Abstract
Learning and memory in the fruit fly, Drosophila melanogaster, is a complex behavior with many parallels to mammalian learning and memory. Although many neurotransmitters including acetylcholine, dopamine, glutamate, and GABA have previously been demonstrated to be involved in aversive olfactory learning and memory, the role of serotonin has not been well defined. Here, we present the first evidence of the involvement of individual serotonin receptors in olfactory learning and memory in the fly. We initially followed a pharmacological approach, utilizing serotonin receptor agonists and antagonists to demonstrate that all serotonin receptor families present in the fly are necessary for short term learning and memory. Isobolographic analysis utilizing combinations of drugs revealed functional interactions are occurring between 5-HT1A-like and 5-HT2, and 5-HT2 and 5-HT7 receptor circuits in mediating short term learning and memory. Examination of long term memory suggest that 5-HT1A-like receptors are necessary for consolidation and important for recall, 5-HT2 receptors are important for consolidation and recall, and 5-HT7 receptors are involved in all three phases. Importantly, we have validated our pharmacological results with genetic experiments, and show that hypomorph strains for 5-HT2Dro and 5-HT1BDro receptors, as well as knockdown of 5-HT7Dro mRNA significantly impair performance in short term memory. Our data highlight the importance of the serotonin system and individual serotonin receptors to influence olfactory learning and memory in the fly, and position the fly as a model system to study the role of serotonin in cognitive processes relevant to mammalian CNS function.
Keywords: Serotonin, learning and memory, ellipsoid body, 5-HT2, 5-HT1A, 5-HT7
The neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) is involved in, among other things, the regulation of mood, cognition, appetite, sleep, aggression, memory, and sexual behavior in mammals (Lucki, 1998). The effects of serotonin are primarily mediated through interactions with several G-protein coupled receptors to initiate multiple signal transduction pathways (Raymond et al., 2001, Nichols and Nichols, 2008). All 5-HT receptor families are present in the human brain, and are believed to play a role in cognition (Barnes and Sharp, 1999, Meneses, 1999). The exploration of serotonin and 5-HT receptors in learning and memory processes has primarily focused on the 5-HT1A and 5-HT2 receptors in mammalian systems. The 5-HT1A receptor is coupled to the Gαi signaling pathway and the inhibition of adenylate cyclase activity, and can regulate presynaptic 5-HT release via autoreceptor activity, as well as modulate postsynaptic neuronal activity via postsynaptically localized receptors. The 5-HT1A receptor influences the activity of glutamatergic, cholinergic, and GABAergic neurons in the cerebral cortex, hippocampus, and septohippocampal projection to affect learning and memory processes (Ogren et al., 2008). The 5-HT7 receptor is coupled to Gαs and activation of adenylate cyclase, and in the mammalian CNS is highly expressed in the suprachiasmatic nucleus of the hypothalamus, thalamus, hippocampus, and cortex (Hedlund and Sutcliffe, 2004). It is known to be involved in regulation of circadian rhythms as well as learning and memory processes (Hedlund and Sutcliffe, 2004). The 5-HT2 receptors are a primary target of classical hallucinogenic drugs like lysergic acid diethylamide, and couple to the Gαq signaling pathway and activation of phospholipase-C β. The 5-HT2 receptor family as a whole is expressed widely throughout the CNS, and contributes to many significant higher order behaviors, including learning and memory (Williams et al., 2002, Nichols and Nichols, 2008).
The homologous serotonin receptor families present in Drosophila are the 5-HT1A-like, 5-HT2, and 5-HT7 receptors (Witz et al, 1990, Saudou et al, 1992, Colas et al, 1995). In flies, the 5-HT1A-like receptors are the 5-HT1ADro and 5-HT1BDro receptors (Witz et al, 1990). The 5-HT1BDro receptor is expressed both presynaptically and postsynaptically, and is highly expressed in brain regions associated with learning and memory like the mushroom bodies (Yuan et al., 2005), which are considered to be the fly equivalent of the mammalian hippocampus (Cayre et al, 2002). The 5-HT1ADro receptor has also been found to be expressed within the mushroom bodies (Yuan et al, 2006). The 5-HT2Dro receptor is expressed in regions distinct from those of the 5-HT1A like receptors, and is detected within the protocerebrum and ellipsoid body (EB) (Nichols, 2007). 5-HT2Dro receptor function has been implicated in circadian rhythms, visual processing, and aggression (Nichols et al, 2002, Nichols, 2007, Johnson et al., 2009). In the adult brain, 5-HT7Dro receptor expression is highly localized to large field R-neurons that innervate the EB, as well as in discreet populations of cells between the central brain and the optic lobes that cluster with but do not express peptide dispersing factor (Becnel et al., 2011).
The study of learning and memory in the fly is a robust field. Drosophila can learn a variety of associative tasks, and several studies have employed discriminative conditioning procedures (McGuire, 1984, Tully, 1984, Tully and Quinn, 1985). These procedures can employ using pairs of odor cues, colored lights or substrate textures as the discriminanda, with shock, quinine, or mechanical shaking as negative reinforcers, and with sucrose or the opportunity to run upwards as the positive reinforcers (Quinn et al., 1974, Menne, 1977, Platt et al., 1980, Tempel et al., 1983). Flies can also be used to study conditioned place preference learning and memory using a heat-box in an operant process in which flies develop spatial preference for one side of an experimental chamber (Putz and Heisenberg, 2002, Sitaraman et al., 2008). Significantly, serotonin has been demonstrated to play a crucial role in place memory in the fly (Sitaraman et al., 2008). The olfactory conditioning paradigm of Quinn et al. (1974) is the most commonly employed procedure in the investigation of learning and memory in Drosophila, and has demonstrated that the fly exhibits several parallels to aspects of mammalian CNS function and behavior. Olfactory learning in Drosophila displays many of the behavioral properties generally described for Pavlovian learning in other animals, including acquisition, extinction, conditioned stimulus/unconditioned stimulus saliency, order dependence, temporal specificity, conditioned excitation, conditioned inhibition, and conditioned stimulus/unconditioned stimulus pre-exposure effects (Dubnau, 2003).
The primary memory circuits in the fly brain are the mushroom bodies (MB), which are discreet structures within the central brain and comprised of different subpopulations of Kenyon cells, whose processes form distinct lobes that perform particular tasks. The mushroom body receives cholinergic inputs from sensory areas of the brain, and depending on the learning task at hand utilize different neurotransmitters to integrate and process the information. Dopamine and dopamine D1 receptors have been demonstrated to be critical for many aspects of learning and memory (Schwaerzel et al., 2003, Kim et al., 2007, Krashes et al., 2009, Waddell, 2010). Octopamine has been shown to be necessary for appetitive and reward conditioning (Schwaerzel et al., 2003). Additional neurotransmitters involved in learning and memory in the fly include GABA (Liu et al., 2007), and glutamate (Xia et al., 2005). The role of serotonin, a key neurotransmitter in mammalian cognitive processes, and its receptors have not been well defined in olfactory learning and memory. Here we follow both pharmacological and genetic approaches to elucidate the role of serotonin receptors in olfactory learning and memory and find all three 5-HT receptor families involved in aspects of both short term and long term learning and memory. Significantly, our data suggest that structures extrinsic to the MBs may be participating in olfactory learning and memory.
EXPERIMENTAL PROCEEDURES
Chemicals
U92016A, Ketanserin, and (R)-3,N-Dimethyl-N-[1-methyl-3-(4-methylpiperidin-1-yl)propyl] benzene sulfonamide (SB 258719) were obtained from Tocris (Ellisville, MO). (R)-1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (R)-DOI and WAY10065 were gifts of Dr. David E. Nichols, Purdue University, West Lafayette, IN, USA. For the olfactory avoidance assays, 4-methylcyclohexanol (MCH) and benzaldehyde (BA) MCH, were purchased from Sigma, and heavy mineral oil from Fisher Scientific (Pittsburgh, PA).
Drosophila rearing and feeding
Canton-S (CS) wild type, 5-HT1BDro-Gal4 (w[1118]; Mi{ET1}5-HT1B[MB05181]), and 5-HT2Dro-GAL4 (w[*];P{w[+mW.hs]=FRT(w[hs])}2AP{ry[+t7.2]=neoFRT}82B PBac{GAL4D,EYFP}5-HT2[PL00052]) transgenic Drosophila strains were obtained from the Bloomington Drosophila Stock Center at Indiana University (Bloomington, IN). The GAL4–5-HT7Dro and UAS-5-HT7DroRNAi transgenic flies were generated as described elsewhere (Becnel et al., 2011). Both the 5-HT1BDro-GAL4 and 5-HT2Dro-GAL4 insertion elements were moved to the CS background by six consecutive rounds of backcrossing before testing. Both male and female adult flies were used for all conditioning procedures. For routine maintenance, flies were grown in 8 oz polypropylene bottles on standard cornmeal-molasses food at 25°C under a 12 hour light/dark cycle until testing. For selection of flies for assays, bottles were cleared, and 48 to 72 hrs later the recently emerged adult flies were transferred to large 64 oz plastic bottles (containing ~2ml of food in the cap) without anesthetization. This prevented over exposure to the food source, and allowed flies to properly groom and feed, resulting clean and dry flies for optimal performance in the conditioning apparatus.
For STM testing, flies were transferred to the 64 oz plastic bottles 48 hrs prior to training. The food cap was filled with ~2 ml of 1% agar + 10% sucrose and drug where appropriate, and the bottles placed at 25°C under 12 hour L/D conditions. After 2 days, flies were assayed for STM performance as described below. As with any pharmacological experiment, an important factor is the concentration of drug used in relation to receptor specificity. Significantly, the maximum drug concentration of 3 mM that we utilized in the food is in accordance with accepted and published drug levels used in multiple Drosophila studies by others (Hendricks et al., 2003, Leal et al., 2004, Hong et al., 2006, Liu et al., 2008, Sitaraman et al., 2008). Furthermore, our specific choice of drug concentration is based upon our previous work with serotonergic agents in the fly (Nichols et al., 2002, Nichols, 2007, Johnson et al., 2009, Becnel et al., 2011). Although 3.0 mM was the highest drug concentration used in the food, behaviors were significantly altered dose dependently down to much lower concentration. We believe that these drugs cross the blood brain barrier and achieve sufficient levels in the brain to activate receptors because feeding these drugs to flies alters CNS mediated behaviors without overtly altering peripheral function as shown here and in our previous work.
Regarding our choice of drugs, we previously have demonstrated the ability of the 5-HT1A selective antagonist WAY100635 to block some of the behavioral effects of the mixed serotonin receptor agonist lysergic acid diethylamide (LSD) (Nichols et al., 2002), as well as the effects of the 5-HT1A agonist 8-OH-DPAT in the fly (Johnson et al., 2009). (R)-DOI is a potent hallucinogenic drug in humans that is a highly selective agonist at the mammalian 5-HT2 receptor family, and has high affinity for the Drosophila 5-HT2Dro receptor (Colas et al., 1995). Ketanserin is a highly selective antagonist for the 5-HT2 receptor family that also has been demonstrated to have affinity for the 5-HT2Dro receptor (Colas et al., 1995). SB258719 is a highly selective antagonist at mammalian 5-HT7 receptors, that also displays partial inverse agonist activity at this receptor (Thomas et al., 1998). U92016A is a potent and highly selective agonist at mammalian 5-HT1A receptors (McCall et al., 1994).
For LTM testing, food and drug was administered according to which aspect of LTM function was explored. To examine acquisition, flies were fed 1% agar + 10% sucrose and drug 48 hrs prior to training. Following training, flies were replaced in 64 oz bottles for 24 hrs where they received 1% agar + 10% sucrose without drug, and were then tested for LTM performance as described below. To examine memory consolidation, flies were placed in 64 oz bottles and fed 1% agar + 10% sucrose without drug for 48 hrs prior to training. Immediately following training, flies were returned to the 64 oz bottles, and fed 1% agar + 10% sucrose and drug for 6 hrs. After 6 hrs, the food cap was replaced with 1% agar + 10% sucrose without drug, and flies were maintained for an additional 18 hrs and then tested. To examine retrieval, flies were placed in 64 oz bottles, fed 1% agar + 10% sucrose without drug 48 hrs prior to training; following training, flies were replaced in the bottles for 22 hrs and fed 1% agar + 10% sucrose without drug. After 22 hrs, the food cap was replaced with 1% agar + 10% sucrose and drug for 2 hrs. After 2 hrs, flies were immediately tested for LTM performance.
Olfactory avoidance
Prior to experimental learning and memory testing, it was important to ensure each of the fly strains, and flies administered drugs, exhibited normal olfactory avoidance to the odors used as cues: 4-methylcyclohexanol (MCH) and benzaldehyde (BA), and to ensure that the drug treatments did not affect odor perception. About 100 untrained flies were transferred to the t-maze of the conditioning apparatus where they received an individual odor paired with room air for 120 s. Flies fed drug were maintained on food + 3.0 mM drug for 48 hours prior to testing. After 120 s flies were trapped in the respective tubes, and collected to calculate performance indices. The performance index was calculated as the number of flies avoiding the aversive odor minus the number of flies not avoiding the odor, divided by the total number of flies. The final effective dilutions of oderant for our system were MCH: 1:100, and BA: 1:75. Air flow through the system was kept constant at 30–40 psi. Conditions in the testing room here, and in all subsequent tests were maintained at 70–80% relative humidity and 25°C.
Shock reactivity and optimization of shock paramaters
About 100 untrained flies were transferred to the t-maze of the conditioning apparatus where electrifiable grids were inserted into both sides of the t-maze instead of the olfactory collection tubes, with only one side receiving shock pulses. Flies fed drug were maintained on food + 3.0 mM drug for 48 hours prior to testing. After 120 s flies were trapped in their respective tubes, and collected to calculate performance indices. The performance indices were calculated as the number of flies avoiding the chamber with shock pulses minus the number of flies entering the electrified chamber divided by the total number of flies. We found that with our system, 75 V (peak current 150 mA) was optimal for shock avoidance.
Conditioning procedures
To assay STM performance, flies received a single training session, and were then tested. Flies fed drug were maintained on food + 3.0 mM drug for 48 hours prior to testing. About 100 flies were transferred to a training chamber containing an electrifiable grid, and allowed to rest for 90 s. Following rest, flies received MCH in the presence of shock for 60 s. A training session consisted of a 75 V (peak current 150 mA) shock pulse for 1.2 s every 5 s. Flies then received 45 s of rest in the absence of both shock and odor. Following rest, flies received 60 s of BA in the absence of shock. After 45 s of rest, flies were then transferred to the holding chamber of the apparatus, and were allowed to rest for 90 s before being transferred to the t-maze (choice point) for testing where they were presented with MCH from one side, and BA from the other. After 120 s, flies were trapped in their respective collection tubes and counted to determine the performance index. The performance index was calculated as the number of flies avoiding the shock paired odor minus the number of flies avoiding the unpaired odor divided by the total number of flies assayed. The entire procedure was then repeated using BA as the shock paired odor, and MCH as the unpaired odor, with both calculated performance indices combined to give the overall PI for the trial.
To assay LTM performance, flies were trained with spaced training. For this, ten training sessions, as described above, were performed with 15-minute rest intervals in between each training session. After the final training session, flies were transferred back to 64 oz bottles containing food (or food with drug at appropriate intervals) as described above, and tested 24 hrs later for LTM performance. The performance index was calculated in the same manner as indicated above.
Isobolar Analysis
Isobolar analysis determines the nature of interactions between individual components of a system, and whether these interactions are synergistic, additive, or disruptive (Tallarida, 2002, Paul, 2011). Isobolar analysis is a much more precise and rigorous method than only adding two sub-effective concentrations of agents together and looking for simple enhancement or suppression of phenotypes. For isobolar analysis, IC50 concentrations for each drug were determined by drug dose-response curves. Next, additional dose-response curves were determined from fixed ratio dose combinations of 2 different drugs simultaneously fed to the flies. These data were then plotted using GraphPad Prism to generate isobolograms and statistical data. The interaction index and statistical data were calculated as described by Tallarida (Tallarida et al., 1989, Tallarida, 2002)
Analysis of 5-HT1BDro mRNA expression
Twenty adult flies from wild type CS, and 5-HT1BDro-GAL4 backcrossed to the same CS strain for six generations, were homogenized in 1.0 ml Tri Reagent RT (Molecular Research Center, Cincinnati, OH) and processed for total RNA following manufacturer's directions. First-strand cDNA was generated using the ImProm-II cDNA synthesis kit (Promega, Madison, WI, USA) following the manufacturer's protocols with 300 ng total RNA per reaction. Quantitative realtime polymerase chain reaction (QPCR) was performed using the Universal ProbeLibrary system from Roche Diagnostics (Indianapolis, IN, USA) in combination with the HotStart-IT Probe qPCR Master Mix from USB (Cleveland, OH, USA) following the manufacturer’s protocols. Reactions were performed in quadruplicate for each RNA sample. Amplicon primers and universal probes utilized for the 5-HT1BDro mRNA and the reference standard, ribosomal protein L32 (RpL32) mRNA were: RpL32 (U#105) F: 5’-CGGATCGATATGCTAAGCTGT-3’, R: 5’-GCGCTTGTTCGATCCGTA-3’; 5-HT1BDro (U#62) F: 5’-CAGCGATGCGGATGATTA-3’, R: 5’-CGAGGCTATCAGATGGTGCT-3’. Relative gene expression levels were calculated using the 2[-⊗⊗C(T)] method.
Statistical analysis
All analysis was performed with GraphPad Prism 4, and used ANOVA with appropriate post-hoc analysis for multiple comparisons unless indicated otherwise.
RESULTS
Olfactory and Shock Avoidance
Olfactory and shock avoidance experiments were performed prior to beginning the learning and memory investigations to determine any adverse effects following drug administration. All drug concentrations were 3.0 mM, which was the maximum concentration administered during learning and memory investigations for any given drug. There were no significant effects observed in olfactory avoidance for any of the drug treatments (Table 1). Furthermore, there were no significant effects observed in shock reactivity for any of the drug treatments (Table 1).
Table 1.
All flies display normal olfactory and shock avoidance. Olfactory and shock avoidance was determined for CS flies fed 3.0 mM drug for 48 hours prior to testing, and for the 5-HT1BDro and 5-HT2Dro receptor mutants backcrossed to CS, as well as the F1 progeny of the 5-HT7Dro-GAL4 driver crossed to the UAS-5-HT7 RNAi (n=4–8) (#, see: Becnel et al, 2011).
| MCH | BA | Shock | |
|---|---|---|---|
| Control | 74±5 | 71±3 | 80±2 |
| U92016A | 69±3 | 67±5 | 71±3 |
| WAY100635 | 63±6 | 62±4 | 70±2 |
| DOI | 67±5 | 58±2 | 82±8 |
| Ketanserin | 66±7 | 61±1 | 69±5 |
| SB258719 | 71±3 | 69±8 | 74±4 |
| 5-HT1BDro mut | 67±7 | 67±7 | 68±3 |
| 5-HT2Dro mut | 68±5 | 70±6 | 71±4 |
| 5-HT7Dro RNAi | # | # | 72±1 |
5-HT Receptor Ligands Disrupt STM Performance
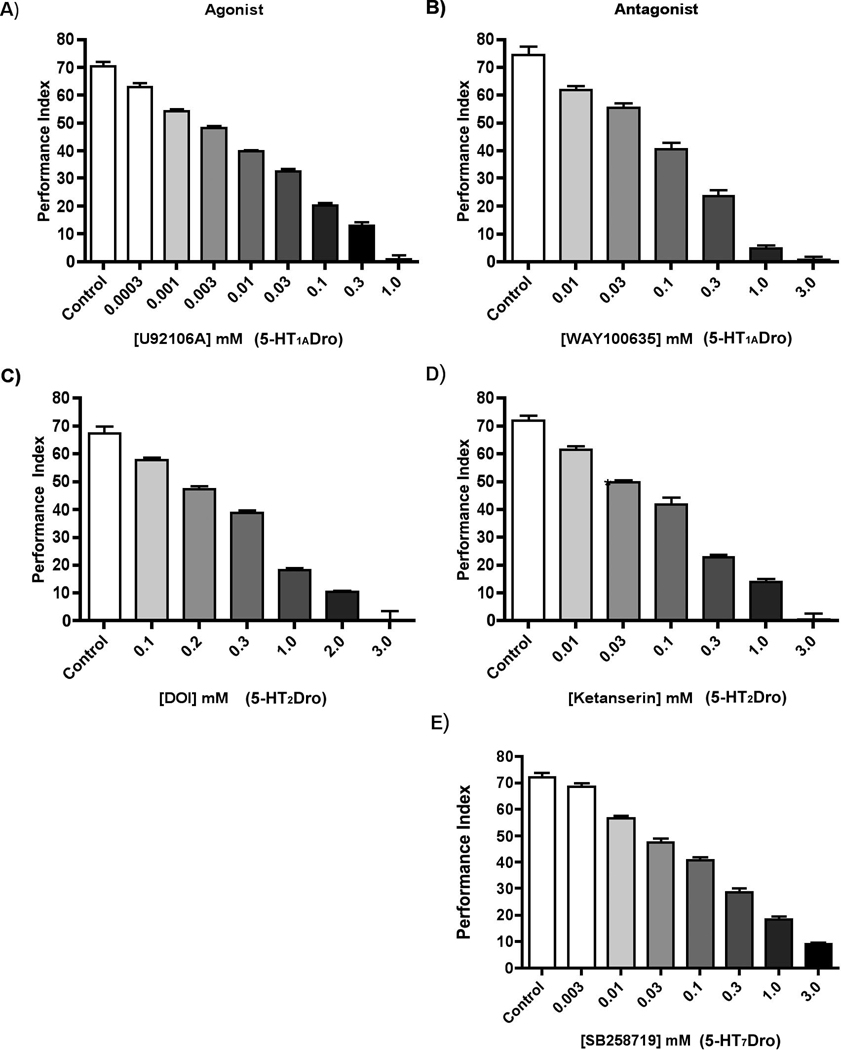
The effect of pharmacologically altering the function of each serotonin receptor on STM performance was examined. Control flies consistently displayed performance indices of about 70 when assayed for STM function, consistent with published PI values in the literature for wild type strains. When we administered the 5-HT1A receptor agonist, U92016A, we observed a concentration-dependent decrease in performance indices with increasing drug concentrations (Figure 1A). The 5-HT1A receptor antagonist WAY100635 also markedly reduced performance indices in a concentration-dependent manner (Figure 1B). Similar concentration-dependent decreases in performance were observed following administration of the 5-HT2 receptor agonist DOI (Figure 1C), and the 5-HT2 receptor antagonist ketanserin (Figure 1D). Administration of the 5-HT7 receptor antagonist SB258719 also revealed a concentration-dependent decrease in performance indices (Figure 1E). There are, unfortunately, no available reliably selective agonists of the 5-HT7 receptor to test the effect of such agents on STM. The effects the 5-HT1A-like receptor agonist appeared to be the most potent. Although the mushroom bodies express both 5-HT1ADro and 5-HT1BDro receptors, and it would be anticipated that alteration of function of receptors directly expressed on these structures would have the greatest effects, it could be that the observed potency of U92106 is simply due to enhanced efficacy of the drug or increased bioavailability compared to the other drugs in the brain. Interestingly, neither 5-HT2Dro nor 5-HT7Dro receptors are known to be expressed in the mushroom bodies (Nichols 2007; Becnel et al, 2011).
Figure 1. 5-HT Receptor Agents Attenuate STM Performance in a Concentration-Dependent Manner.
To determine the effects of 5-HT receptor agents on STM performance levels, 1–3 day old wild-type Canton-S flies were maintained on a solution of 1% agarose and 10% sucrose in the presence or absence of drug for 2 days prior to training. Flies demonstrated a concentration-dependent decrease in performance indices in the presence of all 5-HT receptor agents tested (n=8). The potency of the 5-HT1A agonist to disrupt memory may reflect the high levels of expression of 5-HT1ADro and 5-HT1BDro receptors in the mushroom bodies of the adult brain.
Genetic Validation of STM Results
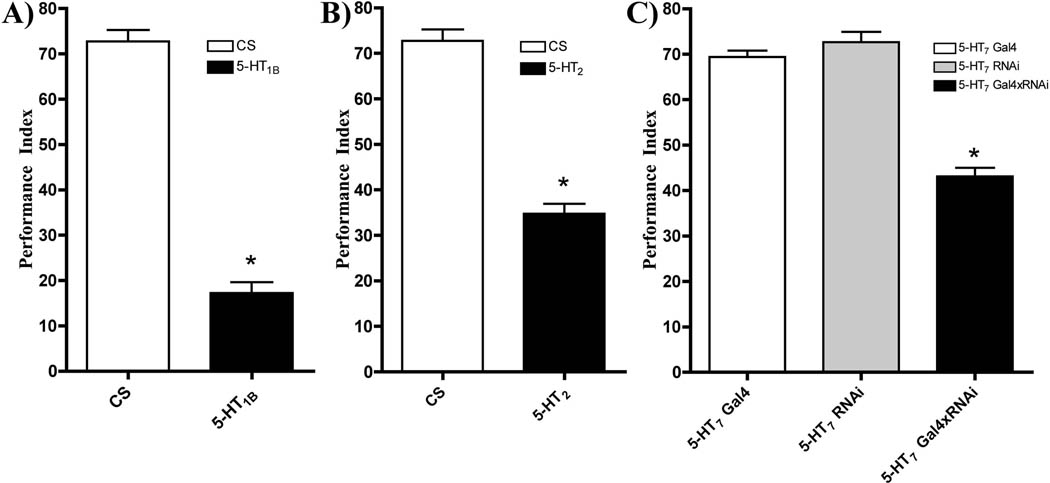
It was critical to validate our findings of serotonin receptor involvement in STM by other means. Therefore, we employed genetic methods to validate our pharmacological results. For the 5-HT1BDro and 5-HT2Dro receptors we used insertion mutant strains. We previously demonstrated that the 5-HT2Dro insertion strain only has 10% of wild type mRNA expression (Nichols, 2007). Our data here indicate that the 5-HT1BDro insertion strain has a 40% decrease in comparison to wild type mRNA expression levels and is also hypormorph (CS: 100±10.92, 5-HT1BDro insertion: 62±5.0), Each of the insertion elements was back crossed into the CS background for 6 generations prior to testing for STM performance. As shown in Table 1, neither insertion resulted in deficits of odor or shock reactivity, but the presence of the insertions caused a reduction in short term memory performance by roughly 75% for the 5-HT1BDro receptor hypormorph (Figure 2A), and 50% for the 5-HT2Dro receptor hypomorph (Figure 2B). These results are consistent with our pharmacological data showing that antagonists of the 5-HT2 and 5-HT1A-like receptors disrupt performance. To test the 5-HT7Dro receptor, we used the fly Gal4/UAS system to express an RNAi transgenic element for the receptor under the control of the a 5-HT7Dro enhancer region GAL4 driver. In the F1 heterozygote, mRNA for the 5-HT7Dro receptor is knocked down about 85% (Becnel et al., 2011). Each parental exhibited normal performance indices, as well as odor and shock reactivity (Figure 2C, Table 1), however the F1 cross demonstrated a 40% reduction in performance for short term memory (Figure 2C). Together, these data support the conclusions of our pharmacological studies that each serotonin receptor family (5-HT1A-like, 5-HT2, and 5-HT7) in the fly is required for normal olfactory learning and memory.
Figure 2. 5-HT receptor mutants and knockdown of gene expression display decreased STM function.
The 5-HT1BDro and 5-HT2Dro receptor hypormorph insertions in the wild-type CS background result in significant impairment of STM compared to CS. There were no significant differences observed among 5-HT7Dro receptor parental Gal4 and RNAi driver lines. F1 progeny had significant impairment of STM. All flies displayed normal olfactory avoidance and shock reactivity. (n=8 for each genotype; *p<0.05, Student’s-t test for A and B, two-way ANOVA with Bonferroni post hoc test for multiple comparison for C).
Drug Interactions Reveal Subadditive, Additive, and Superadditive Relationships
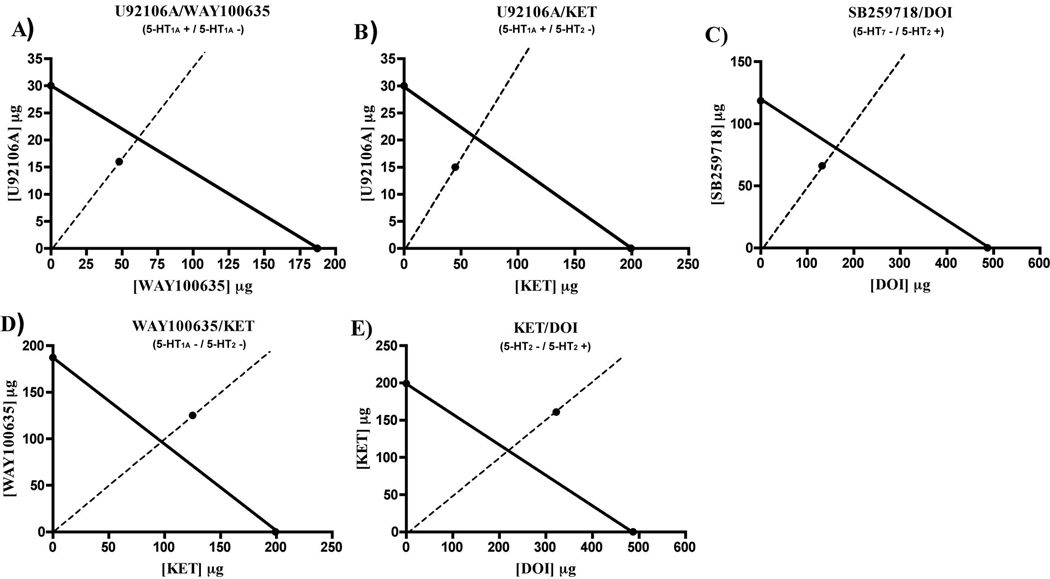
To accurately examine interactions between different 5-HT receptors/circuits we utilized isobolar analysis methods. Individual IC50 (half maximal inhibitory effect) concentrations of each of the serotonergic agents tested above were determined in dose response experiments (Table 2). Next, IC50 values of fixed drug ratios between combinations of ligands were determined (Table 2), and a dose-response curve of fixed drug ratios was generated for each of the drug combinations. This was achieved by simultaneously feeding flies dose ratio combinations of the two drugs being compared, and measuring the resulting performance index of the combination. Isobolograms were created using the individual and drug combination IC50 values, and analyzed for additive properties (Figure 3). The interaction index (γ) was also determined for the various drug combinations (Table 3). If γ=1 the interaction is additive; if γ<1 the interaction is superadditive (synergistic), and if γ>1 the interaction is subadditive (interfering). Superadditive combinations were 5-HT1A+/ 5-HT1A−, 5-HT1A+/ 5-HT2−, and 5-HT2+/ 5-HT7− with interaction indices of 0.80, 0.73, and 0.83, respectively (Figure 3A-3C). Subadditive combinations were 5-HT1A−/ 5-HT2− and 5-HT2+/ 5-HT2− with interaction indices of 1.30 and 1.47, respectively (Figure 3D-3E). 95% confidence intervals were also determined for theoretical and experimental potencies of drug combinations (Table 3). All other drug combinations were additive, indicating no functional interactions.
Table 2. Individual and Drug Combination IC50 Values (mM).
IC50 doses were determined from the dose-response curves of indiv idual drugs and fixed ratio drug mixtures (n=8 at each data point).
| Drug | IC50 Values (A or B) | ||
|---|---|---|---|
| U92016A | 0.03 | ||
| WAY100635 | 0.1872 | ||
| DOI | 0.4875 | ||
| Ketanserin | 0.1992 | ||
| SB258719 | 0.1184 | ||
| Drug Combination | Ratio | Experimental IC50 Combination (a/b) | |
| U92016A/ WAY100635 | 1:3 | (.016, .48) | |
| U92016A/DOI | 1:10 | (.017, .17) | |
| U92016A/Ketanserin | 1:3 | (.015, .045) | |
| U92016A/SB258719 | 1:3 | (.017, .051) | |
| WAY100635/DOI | 1:2 | (.103, .206) | |
| WAY100635/Ketanserin | 1:1 | (.125, .125) | |
| WAY100635/SB258719 | 1:1 | (.077, .077) | |
| DOI/Ketanserin | 2:1 | (.322,.161) | |
| DOI/SB258719 | 2:1 | (.132, .066) | |
| Ketanserin/SB258719 | 1:1 | (.067, .067) | |
Figure 3. Isobolar Analysis of Significant Receptor/Drug Interactions.
Isobolographic analysis determined the nature of interactions between two receptors/drugs and their effects on STM performance. The IC50 performance levels for a given drug at one receptor and that for another drug at a different receptor were compared to the effects on STM of a combination of the drugs. Experimental drug combination IC50 values that were found to lie on the theoretical IC50 isobole were considered additive; values that were above and below the isobole were considered superadditive and subadditive, respectively. Only isoboles for statistically significant interactions are shown.
Table 3. Interaction Index (γ) and Confidence Intervals (mM).
The interaction index, denoted by γ, was determined using Tallarida’s equation:
a/A + b/B = γ. γ<1 = superadditive; γ= 1 additive; γ>1= subadditive. Confidence intervals were determined for superadditive and subadditive mixtures. (−) = antagonist, (+) = agonist.
|
Receptor Treatment Combination |
(γ) | Theoretical IC50 | Experimental IC50 |
|---|---|---|---|
| 5-HT1A+/ 5-HT1A− | 0.80 | .0826 to .1156 | .03347 to .1242 |
| 5-HT1A+/ 5-HT2+ | NS | NS | NS |
| 5-HT1A+/ 5-HT2− | 0.73 | .0814 to .1324 | .0382 to .1002 |
| 5-HT1A+/ 5-HT7− | NS | NS | NS |
| 5-HT1A−/ 5-HT2+ | NS | NS | NS |
| 5-HT1A−/ 5-HT2− | 1.30 | .1492 to .2368 | .2003 to .2578 |
| 5-HT1A−/ 5-HT7− | NS | NS | NS |
| 5-HT2+/ 5-HT2− | 1.47 | .1147 to .5491 | .3201 to .7871 |
| 5-HT2+/ 5-HT7− | 0.83 | .1959 to .2879 | .1208 to .3364 |
| 5-HT2−/ 5-HT7− | NS | NS | NS |
5-HT Receptor ligands Modulate Aspects of LTM
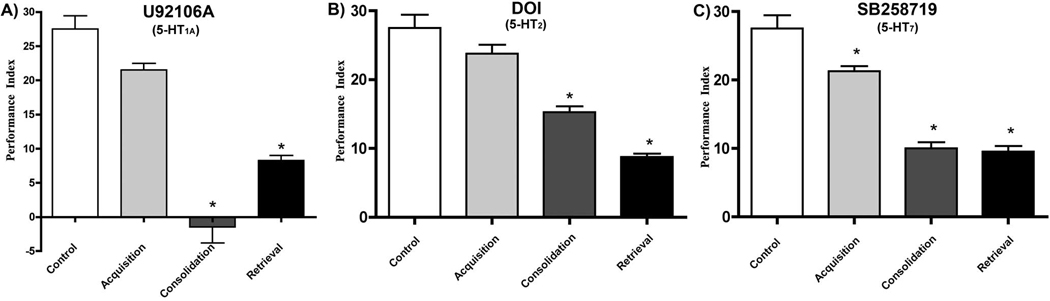
To further explore the role of the fly serotonin receptors in learning and memory processes, we investigated these receptors in long-term memory assays as described in the Methods. We divided our investigations into 3 components: acquisition, consolidation, and retrieval. The 5-HT1A receptor agonist U92016A appeared to completely abolish consolidation, and decreased retrieval of LTM by ~70% (Figure 4A). There were no significant effects on acquisition. Administration of the 5-HT2 receptor agonist (R)-DOI revealed an effect on consolidation and retrieval in the fly (Figure 4B), however, the observed effect was not as great as that produced by 5-HT1A agonist administration. Consolidation decreased by ~50%, and retrieval decreased by ~70%. We did not observe a significant decrease in acquisition. We administered the 5-HT7 receptor antagonist, SB258719, to explore the role of this receptor in each phase of long-term memory. Administration of the agent revealed an effect on all three phases of LTM (Figure 4C). Acquisition decreased by ~25%, and consolidation and retrieval were both attenuated by ~65%.
Figure 4. 5-HT receptors differentially modulate acquisition, consolidation and retrieval of LTM.
Based upon the STM results, 0.03mM U92106, 0.3mM DOI, and 0.3mM SB258719 was administered to 3–5 day old flies according to the above drug treatment protocol. The 5-HT1A agonist had a significant effect on LTM consolidation, and also seems to play a role in retrieval. The 5-HT2 agonist appears to play a role in both LTM consolidation and its retrieval. The 5-HT7 antagonist appears to be important in all three aspects of LTM, and is equally important in consolidation and retrieval. (n=4 trials/treatment;*p<0.05 compared with control, one-way ANOVA with Dunnett’s post test for multiple comparison).
DISCUSSION
We have found that serotonin 5-HT1A/1BDro, 5-HT2Dro, and 5-HT7Dro receptors are involved in aspects of both short term and long term conditioned stimulus olfactory aversive learning and memory processes. One significant finding of our work is that structures extrinsic to the mushroom bodies may be involved at some level in olfactory learning and memory, as 5-HT2Dro and 5-HT7Dro receptors are not known to express in the mushroom bodies. For each of the receptor drugs tested, there are no disruptive effects on lococomotor activity (Johnson et al., 2009), and we have shown here that there are no confounding effects on olfaction or shock reactivity. Importantly, to validate our pharmacological data, we used genetic methods and found that insertion alleles and knockdown of receptor mRNA also produced significant deficits in performance. Together, our genetic data are consistent with our pharmacological data, and demonstrate that each serotonin receptor is necessary for aspects of normal olfactory learning and memory in the fly. For STM, we can not yet say which components require serotonin receptors. They may be only necessary for learning, or memory, or they may be involved in both. Future studies will address this issue in more depth.
Although there are a number of genetic tools that can be used to examine neuronal and receptor function, pharmacological methods can often provide unique and complementing information. Therefore, we followed a pharmacological approach that incorporated dose response strategies to examine relative contributions of each receptor to short term learning and memory, the nature of functional interactions between individual receptor types and circuits, and to begin to dissect out individual roles in specific components of LTM. Our results here support the notion of some degree of receptor selectivity for these drugs, as do our previous works examining serotonin receptors in the fly (Nichols et al., 2002, Nichols, 2007, Johnson et al., 2009) where we have shown that these drugs can have very different behavioral effects from one another in multiple behaviors. Nevertheless, the exact affinities for and selectivity of these drugs at their intended target receptor remain to be fully validated in Drosophila, and some caution must be exercised when interpreting these data.
Interestingly, our data show that both agonists and antagonists at the same receptors disrupt performance. One may predict that if an antagonist is disruptive, then an agonist may be enhancing, and vise versa. This is not always the case with GPCR signaling, and there are reports in the literature of both types of ligands at a given receptor producing disruptive effects (Baker et al., 2003). Because serotonin primarily plays a modulatory role in the CNS, the receptors likely require a dynamic response to properly regulate learning and memory processes. The homologous mammalian receptors each exhibit constitutive activity, and the fly receptors are predicted to have similar constitutive activity associated with them. If a certain level of basal activity and dynamic response to input levels of serotonin is required for normal performance and homeostasis, then both agonists and antagonists (and inverse agonists, as ketanserin, WAY100635, and SB258719 are) would lead to a reduction of the ability of the receptors to dynamically respond to serotonin, leading to a degradation of performance. For example, similar phenomena are seen with increased and decreased receptor activity in human behaviors (Elia et al., 1999). We also observed that whereas drugs were able to completely disrupt STM performance, genetic methods only produced ~50–75% reduction in performance. It is likely that with pharmacological methods, we are able to completely disrupt receptor function as drug levels increase, but for both the hypomorph strains and the RNAi knockdown studies there still exists a population of normal receptors, albeit reduced in expression, that confer some degree of functionality to the circuitry. Another factor may be time of administration. For the STM experiments, drugs were administered for 48 hours to reach steady state levels, which may have greater or even different effects than would be evident with acute administration.
With respect to long term memory processes, the effects of IC50 concentrations of drug in the food were used to assess the role of the serotonin receptors on acquisition, consolidation, and retrieval. Our data suggest that each receptor/circuit has their own unique contribution to LTM where 5-HT1A-like receptors are critical for consolidation and important for retrieval, 5-HT2 receptors are important for consolidation and retrieval, and 5-HT7 receptors are important for all three components. The possibility remains, however, that the differential results we observed on LTM may simply be due to the drugs having differential off-target effects at other GPCRs that are important for LTM in addition to the core response mediated by the individual 5-HT receptors.
Having established the involvement of serotonin receptors in learning and memory, how might they function in this capacity? The 5-HT1A/1BDro receptors are expressed postsynaptically in the mushroom bodies. Localization of these receptors to the mushroom bodies strongly implies that they directly influence MB function. Significantly, the 5-HT1A/1BDro receptors are coupled to Gαi and inhibition of adenylate cyclase activity, and when stimulated lead to a reduction of cAMP levels (Witz et al., 1990). Levels of cAMP have been demonstrated to be extremely important for learning and memory. As in mammals, the 5-HT1A-like receptors are also expressed presynaptically, and are predicted to have autoreceptor function. Our data indicate that administration of the 5-HT1A receptor agonist U92016A in combination with the 5-HT1A receptor antagonist WAY100635 work synergistically to attenuate STM function in the fly. Although these agents are both targeting 5-HT1A receptors, binding affinity and/or functional selectivity at pre- versus postsynaptic receptors could be promoting a superadditive rather than a subadditive relationship between these two agents. For example, the agonist U92016A may have greater affinity or efficacy at presynaptic receptors and reduce 5-HT release through autoreceptor activity, and the antagonist WAY100635 may have greater affinity or efficacy at postsynaptic receptors to block reception of the signal that together produce a superaddative decrease in 5-HT effects. We observed a similar synergistic behavioral effect in previous studies with combinations of agonists and antagonists for 5-HT1A-like receptors with respect to aggressive behaviors (Johnson et al., 2009). Different affinities and efficacies for drugs acting at the same G-protein coupled receptor located at different biological sites is a well established phenomenon termed functional selectivity (Urban et al., 2007), and this pre/post synaptic phenomenon plays a significant role in the action of drugs, such as aripiprazole, in humans (Mailman, 2007).
5-HT2Dro receptors are located postsynaptically on neurons of the protocerebrum that are in close proximity to Kenyon cells of the MBs (Nichols, 2007). These cells may normally serve to influence the function of Kenyon cells, and 5-HT2Dro receptor activity may therefore conceivably be indirectly modulating function of the MBs. These receptors are coupled to Gαq, and are generally stimulatory in nature and in mammalian CNS are involved in cognitive processing and integrating sensory information (reviewed in: (Nichols, 2004). Their role in the fly may be similar, and facilitating the integration of sensory information into the mushroom bodies. Interaction data show that simultaneous administration of 5-HT2 receptor agonist and antagonist are interfering, which would be predicted for a receptor with only postsynaptic localization. Significantly, 5-HT1A antagonists in combination with 5-HT2 antagonists are interfering, indicating a functional interaction between the two receptor circuitries. In mammals, 5-HT1A and 5-HT2 receptors often functionally antagonize one another, and it may be that blockade of 5-HT2Dro receptors counteracts the effects of blockade of 5-HT1ADro receptors in the fly. In addition, there is expression of 5-HT2Dro in a subset of cells of the ellipsoid body that may be contributing to its role in learning and memory. This notion is supported by data indicating functional interactions occurring between 5-HT2Dro and 5-HT7Dro receptor circuitry, which has high expression in the ellipsoid body.
Our results examining the 5-HT7Dro receptor are very intriguing. Although the receptor is expressed weakly in other circuits and areas of the brain (Becnel et al., 2011), its strong expression in all large field R-neurons of the ellipsoid body is suggestive of involvement of the ellipsoid body at some level in olfactory learning and memory processes. Previous attempts to study the role of this structure in olfactory learning and memory have largely been unsuccessful. Walking and flying are mediated by the EB (Strauss, 2002), and the use temperature sensitive off/on shibireTS or TRPM channels to inactivate the entire structure, or mutants that structurally disrupt the EB, have been shown to produce profound coordination and locomotor difficulties, precluding accurate testing of the ellipsoid body’s role in behaviors (Strauss and Heisenberg, 1993, Krashes and Waddell, 2008). An attempt at a more precise analysis examined NMDA receptor function in a subset of ellipsoid body neurons, and a role was proposed for consolidation in long term memory (Wu et al., 2007). Significantly, a subset of large field R-neurons has recently been demonstrated to be necessary for visual pattern memory (Pan et al., 2009). Because there are no direct connections between the central complex and the MB, it remains to be elucidated how structures of the central complex are involved in modulating both olfactory and visual memory. Furthermore, the precise 5-HT7Dro expressing neurons extrinsic to the mushroom bodies, either within the central complex or elsewhere, modulating learning and memory remain to be determined in future studies.
In summary, we provide here the first evidence that serotonin receptors are necessary for normal olfactory learning and memory in the fly. STM is disrupted by both pharmacological agents and by genetic manipulations of serotonin receptor function. The use of pharmacological tools has allowed us to examine receptor-receptor and receptor-circuitry interactions through isobolographic analysis, where we have determined that there are functional interactions between 5-HT1A and 5-HT2 circuitries, as well as 5-HT2 and 5-HT7 receptor circuitries. These interactions may be interpreted in a model such that 5-HT1A-like receptors expressed within the MBs directly influence MB function for STM, and particularly consolidation in LTM, by virtue of their location in MB neurons. The 5-HT2Dro expressing multipolar neurons in close proximity to the Kenyon cells of the protocerebrum, and neurons within the ellipsoid body, may then be modulating the activity of the MBs in STM, and in consolidation and retrieval for LTM. The 5-HT7Dro circuitry may be indirectly influencing MB function through modulation of 5-HT2Dro circuits, or potentially other yet to be identified circuits. In this model, components of the central complex like the EB may be playing a master regulatory role for complex behaviors like STM and all three aspects of LTM, rather than a more specific and direct role in olfactory learning and memory per se. This is consistent with our observations that 5-HT7Dro receptor activity is required for other complex behaviors like normal courtship and mating (Becnel et al., 2011). This work is intended to be presented as an initial characterization of serotonin receptor involvement in olfactory learning and memory, however, additional work remains to fully elucidate the role of serotonin and its receptors in these processes.
ACKNOWLEDGEMENTS
We would like to thank Dr. Shouzhen Xia, Dr. Tim Tulley, and Dr. Josh Dubnau for their assistance and advice in helping to establish the olfactory learning and memory assay in our laboratory; Dr. Dennis Paul for assistance with the isobolographic analysis; Kelly Jean Sherman for Technical assistance. This work was funded by National Institutes of Mental Health grants R21MH078454 and R01MH083689 (CDN); and R21MH078454-S1 (OJ).
ABBREVIATIONS
- (5-HT)
5-hydroxytryptamine
- (EB)
ellipsoid body
- (STM)
short term memory
- (LTM)
long term memory
- ((R)-DOI)
(R)-1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane
- (MCH)
4-methylcyclohexanol
- (BA)
benzaldehyde
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baker JG, Hall IP, Hill SJ. Agonist actions of "beta-blockers" provide evidence for two agonist activation sites or conformations of the human beta1-adrenoceptor. Mol Pharmacol. 2003;63:1312–1321. doi: 10.1124/mol.63.6.1312. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Becnel J, Johnson O, Luo J, Nässel D, D NC. The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS ONE. 2011 doi: 10.1371/journal.pone.0020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayre M, Malaterre J, Scotto-Lomassese S, Strambi C, Strambi A. The common properties of neurogenesis in the adult brain: from invertebrates to vertebrates. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:1–15. doi: 10.1016/s1096-4959(01)00525-5. [DOI] [PubMed] [Google Scholar]
- Colas JF, Launay JM, Kellermann O, Rosay P, Maroteaux L. Drosophila 5-HT2 serotonin receptor: coexpression with fushi-tarazu during segmentation. Proc Natl Acad Sci U S A. 1995;92:5441–5445. doi: 10.1073/pnas.92.12.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J. Neurogenetic dissection of conditioned behavior: evolution by analogy or homology? J Neurogenet. 2003;17:295–326. doi: 10.1080/01677060390441859. [DOI] [PubMed] [Google Scholar]
- Elia J, Ambrosini PJ, Rapoport JL. Treatment of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340:780–788. doi: 10.1056/NEJM199903113401007. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Sutcliffe JG. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol Sci. 2004;25:481. doi: 10.1016/j.tips.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Kirk D, Panckeri K, Miller MS, Pack AI. Modafinil maintains waking in the fruit fly drosophila melanogaster. Sleep. 2003;26:139–146. doi: 10.1093/sleep/26.2.139. [DOI] [PubMed] [Google Scholar]
- Hong ST, Bang S, Paik D, Kang J, Hwang S, Jeon K, Chun B, Hyun S, Lee Y, Kim J. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci. 2006;26:7245–7256. doi: 10.1523/JNEUROSCI.5426-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson O, Becnel J, Nichols CD. Serotonin 5-HT(2) and 5-HT(1A)-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster. Neuroscience. 2009;158:1292–1300. doi: 10.1016/j.neuroscience.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SM, Kumar N, Neckameyer WS. GABAergic modulation of motor-driven behaviors in juvenile Drosophila and evidence for a nonbehavioral role for GABA transport. J Neurobiol. 2004;61:189–208. doi: 10.1002/neu.20061. [DOI] [PubMed] [Google Scholar]
- Liu T, Dartevelle L, Yuan C, Wei H, Wang Y, Ferveur JF, Guo A. Increased dopamine level enhances male-male courtship in Drosophila. J Neurosci. 2008;28:5539–5546. doi: 10.1523/JNEUROSCI.5290-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Krause WC, Davis RL. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron. 2007;56:1090–1102. doi: 10.1016/j.neuron.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall RB, Romero AG, Bienkowski MJ, Harris DW, McGuire JC, Piercey MF, Shuck ME, Smith MW, Svensson KA, Schreur PJ, et al. Characterization of U-92016A as a selective, orally active, high intrinsic activity 5-hydroxytryptamine1A agonist. J Pharmacol Exp Ther. 1994;271:875–883. [PubMed] [Google Scholar]
- McGuire TR. Learning in three species of Diptera: the blow fly Phormia regina, the fruit fly Drosophila melanogaster, and the house fly Musca domestica. Behav Genet. 1984;14:479–526. doi: 10.1007/BF01065445. [DOI] [PubMed] [Google Scholar]
- Meneses A. 5-HT system and cognition. Neurosci Biobehav Rev. 1999;23:1111–1125. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Menne D, Spatz HC. Colour vision in Drosophila melanogaster. J Comp Physiol. 1977:301–312. [Google Scholar]
- Nichols CD. 5-HT2 receptors in Drosophila are expressed in the brain and modulate aspects of circadian behaviors. Developmental Neurobiology. 2007;67:752–763. doi: 10.1002/dneu.20370. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Ronesi J, Pratt W, Sanders-Bush E. Hallucinogens and Drosophila: linking serotonin receptor activation to behavior. Neuroscience. 2002;115:979–984. doi: 10.1016/s0306-4522(02)00354-8. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekstrom JC, Svenningsson P, Meister B, Kehr J, Stiedl O. The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhou Y, Guo C, Gong H, Gong Z, Liu L. Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn Mem. 2009;16:289–295. doi: 10.1101/lm.1331809. [DOI] [PubMed] [Google Scholar]
- Paul D. Experimental Designs for the Study of Receptor-Receptor Interactions. In: Stevens C, editor. Neuromethods: G Protein-Coupled Receptor Technology. NY: Springer-Verlag, N.Y; 2011. [Google Scholar]
- Platt SA, Holliday M, Drudge OW. Discrimination learning of an instrumental response in individual Drosophila melanogaster. J Exp Psychol Anim Behav Process. 1980;6:301–311. [PubMed] [Google Scholar]
- Putz G, Heisenberg M. Memories in drosophila heat-box learning. Learn Mem. 2002;9:349–359. doi: 10.1101/lm.50402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. Embo J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, Kitamoto T, Rottinghaus GE, Zars T. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. The interaction index: a measure of drug synergism. Pain. 2002;98:163–168. doi: 10.1016/s0304-3959(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Porreca F, Cowan A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989;45:947–961. doi: 10.1016/0024-3205(89)90148-3. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Gittins SA, Collin LL, Middlemiss DN, Riley G, Hagan J, Gloger I, Ellis CE, Forbes IT, Brown AM. Functional characterisation of the human cloned 5-HT7 receptor (long form); antagonist profile of SB-258719. Br J Pharmacol. 1998;124:1300–1306. doi: 10.1038/sj.bjp.0701946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T. Drosophila learning: behavior and biochemistry. Behav Genet. 1984;14:527–557. doi: 10.1007/BF01065446. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33:457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witz P, Amlaiky N, Plassat JL, Maroteaux L, Borrelli E, Hen R. Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc Natl Acad Sci U S A. 1990;87:8940–8944. doi: 10.1073/pnas.87.22.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CL, Xia S, Fu TF, Wang H, Chen YH, Leong D, Chiang AS, Tully T. Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat Neurosci. 2007;10:1578–1586. doi: 10.1038/nn2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Miyashita T, Fu TF, Lin WY, Wu CL, Pyzocha L, Lin IR, Saitoe M, Tully T, Chiang AS. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol. 2005;15:603–615. doi: 10.1016/j.cub.2005.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]