Abstract
While stress and stress-induced glucocorticoids are classically considered immunosuppressive, they can also enhance proinflammatory responses to subsequent challenges. Corticosterone (CORT) primes rat immune cells, exacerbating pro-inflammatory responses to subsequent immune challenges. Stress can also sensitize pain. One possibility is that stress primes spinal immune cells, predominantly glia, which are key mediators in pain enhancement through their release of proinflammatory cytokines. Therefore, we aimed to identify whether prior CORT sensitizes spinal cord glia such that a potentiated pro-inflammatory response occurs to later intrathecal (IT) lipopolysaccharide (LPS), thereby enhancing pain. Rats received subcutaneous CORT/vehicle 24 h before IT LPS/vehicle. Hind paw pain thresholds were measured before CORT/vehicle, before and up to 48 h after IT LPS/vehicle. In separate rats treated as above, lumbar spinal cord tissue was collected and processed for proinflammatory mediators. CORT alone had no effect on pain responses, nor on any pro-inflammatory cytokines measured. LPS induced allodynia (decreased pain threshold) lasting <4 h and elevated spinal IL-1β and IL-6 protein. Prior CORT potentiated allodynia, lasting >24 h following LPS and potentiated spinal IL-1 and IL-6 protein. Coadministration of IL-1 receptor antagonist with LPS IT completely blocked the allodynia irrespective of whether the system was primed by CORT or not. At 24 h, TLR2, TLR4, MD2 and CD14 mRNAs were significantly elevated within the spinal cord in the CORT+LPS group compared to all other groups. Prior CORT before a direct spinal immune challenge is able to potentiate pain responses and pro-inflammatory cytokine production.
Keywords: intrathecal, TLR4, cytokines, mechanical allodynia
1. Introduction
Data from diverse animal models support the idea that pain enhancement involves neuroinflammation arising from central immune activation, predominantly from spinal cord glia (Costigan et al., 2009; De Leo et al., 2006; Watkins et al., 2007; Watkins et al., 2001). This spinal neuroinflammation importantly contributes to central sensitization (Watkins et al., 2007). Numerous pain conditions in humans also are associated with spinal neuroinflammation, including multiple sclerosis, peripheral neuropathies, amyotrophic lateral sclerosis, and spinal cord injury, with activation of resident microglia and infiltrating macrophages (Rezai-Zadeh et al., 2009; Watkins and Maier, 2003).
In addition to neuroinflammation, pain patients incur some extent of psychosocial stress as a consequence of limited mobility, impaired functionality, and a reduced quality of life. This stress may exacerbate the pain, resulting in a downward spiral of increased pain and further disability (Elliott et al., 2009; Somers et al., 2009), raising the question of whether stress may interact with ongoing spinal neuroinflammation so as to enhance pain.
Interestingly, animal studies document that acute and chronic stress can indeed enhance neuroinflammation in the central nervous system (CNS). Stress modulation of neuroinflammation has been explored in both the brain and spinal cord. For example, acute stress “primes” later neuroinflammatory responses, such that subsequent immune challenges potentiate proinflammatory cytokine induction in hippocampus (Johnson et al., 2002; Johnson et al., 2003; O’Connor et al., 2004; O’Connor et al., 2003). Stress-induced priming of subsequent CNS pro-inflammatory cytokine production has been documented to worsen outcomes (behavior, infarct size) in a rat model of stroke (Caso et al., 2006) and, in the spinal cord, glial reactivity but not pro-inflammatory cytokine mRNA, is shown to worsen neuropathic pain behaviors (Alexander et al., 2009; Takasaki et al., 2005). Stress also increases white and gray matter tissue loss in spinal cord injury models associated with chronic pain, suggestive of enhanced spinal neuroinflammatory responses leading to cell death (Grau et al., 2004).
While glucocorticoids have long been used as an anti-inflammatory therapy when administered after injury, there is increasing evidence that glucocorticoids (in rat, corticosterone; CORT), a major hormone released during periods of stress, acts as a priming event resulting in potentiation of both central and peripheral proinflammatory cytokine production following a subsequent systemic immune challenge (Frank et al., 2010; Sorrells et al., 2009; Yeager et al., 2009). During periods of stress, glucocorticoids are not the only hormones released that influence immune function (Sorrells et al., 2009). However, it has been demonstrated that the administration of a dose of CORT that leads to blood levels similar to those observed during an acute stressor potentiates the pro-inflammatory cytokine response to a subsequent systemic inflammatory challenge (Frank et al., 2010; O’Connor et al., 2003). That is, CORT was inter-changeable for acute stress. To understand these data it is important to distinguish between the effects of elevated CORT while the elevation is still present, and delayed effects after levels have returned to normal. It is under the latter condition that pro-inflammatory potentiation is observed.
Most of the work conducted regarding the impact of stress or elevated glucocorticoids on subsequent immune challenges has been focused on the brain. However, there have been some studies that show a comparable effect occurs within the spinal cord. For example, both glucocorticoids and acute stress have been documented to potentiate neuropathic pain induced by peripheral nerve injury (Alexander et al., 2009; Takasaki et al., 2005; Wang et al., 2004). However, in all studies to date that investigate the impact of stress or CORT on the spinal cord, the secondary immune challenge occurred in the periphery (peripheral nerve injury or hind paw inflammation). Therefore, the effect of CORT priming in enhancing pain could have been mediated by immune cells in the periphery or in the brain, parallel to previously observed priming of peripheral and brain responses (Frank et al., 2010; Johnson et al., 2004; O’Connor et al., 2004; O’Connor et al., 2003).
Therefore, the aim of this study was to identify whether exogenous CORT can potentiate neuroinflammation-induced pain that occurs as a consequence of a subsequent spinal inflammatory challenge induced by intrathecal (IT) lipopolysaccharide (LPS). It also explores whether the prior CORT-induced pain effects are associated with amplification of LPS-induced spinal proinflammatory cytokine induction and whether blocking IL-1β binding to the receptor within the spinal cord attenuates the allodynia. Lastly, it explores whether prior CORT up regulates expression of the LPS receptor (toll-like receptor 4; TLR4), other components of the TLR4 receptor signaling complex (MD2, CD14), which could potentially account for observed potentiation of LPS-induced proinflammatory cytokine induction by prior CORT.
2. Material and Methods
2.1 Animals
Pathogen-free male Sprague-Dawley rats (300–325 g, Harlan Inc, Madison, WI, USA) were used for all experiments. Rats were housed two per cage in a temperature (23 ± 0.3°C) and light (12:12 light: dark cycle; lights on at 07:00) controlled environment. Rats had free access to tap water and standard rat chow. All behavioral testing was conducted within the lights on period. All animals were allowed 1 week of acclimation to the colony rooms before experimentation. The Institutional Animal Care and Use Committee of the University of Colorado at Boulder approved all procedures.
2.2 Drug administration
CORT (Sigma, St Louis, MO) was dissolved in 100% propylene glycol (Sigma, St Louis, USA). Lipopolysaccharide (LPS, Escherichia Coli, serotype 011:B4, Sigma) was dissolved in sterile 0.9% saline on the day of experiments. IL-1 receptor antagonist (IL-1ra, 100 μg in 1 μl, Kineret, Amgen, Thousand Oaks, CA, USA) was diluted in sterile saline on the day of experiments and co-administered with the LPS (1 μl) and 7 μl sterile saline flush. CORT (2.5 mg/kg) or vehicle was administered subcutaneously under very brief isoflurane anesthesia. 24 h after CORT or vehicle administration, LPS (1 μg in 1 μl followed by 8 μl saline flush) or equivolume vehicle was administered intrathecally. For the intrathecal drug administration, the lumbar region was shaved and cleaned. An 18-gauge guide needle, with the hub removed, was inserted into the L5/6 intervertebral space. A PE-10 catheter was inserted into the guide needle, pre-marked such that the proximal end of the PE-10 tubing rested over the L4 –L6 lumbar dorsal spinal cord. Each animal was anesthetized for a maximum of 5 min, and none incurred observable neurological damage from the procedure.
2.3 von Frey testing for mechanical allodynia
The von Frey test was performed on the plantar surface of each hind paw, as described previously (Milligan et al., 2000). Before testing, rats are habituated to the testing environment for 4 days, 40 min per day. Rats are placed on wire racks, under clear containers large enough to allow turning and a small amount of walking, elevated above the tester’s eye. The rats were allowed 30 min on the racks before testing began. A logarithmic series of 10 calibrated Semmes–Weinstein monofilaments (407 mg to 15.136 g, Stoelting, Wood Dale, IL, USA)was applied randomly to the left and right hind paws, each for 8 sat constant pressure as described previously (Hains et al., 2010; Loram et al., 2010; Loram et al., 2009; Milligan et al., 2005; Milligan et al., 2000). Each rat is tested three times with the middle filament (2 g). If there are two or three responses the tester drops to the lowest hair (407 mg) and tests incrementally upwards from there until three consecutive positive responses are obtained. If less than two responses are obtained with the 2 g filament, then the tester tests incrementally upwards until three consecutive positive responses are identified. The stimulus intensity threshold is determined by three consecutive responses of the same intensity of filament. All behavioral testing was performed blind with respect to the drug administration. The stimulus intensities eliciting paw withdrawal responses were used to calculate the 50%paw withdrawal threshold (absolute threshold) using the maximum likelihood fit method to fit a Gaussian integral psychometric function (Harvey, 1986) and is described as allodynia or mechanical sensitivity throughout the text. This method normalizes the withdrawal threshold to parametric conditions (Harvey, 1986; Milligan et al., 2000). For all groups (n=6 per group), there was no significant difference between the left and the right hind paw values and thus they were averaged for each rat. Behavioral testing was done before CORT, immediately before LPS and then 1, 2, 4, 6, 24, and 48 h after LPS administration. Where IL-1ra was coadministered behavior was measured for the same time points up to 24 h after IT administration.
2.4 Spinal cord tissue and blood collection
In this study, IT LPS or vehicle was administered 24 h after systemic CORT, as described above but with no pain testing performed at any time. Rats (n=6 per group) were deeply anesthetized with sodium pentobarbital 15 min, 1, 4 and 24 h after IT LPS or vehicle. Following cardiac puncture for blood collection, rats were transcardially perfused with ice-cold saline for 2 min. The left (for mRNA analyses) and right (for protein and CORT analyses) L4–L6 dorsal spinalcord were isolated, the meninges removed and separately flash frozen in liquid nitrogen and stored at − 80°C until further analysis. The blood samples were allowed to clot, centrifuged at 14,000 rpm for 10 min. The serum collected and stored until the endotoxin levels were measured.
2.5 Endotoxin measurement
Serum was assayed for gram-negative bacterial endotoxin levels using the limulus amebocyte lysate (LAL) assay (BioWhittaker QCL-1000). The assay was performed according to manufacturer’s instructions, methods detailed previously (O’Connor et al., 2003).
2.6 RNA isolation and cDNA synthesis
RNA from the lumbar spinal cord was extracted using the standard phenol: chloroform extraction with TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s guidelines. Samples were treated with DNase to remove any contaminating DNA (Ambion, Austin, TX). Total RNA was reverse transcribed into cDNA using Superscript II First-Strand Synthesis System (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized using total RNA, random hexamer primer (5 ng/μl), 1 mM dNTP mix, cDNA synthesis buffer (Invitrogen, Carlsbad, CA) and incubated at 65°Cfor 5 min. Following 2 min incubation on ice, a cDNA synthesis buffer (5× RT buffer, Invitrogen, Carlsbad, CA) and dithiothreitol(10 mM) was added and incubated at 25°C for 2 min. Reverse transcriptase (Superscript II, 200 Units, Invitrogen, Carlsbad, CA)was added to a total volume of 20μl and incubated for 10 min at 25°C, 50 min at 42°C and deactivating the enzyme at 70°C for 15 min. cDNA was diluted 2-fold in nuclease-free water and stored at − 80°C until PCR was performed.
2.7 Real-time polymerase chain reaction (PCR)
Primer sequences were obtained from the Genbank at the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov) and displayed in Table 1. Amplification of the cDNA was performed using Quantitect SYBR Green PCR kit (Qiagen, Valenica, CA) in iCycler iQ 96-well PCR plates (Bio-Rad, Hercules, CA) on a MyiQ single Color Real-Time PCR Detection System (Bio-Rad). The reaction mixture (26μl) was composed of QuantiTect SYBR Green (containing fluorescent dye SYBR Green I,2.5 mM MgCl2, dNTP mix and Hotstart Taq Polymerase), 10 nMfluorescein, 500 nM of each forward and reverse primer (Invitrogen, Carslbad, CA), nuclease-free water and 1μl of cDNA from each sample. Each sample was measured in duplicate. The reactions were initiated with a hot start at 95°C for 25 min, followed by 40 cycles of 15 s at 94°C (denaturation), 30 s at 55–60°C (annealing) and 30 s at 72°C (extension). Melt curve analyses were conducted to assess uniformity of product formation, primer–dimer formation and amplification of non-specific products. The PCR product was monitored in real-time, using the SYBR Green I fluorescence, using the MyiQ single Color Real-Time PCR Detection System (Bio-Rad). Threshold for detection of PCR product was set in the log-linear phase of amplification and the threshold cycle (CT) was determined for each reaction. The level of the target mRNA was quantified relative to the housekeeping gene (GAPDH) and presented as percentage of vehicle control. The expression of GAPDH was not significantly different between treatments.
Table 1.
Primer sequences
| Gene | Primer sequence (5′ -3′) | GenBank accession No. |
|---|---|---|
| GAPDH | TCTTCCAGGAGCGAGATCCC (forward) TTCAGGTGAGCCCCAGCCTT (reverse) |
NC_005103.2 |
| TLR4 | TCCCTGCATAGAGGTACTTC (forward) CACACCTGGATAAATCCAGC (reverse) |
NM_019178.1 |
| TLR2 | TGGAGGTCTCCAGGTCAAATC (forward) ACAGAGATGCCTGGGCAGAAT (reverse) |
NM_198769.2 |
| MD2 | ATCTGAGAGGCAACAGTG (forward) CCTCTTGGAATGAACTCAACA (reverse) |
NM_001024279.1 |
| CD14 | ACCGACCATGAAGCTTATGC (forward) CTGAGAAGTTGCAGTAGCAG (reverse) |
NM_021744.1 |
2.8 Protein quantification
2.8.1 Total protein concentration
The protein concentration from each sample was determined using a Bradford protein assay as described previously(Bradford, 1976) and used to normalize the results from the ELISA and CORT assay described below.
2.8.2 Interleukin (IL) 1β and IL-6 ELISA
IL-1β and IL-6 protein in rat dorsal spinal cord was analyzed using a commercially available ELISA kit specific for rat IL-1β and IL-6 (R&D Systems, Minneapolis, MN, USA). The left lumbar dorsal spinalcord was sonicated in 300μl of cold Iscove’s culture medium containing 5% fetal calf serum and a cocktail enzyme inhibitor(100 mM amino-n-caproic acid, 10 mM EDTA, 5 mM benzamidine–HCl, and 0.2 mM phenylmethylsulfonyl fluoride). Sonicated samples were centrifuged at 14,000 rpm at 4°C for 10 min. Supernatants were removed and stored at 4°C overnight until an ELISA was performed the following day. The sensitivity for the rat IL-1β assay is 5 pg/ml and for the IL-6 assay is 21 pg/ml.
2.9 Corticosterone assay
CORT was measured in the lumbar spinal cord homogenate using a competitive immunoassay (Assay designs, Ins., Ann Arbor, MI, USA) according to manufacturer’s guidelines and normalized to total protein content.
2.10 Statistical analysis
Behavioral measures were normalized as described above and analyzed using repeated measures two-way ANOVA with time and treatment as main effects. CORT was analyzed as a 2-way ANOVA with CORT and LPS as main effects. RT-PCR was converted to percent of vehicle control for each time point. Both RT-PCR and ELISA were analyzed as a 2-way ANOVA with CORT and LPS as main effects using Graphpad Prism version 5. Each time point was analyzed separately. Bonferroni post hoc tests were used where appropriate and P< 0.05 was considered statistically significant.
3. Results
3.1 IT LPS does not lead to measurable levels of endotoxin in the systemic circulation
To test whether intrathecal LPS injections result in measurable endotoxin elevations in the systemic circulation, endotoxin was measured by LAL assay in the serum 15 min, 1 h and 4 h after intrathecal LPS (1 μg) or vehicle. There was no significant difference between groups at any of the time points measured and the endotoxin levels were less than 1 EU/ml. Therefore, the effects reported in the studies below cannot be attributable to elevated systemic endotoxin levels.
3.2 Glucocorticoids potentiate intrathecal LPS-mediated mechanical allodynia
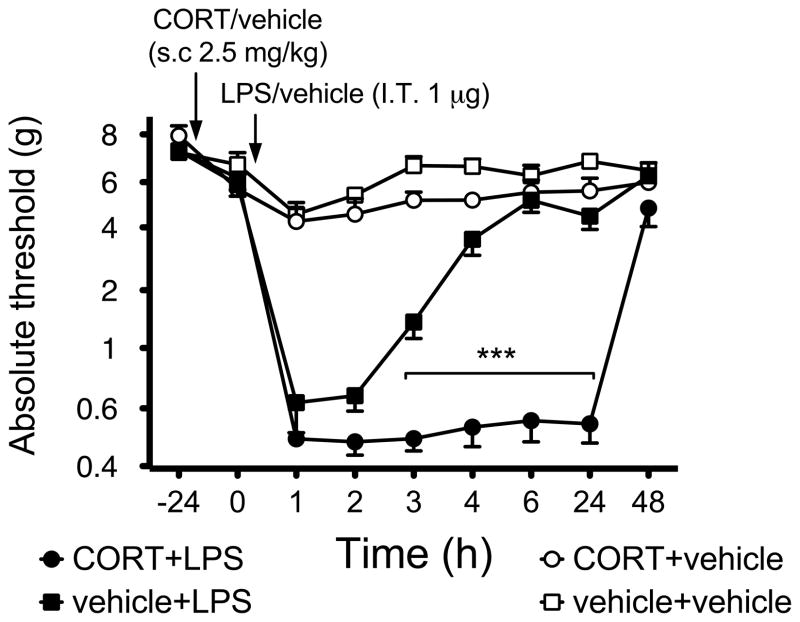
Previous studies have shown that systemic CORT is able to prime hippocampal microglial proinflammatory cytokine production in response to subsequent systemic LPS (Frank et al., 2010). A few studies have demonstrated CORT priming to be sufficient to result in observable behavioral changes, following neuropathic injury, prostaglandin and epinephrine induced hyperalgesia (Alexander et al., 2009; Takasaki et al., 2005). However none have identified prior CORT effects to a later challenge by LPS. Therefore, we assessed the effect of CORT and subsequent IT LPS on pain thresholds assessed on the hind paws, areas innervated by the spinal region exposed to LPS. Figure 1 shows the behavioral response to mechanical stimulation applied to the hind paw before systemic CORT or vehicle and before and after IT LPS or vehicle. 1 μg of LPS administered IT induced mechanical allodynia in the absence of CORT that persisted at least 3 h, but not 4 h, compared to vehicle controls (P<0.0001, main effect of drug F3,24=310, P<0.001, main effect time: F8,24=80.92, P<0.0001, interaction: F24,178=22.03, P<0.0001). Importantly, prior systemic CORT potentiated the duration of allodynia induced by 1 μg LPS such that allodynia now lasted at least 24 h, but not 48 h, compared to vehicle control (P<0.0001). CORT alone had no significant effect on the behavioral response (P>0.05).
Figure 1.
Intrathecal administration of 1 μg LPS induces allodynia, which is potentiated by prior administration of subcutaneous CORT (2.5 mg/kg). Mechanical sensitivity was tested using the von Frey test, before (baseline, BL) CORT or vehicle, before (0) and 1–48 h after intrathecal administration LPS (1 μl with 8 μl saline flush) or equivolume vehicle. Time is reflected as post intrathecal administration. Solid circle depicts CORT+LPS(n= 6), black squares depict vehicle+LPS (n=6), grey circles depict CORT+LPS (n=6) and open circles depicts vehicle+vehicle (n= 6). Data are presented as average absolute thresholds of left and right hind paws (mean ± SEM).
***P< 0.001, against other groups at the same time point.
3.3 Prior exogenous CORT has no effect on spinal cord CORT levels measured after IT LPS or vehicle
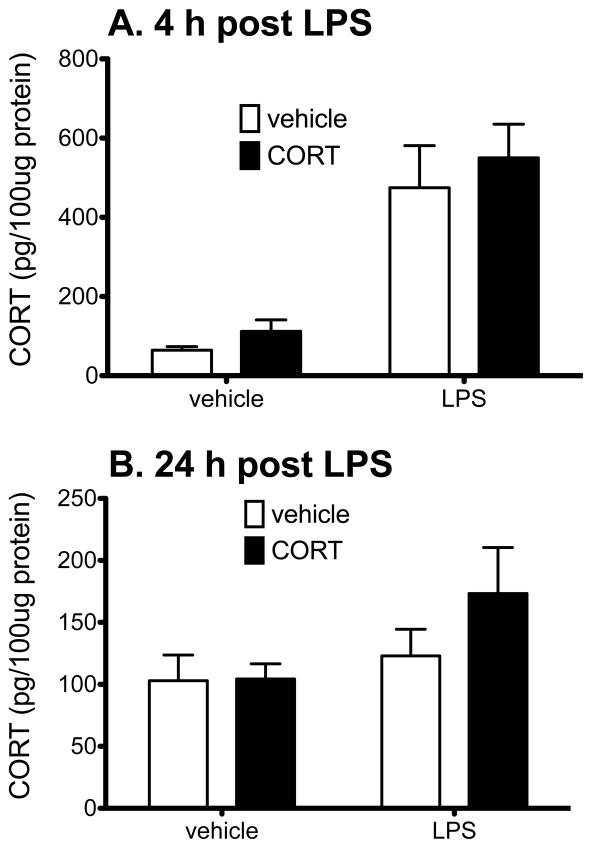
In the present studies exogenous CORT was administered systemically 24 h before IT LPS. In order to identify whether the prior exogenous CORT affects the endogenous CORT produced in response to subsequent IT LPS or vehicle injections, CORT levels were measured within the spinal cord 1, 4, and 24 h after IT LPS or vehicle (that is, 25, 28, and 48 h after exogenous CORT or vehicle so that the CORT measured here is not the administered CORT given these protracted time differences). The 1 h time point showed elevated levels of CORT in all 4 groups, most likely as a result of the anesthesia and intrathecal injection (data not shown), elevation in CORT only in the two LPS groups at 4 h (main effect of LPS: F1,19=41.32, P<0.0001, main effect of CORT: F1,19=0.87, P=0.36, interaction: F1,19=0.04, P=0.84), and return of CORT measured in the LPS groups to basal levels by 24 h (main effect of CORT: F1,19=1.06, P=0.32); main effect of LPS: F1,19=3.16, P=0.09; Interaction: F1,19=0.96, P=0.34) resulting in no significant differences between groups. Figure 2 shows the CORT levels within the spinal cord 4 h (Fig. 2A) and 24 h (Fig. 2B) after LPS or vehicle IT administration (28 h and 48 h after exogenous CORT administration). Prior CORT did not affect CORT levels induced by LPS. IT LPS significantly elevated CORT levels within the spinal cord at 4 h but not 24 h after LPS administration. There was no statistically significant difference between CORT+LPS and veh+LPS at the 4 h time point (P>0.05).
Figure 2.
CORT levels (pg per 100 μg of total protein) within the lumbar spinal cord 4 h (panel A) and 24 h (panel B) after LPS/vehicle administration (28 h and 48 h after CORT/vehicle administration, n=6/7 per group). There is a significant LPS effect (P<0.0001) but no CORT effect at 4 h. There is no difference between the groups at 24 h after LPS/veh administration. Prior exogenous CORT did not affect lumbar spinal cord CORT levels at either time point. Solid bars reflect CORT and open bars reflect vehicle injections.
3.4 Glucocorticoids potentiate pro-inflammatory cytokine IL-1 and IL-6
We have shown previously that systemic CORT is able to sensitize hippocampal microglial induction of proinflammatory cytokines in response to LPS ex vivo(Frank et al., 2010). The aim of this study was to determine whether systemic CORT is also able to sensitize proinflammatory cytokine response within the spinal cord such that a later response to local administration of LPS is potentiated by prior systemic CORT. Figure 3 demonstrates that prior systemic CORT significantly potentiates both LPS-induced IL-1β (Fig. 3A) and IL-6 (Fig. 3B) within the lumbar spinal cord, relative to prior systemic vehicle followed by IT LPS, 4 h after LPS administration (P<0.001) for IL-1β and 4 h (P<0.01) and 24 h (P<0.001) post LPS for IL-6. There was no significant effect of prior CORT followed later by vehicle on either IL-1β or IL-6, but prior vehicle followed by later LPS significantly increased IL-1 β at 1 h, (Interaction: F1,20=4.54, P<0.05) 4 h (Interaction: F1,20=9.68, P<0.0001) and 24 h (main effect of LPS: F1,20=86.57, P<0.0001) but no effect of CORT (P=0.93) or interaction (P=0.63) compared to all other groups. For IL-1β, there was no significant effect between groups at 15 min post LPS. For IL-6 there was a significant effect of LPS at 15 min (F1,20=152.5, P<0.0001) and 1 h post LPS (F1,20=12.74, P<0.01). There was a significant interaction at 4 h (F1,20=5.95, P<0.0001)and 24 h post LPS (F1,20=54.39, P<0.0001)with CORT+LPS being higher than vehicle+LPS (P<0.01).
Figure 3.
The effect of CORT, administered systemically 24 h before intrathecal LPS/vehicle on IL-1β (A.) and IL-6 (B.) protein within the lumbar dorsal spinal cord, 15 min, 1 h, 4 h and 24 h after LPS/vehicle administration. Intrathecal LPS significantly increased IL-1β and IL-6 compared to vehicle controls. CORT potentiated the IL-1β and IL-6 production at 4 h after LPS administration. CORT alone had no effect on IL-1β or IL-6 production. Data are presented mean ± SEM, n=6 per group. *P<0.001 compared to vehicle+LPS, #P<0.001 compared to vehicle+vehicle at the same time point.
3.5 Coadministration of IL-1ra with LPS attenuates the allodynia induced by LPS
In order to determine if the elevated IL-1β contributes to the allodynia induced by LPS and potentiated by CORT, we co-administered 100 μg IL-1ra with LPS IT and measured the behavioral changes subsequent to drug administration. Figure 4 demonstrates that IL-1ra completely blocked the allodynia induced by LPS, irrespective of whether prior CORT was administered or not, from 1 h (P<0.001) until the last time measured at 24 h (P<0.001) post LPS administration compared to CORT+LPS+vehicle group (Interaction: F12,90=8.83, P<0.0001). There was no significant difference at any time point measured between CORT+LPS+IL-1ra and vehicle+LPS+IL-1ra (P>0.05).
Figure 4.
Intrathecal administration of 1 μg LPS + prior administration of subcutaneous CORT (2.5 mg/kg) or vehicle. IL-1ra (100 μg in 1 μl) or equivolume vehicle was coadministered intrathecally with the LPS. Mechanical sensitivity was tested using the von Frey test, before (baseline, BL) CORT or vehicle, before (0) and 1–24 h after intrathecal administration. Time is reflected as post intrathecal administration. Solid circle depicts CORT+LPS+vehicle(n= 6), black squares depict vehicle+LPS+IL-1ra (n=6) and open circles depicts CORT+LPS+IL-1ra (n= 6). Data are presented as average absolute thresholds of left and right hind paws (mean ± SEM).
***P< 0.001, against CORT+LPS+vehicle.
3.6 Glucocorticoids potentiate components of toll-like receptor signaling
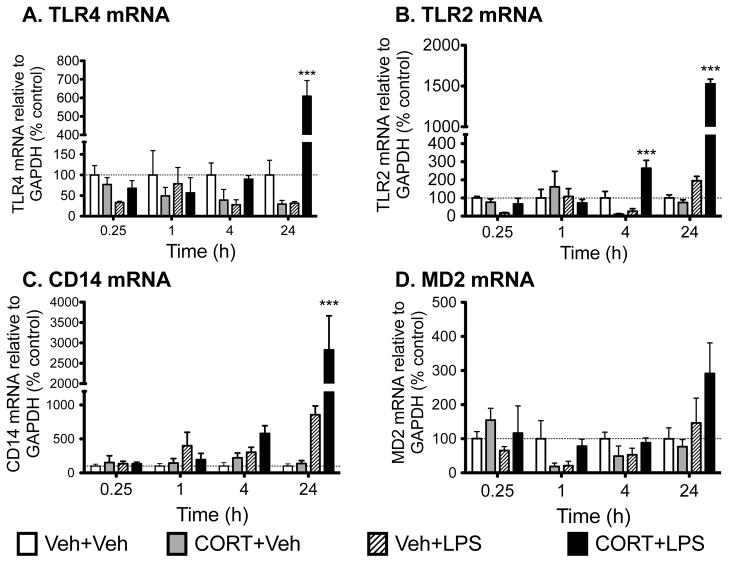
TLR4 is the receptor activated by LPS (Akira and Takeda, 2004). The literature supports that activation of TLR4 can up regulate expression of a closely related TLR, namely TLR2 (Fan et al., 2003). These TLRs are related in that both have common functions in responding to endogenous danger signals (Kawai and Akira, 2010), substances released in response to tissue stress/injury (Kawai and Akira, 2010). Hence, given their linkages, we explored the potential for CORT priming of later LPS-induced changes in expression of TLR2 as well as the endogenous receptor for LPS, TLR4 and its co-signaling molecules (MD2, CD14). Figure 4 shows the mRNA changes of TLR4 (Fig. 5A), TLR2 (Fig. 5B), MD2 (Fig. 5C) and CD14 (Fig. 5D) within the spinal cord 1 h, 4 h and 24 h after LPS or vehicle administration. There was a significant main effect of LPS at 15 min post LPS on TLR4 (F1,20=5.008, P<0.05) and TLR2 (F1,20=6.158, P<0.05). At 4 h there is a significant interaction on TLR4 (F1,20=7.15, P<0.05) and TLR2 (F1,20=37.66, P<0.0001), and a main effect of CORT (F1,19=5.95, P<0.05) and LPS (F1,19=11.89, P<0.01) on CD14. At 24 h there was a significant interaction on TLR4 (F1,19=47.03, P<0.0001), TLR2 (F1,17=10.62, P<0.0001) and CD14 (F1,18=10.04, P<0.01) with CORT+LPS significantly higher than the other groups. There was a significant main effect of LPS on MD2 comparisons show that TLR4 (P<0.0001) (F1,20=4.60, P<0.05) at 24 h post LPS. Post hoc and CD14 mRNA (P<0.001) for CORT+LPS is significantly different to all other groups at 24 h and TLR2 mRNA from the CORT+LPS group was significantly different to all other groups at 4 h (P<0.0001) and 24 h (P<0.01).
Figure 5.
Gene expression in dorsal spinal cord tissue collected 15 min, 1 h, 4 h and 24 h after IT LPS/vehicle expressed as percent of vehicle+vehicle control. CORT or vehicle was administered systemically 24 h before intrathecal LPS or vehicle administration. TLR2, TLR4 and CD14 mRNA were all significantly elevated 24 h after CORT+LPS compared to any of the other groups with CORT+LPS showing an elevated TLR2 mRNA compared to other groups at 4 h. Data are presented as mean ± SEM, n=6 per group. *** P < 0.01 compared to other groups at the same time point.
4. Discussion
The present study documents that systemic CORT administered 24 h before IT LPS potentiated the duration of mechanical allodynia induced by this later IT LPS. In addition, spinal cord IL-1β protein was significantly elevated 1, 4, and 24 h following IT LPS compared to vehicle controls, with CORT potentiating the LPS-induced IL-1β protein response at 4 h. Blocking IL-1 receptors in the spinal cord with IL-1ra abolished the allodynia induced by LPS irrespective of whether prior CORT was administered or not. Spinal IL-6 protein was elevated at 15 min, 1 h and 4 h post IT LPS compared to vehicle controls. This IL-6 response also was significantly potentiated by prior systemic CORT administration at 4 h and 24 h. CORT alone had no effect on mechanical allodynia, spinal IL-1β or spinal IL-6 production at any time point measured. Interestingly TLR4, MD2 and CD14 spinal cord mRNAs were increased 24 h after IT LPS when rats had been primed by CORT 24 h before. TLR2 mRNA was potentiated by prior CORT at both 4 h and 24 h post IT LPS administration. CORT was elevated within the spinal cord when stimulated by local LPS, which was independent of prior CORT. Taken together these data support that prior systemic CORT directly sensitizes the lumbar spinal cord, resulting in elevated cytokine production and potentiated allodynia following a direct spinal cord immune challenge. It should be highlighted that the amount of CORT administered mimics stress levels (Fleshner et al., 1995; Johnson et al., 2002), and so produces physiological rather than pharmacological stimulation of glucocorticoid receptors. In addition, even though the CORT levels were not changed by the prior CORT at the time points tested, it remains to be elucidated whether the glucocorticoid receptor expression or responsivity may have been altered by the prior exogenous CORT exposure.
Glucocorticoids, while classically thought of as anti-inflammatory, can serve as a priming event when administered before a systemic inflammatory challenge (Frank et al., 2010; Sorrells et al., 2009). Previous studies have shown that CORT in vivo sensitized hippocampal microglia to a subsequent LPS challenge ex vivo(Frank et al., 2010). However, other central immunocompetent cell types such as endothelial cells, oligodendrocytes and astrocytes have not been explored and may be involved and contribute to the results presented in this series of studies. We have shown that prior CORT is able to also sensitize immunocompetent cells, within the spinal cord, resulting in an exaggerated pro-inflammatory response to local LPS in vivo. This response was unlikely to arise from peripheral immune activation, as IT LPS did not appear to escape the intrathecal space. This conclusion is based on the fact that no endotoxin was detected in peripheral blood at any time measured after IT LPS.
The priming by prior CORT resulted in not only a potentiated pro-inflammatory response within the spinal cord, but also an exaggerated mechanical allodynia following IT LPS administration. While this is the first time CORT has been demonstrated to potentiate LPS-induced allodynia, a few studies have shown that prior stress or CORT is able to potentiate neuropathic pain, associated with an upregulation of glial activity, (Alexander et al., 2009; Takasaki et al., 2005) as well as epinephrine and prostaglandin mediated hind paw hyperalgesia (Khasar et al., 2008). In addition, intrathecal and systemic glucocorticoid receptor antagonist administration reversed neuropathic pain (Takasaki et al., 2005) and epinephrine-induced hyperalgesia (Khasar et al., 2008). While IL-1ra was able to effectively block the allodynia induced most likely induced by glial cells, whether these phenomena are explained by alterations in spinal glial function or other immunocompetent cells remains to be tested.
Pain enhancement in diverse animal models involves production of pro-inflammatory cytokines produced within the spinal cord, mostly by glial cells (Watkins et al., 2001). This conclusion is based on the fact that various drug candidates known to inhibit glial cells and subsequent proinflammatory cytokine production reverse mechanical allodynia and thermal hyperalgesia (Romero-Sandoval et al., 2008; Watkins et al., 2009; Watkins and Maier, 2003). Both IL-1β and IL-6 have been implicated in such spinally mediated pain enhancement (Arruda et al., 2000; DeLeo et al., 1996; Milligan et al., 2005). Therefore, given the known pain-enhancing effects of IL-1β in spinal cord, and the complete attenuation of allodynia by IL-1ra, identified in our study, the allodynia induced by intrathecal LPS is produced by IL-1β. Whether blocking IL-6 would produce the same effect is as yet unknown. However, it is notable that spinal cord tissue IL-1β and IL-6 levels were elevated in the veh+LPS group at 4 hours after LPS, a timepoint where allodynia had resolved. While speculative, one potential explanation for the apparent disconnect between cytokine elevations in spinal tissue and the loss of allodynia at that same timepoint may be that IL-1β and IL-6 release in pain-modulatory laminae in dorsal horn has ended by 4 hours after LPS, but intracellular production and tissue clearance is ongoing. Alternatively, the apparent disconnect may point to the involvement of other glial products as being responsible for the pain enhancement. For example, inhibiting TNF release by such manipulations as a TNF antagonist and various glial activation inhibitors (minocycline and pentoxifylline) attenuated intrathecal LPS-induced allodynia (Saito et al., 2010).
Numerous studies have demonstrated that exposure to either acute stress or CORT lead, after a delay, in a potentiated elevation of both peripheral and brain IL-1β and IL-6 production following a subsequent systemic LPS challenge (Frank et al., 2010; Johnson et al., 2002; O’Connor et al., 2003). In none of these studies was the spinal cord evaluated. While regional brain differences in effects of CORT have been identified (Munhoz et al., 2006), it does appear that CORT is able to prime immune cells within the spinal cord resulting in exaggerated pro-inflammatory cytokine production when stimulated directly with a subsequent immune challenge. How long the CORT priming effect lasts is not yet known.
The underlying mechanism for CORT priming the immune system remains to be elucidated. However, recent studies suggest that TLR signaling may be involved. Prior CORT resulted in elevated TLR2 and TLR4 mRNA in the hippocampus 4 h after subsequent LPS administration (Frank et al., 2010). In addition, TLR4 mRNA also was elevated within the spinal cord of a spared nerve injury model (Alexander et al., 2009), subsequent to CORT priming. LPS, derived from the cell wall of gram-negative bacteria is recognized by TLR4 (Akira et al., 2006), and leads to activation of the NFκB pathway (Akira and Takeda, 2004). NFκB activation results in pro-inflammatory cytokine production (Akira and Takeda, 2004). Endogenous danger signals (substances released from stress/damaged host cells) in spinal cord have also been recently implicated as important mediators of neuropathic pain through their activation of TLR4 (Hutchinson et al., 2008) and TLR2 (Kim et al., 2007). Therefore, should TLR2 and/or TLR4 be upregulated following CORT administration, LPS and endogenous danger signals would be prime agonists whose effects could be potentiated by such upregulation. Notably, in our study, the TLR2 and TLR4 receptors were not elevated by CORT alone but prior CORT exposure led to elevations in IT LPS-induced TLR4 mRNA at 24 h and TLR2 mRNA at 4 h and 24 h post IT LPS. In addition, the co-receptors required for optimal activation of TLR2 and/or TLR4, CD14 and MD2 (trending but not significant), were elevated 24 h after IT LPS administration in rats primed with prior CORT, but not elevated by CORT alone. Therefore, our proposed hypothesis of prior CORT priming the immune response via upregulated TLR system proved incorrect. If CORT does prime TLR2 and TLR4 signaling, it is possibly via a sensitization of the pathway downstream of the TLR receptor, possibly on NFκB. While the TLR complex gene expression is not altered following CORT, this priming event may result in a rebound upregulation of the TLR complex. This delayed elevation of TLR mRNA in the CORT+LPS group requires further investigation to elucidate the possible link to the potentiated duration of allodynia.
Stress has been implicated in numerous psychiatric disorders, and has clinically been implicated in potentiating pain disorders such as fibromyalgia and rheumatoid arthritis (Nilsen et al., 2007; Zautra et al., 2007). However, the underlying mechanism for stress potentiating pain is not known. Most animal studies investigating the role of prior stress on a subsequent immune challenge have done so focusing on the brain. The present study is novel in that it explores the effect of prior elevated CORT on spinal inflammation. Our results suggest that prior elevated glucocorticoids, equivalent to that produced by a stressor, results in a primed inflammatory response such that subsequent neuroinflammation is potentiated and the allodynia is prolonged.
Acknowledgments
Financial support for these studies was provided by NIH grants DA024044, DE107782, DE020247 and DA023132.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Alexander JK, DeVries AC, Kigerl KA, Dahlman JM, Popovich PG. Stress exacerbates neuropathic pain via glucocorticoid and NMDA receptor activation. Brain Behav Immun. 2009;23:851–860. doi: 10.1016/j.bbi.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda JL, Sweitzer S, Rutkowski MD, DeLeo JA. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain. Brain Research. 2000;879:216–225. doi: 10.1016/s0006-8993(00)02807-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caso JR, Lizasoain I, Lorenzo P, Moro MA, Leza JC. The role of tumor necrosis factor-alpha in stress-induced worsening of cerebral ischemia in rats. Neuroscience. 2006;142:59–69. doi: 10.1016/j.neuroscience.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Colburn RW, Nichols M, Malhotra A. Interleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy model. J Interferon Cytokine Res. 1996;16:695–700. doi: 10.1089/jir.1996.16.695. [DOI] [PubMed] [Google Scholar]
- Elliott JM, Noteboom JT, Flynn TW, Sterling M. Characterization of acute and chronic whiplash-associated disorders. J Orthop Sports Phys Ther. 2009;39:312–323. doi: 10.2519/jospt.2009.2826. [DOI] [PubMed] [Google Scholar]
- Fan J, Frey RS, Malik AB. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest. 2003;112:1234–1243. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Deak T, Spencer RL, Laudenslager ML, Watkins LR, Maier SF. A long-term increase in basal levels of corticosterone and a decrease in corticosteroid-binding globulin after acute stressor exposure. Endocrinology. 1995;136:5336–5342. doi: 10.1210/endo.136.12.7588279. [DOI] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Grau JW, Washburn SN, Hook MA, Ferguson AR, Crown ED, Garcia G, Bolding KA, Miranda RC. Uncontrollable stimulation undermines recovery after spinal cord injury. J Neurotrauma. 2004;21:1795–1817. doi: 10.1089/neu.2004.21.1795. [DOI] [PubMed] [Google Scholar]
- Hains LE, Loram LC, Weiseler JL, Frank MG, Bloss EB, Sholar P, Taylor FR, Harrison JA, Martin TJ, Eisenach JC, Maier SF, Watkins LR. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. J Pain. 2010;11:1004–1014. doi: 10.1016/j.jpain.2010.01.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput. 1986;18:623–632. [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:R422–432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- Loram LC, Harrison JA, Chao L, Taylor FR, Reddy A, Travis CL, Giffard R, Al-Abed Y, Tracey K, Maier SF, Watkins LR. Intrathecal injection of an alpha seven nicotinic acetylcholine receptor agonist attenuates gp120-induced mechanical allodynia and spinal pro-inflammatory cytokine profiles in rats. Brain Behav Immun. 2010;24:959–967. doi: 10.1016/j.bbi.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram LC, Harrison JA, Sloane EM, Hutchinson MR, Sholar P, Taylor FR, Berkelhammer D, Coats BD, Poole S, Milligan ED, Maier SF, Rieger J, Watkins LR. Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J Neurosci. 2009;29:14015–14025. doi: 10.1523/JNEUROSCI.3447-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O’Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, Chavez RA, Leinwand LA, Watkins LR. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci. 2005;21:2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen KB, Sand T, Westgaard RH, Stovner LJ, White LR, Bang Leistad R, Helde G, Ro M. Autonomic activation and pain in response to low-grade mental stress in fibromyalgia and shoulder/neck pain patients. Eur J Pain. 2007;11:743–755. doi: 10.1016/j.ejpain.2006.11.004. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Ginsberg AB, Maksimova E, Wieseler Frank JL, Johnson JD, Spencer RL, Campeau S, Watkins LR, Maier SF. Stress-induced sensitization of the hypothalamic-pituitary adrenal axis is associated with alterations of hypothalamic and pituitary gene expression. Neuroendocrinology. 2004;80:252–263. doi: 10.1159/000082876. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J Neuroimmune Pharmacol. 2009;4:462–475. doi: 10.1007/s11481-009-9166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sandoval EA, Horvath RJ, DeLeo JA. Neuroimmune interactions and pain: focus on glial-modulating targets. Curr Opin Investig Drugs. 2008;9:726–734. [PMC free article] [PubMed] [Google Scholar]
- Saito O, Svensson CI, Buczynski MW, Wegner K, Hua XY, Codeluppi S, Schaloske RH, Deems RA, Dennis EA, Yaksh TL. Spinal glial TLR4-mediated nociception and production of prostaglandin E(2) and TNF. Br J Pharmacol. 2010;160:1754–1764. doi: 10.1111/j.1476-5381.2010.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers TJ, Keefe FJ, Godiwala N, Hoyler GH. Psychosocial factors and the pain experience of osteoarthritis patients: new findings and new directions. Curr Opin Rheumatol. 2009;21:501–506. doi: 10.1097/BOR.0b013e32832ed704. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki I, Kurihara T, Saegusa H, Zong S, Tanabe T. Effects of glucocorticoid receptor antagonists on allodynia and hyperalgesia in mouse model of neuropathic pain. Eur J Pharmacol. 2005;524:80–83. doi: 10.1016/j.ejphar.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo G, Yang L, Mao J. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. J Neurosci. 2004;24:8595–8605. doi: 10.1523/JNEUROSCI.3058-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain. 2001;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- Yeager MP, Rassias AJ, Pioli PA, Beach ML, Wardwell K, Collins JE, Lee HK, Guyre PM. Pretreatment with stress cortisol enhances the human systemic inflammatory response to bacterial endotoxin. Crit Care Med. 2009;37:2727–2732. doi: 10.1097/ccm.0b013e3181a592b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Parrish BP, Van Puymbroeck CM, Tennen H, Davis MC, Reich JW, Irwin M. Depression history, stress, and pain in rheumatoid arthritis patients. J Behav Med. 2007;30:187–197. doi: 10.1007/s10865-007-9097-4. [DOI] [PubMed] [Google Scholar]