Abstract
Excitotoxic neuronal damage via over-activation of the NMDA receptor has been implicated in many neurodegenerative diseases. In vitro modeling of excitotoxic injury has shown that activation of G-protein coupled receptors (GPCRs) counteracts such injury through modulation of neuronal pro-survival pathways and/or NMDA receptor signaling. We have previously demonstrated that the GPCR APJ and its endogenous neuropeptide ligand apelin can protect neurons against excitotoxicity, but the mechanism(s) of this neuroprotection remain incompletely understood. We hypothesized that apelin can promote neuronal survival by activating pro-survival signaling as well as inhibiting NMDA receptor-mediated excitotoxic signaling cascades. Our results demonstrate that (i) apelin activates pro-survival signaling via inositol trisphosphate (IP3), protein kinase C (PKC), mitogen-activated protein kinase kinase 1/2 (MEK1/2), and extracellular signal-regulated kinase-1/2 (ERK1/2) to protect against excitotoxicity, and (ii) apelin inhibits excitotoxic signaling by attenuating NMDA receptor and calpain activity, and by modulating NMDA receptor subunit NR2B phosphorylation at serine 1480. These studies delineate a novel apelinergic signaling pathway that concurrently promotes survival and limits NMDA receptor-mediated injury to protect neurons against excitotoxicity. Defining apelin-mediated neuroprotection advances our understanding of neuroprotective pathways and will potentially improve our ability to develop therapeutics for excitotoxicity-associated neurodegenerative disorders.
Keywords: apelin, NMDA receptor, HIV, neuroprotection, excitotoxicity
Introduction
Excitotoxic neuronal damage via over-activation of the NMDA receptor has been implicated in many neurodegenerative diseases, and further understanding of endogenous mechanisms that regulate NMDA receptor signaling is critical for the development of effective therapeutics (Hardingham & Bading 2010). NMDA receptors include two glycine-binding NR1 subunits and two glutamate-binding NR2 subunits of four types (NR2A - NR2D; (Cull-Candy & Leszkiewicz 2004), and these subunits demonstrate well-defined developmental and regional distribution in rodent models and in humans (Monyer et al. 1994, Standaert et al. 1996, Conti et al. 1999, Law et al. 2003). Phosphorylation of these subunits, particularly within the C-terminus of NR2B, can differentially regulate receptor function and susceptibility to excitotoxicity (Waxman & Lynch 2005, Chen & Roche 2007). Specifically, Src family kinases phosphorylate NR2B tyrosine residues Y1336 and Y1472, and in general, potentiate NMDA receptor currents to enhance excitotoxicity (Salter & Kalia 2004). Casein kinase-2 (CK2) phosphorylates NR2B serine residue S1480 (Chung et al. 2004, Sanz-Clemente et al. 2010), which has been indirectly implicated in neuroprotection against excitotoxicity (Clapp et al. 2009). Our studies have focused on HIV infection of the CNS as an excitotoxicity disease model, and we have shown that neuronal vulnerability to HIV neurotoxicity is dependent upon developmentally-regulated expression of NR2B (O’Donnell et al. 2006). Additionally, NR2B phosphorylation has been implicated in susceptibility to excitotoxicity in various HIV neurotoxicity models (Haughey et al. 2001, Viviani et al. 2006, Eugenin et al. 2007).
Accordingly, G-protein coupled receptors (GPCRs) may protect against HIV-induced excitotoxicity via NMDA receptor modulation (Kaul & Lipton 1999, Meucci et al. 2000, Bruno et al. 2000). The GPCR APJ receptor and its endogenous neuropeptide ligand apelin are highly expressed in the CNS, especially in cortical, hippocampal, and hypothalamic neurons, with comparable distribution between rodents and humans (De Mota et al. 2000, Lee et al. 2000, Reaux et al. 2002, Kleinz & Davenport 2005). We have shown that treatment of rodent hippocampal neuronal cultures with several native apelin isoforms, including apelin-36, phosphorylates extracellular signal-regulated kinase-1/2 (ERK1/2) and protects against excitotoxicity (O’Donnell et al. 2007). However, the mechanism(s) of apelin-mediated neuroprotection remain largely unknown, including clear delineation of the neuronal apelinergic G-protein coupled signaling pathway and potential modulation of NMDA receptors. We hypothesized that apelin can promote neuronal survival by activating pro-survival signaling as well as inhibiting NMDA receptor-mediated excitotoxic signaling cascades. Our results demonstrate that apelinergic signaling concurrently promotes survival via inositol trisphosphate (IP3), protein kinase C (PKC), mitogen-activated protein kinase kinase 1/2 (MEK1/2), and ERK1/2 activation and limits excitotoxicity by modulating NR2B S1480 phosphorylation and attenuating NMDA receptor-mediated ionic currents, Ca2+ accumulation, and calpain activation. Targeting apelinergic signaling may have therapeutic value for disorders involving excitotoxicity, including HIV-associated neurocognitive disorders, ischemia, epilepsy, Huntington’s disease, Parkinson’s disease and Alzheimer’s disease (Waxman & Lynch 2005, Hardingham & Bading 2010).
Materials and Methods
Detailed information regarding materials, quantification of excitotoxicity in primary rat brain cultures, calcium imaging, electrophysiology and Western blotting can be found as Supplemental Methods.
Preparation of primary rat brain cultures
Primary rat cerebrocortical cultures were prepared from embryonic day 17 Sprague-Dawley rat pups as previously described (Brewer 1995, Wilcox et al. 1994). All procedures were within the ARRIVE guidelines for animal research, and in accordance with protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Cells were plated in tissue culture dishes pre-coated with poly-L-lysine (Peptides International, Louisville, KY) and maintained in neurobasal media plus B27 supplement (Invitrogen, Carlsbad, CA) at 37 °C/5% CO2. Half of the media was replaced every 7 days, and cultures were used between 14 days in vitro (DIV) and 18 DIV.
Preparation of human monocyte-derived macrophages and HIV infections
HIV infection of monocyte-derived macrophages was performed as previously described (Chen et al. 2002, O’Donnell et al. 2006), and in accordance with protocols approved by the University of Pennsylvania Committee on Studies Involving Human Beings. Briefly, macrophages were infected with a CSF-derived, macrophage-tropic HIV-1 isolate (HIV-Jago) from a patient with confirmed HIV-associated dementia. Viral stocks were prepared by the University of Pennsylvania Center for AIDS Research Virology Core. Supernatants from HIV-infected or non-infected (vehicle) macrophages were collected and monitored for HIV replication by quantifying viral reverse transcriptase activity as the amount of radiolabeled deoxythymidine incorporation (Ho et al. 1992).
Quantification of excitotoxicity in primary rat brain cultures
Cell survival was quantified by three methods: (i) cell counting of microtubule associated protein-2 (MAP-2)- and glial fibrillary acidic protein (GFAP)-reactive cells (O’Donnell et al. 2006); (ii) cell-based MAP-2 ELISA assay (Wang et al. 2007, White et al. 2011); and (iii) lactate dehydrogenase (LDH) assay.
Calcium imaging
Primary rat cerebrocortical cells were plated at a density of 2×105 cells per 35mm glass bottom dish (MatTek Corporation, Ashland, MD), and intracellular neuronal Ca2+ concentration was determined as previously described (Wu et al. 2007, Lynch et al. 2001).
Electrophysiology
Primary rat cerebrocortical cells were plated at a density of 2–4×105 cells per 35mm dish with glass coverslips, and standard whole-cell voltage clamp techniques were used for recording NMDA-induced ionic currents. Treatments applications were performed under the following paradigm: 2sec agonist, 1min wash, 2min treatment, 2sec agonist, 3min wash.
Site-directed mutagenesis of NR2B
NR2B at serine 1480 was mutated to alanine (S1480A) in the mammalian expression vector pRK7-NR2B using the QuikChange II Site-Directed Mutagenesis kit (Agilent, Santa Clara, CA) as previously described (Wu et al. 2007) using published primers (Chung et al. 2004).
Transfection of HEK293 cells
HEK293 cells were plated in tissue culture dishes pre-coated with poly-D-lysine (Sigma) and maintained in minimum essential media plus 7.5% fetal bovine serum, 2.5% horse serum, 1% penicillin/streptomycin, and 1% L-glutamine at 37 °C/5% CO2. Transfections were performed using calcium phosphate in the presence of ketamine (500μM, Sigma) to prevent NMDA receptor activation (Grant et al. 1997). Treatments were administered 16–18hr post-transfection.
Quantification of excitotoxicity in NMDA receptor-transfected HEK293 cells
HEK293 cells were plated in 24-well plates with glass coverslips, and following transfection and experimental treatments, cultures were incubated in Hoescht 33342 (5μg/ml) for 20min at room temperature and fixed in 4% paraformaldehyde in PBS for 20min at 4°C. The number of surviving GFP-positive cells was estimated by blinded counting of GFP- and Hoescht-positive cells from 3–5 randomly selected fields per coverslip at 20x magnification.
Western blotting
HEK293 cells or primary rat cerebrocortical cells were plated at a density of 1×106 cells per 60mm dish or 4×105 cells per 35mm dish. Following experimental treatments, whole cell lysates were subjected to SDS-PAGE as previously described (O’Donnell et al. 2006).
Statistical analysis
For quantification of excitotoxicity, Western blotting, and electrophysiology, values are expressed as mean ± standard error of mean, and statistical comparisons were made by Student’s t-test or one-way ANOVA plus Newman-Keuls post hoc testing as indicated in the figure legends. For Ca2+ imaging, statistical comparisons for populations of individuals neurons were made by the Kruskal-Wallis test plus Dunn’s Multiple Comparison post hoc testing, and correlations were made by Spearman’s test. All graphs were generated and statistical analyses were performed using GraphPad Prism software (San Diego, CA), and values of p<0.05 were considered significant.
Results
Apelin protects cerebrocortical neurons against glutamate- or HIV-induced excitotoxicity via IP3-, PKC-, MEK1/2, and ERK1/2-mediated signaling pathways
Several in vitro models have shown that HIV infection in the CNS causes release of soluble excitotoxins, especially glutamate, from productively infected macrophages (Kaul et al. 2005). Glutamate and supernatants from HIV-infected macrophages cause neurotoxicity in primary rat hippocampal and cerebrocortical cultures dependent upon developmentally-regulated expression of NR2B (14 days in vitro and older; (O’Donnell et al. 2006)). To define the mechanism(s) of apelin-mediated neuroprotection, we used 14–18 days in vitro fetal rat cerebrocortical neuronal/glial cultures exposed to glutamate or HIV-infected macrophage supernatants, each of which caused dose-dependent excitotoxicity (Fig. 1; Supplemental Fig. 1; Supplemental Fig. 2). Treatment with a native apelin peptide (apelin-36), but not a negative control peptide (apelin-36 scramble), showed robust, dose-dependent neuroprotection against both glutamate and HIV supernatant insults, while apelin in the absence of insult had no effect on neuronal survival (Fig. 1b, 1d). Interestingly, MK801 in the absence of insult induced some neurotoxicity (Fig. 1d), consistent with studies suggesting that elimination of all NMDA receptor activity can cause cell death (Papadia et al. 2005). Together, these results demonstrate that apelin protects cerebrocortical neurons against excitotoxicity in a dose-dependent manner.
Fig 1.
Apelin protects cerebrocortical neurons from HIV-and glutamate-induced excitotoxicity in a dose-dependent manner. (a) Cultures were exposed to HIV-infected macrophage supernatant for 24h (1:20 dilution). Cell survival was assessed by immunofluorescent staining for neuronal marker MAP-2 (red) and nuclear marker Hoescht 33324 (blue). Magnification for fluorescent micrographs is 20x (scale bar: 20μM). (b) Cultures were treated with apelin-36 (0.2–20μM), apelin-36 scramble (20μM), or MK801 (10μM) for 45 minutes, followed by exposure to HIV supernatant for 24hr. Cell survival was quantified by MAP-2 ELISA and expressed as a percentage of untreated (UT) cultures (n = 12; *p<0.05, **p<0.01, ***p<0.01 vs. HIV). (c) Cultures were exposed to glutamate for 24h (50μM). Cell survival was assessed by immunofluorescent staining for MAP-2 (green) and Hoescht 33324 (blue). (d) Cultures were treated as in (b), followed by exposure to glutamate for 24hr. Cell survival was quantified by MAP-2 ELISA (n = 11–17; ###p<0.001 vs. vehicle; **p<0.01, ***p<0.001 vs. glutamate). All statistical comparisons were made by one-way ANOVA plus Newman-Keuls post hoc testing.
We next sought to identify signaling pathways underlying apelin-mediated neuroprotection using specific pharmacologic inhibitors (Fig. 2). Apelin caused phosphorylation of ERK1/2 at the kinases’ active sites (Fig. 2a). Apelin-induced ERK1/2 phosphorylation was blocked by 2-APB, which inhibits Ca2+ release from IP3 receptors, and by MEK1/2 inhibitors U0126 and PD98059, consistent with Ca2+-dependent GPCR activation of ERK1/2 (Fig. 2a, Fig. 8, (Gutkind 2000)). Apelin also caused PKC phosphorylation at its active site, which is required prior to Ca2+ and/or lipid second messenger binding for PKC activation (Gould & Newton 2008). As expected, apelin-induced PKC phosphorylation was blocked by GF109203X and chelerythrine chloride, which inhibit conventional and novel PKC isoforms, but not by 2-APB, as PKC phosphorylation occurs before its activation by Ca2+ (Fig. 2b). Notably, 2-APB, U0126, PD98059, GF109203X, and chelerythrine chloride all blocked apelin neuroprotection against glutamate, while a negative control epidermal growth factor tyrosine kinase inhibitor AG1478 did not (Fig. 2c). Notably, similar effects were seen at apelin concentrations of 20μM (Fig. 2c) and 2μM (data not shown). Together, these studies demonstrate that apelin promotes neuronal survival against excitotoxicity by activating IP3, PKC, MEK1/2, and ERK1/2 intermediaries.
Fig 2.
Apelin protects cerebrocortical neurons from glutamate-induced excitotoxicity in an IP3-, PKC-, and MEK1/2-dependent manner. (a–b) Cultures were exposed to 2-APB (50μM, 5min), U0126 (500nM, 1hr), PD98059 (20μM, 1hr), GF109203X (1μM, 1hr), or chelerythrine chloride (5μM, 30min), then treated with apelin-36 (20μM) for 5min. Phosphorylation of ERK1/2 (threonine 202/ tyrosine 204; (a)), or PKCγ (threonine 514 and homologous residues; (b)) was assessed by Western blot and quantified by densitometry of phospho- over total kinase normalized to β-tubulin (n = 3; *p<0.05, **p<0.01 vs. vehicle). (c) Cultures were exposed to inhibitors as in (a–b) or AG1478 (250nM, 30min), then treated with apelin-36 (20μM) for 45min, followed by glutamate (50μM) for 24hr. Cell survival was quantified by MAP-2 ELISA (n = 6–30; **p<0.01, ***p<0.001 vs. glutamate). All statistical comparisons were made by one-way ANOVA plus Newman-Keuls post hoc testing.
Fig 8.

Proposed mechanism of apelin-mediated neuroprotection. Apelin binds to APJ and triggers a GPCR signaling cascade through IP3, Ca2+, PKC, MEK1/2, and ERK1/2 to prevent neurotoxicity. Apelin/APJ signaling can also prevent neurotoxicity through Ca2+-dependent CK2 phosphorylation of NR2B S1480. NR2B S1480 phosphorylation may provide the molecular mechanism for apelin-mediated attenuation of NMDA receptor and calpain activation. Inhibitors used in these studies are shown in boxes.
Additional studies investigated other pathways implicated in apelinergic signaling, including the phosphoinositide 3-kinase–Akt–mammalian target of rapamycin pathway (Masri et al. 2005, Japp & Newby 2008), but pharmacologic inhibitors caused significant neurotoxicity (data not shown), thereby preventing conclusive analysis.
Apelin attenuates NMDA receptor activity and calpain activation in cerebrocortical neurons
In addition to activation of pro-survival signaling pathways, some GPCR ligands can induce neuronal Ca2+ transients and inhibit NMDA receptor-mediated Ca2+ influx and accumulation to protect against excitotoxicity (Meucci et al. 2000, Deiva et al. 2004, Limatola et al. 2005, Yao et al. 2009). Similarly, apelin induced dose-dependent, oscillatory Ca2+ transients in cerebrocortical neurons (Fig. 3a-d), consistent with our previous findings in human NT2.N neuronal cell lines (Choe et al. 2000). Expanding on these findings, apelin-induced Ca2+ transients were below the maximum Ca2+ concentration, as determined by exposure to the Ca2+ ionophore ionomycin (Fig. 3a-d), and decayed within 10min post-treatment (data not shown), suggesting that apelin causes sub-maximal and reversible Ca2+ accumulation. Furthermore, apelin inhibited Ca2+ accumulation induced by NMDA plus glycine (Fig. 3e), and neurons with the largest Ca2+ response to apelin showed the greatest attenuation of NMDA receptor-mediated Ca2+ accumulation (Fig. 3f). This result directly links apelin induction of Ca2+ transients with attenuation of NMDA receptor-mediated Ca2+ accumulation. Additional studies demonstrated that apelin can attenuate Ca2+ accumulation at higher NMDA receptor agonist concentrations (200μM NMDA plus 100μM glycine), and that apelin did not affect potassium-depolarization-induced Ca2+ accumulation (data not shown), suggesting that apelin modulation of NMDA receptors is not due to non-specific inhibition of neuronal Ca2+ responses.
Fig 3.
Apelin induces Ca2+ transients and attenuates NMDA-induced Ca2+ accumulation in cerebrocortical neurons. (a–c) Cultures were loaded with Fura-2/AM and intracellular Ca2+ concentration ([Ca2+]in) was recorded during treatment with apelin-36 as in Fig. 1, followed by exposure to NMDA (50μM) + glycine (10μM), then ionomycin (1μM). Representative recordings from individual neurons are shown for treatment with vehicle (a), apelin-36 (20μM; (b)), or apelin-36 scramble (20μM; (c)), expressed as a percent change from baseline. (d–e) Quantification of peak [Ca2+]in after each treatment (d) and after NMDA + glycine ((e); n= 3–5 cultures per condition, 6–10 neurons per culture; ***p<0.001 vs. vehicle by Kruskal-Wallis test plus Dunn’s post hoc testing). (f) Correlation between peak [Ca2+]in induced by treatment with 20μM apelin-36 and by NMDA + glycine (n = 5 cultures, 40 neurons total; r = −0.4891, p = 0.0007 by Spearman’s test).
As apelin effectively blocks NMDA receptor-mediated Ca2+ accumulation, we reasoned that apelin might also directly attenuate NMDA receptor-mediated ionic currents. Using whole-cell voltage clamp electrophysiology in cerebrocortical neurons, apelin demonstrated a dose-dependent inhibition of NMDA-induced currents, while apelin-36 scramble showed no effect (Fig. 4). These studies demonstrate that rapid apelin-mediated signaling events can directly inhibit NMDA receptor activity as measured by ionic currents and Ca2+ accumulation.
Fig 4.
Apelin attenuates NMDA-induced whole cell currents in cerebrocortical neurons. (a–c) Individual neurons were recorded in response to NMDA (50μM) + glycine (10μM) before and after 2min treatment with apelin-36 as in Fig. 1. Representative recordings are shown for treatment with vehicle (a), apelin-36 (20μM; (b)), or apelin-36 scramble (20μM; (c)). (d) Quantification of peak current after each treatment, expressed as a percent change from before treatment (n=6–11; *p<0.05 vs. 20μM apelin-36 by one-way ANOVA plus Newman-Keuls post hoc testing).
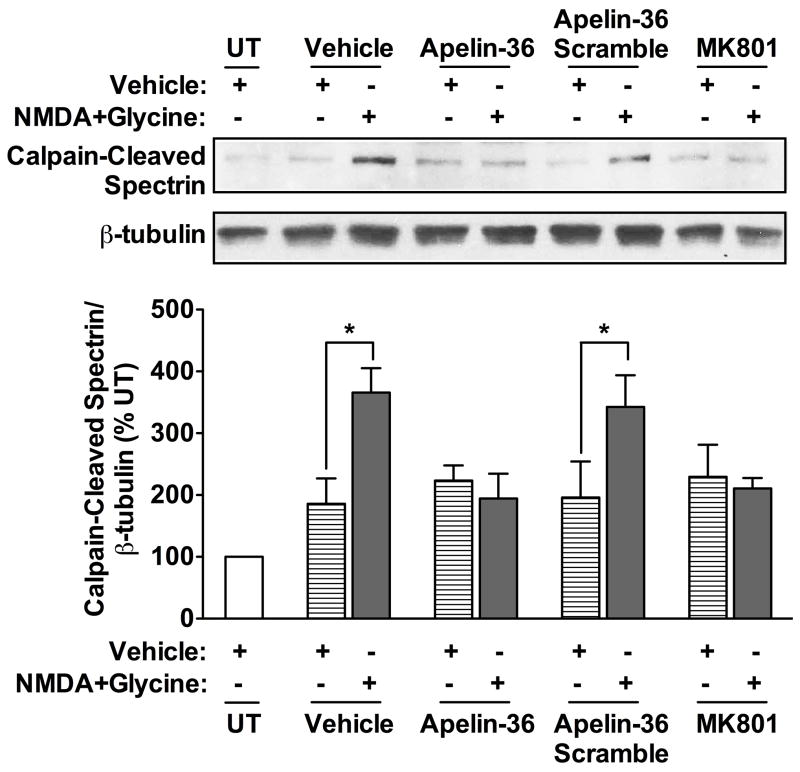
Excessive NMDA receptor activity can ultimately lead to Ca2+-dependent activation of calpains (Goll et al. 2003), and calpain inhibitors can provide neuroprotection against glutamate- or HIV-induced excitotoxicity (Supplemental Fig. 3a, (O’Donnell et al. 2006, Wang et al. 2007)). As expected, treatment with apelin inhibited NMDA-induced calpain activation, as measured by calpain-specific cleavage of the cytoskeletal protein spectrin (Fig. 5). Moreover, apelin in the absence of NMDA did not modulate calpain activation (Fig. 5), again suggesting that apelin causes sub-maximal and reversible Ca2+ accumulation that is insufficient to either activate calpain or cause excitotoxicity. Together, these studies demonstrate that apelin attenuates excessive NMDAR activity and calpain activation, suggesting that apelin promotes neuronal survival by inhibiting excitotoxic NMDA receptor signaling.
Fig 5.
Apelin inhibits NMDA-induced calpain activation in cerebrocortical cultures. Cultures were treated with apelin-36 (20μM), apelin-36 scramble (20μM), or MK801 (10μM) for 5min, followed by exposure to NMDA (50μM) + glycine (10μM) for 5min. Expression of calpain-cleaved spectrin was assessed by Western blot and quantified by densitometry normalized to β-tubulin (n = 3; *p<0.05 vs. vehicle by Student’s t-test).
Apelin-induced phosphorylation of NR2B S1480 mediates protection against excitotoxicity in cerebrocortical neurons and NMDA receptor-transfected HEK293 cells
NR2B-containing NMDA receptors are the primary mediators of excitotoxicity in our model system (Supplemental Fig. 3b–c, (O’Donnell et al. 2006)), and NR2B phosphorylation can differentially regulate susceptibility to excitotoxicity (Salter & Kalia 2004, Chen & Roche 2007). Thus, we sought to identify the effects of apelin on NR2B phosphorylation as a mechanism for neuroprotection. Apelin increased NR2B phosphorylation at S1480 in the presence of NMDA (Fig. 6a) without altering NR2B phosphorylation at Y1336 or Y1472 (data not shown). Treatment with 2-APB or TBB, a competitive inhibitor of CK2, prior to apelin and NMDA exposure blocked S1480 phosphorylation (Fig. 6b), consistent with Ca2+-dependent CK2 phosphorylation of S1480 (Chung et al. 2004, Sanz-Clemente et al. 2010). Neither PKC inhibitor GF109203X nor MEK1/2 inhibitor PD98059 blocked apelin-induced phosphorylation of S1480 (Fig. 6b). Additionally, TBB blocked apelin-mediated neuroprotection against glutamate, while the Src family kinase inhibitor PP2, which blocks phosphorylation at Y1336 and Y1472, had no effect (Fig. 6c). Similar effects were seen at apelin concentrations of 20μM (Fig. 2c) and 2μM (data not shown). Together, these studies demonstrate that apelin-induced phosphorylation of NR2B S1480 during NMDA receptor activation may contribute to apelin neuroprotection.
Fig 6.
Apelin induces phosphorylation of NR2B S1480, and inhibition of NR2B S1480 phosphorylation blocks apelin-mediated neuroprotection in cerebrocortical cultures. (a) Cultures were treated with apelin-36 (20μM), or apelin-36 scramble (20μM) for 5min, followed by exposure to NMDA (50μM) + glycine (10μM) for 5min. Phosphorylation of NR2B at S1480 was assessed by Western blot (n = 3). (b) Cultures were exposed to 2-APB (50μM, 5min), TBB (20μM, 1hr), GF109203X (1μM, 1hr), or PD98059 (20μM, 1hr), then treated with apelin-36 (20μM) for 5min, followed by NMDA + glycine for 5min. Western blots were performed as in (a) (n = 3). (c) Culture were exposed to TBB as in (b) or PP2 (10μM, 1hr), then treated with apelin-36 (20μM) for 45min, followed by glutamate (50μM) for 24hr. Cell survival was quantified by MAP-2 ELISA (n = 12–18; *p<0.05, **p<0.01, ***p<0.001 vs. Veh by one-way ANOVA plus Newman-Keuls post hoc testing).
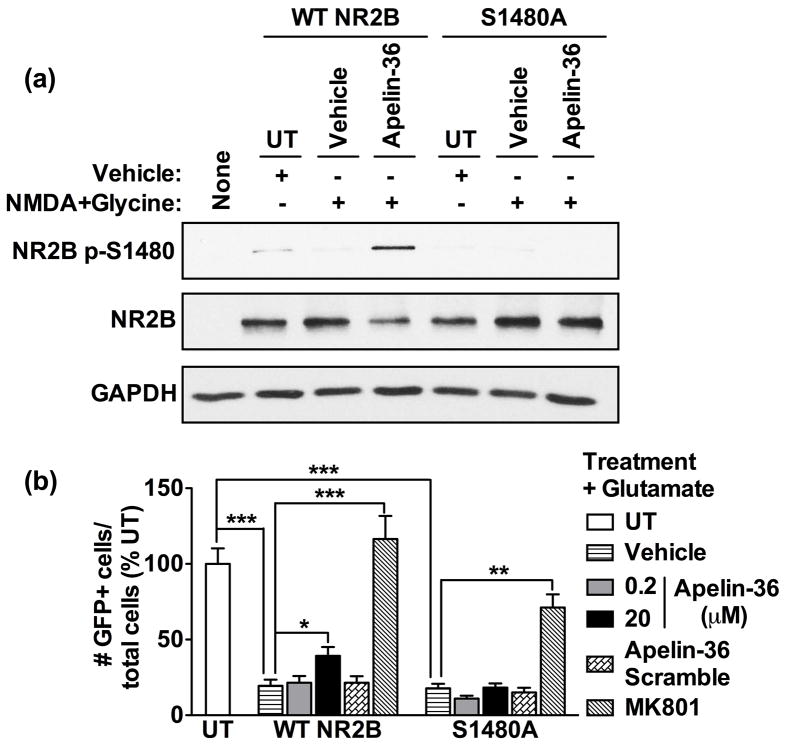
To further validate the role of NR2B S1480 phosphorylation in apelin-mediated neuroprotection, we transiently transfected HEK293 cells with expression plasmids containing green fluorescent protein (GFP), APJ, NR1, postsynaptic density-95 (PSD-95), and wild-type (WT) NR2B or NR2B with S1480 mutated to alanine (S1480A), which cannot be phosphorylated (Lim et al. 2002). Consistent with our studies in neurons, exposure to apelin and NMDA induced S1480 phosphorylation in WT NR2B-expressing cells, but not S1480A-expressing cells (Fig. 7a). Similarly, apelin protected WT NR2B-expressing cells, but not S1480A-expressing cells, against glutamate-induced excitotoxicity (Fig. 7b). These studies further suggest that apelin-induced phosphorylation of NR2B S1480 contributes to apelin neuroprotection.
Fig 7.
Apelin-induced phosphorylation of NR2B S1480 mediates protection against excitotoxicity in HEK293 cells. (a) Cultures transfected with APJ, NR1, PSD-95, GFP, and wild-type (WT) NR2B or NR2B S1480A-expressing plasmids were treated with apelin-36 (20μM) for 5min, followed by NMDA (50μM) + glycine (10μM) for 5min. Phosphorylation of NR2B at S1480 was assessed by Western blot (n = 3). (b) Cultures were treated with apelin-36 (0.2, 20μM), apelin-36 scramble (20μM), or MK801 (10μM) for 45min, followed by glutamate (50μM) for 24hr. Transfected cell survival was quantified by counting GFP-positive cells and expressed as the ratio of GFP-positive cells to the total number of cells (n = 5–6 coverslips per treatment condition, 25–30 fields total; *p<0.05, **p<0.01, ***p<0.001 vs. vehicle by one-way ANOVA plus Newman-Keuls post hoc testing).
Discussion
Previous work in our laboratory demonstrated that apelin can protect neurons against excitotoxicity (O’Donnell et al. 2007), and in this study, we sought to more fully define the mechanism(s) of this neuroprotection. We now propose a novel pathway in which apelin triggers signaling through IP3, Ca2+, CK2, PKC, MEK1/2, ERK1/2, and NR2B S1480 phosphorylation to concurrently promote survival and limit NMDA receptor-mediated excitotoxic activity (Fig. 8). Therefore, targeting the apelinergic system in the CNS may have significant and novel therapeutic value, especially for HIV and other neurodegenerative disorders in which excitotoxicity is implicated.
Apelinergic activation of pro-survival signaling
We have shown that apelin dose-dependently protects cerebrocortical neurons against HIV- or glutamate-induced excitotoxicity via IP3-, PKC-, MEK1/2- and ERK1/2-mediated signaling pathways (Fig. 1–2). Although the G-protein coupling of APJ, apelin’s receptor, is unknown in neurons, apelinergic signaling in heterologous systems and in non-neuronal cells occurs through Gi/o or Gq (Masri et al. 2005, Japp & Newby 2008). Gi/o and Gq can activate phospholipase C (PLC) to hydrolyze phosphatidylinositol bisphosphate (PIP2) into IP3 and diacylglcerol (DAG), causing Ca2+ release through endoplasmic reticulum IP3 receptors and subsequent activation of several PKC isoforms, MEK1/2, and ERK1/2 (Fig. 8; (Gutkind 2000)). Thus, our findings are consistent with Gi/o- or Gq-coupling of apelinergic signaling in primary neurons (Fig. 2).
Apelin triggers neuronal Ca2+ transients typical of GPCR signaling, and our inhibitor studies indirectly implicate IP3 receptor activation in these transients (Fig. 2–3). However, we have not ruled out the contribution of other Ca2+ channels in apelinergic signaling. Recent studies suggest that neuroprotective chemokine GPCR ligands that trigger IP3 signaling can activate transient receptor potential canonical channels, a superfamily of Ca2+-permeable channels that shunt out intracellular Ca2+ during NMDA receptor activation (Yao et al. 2009). Apelin modulation of such channels remains a possibility for further investigation.
Our inhibitor studies also indicate apelin activation of conventional (α, βI/βII, γ) and/or novel (δ, ε, η, θ) PKC isoforms (Fig. 2). PKC activation requires sequential phosphorylation at three sites followed by binding of Ca2+ and/or lipid second messengers, such as DAG. Conventional PKC isoforms require Ca2+ and DAG for activation, novel isoforms require DAG only, and atypical isoforms (ι/λ, ζ) require neither (Gould & Newton 2008). Apelin-mediated PKC phosphorylation was blocked by inhibitors of conventional and novel PKC isoforms, GF109203X and chelerythrine chloride, but not by 2-APB, since PKC phosphorylation occurs prior to its activation by Ca2+ (Fig. 2). However, 2-APB blocked apelin-mediated ERK1/2 phosphorylation (Fig. 2), suggesting that Ca2+-dependent conventional PKC isoforms may underlie apelin activation of ERK1/2 (Fig. 8). These findings are inconsistent with studies in APJ-transfected cells in which an inhibitor of atypical PKCs blocked apelin-stimulated ERK1/2 activation (Masri et al. 2004), indicating that apelinergic signaling in primary neurons might be distinct from that observed in cell lines.
Apelinergic inhibition of excitotoxic signaling
We have also shown that apelin induces Ca2+ transients and attenuates excitotoxic NMDA receptor and calpain activity (Fig. 3–5). Furthermore, we correlate increased Ca2+ response to apelin with decreased response to NMDA, directly linking apelin-induced Ca2+ transients to NMDA receptor attenuation (Fig. 3f). Recent work has implicated apelin at lower concentrations than used in our study in modulation of NMDA-mediated Ca2+ accumulation and injury (Zeng et al. 2010). However, several key disparities suggest a substantially different mechanism of low-dose apelin neuroprotection, including no apelin-induced Ca2+ transients, greatly delayed kinetics of NMDA-mediated Ca2+ accumulation, decreased severity of the excitotoxic insult, and diminished rescue with apelin (Zeng et al. 2010). Moreover, neuronal signaling pathways activated by low-dose apelin have not been addressed, whereas our study outlines a detailed cascade linked to direct modulation of NMDA-induced Ca2+ accumulation as well as ionic currents, calpain activity and phosphorylation state (Fig. 8). Nonetheless, difference in dose-dependent apelin signaling can inform development of apelinergic therapeutics and remain an area of ongoing study in our laboratory.
Apelin can induce phosphorylation of NR2B S1480 through Ca2+- and CK2-dependent mechanisms to provide protection against excitotoxicity (Fig. 6–7), and the phosphorylation of S1480 is of emerging importance in the regulation of synaptic plasticity and excitability (Chung et al. 2004, Clapp et al. 2009, Li et al. 2009, Sanz-Clemente et al. 2010). Recent work demonstrates that CK2 preferentially phosphorylates S1480, disrupting NR2B-PSD-95 interactions and causing decreased surface NR2B expression (Chung et al. 2004, Sanz-Clemente et al. 2010). Moreover, S1480 phosphorylation has been indirectly implicated in neuroprotection against excitotoxicity following ethanol withdrawal (Clapp et al. 2009). Therefore, S1480 phosphorylation may decrease NR2B surface expression to limit NMDA receptor-mediated injury, although the specific effects of S1480 phosphorylation on NMDA receptor and calpain activity are yet to be defined. An alternative hypothesis is that CK2 may have direct, PSD-95-independent effects on NMDA receptor-mediated Ca2+ permeability and conductance, as has been shown for other serine/threonine kinases like PKA (Skeberdis et al. 2006). Whether apelin-mediated S1480 phosphorylation modulates NR2B trafficking and/or activity is unknown, and our preliminary studies indicate no consistent effect of apelin on NR2B surface expression (Cook DR and Gleichman AJ, unpublished observations). Studies are currently underway in our laboratory to define the functional consequences of apelin-induced S1480 phosphorylation on NMDA receptors.
The relative contributions of increased pro-survival signaling and decreased excitotoxic NMDA receptor signaling are not clearly distinguished in our current study, and indeed they could be directly linked. Our proposed mechanism suggests that apelin-induced Ca2+ transients can activate canonical GPCR pro-survival signaling through PKC, MEK1/2, and ERK1/2 in conjunction with activation of CK2 and S1480 phosphorylation which may attenuate excitotoxic signaling (Fig. 8). Moreover, events such as PKC and ERK1/2 activation have pleiotropic effects on multiple signaling pathways, and these could certainly impact NMDA receptor modulation, either directly or indirectly. Excitotoxic NMDA receptor activity has also been shown to directly inhibit pro-survival signaling pathways, including ERK1/2 activation (Soriano & Hardingham 2007). Together, these findings suggest that increased pro-survival signaling and decreased excitotoxic NMDA receptor activity are overlapping pathways for apelin-mediated neuroprotection that combine to prevent neuronal injury.
Therapeutic considerations for apelinergic signaling
Our study focuses on apelin modulation of excitotoxic NMDA receptor activity, and we have no evidence for complete suppression of NMDA receptor activity by apelin. Indeed, NMDA receptor activity is essential for normal neuronal function and survival, and preservation of physiologic activity is a key therapeutic consideration (Hardingham & Bading 2010). Moreover, physiologic NMDAR activity can actually mitigate excitotoxic cell death. Referred to as preconditioning, a low dose of NMDA (~10μM) prior to an excitotoxic dose (≥20μM) promotes neuronal survival by preferentially activating synaptic NMDA receptors to cause ERK1/2 phosphorylation (Ivanov et al. 2006, Soriano et al. 2006) and attenuation of Ca2+ accumulation and calpain activity caused by the excitotoxic dose (Tauskela et al. 2001). NMDA preconditioning is contingent on the neuroprotective dose enhancing neuronal excitability, characterized by oscillatory, sub-maximal Ca2+ transients, while the toxic NMDA dose triggers non-oscillatory, maximum, and sustained Ca2+ accumulation (Soriano et al. 2006). These preconditioning mechanisms are strikingly similar to our findings with apelin (Fig. 2–5), including our observation that apelin only decreases calpain activity or increases NR2B S1480 phosphorylation in the presence of NMDA (50μM; Fig. 5–7). Furthermore, preliminary studies indicate that apelin increases excitatory postsynaptic potential frequency in a dose-dependent and reversible manner (Cook DR and Gleichman AJ, unpublished observations), suggesting that apelin may cause a large but transient increase in synaptic glutamate that preconditions neurons against excitotoxicity. Overall, our results suggest that apelin does not diminish NMDA receptor activity under physiologic conditions, but only under conditions of excitotoxicity. Further studies to determine the role of apelin in pro-survival NMDA receptor activity are crucial, as effective therapeutics must selectively inhibit excitotoxic signaling while preserving cell survival signaling.
Much current effort is directed towards targeting ubiquitous GPCRs for therapeutics against both CNS and systemic diseases, and similar efforts are underway for targeting the apelinergic system (Lee et al. 2006, Sorli et al. 2006, Ladeiras-Lopes et al. 2008). Because of the remarkable redundancy of GPCR/ligand expression and function, ‘off target effects’ for any such pharmacologic activation of GPCRs are being considered, but are not seen as deterrents for investigating APJ agonists as therapeutics. Further studies in vitro and in vivo are needed to determine the effects of longer-term APJ activation, and we hypothesize that chronic APJ activation would provide persistent neuroprotection via upregulation of pro-survival genes without prolonged and potentially detrimental induction of ERK1/2 signaling or suppression of NMDA receptor signaling due to ligand-induced APJ internalization (Zhou et al. 2003, Lee et al. 2010). Additionally, a neuroactive, nonpeptidic APJ agonist has recently been identified (Iturrioz et al. 2009), and our studies suggest that such small molecule agonists should be considered as potential neuroprotectants for excitotoxicity-associated neurodegenerative disorders.
Supplementary Material
Acknowledgments
This work was supported by NIH grants NS043994 (DLK), NS27405 (DLK), NS45956 (DRL), and MH083395 (AJG). DRC was supported by the University of Pennsylvania Training Grant T32 AI07632 in HIV Pathogenesis and by NIH grant F31 NS066791. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The authors declare no conflicts of interest. We are grateful to Patricia Vance and Lorraine Kolson for expert preparation of human monocyte-derived macrophages and HIV infections, Margaret Maronski for expert preparation of primary rodent cultures, Evan Eisler and Siddharth Kishore for technical assistance with the HEK293 studies, and Samantha Soldan and Lauren O’Donnell for critical review of the manuscript.
Abbreviations used
- Ca2+
calcium
- CK2
casein kinase-2
- DAG
diacylglcerol
- DIV
days in vitro
- ERK1/2
extracellular signal-regulated kinase-1/2
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- GPCR
G-protein coupled receptor
- HEK293
human embryonic kidney cells
- HIV
human immunodeficiency virus
- IP3
inositol trisphosphate
- LDH
lactate dehydrogenase
- MAP-2
microtubule associated protein-2
- MEK1/2
mitogen-activated protein kinase kinase 1/2
- PIP2
phosphatidylinositol bisphosphate
- PKC
protein kinase C
- PLC
phospholipase C
- PSD-95
postsynaptic density-95
- UT
untreated
- WT
wildtype
References
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- Bruno V, Copani A, Besong G, Scoto G, Nicoletti F. Neuroprotective activity of chemokines against N-methyl-D-aspartate or beta-amyloid-induced toxicity in culture. Eur J Pharmacol. 2000;399:117–121. doi: 10.1016/s0014-2999(00)00367-8. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Sulcove J, Frank I, Jaffer S, Ozdener H, Kolson DL. Development of a human neuronal cell model for human immunodeficiency virus (HIV)-infected macrophage-induced neurotoxicity: apoptosis induced by HIV type 1 primary isolates and evidence for involvement of the Bcl-2/Bcl-xL-sensitive intrinsic apoptosis pathway. J Virol. 2002;76:9407–9419. doi: 10.1128/JVI.76.18.9407-9419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe W, Albright A, Sulcove J, Jaffer S, Hesselgesser J, Lavi E, Crino P, Kolson DL. Functional expression of the seven-transmembrane HIV-1 co-receptor APJ in neural cells. J Neurovirol. 2000;6(Suppl 1):S61–69. [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp P, Gibson ES, Dell’acqua ML, Hoffman PL. Phosphorylation regulates removal of synaptic N-methyl-d-aspartate receptors after withdrawal from chronic ethanol exposure. J Pharmacol Exp Ther. 2009;332:720–729. doi: 10.1124/jpet.109.158741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F, Barbaresi P, Melone M, Ducati A. Neuronal and glial localization of NR1 and NR2A/B subunits of the NMDA receptor in the human cerebral cortex. Cereb Cortex. 1999;9:110–120. doi: 10.1093/cercor/9.2.110. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- De Mota N, Lenkei Z, Llorens-Cortes C. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology. 2000;72:400–407. doi: 10.1159/000054609. [DOI] [PubMed] [Google Scholar]
- Deiva K, Geeraerts T, Salim H, Leclerc P, Hery C, Hugel B, Freyssinet JM, Tardieu M. Fractalkine reduces N-methyl-d-aspartate-induced calcium flux and apoptosis in human neurons through extracellular signal-regulated kinase activation. Eur J Neurosci. 2004;20:3222–3232. doi: 10.1111/j.1460-9568.2004.03800.x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Gould CM, Newton AC. The life and death of protein kinase C. Curr Drug Targets. 2008;9:614–625. doi: 10.2174/138945008785132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant ER, Bacskai BJ, Pleasure DE, Pritchett DB, Gallagher MJ, Kendrick SJ, Kricka LJ, Lynch DR. N-methyl-D-aspartate receptors expressed in a nonneuronal cell line mediate subunit-specific increases in free intracellular calcium. J Biol Chem. 1997;272:647–656. doi: 10.1074/jbc.272.1.647. [DOI] [PubMed] [Google Scholar]
- Gutkind JS. Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci STKE. 2000;2000:RE1. doi: 10.1126/stke.2000.40.re1. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Lioy J, Song L, Cutilli JR, Polin RA, Douglas SD. Infection of cord blood monocyte-derived macrophages with human immunodeficiency virus type 1. J Virol. 1992;66:573–579. doi: 10.1128/jvi.66.1.573-579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturrioz X, Alvear-Perez R, De Mota N, et al. Identification and pharmacological properties of E339-3D6, the first nonpeptidic apelin receptor agonist. FASEB J. 2009 doi: 10.1096/fj.09-140715. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japp AG, Newby DE. The apelin-APJ system in heart failure Pathophysiologic relevance and therapeutic potential. Biochem Pharmacol. 2008 doi: 10.1016/j.bcp.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ladeiras-Lopes R, Ferreira-Martins J, Leite-Moreira AF. The apelinergic system: the role played in human physiology and pathology and potential therapeutic applications. Arq Bras Cardiol. 2008;90:343–349. doi: 10.1590/s0066-782x2008000500012. [DOI] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Webster MJ, Herman MM, Kleinman JE, Harrison PJ. Expression of NMDA receptor NR1, NR2A and NR2B subunit mRNAs during development of the human hippocampal formation. Eur J Neurosci. 2003;18:1197–1205. doi: 10.1046/j.1460-9568.2003.02850.x. [DOI] [PubMed] [Google Scholar]
- Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O’Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- Lee DK, Ferguson SS, George SR, O’Dowd BF. The fate of the internalized apelin receptor is determined by different isoforms of apelin mediating differential interaction with beta-arrestin. Biochem Biophys Res Commun. 2010;395:185–189. doi: 10.1016/j.bbrc.2010.03.151. [DOI] [PubMed] [Google Scholar]
- Lee DK, George SR, O’Dowd BF. Unravelling the roles of the apelin system: prospective therapeutic applications in heart failure and obesity. Trends Pharmacol Sci. 2006;27:190–194. doi: 10.1016/j.tips.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Li YC, Xi D, Roman J, Huang YQ, Gao WJ. Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J Neurosci. 2009;29:15551–15563. doi: 10.1523/JNEUROSCI.3336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim IA, Hall DD, Hell JW. Selectivity and promiscuity of the first and second PDZ domains of PSD-95 and synapse-associated protein 102. J Biol Chem. 2002;277:21697–21711. doi: 10.1074/jbc.M112339200. [DOI] [PubMed] [Google Scholar]
- Limatola C, Lauro C, Catalano M, Ciotti MT, Bertollini C, Di Angelantonio S, Ragozzino D, Eusebi F. Chemokine CX3CL1 protects rat hippocampal neurons against glutamate-mediated excitotoxicity. J Neuroimmunol. 2005;166:19–28. doi: 10.1016/j.jneuroim.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Lynch DR, Shim SS, Seifert KM, Kurapathi S, Mutel V, Gallagher MJ, Guttmann RP. Pharmacological characterization of interactions of RO 25–6981 with the NR2B (epsilon2) subunit. Eur J Pharmacol. 2001;416:185–195. doi: 10.1016/s0014-2999(01)00868-8. [DOI] [PubMed] [Google Scholar]
- Masri B, Knibiehler B, Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell Signal. 2005;17:415–426. doi: 10.1016/j.cellsig.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65–77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J. 2004;18:1909–1911. doi: 10.1096/fj.04-1930fje. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- O’Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J Neurosci. 2006;26:981–990. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell LA, Agrawal A, Sabnekar P, Dichter MA, Lynch DR, Kolson DL. Apelin, an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem. 2007;102:1905–1917. doi: 10.1111/j.1471-4159.2007.04645.x. [DOI] [PubMed] [Google Scholar]
- Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005;25:4279–4287. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaux A, Gallatz K, Palkovits M, Llorens-Cortes C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience. 2002;113:653–662. doi: 10.1016/s0306-4522(02)00192-6. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein Kinase 2 Regulates the NR2 Subunit Composition of Synaptic NMDA Receptors. Neuron. 2010;67:984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Hardingham GE. Compartmentalized NMDA receptor signalling to survival and death. J Physiol. 2007;584:381–387. doi: 10.1113/jphysiol.2007.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FX, Papadia S, Hofmann F, Hardingham NR, Bading H, Hardingham GE. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J Neurosci. 2006;26:4509–4518. doi: 10.1523/JNEUROSCI.0455-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorli SC, van den Berghe L, Masri B, Knibiehler B, Audigier Y. Therapeutic potential of interfering with apelin signalling. Drug Discov Today. 2006;11:1100–1106. doi: 10.1016/j.drudis.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Landwehrmeyer GB, Kerner JA, Penney JB, Jr, Young AB. Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex and hippocampus. Brain Res Mol Brain Res. 1996;42:89–102. doi: 10.1016/s0169-328x(96)00117-9. [DOI] [PubMed] [Google Scholar]
- Tauskela JS, Comas T, Hewitt K, Monette R, Paris J, Hogan M, Morley P. Cross-tolerance to otherwise lethal N-methyl-D-aspartate and oxygen-glucose deprivation in preconditioned cortical cultures. Neuroscience. 2001;107:571–584. doi: 10.1016/s0306-4522(01)00381-5. [DOI] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M. IL-1beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem. 2006 doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- Wang Y, White MG, Akay C, et al. Activation of cyclin-dependent kinase 5 by calpains contributes to human immunodeficiency virus-induced neurotoxicity. J Neurochem. 2007;103:439–455. doi: 10.1111/j.1471-4159.2007.04746.x. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- White MG, Wang Y, Akay C, Lindl KA, Kolson DL, Jordan-Sciutto KL. Parallel high throughput neuronal toxicity assays demonstrate uncoupling between loss of mitochondrial membrane potential and neuronal damage in a model of HIV-induced neurodegeneration. Neurosci Res. 2011 doi: 10.1016/j.neures.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox KS, Buchhalter J, Dichter MA. Properties of inhibitory and excitatory synapses between hippocampal neurons in very low density cultures. Synapse. 1994;18:128–151. doi: 10.1002/syn.890180206. [DOI] [PubMed] [Google Scholar]
- Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter DA, Lynch DR. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007;282:20075–20087. doi: 10.1074/jbc.M700624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Peng F, Dhillon N, Callen S, Bokhari S, Stehno-Bittel L, Ahmad SO, Wang JQ, Buch S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29:1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XJ, Yu SP, Zhang L, Wei L. Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp Cell Res. 2010;316:1773–1783. doi: 10.1016/j.yexcr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Fan X, Mukhtar M, Fang J, Patel CA, DuBois GC, Pomerantz RJ. Cell-cell fusion and internalization of the CNS-based, HIV-1 co-receptor, APJ. Virology. 2003;307:22–36. doi: 10.1016/s0042-6822(02)00021-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.