Abstract
Professional boxers and other contact sport athletes are exposed to repetitive brain trauma that may affect motor functions, cognitive performance, emotional regulation and social awareness. The term of chronic traumatic encephalopathy (CTE) was recently introduced to regroup a wide spectrum of symptoms such as cerebellar, pyramidal, and extrapyramidal syndromes, impairments in orientation, memory, language, attention, information processing and frontal executive functions, as well as personality changes and behavioural and psychiatric symptoms. Magnetic resonance imaging (MRI) usually reveals hippocampal and vermis atrophy, a cavum septum pellucidum (CSP), signs of diffuse axonal injury, pituitary gland atrophy, dilated perivascular spaces, and periventricular white matter disease. Given the partial overlapping of the clinical expression, epidemiology, and pathogenesis of CTE and Alzheimer’s disease (AD), as well as the close association between traumatic brain injuries (TBIs) and neurofibrillary tangle formation, a mixed pathology promoted by pathogenetic cascades resulting in either CTE or AD has been postulated. Molecular studies suggested that TBIs increase the neurotoxicity of the TAR DNA-binding protein 43 (TDP-43) that is a key pathological marker of ubiquitin-positive forms of frontotemporal dementia (FTLD-TDP) associated or not with motor neuron disease/amyotrophic lateral sclerosis (MND/ALS). Similar patterns of immunoreactivity for TDP-43 in CTE, FTLD-TDP, and ALS as well as epidemiological correlations support the presence of common pathogenetic mechanisms. The present review provides a critical update of the evolution of the concept of CTE with reference to its neuropathological definition together with an in depth discussion of the differential diagnosis between this entity, AD and frontotemporal dementia.
Keywords: chronic traumatic encephalopathy, traumatic brain injuries, boxing, contact sports, Alzheimer’s disease, frontotemporal dementia, amyotrophic lateral sclerosis
Introduction
The expression “punch-drunk syndrome”, used in lay boxing circles [1] was renamed dementia pugilistica (DP) in the late 1930s [2], emphasizing the severity of cognitive sequels. Later on, this concept was revised to include three temporally distinct types of boxing-related traumatic events. Critchley, in 1957, examined the entire clinical spectrum: acute “knock-out” and amnesia, intermediate reactions such as the “groggy state”, and chronic conditions referred to as chronic progressive traumatic encephalopathy [3]. More recently, the terms of chronic traumatic encephalopathy (CTE) [4–10], or chronic traumatic brain injury (CTBI) [10–13] were proposed for the same condition. CTE, CTBI, and DP as such encompass the complex constellation of the long-term cognitive, motor, and psychiatric repercussions of repetitive head trauma not only in boxers [14–24], but also in other contact sports [8,9,25,26] (Table 1).
Table 1.
Boxing-related CTE over more representative reports in literature
| Authors / years | Type of study / patients | Main observations |
|---|---|---|
| Martland, 1928 | 23 cases of retired boxers (clinical details in the case-report of a 38 year-old retired professional boxer) | “Punch-drunk syndrome” (“the occurrence of the symptoms in almost 50% of fighters seems to be good evidence that some special brain injury due their occupation exist”) |
| Millspaugh, 1937 | - | “Dementia pugilistica” |
| Critchley, 1957 | 21 case reports of boxers (16 professional), at different stages of their sportive activity | Panel of possible boxing-related neuro-psychiatric injuries over the time (knock-out/amnesias → “Groggy state” → “Punch-drunk state”); “Chronic progressive encephalopathy” (cumulative brain damage) |
| Roberts, 1969 | Prospective study: randomly sampled 250 retired professional boxers (registered in British Boxing Council between 1929–1955) of which 224 formally studied (physical examinations, psychiatric interviews, neuropsychological assessment, EEG and pneumoencephalography) | Prevalence of clinically defined boxing-related CTE among British retired professional boxers in the percentage of the 17% |
| Corsellis et al., 1973 | Neuropathological examinations in 15 cases of retired boxers with CTE | Characteristic neuropathological findings: NFT spreaded diffusely through both the cerebral cortex and brain-stem, CSP with fenestrations, cerebellar and cerebral scarring and degeneration of the substantia nigra; absence of SP |
| Guterman and Smith, 1987 | Literature review (physiopathology of brain injury; neuropathological, clinical and neuroradiological aspects; analyson contributions of electrophysiological studies and other laboratory methods; questioning on safety of boxing) | Correlation between EEG abnormalities in boxers with encephalopathy (while present in approximately 60% of cases) and encephalopathy is low: role of EEG in prevention, but not in diagnosis |
| Roberts et al., 1990 | Neuropathological examinations of 20 CTE cases in ex-boxers (15 professionals and 5 amateurs, of which 14 cases from archival formalin-fixed material of original study of Corsellis et al., 1973) + 20 Alzheimer’s Disease cases + 20 cases-controls | At the immunocytochemical investigation, presence in all the DP cases of extensive β-amyloid deposits (diffuse SP) |
| Hof et al., 1992 | Neuropathological examinations of 3 cases of CTE (in 3 retired professional boxers) + 8 cases of AD | Association cortex of brains in DP demonstrates an inverse NFT distribution as compared to AD |
| Unterharnscheidt, 1995 I,II,III,IV,V | Literature review (neurological and neuropathological aspects) | Permanent brain damage in boxers (both professional and amateur) |
| Mendez, 1995 | Literature review of studies on 274 professional boxers (neurobiological, clinical, neuropsychological, diagnostic, and management aspects of boxing-related brain injuries) | Clinical expression of CTE may results in a spectrum (mild, non-progressive motor changes → DP). Safety’s measures and rehabilitation techniques do not eliminate risk of CTE |
| Jordan, 2000 | Literature review (clinical, diagnostic, neuropathological and treatments aspects) | Main risk factors for CTBI: increased exposure (i.e., duration of career, age of retirement, total number of bouts), poor performance, increased sparring, ApoE-ε4. Mainstay of treatment is prevention; medications used in AD and/or parkinsonism may be utilized |
| Moseley, 2000 | Literature review (significance analysis of structural and functional neuroimaging techniques) | Significant correlations between imaging abnormalities and clinical evidence of brain damage not attainable by most morphological studies; large informative data in functional imaging not yet available |
| Clausen, 2005 | Literature review on retired professional boxers and data analysis of active professional boxers (636 subjects since 1930s to present, in the U. K. and Australia) | Exposure in professional boxing (measured by bouts and length of career) has decreased significantly over the century → incidence of boxing related CTBI should diminish in the current era |
| McCrory et al., 2007 | Literature review (definition, pathophysiology, clinical assessment, epidemiological data and neuroanatomical/neuroradiological aspects) | Attempt of establishing clinical evidence basis for boxing-related CTE |
| Areza-Fegyveres et al., 2007 | Case report (a 61 year-old retired boxer, amateur for 14 years and professional for 3 years) | A case of DP diagnosed on neuroimaging and neuropathology data with practically undistinguishable clinical features from AD |
| Loosemore et al., 2008 | Review of observational studies on clinically defined boxing-related CTE (1950 to 2007) | There is no strong evidence to associate CTE with amateur boxing |
| Heilbronner et al., 2009 | Literature review (neuropsychological aspect and recommendations to improve safety standards) | Recommendation of a systematic monitoring of the neurocognitive status throughout a boxer’s career (baseline and serial neuropsychological assessments) |
| Nowak et al., 2009 | Case report and literature review (a retired world boxing champion; correlation of clinical features with histochemical and immunohistochemical changes) | Dementia in retired boxers could be exacerbated by others etiologic factors than those typical in DP (such as multiple cerebral infarcts and Wernicke-Korsakoff syndrome) |
| McKee et al., 2009 | Review of 48 cases of neuropathologically verified CTE recorded in literature (37 retired boxers) and clinical findings of 3 case reports (2 retired boxers) | CTE as a neuropathologically distinct slowing progressive tauopathy with a clear environmental etiology; hypothesis of interactions with the pathogenetic cascade of AD |
| Orrison et al., 2009 | Literature review on MRI findings and assessment of 100 unselected consecutive 1.5- and 3.0-Tesla MRI examinations of professional unarmed combatants (boxers and mixed martial arts fighters) | Literature-based checklist approach by high-field MRI showed that 76% of the unarmed combatants had at least one finding that may be associated with Traumatic Brain Injury: 59% hippocampal atrophy, 43% CSP, 32% dilated perivascular spaces, 29% diffuse axonal injuries, 24% cerebral atrophy, 19% increased lateral ventricular size, 14% pituitary gland atrophy; 5% arachnoid cysts and 2% contusions. Possible role in prevention of a systematic assessment |
| Handratta et al., 2010 | Case report (a retired professional boxer, 47-years-old, with double diagnoses of schizophrenia and CTBI) and literature review | Hypotheses about pathophysiology of neuroimaging findings and their relationship to neuropsychiatric symptoms |
| King et al., 2010 | Neuropathological examinations of 59 cases of a variety of neurodegenerative conditions (of which 3 cases of boxing-related CTE) | Peculiar pattern of abnormal expression of TDP-43 in the neocortex of boxing-related CTE cases |
| Omalu et al., 2010 | Clinical findings + neuropathological examinations of 5 cases of CTE in professional contact sports athletes | Suicidal and para-suicidal behaviors |
| McKee et al., 2010 | Clinical findings + neuropathological examinations of 12 cases of CTE, of whom 3 with MND (4 professional boxers) | TDP-43 proteinopathy in brain and spinal cord: clinical and pathological link between CTE, FTD and MND |
Abbreviations: AD: Alzheimer’s disease; CTBI: chronic traumatic brain injury; CSP: cavum septum pellucidum; CTE: chronic traumatic encephalopathy; DP: dementia pugilistica; FTD: frontotemporal dementia; MCI: minimal cognitive impairment; MND: motor neuron disease; MRI: magnetic resonance imaging; NFT: neurofibrillary tangles
Clinically, the diagnosis of CTE depends on the presence of progressively evolving neuropsychiatric symptoms attributable to repeated brain injuries that cannot be attributed to other pathological processes [10]. Alzheimer’s disease (AD) shares several similarities with CTE both in terms of clinical expression [17,22] and structural neuroimaging changes [27]. Post-mortem analyses may often permit to define CTE [22,28], yet several reports have questioned the definite neuropathological distinction between CTE and AD [8,10,12,16,17,21]. In this line, recent molecular and neuropathological findings indicate a close relationship between CTE and frontotemporal dementia (FTD) spectrum [9,24].
The present review summarizes the current status of knowledge on the clinical expression and pathophysiology of boxing and contact sports-related CTE, with particular reference to the place of neuropathology in the differential diagnosis between this condition, AD, and FTD. We focus on boxing-related CTE because it has been more consistently described in the medical literature. However, recent observations show that the expression of CTE and its pathological determinants is quite similar in other contact sports [8,27,29]. In order to illustrate the diagnostic challenge related to the distinction of these disorders, we also provide the case report of a professional ex-boxer, who developed a rapidly progressive cognitive decline long after his retirement from competitions.
From concussions to CTE
Concussions are usually triggered by traumatic biomechanical forces (a direct blow to the head or elsewhere on the body with an impulsive force transmitted to the head) that induce functional alterations rather than structural injuries and result in a graded set of neurological symptoms with or without loss of consciousness [30]. Contact sports athletes are commonly exposed to concussions [30–39]; the frequency in professional boxers is estimated to be 0.8 brain injuries per 10 rounds and 2.9 brain injuries per 10 boxers [7]. After a first episode, a higher susceptibility to subsequent concussions of increased severity leading to cumulative and often severe brain damage was reported [3,32,40–44]. This chronic damage was associated with a variety of sports that expose to concussions such as boxing, football, soccer, rugby, martial arts, wrestling, and ice-hockey [8,9,25,26,29,37,40,42,43,45–51]. The expression “groggy state” designs the intermediate condition between knock-out/amnesia and boxing-related CTE [3,6]. Boxers present with impairment of movements and fighting skills, become increasingly vulnerable to blows and take longer to recover after the bouts. At this stage, the most common neurological symptoms include slurring dysarthria, dysequilibrium, gait ataxia and less frequently a fine intermittent tremor in the absence of other extrapyramidal signs. The cognitive deficits concern only directed attention. Changes in personality have been reported along with various behavioural and psychiatric symptoms such as emotional lability, episodes of hypomania, and pathological sensitivity to alcohol [3,6,52,53] .
Epidemiological data on boxing-related CTE are surprisingly scarce [6,11]. Only one well-designed study reported prevalence rates close to 17% in British professional boxers who had careers in the 1930–1950s [53]. Although published almost 50 years ago, this study remains the best estimate of the prevalence of CTE in this contact sport. An analysis among the population of professional boxers over the century (from 1900 to 2005) revealed a significant decrease in their exposure (measured by the length of career, mean of 19 years reduced to 5, and bout numbers, mean of 336 reduced to 13) predicting a significant decrease of CTE in the next decades [11]. Better medical care may also contribute to this trend [6,11]. Besides repeated concussive head impacts, estimated by the length of career and total number of bouts [4,10,53–56], age of retirement, increased sparring, Caucasian race, poor performances and slugging type fighters, as well as ApoE ε4 genotype, are among the most frequently cited risk factors for this condition [3,5,8,10,52,57–60].
Clinically, CTE has an insidious onset [61] and approximately one third of the cases are progressive [53]. Evolution is unusually rapid with only a 2 to 3 years period between clinical onset and late manifestations [52]. Although three stages of clinical deterioration characterised by specific neuropsychiatric features were initially proposed [52], recent evidences do not support the concept of a sequential clinical evolution [6,11]. Depending on the lesion location, a combination of various cerebellar, pyramidal, and extrapyramidal syndromes including traumatic parkinsonism (in its complete or more often abortive form) may occur [3,4,6,11,52,26,62–65]. However, a few cases without motor signs have been also described [22,66,67]. The progressive cognitive decline predominates in the later phases of the disorder [10,52]. It usually evolves to clinically overt dementia 10 to 30 years after retirement from competitions [7,10,20] with an unknown percentage of cases experiencing stable mild cognitive impairment [20]. Neuropsychological tests revealed impairments in memory, attention and concentration, information processing and finger tapping speed, sequencing abilities, and frontal executive functions [4,7], as well as difficulties in maintaining an effective action strategy, mental inflexibility, perseveration, anomia [66], and spatial disorientation [68]. Athletes who sustained their last sport concussions more than 30 years ago exhibit neuropsychological deficits that affect episodic memory and attention/executive functions similar to those reported in mild cognitive impairment and AD [50]. Personality changes and behavioural and psychiatric symptoms are among the cardinal features of the disease [6,10,53]. Exacerbation of premorbid personality traits and appearance of suspiciousness, restlessness, impulsivity, disinhibition, irritability, violent behaviours with explosive outburst of aggression, transient cheerful or fatuous attitude (the “fatuous or euphoric dementia” described by Critchley, 1957), mood fluctuations, clinically overt depression and hypomanic episodes, morbid jealousy, paranoia, and drug abuse are among the most frequently encountered symptoms [3,4,6,10,53,66,69]. More rarely, patients display hyperorality and hypersexuality as elements of the Klüver-Bucy syndrome [53,69–72]. Multiple cerebral infarcts and Wernicke-Korsakoff syndrome often exacerbate CTE neuropsychiatric expression [23].
Structurally, magnetic resonance imaging (MRI) is more sensitive than computed tomography (CT) for the detection of boxing-related chronic sequelae [10,73]. Commonly observed abnormalities include cortical atrophy with enlargement of the sulci and lateral ventricles, hippocampal and vermis atrophy, presence of a cavum septum pellucidum (CSP), signs of diffuse axonal injury, pituitary gland atrophy, dilated perivascular spaces, and periventricular white matter disease [6,13,27,74,75]. A study on 100 cases of professional combatants (boxers and mixed martial arts fighters) using high-field MRI revealed that 76% of the subjects had at least one among these findings with a clear predominance of the hippocampal atrophy (59%) and CSP (43%) [27]. MR spectroscopy has been also used to examine the reduction of N-acetyl-aspartate in the lentiform nucleus but its specificity in this context remains unknown [6,74,76,77].
Unlike MRI, data on functional imaging changes in CTE are rare. Most studies included limited samples lacking appropriate clinical documentation [6,74]. The pattern of hypoperfusion is quite similar to that observed in AD cases with a predominant involvement of the parietal and temporal cortex [13,78–80]. Early electrophysiological studies at rest as well as event-related potentials did not allow for distinguishing CTE from AD [for review see 6]. Increased phasic theta latencies were reported only in one-third to one-half of professional boxers with CTE possibly reflecting directed attention deficits [52,53,69,72,81–83]. When present, these EEG changes correlate with age, number of bouts, and history of knockouts [72,82,84].
CTE, AD, and FTD: pathogenetic issues
The overlap between AD and CTE is not new and concerns not only the similarities in the clinical expression of these conditions but also their epidemiology and pathogenesis. More recently, a similar debate has emerged in respect to the complex relationships between CTE and FTD. In order to illustrate the diagnostic challenge of CTE, we provided a case report of a 83-year old ex-boxer with detailed neuropsychological, neuroimaging and psychiatric documentation as online supporting information.
From an epidemiological viewpoint, both positive [46,85–92] and negative [93,94] data were reported in respect to the association between a history of previous head trauma and AD risk. The severity of brain injury mostly assessed by the loss of consciousness was described as one among the rare robust environmental risk factors for AD [46,85–87,89]. Conversely, data for repetitive head injuries are limited; a study in former athletes who suffered multiple concussions reported a 5-fold increase in the prevalence of mild cognitive impairment as well an earlier onset of AD compared to age-matched controls [49]. A meta-analysis conducted up to 2001 [88] replicated the results of a first meta-analysis [85] that supported a significant association between head injury and AD, but only in males. How traumatic brain injury (TBIs) can trigger the neurodegenerative cascade of events resulting in AD is still controversial [92]. Moreover, AD-like neurodegenerative changes following head injuries or repetitive mild trauma may be present alone or in conjunction with other types of neurodegenerative lesions [95]. In the same line, ApoE ε4 genotype, the major risk factor for AD, is associated with increased risk for CTE [5,10,57,96]. In a recent study of 10 cases with autopsy-confirmed CTE, five subjects carried at least one ApoE ε4 allele and one was homozygous for ApoE ε4 [8]. Recent genetic association studies explored the possible role of several gene polymorphisms in the neuropsychological, neuropathological or neuroimaging phenotypes of TBI [96–98]. While correlation with APOE is the strongest [96,98], other data emerged: neprilysin polymorphism may make TBI patients more vulnerable to amyloid-beta plaque formation [99]; ACE polymorphism may be associated with worse neuropsychological sub-acute performances in moderate and severe TBI [100]; COMT and DRD2 polymorphisms may influence dopamine dependent cognitive processes as executive/frontal lobe functions [96]; Val66Met BDNF polymorphism affects the recovery of executive functions after combat-related TBI [101]; BCL2 [102] and neuroglobin [103] polymorphisms influence functional outcome (also neurobehavioural outcome for BCL2). The role of interleukins (Il-1α, Il-1β, Il-6) [96,98,104,105] and p53 [96,98,106] polymorphisms are still debated. Finally, the interaction between TBI and APOE gene status in respect to AD risk reported conflicting results [89,92,96]. In a large population-based prospective historical cohort study, a non-significant trend toward a stronger association between AD and early adulthood head injury in subjects with more epsilon4 alleles were reported [46]. In an extensive multi-centre epidemiological study [86] and in a retrospective autopsy study [87] the influence of severe TBI on the risk of AD appears to be higher in subjects lacking APOE ε4 alleles. On the contrary, two studies evoked the possibility of a synergistic interaction between these alleles and TBI (91,107). In the same line, a recent experimental study demonstrated that TBI accelerates neurodegenerative pathology in double-transgenic animals expressing the common human apoE alleles and mutated amyloid precursor protein, and that pathology is exacerbated in the presence of the apoE4 allele [108].
Unlike AD, the possible links between CTE and FTD have aroused growing attention only in recent years. The TAR DNA-binding protein 43 (TDP-43), a neurofilament-binding and mRNA-stabilizing transcript protein [109], was recently established as the major pathological protein in FTD with ubiquitin-positive and tau-negative inclusions (FTLD-U, renamed FTLD-TDP), with or without motor neuron disease/amyotrophic lateral sclerosis (MND/ALS), and in sporadic ALS, setting the concept of a spectrum of “TDP-43 proteinopathies” [110]. This protein is also present in a number of other neurodegenerative diseases, including AD and boxing-related CTE [9,24]. In some individuals with CTE, the TDP-43 proteinopathy involves the spinal cord and may be associated with clinically overt ALS [9]. Some epidemiological studies also showed a robust association between ALS and head injury [111,112], but this position was challenged by Turner and collaborators [113] who suggested the possibility of interference by recall or reporting bias. It has been postulated that TDP-43 plays a critical role in mediating the response of the neuronal cytoskeleton to axonal injury [114] and therefore may be encountered among the triggers of neuronal degeneration induced by cumulative head trauma [9]. In agreement with this model, during a traumatic brain injury the brain and spinal cord undergo shear deformation, producing a transient stretch of axons and consequent perturbation of cytoskeleton, consisting in dissolution of microtubules/neurofilaments and pathological reorganisation of neurofilaments [115,116]. This process accelerates the accumulation, aggregation, and mislocation to the cytoplasm of TDP-43, with consequent enhancement of its neurotoxicity.
Differential diagnosis between CTE, AD, and FTD: the place of neuropathology
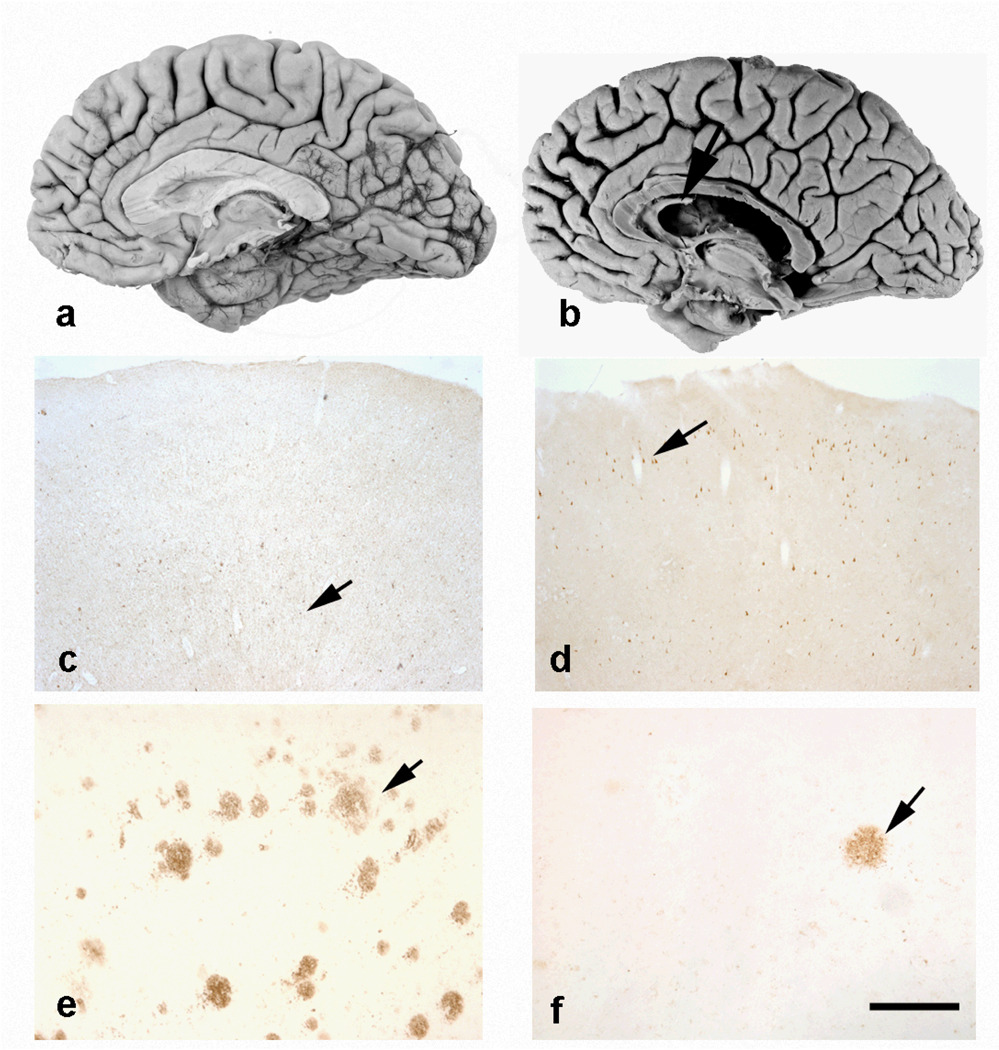
Neuropathological examinations in boxers with CTE revealed fenestrations in septum pellucidum, loss of large pyramidal neurons and Purkinje cells, degeneration of the substantia nigra pigmented cells, and formation of neurofibrillary tangles (NFT) and in some cases senile plaques (SP) or diffuse amyloid deposits (Fig 1), which then may reflect the presence of concomitant AD [8,14,15,17–19,22,28,53,65,117–119].
Figure 1.
Mild cortical atrophy in Alzheimer’s disease (AD) (a) and chronic traumatic encephalopathy (CTE) (b) with fenestration of the septum in CTE (arrow). Note the differences in the laminar distribution of NFTs: in AD (c), they are mainly in the deep layers, whereas in CTE (d) the superficial layers contain tau-immunoreactive inclusions (arrows). Numerous senile plaques are seen in AD (e) but they are very rare in CTE (f) (arrows). c and d: immunohistochemistry with anti-tau antibody AT8 (Innogenetics, Gent, Belgium; 1:3,000), e and f: with anti-amyloid beta antibody 4G8 (Signet laboratories, Dedham, MA, USA; 1:2,000). Scale bar: c and d = 500 mm, e and f = 125 mm.
In an early clinicopathological case report, we described the clinical evolution of a Caucasian former professional boxer who developed dementia pugilistica. [18,120,121]. The patient started boxing at 17 and at 22 he was at European championship level. At the age of 24 he began to exhibit behaviour described as bizarre, that led to the supposition of emerging intellectual deficit. From the age of 25, he developed a progressive neuropsychiatric symptomatology, characterized by extra-pyramidal hyperkinetic-rigid syndrome, pyramidal signs, epileptic manifestations (generalized tonic-clonic seizures, absences and focal manifestations) as well cognitive impairment (memory disturbances, temporospatial disorientation, deficit of judgment/abstract thinking with agnosognosia) and psychiatric/behavioral symptoms (dysphoria, megalomania, paranoid attitudes and aggressiveness). He was hospitalized in the psychiatric Hospital of Geneva twice; he died at the age of 58 of a pulmonary embolism. The neuropathological analysis revealed no fibrillar amyloid deposits, but large numbers of NFT concentrated in the supragranular layers of the neocortex (Fig. 1). NFT densities in the neocortex but not in the hippocampal formation were significantly higher in this boxing-related CTE than in typical AD cases [18]. Overall, a distinct pattern of NFT distribution within neocortical association areas was described in CTE compared to AD cases. NFT in CTE were mainly located in superficial layers (II and upper III; Fig. 1) whereas in AD they predominate in deep layers (V and VI), correlating with the location of neurons forming specific corticocortical connections. In the same line, a case of head banging in a young adult patient with autism showed high numbers of NFT in layers II and III of the inferior temporal cortex, aggregating in large clusters, without amyloid deposition [122]. This finding suggests that a more circumscribed population of cortical pyramidal neurons might be affected in CTE than in AD; in the hippocampus and entorhinal cortex, on the contrary, the NFT distribution is comparable to that observed in AD (with numerous NFT in the CA1 field, subiculum, and layers II and V of the entorhinal cortex) [18]. NFT in boxing-related CTE are strongly immunolabeled by antibodies to hyperphosphorylated tau proteins [18] and show biochemical modifications comparable to those observed in AD [16,18,19,118,119,123,124]. Compared with others tauopathies, neurofibrillary degeneration in CTE is distinguished by preferential involvement of the superficial cortical layers, irregular patchy distribution in the frontal and temporal cortices, propensity for sulcal depths, prominent perivascular, periventricular, and subpial distribution, and marked accumulation of tau-immunoreactive astrocytes [8,18,115,119]. Diffuse amyloid deposits and SP formation have been reported in the brains of boxing-related CTE and post-traumatic AD cases [17,19,118,125–130]. These studies also pointed to the fact that deposition of amyloid and amyloid precursor protein may occur very rapidly following TBI leading to an extensive cortical amyloid deposition in most of these cases. However, the impact of this phenomenon on the clinical expression of CTE remains controversial [8,10,17,18]. In this respect, boxing-related CTE seems more comparable to amyotrophic lateral sclerosis/Parkinsonism-dementia complex of Guam (Guamanian ALS/PDC) where amyloid deposition marginally influences the cognitive performances [131–133]. Observations of amyloid-containing NFT have been reported in AD, Guamanian ALS/PDC and boxing-related CTE [18,134–137], although this finding is more frequent in the first two illnesses. Variable amounts of neuropil threads have been observed in boxing-related CTE using classical histological stains or immunohistochemistry against tau protein isoforms [18,19]. More recently, using TPD-43 immunocytochemistry, McKee and colleagues [8,9] reported a profusion of thread-like structures in these brains that probably have a complex cellular origin.
NFT in CTE are primarily distributed in regions functionally related to the limbic structures (perirhinal and entorhinal cortex, amygdala, hippocampo-septo-hypothalamic pathway, piriform and orbitofrontal cortex) pointing to a possible olfactory system-related origin of the pathology [8,122].
Abnormal expression of TDP-43 (usually confined to limbic areas in moderate to severe AD cases) is more frequent in the neocortex of boxing-related CTE cases [9,24]. The pattern of distribution of TDP-43 inclusions here is similar to that observed in FTLD-TDP, in that widespread regions of the brain are affected [9]. This widespread TDP-43 proteinopathy in CTE may affect the brainstem, basal ganglia, diencephalon, medial temporal lobe, frontal, temporal, and insular cortices, and subcortical white matter in most cases [9]. FTLD-TDP is also associated with MND/ALS and TDP-43 immunolabeling, but it is often characterised by tau-negative neuronal inclusions that are immunoreactive for ubiquitin. Conversely, the TDP-43 immunoreactivity found in CTE is associated with an extensive tauopathy [9].
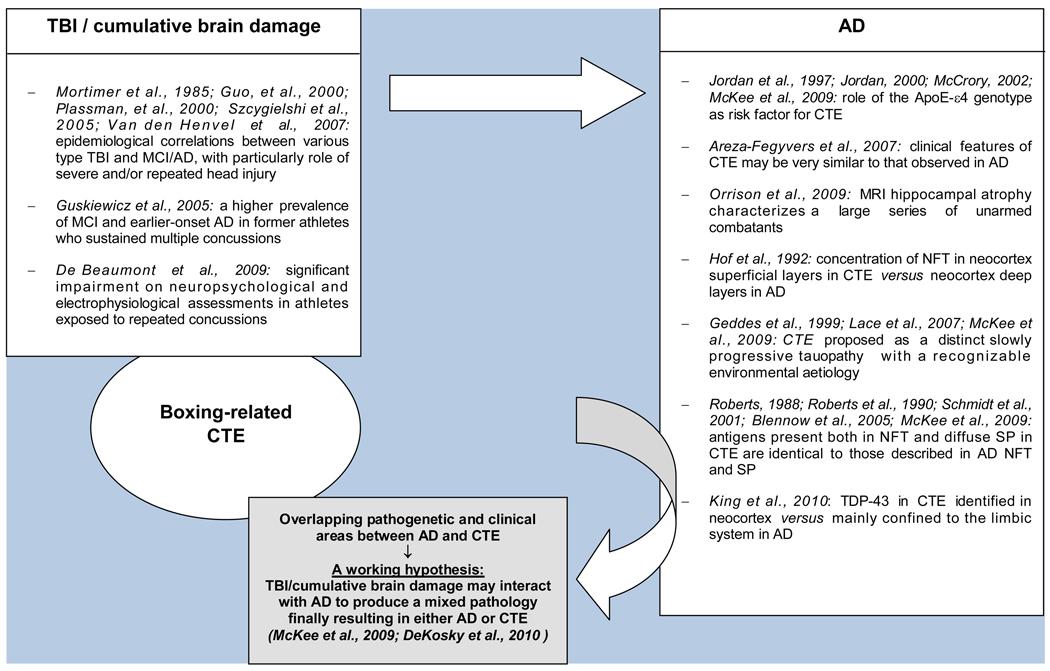
Two main hypotheses have been proposed to explain the pathogenesis of CTE. This disorder may be seen as a slowly progressive tauopathy with a recognizable environmental aetiology [8,115,138]. The fact that CTE shares some features of AD, in particular immunocytochemically identical NFT and in a minority of cases diffuse Aβ deposits and SP suggest an at least partly similar biological background [8,12,16,17,21,126]. Taking into account the clinical and neuroradiological similarities between CTE and AD reported above [17,22,27] as well their epidemiological and neuropathological relationships, it has been recently proposed that just as acquired vascular injuries may interact additively or synergistically with AD, traumatic injuries may produce a mixed pathology by promoting cascades resulting in either AD or CTE (Fig 2) [8,51]. Alternatively, the positive immunoreactivity for TDP-43 in CTE, FTLD-TDP, and ALS, as well as the frequency occurrence of TBI in ALS indicate that these entities may share some pathogenetic mechanisms related to pathological expression of this protein [24,110].
Figure 2.
Cumulative brain damage, CTE, and AD: findings from epidemiologic, genetic, and neuropathological/immunocytochemical studies
Abbreviations: AD: Alzheimer’s disease; CTE: chronic traumatic encephalopathy; MCI: minimal cognitive impairment; NFT: neurofibrillary tangles; SP: senile plaques; TBI: traumatic brain injury; TDP-43: TAR DNA-binding protein 43
Conclusions
In a boxing-exposed population but also in other contact sports involving the head, effective measures of prevention are still rare [6,10,11]. A number of early “return to play” (RTP) guidelines have been proposed to decrease the occurrence of concussions but their scientific validity is still questionable [139]. The most widely used protocols are the Cantu guidelines [140], the Colorado guidelines [141], and the American Academy of Neurology practice parameters for concussion management [32]. More recently, a more complex RTP assessment was conceived as a dynamic model taking into account the interactions among several variables related to athletes, nature of traumatic events, medical presence, neuropsychological assessment, and others extraneous factors [142]. In 2009, a National Academy of Neuropsychology Education Paper emphasized the role of a baseline and serial neuropsychological evaluations and proposed ad hoc parameters for ringside evaluation, post-injury neuropsychological evaluation, return-to-ring and retirement decision [7]. Other contributions argued that neuropsychology has a unique, but not exclusive, role in the decision making process [143–145]. Most consensus statement papers suggest the utility of a multidisciplinary approach, including periodic medical check-ups and neuroimaging assessment, such as baseline and follow-up CT as well as more sensitive MRI [6,30,31,74,146].
The most interesting perspectives in this field concern the possibility of identifying structural and functional alterations that would predict future CTE in asymptomatic athletes. Conventional 1.5 T MR imaging detects only a small part of the structural changes due to concussions. The availability and use of new techniques will certainly change this situation. The improved resolution and increased signal-to-noise ratio on 3 T MRI systems could help to detect very small gray matter changes that take place long before the emergence of first symptoms [27]. In the same line, the use of an MRI technique more sensitive to microstructural changes such as diffusion tensor imaging (DTI) could reveal earlier diffuse brain damage in boxers [147]. A first neurophysiological study in asymptomatic concussed athletes has demonstrated a significant decrease in P300 amplitudes in response to an auditory stimulus at least 5 weeks post-concussion [148]. These data support the idea of a recovery period of at least 4 to 6 weeks before returning to the ring and question the validity of the absence of symptoms as a guidepost in RTP assessments [8,149]. More recently, an EEG study of 12 professional concussed boxers (without a CTE diagnosis) showed increased P300 latency and reduced amplitude in a Go/No-Go task (that mimics fast stimulus discrimination, response selection, and motor reaction or inhibition, that are particularly relevant in a combat sport), that correlated with executive processing impairment. Based on this observation, the authors proposed that event-related potentials might be a tool for early detection of boxing-related brain dysfunction [150]. Overall, these first attempts to identify biological markers of future cognitive decline among boxers parallel with a 10 year delay the progressive change of focus of the clinical research in AD from the treatment of clinically overt cases to the identification of at risk individuals on the basis of their biological vulnerability. Future investigations should aim to identify early biochemical, structural and functional hallmarks of CTE and test their predictive validity in large cohorts of contact sport athletes.
Supplementary Material
Acknowledgements
We thank all the technical staff members of the Division of Neuropsychiatry of the University of Geneva Hospitals for expert assistance. This work was supported in part by NIH grants AG02219 and AG05138 (PRH, SG).
List of abbreviations
- ACE
angiotensin converting enzyme
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- BCL2
B-cell lymphoma 2 proto-oncogene anti-apoptotic protein
- BDNF
brain-derived neurotrophic factor
- COMT
catechol-o-methyltransferase
- CTBI
chronic traumatic brain injury
- CSP
cavum septum pellucidum
- CTE
chronic traumatic encephalopathy
- DP
dementia pugilistica
- DRD2
dopamine D2 receptor gene
- FTD
frontotemporal dementia
- Il-1α
interleukin-1α
- Il-1β
interleukin-1β
- Il-6
interleukin-6
- MCI
minimal cognitive impairment
- MND
motor neuron disease
- MRI
magnetic resonance imaging
- NFT
neurofibrillary tangles
- p53
apoptosis-inducing protein 53
- SP
senile plaques
- TBI
traumatic brain injury
- TDP-43
TAR DNA-binding protein 43
Contributor Information
Alessandra Costanza, Department of Psychiatry, University of Geneva School of Medicine, Geneva, Switzerland, Tel.: +41-22-3055361, Fax: +41-22-3055350.
Kerstin Weber, Department of Psychiatry, University of Geneva School of Medicine, Geneva, Switzerland, Tel.: +41-22-3055361, Fax: +41-22-3055350.
Samuel Gandy, Departments of Neurology and Psychiatry and Alzheimer's Disease Research Center, Mount Sinai School of Medicine, New York, NY 10029, and James J. Peters VA Medical Center, Bronx, NY 10461, USA.
Constantin Bouras, Department of Psychiatry, University of Geneva School of Medicine, Geneva, Switzerland, Tel.: +41-22-3055361, Fax: +41-22-3055350.
Patrick R. Hof, Department of Neuroscience and Alzheimer's Disease Research Center, Mount Sinai School of Medicine, New York, NY 10029, USA
Panteleimon Giannakopoulos, Department of Psychiatry, University Hospitals and Faculty of Medicine of the University of Geneva, 1225 Geneva and Department of Psychiatry, Division of Old Age Psychiatry, Hospices-CHUV, 1008 Lausanne, Switzerland, Panteleimon.Giannakopoulos@unige.ch, Tel: +41-22-3055001, Fax:41-22-3055044.
Alessandra Canuto, Department of Psychiatry, University of Geneva School of Medicine, Geneva, Switzerland, Tel.: +41-22-3055361, Fax: +41-22-3055350.
REFERENCES
- 1.Martland HS. Punch-drunk. J Am Med Assoc. 1928;19:1103–1107. [Google Scholar]
- 2.Millspaugh JA. Dementia Pugilistica. U.S. Nav Med Bull. 1937;35:297–303. [Google Scholar]
- 3.Critchley M. Medical aspects of boxing, particularly from a neurological standpoint. Br Med J. 1957;1:357–362. doi: 10.1136/bmj.1.5015.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendez MF. The neuropsychiatric aspects of boxing. Int J Psychiatry Med. 1995;25:249–262. doi: 10.2190/CUMK-THT1-X98M-WB4C. [DOI] [PubMed] [Google Scholar]
- 5.McCrory P. Boxing and the brain. Revisiting chronic traumatic encephalopathy. Br J Sports Med. 2002;36:2. doi: 10.1136/bjsm.36.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCrory P, Zazryn T, Cameron P. The evidence for chronic traumatic encephalopathy in boxing. Sports Med. 2007;37:467–476. doi: 10.2165/00007256-200737060-00001. [DOI] [PubMed] [Google Scholar]
- 7.Heilbronner RL, Bush SS, Ravdin LD, Barth JT, Iverson GL, Ruff RM, Lovell MR, Barr WB, Echemendia RJ, Broshek DK. Neuropsychological consequences of boxing and recommendations to improve safety: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol. 2009;24:11–19. doi: 10.1093/arclin/acp005. [DOI] [PubMed] [Google Scholar]
- 8.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, Morin P, Lee HS, Kubilus CA, Daneshvar DH, Wulff M, Budson AE. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan BD. Chronic traumatic brain injury associated with boxing. Semin Neurol. 2000;20:179–185. doi: 10.1055/s-2000-9826. [DOI] [PubMed] [Google Scholar]
- 11.Clausen H, McCrory P, Anderson V. The risk of chronic traumatic brain injury in professional boxing: change in exposure variables over the past century. Br J Sports Med. 2005;39:661–664. doi: 10.1136/bjsm.2004.017046. discussion 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 13.Handratta V, Hsu E, Vento J, Yang C, Tanev K. Neuroimaging findings and brain-behavioral correlates in a former boxer with chronic traumatic brain injury. Neurocase. 2010;16:125–134. doi: 10.1080/13554790903329166. [DOI] [PubMed] [Google Scholar]
- 14.Brandenburg W, Hallervorden J. Dementia pugilistica with anatomical findings. Virchows Arch. 1954;325:680–709. doi: 10.1007/BF00955101. [DOI] [PubMed] [Google Scholar]
- 15.Grahmann H, Ule G. Beitrag zur Kenntnis der chronischen cerebralen Krankheitsbilder bei Boxern (Dementia pugilistica und traumatische Boxer-Encephalopathie) Psychiat Neurol. 1957;134:261–283. [PubMed] [Google Scholar]
- 16.Roberts GW. Immunocytochemistry of neurofibrillary tangles in dementia pugilistica and Alzheimer's disease: evidence for common genesis. Lancet. 1988;2:1456–1458. doi: 10.1016/s0140-6736(88)90934-8. [DOI] [PubMed] [Google Scholar]
- 17.Roberts GW, Allsop D, Bruton C. The occult aftermath of boxing. J Neurol Neurosurg Psychiatry. 1990;53:373–378. doi: 10.1136/jnnp.53.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hof PR, Bouras C, Buée L, Delacourte A, Perl DP, Morrison JH. Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer's disease cases. Acta Neuropathol. 1992;85:23–30. doi: 10.1007/BF00304630. [DOI] [PubMed] [Google Scholar]
- 19.McKenzie JE, Roberts GW, Royston MC. Comparative investigation of neurofibrillary damage in the temporal lobe in Alzheimer's disease, Down's syndrome and dementia pugilistica. Neurodegeneration. 1996;5:259–264. doi: 10.1006/neur.1996.0034. [DOI] [PubMed] [Google Scholar]
- 20.Erlanger DM, Kutner KC, Barth JT, Barnes R. Neuropsychology of sports-related head injury: Dementia Pugilistica to Post Concussion Syndrome. Clin Neuropsychol. 1999;13:193–209. doi: 10.1076/clin.13.2.193.1963. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt ML, Zhukareva V, Newell KL, Lee VM, Trojanowski JQ. Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer's disease. Acta Neuropathol. 2001;101:518–524. doi: 10.1007/s004010000330. [DOI] [PubMed] [Google Scholar]
- 22.Areza-Fegyveres R, Rosemberg S, Castro RM, Porto CS, Bahia VS, Caramelli P, Nitrini R. Dementia pugilistica with clinical features of Alzheimer's disease. Arq Neuropsiquiatr. 2007;65:830–833. doi: 10.1590/s0004-282x2007000500019. [DOI] [PubMed] [Google Scholar]
- 23.Nowak LA, Smith GG, Reyes PF. Dementia in a retired world boxing champion: case report and literature review. Clin Neuropathol. 2009;28:275–280. [PubMed] [Google Scholar]
- 24.King A, Sweeney F, Bodi I, Troakes C, Maekawa S, Al-Sarraj S. Abnormal TDP-43 expression is identified in the neocortex in cases of dementia pugilistica, but is mainly confined to the limbic system when identified in high and moderate stages of Alzheimer's disease. Neuropathology. 2010;30:408–419. doi: 10.1111/j.1440-1789.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 25.Unterharnscheidt F, Sellier K. Boxing. Mechanics, pathomorphology and clinical picture of traumatic lesions of the CNS in boxers. Fortschr Neurol Psychiatr Grenzgeb. 1971;39:109–151. [PubMed] [Google Scholar]
- 26.Unterharnscheidt F. A neurologist's reflections on boxing. V. Conclude remarks. Rev Neurol. 1995;23:1027–1032. [PubMed] [Google Scholar]
- 27.Orrison WW, Hanson EH, Alamo T, Watson D, Sharma M, Perkins TG, Tandy RD. Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J Neurotrauma. 2009;26:689–701. doi: 10.1089/neu.2008.0636. [DOI] [PubMed] [Google Scholar]
- 28.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 29.Omalu BI, Bailes J, Hammers JL, Fitzsimmons RP. Chronic traumatic encephalopathy, suicides and parasuicides in professional American athletes: the role of the forensic pathologist. Am J Forensic Med Pathol. 2010;31:130–132. doi: 10.1097/PAF.0b013e3181ca7f35. [DOI] [PubMed] [Google Scholar]
- 30.McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, Cantu R. Consensus statement on Concussion in Sport--the 3rd International Conference on Concussion in Sport held in Zürich, November 2008. J Sci Med Sport. 2009;12:340–351. doi: 10.1016/j.jsams.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Cantu RC. Cerebral concussion in sport: management and prevention. Sports Med. 1992;14:64–74. doi: 10.2165/00007256-199214010-00005. [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Neurology. Report of the Quality Standards Subcommittee. Practice parameter: the management of concussion in sports (Summary Statement) Neurology. 1997;48:581–585. doi: 10.1212/wnl.48.3.581. [DOI] [PubMed] [Google Scholar]
- 33.Kelly JP, Rosenberg JH. Diagnosis and management of concussion in sports. Neurology. 1997;48:575–580. doi: 10.1212/wnl.48.3.575. [DOI] [PubMed] [Google Scholar]
- 34.Kelly JP. Traumatic brain injury and concussion in sports. J Am Med Assoc. 1999;282:989–991. doi: 10.1001/jama.282.10.989. [DOI] [PubMed] [Google Scholar]
- 35.Scott Delaney J, Puni V, Ronah F. Mechanisms of injury for concussions in university football, ice hockey and soccer: a pilot study. Clin J Sport Med. 2006;16:162–165. doi: 10.1097/00042752-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Moser RS, Iverson GL, Echemendia RJ, Lovell MR, Schatz P, Webbe FM, Ruff RM, Barth JT. Neuropsychological evaluation in the diagnosis and management of sports-related concussion. Arch Clinical Neuropsychol. 2007;22:909–916. doi: 10.1016/j.acn.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Zazryn TR, McCrory PR, Cameron PA. Neurologic injuries in boxing and other combat sports. Neurol Clin. 2008;26:257–270. doi: 10.1016/j.ncl.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Meehan WP, 3rd, Bachur RG. Sport-related concussion. Pediatrics. 2009;123:114–123. doi: 10.1542/peds.2008-0309. [DOI] [PubMed] [Google Scholar]
- 39.Reddy CC, Collins MW. Sports concussion: management and predictors of outcome. Curr Sports Med Rep. 2009;8:10–15. doi: 10.1249/JSR.0b013e31819539ca. [DOI] [PubMed] [Google Scholar]
- 40.Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, Kelly JP. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. J Am Med Assoc. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- 41.Sercl M, Jaros O. The mechanisms of cerebral concussion in boxing and their consequences. World Neurol. 1962;3:351–358. [PubMed] [Google Scholar]
- 42.Gronwall D, Wrightson P. Cumulative effect of concussion. Lancet. 1975;2:995–997. doi: 10.1016/s0140-6736(75)90288-3. [DOI] [PubMed] [Google Scholar]
- 43.Lundberg GD. Brain injury in boxing. Am J Forensic Med Pathol. 1985;6:192–198. doi: 10.1097/00000433-198509000-00004. [DOI] [PubMed] [Google Scholar]
- 44.NIH. Consensus conferences. Consensus development panel on rehabilitation of persons with traumatic brain injury. J Am Med Assoc. 1999;282:974–983. [PubMed] [Google Scholar]
- 45.Corsellis JA. Brain damage in sport. Lancet. 1976;1:401–402. [PubMed] [Google Scholar]
- 46.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 47.Rabadi MH, Jordan BD. The cumulative effect of repetitive concussion in sports. Clin J Sport Med. 2001;11:194–198. doi: 10.1097/00042752-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–134. doi: 10.1227/01.neu.0000163407.92769.ed. discussion 128–134. [DOI] [PubMed] [Google Scholar]
- 49.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. discussion 719–726. [DOI] [PubMed] [Google Scholar]
- 50.De Beaumont L, Theoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, Ellemberg D, Lassonde M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- 51.DeKosky ST, Ikonomovic MD, Gandy S. Traumatic brain injury - Football, warfare, and long-term effects. N Engl J Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- 52.Guterman A, Smith RW. Neurological sequelae of boxing. Sports Med. 1987;4:194–210. doi: 10.2165/00007256-198704030-00004. [DOI] [PubMed] [Google Scholar]
- 53.Roberts AH. Pitman. London: 1969. Brain damage in boxers: a study of the prevalence of traumatic encephalopathy among ex-professional boxers. [Google Scholar]
- 54.Blennow K, Popa C, Rasulzada A, Minthon L, Wallin A, Zetterberg H. There is a strong evidence that professional boxing results in chronic brain damage. The more head punches during a boxer's career, the bigger is the risk. Lakartidningen. 2005;102:2468–2470. 2472-2465. [PubMed] [Google Scholar]
- 55.Loosemore M, Knowles CH, Whyte GP. Amateur boxing and risk of chronic traumatic brain injury: systematic review of observational studies. Br J Sports Med. 2008;42:564–567. [PubMed] [Google Scholar]
- 56.Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24:439–451. doi: 10.1097/HTR.0b013e3181c15600. [DOI] [PubMed] [Google Scholar]
- 57.Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. J Am Med Assoc. 1997;278:136–140. [PubMed] [Google Scholar]
- 58.Berker E. Diagnosis, physiology, pathology and rehabilitation of traumatic brain injuries. Int J Neurosci. 1996;85:195–220. doi: 10.3109/00207459608986683. [DOI] [PubMed] [Google Scholar]
- 59.Stern Y. Cognitive reserve: theory and application. N.Y: Taylor & Francis; 2007. [Google Scholar]
- 60.Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc. 2008;14:1–22. doi: 10.1017/S135561770808017X. [DOI] [PubMed] [Google Scholar]
- 61.Jordan BD. Neurologic aspects of boxing. Arch Neurol. 1987;44:453–459. doi: 10.1001/archneur.1987.00520160083020. [DOI] [PubMed] [Google Scholar]
- 62.Unterharnscheidt F. A neurologist's reflections on boxing. I: Impact mechanics in boxing and injuries other than central nervous system damage. Rev Neurol. 1995;23:661–674. [PubMed] [Google Scholar]
- 63.Unterharnscheidt F. A neurologist's reflections on boxing. II. Acute and chronic clinical findings secondary to central nervous system damage. Rev Neurol. 1995;23:833–846. [PubMed] [Google Scholar]
- 64.Unterharnscheidt F. A neurologist's reflections on boxing. III. Vascular injuries. Rev Neurol. 1995;23:847–855. [PubMed] [Google Scholar]
- 65.Unterharnscheidt F. A neurologist's reflections on boxing. IV. Late and permanent brain damage. Rev Neurol. 1995;23:1013–1026. [PubMed] [Google Scholar]
- 66.Rochon M. Présentation d'un cas: l'encéphalopathie des boxeurs. Can J Psychiatry. 1994;39:211–214. doi: 10.1177/070674379403900403. [DOI] [PubMed] [Google Scholar]
- 67.Naccache L, Slachevsky A, Deweer B, Habert MO, Dubois B. Boxer's dementia without motor signs. Presse Med. 1999;28:1352–1354. [PubMed] [Google Scholar]
- 68.Cordeiro Q, Jr, de Oliveira AM. Parkinsonian, cerebellar, psychotic and demential symptoms in ex-boxer: case report. Arq Neuropsiquiatr. 2001;59:283–285. [PubMed] [Google Scholar]
- 69.Johnson J. Organic psychosyndromes due to boxing. Br J Psychiatry. 1991;15:45–53. doi: 10.1192/bjp.115.518.45. [DOI] [PubMed] [Google Scholar]
- 70.Critchley E. Nervous disorders in boxers. Medical Annual. 1937:318–320. [Google Scholar]
- 71.Spillane JD. Five boxers. Br Med J. 1962;2:1205–1210. doi: 10.1136/bmj.2.5314.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sironi VA, Scotti G, Ravagnati L, Franzini A, Marossero F. CT-scan and EEG findings in professional pugilists: early detection of cerebral atrophy in young boxers. J Neurosurg Sci. 1982;26:165–168. [PubMed] [Google Scholar]
- 73.Jordan BD, Zimmerman RD. Computed tomography and magnetic resonance imaging comparisons in boxers. J Am Med Assoc. 1990;263:1670–1674. [PubMed] [Google Scholar]
- 74.Moseley IF. The neuroimaging evidence for chronic brain damage due to boxing. Neuroradiology. 2000;42:1–8. doi: 10.1007/s002340050001. [DOI] [PubMed] [Google Scholar]
- 75.Zhang L, Ravdin LD, Relkin N, Zimmerman RD, Jordan B, Lathan WE, Ulug AM. Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? Am J Neuroradiol. 2003;24:52–57. [PMC free article] [PubMed] [Google Scholar]
- 76.Davie CA, Pirtosek Z, Barker GJ, Kingsley DP, Miller PH, Lees AJ. Magnetic resonance spectroscopic study of parkinsonism related to boxing. J Neurol Neurosurg Psychiatry. 1995;58:688–691. doi: 10.1136/jnnp.58.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaudhuri KR, Lemmens G, Williams SC, Leigh PN. Magnetic resonance spectroscopic study of parkinsonism related to boxing. J Neurol Neurosurg Psychiatry. 1995;59:561–562. doi: 10.1136/jnnp.59.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez G, Ferrillo F, Montano V, Rosadini G, Sannita WG. Regional cerebral blood flow in boxers. Lancet. 1983;322:858. doi: 10.1016/s0140-6736(83)90782-1. [DOI] [PubMed] [Google Scholar]
- 79.Kemp PM, Houston AS, Macleod MA, Pethybridge RJ. Cerebral perfusion and psychometric testing in military amateur boxers and controls. J Neurol Neurosurg Psychiatry. 1995;59:368–374. doi: 10.1136/jnnp.59.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Houston AS, Kemp PM, Macleod MA, Francis JR, Colohan HA, Matthews HP. Use of significance image to determine patterns of cortical blood flow abnormality in pathological and at-risk groups. J Nucl Med. 1998;39:425–430. [PubMed] [Google Scholar]
- 81.Busse EW, Silverman AJ. Electroencephalographic changes in professional boxers. J Am Med Assoc. 1952;149:1522–1525. doi: 10.1001/jama.1952.02930340006003. [DOI] [PubMed] [Google Scholar]
- 82.Ross RJ, Cole M, Thompson JS, Kim KH. Boxers — computed tomography, EEG, and neurological evaluation. J Am Med Assoc. 1983;249:211–213. doi: 10.1001/jama.249.2.211. [DOI] [PubMed] [Google Scholar]
- 83.Kaplan HA, Browder J. Observations on the clinical and brain wave patterns of professional boxers. J Am Med Assoc. 1954;156:1138–1144. doi: 10.1001/jama.1954.02950120012004. [DOI] [PubMed] [Google Scholar]
- 84.Brookler K, Itil T, Jordan B. Electro-physiologic testing in boxers. In: Jordan B, editor. Medical aspects of boxing. Boca Raton (FL): CRC Press; 1993. pp. 207–214. [Google Scholar]
- 85.Mortimer JA, French LR, Hutton JT, Schuman LM. Head injury as a risk factor for Alzheimer's disease. Neurology. 1985;35:264–267. doi: 10.1212/wnl.35.2.264. [DOI] [PubMed] [Google Scholar]
- 86.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, Johnson K, Go RC, Growdon JH, Haines JL, Kukull WA, Farrer LA. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 87.Jellinger KA, Paulus W, Wrocklage C, Litvan I. Traumatic brain injury as a risk factor for Alzheimer disease. Comparison of two retrospective autopsy cohorts with evaluation of ApoE genotype. BMC Neurol. 2001;1:3. doi: 10.1186/1471-2377-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jellinger KA. Head injury and dementia. Curr Opin Neurol. 2004;17:719–723. doi: 10.1097/00019052-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 90.Szczygielski J, Mautes A, Steudel WI, Falkai P, Bayer TA, Wirths O. Traumatic brain injury: cause or risk of Alzheimer's disease? A review of experimental studies. J Neural Transm. 2005;112:1547–1564. doi: 10.1007/s00702-005-0326-0. [DOI] [PubMed] [Google Scholar]
- 91.Mauri M, Sinforiani E, Bono G, Cittadella R, Quattrone A, Boller F, Nappi G. Interaction between Apolipoprotein epsilon 4 and traumatic brain injury in patients with Alzheimer's disease and Mild Cognitive Impairment. Funct Neurol. 2006;21:223–228. [PubMed] [Google Scholar]
- 92.Van den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer's disease: a review. Prog Brain Res. 2007;161:303–316. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- 93.Fratiglioni L, Ahlbom A, Viitanen M, Winblad B. Risk factors for late-onset Alzheimer’s disease: a population-based, case-control study. Ann Neurol. 1993;33:258–266. doi: 10.1002/ana.410330306. [DOI] [PubMed] [Google Scholar]
- 94.Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JRM, Dartigues JF, Kragh-Sorenses P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A EURODEM Incidence Research Group and Work Groups. Rates and risk factors for dementia and Alzheimer’s disease. Results from EURODEM pooled analyses. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 95.Gavett BE, Stern RA, Cantu RC, Nowinski CJ, McKee AC. Mild traumatic brain injury: a risk factor for neurodegeneration. Alzheimers Res Ther. 2010;2:18. doi: 10.1186/alzrt42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jordan BD. Genetic influences on outcome folowing traumatic brain injury. Neurochem Res. 2007;32:905–915. doi: 10.1007/s11064-006-9251-3. [DOI] [PubMed] [Google Scholar]
- 97.Diaz-Arrastia R, Baxter V. Genetic factors in outcome after traumatic brain injury: what the human genome project can teach us about brain trauma. J Head Trauma Rehabil. 2006;21:361–374. doi: 10.1097/00001199-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Dardiotis E, Fountas KN, Dardioti M, Xiromerisiou G, Kapsalaki E, Tasiou A, Hadjigeourgiou GM. Genetic association studies in patients with traumatic brain injury. Neurosurg Focus. 2010;28:1–12. doi: 10.3171/2009.10.FOCUS09215. [DOI] [PubMed] [Google Scholar]
- 99.Johnson VE, Stewart W, Stewart JE, Graham DI, Praestgaard AH, Smith DH. A neprilysin polymorphism and amyloid-beta plaques following traumatic brain injury. J Neurotrauma. 2009;26:1197–1202. doi: 10.1089/neu.2008.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ariza M, Matarin MD, Junqué C, Mataró M, Clemente I, Moral P, Antonia Poca M, Garnacho A, Sahuquillo J. Influence of Angiotensin-converting enzyme polymorphism on neuropsychological subacute performance in moderate and severe traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2006;18:39–44. doi: 10.1176/jnp.18.1.39. [DOI] [PubMed] [Google Scholar]
- 101.Krueger F, Pardini M, Huey ED, Raymont V, Solomon J, Lipsky RH, Hodgkinson CA, Goldman D, Grafman J. The role of the Met66 Brain-Derived Neurotrophic Factor allele in the recovery of executive functioning after combat-related traumatic brain injury. J Neurosci. 2011;31:598–606. doi: 10.1523/JNEUROSCI.1399-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zangrilli Hoh N, Wagner AK, Alexander SA, Clark RB, Beers SR, Okonkwo DO, Ren D, Conley YP. BCL2 genotypes. Functional and neurobehavioral outcomes after seere traumatic brain injury. J Neurotrauma. 2010;27:1413–1427. doi: 10.1089/neu.2009.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chuang PY, Conley YP, Poloyac SM, Okonkwo DO, Ren D, Sherwood PR, Hravnak M, Alexander S. Neuroglobin genetic polymorphisms and their relationship to functional outcomes after traumatic brain injury. J Neurotrauma. 2010;27:999–1006. doi: 10.1089/neu.2009.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Uzan M, Tanriverdi T, Baykara O, Kafadar A, Sanus GZ, Tureci E, Ozkara C, Uysal O, Buyra N. Association between interleukin-1 beta (IL-1 beta) gene polymorphism and outcome after head injury: an early report. Acta Neurochir (Wien) 2005;147:715–720. doi: 10.1007/s00701-005-0529-z. [DOI] [PubMed] [Google Scholar]
- 105.Tanriverdi T, Uzan M, Sanus GZ, Baykara O, Is M, Ozkara C, Buyra N. Lack of association between the IL1A gene (−889) polymorphism and outcome after head injury. Surg Neurol. 2006;65:7–10. doi: 10.1016/j.surneu.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 106.Martínez-Lucas P, Moreno-Cuesta J, García-Olmo DC, Sánchez-Sánchez F, Escribano-Martínez J, del Pozo AC, Lizán-García M, García-Olmo D. Relationship between the Arg72Pro polymorphism of p53 and outcome for patients with traumatic brain injury. Intensive Care Med. 2005;31:1168–1173. doi: 10.1007/s00134-005-2715-0. [DOI] [PubMed] [Google Scholar]
- 107.Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, Chun M, Tycko B, Shelanski M. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer's disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 108.Laskowitz DT, Song P, Wang H, Mace B, Sullivan PM, Vitek MP, Dawson HN. Traumatic brain injury exacerbates neurodegenerative pathology: improvement with an apolipoprotein E-based therapeutic. J Neurotrauma. 2010;27:1983–1995. doi: 10.1089/neu.2010.1396. [DOI] [PubMed] [Google Scholar]
- 109.Strong MJ, Volkening K, Hammond R, Yang W, Strong W, Leystra-Lantz C, Shoesmith C. TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol Cell Neurosci. 2007;35:320–327. doi: 10.1016/j.mcn.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 110.Geser F, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: a spectrum of TDP-43 proteinopathies. Neuropathology. 2010;30:103–112. doi: 10.1111/j.1440-1789.2009.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol. 2007;166:810–816. doi: 10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmidt S, Kwee LC, Allen KD, Oddone E. Association of ALS with head injury, cigarette smoking and APOE genotypes. J Neurol Sci. 2010;291:22–29. doi: 10.1016/j.jns.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turner MR, Abisgold J, Yeates DGR, Talbot K, Goldacre MJ. Head and other physical trauma requiring hospitalization is not a significant risk factor in the development of ALS. J Neurol Neurosci. 2010;288:45–48. doi: 10.1016/j.jns.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 114.Moisse K, Mepham J, Volkening K, Welch I, Hill T, Strong MJ. Cytosolic TDP-43 expression following axotomy is associated with caspase 3 activation in NFL−/− mice: support for a role for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009;1296:176–186. doi: 10.1016/j.brainres.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 115.Geddes JF, Vowles GH, Nicoll JA, Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- 117.Serbest G, Burkhardt MF, Siman R, Raghupathi R, Saatman KE. Temporal profiles of cytoskeletal protein loss following traumatic axonal injury in mice. Neurochem Res. 2007;32:2006–2014. doi: 10.1007/s11064-007-9318-9. [DOI] [PubMed] [Google Scholar]
- 117.Lampert PW, Hardman JM. Morphological changes in brains of boxers. J Am Med Assoc. 1984;251:2676–2679. [PubMed] [Google Scholar]
- 118.Tokuda T, Ikeda S, Yanagisawa N, Ihara Y, Glenner GG. Re-examination of ex-boxers' brains using immunohistochemistry with antibodies to amyloid beta-protein and tau protein. Acta Neuropathol. 1991;82:280–285. doi: 10.1007/BF00308813. [DOI] [PubMed] [Google Scholar]
- 119.Geddes JF, Vowles GH, Robinson SF, Sutcliffe JC. Neurofibrillary tangles, but not Alzheimer-type pathology, in a young boxer. Neuropathol Appl Neurobiol. 1996;22:12–16. [PubMed] [Google Scholar]
- 120.Constantinidis J, Tissot R. Lésions neurofibrillaires d’Alzheimer généralisées sans plaques séniles. Schweiz Arch Neurol Neurochir Psychiatr. 1967;100:117–130. [PubMed] [Google Scholar]
- 121.Bouras C, Hof PR, Guntern R, Morrison JH. Down’s syndrome (DS), dementia pugilistica (DP), and Alzheimer’s disease (AD): a quantitative neuropathologic comparison. Proc Soc Neurosci. 1990;16:1264. [Google Scholar]
- 122.Hof PR, Knabe R, Bovier P, Bouras C. Neuropathological observations in a case of autism presenting with self-injury behavior. Acta Neuropathol. 1991;82:321–326. doi: 10.1007/BF00308819. [DOI] [PubMed] [Google Scholar]
- 123.Dale GE, Leigh PN, Luthert P, Anderton BH, Roberts GW. Neurofibrillary tangles in dementia pugilistica are ubiquitinated. J Neurol Neurosurg Psychiatry. 1991;54:116–118. doi: 10.1136/jnnp.54.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 125.Rudelli R, Strom JO, Welch PT, Ambler MW. Posttraumatic premature Alzheimer's disease. Neuropathologic findings and pathogenetic considerations. Arch Neurol. 1982;39:570–575. doi: 10.1001/archneur.1982.00510210040009. [DOI] [PubMed] [Google Scholar]
- 126.Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1994;57:419–425. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Clinton J, Ambler MW, Roberts GW. Post-traumatic Alzheimer's disease: preponderance of a single plaque type. Neuropathol Appl Neurobiol. 1991;17:69–74. doi: 10.1111/j.1365-2990.1991.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 128.McKenzie JE, Gentleman SM, Roberts GW, Graham DI, Royston MC. Increased numbers of βAPP-immunoreactive neurons in the entorhinal cortex after head injury. NeuroReport. 1994;6:161–164. doi: 10.1097/00001756-199412300-00041. [DOI] [PubMed] [Google Scholar]
- 129.Sherriff FE, Bridges LR, Sivaloganathan S. Early detection of axonal injury after human head trauma using immunocytochemistry for beta-amyloid precursor protein. Acta Neuropathol. 1994;87:55–62. doi: 10.1007/BF00386254. [DOI] [PubMed] [Google Scholar]
- 130.Graham DI, Gentleman SM, Lynch A, Roberts GW. Distribution of beta-amyloid protein in the brain following severe head injury. Neuropathol Appl Neurobiol. 1995;21:27–34. doi: 10.1111/j.1365-2990.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 131.Hirano A, Llena J. Neuropathological features of Parkinson-dementia complex on Guam: reappraisal and comparative study with Alzheimer’s disease and Parkinson’s disease. In: Zimmerman HM, editor. Progress in neuropathology. Vol. 6. New York: Raven Press; 1986. pp. 17–31. [Google Scholar]
- 132.Hof PR, Perl DP, Loerzel AJ, Morrison JH. Neurofibrillary tangle distribution in the cerebral cortex of parkinsonism-dementia cases from Guam: differences with Alzheimer's disease. Brain Res. 1991;564:306–313. doi: 10.1016/0006-8993(91)91467-f. [DOI] [PubMed] [Google Scholar]
- 133.Hof PR, Perl DP, Loerzel AJ, Steele JC, Morrison JH. Amyotrophic lateral sclerosis and parkinsonism-dementia from Guam: differences in neurofibrillary tangle distribution and density in the hippocampal formation and neocortex. Brain Res. 1994;650:107–116. doi: 10.1016/0006-8993(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 134.Allsop D, Haga S, Bruton C, Ishii T, Roberts GW. Neurofibrillary tangles in some cases of dementia pugilistica share antigens with amyloid beta-protein of Alzheimer's disease. Am J Pathol. 1990;136:255–260. [PMC free article] [PubMed] [Google Scholar]
- 135.Ito H, Hirano H, Yen SH, Kato S. Demonstration of beta amyloid protein-containing neurofibrillary tangles in parkinsonism-dementia complex on Guam. Neuropathol Appl Neurobiol. 1991;17:365–373. doi: 10.1111/j.1365-2990.1991.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 136.Nakazato Y, Shoji M, Okamoto K, Ihara Y, Morimatsu M, Hirai S. Secondary deposition of beta amyloid within extracellular neurofibrillary tangles in Alzheimer-type dementia. Am J Pathol. 1991;138:699–705. [PMC free article] [PubMed] [Google Scholar]
- 137.Steele JC, Akiyama H, McGeer EG, McGeer PL. Relationship of amyloid beta/A4 protein to the neurofibrillary tangles in Guamanian parkinsonism-dementia. Acta Neuropathol. 1995;90:287–298. doi: 10.1007/BF00296513. [DOI] [PubMed] [Google Scholar]
- 138.Lace GL, Wharton SB, Ince PG. A brief history of tau: the evolving view of the microtubule-associated protein tau in neurodegenerative diseases. Clin Neuropathol. 2007;26:43–58. doi: 10.5414/npp26043. [DOI] [PubMed] [Google Scholar]
- 139.Collins MW, Lovell MR, McKeag DB. Current issues in managing sports-related concussion. J Am Med Assoc. 1999;282:2283–2285. doi: 10.1001/jama.282.24.2283. [DOI] [PubMed] [Google Scholar]
- 140.Cantu RC. Guidelines for return to contact sports after a cerebral concussion. Physician Sports Med. 1986;14:75. doi: 10.1080/00913847.1986.11709197. [DOI] [PubMed] [Google Scholar]
- 141.Colorado Medical Society. Report of the Sports Medicine Committee: guidelines for the management of concussion in sports. Denver: Colorado Medical Society; 1991. [Google Scholar]
- 142.Echemendia RJ, Cantu RC. Return to play following sports-related mild traumatic brain injury: the role for neuropsychology. Appl Neuropsychol. 2003;10:48–55. doi: 10.1207/S15324826AN1001_7. [DOI] [PubMed] [Google Scholar]
- 143.Echemendia RJ, Herring S, Bailes J. Who should conduct and interpret the neuropsychological assessment in sports-related concussion? Br J Sports Med. 2009;43 Suppl. 1:i32–i35. doi: 10.1136/bjsm.2009.058164. [DOI] [PubMed] [Google Scholar]
- 144.Ruff R. Best practice guidelines for forensic neuropsychological examinations of patients with traumatic brain injury. J Head Trauma Rehabil. 2009;24:131–140. doi: 10.1097/01.HTR.0000348755.42649.e9. [DOI] [PubMed] [Google Scholar]
- 145.Bailey CM, Barth JT, Bender SD. SLAM on the stand: how the sports-related concussion literature can inform the expert witness. J Head Trauma Rehabil. 2009;24:123–130. doi: 10.1097/HTR.0b013e31819c1caa. [DOI] [PubMed] [Google Scholar]
- 146.Guskiewicz KM, Bruce SL, Cantu RC, Ferrara MS, Kelly JP, McCrea M, Putukian M, McLeod TC. Research based recommendations on management of sport related concussion: summary of the National Athletic Trainers' Association position statement. Br J Sports Med. 2006;40:6–10. doi: 10.1136/bjsm.2005.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chappell MH, Ulug AM, Zhang L, Heitger MH, Jordan BD, Zimmerman RD, Watts R. Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study. J Magn Reson Imaging. 2006;24:537–542. doi: 10.1002/jmri.20656. [DOI] [PubMed] [Google Scholar]
- 148.Gosselin N, Theriault M, Leclerc S, Montplaisir J, Lassonde M. Neurophysiological anomalies in symptomatic and asymptomatic concussed athletes. Neurosurgery. 2006;58:1151–1161. doi: 10.1227/01.NEU.0000215953.44097.FA. discussion 1151–1161. [DOI] [PubMed] [Google Scholar]
- 149.Mayers L. Return-to-play criteria after athletic concussion: a need for revision. Arch Neurol. 2008;65:1158–1161. doi: 10.1001/archneur.65.9.1158. [DOI] [PubMed] [Google Scholar]
- 150.Di Russo F, Spinelli D. Sport is not always healthy: Executive brain dysfunction in professional boxers. Psychophysiology. 2010;47:425–434. doi: 10.1111/j.1469-8986.2009.00950.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.