Abstract
Repeated brief maternal separation (i.e., 15 minutes daily, MS15) of rat pups during the first one to two postnatal weeks enhances active maternal care received by the pups and attenuates their later behavioral and neuroendocrine responses to stress. In previous work, we found that MS15 also alters the developmental assembly and later structure of central neural circuits that control autonomic outflow to the viscera, suggesting that MS15 may alter central visceral circuit responses to stress. To examine this, juvenile rats with a developmental history of either MS15 or no separation (NS) received microinjection of retrograde neural tracer, FluoroGold (FG), into the hindbrain dorsal vagal complex (DVC). After one week, FG-injected rats and surgically intact littermates were exposed to either a 15-minute restraint stress or an unrestrained control condition, and then perfused one hour later. Brain tissue sections from surgically-intact littermates were processed for Fos alone or in combination with phenotypic markers to examine stress-induced activation of neurons within the paraventricular nucleus of the hypothalamus (PVN), bed nucleus of the stria terminalis (BNST), and hindbrain DVC. Compared to NS controls, MS15 rats displayed less restraint-induced Fos activation within the dorsolateral BNST (dBNST), the caudal PVN, and noradrenergic neurons within the caudal DVC. To examine whether these differences corresponded with altered neural inputs to the DVC, sections from tracer-injected rats were double-labeled for FG and Fos to quantify retrogradely-labeled neurons within hypothalamic and limbic forebrain regions of interest, and the proportion of these neurons activated after restraint. Only the dBNST displayed a significant effect of postnatal experience on restraint-induced Fos activation of DVC-projecting neurons. The distinct regional effects of MS15 on stress-induced recruitment of neurons within hypothalamic, limbic forebrain, and hindbrain regions has interesting implications for understanding how early life experience shapes the functional organization of stress-responsive circuits.
Keywords: postnatal handling, central nucleus of the amygdala, medial prefrontal cortex, Fos, preautonomic, autonomic
INTRODUCTION
Early life experience is an important factor in shaping later stress reactivity in humans and animals. For example, paradigms that alter the amount and quality of maternal care received by rat pups during the early postnatal period result in life-long alterations in their behavioral and hypothalamic-pituitary-adrenal (HPA) stress responses (Plotsky and Meaney, 1993, Liu et al., 1997, Caldji et al., 2000, Macri et al., 2008). In one widely-used experimental model, rat pups undergo a brief period (i.e., 15 minutes) of maternal separation (MS15) each day for the first 10-14 days after birth. This procedure has been reported to enhance the active maternal care received by pups after they are reunited with their dam, which has in turn been linked to reduced HPA axis stress reactivity and reduced anxiety-like behavior in the offspring when they are tested as adults (Macri et al., 2004, Macri et al., 2008).
While the impact of early maternal care on life-long stress responsiveness is relatively well established, few studies have examined how early life experience shapes the structural and functional organization of central neural circuits that govern physiological responses to stress. These circuits include hypothalamic and limbic forebrain regions such as the paraventricular nucleus of the hypothalamus (PVN), bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (CeA), and medial prefrontal cortex (mPFC). Preautonomic neurons within each of these regions are recruited during stress, and are known to modulate parasympathetic vagal motor outflow to the viscera via direct projections to the hindbrain dorsal vagal complex (DVC) (McCann and Rogers, 1991, Flanagan et al., 1992a, Rinaman et al., 1999, Yang et al., 1999, Zhang et al., 1999, Rinaman et al., 2000). Projections from these regions converge within the DVC in the caudal visceral nucleus of the solitary tract (NST) (Terreberry and Neafsey, 1983, Pickel et al., 1995, Saha et al., 2000, Dong et al., 2001), which contains noradrenergic (NA) neurons that are activated during stress and contribute to the stress response. For example, stress-induced inhibition of gastric motility (Iwa et al., 2006, Travagli et al., 2006, Herman et al., 2009) is due in part to direct NA innervation of nearby gastric dorsal motor nucleus of the vagus (DMV) neurons (Nagata and Osumi, 1993, Rogers et al., 2003, Pearson et al., 2007, Herman et al., 2008).
Prior research from our laboratory revealed that MS15 alters the ongoing assembly of gastric preautonomic circuits during their development in neonatal rats (Card et al., 2005). In a more recent study (Banihashemi and Rinaman, 2010), we tested the hypothesis that the developmental effect of MS15 on circuit assembly would manifest as altered circuit later in development. We used the retrograde transynaptic tracer pseudorabies virus (PRV) to demonstrate that gastric preautonomic circuits differed in juvenile rats with a developmental history of MS15 compared to rats raised in non-separated (NS) control litters (Banihashemi and Rinaman, 2010). Compared to NS controls, MS15 rats displayed a significant increase in the amount of transynaptic PRV labeling within the PVN, evidence for an increased number of synaptically-linked neurons and/or an increase in innervation density between PVN gastric preautonomic neurons and their postsynaptic targets (Banihashemi and Rinaman, 2010). These results support the view that early life experience may shape later stress responsiveness, at least in part, by shaping the structure of central preautonomic circuits that orchestrate visceral responses to stress.
The present study was designed to extend our previous findings by examining how MS15-related structural alterations in preautonomic circuitry might translate into functional differences at the same juvenile stage of development. To this end, we examined the stress-induced recruitment of central preautonomic regions, as assessed by neuronal Fos expression. Because stimulus-induced Fos activation cannot be accurately examined in combination with transneuronal viral tracing, visceral-related hypothalamic and limbic forebrain neurons were identified using a standard retrograde tracer (i.e., FluoroGold, FG) microinjected into the DVC in male juvenile rats from MS15 and NS litters. Restraint was selected as the acute stressor of choice for this study, because its ability to activate DVC neurons, including stress-responsive NA neurons within the caudal NST, depends on descending projections from the hypothalamus and limbic forebrain (Dayas et al., 2004). MS15 and NS littermates that did not receive FG tracer injections (i.e., surgically-intact) were used to assess the ability of restraint to activate specific brain regions and neuronal phenotypes, including corticotropin-releasing hormone (CRH)-positive neurons within the PVN, and NA neurons within the NST.
We hypothesized that rats with a developmental history of MS15 would display altered restraint-induced neural activation within limbic forebrain, hypothalamic, and brainstem regions of interest, including altered activation of DVC-projecting neurons within these regions that would be consistent with their phenotype of less stress reactivity (Plotsky and Meaney, 1993, Liu et al., 2000).
EXPERIMENTAL PROCEDURES
Animals
Experimental protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Animals were held in a controlled environment (20-22°C) with a 12-hour light dark cycle (lights on at 0700 hr) with ad libitum access to water and pelleted rat chow (Purina #5001, Bethlehem, PA).
The progeny of 12 pregnant multiparous Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN) were used in this study. Pregnant rats arrived at the animal facility at a gestational stage between embryonic days 16 and 17 and were subsequently housed singly in opaque polyethylene cages with soft woodchip bedding and a wire lid. Pregnant rats were checked daily to determine their pups’ date of birth, designated postnatal day (P)0. On P1, the anogenital distance of each pup was measured in order to determine sex (Jackson, 1912). Litters were then culled to eight pups with a majority of males but with at least two female pups per litter; however, only males were used for experiments in the present report. All pups within each litter underwent the same postnatal treatment. Litters were designated as either NS controls (6 litters) or MS15 (6 litters).
Experimental design
On P1, NS litters were returned to their dam promptly after the litter was sexed and culled, whereas MS15 litters were placed in an incubator for the remainder of their first daily 15-minute maternal separation period. As in our previous studies (Card et al., 2005, Banihashemi and Rinaman, 2010), pups in MS15 litters were again separated from their dam for 15 minutes on P2 and on each subsequent day through P10, at approximately the same time each day (i.e., ~1445 -1500 hr). At the beginning of each daily MS15, the dam was briefly removed from the home cage and placed into a dedicated polyethylene tub. Using gloved hands, all of the pups were removed from their home cage along with a handful of home cage bedding and transferred together into a smaller polyethylene tub (26.7 × 15.2 × 12.7 cm). The dam was then returned to her home cage and the small tub containing the pups was promptly placed into an incubator (~36°C, ~30-50% humidity) in an adjacent room. MS15 litters remained in the incubator for 15 minutes and were then returned simultaneously to their dam. NS and MS15 litters and dams underwent a weekly move to a clean cage with fresh bedding, consistent with standard animal housing procedures.
Rats were weaned on P21 and group-housed thereafter with same-sex littermates. On P30, a subset of male rats from each litter underwent surgery to microinject FG retrograde tracer into the DVC, as described below (see “DVC FG injections”; NS n = 18, MS15 n = 21). The remaining male rats from each litter were left surgically intact (NS n = 11, MS15 n = 10) and were used to assess stress-induced Fos expression within the DVC and additional brain regions. On P37, FG-injected rats and surgically-intact littermates were exposed to a 15-minute restraint stress or were assigned to the unrestrained control condition (see “Restraint stress exposure,” below). Experimental groups were typically derived from four to five litters with one to two pups per litter for each group to minimize potential litter effects on experimental outcomes. Our rationale for selecting this “juvenile” developmental stage was to choose a time-point that was post-weaning and prior to sexual maturation (between P45 and P48, Harlan) and to extend our previous findings in male juvenile rats (Banihashemi and Rinaman, 2010).
DVC FG injections
Rats were anesthetized by isoflurane inhalation (~2% in oxygen) and mounted into a stereotaxic frame with the neck ventroflexed. A longitudinal incision was made through the skin overlying the occipital ridge. Dorsal neck muscles were retracted to reveal the atlanto-occipital membrane overlying the roof of the fourth ventricle. The membrane was opened to reveal the area postrema (AP) on the caudal floor of the fourth ventricle. A 1.0-μl Hamilton syringe equipped with a fine glass tip filled with FG (Fluorochrome, Inc., Englewood, CO; 2.0% in 0.15 M NaCl) was positioned on one lateral edge of the AP at its mid-rostrocaudal extent (corresponding to bregma level -13.80 mm), and then lowered 0.25 mm into the medial NST-DMV. Once lowered, the syringe was left in place for two minutes, and then a total volume of 50 nl of FG was pressure-injected over 2.5 minutes. The syringe was left in place for two minutes after tracer injection and then withdrawn. The incision sites were closed and rats were returned to their home cage after recovery from anesthesia.
Restraint stress exposure
In terminal studies on P37, a subset of FG-injected and surgically-intact rats were exposed to 15 minutes of restraint stress in a clear plastic cylindrical tube (Kent Scientific Corporation, Torrington, CT), while the remaining rats served as unrestrained controls [DVC FG injections: NS-unrestrained n = 7, NS-restrained n = 11, MS15-unrestrained n = 7, MS15-restrained n = 14 (see Results for final group sizes); Surgically-intact: NS-unrestrained n = 5, NS-restrained n= 6, MS15-unrestrained n = 5, MS15-restrained n = 5]. Experiments were performed in six replicate cohorts, with each cohort including rats derived from one NS and one MS15 litter. During each experiment, rats designated to receive restraint were placed individually into a polyethylene cage with fresh bedding, transported to an adjacent room, put into the restrainer, and then left undisturbed in the restrainer within the fresh cage for 15 minutes. At the end of restraint, the rat crawled out of the restrainer and the restrainer was removed from the cage. Rats remained in the same cage, which was returned to a shelf in the adjacent animal facility for an additional 60 minutes. Rats were then anesthetized and perfused with fixative (see “Perfusion and histology,” below) in order to capture maximal stress-induced neural Fos expression, which typically peaks 60 minutes after treatment-induced neural stimulation (for review see Kovacs, 1998). Restraints were staggered such that perfusions began every 20 minutes. Rats designated as unrestrained controls were perfused during each experiment either before or after restraint procedures and perfusions were complete, and this order was counterbalanced across cohorts. Restraints and perfusions were performed beginning in the morning and ending approximately 3 hours later (~0900 – 1200 hr).
Perfusion and histology
Rats were anesthetized by isoflurane inhalation (5% in oxygen) followed by sodium pentobarbital (Nembutal, 100 mg/kg BW, i.p.). Rats were then perfused transcardially with a brief saline rinse followed by 250 ml of fixative (4% paraformaldehyde in 0.1 M phosphate buffer with L-lysine and sodium metaperiodate) (McLean and Nakane, 1974). Brains were removed from the skull, postfixed overnight at 4°C, and then cryoprotected for 24-72 hours in 20% sucrose solution at 4°C. Coronal 35 μm-thick tissue sections were cut from the spinomedullary junction through the rostral extent of the corpus callosum using a sliding, freezing microtome. Sections were collected serially in six adjacent sets (sampling frequency of 210 μm within each set) and stored at -20°C in a cryopreservant solution (Watson et al., 1986).
Immunocytochemistry
One set of sections from each FG-injected rat and two sets from each intact rat were removed from storage and rinsed in buffer (0.1 M sodium phosphate, pH 7.4). Antisera were diluted in buffer containing 1% normal donkey serum and 0.3% Triton-X100. All tissue sections were initially processed for immunoperoxidase localization of Fos protein using a rabbit polyclonal antiserum (1:50,000; provided by Dr. Philip Larsen, Denmark) and Vectastain Elite ABC reagents (Vector Laboratories, Burlingame, CA). The specificity of this antibody for Fos has been reported (Rinaman et al., 1997). Sections were reacted with nickel sulfate-intensified diaminobenzidine (DAB) to generate a blue-black reaction product in the nuclei of Fos-positive cells. In surgically intact rats, one of the two sets of Fos-reacted sections was used for regional quantitative analyses of treatment-induced Fos activation, as described further below. From the second set of Fos-reacted sections from intact rats, forebrain sections were subsequently processed for dual immunoperoxidase localization of CRH using a polyclonal rabbit anti-CRH antiserum (1:15,000; Peninsula, Belmont, CA), while brainstem sections were processed for dual immunoperoxidase localization of the NA synthetic enzyme, dopamine-beta-hydroxylase (DbH), using a monoclonal mouse anti-DbH antibody (1:30,000; Millipore, Temecula, CA). The specificity of the CRH and DbH antibodies for their respective antigens has been reported (Rinaman, 2001, Tagliaferro and Morales, 2008). In FG-injected rats, Fos-labeled tissue sections were subsequently processed for dual immunoperoxidase localization of FG (rabbit anti-FG: Chemicon; 1:30,000). Non-intensified DAB was used to generate a brown reaction product to reveal CRH, DbH, and FG immunolabeling. Single and dual-peroxidase labeled sections were mounted onto Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA), dehydrated in graded alcohols, cleared in xylene, and coverslipped using Cytoseal 60 (VWR, West Chester, PA).
Microscopic analysis and data collection
Quantification of Fos activation within the PVN, BNST, PFC, and NST in surgically-intact rats
The total amount of Fos labeling in each region of interest (ROI) was quantified in single-labeled tissue sections from unrestrained and restrained surgically-intact rats. In each experimental case, two PVN sections (bregma levels -1.88 through -1.80 mm; Paxinos and Watson, 1998), two BNST sections (bregma levels -0.40 through -0.26 mm), two medial PFC sections (bregma levels 2.20 through 2.70 mm), and three sections through the caudal visceral NST (i.e., through three rostrocaudal levels of the AP; bregma levels -14.08 through -13.68 mm) were analyzed bilaterally. For each brain region, rostrocaudal levels selected for analysis were those that contained the densest distribution of FG labeling from the DVC in rats with tracer injections, and/or contained the most robust Fos labeling after restraint (see Results). Fos labeling within dorsolateral and ventrolateral subnuclei of the BNST was quantified separately. The CeA was excluded from the analysis of single-Fos labeling because restraint does not induce robust Fos activation within this region (Crane et al., 2005), a conclusion supported by qualitative observations in the present study (see Results). However, the CeA was included in quantification of FG retrograde labeling and FG+Fos double labeling to test the hypothesis that MS15 alters stress-induced recruitment of DVC-projecting CeA neurons (see below, “Quantification of FG and Fos labeling in DVC tracer rats”).
ROIs within each tissue section were photographed using an Olympus photomicroscope and a Hamamatsu digital camera (Hamamatsu Photonics, Hamamatsu, Japan). Image analysis was performed using SimplePCI imaging software (Hamamatsu Corporation, Sewickly, PA, USA) as previously described (Bienkowski and Rinaman, 2008, Banihashemi and Rinaman, 2010). Briefly, the ROI was manually outlined and then two thresholds were set, one for the detection of Fos labeling intensity and another for the detection of the size of individual Fos-positive profiles. The threshold for labeling intensity was set to exclude Fos-positive profiles that were very lightly labeled to avoid detection of false-positives. The threshold for size was set to exclude objects too small to be considered a Fos-positive nucleus (e.g., peroxidase-labeled red blood cells or immunoperoxidase reaction artifacts). The size threshold was also intended to avoid situations in which a single Fos-positive nucleus could be quantified as multiple “objects.” Thus, labeling that met threshold for intensity would be excluded if sub-threshold for size. The final “object count” for each ROI was equal to the number of Fos-positive profiles that exceeded both detection thresholds. Established thresholds were held constant for all analyses within each brain region and across all cases.
Quantification of double labeling in surgically-intact rats
Criteria for counting a neuron as DbH- or CRH-positive included the presence of brown cytoplasmic immunoreactivity and a visible nucleus. Neurons were considered Fos-positive (i.e., double-labeled) if their nucleus contained blue-black immunolabeling, regardless of intensity, and Fos-negative if their nucleus was unlabeled. Hindbrain tissue sections were analyzed with a light microscope at 40X magnification to determine the number of double-labeled, DbH+Fos-positive NA neurons within the NST and ventrolateral medulla (VLM). As for the single-label Fos analysis described above, double labeled DbH+Fos-positive NST neurons were quantified bilaterally through three rostrocaudal levels of the AP, where restraint-induced Fos activation was maximal. Counts of DbH+Fos double-labeled neurons within the VLM were made bilaterally in the same sections used for NST labeling analysis.
Forebrain tissue sections were analyzed with a light microscope at 40x magnification to determine the number of double-labeled CRH+Fos-positive neurons within the PVN. In each experimental case, two PVN sections (bregma levels -2.12 through -1.80 mm) were analyzed bilaterally.
Quantification of FG and Fos labeling in tracer-injected rats
Tissue sections from DVC tracer-injected rats were analyzed with a light microscope to determine the number of retrogradely-labeled, FG-positive neurons within each brain ROI, and the proportion of FG-labeled neurons that were Fos-positive after restraint or the unrestrained control condition. FG-positive neurons were counted bilaterally in two sections through the PVN (bregma levels -2.12 through -1.80 mm) where their distribution was most dense. PVN labeling was quantified by mapping the distribution of single- and double-labeled FG-positive neurons using an image analysis system and StereoInvestigator software (Microbrightfield, Inc., Williston, VT). Tissue section boundaries and landmarks were outlined at low magnification (2.5X objective). At higher magnification (40X objective), each section was systematically examined and each FG-positive neuron was plotted individually to indicate whether it was single-labeled (i.e., Fos-negative), or double-labeled (i.e., Fos-positive). The Stereoinvestigator software tallied the number of single- and double-labeled neurons plotted within each section.
Retrograde labeling within the dBNST, CeA, and mPFC was less dense compared to labeling within the PVN; thus, FG-positive neurons in these structures were not plotted but were instead quantified visually using a light microscope to record the total number of retrogradely-labeled neurons and the proportion that were double-labeled (i.e., FG+Fos-positive). In each experimental case, labeling was quantified bilaterally in two dBNST sections (bregma levels -0.40 through -0.26 mm), five CeA sections (bregma levels -2.80 through -1.80 mm), and two mPFC sections (bregma levels 2.20 through 2.70 mm)(Paxinos and Watson, 1998).
Data analysis
Rostrocaudal assessments of labeling were planned a priori for the PVN and NST, as each region contains distinct subdivisions and topography that are differentially evident at discrete rostrocaudal levels (Paxinos, 2004, Simmons and Swanson, 2009). For example, our previous report demonstrated a significant effect of MS15 to alter transneuronal viral labeling of preautonomic neurons at only one distinct rostrocaudal level of the PVN (Banihashemi and Rinaman, 2010). Thus, single- and double-labeling data within the PVN and NST regions were analyzed initially by separate ANOVAs organized by rostrocaudal level. If no rostrocaudal level-specific differences were revealed, data from each brain region were collapsed across rostrocaudal levels and then re-examined collectively for the entire region.
Within each brain ROI (and initially within each rostrocaudal level of the PVN and NST, as described above), two-way ANOVAs were used to reveal potential main effects and interactions of postnatal experience (MS15 vs. NS) and/or stress condition (restraint vs. unrestrained control) on Fos activation in single- and double-labeled tissue sections. Total counts of FG-positive neurons within each region were similarly examined to determine potential group differences in retrogradely-labeled neurons from DVC injection sites. When F values indicated significant main effects and/or interactions of stress condition or postnatal experience, ANOVAs were followed by independent samples t-tests. Differences were considered statistically significant when p ≤ 0.05. Data were also evaluated to ensure the lack of litter effects on experimental outcomes.
RESULTS
Effect of postnatal experience on stress-induced Fos activation in the forebrain
Paraventricular nucleus of the hypothalamus
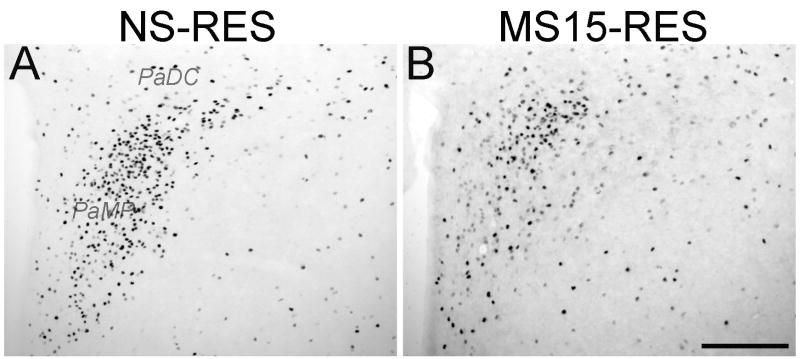
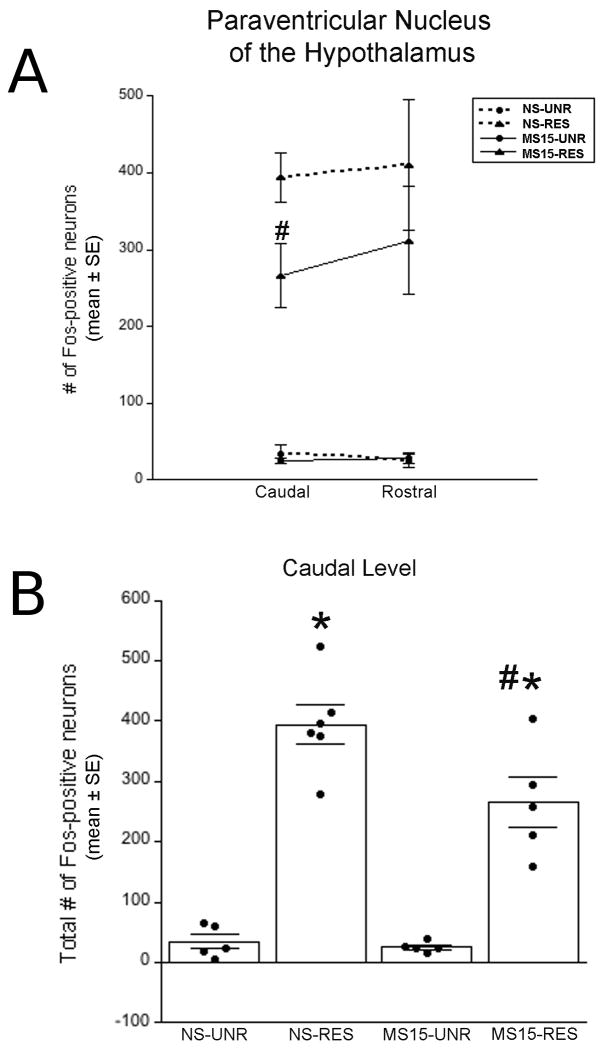
Fos labeling data were analyzed in 21 surgically-intact rats (NS-unrestrained n = 5, NS-restrained n= 6, MS15-unrestrained n = 5, MS15-restrained n = 5). Restraint-induced Fos labeling within the PVN was most prevalent in sections (corresponding to bregma levels -1.88 through -1.80 mm) that contained both the medial parvocellular (PaMP) and dorsal cap subnuclei (PaDC), and appeared to be densest within the PaMP subdivision (Fig. 1). Unrestrained control rats from both postnatal groups displayed very little PVN Fos activation (Figs. 2A and B), with no difference between postnatal groups. There was a clear trend for MS15-restrained rats to display fewer Fos-positive PVN neurons compared to NS-restrained rats at both rostrocaudal levels examined (Fig. 2A). However, this trend was statistically significant only at the caudal PVN level (Fig. 2A). At this level, two-way ANOVA revealed a significant interaction between postnatal group and stress condition on the number of Fos-positive neurons [F(1,20) = 4.59; p = 0.05; Fig. 2B]. Independent t-tests at the caudal PVN level revealed that while restraint significantly increased the number of Fos-positive neurons in both NS and MS15 groups compared to Fos activation in unrestrained controls (p < 0.01 for each comparison; Fig. 2B), restraint-induced Fos labeling was significantly less pronounced in MS15 rats compared to NS rats (p = 0.04; Figs. 1 and 2B).
Fig. 1. Photomicrographs depicting restraint-induced Fos activation within the PVN in (A) NS and (B) MS15 cases.
MS15 rats displayed less restraint (RES)-induced Fos activation than NS rats. This difference was significant at the rostrocaudal level depicted in these photomicrographs (see Fig. 2). Scale bar (in B) = 200 μm, applies to both panels. PaDC; PVN dorsal cap: PaMP: PVN medial parvocellular.
Fig. 2. Quantification of Fos-positive PVN neurons.
(A) Line graph depicting the number of Fos-positive PVN neurons counted at two rostrocaudal levels. Compared to NS rats, MS15 rats displayed significantly less Fos activation at the caudal level of the PVN after restraint (RES). (B) Bar graph depicting the total number of Fos-positive neurons within the caudal PVN in unrestrained (UNR) control and RES cases within both postnatal groups (overlaid dot plots depict data from individual cases). RES significantly increased the number of Fos-positive PVN neurons in both postnatal groups; however, this effect was significantly attenuated in the MS15 group. # p < 0.05 between postnatal groups, * p < 0.05 within postnatal group
In an effort to identify a phenotypic population of PVN neurons that might contribute to the postnatal group difference in restraint-induced Fos labeling, we examined Fos expression within immunocytochemically-identified CRH-positive PVN neurons. Two-way ANOVA revealed no interaction between postnatal group and stress condition on the number of CRH-positive PVN neurons that were double-labeled for Fos [F(1,20) = 0.06; p = 0.80]. However, a significant main effect of stress condition (unrestrained vs. restrained) was revealed [F(1,20) = 71.34; p < 0.01], with no main effect of postnatal group [F(1,20) = 0.04; p = 0.85]. Thus, restraint significantly increased the number of CRH+Fos-positive neurons equivalently within both NS and MS15 groups compared to unrestrained controls (p < 0.01 for both comparisons; NS-unrestrained: 3.8 ± 1.3 vs. NS-restrained: 173.5 ± 32.7, MS15-unrestrained: 2.6 ± 0.5 vs. MS15-restrained: 182.8 ± 17.2). Thus, postnatal experience did not alter the ability of restraint to activate CRH-positive PVN neurons.
Bed Nucleus of the Stria Terminalis
Dorsolateral BNST (dBNST)
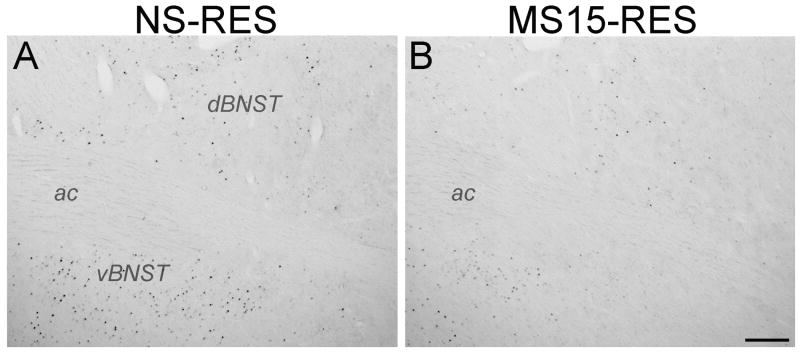
Two-way ANOVA revealed a significant interaction between postnatal group and stress condition on dBNST Fos labeling [F(1,20) = 6.01; p = 0.03, Figs. 3 and 4A]. Independent samples t-tests demonstrated that restraint increased the number of Fos-positive dBNST neurons in both postnatal groups (NS-unrestrained vs. NS-restrained: p < 0.01; MS15-unrestrained vs. MS15-restrained: p = 0.04). However, MS15 rats displayed significantly less restraint-induced Fos expression within the dBNST compared to NS controls (NS-restrained vs. MS15-restrained: p = 0.01, Figs. 3 and 4A).
Fig. 3. Photomicrographs depicting restraint stress-induced Fos activation within the BNST in (A) NS and (B) MS15 cases.
MS15 rats displayed less restraint (RES)-induced Fos activation within the dorsolateral and ventrolateral BNST (dBNST and vBNST, respectively) compared to NS controls. Scale bar (in B) = 200 μm, applies to both panels. ac, anterior commissure.
Fig. 4. Fos-positive neurons within the BNST.
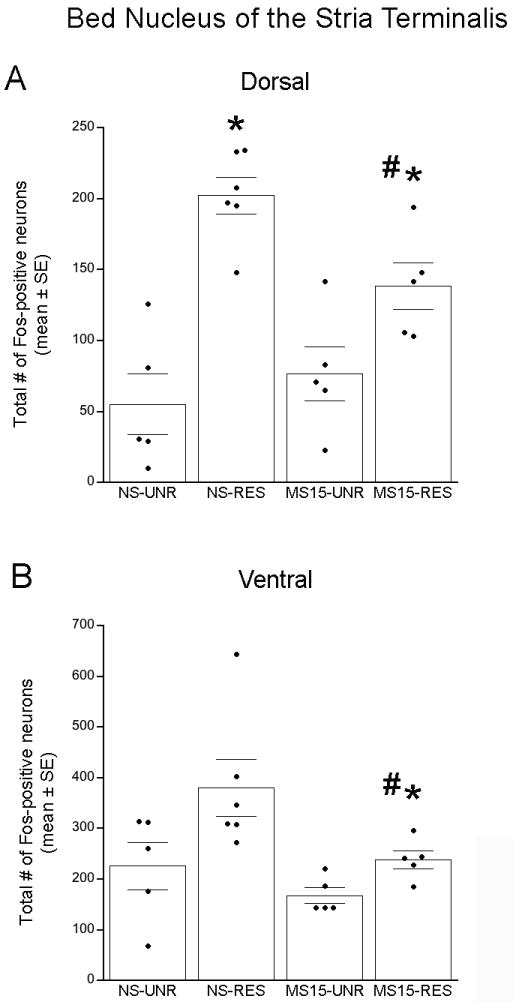
(A) Restraint (RES) significantly increased the number of Fos-positive neurons within the dorsolateral BNST in both postnatal groups; however, the effect of RES was significantly attenuated in the MS15 group. (B) There was a similar trend in the ventrolateral BNST, in which MS15-RS rats displayed significantly less RES-induced Fos activation compared to the NS-RES group. # p < 0.05 between postnatal groups; * p < 0.05 within postnatal group).
Ventrolateral BNST (vBNST)
Two-way ANOVA revealed no significant interaction [F(1,20) = 1.02; p = 0.33] on Fos labeling within the vBNST (Figs. 3 and 4B) but did reveal significant main effects of both postnatal group [F(1,20) = 5.97; p = 0.03] and stress condition [F(1,20) = 7.57; p = 0.01]. Independent samples t-tests demonstrated that, compared to Fos labeling in unrestrained controls, restraint increased the number of Fos-positive vBNST neurons within the MS15 group, but not within the NS group (see Fig. 4B). Further, there was a significant difference between postnatal groups after restraint (p = 0.05; Figs. 3 and 4B), with MS15 rats displaying less restraint-induced Fos expression within the vBNST compared to NS rats.
Prefrontal Cortex
Two-way ANOVA revealed no interaction between postnatal group and stress condition [F(1,20) = 0.18; p = 0.68] and no main effect of postnatal group [F(1,20) = 0.46; p = 0.51]. However, there was a significant main effect of stress condition (unrestrained vs. restrained) on the number of Fos-positive neurons within the mPFC [F(1,20) = 43.49; p < 0.01]. Thus, postnatal experience did not alter the ability of restraint to activate Fos expression within the mPFC (MS15-unrestrained vs. MS15-restrained: p = 0.01; NS-unrestrained: 615.8 ± 234.5, NS-restrained: 2534.67 ± 272.2, MS15-unrestrained: 544.60 ± 119.6, MS15-restrained: 2234.6 ± 389.4).
Effect of postnatal experience on stress-induced Fos activation in the hindbrain
Nucleus of the solitary tract
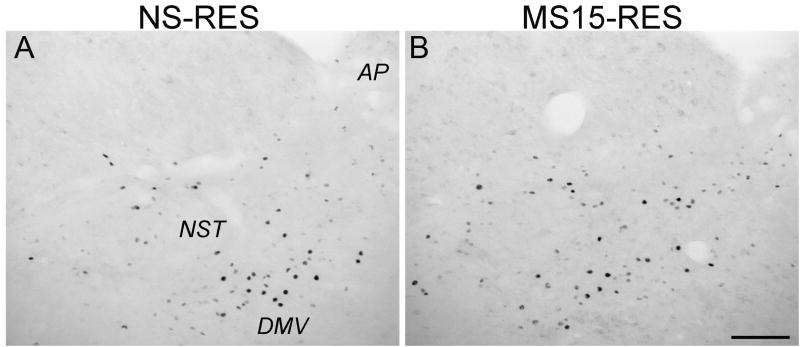
Restraint-induced DVC Fos activation was restricted primarily to the NST in both postnatal groups, with little to no Fos labeling within the DMV (Fig. 5). Unrestrained control cases within both postnatal groups displayed relatively little Fos within the NST or other DVC subregions. Two-way ANOVA revealed no interaction between postnatal group and stress condition [F(1,20) = 0.00; p = 0.97; Fig. 5] and no effect of postnatal group on NST Fos activation [F(1,20) = 0.68; p = 0.42]. However, there was a significant main effect of stress condition (unrestrained vs. restrained) on the number of Fos-positive neurons within the NST [F(1,20) = 41.11; p < 0.01]. T-tests confirmed significant and equivalent effects of restraint to increase NST Fos labeling in both postnatal groups compared to unrestrained controls (p < 0.01 for both comparisons; NS-unrestrained: 36.20 ± 17.30 vs. NS-restrained: 132.50 ± 11.36, MS15-unrestrained: 24.40 ± 8.35 vs. MS15-restrained: 119.60 ± 20.65).
Fig. 5. Photomicrographs depicting restraint-induced Fos activation within the NST in (A) NS and (B) MS15 cases.
There was no postnatal group difference in restraint (RES)-induced Fos activation within the NST. Scale bar (in B) = 200 μm, applies to both panels. AP, area postrema; DMV, dorsal motor nucleus of the vagus; NST, nucleus of the solitary tract.
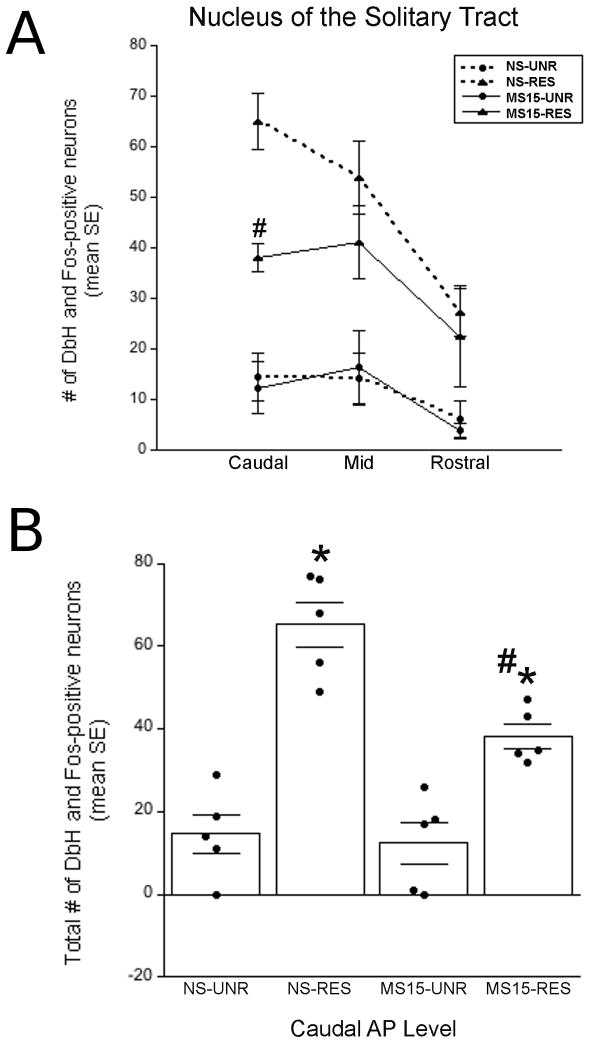
Despite the overall lack of postnatal group effect on restraint-induced Fos activation within the NST, we hypothesized that postnatal experience might differentially affect the ability of restraint to recruit a subpopulation of neurons comprising the NA A2 cell group that occupies the caudal visceral NST. To test this hypothesis, we counted the number of DbH+Fos-positive (i.e., double-labeled) neurons within the NST. Few DbH-positive NST neurons expressed Fos in unrestrained controls from either postnatal group (Fig. 6A and B; Fig. 7A and B). Compared to rats from NS litters, rats from MS15 litters displayed a trend towards less restraint-induced activation of DbH-positive neurons at all three rostrocaudal NST levels examined (Fig. 7A). However, the effect of postnatal group was significant only at the most caudal level of the AP. At this level, two-way ANOVA revealed a significant interaction between postnatal group and stress condition [F(1,19) = 7.02; p = 0.02; Figs. 6 and 7B]. T-tests at this caudal NST level revealed that while restraint significantly increased the number of DbH+Fos-positive neurons in both postnatal groups (p < 0.01 for both comparisons), the restraint-induced activation of DbH-positive neurons was significantly attenuated in MS15 rats compared to NS rats (p < 0.01; Figs. 6 and 7B).
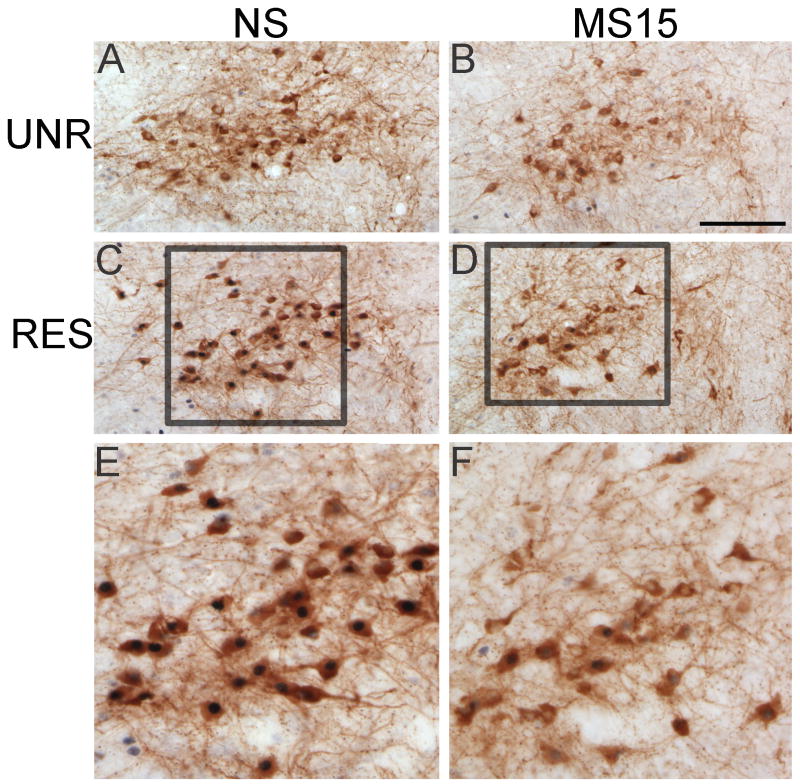
Fig. 6. Photomicrographs of DbH and Fos labeling within the NST in NS (A and C) and MS15 (B and D) cases.
There was no postnatal group difference in the number of double-labeled neurons among unrestrained (UNR) controls (A vs. B). However, MS15 rats displayed significantly fewer double-labeled neurons in response to restraint (RES) compared to NS controls (C vs. D). See E and F for higher-magnification views of the outlined areas in C and D. Scale bar (in B) = 200 μm, applies to panels A-D.
Fig. 7. Fos and DbH-positive neurons within the NST.
(A) Line graph depicting double-labeled neurons at three rostrocaudal levels of the NST. At the caudal level, there was a significant difference between the NS and MS15 groups in the number of DbH neurons activated to express Fos after restraint (RES). (B) Bar graph depicting the total number of double-labeled (DbH+Fos) neurons within the caudal level of the NST in unrestrained (UNR) control and RES rats from both postnatal groups. RES significantly increased the number of double-labeled neurons in both postnatal groups; however, this effect was attenuated in the MS15 group. Overlaid dot plots depict data from individual cases. # p < 0.05 between postnatal groups; * p< 0.05 within postnatal group.
Ventrolateral Medulla
Two-way ANOVA revealed no interaction between postnatal group and stress condition on the number of DbH+Fos-positive VLM neurons comprising the A1 NA cell group [F(1,20) = 1.23; p = 0.28] and no main effect of postnatal group [F(1,20) = 1.44; p = 0.25]. However, there was a significant main effect of stress condition (unrestrained vs. restrained) [F(1,20) = 183.00; p < 0.01]. T-tests demonstrated that, compared to the unrestrained control condition, restraint significantly and equivalently increased the number of DbH+Fos-positive VLM neurons within both postnatal groups (p < 0.01 for both comparisons; NS-unrestrained: 3.6 ± 1.4 vs. NS-restrained: 101.7 ± 7.7, MS15-unrestrained: 3.0 ± 1.8 vs. MS15-restrained: 82.5 ± 12.4).
Effect of postnatal experience on stress-induced activation of DVC-projecting forebrain neurons
DVC FluoroGold injection sites
Of the 39 rats that underwent DVC FG injection, 11 were excluded as missed injections. Tissue sections from the remaining 28 rats were included in data analyses (NS-unrestrained n = 7, NS-restrained n= 7, MS15-unrestrained n = 7, MS15-restrained n = 7). Tracer injection sites targeted the mid-NST/DMV and were intentionally large in order to maximize the amount of retrograde labeling achieved. Previous reports have demonstrated that the PVN, BNST, CeA, and mPFC provide little or no direct input to hindbrain regions immediately surrounding the DVC (Luiten et al., 1985, Saha et al., 2000, Dong et al., 2001, Gabbott et al., 2007). Labeling results from missed injections in our study confirmed this by producing little to no retrograde labeling within the PVN or other forebrain regions of interest when the FG injection site targeted structures situated dorsal (i.e., gracile or cuneate nuclei) or ventral (i.e., hypoglossal nucleus) to the mid-NST/DMV. Conversely, FG tracer injections centered within the mid-NST/DMV produced retrograde labeling in regions previously reported to project to the DVC (Gray and Magnuson, 1987, Danielsen et al., 1989, Rinaman, 2003b, Gabbott et al., 2007), including the mPFC (prelimbic and infralimbic cortices), CeA (primarily its lateral region), PVN (primarily its ventral parvicellular subdivision; see Fig. 8), and BNST (primarily its dorsolateral region; see Fig. 9).
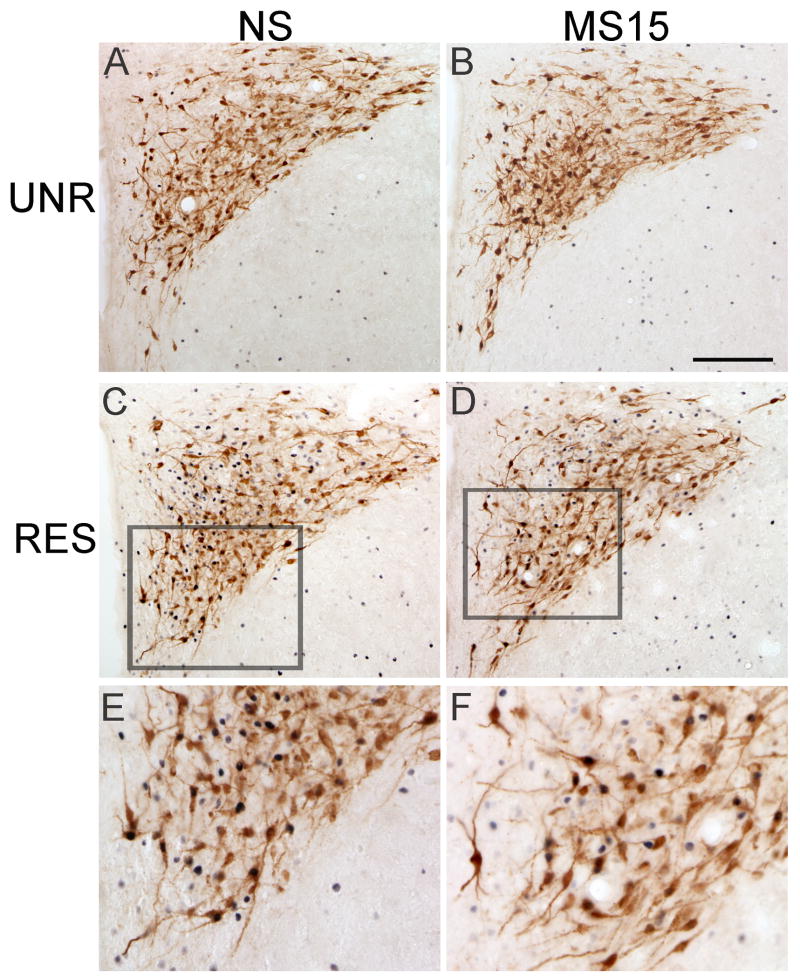
Fig. 8. Photomicrographs of FluoroGold and Fos labeling within the PVN in NS (A and C) and MS15 (B and D) cases.
There was no postnatal group difference in the number of FG-positive PVN neurons projecting to the DVC (A vs. B), or in the number of FG-positive neurons expressing Fos in response to restraint (RES, C vs. D). See panels E and F for higher magnification views of the outlined areas in C and D. Scale bar (in B) = 200 μm, applies to panels A-D.
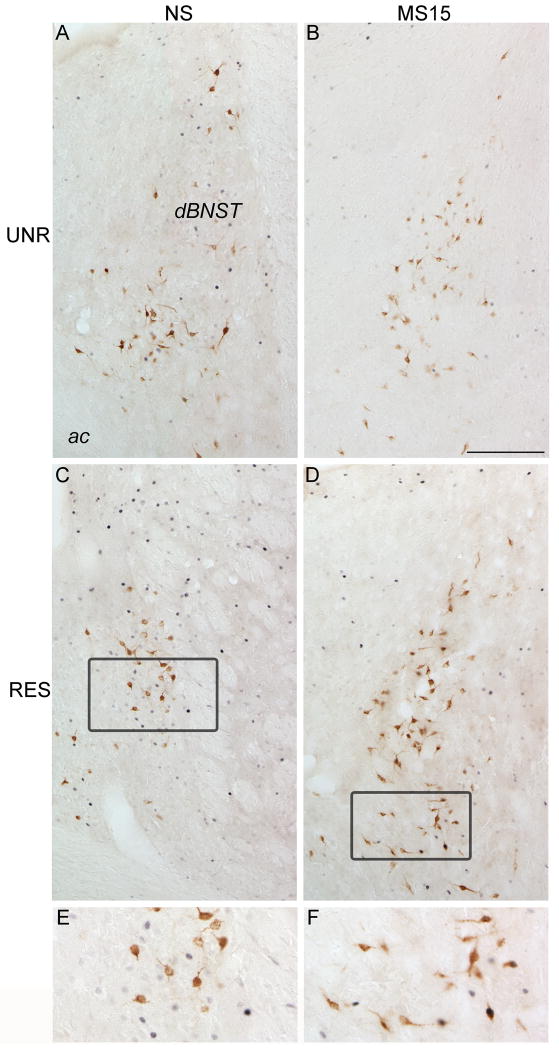
Fig. 9. Photomicrographs of FluoroGold and Fos labeling within the dosolateral BNST in NS (A and C) and MS15 (B and D) cases.
MS15-restrained (RES) rats displayed a greater proportion of DVC-projecting, FG-positive neurons that expressed Fos compared to NS-RES rats (C vs. D). See panels E and F for higher magnification views of the outlined areas in C and D. Scale bar (in B) = 200 μm, applies to panels A-D.
There was no significant effect of postnatal group on the total number of retrogradely-labeled, DVC-projecting (i.e., FG-positive) neurons within any forebrain region analyzed, including no significant differences between rats assigned to the unrestrained control vs. restraint stress conditions. Thus, early life experience had no discernable effect on the number of hypothalamic and limbic forebrain neurons projecting to the DVC. Retrograde labeling and Fos data are summarized in Table 1.
Table 1.
Number of FG-positive neurons and the number and proportion of these expressing Fos across forebrain regions of interest, reported by postnatal group (NS vs. MS15) and stress condition (unrestrained, UNR vs. restrained, RES). All values represent group mean ± SE. n=14 rats per postnatal group and 7 per stress condition.
| Brain region | Postnatal Group | Stress Condition | # of FG-positive neurons | # of FG+Fos-positive neurons | Proportion of FG-positive neurons expressing Fos |
|---|---|---|---|---|---|
| PVN | NS | UNR | 808.0 ± 46.9 | 40.0 ± 5.0 | 5.0% ± 0.5 |
| RES | 778.7 ± 38.8 | 119.7 ± 15.7 * | 15.4% ± 1.9 * | ||
| MS15 | UNR | 811.4 ± 60.4 | 40.6 ± 11.3 | 4.7% ± 1.3 | |
| RES | 780.7 ± 62.7 | 109.3 ± 19.3 * | 14.1% ± 1.9 * | ||
| dBNST | NS | UNR | 73.0 ± 11.2 | 1.4 ± 0.8 | 1.5% ± 0.8 |
| RES | 71.9 ± 13.3 | 0.4 ± 0.2 | 0.5% ± 0.2 | ||
| MS15 | UNR | 83.3 ± 9.4 | 0.6 ± 0.4 | 0.8% ± 0.5 | |
| RES | 67.0 ± 17.0 | 1.9 ± 0.9 | 3.3% ± 1.3 # | ||
| CeA | NS | UNR | 589.3 ± 56.4 | 5.0 ± 1.8 | 0.8% ± 0.3 |
| RES | 711.4 ± 115.5 | 5.4 ± 2.1 | 0.6% ± 0.2 | ||
| MS15 | UNR | 758.4 ± 72.4 | 4.4 ± 0.9 | 0.7% ± 0.2 | |
| RES | 638.9 ± 117.7 | 7.6 ± 3.3 | 1.3% ± 0.4 | ||
| mPFC | NS | UNR | 318.9 ± 50.2 | 15.1 ± 4.6 | 4.9% ± 1.1 |
| RES | 316.6 ± 33.9 | 43.3 ± 7.2 * | 13.3% ± 1.1 * | ||
| MS15 | UNR | 371.0 ± 38.4 | 13.4 ± 3.2 | 3.6% ± 0.7 | |
| RES | 326.9 ± 43.7 | 46.6 ± 8.1 * | 14.0% ± 0.6 * |
p < 0.05, significant difference within postnatal group, between stress conditions;
p < 0.05, significant difference between postnatal groups, within stress condition
As described in more detail below, restraint significantly increased both the total number of FG+Fos-positive neurons and proportion of FG-positive neurons that were double-labeled for Fos within the PVN and mPFC in both postnatal groups (Table 1). However, there was no additional effect of postnatal group on the total number of FG+Fos-positive neurons in any brain region, and no significant interactions between postnatal group and stress condition. However, when the proportion of FG-positive neurons that were also Fos-positive was compared within each brain region, a significant interaction between postnatal group and stress condition was revealed exclusively within the dBNST.
Paraventricular nucleus of the hypothalamus
Retrograde FG labeling within the PVN was predominantly localized to the same regions (corresponding to bregma levels -2.12 through -1.80 mm) that contained the most robust Fos labeling after restraint, and was heaviest in the ventral “pre-parasympathetic” subnucleus (Luiten et al., 1985) (Fig. 8). Overall, FG labeling within the PVN appeared remarkably similar between NS and MS15 groups (see Fig. 8, A vs. B or C vs. D), an observation that was confirmed quantitatively (Table 1).
Two-way ANOVA revealed no interaction between postnatal group and stress condition [F(1,27) = 0.11; p = 0.75] on the proportion of FG-positive PVN neurons that expressed Fos and no main effect of postnatal group [F(1,27) = 0.26; p = 0.61]. However, there was a significant main effect of stress condition (unrestrained vs. restrained) [F(1,27) = 37.52; p < 0.01]. T-tests confirmed that restraint significantly increased the proportion of FG-positive neurons expressing Fos compared to unrestrained controls equivalently within both NS and MS15 groups (p < 0.01 for each comparison; Table 1).
Bed nucleus of the stria terminalis
The large majority of DVC-projecting BNST neurons were located within the dBNST (Fig. 9), where we focused our quantitative analyses (Fig. 10). Two-way ANOVA revealed a significant interaction between postnatal group and stress condition [F(1,27) = 4.91; p = 0.04] on the proportion of FG-positive neurons expressing Fos (Fig. 10; Table 1). Independent samples t-tests demonstrated that restraint did not significantly increase the proportion of retrogradely-labeled dBNST neurons that were activated within either postnatal group compared to the unrestrained control condition (Fig. 10; Table 1). However, restraint activated a small, yet significantly greater proportion of retrogradely labeled neurons in the MS15 postnatal group compared to the NS postnatal group (p = 0.05; Figs. 9 C vs. D, and 10; Table 1). Thus, MS15 increased the ability of restraint to activate DVC-projecting dBNST neurons.
Fig. 10. Bar graph depicting the percentage of DVC-projecting dosolateral BNST neurons expressing Fos.
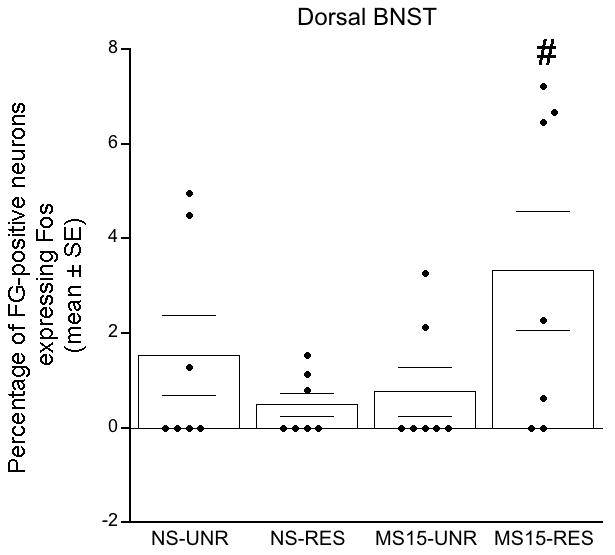
The MS15-restrained (RES) group displayed a significantly greater percentage of FG-positive neurons that expressed Fos compared to the NS-RES group. Overlaid dot plots depict data from individual cases.
Central nucleus of the amygdala
Two-way ANOVA revealed no interaction between postnatal group and stress condition on the proportion of FG-positive CeA neurons expressing Fos [F(1,27) = 2.29; p = 0.14] and no significant main effects of either postnatal group [F(1,27) = 0.90; p = 0.35] or stress condition [F(1,27) = 0.53; p = 0.47] (Table 1). Thus, postnatal experience did not significantly alter the overall activation of DVC-projecting CeA neurons in either stress condition.
Medial prefrontal cortex
Two-way ANOVA revealed no interaction between postnatal group and stress condition on the proportion of FG-positive mPFC neurons expressing Fos [F(1,27) = 1.15; p = 0.30] and no main effect of postnatal group [F(1,27) = 0.12; p = 0.73]. However, there was a significant main effect of stress condition (unrestrained vs. restrained) [F(1,27) = 102.9; p < 0.01]. T-tests confirmed that restraint significantly increased the proportion of FG-labeled neurons expressing Fos compared to the unrestrained condition equivalently within both NS and MS15 groups (Table 1).
DISCUSSION
Our previous study demonstrated that a developmental history of MS15 is associated with enhanced synaptic connections between the PVN and gastric autonomic output neurons in male juvenile rats (Banihashemi and Rinaman, 2010). This finding led us to hypothesize that MS15 would also alter the ability of stress to activate neuronal Fos expression within central preautonomic regions in male rats examined at the same developmental time-point. Results from the present study demonstrated that MS15 rats displayed less restraint stress-induced Fos activation of a specific population of NA neurons within the caudal visceral NST, without a decrease in overall Fos activation within the NST. Interestingly, MS15 rats also displayed increased stress-induced Fos activation within DVC-projecting dBNST neurons, but less stress-induced Fos activation within the BNST overall. Finally, MS15 rats showed less restraint stress-induced Fos activation within the caudal PVN, although this was not accompanied by decreased activation of CRH-positive or DVC-projecting PVN neurons.
MS15 alters NST, BNST, and PVN responses to restraint stress
MS15 rats displayed less restraint stress-induced Fos activation of NA neurons within the caudal NST compared to NS controls. Given the role of NA signaling within the DVC in stress-induced inhibition of gastric motility (Nagata and Osumi, 1993, Rogers et al., 2003, Pearson et al., 2007, Herman et al., 2008), less activation of these NA neurons after restraint suggests that stress-induced inhibition of gastric motility may be attenuated, which would be consistent with the MS15 phenotype of decreased stress reactivity. This effect of MS15 to reduce NA neuronal activation after restraint was predominantly localized to the caudal level of the AP, where DVC neurons receive vagal sensory input from the stomach and other viscera (Altschuler et al., 1989) together with descending input from the PVN, mPFC, CeA, BNST, and other stress-responsive brain regions (cf. Banihashemi and Rinaman, 2010).
Interestingly, the BNST sends primarily inhibitory projections to the DVC (Pickel et al., 1995, Saha et al., 2000, Dong et al., 2001) and stimulation of the BNST increases vagally-mediated gastric motility (Henke, 1982, Hermann et al., 1990). The decreased ability of restraint to activate Fos expression in NA neurons in MS15 rats could be due in part, to the observed increase in activation of DVC-projecting BNST neurons after restraint. This increase in stress-induced BNST activation in MS15 rats is selective for the DVC-projecting population, given the observed decrease in overall stress-induced BNST activation. Thus, increased recruitment of DVC-projecting GABAergic BNST neurons (Dong et al., 2001) may directly inhibit NA NST neurons, which would potentially attenuate stress-induced inhibition of gastric motility (Fig. 11). This is consistent with Henke’s theory that the lateral BNST acts to constrain autonomic responses to stress (Henke, 1984).
Fig. 11. Proposed model for MS15-related alterations in stress-related neural circuits and their potential functional consequences.
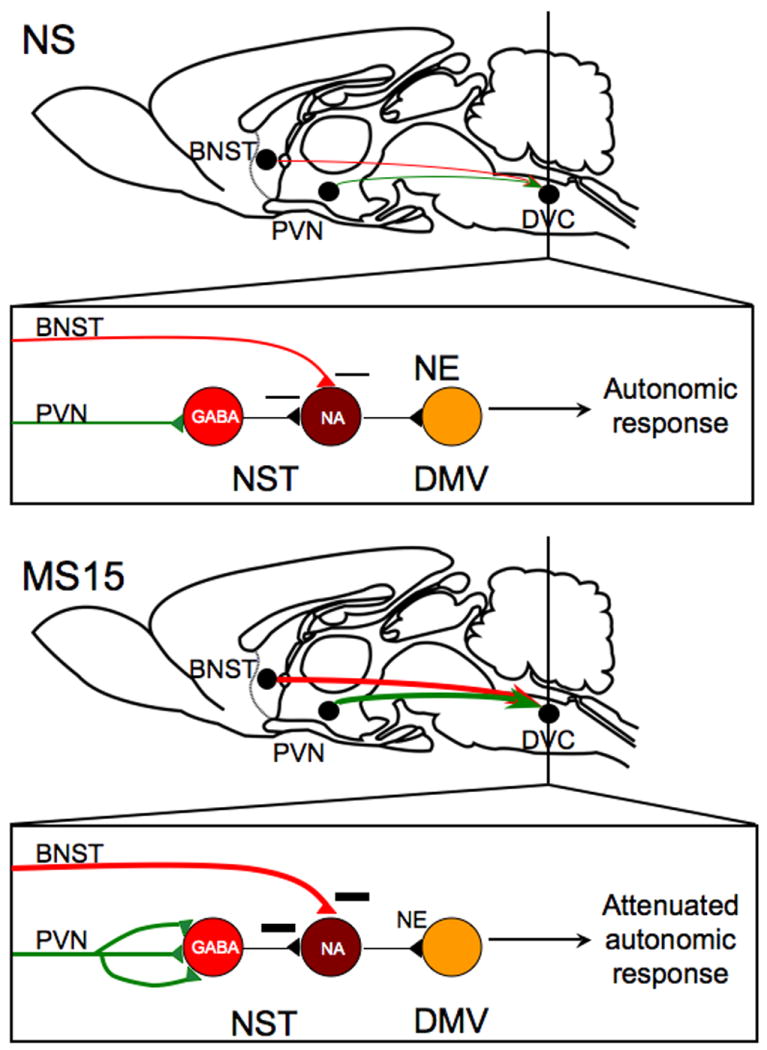
Our previous finding that MS15 rats display enhanced circuit strength of PVN gastric preautonomic circuits, taken together with our current finding that MS15 rats display decreased restraint-induced activation of noradrenergic (NA) neurons within the nucleus of the solitary tract (NST), suggests that increased synaptic contacts from the paraventricular nucleus of the hypothalamus (PVN) may primarily target local GABAergic neurons within the NST. Further, our current finding that MS15 rats display increased restraint-induced activation of dorsal vagal complex (DVC)-projecting dorsolateral bed nucleus of the stria terminalis (dBNST) neurons suggests that this descending projection might contribute to decreased activation of NA NST neurons after restraint. These findings support the view that restraint stress may cause less norepinephrine (NE) release within the dorsal motor nucleus of the vagus (DMV) in MS15 rats, which may result in attenuated autonomic responses to stress, perhaps including attenuated inhibition of gastric motility.
Our findings that juvenile rats with a developmental history of MS15 displayed less stress-induced Fos activation within the caudal PVN and dBNST concur with previous reports of attenuated Fos activation of hypothalamic and limbic forebrain regions in adult MS15 rats after stress (Abraham and Kovacs, 2000, Koehnle and Rinaman, 2010). Interestingly, the PVN region in which juvenile MS15 rats displayed a decrease in restraint-induced Fos is the same region in which juvenile MS15 rats display enhanced retrograde transynaptic viral transport from the stomach wall (Banihashemi and Rinaman, 2010). This PVN region also houses endocrine CRH neurons at the apex of the HPA axis. However, in the present study, there were no postnatal group differences in restraint-induced activation of CRH-positive PVN neurons. This finding conflicts with previous work demonstrating blunted HPA axis responses to acute stress in adult MS15 rats (Plotsky and Meaney, 1993; Macri et al., 2008), although double CRH+Fos immunolabeling data comparable to ours have not previously been reported. In addition, rats were examined as juveniles in the present study, and a postnatal group difference in restraint-induced Fos activation of CRH-containing PVN neurons might emerge later in development. Although there was no significant difference in Fos activation of DVC-projecting PVN neurons in MS15 rats compared to NS controls after restraint, decreased activation of spinally-projecting pre-sympathetic neurons within the dorsal cap subnuclei of the PVN (Luiten et al., 1985) may have contributed to the reduced Fos activation in MS15 rats.
Experimental stimulation of the PVN (which send primarily excitatory projections to the DVC) promotes inhibition of vagally-mediated gastric motility (Henke, 1982, Hurley-Gius and Neafsey, 1986, McCann and Rogers, 1991, Panteleev and Grundy, 2000), which mimics the effect of acute restraint stress in rats (Tache and Bonaz, 2007, Zheng et al., 2009). Thus, we hypothesized that MS15 rats would display less stress-induced activation of DVC-projecting PVN neurons. However, the ability of restraint stress to recruit DVC-projecting PVN neurons did not differ between these male juvenile MS15 and NS rats. The possibility remains open that stress-induced activation of specific phenotypic sub-populations of DVC-projecting neurons differs between postnatal groups. For example, DVC-projecting oxytocin and CRH neurons within the PVN contribute to DVC neuron activity, and have important influences on gastric autonomic outflow (Gunion and Tache, 1987, McCann and Rogers, 1990, Flanagan et al., 1992b, Monnikes et al., 1992). It is also possible that a postnatal group difference in the stress-induced activation of DVC-projecting PVN neurons would be detected at a later developmental time-point, although the proportion of DVC-projecting PVN neurons activated in response to restraint stress in these juvenile rats is similar to that previously reported in adult rats (Dayas et al., 2004). Additional work will be needed to determine whether MS15 exerts a specific effect on the stress responsiveness of these or other phenotypic subsets of neurons at this and later developmental time-points.
MS15 may increase synaptic contacts between the PVN and DVC
We previously reported that, compared to NS controls, MS15 rats display increased retrograde transynaptic viral labeling from the stomach to the PVN. The increased labeling could result from an increased number of PVN neurons in synaptic contact with DVC (or spinal) preganglionic neurons, an increase in the number of synaptic contacts formed by individual PVN neurons with infected postsynaptic targets, or both (Banihashemi and Rinaman, 2010). In the present study, retrograde FG labeling within the PVN and other forebrain regions of interest was remarkably similar between MS15 and NS rats, suggesting that the number of DVC-projecting neurons within the PVN and other brain regions examined does not differ in this model of altered early life experience. Thus, we can now propose that increased viral labeling within the PVN of MS15 rats is due to an increase in the number of synaptic contacts between individual PVN neurons and virally-infected postsynaptic target neurons. However, it is important to note that retrogradely-labeled neurons in the current study are a general population of DVC-projecting neurons, only a subset of which are directly preautonomic and/or pre-gastric, and that spinally-projecting pre-sympathetic forebrain neurons were not examined. It is possible that there are differences in the number of preautonomic/pre-gastric forebrain neurons in MS15 rats that are not revealed by the DVC retrograde tracing approach used in this study.
Previous research has emphasized the importance of the PVN in restraint stress-induced activation of NA neurons within the NST (Buller et al., 2003, Dayas et al., 2004). Thus, one might predict that the previously-reported enhancement of PVN inputs to gastric-related DVC neurons in MS15 rats (Banihashemi and Rinaman, 2010) would result in increased, rather than decreased, activation of NST NA neurons after restraint. One possible interpretation of our finding that restraint-induced NA activation is lower in MS15 rats compared to NS controls is that the postsynaptic targets of increased input from the PVN in MS15 rats may primarily include a known population of GABAergic interneurons within the NST (Meeley et al., 1985, Pickel et al., 1996), some of which inhibit NA neurons (Fig. 11). Blockade of GABAA receptors within the DVC produces robust decreases in vagally-mediated gastric tone, whereas GABAA receptor agonists increase gastric tone (Herman et al, 2009). Thus, in concert with reduced NA neuronal activation, restraint stress may activate more GABAergic NST neurons in MS15 rats compared to NS rats. This could account for our present finding that the total number of NST neurons activated to express Fos after restraint was not different between MS15 and NS rats. Previous studies have demonstrated that, compared to adult NS rats, adult MS15 rats have substantially increased levels of NST α2 adrenergic receptor binding (Liu et al., 2000) and increased NST GABAA receptor mRNA and binding (Caldji et al., 2000). The adrenergic α2 receptor acts presynaptically to reduce transmitter release from nerve terminals, and also acts as an inhibitory postsynaptic receptor (Wozniak et al., 1995). Previous work from our laboratory suggests that NA NST neurons collateralize locally (Rinaman, 2003a); thus, MS15 rats potentially release less norepinephrine (NE) within the NST during restraint stress, which could attenuate stress-induced inhibition of gastric motility. Overall, the evidence suggests that MS15 rats display increased GABAergic and decreased NA tone within the NST. Additional work will be needed to determine the effects of MS15 on stress-induced GABAergic NST neuron activation and whether gastric motor responses to restraint stress are indeed blunted in rats with an early life history of MS15.
Potential effect of MS15 to alter ascending NA signaling pathways
Ascending NA projections from the NST and the VLM densely innervate the PVN (Ter Horst et al., 1989, Rinaman, 2001), vBNST (Aston-Jones et al., 1999, Fendt et al., 2005, Banihashemi and Rinaman, 2006), and other brain regions (Rinaman, 2011), and stress significantly increases extracellular levels of NE in these regions (Pacak et al., 1995, Fuentealba et al., 2000, Cecchi et al., 2002). NA signaling within the BNST and PVN induces stress-like activation of HPA axis and autonomic responses, whereas interruption of NA signaling in these regions blunts the ability of stress to induce these responses (Bachelard et al., 1992, Pacak et al., 1995, Banihashemi and Rinaman, 2006, Bienkowski and Rinaman, 2008). Interestingly, many of the hindbrain NA neurons that innervate the BNST have axon collaterals that also project to the PVN (Banihashemi and Rinaman, 2006, Bienkowski and Rinaman, 2008) evidence that ascending NA projections from the caudal brainstem co-regulate BNST and PVN neural activity. Interestingly, MS15 rats display markedly lower levels of NE release within the PVN during and after restraint (Liu et al., 2000), which may be due to the reduced ability of restraint to activate NA NST neurons reported in the present study. Thus, potentially reduced stress-induced NA signaling from the caudal NST in MS15 rats may contribute to the overall decrease in restraint-induced Fos activation observed in the caudal PVN and vBNST after restraint in the present study. Our results did not reveal a similar effect of MS15 to reduce Fos activation in NA VLM neurons after restraint, suggesting that MS15-induced alterations in the NST contribute more importantly to alterations in stress-related circuitry and reactivity.
Summary and future directions
In summary, juvenile rats with a developmental history of MS15 display less stress-induced Fos activation in NA neurons within the caudal visceral NST compared to NS controls. Based on this and other findings in the present study, we speculate that the increased transneuronal viral labeling of PVN gastric preautonomic circuits observed in our previous report (Banihashemi and Rinaman, 2010) is due to increased synaptic inputs from the PVN to GABAergic neurons within the NST, thereby activating these neurons more robustly during restraint stress to promote increased local GABAergic inhibition of stress-responsive NA neurons. Further, stress-induced recruitment of inhibitory inputs from the dBNST to the DVC is also enhanced in MS15 rats, which could further contribute to a reduced ability of restraint to activate Fos expression within NA NST neurons (Fig. 11). We predict that these alterations in stress-related circuits will correspond to reduced autonomic responses to stress, e.g., reduced inhibition of gastric motility. Further research will be necessary to examine these hypotheses and predictions. The present study is the first to examine the long-term consequences of MS15 on the ability of stress to recruit neural activation within anatomically-defined central visceral circuits, and our results may provide insights into how early life experience shapes individual differences in life-long stress reactivity.
Highlights.
Repeated brief maternal separation of rat pups (MS15) suppresses later stress responsiveness
MS15 alters restraint stress-induced neural Fos expression in juvenile rats
Alterations occur in the hindbrain, hypothalamus, and limbic forebrain
Acknowledgments
We thank Victoria Maldovan Dzmura and Li Cai for their expert technical assistance. This work was supported by a University of Pittsburgh Andrew Mellon Predoctoral Fellowship to L.B., and by NIH grant #MH081817 (L.R.).
Abbreviations
- AP
Area postrema
- BNST
Bed nucleus of the stria terminalis
- dBNST
Bed nucleus of the stria terminalis, dorsolateral
- vBNST
Bed nucleus of the stria terminalis, ventrolateral
- CeA
Central nucleus of the amygdala
- CRH
Corticotropin-releasing hormone
- DbH
Dopamine-beta-hydroxylase
- DMV
Dorsal motor nucleus of the vagus
- DVC
Dorsal vagal complex
- FG
FluoroGold
- HPA
Hypothalamic-pituitary-adrenal
- MS15
Maternal separation, repeated brief
- mPFC
Medial prefrontal cortex
- NA
Noradrenergic
- NE
Norepinephrine
- NS
No separation
- NST
Nucleus of the solitary tract
- PVN
Paraventricular nucleus of the hypothalamus
- PaDC
Paraventricular nucleus of the hypothalamus, dorsal cap
- PaMP
Paraventricular nucleus of the hypothalamus, medial parvocellular
- P
Postnatal day
- PRV
Pseudorabies virus
- RES
Restrained/restraint
- ROI
Region of interest
- UNR
Unrestrained
- VLM
Ventrolateral medulla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham IM, Kovacs KJ. Postnatal handling alters the activation of stress-related neuronal circuitries. European Journal of Neuroscience. 2000;12:3003–3014. doi: 10.1046/j.1460-9568.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. The Journal of Comparative Neurology. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Annals of the New York Academy of Sciences. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Bachelard H, Harland D, Gardiner SM, Kemp PA, Bennett T. Regional haemodynamic effects of noradrenaline injected into the hypothalamic paraventricular nuclei of conscious, unrestrained rats: possible mechanisms of action. Neuroscience. 1992;47:941. doi: 10.1016/0306-4522(92)90042-z. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. The Journal of Neuroscience. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Repeated brief postnatal maternal separation enhances hypothalamic gastric autonomic circuits in juvenile rats. Neuroscience. 2010;165:265–277. doi: 10.1016/j.neuroscience.2009.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience. 2008;156:1093–1102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller KM, Dayas CV, Day TA. Descending pathways from the paraventricular nucleus contribute to the recruitment of brainstem nuclei following a systemic immune challenge. Neuroscience. 2003;118:189–203. doi: 10.1016/s0306-4522(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis DD, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABA A and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. The Journal of Neuroscience. 2005;25:9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Crane JW, French KR, Buller KM. Patterns of neuronal activation in the rat brain and spinal cord in response to increasing durations of restraint stress. Stress: The International Journal on the Biology of Stress. 2005;8:199–211. doi: 10.1080/10253890500333817. [DOI] [PubMed] [Google Scholar]
- Danielsen EH, Magnuson DJ, Gray TS. The central amygdaloid nucleus innervation of the dorsal vagal complex in rat: a phaseolus vulgaris leucoagglutinin lectin anterograde tracing study. Brain Research Bulletin. 1989;22:705–715. doi: 10.1016/0361-9230(89)90090-7. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Hypothalamic paraventricular nucleus neurons regulate medullary catecholamine cell responses to restraint strses. The Journal of Comparative Neurology. 2004;478:22–34. doi: 10.1002/cne.20259. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of Comparative Neurology. 2001;436 doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. The Journal of Neuroscience. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan LM, Dohanics J, Verbalis JG, Stricker EM. Gastric motility and food intake in rats after lesions of hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 1992a;263:R39–44. doi: 10.1152/ajpregu.1992.263.1.R39. [DOI] [PubMed] [Google Scholar]
- Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Research. 1992b;578:256–260. doi: 10.1016/0006-8993(92)90255-8. [DOI] [PubMed] [Google Scholar]
- Fuentealba JA, Forray MI, Gysling K. Chronic morphine treatment and withdrawal increase extracellular levels of norepinephrine in the rat bed nucleus of the stria terminalis. Journal of Neurochemistry. 2000;75:741–748. doi: 10.1046/j.1471-4159.2000.0750741.x. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner T, Busby SJ. Catecholaminergic neurons in medullary nuclei are among the post-synaptic targets of descending projections from infralimbic area 25 of the rat medial prefrontal cortex. Neuroscience. 2007;144:623–635. doi: 10.1016/j.neuroscience.2006.09.048. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. The Journal of Comparative Neurology. 1987;262:365–374. doi: 10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- Gunion MW, Tache Y. Intrahypothalamic microinfusion of corticotropin-releasing factor inhibits gastric acid secretion but increases secretion volume in rats. Brain Research. 1987;411:156–161. doi: 10.1016/0006-8993(87)90693-7. [DOI] [PubMed] [Google Scholar]
- Henke PG. The telencephalic limbic system and experimental gastric pathology: A review* 1. Neuroscience & Biobehavioral Reviews. 1982;6:381–390. doi: 10.1016/0149-7634(82)90047-1. [DOI] [PubMed] [Google Scholar]
- Herman MA, Cruz MT, Sahibzada N, Verbalis J, Gillis RA. GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296:G101. doi: 10.1152/ajpgi.90504.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Niedringhaus M, Alayan A, Verbalis JG, Sahibzada N, Gillis RA. Characterization of noradrenergic transmission at the dorsal motor nucleus of the vagus involved in reflex control of fundus tone. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology. 2008;294:R720. doi: 10.1152/ajpregu.00630.2007. [DOI] [PubMed] [Google Scholar]
- Hermann GE, McCann MJ, Rogers RC. Activation of the bed nucleus of the stria terminalis increases gastric motility in the rat. Journal of the Autonomic Nervous System. 1990;30:123–128. doi: 10.1016/0165-1838(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Hurley-Gius KM, Neafsey EJ. The medial frontal cortex and gastric motility: microstimulation results and their possible significance for the overall pattern of organization of rat frontal and parietal cortex. Brain Research. 1986;365:241–248. doi: 10.1016/0006-8993(86)91635-5. [DOI] [PubMed] [Google Scholar]
- Iwa M, Nakade Y, Pappas TN, Takahashi T. Electroacupuncture improves restraint stress-induced delay of gastric emptying via central glutaminergic pathways in conscious rats. Neuroscience Letters. 2006;399:6–10. doi: 10.1016/j.neulet.2005.11.069. [DOI] [PubMed] [Google Scholar]
- Jackson CM. On the recognition of sex through external characters in the young rat. Biological Bulletin. 1912;23 [Google Scholar]
- Koehnle TJ, Rinaman L. Early experience alters limbic forebrain Fos responses to a stressful interoceptive stimulus in young adult rats. Physiol Behav. 2010;100:105–115. doi: 10.1016/j.physbeh.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ. Invited review c-Fos as a transcription factor: a stressful (re) view from a functional map. Neurochemistry International. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinephrine release in the hypothalamic paraventricular nucleus. Journal of Neuroendocrinology. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic-Pituitary-Adrenal Responses to Stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Luiten PGM, Ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Research. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Macri S, Chiarotti F, Wurbel H. Maternal separation and maternal care act independently on the development of HPA responses in male rats. Behav Brain Res. 2008;191:227–234. doi: 10.1016/j.bbr.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. European Journal of Neuroscience. 2004;20:1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. Journal of Physiology. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Brain-Gut Interactions. CRC Press; 1991. Central modulation of the vagovagal reflex: influence on gastric function; pp. 58–67. [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformadehyde fixative. A new fixative for immunoelectron microscopy. Journal of Histochemistry and Cytochemistry. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Meeley MP, Ruggiero DA, Ishitsuka T, Reis DJ. Intrinsic gamma-aminobutyric acid neurons in the nucleus of the solitary tract and the rostral ventrolateral medulla of the rat: an immunocytochemical and biochemical study. Neuroscience Letters. 1985;58:83–89. doi: 10.1016/0304-3940(85)90333-7. [DOI] [PubMed] [Google Scholar]
- Monnikes H, Schmidt BG, Raybould HE, Tache Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol Gastrointest Liver Physiol. 1992;262:G137–143. doi: 10.1152/ajpgi.1992.262.1.G137. [DOI] [PubMed] [Google Scholar]
- Nagata M, Osumi Y. Central 2-adrenoceptor-mediated inhibition of gastric motility in rats. Japanese journal of pharmacology. 1993;62:329–330. doi: 10.1254/jjp.62.329. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Frontiers in Neuroendocrinology. 1995;16:89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- Panteleev S, Grundy D. Descending influences from the infralimbic cortex on vago-vagal reflex control of gastric motor activity in the rat. Autonomic Neuroscience. 2000;86:78–83. doi: 10.1016/S1566-0702(00)00249-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G. The rat nervous system. Academic Press; 2004. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pearson RJ, Gatti PJ, Sahibzada N, Massari VJ, Gillis RA. Ultrastructural evidence for selective noradrenergic innervation of CNS vagal projections to the fundus of the rat. Autonomic Neuroscience: Basic and Clinical. 2007;136:31–42. doi: 10.1016/j.autneu.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel V, Van Bockstaele EJ, Chan J, Cestari D. Amygdala efferents form inhibitory-type synapses with a subpopulation of catecholaminergic neurons in the rat nucleus tractus solitarius. The Journal of Comparative Neurology. 1995;362:510–523. doi: 10.1002/cne.903620406. [DOI] [PubMed] [Google Scholar]
- Pickel V, Van Bockstaele EJ, Chan J, Cestari D. GABAergic neurons in rat nuclei of solitary tracts receive inhibitory-type synapses from amygdaloid efferents lacking detectable GABA-immunoreactivity. Journal of Neuroscience Research. 1996;44:446–458. doi: 10.1002/(SICI)1097-4547(19960601)44:5<446::AID-JNR5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Postnatal development of catecholamine inputs to the paraventricular nucleus of the hypothalamus in rats. The Journal of Comparative Neurology. 2001;438:411–422. doi: 10.1002/cne.1324. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. The Journal of Neuroscience. 2003a;23:10084–10092. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiology & Behavior. 2003b;79:65–70. doi: 10.1016/s0031-9384(03)00105-7. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–R235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Levitt P, Card JP. Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus. The Journal of Neuroscience. 2000;20:2731–2741. doi: 10.1523/JNEUROSCI.20-07-02731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Roesch MR, Card JP. Retrograde transynaptic pseudorabies virus infection of central autonomic circuits in neonatal rats. Developmental Brain Research. 1999;114:207–216. doi: 10.1016/s0165-3806(99)00039-5. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Stricker EM, Hoffman GE, Verbalis JG. Central c-fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline. Neuroscience. 1997;79:1165–1175. doi: 10.1016/s0306-4522(97)00022-5. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2003;285:479. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Batten TFC, Henderson Z. A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience. 2000;99:613–626. doi: 10.1016/s0306-4522(00)00240-2. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: Toward a global 3D model. The Journal of Comparative Neurology. 2009;516 doi: 10.1002/cne.22126. [DOI] [PubMed] [Google Scholar]
- Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor functions. The Journal of Clinical Investigation. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. The Journal of Comparative Neurology. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst GJ, De Boer P, Luiten PGM, Van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31:785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. Rat medial frontal cortex: a visceral motor region with a direct projection to the solitary nucleus. Brain Research. 1983;278:245–249. doi: 10.1016/0006-8993(83)90246-9. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. 2006 doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Jr, Weigand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Schramm NL, Limbird LE. The noradrenergic receptor subtypes. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. [Google Scholar]
- Yang M, Card JP, Tirabassi RS, Miselis RR, Enquist LW. Retrograde, transneuronal spread of pseudorabies virus in defined neuronal circuitry of the rat brain is facilitated by gE mutations that reduce virulence. Journal of Virology. 1999;73:4350–4359. doi: 10.1128/jvi.73.5.4350-4359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Fogel R, Renehan WE. Stimulation of the paraventricular nucleus modulates the activity of gut-sensitive neurons in the vagal complex. American Journal of Physiology Gastrointestinal and Liver Physiology. 1999;277 doi: 10.1152/ajpgi.1999.277.1.G79. [DOI] [PubMed] [Google Scholar]
- Zheng J, Dobner A, Babygirija R, Ludwig K, Takahashi T. Effects of repeated restraint stress on gastric motility in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2009;296:R1358. doi: 10.1152/ajpregu.90928.2008. [DOI] [PubMed] [Google Scholar]