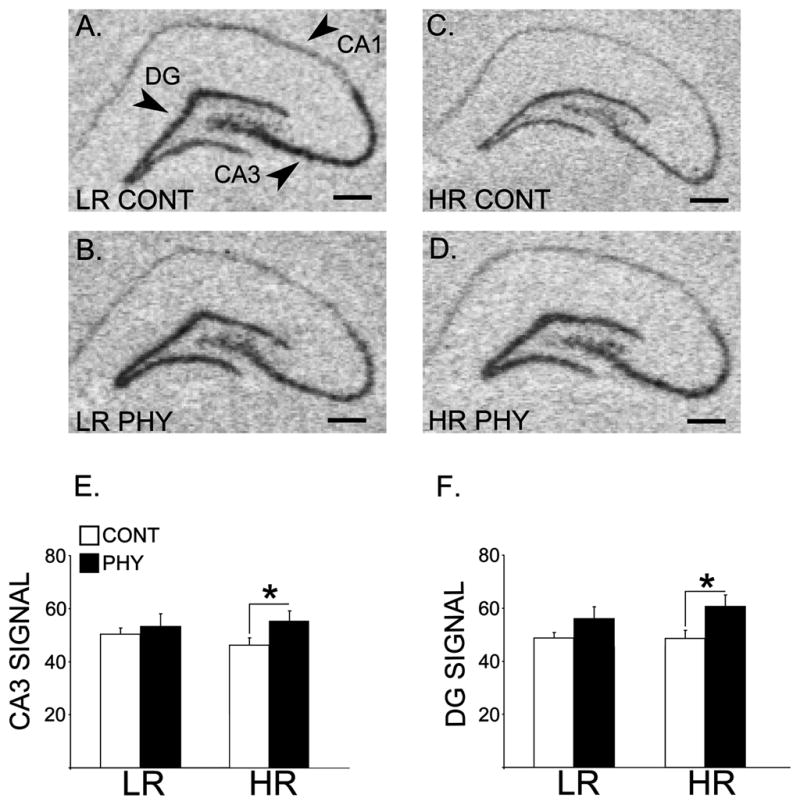
Panels A, B, C and D constitute x-ray film-exposed images of representative coronal hemisections of the hippocampus that were radioactively labeled with an antisense cRNA probe against the BDNF mRNA in the LRHR rats following chronic variable physical stress or control handling (n=6 per experimental group). Means for optical densities ± SEMs are plotted by bar graphs for signal in the CA3 field (E) and the DG (F) of the hippocampus. *: p ≤ 0.05. Scale bar = 250 μm.
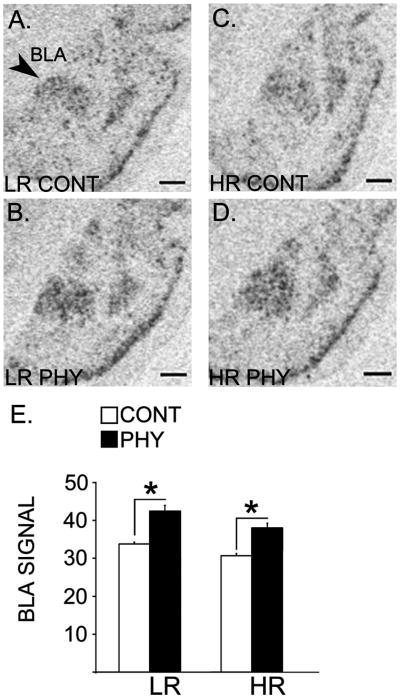
Figure 4 shows expression of the BDNF mRNA in the BLA of the LRHR rats. A two-way ANOVA showed a significant main effect of Stress (PHY, CONT) in the BDNF mRNA in the BLA [F(1,20) = 27.771, p = 0.001]. Specific post-hoc comparisons showed that BDNF mRNA levels were upregulated following the chronic variable physical stress exposure in both the LR and HR rats compared to their respective handled controls [ps ≤ 0.003].
Figure 4.
Panels A, B, C and D constitute x-ray film-exposed images of representative sections showing basolateral amygdala that were radioactively labeled with an antisense cRNA probe against the BDNF mRNA in the LRHR rats following chronic variable physical stress or control handling (n=6 per experimental group). Means for optical densities ± SEMs are plotted by a bar graph (E). *: p ≤ 0.05. Scale bar = 250 μm.
Stereological estimation of mossy fiber terminal field volumes
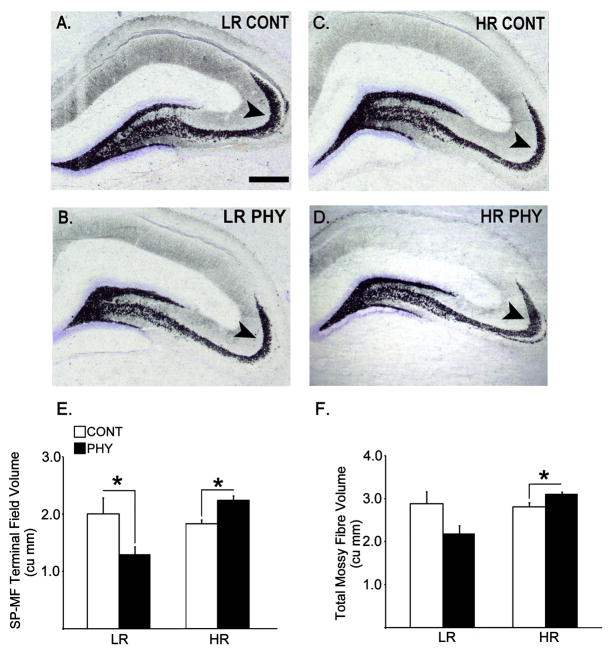
The two major components of the mossy fiber system (SP-MF and IIP-MF) in which quantitative estimates were performed are shown in Figure 5. Figure 6 depicts representative sections of the Timm-stained septal hippocampi from experimental groups, and estimates of terminal field volumes for SP-MF and total mossy fibres (SP-MF + IIP-MF). Two-way ANOVAs showed significant interactions between Phenotype (LR, HR) and Stress (PHY, CONT), and main effects of Phenotype in the SP-MF and total mossy fibre terminal field volumes [Fs(1,20) ≥ 3.572, ps ≤ 0.048], whereas no significant effects were detected in the IIP-MF terminal field volume (data not shown). Subsequent posthoc comparisons showed a significant increase in the SP-MF and total mossy fibre terminal field volumes in HRs exposed to chronic physical stress compared to handled controls, and a significant decrease in the SP-MF volume in LRs exposed to chronic variable physical stress compared to handled controls [ps ≤ 0.045]. Insert Figures 5 and 6 about here
Figure 5.
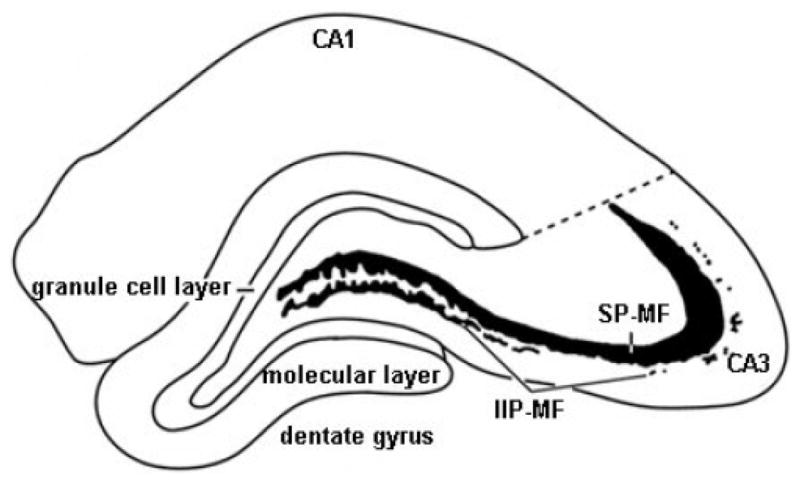
An illustration of a coronal hemisection of the dorsal hippocampus depicting the major hippocampal subdivisions and the two compartments of the mossy fibre system (SP-MF and IIP-MF) in which quantitative estimates were performed in the Timm-stained tissue.
Figure 6.
Coronal hemisections of the dorsal hippocampus showing Timm-stained mossy fibre terminal fields that are counterstained by cresyl violet representing LR control (A), LR chronic variable physical stress (B), HR control (C) and HR chronic variable physical stress (D) groups (n=6 per experimental group). Arrows are pointing at the SP-MF compartment of the mossy fibre projections. Data pertaining to estimated SP-MF terminal field volume (E) and total mossy fibre terminal field volume (F, SP-MF + IIP-MF) are expressed as means ± SEMs in bar graphs. *: p ≤ 0.05. Scale bar = 250 μm.
DISCUSSION
Our results showed a dissociation in the depressive- and social anxiety-like behaviors in the HR rats following chronic variable physical stress during the peripubertal-juvenile period, in that a decrease in immobility (i.e., antidepressive-like effect) was detected on the forced swim test along with a decrease in the amount of social interaction (i.e., anxiogenic-like effect). In the LR phenotype, chronic variable physical stress led to an increase in immobility while also causing an increase in the social anxiety-like behavior. These behavioral findings reflect a phenotype non-specific emergence of social anxiety-like behavior with the peripubertal-juvenile chronic physical stress exposure. Simultaneously a phenotype-specific regulation in the depressive-like behavior is observed with the peripubertal-juvenile chronic physical stress in that an increase in depressive-like behavior is observed in the LR phenotype in agreement with the widely reported effects in the adult animals, whereas a surprising decrease in depressive-like behavior is observed in the HR phenotype. Moreover, although basal levels of histone acetylation at the BDNF gene was lower in HR compared to LR hippocampi between respective handled controls, levels of acetylated H3 and H4 at the BDNF promoters P2 and P4 respectively were increased with physical stress in the HR hippocampus compared to levels observed in handled controls. In contrast, a significant decrease in levels of acetylated H4 was observed at the BDNF promoter P4 in the LR hippocampus following the physical stress regimen. These findings suggest increased epigenetic activation of the hippocampal BDNF gene in HRs and in contrast a decreased epigenetic activation of the hippocampal BDNF gene in LRs with chronic variable physical stress. Furthermore, upregulation in the BDNF mRNA was observed in the HR hippocampus (CA3 and DG) following physical stress compared to levels observed in handled controls. These effects in the BDNF mRNA are in the same direction as the effects observed in acetylated histone levels at the BDNF promoters in the HR phenotype, namely both indicating increased hippocampal BDNF transcription. Concurrent with findings on the hippocampal BDNF levels, our results showed a significant increase in total mossy fibre volume, particularly in SP-MF terminal field size in HRs and a significant decrease in SP-MF terminal field size was observed in LRs following the chronic variable physical stress regimen. These findings suggest that fluctuations in the hippocampal BDNF levels induced by chronic physical stress during the peripubertal-juvenile period, particularly those that are mediated by histone modifications at the BDNF gene, may sustain observed structural plasticity at the mossy fibre terminals, which may mediate depressive-like behavior in the LRHR rats. Lastly, the emergence of social anxiety-like behavior in both phenotypes was associated with the upregulation of the BDNF mRNA in the BLA, indicating that stress-induced increase in BDNF levels in the amygdala may be mediating a general anxiogenic effect in the juvenile LRHR rats.
Depressive-like behavior and hippocampal BDNF
Our results showed an opposite regulatory pattern for LRHR rats in depressive-like behavior measured as immobility on the forced swim test following the peripubertal-juvenile exposure of chronic physical stress, in that LR rats had increased amount of immobility whereas HR rats had decreased amount of immobility compared to levels observed in handled controls. Stress-induced increase in depressive-like behavior observed in the juvenile LR rats is in line with numerous reports showing increased depressive-like behavior following chronic stress in adulthood. In fact, paradigms of chronic stress are widely accepted as animal models for studying enhanced depressive-like state (see review; Duman, 2010). Interestingly, the same chronic stress paradigm applied during the peripubertal-juvenile period resulted in an antidepressive-like effect in the HR rats. Although individual components of our chronic variable physical stress regimen are commonly employed as stressors in the literature such as restraint (Andrus et al., 2010), and generally considered as “negative” experiences, a regimen of combined stressors applied intermittently at varying times of day and at varying durations during the peripubertal-juvenile period may indeed mimic an enrichment-like effect, especially in the stress responsive HR phenotype. It is not uncommon to induce a robust physiological stress response in conjunction with rewarding environmental stimuli during adolescence. For example, rough- and tumble-play in juveniles induces positive experiences and is rewarding (Burgdorf and Panksepp, 2001), although marked with increased levels of glucocorticoids (Terranova et al., 1999). In addition to playful social interactions, positive reinforcing activities such as feeding and mating have also been shown to associate with increased levels of glucocorticoids (Dallman et al., 1995; Frye et al., 1996). Classic postweaning enrichment paradigm produces an antidepressive-like effect on the forced swim test (Brenes et al., 2009), such as the one we report in the HR rats following the chronic physical stress regimen applied postweaning into juvenile period.
In parallel with the opposite regulatory pattern in depressive-like behavior following chronic stress, an accompanying regulation is observed in the chromatin plasticity associated with the hippocampal BDNF gene in the LRHR rats, namely increased depressive-like behavior is associated with decreased acH4 levels in the P4 promoter of the BDNF gene in LRs, whereas antidepressive-like effect in HRs is associated with increased acH3 and acH4 at the P2 and P4 promoters of the BDNF gene respectively. Hippocampal BDNF levels are traditionally shown to be reduced following chronic stress such as social defeat (Tsankova et al., 2006) and immobilization (Smith et al., 1995; Vollmayr et al., 2001; Roceri et al., 2002; Nair et al., 2007); effects that are in agreement with the data presented here from the juvenile LR rats. Chromatin plasticity in terms of increased histone methylation at the BDNF promoter is previously linked to stress-induced suppression of the hippocampal BDNF gene in adult animals (Tsankova et al., 2006). Here we show, at least in the juvenile LR rats, chromatin plasticity in the form of decreased H4 acetylation at the hippocampal BDNF gene. Stress-induced decrease in acetylation is confined to the H4 at the P4 promoter in the LRs with no effects at the level of the total BDNF mRNA in the hippocampus. Investigation of particular splice variants of the BDNF mRNA may be useful to specify where stress-induced regulatory effects may reside in the LR phenotype. In a previous report, antidepressant treatments are shown to induce hyperacetylation of H3 at the BDNF promoter (Tsankova et al., 2006). Our juvenile stress exposure in the HR phenotype appears to mimic previously reported effects of antidepressants on histone acetylation at the hippocampal BDNF gene, depicting a novel mechanism for environmental-induction of the BDNF. Moreover, an upregulation of the BDNF mRNA in the DG and the CA3 fields of the hippocampus were detected in the HR animals following chronic variable physical stress regimen; suggesting, along with the chromatin plasticity, there may be classic transcriptional regulatory mechanisms responsible for stress-induced changes in the BDNF levels. These data are in agreement with recent reports showing that while four weeks of unpredictable mild stress applied at adulthood induced anhedonia and decreased hippocampal BDNF expression, same chronic stress applied at PN 30 resulted in increased hippocampal BDNF expression (Toth et al., 2008), suggesting age-dependent effects of stress on depressive symptoms and hippocampal BDNF. In addition, a recent report showed that the classic environmental enrichment procedure in adolescence can stimulate expression of the hippocampal BDNF gene through chromatin-specific events (Kuzumaki et al., 2010), providing further support for enriching-like quality of our stress paradigm in the HR rats. We should note that our previously published report utilized 28 days of stress exposure spanning between postweaning (PN 28) to young adulthood (PN 56; Isgor et al., 2004a), and therefore results obtained here with shorter procedure (i.e., 14 days) may not necessarily apply to longer procedure or vice versa.
It is interesting to note that gender differences in responsiveness to chronic stress are linked to differences in hippocampal BDNF levels. BDNF deletion in the forebrain has been shown to induce anxiogenic and anhedonic behaviors in chronic stress-exposed females compared to control counterparts. However, loss of BDNF in the same region does not increase the vulnerability to depression in males (Autry et al., 2009). Therefore, it is plausible to extrapolate that female rats would act similar to the stress-exposed LR males in response to our CVP paradigm with increased susceptibility to depressive-like behavior associated with decreased BDNF levels in the hippocampus.
Anxiety-like behavior and amgdalar BDNF
LRHR animals showed increased anxiety-like behavior following chronic variable physical stress, measured as reduced social contact in the social interaction test. Emergence of social anxiety-like behavior in response to chronic variable physical stress was marked with upregulation in the BDNF mRNA in the BLA of juvenile LRHR rats. Increase in anxiety-like behavior in a social context as well as in traditional indices of anxiety such as open field behavior has been previously reported following adolescent social isolation (Lukkes et al., 2009), early life maternal separation and chronic restraint stress (Eiland and McEwen, 2010). Furthermore, previous studies also linked chronic stress-induced anxiety with increase in spine density in the BLA (Vyas et al., 2002; Mitra et al., 2005), together with a long-term increase in amygdalar BDNF (Fanous et al., 2010) that may mediate the synaptic plasticity. What is intriguing is the question how dissociation between the depressive- and anxiety-like behaviors may develop in the HR rats, and whether there are molecular models that can mimic this effect. One study showed that transgenic mice overexpressing BDNF in the hippocampus and the amygdala display both anxiogenic and antidepressant effects (Govindarajan et al., 2006). Specifically these authors showed that BDNF overexpression led to increased anxiety which is paralleled by an increase in spine density in the BLA, similar to chronic stress-induced effects reported in the literature (Duman et al., 2000; Vyas et al., 2002). Also BDNF overexpression in the hippocampus was proposed as a mechanism to prevent chronic stress-induced cellular atrophy together with improved performance on forced swim test. In conclusion, these authors stated that BDNF locally in the amygdala may facilitate the anxiety-like symptoms, whereas BDNF locally in the hippocampus may attenuate depressive-like symptoms (Govindarajan et al., 2006). Juvenile physical stress regimen applied here leads to a similar regulatory pattern between depressive- and anxiety-like behaviors in the HR phenotype by way of inducing BDNF expression in both the hippocampus and the BLA. By the same token, same chronic stress regimen, by way of inducing BDNF expression only in the BLA of the LR phenotype, could lead to emergence of only the anxiogenic phenotype leaving depressive-like behavior unchanged.
Mossy fibre terminal fields
Chromatin modifications in the form of histone acetylation at the BDNF promoters were closely associated with mossy fiber remodeling in the LRHR hippocampi following chronic variable physical stress exposure during the peripubertal-juvenile period. Following stress exposure, an increase in mossy fibre terminal field size, particularly the SP-MF volume, in the HR animals was associated with increases in H3 and H4 acetylation at the P2 and P4 promoters respectively; and a decrease in SP-MF volume in the LR animals was associated with a decrease in H4 acetylation at the P4 promoter. These findings strongly suggest BDNF-mediated synaptic plasticity at the mossy fibre terminals of the LRHR rats following the peripubertal-juvenile stress.
Morpho-behavioral correlations in genetically-altered mice implicated hippocampal mossy fibres with exploratory behavior (Roullet and Lasalle, 1993; Ivanco and Greenough, 2002; Mineur and Crusio, 2002). The mossy fibre projection relays multimodal sensory information from the entorhinal cortex to the hippocampus proper, which upon receipt is compared with previously stored information (Amaral and Witter, 1989; Witter et al., 1989; Vinogradova, 2001). Such a mechanism to detect novelty within the environment must be a necessary component of the novelty-seeking phenotype (i.e., LRHR phenotype). Moreover, the peripubertal-juvenile period is a critical period for mossy fibre remodeling (Mori-Kawakami et al., 2003), and amount of mossy fibre content is highly dependent on experiential factors (Gomez-Di Cesare et al., 1997). In the face of high level of late developmental neuroplasticity in the mossy fibre system, maintaining normal hippocampal function would require correct mossy fibre path finding and precise synaptic connections (Parent et al., 1997), which are likely affected by stressful environmental stimulation during adolescence. Since hippocampal BDNF is primarily stored in the axon terminals of the mossy fibres, stress-induced changes in the hippocampal BDNF levels could mediate structural changes in the mossy fibre-CA3 synapse. Intrahippacampal microinfusion of the BDNF is shown to increase mossy fibre content at the stratum oriens of the CA3 (Schjetnan and Escobar, 2010). Hence, it is plausible that the increase in the hippocampal BDNF in the juvenile HR rats following chronic physical stress may lead to increased mossy fibre innervation of the CA3 neurons, and may function as an attempt to rescue CA3 neurons from stress-induced atrophy (Watanabe et al., 1992; Magarinos et al., 1996).
Although mossy fibre plasticity is linked to cognitive function (Notenboom et al., 2010), implications in depressive-like behavior are, for the most part, unexplored. Functional maturation of the DG and its output mossy fibres are implicated in pathophysiology of depression. Particularly antidepressants are shown to increase adult neurogenesis in the DG (Malberg et al., 2000), whereas stress exposure results in decrease in neurogenesis (Warner-Schmidt and Duman, 2006), suggesting granule neurons and their axonal projections onto CA3 may be critically involved in pathogenesis of depression and antidepressant effects (Kobayashi, 2009). In support of this, repeated electroconvulsive stimulation (ECS) is shown to induce mossy fibre sprouting (Vaidya et al., 1999). Similar to ECS, we report increased mossy fibre terminal fields in the HR phenotype following chronic stress and accompanying antidepressive-like effects on forced swim test. In contrast, the stress-induced atrophy observed in the mossy fibre terminal fields in the LR animals accompany depressive-like effects on forced swim test, which implicate the mossy fibre system as a target for neuroadaptations induced by stressful stimulation during the peripubertal-juvenile period.
It should be noted that brain areas involved in emotional behavior continue to grow across peripubertal-juvenile period into young adulthood (Bayer et al., 1982; Sousa et al., 1998; Isgor et al., 2004a). Curiously adolescence is also the period when the novelty-seeking phenotype (i.e., sensation seeking trait in humans) first emerges (Martin et al., 2002; Butkovic and Bratko, 2003; Zuckerman, 2004). Both heightened responsiveness to environmental perturbations and emergence of phenotypic disposition during adolescence are likely consequences of a phase of rapid neural growth. Indeed there are both progressive (i.e., axonal growth, myelination) as well as regressive (i.e., axonal and synaptic pruning) mechanisms at work during adolescence, suggesting that this period marks the maturation of neuronal connectivity (Pffeferbaum et al., 1994; Caviness et al., 1996; Reiss et al., 1996). Emerging clinical data confirm increased HPA activity and heightened emotional response during adolescence compared to adulthood (Gunnar et al., 2009; Stroud et al., 2009) associated with puberty-specific maturation in affective systems (Dahl, 2004; Dahl and Gunnar, 2009). For this purpose, it is highly relevant to focus on structural plasticity induced by chronic stress during the peripubertal-juvenile period in brain emotional circuitry (i.e., amygdala and hippocampus) to delineate cellular substrates that may mediate the depressive and anxiety states.
In conclusion, we report depressive and anxiogenic behavioral effects emerging with juvenile exposure of chronic physical stress in the LR rats; and an antidepressive and anxiogenic behavioral effects in the HR rats following the same peripubertal-juvenile stress exposure. Accompanying the behavioral effects, differential epigenetic regulation of the hippocampal BDNF gene is observed that may mediate synaptic plasticity at the mossy fibre terminals. The phenotype nonspecific induction of social anxiety-like behavior in the LRHR rats following the chronic variable physical stress is accompanied by the upregulation of the BDNF mRNA in the BLA. These findings assign differential functional significance to the induction of hippocampal and amygdalar BDNF by chronic variable physical stress in the juvenile HR phenotype.
Highlights.
Chronic stress exposed HRs showed social anxiety- and antidepressant-like behaviors.
Epigenetic activation of BDNF gene was increased in HRs exposed to chronic stress.
Chronic stress induced upregulation of BDNF mRNA in hippocampus and amygdala in HRs.
Chronic variable stress resulted in increased mossy fibre terminal field size in HRs.
Abbreviations
- acH3
acetylated histone 3
- acH4
acetylated histone 4
- BDNF
brain-derived neurotrophic factor
- BLA
basolateral amygdala
- CRF
corticotropin releasing factor
- DG
dentate gyrus
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- HR
high responders
- IIP-MF
intra/infra pyramidal mossy fibres
- LR
low responders
- SP-MF
suprapyramidal mossy fibres
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–91. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andrus BM, Blizinsky K, Vedell PT, Dennis K, Shukla PK, Schaffer DJ, Radulovic J, Churchill GA, Redei EE. Gene expression patterns in the hippocampus and amygdala of endogenous depression and chronic stress models. Mol Psychiatry. 2010 Nov 16; doi: 10.1038/mp.2010.119. (Epub ahead of printing) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Cheng P, Monteggia LM. Gender-specific impact on brain-derived neurotrophic factor signaling on stress-induced depression-like behavior. Biol Pschiatry. 2009;66:84–90. doi: 10.1016/j.biopsych.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Vulnerability to nicotine abstinence-related social anxiety-like behavior: Molecular correlates in neuropeptide Y, Y2 receptor and corticotropin releasing factor. Neurosci Lett. 2010;490(3):220–5. doi: 10.1016/j.neulet.2010.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Effects of a selective Y2R antagonist, JNJ-31020028, on nicotine abstinence-related social anxiety-like behavior, neuropeptide Y and corticotropin releasing factor mRNA levels in the novelty-seeking phenotype. Behav Brain Res. 2011;222(2):332–41. doi: 10.1016/j.bbr.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Padilla M, Fornaguera J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav Brain Res. 2009;197(1):125–37. doi: 10.1016/j.bbr.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72:167–73. doi: 10.1016/s0031-9384(00)00411-x. [DOI] [PubMed] [Google Scholar]
- Butkovic A, Bratko D. Generation and sex differences in sensation-seeking: results of the family study. Percept Mot Skills. 2003;97:965–970. doi: 10.2466/pms.2003.97.3.965. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Dahl R. Adolescent brain development: A period of opportunities and vulnerabilities. Ann NY Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Paychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Danscher G. Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry. 1981;71:1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann NY Acad Sci. 1995;771:730–42. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- Duman CH. Models of Depression. Vitam Horm. 2010;82:1–21. doi: 10.1016/S0083-6729(10)82001-1. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neural plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–9. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2010 Sep 16; doi: 10.1002/hipo.20862. [Epub ahead of printing] [DOI] [PubMed] [Google Scholar]
- Fanous S, Hammer RP, Jr, Nikulina EM. Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience. 2010;167(3):598–607. doi: 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–38. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- Frye CA, McCormick CM, Coopersmith C, Erskine MS. Effects of paced and non-paced mating stimulation on plasma progesterone, 3 alpha-diol and corticosterone. Psychoneuroendocrinology. 1996;21:431–9. doi: 10.1016/0306-4530(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Geneser FA, Holm IE, Slomianka L. Application of the Timm and selenium methods to the central nervous system. Neurosci Protocols. 1993;50–15:1–14. [Google Scholar]
- Gomez-Di Cesare CM, Smith KL, Rice FL, Swann JW. Axonal remodeling during postnatal maturation of CA3 hippocampal pyramidal neurons. J Comp Neurol. 1997;384:165–80. [PubMed] [Google Scholar]
- Gomez-Palacio-Schjetnan A, Escobar ML. In vivo BDNF modulation of adult functional and morphological synaptic plasticity at hippocampal mossy fibers. Neurosci Lett. 2008;445(1):62–7. doi: 10.1016/j.neulet.2008.08.069. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA. 2006;103:13208–13. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Dev Psychopathol. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004a;14(5):636–48. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Isgor C, Slomianka L, Watson SJ. Hippocampal mossy fibre terminal field size is differentially affected in a rat model of risk-taking behavior. Behav Brain Res. 2004b;153(1):7–14. doi: 10.1016/j.bbr.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Ivanco TL, Greenough WT. Altered mossy fibre distribution in adult Fmr1 (FVB) knockout mice. Hippocampus. 2002;12:47–54. doi: 10.1002/hipo.10004. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Isgor C. Effects of chronic environmental and social stimuli during adolescence on mesolimbic dopaminergic circuitry markers. Neurosci Lett. 2007;422(1):7–12. doi: 10.1016/j.neulet.2007.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol. 2009;39(1):24–36. doi: 10.1007/s12035-008-8049-5. [DOI] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Tamura R, Hareyama N, Imai S, Narita M, Torigoe K, Niikura K, et al. Hippocampal epigenetic modification at the brain-derived neurotrophic factor gene induced by an enriching environment. Hippocampus. 2010 Mar 15; doi: 10.1002/hipo.20775. [Epub ahead of printing] [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress response. Horm Behav. 2009;55(1):248–56. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16(10):3534–40. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation-seeking, puberty and nicotine, alcohol and marijuana use in adolescence. J Am Acad Child Adolescent Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Crusio WE. Behavioral and neuroanatomical characterization of FVB/N inbred mice. Brain Res Bull. 2002;57:41–7. doi: 10.1016/s0361-9230(01)00635-9. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102(26):9371–6. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori-Kawakami F, Kobayashi K, Takahashi T. Developmental decrease in synaptic facilitation at the mouse hippocampal mossy fibre synapse. J Physiol. 2003;553:37–48. doi: 10.1113/jphysiol.2003.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Vadodaria KC, Banerjee SB, Benekareddy M, Dias BG, Duman RS, et al. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology. 2007;32:1504–19. doi: 10.1038/sj.npp.1301276. [DOI] [PubMed] [Google Scholar]
- Notenboom RG, Ramakers GM, Kamal A, Spruijt BM, de Graan PN. Long-lasting modulation of synaptic plasticity in rat hippocampus after early-life complex febrile seizures. Eur J Neurosci. 2010;32(5):749–58. doi: 10.1111/j.1460-9568.2010.07321.x. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geshwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesisis increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–38. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. San Diego, CA: Academic Press; 1982. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Wecker L. Dependence of adolescent novelty-seeking behavior on response phenotype and effects of apparatus scaling. Behav Neurosci. 2008;4:861–875. doi: 10.1037/0735-7044.122.4.861. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–78. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla M. Brain development, gender, and IQ in children: a volumetric study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry. 2002;7:609–16. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- Roullet P, Lassalle JM. Spontaneous exploration, response plus position learning and hippocampal mossy fibre distribution: a correlational study. Behav Process. 1993;29:217–28. doi: 10.1016/0376-6357(93)90125-B. [DOI] [PubMed] [Google Scholar]
- Schjetnan AG, Escobar ML. In vivo BDNF modulation of hippocampal mossy fiber plasticity induced by high frequency stimulation. Hippocampus. 2010 Sept 16; doi: 10.1002/hipo.20866. (Epub ahead of printing) [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain derived neurotrophic factor and neurotrophin-3 mRNA’s in the hippocampus. J Neurosci. 1995;15:1766–77. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Madeira MD, Paula-Barbosa MM. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res. 1998;794:199–210. doi: 10.1016/s0006-8993(98)00218-2. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Forster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stress. Dev Payshopathol. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova ML, Cirulli F, Laviola G. Behavioral and hormonal effects of partner familiarity in periadolescent rat pairs upon novelty exposure. Psychoneuroendocrinology. 1999;24:639–56. doi: 10.1016/s0306-4530(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, et al. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–32. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9(4):519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–10. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999;89:157–66. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–98. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Faust H, Lewicka S, Henn FA. Brain-derived-neurotrophic-factor (BDNF) stress response in rats bred for learned helplessness. Mol Psychiatry. 2001;358:471–4. doi: 10.1038/sj.mp.4000907. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic modeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–18. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–49. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2(4):431–5. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. The shaping of personality: genes, environments, and chance encounters. J Pers Assess. 2004;82:11–22. doi: 10.1207/s15327752jpa8201_3. [DOI] [PubMed] [Google Scholar]