Abstract
In the past few decades it has become clear that estrogen signaling plays a much larger role in modulating the cognitive centers of the brain than previously thought possible. We have developed a nonhuman primate (NHP) model to investigate the relationships between estradiol (E) and cognitive aging. Our studies of cyclical E treatment in ovariectomized (OVX) young and aged rhesus monkeys have revealed compelling cognitive and synaptic effects of E in the context of aging. Delayed response (DR), a task that is particularly dependent on integrity of dorsolateral prefrontal cortex (dlPFC) area 46 revealed the following: 1) that young OVX rhesus monkeys perform equally well whether treated with E or vehicle (V), and 2) that aged OVX animals given E perform as well as young adults with or without E, whereas OVX V-treated aged animals display significant DR impairment. We have analyzed the structure of layer III pyramidal cells in area 46 in these same monkeys. We found both age and treatment effects on these neurons that are consistent with behavioral data. Briefly, reconstructions of pyramidal neurons in area 46 from these monkeys showed that cyclical E increased the density of small, thin spines in both young and aged monkeys. However, this effect of E was against a background of age-related loss of small, thin spines, leaving aged V-treated monkeys with a particularly low density of these highly plastic spines and vulnerable to cognitive decline. Our current interpretation is that E not only plays a critically important role in maintaining spine number, but also enables synaptic plasticity through a cyclical increase in small highly plastic spines that may be stabilized in the context of learning. Interestingly, recent studies demonstrate that chronic E is less effective at inducing spinogenesis than cyclical E. We have begun to link certain molecular attributes of excitatory synapses in area 46 to E effects and cognitive performance in these monkeys. Given the importance of synaptic estrogen receptor α (ER-α) in rat hippocampus, we focused our initial studies on synaptic ER-α in area 46. Three key findings have emerged from these studies: 1) synaptic ER-α is present in axospinous synapses in area 46; 2) it is stable across treatment and age groups (which is not the case in rat hippocampus); and 3) the abundance and distribution of synaptic ER-α is a key correlate of individual variation in cognitive performance in certain age and treatment groups. These findings have important implications for the design of hormone treatment strategies for both surgically and naturally menopausal women.
Keywords: Prefrontal cortex, estrogen, aging, primate, cognition, hormone replacement therapy
1. Introduction
This year, the first members of the “baby boomer” generation will turn 65, after which the risk of developing dementia increases exponentially (Bermejo-Pareja et al., 2008). At the same time, the youngest members of this generation are fast approaching 51, the average age of menopause in the United States (Kato et al., 1998). In the past few decades, it has become clear that estrogen signaling plays a significant role in modulating areas of the brain associated with higher cognitive functions, and can have profound neurotrophic and neuroprotective effects in models of injury and disease. The resulting question of how the precipitous drop in circulating estrogen levels during menopause affects the course of cognitive aging in women has become a major focus of research.
Despite this interest, there is still considerable debate as to whether hormone replacement therapy can help to prevent age-related cognitive decline in postmenopausal women. Several laboratory studies in young, cycling women have found that cognitive performance fluctuates with levels of circulating estrogen (E) across the menstrual cycle (Hampson et al., 1990; Phillips and Silverman, 1997; Portin et al., 1999; Postma et al., 1999; Hausmann et al., 2000; Maki et al., 2002; Rosenberg et al., 2002) and across the different phases of birth control (Mordecai et al., 2008), though some studies have failed to replicate these results (Epting and Overman, 1998). Others have found that pharmacological E suppression in premenopausal women can produce measurable cognitive deficits, which are reversed by add-back E treatment (Sherwin and Tulandi, 1996).
Surgical menopause, induced through bilateral oophorectomy, causes a sharp drop in circulating estrogen levels when performed in premenopausal women. Several studies of the effect of bilateral oophorectomy on cognitive function in premenopausal women have found that surgical menopause produces marked deficits across several cognitive domains, which could be prevented by the timely initiation of estrogen replacement therapy in those studies that contained a hormone therapy component (Phillips and Sherwin, 1992; Kimura, 1995; Szklo et al., 1996; Nappi et al., 1999; Verghese et al., 2000; Farrag et al., 2002). However, many clinical studies have failed to reproduce this effect, and it is difficult to determine whether this dichotomy represents a failure of the small sample sizes in the laboratory studies to accurately represent the larger population of surgically menopausal women or a failure of the less-sensitive cognitive assessments performed in the larger studies to detect more subtle declines in cognitive function (Vearncombe and Pachana, 2009).
Evidence suggests that natural menopause does not produce a substantial decline in cognitive function between pre- and post-menopausal women, though a slight dip in learning ability has been reported during perimenopause (Herlitz et al., 2007; Greendale et al., 2009). However, several laboratory studies and randomized clinical trials have found that initiation of hormone replacement therapy (HRT) during perimenopause or soon after the menopausal transition can improve cognitive function (Carlson et al., 2001; Keenan et al., 2001) and reduce a woman’s risk of developing cognitive impairment or dementia later in life (Kimura, 1995; Matthews et al., 1999; Carlson et al., 2001; Zandi et al., 2002; Henderson et al., 2003; Bagger et al., 2005; Henderson et al., 2005; Henderson et al., 2007; Greendale et al., 2009; Rocca et al., 2011).
The literature in this area, however, is far from consistent. Initiation of HRT more than a few years after menopause has been linked to an unchanged or increased risk of dementia and age-associated cognitive decline (Matthews et al, 1999; S.R. Rapp et al., 2003; Shumaker et al., 2003; Henderson et al., 2005; MacLennan et al., 2006). Moreover, several randomized clinical trials have found equivocal or negative effects of HRT on cognitive function, even when initiated soon after menopause (reviewed in Maki and Sundermann, 2009). This inconsistency has left women and their physicians unsure whether and how estrogen replacement therapy should be used in the context of age-related cognitive decline (Buist et al., 2004; Hersh et al., 2004).
Several factors, including hormone formulation, treatment schedule and time between menopause and treatment, have been identified as likely contributors to these discrepancies (reviewed in Sherwin and Henry, 2008). However, determining the optimal parameters for a successful estrogen replacement therapy has been difficult, as the biological mechanisms underpinning the interplay between estrogen, aging and cognitive function are not well understood. The use of an animal model permits the investigation of the effects of estrogen on cognition in tandem with the neurobiological bases of those effects. The nonhuman primate (NHP) is a particularly attractive model in this case, due to the similarity of their reproductive physiology to that of women (Van Esch et al., 2008). These primates have a 28-day menstrual cycle with similar ovarian hormone fluctuations (Dufau et al., 1977) and experience a low-estrogen menopause in their third decade of life (Nichols et al., 2005).
It is worth noting that most research about the effect of estrogen replacement therapy in NHPs is performed on surgically menopausal monkeys. Though there is some evidence that the effect of estrogen therapy may be more robust in women that are surgically menopausal than in naturally menopausal women (Szklo et al., 1996), this is still a matter of considerable debate (reviewed in Vearncombe and Pachana, 2009). Moreover, the same cognitive functions tend to be implicated in both groups as being affected by menopause and by HRT. In NHPs, this same pattern is evident; performance on many of the same tasks is affected by natural and by surgical menopause in aged animals (Roberts et al., 1998; Rapp et al., 2003) and the corresponding changes in the structure of the prefrontal cortex involve many identical features (Hao et al., 2007; Dumitriu et al., 2010). Research in surgically menopausal NHPs, therefore, can provide valuable insight into the mechanisms by which estrogen withdrawal and replacement can affect the function of the brain in order to produce the types of cognitive changes observed after natural menopause.
An additional benefit of the NHP model is the structural and functional similarity of the NHP prefrontal cortex to that of humans (Petrides and Pandya, 1999). The dorsolateral prefrontal cortex (dlPFC) has received increasing interest as a critical site of estrogen’s effects on cognitive function. Several studies in young and middle-aged women demonstrate that estrogen improves performance specifically on functions sensitive to the integrity of the dlPFC, such as verbal learning, fluency, and memory, and switching of attention and strategy. Such improvements have been noted during high-estrogen periods of the ovarian cycle (Maki et al., 2002; Rosenberg et al., 2002) and during the estrogen phase of birth control (Mordecai et al., 2008) in premenopausal women. Conversely, estrogen deprivation due to pharmacological blockade (Sherwin and Tulandi, 1996; Berman et al., 1997) or to surgical (Phillips et al., 1992) or natural menopause (Wolf et al., 1999; Keenan et al., 2001) can cause deficits in executive function, which are reversed following add-back estrogen treatment. Such results have led some researchers to propose that the cognitive deficits experienced by postmenopausal women are due to executive dysfunction, and that the PFC, rather than the hippocampus, is the “site of estrogen’s effect on cognition” (Keenan et al., 2001).
This review will highlight findings from this NHP model on the interactions among estrogen, the morphological and molecular profiles of dlPFC neurons, and age-related cognitive decline.
2. Cognition
2.1. Measures of cognitive aging in nonhuman primates
Studies have shown that aged NHPs become impaired in both acquiring and performing many dlPFC-dependent cognitive tasks. It is worth noting, however, that the degree of impairment varies considerably among individuals (Presty et al., 1987; Rapp and Amaral, 1991; Herndon et al., 1997), and as such not every cohort is impaired on every task (Rapp et al., 1997; Dumitriu et al., 2010).
Several well-characterized cognitive tasks are commonly used to assess the function of the dorsolateral prefrontal cortex in nonhuman primates. In the delayed nonmatching-to-sample recognition memory task (DNMS), the subject is shown an object, which is removed for a measured delay interval. After this period, the monkey is presented simultaneously with the same object and a new object, and a reward is given for selection of the new object (Rapp et al., 2003). The delayed response test of visuospatial working memory involves two empty wells. The monkey watches as one of the wells is baited, and then a screen descends to hide the wells from the monkey’s view for a specified delay interval. After the delay, the screen is raised and the monkey must remember and select the baited well to obtain a reward (Rapp et al., 2003). Given that DR performance critically relies on the integrity of the dlPFC (Gross and Weiskrantz, 1962; Divac and Warren, 1971), and that changes in the morphological and electrophysiological characteristics of area 46 have been demonstrated to correlate with changes in DNMS performance (Peters et al., 1998; Chang et al., 2005; Shamy et al., 2010), these tasks are often used in assessing the effects of aging on the dlPFC in NHPs (Luebke et al., 2010).
In general, aged monkeys have difficulty reversing visual (Rapp, 1990; Voytko, 1999) and spatial (Lai et al., 1995; Herndon et al., 1997) discriminations, performing a monkey version of the WCST, (Moore et al., 2003), and in acquiring (Herndon et al., 1997; Rapp et al., 1997; Dumitriu et al., 2010) and performing (Presty et al., 1987; Rapp and Amaral, 1989; Rapp and Amaral, 1991; Herndon et al., 1997; Rapp et al., 2003; Dumitriu et al., 2010) the DNMS task, especially when the demand on the dlPFC is increased through the use of repeated objects (Rapp and Amaral, 1989). Tests of visuospatial working memory, such as the delayed response task (DR) (Rapp and Amaral, 1989; Roberts et al., 1997; Rapp et al., 2003) and the spatial condition of the delayed recognition span test (spatial-DRST) (Herndon et al., 1997; Moss et al., 1997; Lacreuse et al., 2005), are also highly sensitive to the effects of cognitive aging.
2.2. Effects of estrogen on executive function in nonhuman primates
In intact rhesus monkeys, menopause compounds the effect of aging on cognitive function. Though aging alone affects DR and DNMS task performance, premenopausal monkeys are significantly less impaired than age-matched peri-/postmenopausal animals [see Fig. 1] (Roberts et al., 1997; Hara et al., in press). This pattern is also evident after surgical menopause. Aged intact animals perform substantially better on DNMS than ovariectomized animals of the same age when tested at longer delays (Lacreuse et al., 2000). Estrogen treatment reverses this impairment; aged ovariectomized animals given long-term cyclical estrogen treatment perform the DR task at a level indistinguishable from that of young animals, while those not so supplemented are substantially impaired [see Fig. 2] (Rapp et al., 2003). Estrogen replacement also improves the performance of aged OVX animals on several other age-sensitive tasks, including DNMS (Rapp et al., 2003), the primate analogue of the WCST (Voytko et al., 2009), and some aspects of a visuospatial attention task (Tinkler and Voytko, 2005; Voytko et al., 2009). In contrast, young animals are resilient to the cognitive effects of estrogen deprivation, and their performance on many of the same visual and spatial memory tasks is unaffected by ovariectomy or estrogen replacement [see Fig. 2] (Voytko et al., 2000; Lacreuse and Herndon, 2003; Hao et al., 2007).
Figure 1. DR performance in young and aged surgically-intact Rhesus monkeys.
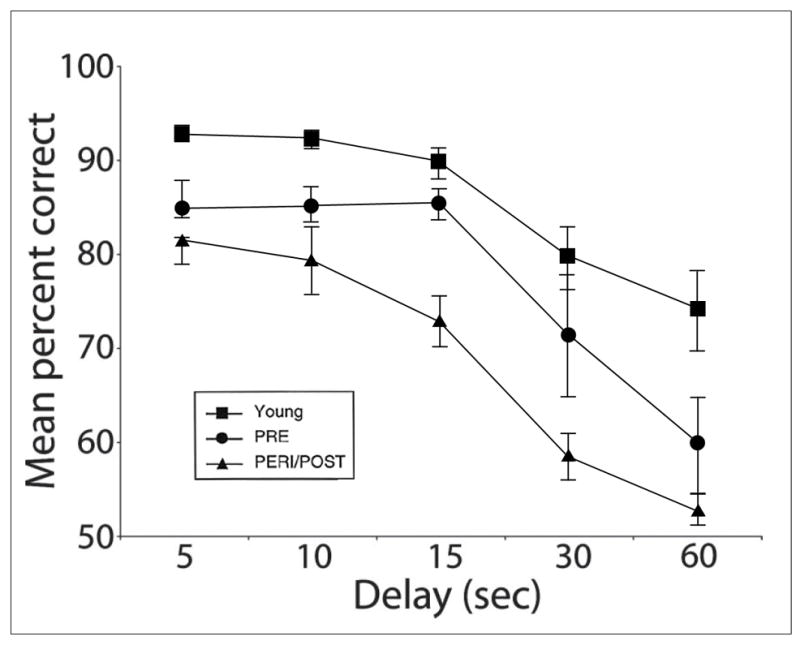
Young monkeys perform the DR task with higher average accuracy than aged animals (p < 0.05). Among aged animals, pre-menopausal monkeys perform more accurately than age-matched peri-/postmenopausal monkeys (p < 0.05). (PRE = pre-menopausal, PERI/POST = peri-/postmenopausal; reproduced from Roberts et al., 1997)
Figure 2. DR performance in young and aged OVX Rhesus monkeys with and without E replacement.
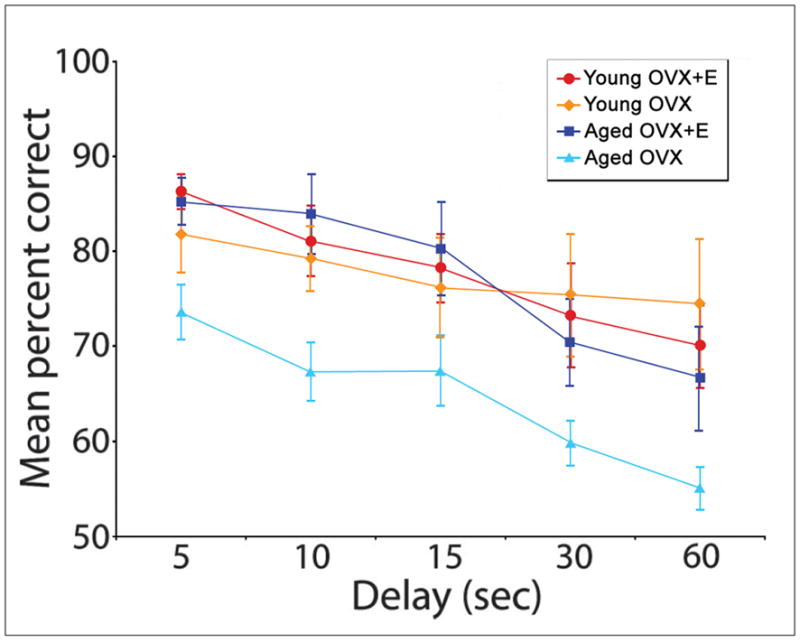
Young OVX animals perform the DR task with equivalent average accuracy whether treated with cyclical estrogen therapy or with vehicle alone. Aged OVX monkeys perform significantly less accurately than young animals (p < 0.03 at all time points), but this deficit is reversed by cyclical estrogen treatment. (E = estrogen-treated, other animals are treated with vehicle alone; reproduced from Hao et al., 2007)
3. Neuronal morphology
It has been demonstrated in recent years that cognitive aging, in the absence of neurodegenerative disorders like Alzheimer’s disease, is not associated with significant neuronal loss in the human (Pakkenberg and Gundersen, 1997) or NHP neocortex (Peters et al., 1998). Age-related cognitive decline is thought to result instead from more subtle morphological and molecular changes in the neurons mediating these processes and in the connections between them (Morrison and Hof, 1997; Hof and Morrison, 2004).
3.1. Spine/synapse density
3.1.1. Effect of aging on prefrontal spine/synapse density
The great majority of excitatory synapses between cortical neurons occur on tiny dendritic protrusions called spines (Nimchinsky et al., 2002). These excitatory synapses are referred to as “asymmetric” based upon their appearance when viewed under an electron microscope. Pyramidal neurons in the human PFC lose a significant proportion of their dendritic spines with age (Jacobs et al., 1997), both in terms of the number of spines per neuron and the density of spines per unit dendritic length. The same pattern is evident in NHPs. Between young adulthood and old age there is a significant decrease in the density of both dendritic spines and asymmetric synapses in the dlPFC of rhesus monkeys. This loss is evident in the NHP dlPFC in layers II/III and V (Uemura, 1980; Duan et al., 2003; Peters et al., 2008), across the apical and basal dendritic trees of the pyramidal neurons in layers III and IV (Uemura, 1980; Duan et al., 2003; Hao et al., 2007; Dumitriu et al., 2010), and is especially profound in layer I, where 50% of axospinous synapses may be lost (Peters et al., 1998).
This dramatic decrease in prefrontal connectivity has a measurable impact on cognitive performance. In fact, the degree of spine or synapse loss in the dlPFC has been found to correlate with individual age-related cognitive impairment on several tasks, including DNMS acquisition (Peters et al., 1998; Dumitriu et al., 2010) and recognition accuracy (Peters et al., 1998) and performance on the spatial condition of the DRST (Peters et al., 1998).
3.1.2. Effect of estrogen on prefrontal spine/synapse density
In contrast to the effect of aging, cyclical estrogen treatment in the form of a single injection of 17β-estradiol administered every three weeks substantially increases dendritic spine density in the dlPFC in both young and aged OVX monkeys [Fig. 2A] (Tang et al., 2004; Hao et al., 2006; Hao et al., 2007). Estrogen also increases asymmetric synapse density in this area, suggesting that many of these new spines form synaptic contacts (Leranth et al., 2008). In aged animals, this increase is accompanied by improvements in DR and DNMS performance compared to untreated controls (Rapp et al., 2003). Interestingly, dendritic length increases in young OVX animals without estrogen treatment, such that the number of spines per neuron remains approximately stable despite the drop in spine density (Hao et al., 2007). No such outgrowth occurs in estrogen-deprived aged animals. It is possible that this represents one mechanism by which the young brain is capable of adapting to the lack of estrogen and preserving cognitive function.
3.2. Effects of aging and estrogen on dendritic spine morphology
Dendritic spines are highly variable in shape and size (Harris et al., 2002). Studies using in vivo time-lapse imaging have shown that it is possible to separate these spines into independent populations based on their morphology and average lifespan (Holtmaat et al., 2005). Large mushroom spines are remarkably persistent in vivo, lasting for months, years, or potentially for the life of the animal (Kasai et al., 2003; Holtmaat et al., 2005). These spines are characterized by large postsynaptic densities (Harris et al., 1992), with large numbers of AMPA receptors (Matsuzaki et al., 2001). In contrast, long, thin spines have a high rate of turnover, and are continually formed both at rest and during activity-dependent processes (Kasai et al., 2003). These spines have few AMPA receptors (Matsuzaki et al., 2001) but abundant NMDA receptors (Noguchi et al., 2005) and are highly motile, capable of retracting back into the dendrite or stabilizing and expanding to form new mushroom spines (Kasai et al., 2003; Holtmaat et al., 2005). Long-term potentiation (LTP) and long-term depression (LTD) provide a mechanism for conversion between these two classes of spines. LTP induction causes a rapid and persistent enlargement of thin spines and an increase in functional AMPA receptor expression (Matsuzaki et al., 2004), while LTD leads to spine shrinkage and retraction (Zhou et al., 2004).
It has been proposed that mushroom and thin spines serve as “write-protected” and “write-enabled” bits of memory, respectively (Kasai et al., 2003). The stability and strength of the mushroom spine makes it a good candidate for the storage of long-term memories, while the labile, dynamic thin spine population provides an ever-refreshing candidate pool for the formation of new connections.
3.2.1. Effects of aging on spine morphology
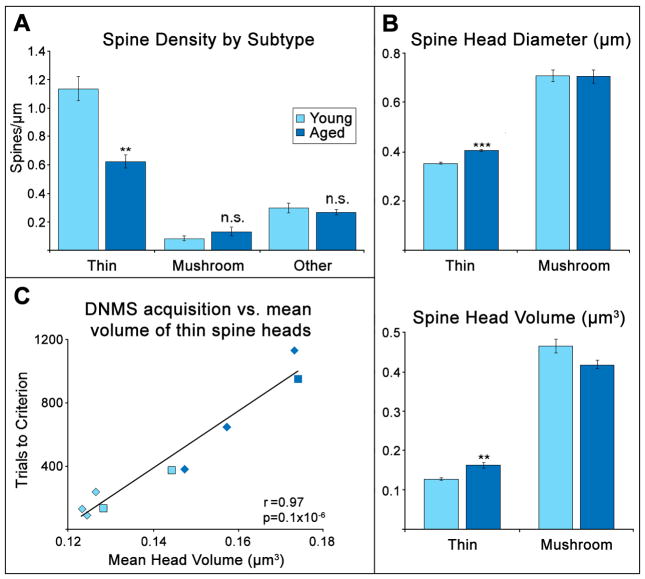
Not all spine types are lost equally with age; in fact, the density of mushroom spines, defined as those with a head diameter greater than 0.6 μm, does not decrease with age in NHP dlPFC (Hao et al., 2007; Dumitriu et al., 2010). Instead, the age-related decrease in spine density is driven almost entirely by the loss of thin spines, in both intact and ovariectomized animals [Fig 3B, Fig 4A] (Hao et al., 2007; Dumitriu et al., 2010). It is the density of these thin spines, in particular, that drives the correlation between spine density and DNMS acquisition (Dumitriu et al., 2010).
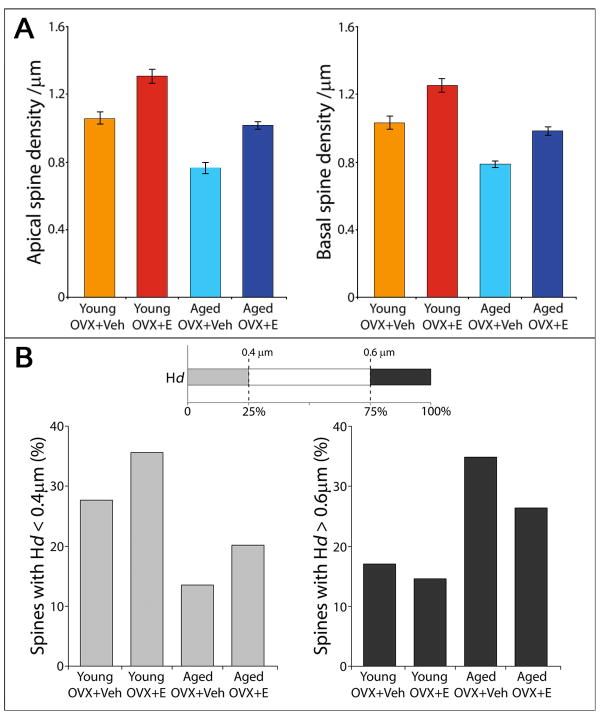
Figure 3. Spine density and head diameter in young and aged OVX Rhesus monkeys with and without E replacement.
Effect of estrogen treatment on dendritic spines on dlPFC pyramidal neurons in young and aged OVX monkeys. A. Estrogen treatment increases spine density on dlPFC neurons in OVX monkeys. B. Estrogen treatment also increases the proportion of smaller spines in young and aged OVX monkeys. (E = estrogen-treated, Veh = vehicle-treated; adapted from Hao et al., 2007)
Figure 4. Density and size of thin and mushroom spines in young and aged surgically-intact Rhesus monkeys and their correlation with the speed of DNMS acquisition.
A. The density of thin spines, but not of mushroom spines, is decreased in the dlPFC of aged monkeys. B. Thin spines in the aged NHP dlPFC are larger on average than those in young monkeys. C. The mean volume of thin spines is highly correlated with the number of trials required to learn the DNMS task in both aged and young monkeys. (adapted from Dumitriu et al., 2010)
It has also been noted that those thin spines which remain in aged animals are larger than those present in younger animals [Fig. 4B] (Dumitriu et al., 2010). Thin spines grow larger slowly after their formation, and so the size of these spines may reflect the rate of their turnover (Yasumatsu et al., 2008), with larger thin spines indicating a slower rate. The size of these spines is the strongest predictor of individual cognitive performance in aged surgically-intact animals [Fig. 4C] (Dumitriu et al., 2010), suggesting that maintaining a high rate of turnover in the thin spine population is particularly critical for optimal function of the dlPFC.
3.2.2. Effects of E on spine morphology
Estrogen treatment selectively increases the density of these vulnerable thin spines both in vitro and in vivo. Addition of estrogen to rat CA1 hippocampal neurons increases the number of thin spines and filipodia without changing the numbers of mushroom or stubby spines (Mukai et al., 2007). In NHPs, the increase in spine density on layer III pyramidal neurons in the dlPFC following cyclical E replacement occurs primarily in the smallest 25% of spines, restoring the pool of thin “learning” spines that are vulnerable to aging and connected to age-related cognitive impairment [Fig. 3B] (Hao et al., 2006, 2007). The cyclical increase and loss of spines with naturally fluctuating estrogen levels (Woolley et al., 1990) may serve to maintain a high turnover rate in this spine population, refreshing the pool of potential synaptic contacts.
These results highlight the increased vulnerability of aged animals to the loss of circulating estrogen. As aging and estrogen deprivation both reduce the population of thin spines in the dlPFC, the combination of these states produces a “double hit” effect that results in a 3-fold difference in the density of thin spines between young estrogen-treated animals and aged animals lacking E [Fig. 3B] (Hao et al., 2007).
4. Effects of aging and estrogen on the synaptic molecular profile
In addition to understanding the effects of estrogen on connectivity in the PFC, it is critical to understand the mechanisms by which these effects are produced if the cognitive benefits of estrogen therapy seen in primate studies are to be successfully brought to clinical practice. Using postembedding immunogold electron microscopy, it is possible to view the distribution of a protein in individual synapses through the use of antibodies linked to electron-dense gold particles (see Wang et al., 2010 for detailed methods). We have begun to link certain molecular attributes of excitatory synapses in the dlPFC to E effects and cognitive performance in young and aged rhesus monkeys.
4.1. The role of synaptic estrogen receptor α in estrogen signaling and cognition
The classical estrogen receptors, ER-α and ER-β, are DNA-binding transcription factors which are generally located in the nucleus and cytosol (Htun et al., 1999). In recent years it has become clear that membrane-bound forms of both ER-α (Milner et al., 2001; Adams et al., 2002) and ER-β (Milner et al., 2005) are present in dendritic spines and presynaptic terminals, strategically positioned to modulate both transmitter release and spine dynamics. These receptors can rapidly modulate synaptic structure and function through the activation of second-messenger systems (Toran-Allerand, 2000; Dominguez et al., 2007), and can be activated both by circulating gonadal estrogen and estrogen synthesized locally within neurons (Hojo et al., 2008).
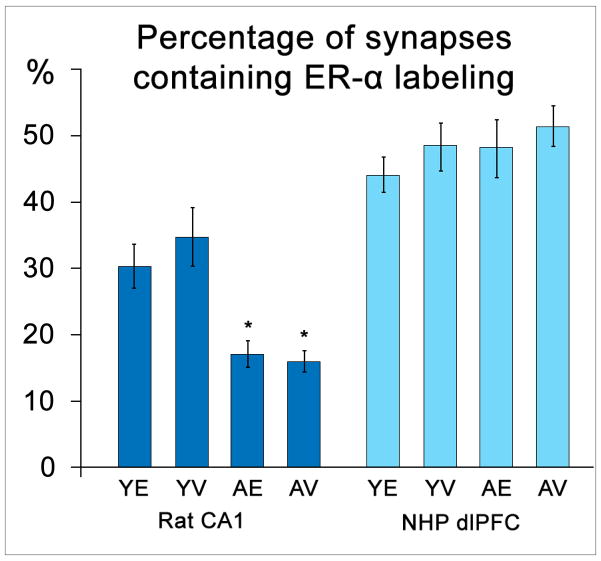
Activation of synaptic ER-α appears to be necessary for the effect of estrogen on the volumetric density of spine and synapse in the rat hippocampus. Selective ER-α activation is significantly more effective than that of ER-β at mimicking estrogen’s spinogenic effect in CA1 neurons, and the addition of an ER-α antagonist blocks this spinogenic response (Mukai et al., 2007). In female rats, the percentage of hippocampal synapses containing ER-α decreases sharply with age [Fig. 5] (Adams et al., 2002), as does the effect of estrogen on hippocampal spine density (Adams et al., 2001). Estrogen treatment has no effect on hippocampal ER-α levels in either young or aged OVX rats [Fig. 5] (Adams et al., 2002).
Figure 5.
Percentage of synapses labeled for ER-α in the rat CA1 and NHP dlPFC in the context of age and estrogen status. Unlike levels in the rat hippocampus (p < 0.0001 for both aged groups), ER-α levels in the monkey dlPFC do not decline with age (p > 0.05). (Y = young, A = aged, E = estrogen-treated, V = vehicle-treated; adapted from Adams et al., 2002 and Wang et al., 2010)
ER-α is also located both pre- and postsynaptically in the NHP dlPFC (Wang et al., 2010). In contrast to the effect in the rat hippocampus, synaptic ER-α levels in the NHP remain stable with age, though these levels are similarly unaffected by estrogen status [Fig. 5] (Wang et al., 2010). Despite the stability in overall ER-α prevalence across age and estrogen treatment groups, examination of the synaptic localization and abundance of this receptor in the context of individual cognitive performance reveals striking correlations.
4.2. ER-α and cognition in young OVX animals in the absence of estrogen
The distribution of synaptic ER-α in the dlPFC of young rhesus monkeys predicts individual cognitive resilience to estrogen withdrawal after surgical menopause. Higher levels of ER-α in the presynaptic terminal are strongly correlated with higher average accuracy on the DR task in young animals not given estrogen treatment after OVX (Wang et al., 2010). This additional presynaptic ER-α may allow young animals to make the best possible use of the more limited amounts of estrogen available in the absence of circulating gonadal estrogen. In addition, the presence of ER-α in perforated synapses appears to be of particular significance. Perforated synapses are those marked by the presence of a discontinuity within the postsynaptic density. Synapses with perforations tend to be particularly large, stable, and strong, with a significantly higher density of synaptic AMPA receptors than non-perforated synapses of the same size (Ganeshina et al., 2004). The percentage of perforated synapses containing ER-α correlates strongly with DR performance in estrogen-deprived young animals (Wang et al., 2010), with higher percentages predicting better accuracy. Perforated synapses tend to be very large and stable, with a higher density of AMPA receptors than non-perforated synapses (Ganeshina et al., 2004). Thus, perforated synapses are proposed to be a structural correlate of enhanced synaptic efficacy (Peters and Kaiserman-Abramof, 1969; Greenough et al., 1978; Geinisman et al., 1991). This may indicate that, when circulating estrogen is unavailable, young animals are able to compensate for the unavailability of thin spines by strengthening existing large, stable synapses (Wang et al., 2010). The absence of such correlations in the aged estrogen-deprived animals (Wang et al., 2010) may reflect the inability of these animals to compensate for the loss of E, as evidenced by their poor DR performance (Hao et al., 2007).
4.3. ER-α and cognition in aged OVX animals after estrogen treatment
Synaptic ER-α distribution also predicts the magnitude of cognitive improvement experienced by individual aged animals given estrogen replacement therapy, as compared to aged animals without E. Increased abundance of postsynaptic ER-α located 30–60 nm from the synaptic membrane is highly correlated with improved DR performance in those aged animals given estrogen treatment after OVX (Wang et al., 2010). Receptors located in this region are well-positioned to activate the second messenger cascades involved in ER-mediated spine and synapse formation (Spencer et al., 2008).
4.4. Implications for hormone replacement therapy in women
There is considerable variability in the cognitive response of individual women to both estrogen depletion and estrogen replacement therapies (Ancelin and Ritchie, 2005). These findings open up a new avenue by which to understand the mechanisms behind that variability, which seems especially promising in light of recent studies linking polymorphisms of the ER-α gene ESR1 to differential risk of developing dementia (Sundermann et al., 2010).
In addition to ER-α, the estrogen receptors ER-β and GPR30 have been implicated in estrogen’s effects on cognitive function in rodent models. As mentioned above, ER-β is also localized in rat hippocampal spines and presynaptic terminals (Milner et al., 2005). Administration of specific ER-β agonists to OVX rats replicates the effects of estradiol on performance of several cognitive tasks (Jacome et al., 2010; Neese et al., 2010), and ER-β signaling may be especially important in mediating the anxiolytic effects of estrogen (reviewed in ter Horst, 2010). GPR30, a recently characterized estrogen receptor, is present in the rat hippocampus and frontal cortex (Hazell et al., 2009; Hammond et al., 2011), and localizes to the plasma membrane in rat hippocampal pyramidal neurons (Funakoshi et al., 2006). GPR30 signaling has been implicated in estrogen’s neuroprotective effects in hippocampal cells (Gingerich et al., 2010). Specific agonists of ER-α, ER-β and GPR30 have all been reported to replicate the beneficial effect of estradiol on acquisition of a spatial learning task when administered to OVX rats (Hammond et al., 2009).
These results suggest that targeting ER-α, and perhaps other synaptic estrogen receptors, may provide a promising alternative to global estrogen replacement as a means to preserve cognitive function in naturally and surgically menopausal women.
5. Conclusion
The work described above has led to a useful framework and set of hypotheses regarding NHP dlPFC that links synaptic plasticity, cognitive aging, and interactive effects of estrogen. We hypothesize that the cognitive domains mediated by dlPFC require a particularly high degree of synaptic plasticity for axospinous synapses. We hypothesize further that a key element of such plasticity is the outgrowth of new, thin, highly motile, potentially transient spines that are available for synaptic stabilization in the context of learning. Cognitive decline is linked to age-related loss of such spines and E-enhanced cognition in aged monkeys occurs through partial restoration of this spine/synapse class. Importantly, the effect of E on this class of synapses suggests that a substantial proportion of age-related spine loss is not inevitable and may be responsive to pharmaceutical intervention. Such approaches may be particularly important if age-related synaptic alterations such as those described here leave these neurons vulnerable to the degenerative cascade that occurs in AD in humans. In addition, the findings regarding synaptic ER-α suggest that strategies targeting the synaptic ERs directly rather than relying exclusively on E replacement may be useful for cognitive enhancement in women.
The NHP model has provided several novel insights into our understanding of the effects of estrogen on the structure and function of the dlPFC in the context of aging. This research has already led to promising new directions for research into safer, better-targeted therapies for menopausal women, as well as to a new understanding of the mechanisms behind individual variation in cognitive ability. Our current and future research with the NHP model will proceed along several related paths. For example, great effort will be directed toward defining the molecular profile of thin vs. mushroom spines to reveal potential targets to enhance plasticity and protect against age-related cognitive decline. In addition, other treatment regimens (e.g., chronic, combined with progesterone, etc.) are being tested to determine if they are as effective at rescuing the target spine class and sustaining cognitive function as is cyclical, unopposed E, which was the treatment used in the studies described above. In addition, we will investigate the importance of the timing of E treatment with respect to both a potential window of opportunity after the cessation of ovarian function and the duration of cognitive enhancement once treatment is discontinued. These NHP studies will have a particularly high degree of translational power and will directly inform treatment issues in women.
Research Highlights.
Young monkeys lacking estradiol perform as well as estradiol-treated monkeys.
Aged OVX animals given estradiol perform as well as young adults.
Aging decreases thin spines on pyramidal neurons in the prefrontal cortex.
Estradiol increases the proportion of thin spines in the prefrontal cortex.
Thin spines correlate with cognitive performance.
Acknowledgments
This work was supported by National Institutes of Health Grants AG16765, AG10606, and AG06647. We would like to thank Dr. Peter Rapp, our colleagues at Mount Sinai School of Medicine, Rockefeller University, the Intramural Research Program at NIA, The California National Primate Research Center, the University of Texas, and Rush University for valuable discussions and insights regarding the work presented in this article.
Abbreviations
- NHP
nonhuman primate
- E
estrogen/estradiol
- DR
delayed response
- DNMS
delayed nonmatching to sample
- DRST
delayed recognition span test
- OVX
ovariectomized
- dlPFC
dorsolateral prefrontal cortex
- ER-α
estrogen receptor alpha
- WCST
Wisconsin card sorting task
- PFC
prefrontal cortex
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
n-methyl-d-aspartic acid
- LTP
long-term potentiation
- LTD
long-term depression
- CA1
Cornu Ammonis area 1
- ER-β
estrogen receptor beta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin ML, Ritchie K. Lifelong endocrine fluctuations and related cognitive disorders. Curr Pharm Des. 2005;11:4229–4252. doi: 10.2174/138161205774913228. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C PERF Study Group. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause. 2005;12:12–17. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Pareja F, Benito-León J, Vega S, Medrano MJ, Román GC Neurological Disorders in Central Spain (NEDICES) Study Group . Incidence and subtypes of dementia in three elderly populations of central Spain. J Neurol Sci. 2008;264:63–72. doi: 10.1016/j.jns.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Buist DS, Newton KM, Miglioretti DL, Beverly K, Connelly MT, Andrade S, Hartsfield CL, Wei F, Chan KA, Kessler L. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042–1050. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Zandi PP, Plassman BL, Tschanz JT, Welsh-Bohmer KA, Steffens DC, Bastian LA, Mehta KM, Breitner JC Cache County Study Group . Hormone replacement therapy and reduced cognitive decline in older women: the Cache County Study. Neurology. 2001;57:2210–2216. doi: 10.1212/wnl.57.12.2210. [DOI] [PubMed] [Google Scholar]
- Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex. 2005;15:409–418. doi: 10.1093/cercor/bhh144. [DOI] [PubMed] [Google Scholar]
- Divac I, Warren JM. Delayed response by frontal monkeys in the Nencki testing situation. Neuropsychologia. 1971;9:209–217. doi: 10.1016/0028-3932(71)90045-5. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Liu R, Baudry M. 17-Beta-estradiol-mediated activation of extracellular-signal regulated kinase, phosphatidylinositol 3-kinase/protein kinase B-Akt and N-methyl-D-aspartate receptor phosphorylation in cortical synaptoneurosomes. J Neurochem. 2007;101:232–240. doi: 10.1111/j.1471-4159.2006.04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau ML, Hodgen GD, Goodman AL, Catt KJ. Bioassay of circulating luteinizing hormone in the rhesus monkey: comparison with radioimmunoassay during physiological changes. Endocrinology. 1977;100:1557–1565. doi: 10.1210/endo-100-6-1557. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrag AK, Khedr EM, Abdel-Aleem H, Rageh TA. Effect of surgical menopause on cognitive functions. Dement Geriatr Cogn Disord. 2002;13:193–198. doi: 10.1159/000048652. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J Comp Neurol. 2004;468:86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res. 1991;566:77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- Gingerich S, Kim GL, Chalmers JA, Koletar MM, Wang X, Wang Y, Belsham DD. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience. 2010;170:54–66. doi: 10.1016/j.neuroscience.2010.06.076. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–1857. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, West RW, DeVoogd TJ. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978;202:1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Gross CG, Weiskrantz L. Evidence for dissociation of impairment on auditory discrimination and delayed response following lateral frontal lesions in monkeys. Exp Neurol. 1962;5:453–476. doi: 10.1016/0014-4886(62)90057-2. [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology. 2011;36:182–192. doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn. 1990;14:26–43. doi: 10.1016/0278-2626(90)90058-v. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WG, Roberts MT, Morrison JH, Rapp PR. Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.09.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann M, Slabbekoorn D, Van Goozen SH, Cohen-Kettenis PT, Güntürkün O. Sex hormones affect spatial abilities during the menstrual cycle. Behav Neurosci. 2000;114:1245–1250. doi: 10.1037//0735-7044.114.6.1245. [DOI] [PubMed] [Google Scholar]
- Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202:223–236. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Estrogen exposures and memory at midlife: a population-based study of women. Neurology. 2003;60:1369–1371. doi: 10.1212/01.wnl.0000059413.75888.be. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA MIRAGE Study Group . Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitz A, Thilers P, Habib R. Endogenous estrogen is not associated with cognitive performance before, during, or after menopause. Menopause. 2007;14:425–431. doi: 10.1097/01.gme.0000247019.86748.e3. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda M, Ishii H, Kimoto T, Kawato S. Estrogen synthesis in the brain--role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008;290:31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Htun H, Holth LT, Walker D, Davie JR, Hager GL. Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor. Mol Biol Cell. 1999;10:471–486. doi: 10.1091/mbc.10.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol. 1998;51:1271–1276. doi: 10.1016/s0895-4356(98)00119-x. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- Kimura D. Estrogen replacement therapy may protect against intellectual decline in postmenopausal women. Horm Behav. 1995;29:312–321. doi: 10.1006/hbeh.1995.1022. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG, Moss MB. Cognitive function in aged ovariectomized female rhesus monkeys. Behav Neurosci. 2000;114:506–513. doi: 10.1037//0735-7044.114.3.506. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG. Estradiol selectively affects processing of conspecifics’ faces in female rhesus monkeys. Psychoneuroendocrinology. 2003;28:885–905. doi: 10.1016/s0306-4530(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Kim CB, Rosene DL, Killiany RJ, Moss MB, Moore TL, Chennareddi L, Herndon JG. Sex, age, and training modulate spatial memory in the rhesus monkey (Macaca mulatta) Behav Neurosci. 2005;119:118–126. doi: 10.1037/0735-7044.119.1.118. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiol Aging. 1995;16:947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Luebke J, Barbas H, Peters A. Effects of normal aging on prefrontal area 46 in the rhesus monkey. Brain Res Rev. 2010;62:212–232. doi: 10.1016/j.brainresrev.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, Stocks NP, Taylor AW. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13:28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia. 2002;40:518–29. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- Maki PM, Sundermann E. Hormone therapy and cognitive function. Hum Reprod Update. 2009;15:667–681. doi: 10.1093/humupd/dmp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Cauley J, Yaffe K, Zmuda JM. Estrogen replacement therapy and cognitive decline in older community women. J Am Geriatr Soc. 1999;47:518–523. doi: 10.1111/j.1532-5415.1999.tb02563.x. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol Aging. 2003;24:125–134. doi: 10.1016/s0197-4580(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Horm Behav. 2008;54:286–293. doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Moss MB, Killiany RJ, Lai ZC, Rosene DL, Herndon JG. Recognition memory span in rhesus monkeys of advanced age. Neurobiol Aging. 1997;8:13–19. doi: 10.1016/s0197-4580(96)00211-4. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WG, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47:29–36. doi: 10.1159/000010058. [DOI] [PubMed] [Google Scholar]
- Neese SL, Korol DL, Katzenellenbogen JA, Schantz SL. Impact of estrogen receptor alpha and beta agonists on delayed alternation in middle-aged rats. Horm Behav. 2010;58:878–890. doi: 10.1016/j.yhbeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM. Ovarian senescence in the rhesus monkey (Macaca mulatta) Hum Reprod. 2005;20:79–83. doi: 10.1093/humrep/deh576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The synapses upon dendritic spines. Z Zellforsch Mikrosk Anat. 1969;100:487–506. doi: 10.1007/BF00344370. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8:671–684. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Phillips K, Silverman I. Differences in the relationship of menstrual cycle phase to spatial performance on two- and three-dimensional tasks. Horm Behav. 1997;32:167–175. doi: 10.1006/hbeh.1997.1418. [DOI] [PubMed] [Google Scholar]
- Portin R, Polo-Kantola P, Polo O, Koskinen T, Revonsuo A, Irjala K, Erkkola R. Serum estrogen level, attention, memory and other cognitive functions in middle-aged women. Climacteric. 1999;2:115–123. doi: 10.3109/13697139909025575. [DOI] [PubMed] [Google Scholar]
- Postma A, Winkel J, Tuiten A, van Honk J. Sex differences and menstrual cycle effects in human spatial memory. Psychoneuroendocrinology. 1999;24:175–192. doi: 10.1016/s0306-4530(98)00073-0. [DOI] [PubMed] [Google Scholar]
- Presty SK, Bachevalier J, Walker LC, Struble RG, Price DL, Mishkin M, Cork LC. Age differences in recognition memory of the rhesus monkey (Macaca mulatta) Neurobiol Aging. 1987;8:435–440. doi: 10.1016/0197-4580(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta) Behav Neurosci. 1990;104:876–884. doi: 10.1037//0735-7044.104.6.876. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging. 1991;12:481–486. doi: 10.1016/0197-4580(91)90077-w. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport. 1997;8:1923–1928. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D WHIMS Investigators . Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. J Am Med Assoc. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Gilardi KV, Lasley B, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. Neuroreport. 1997;8:2047–2051. doi: 10.1097/00001756-199705260-00048. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Park S. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology. 2002;27:835–841. doi: 10.1016/s0306-4530(01)00083-x. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Habeck C, Hof PR, Amaral DG, Fong SG, Buonocore MH, Stern Y, Barnes CA, Rapp PR. Volumetric Correlates of Spatiotemporal Working and Recognition Memory Impairment in Aged Rhesus Monkeys. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq210. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J WHIMS Investigators . Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. J Am Med Assoc. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Maki PM, Bishop JR. A review of estrogen receptor alpha gene (ESR1) polymorphisms, mood, and cognition. Menopause. 2010;17:874–886. doi: 10.1097/gme.0b013e3181df4a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklo M, Cerhan J, Diez-Roux AV, Chambless L, Cooper L, Folsom AR, Fried LP, Knopman D, Nieto FJ. Estrogen replacement therapy and cognitive functioning in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1996;144:1048–1057. doi: 10.1093/oxfordjournals.aje.a008877. [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- ter Horst GJ. Estrogen in the limbic system. Vitam Horm. 2010;82:319–338. doi: 10.1016/S0083-6729(10)82017-5. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Voytko ML. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:423–431. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Novel sites and mechanisms of oestrogen action in the brain. Novartis Found Symp. 2000;230:56–69. doi: 10.1002/0470870818.ch6. [DOI] [PubMed] [Google Scholar]
- Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: synaptic density. Exp Neurol. 1980;69:164–172. doi: 10.1016/0014-4886(80)90151-x. [DOI] [PubMed] [Google Scholar]
- Van Esch E, Cline JM, Buse E, Wood CE, de Rijk EPCT, Weinbauer GF. Summary comparison of female reproductive system in human and the Cynomolgus monkey (Macaca fascicularis) Toxicol Pathol. 2008;36:171S–172S. [Google Scholar]
- Vearncombe KJ, Pachana NA. Is cognitive functioning detrimentally affected after early, induced menopause? Menopause. 2009;16:188–198. doi: 10.1097/gme.0b013e3181775eb4. [DOI] [PubMed] [Google Scholar]
- Verghese J, Kuslansky G, Katz MJ, Sliwinski M, Crystal HA, Buschke H, Lipton RB. Cognitive performance in surgically menopausal women on estrogen. Neurology. 2000;55:872–874. doi: 10.1212/wnl.55.6.872. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Impairments in acquisition and reversals of two-choice discriminations by aged rhesus monkeys. Neurobiol Aging. 1999;20:617–627. doi: 10.1016/s0197-4580(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Murray R, Higgs CJ. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J Neurosci. 2009;29:10362–10370. doi: 10.1523/JNEUROSCI.1591-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770–12776. doi: 10.1523/JNEUROSCI.3192-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, Törber S, McEwen BS, Kirschbaum C. Two weeks of transdermal estradiol treatment in postmenopausal elderly women and its effect on memory and mood: verbal memory changes are associated with the treatment induced estradiol levels. Psychoneuroendocrinology. 1999;24:727–41. doi: 10.1016/s0306-4530(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J, Kasai H. Principles of long-term dynamics of dendritic spines. J Neurosci. 2008;28:13592–13608. doi: 10.1523/JNEUROSCI.0603-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC Cache County Memory Study Investigators . Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]