Abstract
In rats, hedonic USVs is a validated model of positive affect and is best elicited by rough-and-tumble play. Here we report that modulation of GluN2B-containing NMDA receptors (NMDAR) in the medial prefrontal cortex (MPFC) is involved in positive emotional learning. Rough and tumble play increased both GluN1 and GluN2B NMDAR subunit mRNA and protein levels in the frontal cortex. GLYX-13, a GluN2B-preferring, NMDAR glycine-site partial agonist (1 mg / kg i.v.) significantly increased positive emotional learning whereas the GluN2B receptor-specific antagonist, ifenprodil (10 mg/kg i.p.), inhibited positive emotional learning. Animals selectively bred for low rates of hedonic USVs were returned to wild-type levels of positive emotional learning following GLYX-13 treatment. MPFC microinjections of GLYX-13 (0.1–10 μg / side) significantly increased rates of positive emotional learning. Thus GluN2B-containing NMDARs may be involved in positive emotional learning in the MPFC by similar mechanisms as spatial / temporal learning in the hippocampus.
Keywords: GLYX-13, GluN2B, medial prefrontal cortex, positive emotion, learning, vocalizations
Positive affect induces resilience to depression and is an under-examined area for the development of novel therapeutics. Positive affective states, as studied longitudinally in humans, confer resilience to depression and anxiety, and lead to an increase in overall health and a decrease in mortality from all causes (Lyubomirsky et al., 2005). Following a major life stressor, individuals with high positive affect are less likely to develop psychological disorders such as anxiety and depression (Fredrickson et al., 2003). Conversely, individuals who have low levels of positive emotion are at greater risk of developing anxiety disorders, depression, and global health problems (Lyubomirsky et al., 2005). The molecular mechanisms underlying positive affective states are poorly understood and require efficient animal models.
Rat 50-kHz ultrasonic vocalization (hedonic USVs) is a validated model for the study of positive affective states and is best elicited by rough-and-tumble play. Human positive affective states and rat hedonic USVs are elicited by the same stimuli and have homologous neuroanatomical and molecular substrates and alternative non-hedonic interpretations of 50-kHz USVs are not supported by the available data (Burgdorf and Panksepp, 2006, Burgdorf et al., 2008).
GLYX-13 is a tetrapeptide (threonine-proline-proline-threonine) derived from one of the hypervariable regions of the monoclonal antibody, B6B21, and has been shown to act as a partial agonist at the glycine site of the NMDAR (Haring et al., 1991, Thompson et al., 1992, Moskal et al., 2005, Zhang et al., 2008, Burgdorf et al., 2009b). GLYX-13 shows preferential modulation of GluN2B containing NMDARs (Zhang et al., 2008). To date, GLYX-13 has been reported to: (1) enhance the magnitude of long-term potentiation of synaptic transmission while reducing long-term depression (Zhang et al., 2008); (2) significantly increase learning in a variety of hippocampus-dependent learning tasks including trace eyeblink conditioning and the Morris water maze in both young adult and learning-impaired aging rats (Burgdorf et al., 2009b); (3) markedly reduce delayed (24 hr) CA1 pyramidal neuronal cell death produced by bilateral carotid occlusion in Mongolian gerbils when administered up to 5 hr post-ischemia (Stanton et al., 2009); and (4) produce a significant antidepressant-like effect in the rat forced swim test (Burgdorf et al., 2010c) .
The present study was designed to evaluate the ability of GLYX-13 to facilitate play-induced hedonic 50-kHz USVs, as well as to examine the functional role of GluN2B-containing NMDARs for the generation of hedonic 50-kHz USVs in rats. Further, we evaluated the ability of GLYX-13 microinjections into the medial prefrontal cortex to facilitate positive emotional learning.
Experimental Procedures
Subjects
Rats were purchased from Harlan Sprague Dawley (USA) and housed in Lucite cages with corn cob or sawdust bedding, maintained on a 14:10 light:dark cycle (lights on 8 AM), and given ad libitum access to Purina lab chow and tap water throughout the study. Three different strains of rats were used in these studies. For the conspecific rough-and-tumble play studies, adolescent (1 month old) Long Evans (LE) rats were used given that such young rats exhibit the highest rates of social rough-and-tumble play behavior from among all rat strains tested (Panksepp et al., 1984, Burgdorf et al., 2010a). For analysis of well-controlled heterospecific play (i.e., ‘tickling’ by a human hand, simulating the play of rats, which can bring 50 kHz USVs under ‘stimulus-control’), 2–3 month old Sprague Dawley or F1 Fisher 344 X Brown Norway Rats (FBNF1) were used given that they exhibit especially high rates of adult heterospecific play induced 50-kHz USVs in adulthood (Burgdorf et al., 2010a). Adolescent heterospecific play was also examined in 1 month old LE rats selectively bred for high or low rates of 50-kHz USVs (Burgdorf et al., 2009a). Male 2–3 month old FBNF1 rats were used for the eyeblink conditioning studies given that they are the most widely used strain for such studies (Weiss et al., 1999a, Weiss et al., 1999b, Moskal et al., 2005, Burgdorf et al., 2009b). All experiments were approved by either Bowling Green State University or Northwestern University Animal Care and Use Committees.
Experiment 1. Effect of rough-and-tumble play on NMDA GluN1 and GluN2B mRNA and protein levels
We examined GluN1, GluN2A and GluN2B NMDA receptor subunit mRNA and protein levels in the frontal cortex (frontal pole anterior to +.2.70 mm from bregma excluding the olfactory bulb) and posterior cortex (dorsal cortex from −0.8 to −5.30 mm from bregma) following conspecific rough-and-tumble play or heterospecific rough-and-tumble play. Six hours after behavioral testing, animals were decapitated and their brains removed for subsequent determination of NMDA receptor mRNA levels by microarray and qRT-PCR and protein levels by Western analysis.
Rough-and-tumble play testing
Conspecific play and heterospecific play were conducted as previously described (Burgdorf et al., 2010a). For conspecific play studies, male or female 32-day old Long Evans rats were assigned to play pairs based on gender and weight matching and were individually placed in the homecage of a conspecific rat for 30 min (experimental group n = 12) or alone in a conspecific’s homecage (control group n = 6). During testing, animals were videotaped and high frequency ultrasonic vocalizations were recorded. Rates of play behavior (dorsal contacts and pins) as well as rates of ultrasonic vocalizations were scored by a blind experimenter with an inter-rater reliability of r > 0.9. All animals were sacrificed 6 hrs after testing, the brains rapidly dissected (~90 sec), frozen on dry ice and stored at -80°C until assayed as described in (Burgdorf et al., 2006). Conspecific play animals used for mRNA and protein studies represent the upper quartile of a larger group of animals based on high rates of rough-and-tumble play behavior (pins and dorsal contacts), high rates of hedonic 50-kHz USVs, and low rates of aversive 20-kHz USVs (Burgdorf et al., 2010a). The 6 hr time point was chosen given that we had previously reported robust changes in brain mRNA and protein levels following play (Burgdorf and Panksepp, 2006, Burgdorf et al., 2006). To control for social and somatosensory stimulation associated with play, 32 day old LE rats of both genders selectively bred for high rates of hedonic 50-kHz USVs and shown to exhibit a depression resilient phenotype (Burgdorf et al., 2009a) were used. Animals received 20 min of experimenter administered rough-and-tumble play stimulation (heterospecific play; experimental group n = 10) or 20 min of experimenter administered light touch stimulation (light touch; control group n = 8) using trials of 15 s stimulation followed by 15 s of no stimulation across the 20 min test session as described previously (Burgdorf and Panksepp, 2001). Animals were sacrificed 6 hrs after testing, the brains rapidly dissected (~90 s), frozen on dry ice and stored at −80oC until assayed.
Trace Eyeblink conditioning
Trace eyeblink conditioning (tEBC) was conducted exactly as described in(Burgdorf et al., 2009b). Male, 2–3 month old, FBNF1 rats were anesthetized with either a combination of xylazine (13 mg/kg, i.p.) and ketamine (87 mg/kg, i.p.), or isoflurane (to effect) and placed in a stereotaxic device. An incision was made on the top of the skull allowing for retraction of the periosteum. A total of six bilateral holes were drilled into the skull for insertion of stainless steel screws. A strip connector with two Teflon coated stainless steel wires and a non-insulated wire for an animal ground was then placed on the skull. EMG activity was recorded from the orbicularis oculi muscle via the recording wires, which were inserted underneath the skin until they penetrated the upper eyelid of the right eye. A tether holding a connector for relaying EMG activity and a tube for air puff delivery was attached to the strip connector. Dental cement was then placed around the connector and over the screws until the connector was firmly in place. Following surgery, animals were placed on a heating pad and given Buprenex (0.5 mg/kg, s.c.) to alleviate possible discomfort due to surgery. Animals were given a minimum of 5 days to recover before beginning the behavioral training. Rats received injections of 0.9% saline 10 min prior to habituation and each daily training session. All injections were given in a volume of 1 ml/kg. Animals were then subjected to trace or pseudo-conditioning paradigms, as described below. A habituation session preceded testing and was of equal duration to the training sessions. No stimuli were presented during the habituation session. During tEBC, rats received 10 sessions (1 session/day) of 30 paired presentations per session of an auditory conditioned stimulus (CS, 250 ms, 8 kHz, 85 dB, 5ms rise/fall) and, following a 250 ms trace interval, an air puff unconditioned stimulus (US, 100 ms, 4.5 psi [0.31 kg/cm2] corneal airpuff), with an inter-trial interval (ITI) of 20–40 s (30 s average). Testing for the pseudo conditioning group was identical to the tEBC animals except that the CS and USC stimuli were unpaired. Data were collected and analyzed as described previously (Weiss et al., 1999a, Weiss et al., 1999b, Burgdorf et al., 2009b).
Microarray analysis of gene expression
Microarray and data analysis were conducted as previously described (Kroes et al., 2006, Burgdorf et al., 2010a). Duplicate microarray analyses using frontal cortex (all cortical tissue anterior to +1 mm bregma) and posterior cortex (cortical tissue dorsal to hippocampus posterior to -1 mm bregma) isolated from individual rats 6 hrs after a 30 min play session (n = 6 play, n = 6 controls) were performed in a blind manner. Conspecific play animals were selected for the microarray study based on high rates of play behavior (pins and dorsal contacts), high rates of hedonic 50-kHz USVs and low rates of aversive 20-kHz USVs, with ~40% of all animals tested meeting these criterion. The 6 h time point was chosen given that we have previously reported robust changes in brain mRNA and protein levels following play (Burgdorf et al., 2006, Burgdorf et al., 2010a).
Individual 45-mer oligonucleotides complementary to sequences of 1178 cloned rat CNS mRNAs were synthesized on a PolyPlexTM 96-well oligonucleotide synthesizer (GeneMachines R, USA) and spotted in triplicate onto epoxy coated slides (Telechem, USA) using a OmniGridTM robotic microarrayer (GeneMachinesR). Total RNA was extracted (RNeasy, Qiagen, USA) and used as the substrate for RNA amplification and labeling using the Eberwine protocol (Van Gelder et al., 1990). Two micrograms of Cy5-labeled (experimental) and Cy3-labeled (universal rat reference, Stratagene, USA) amplified RNA (aRNA) were cohybridized on individual arrays at 46°C for 16 hours. Arrays were scanned using two lasers (633 nm and 543 nm) at 5 μm resolution on the ScanArray 4000XL (Packard Biochip Technologies, USA). Raw image files were normalized using locally weighted scatter plot smoothing (LOWESS) curve-fitting (GeneTraffic, USA). The data was analyzed by Significance Analysis of Microarrays (SAM) followed by data mining with Gene Ontology Miner (GoMiner) as described in (Burgdorf et al., 2006, Kroes et al., 2006).
Quantitative real-time PCR analysis
qRT-PCR was conducted as previously described (Burgdorf et al., 2006, Kroes et al., 2006). qRT-PCR was performed in a blind manner on frontal and posterior cortex of individual rats following play (n = 6 play, n = 6 controls) and dorsal hippocampus of individual rats receiving trace eyeblink conditioning (n = 9), pseudo eyeblink conditioning (n = 6), or naive controls (n = 6). The sequences of the qRT-PCR primers used in the study were as follows: GluN1 (NM_017010), forward 5'-ATGGCTTCTGCATAGACC-3' and reverse 5'-GTTGTTTACCCGCTCCTG-3'. GluN2A (NM_012573), forward 5'-AGTTCACCTATGACCTCTACC-3' and reverse 5'-GTTGATAGACCACTTCACCT-3'. GluN2B (NM_012574), forward 5'-AAGTTCACCTATGACCTTTACC-3' and reverse 5'-CATGACCACCTCACCGAT-3'.
Western blot assays
Western analyses were performed as previously described (Burgdorf et al., 2010a). Frontal and posterior cortex protein samples following either conspecific or heterospecific rough-and-tumble plays were separated by SDS-PAGE. Membranes were probed with GluN1 (sc-1467, 1:5000), GluN2A (sc-1468, 1:1000), GluN2B (sc-1469, 1:500 ), antibodies overnight at 4°C, followed by a 1 hr incubation at 25°C with an HRP conjugated secondary antibody (Santa Cruz Biotechnology, USA). Immunoreactive bands were visualized by enhanced chemiluminescence (Immun-Star HRP, Bio-Rad, USA) and developed on film (BioMax, Kodak, USA). Membranes were stained with Ponceau S (Sigma, USA) to ensure equal protein loading. Sample NMDA subunit protein levels were quantitated by comparison to the pooled dilution series using linear regression. All images were quantified by Image J (NIH, USA).
Experiment 2. Effect of NMDA GluN2B modulation on hedonic USVs
We examined the effect of the GluN2B preferring glycine site functional partial agonist (GFPA) GLYX-13, the GluN2B antagonist Ifenprodil and MK-801 on the acquisition of heterospecific play induced hedonic USVs. Of the two NMDAR glycine site partial agonists with therapeutic potential, GLYX-13 was used because it can selectively enhance GluN2B-containing NMDAR activity. During the intervening no-stimuli intervals, locomotor activity, as measured by line crosses, was also collected.
Heterospecific play testing
Male Sprague Dawley rats (250 g) housed 3 rats per cage were used in these studies. Twenty minutes before the start of testing, animals were given injections of GLYX-13 (1, 3, 10 mg/kg i.v.) or 0.9% sterile saline vehicle (1 ml/kg); MK-801 (0.025, 0.1, 0.25 mg/kg i.p.; Sigma, USA) or 0.9% sterile saline vehicle (1 ml/kg); the GluN2B selective antagonist Ifenprodil (1 mg / kg i.p.; Sigma, USA); (Williams, 1993) or DMSO vehicle (1 ml/kg) using a blind between subjects design (N = 7 – 10 per group).
Heterospecific rough-and-tumble play was conducted as previously described (Burgdorf and Panksepp, 2001, Burgdorf et al., 2010a), and consisted of vigorous whole-body playful stimulation that included repeated pinning of the animal. Heterospecific rough-and-tumble play stimulation was administered by the experimenter’s right hand. The experimenter was blind to the drug condition. Animals received 2–3 min of heterospecific rough-and-tumble play consisting of alternating 15 s blocks of heterospecific play (UCS) preceded with 15 s block with no play stimulation in which a audible click CS ( ~60 ms, ~ 15 db above baseline) that predicts the UCS was presented at the start of this no stimulation period. In a subset of animals, the experimenters hand was placed ~3 cm from the rat’s nose during the no stimulation period to provide an additional visual / olfactory CS. During the extinction study (figure 2B), animals received 3 min of heterospecific play as described above (acquisition) followed immediately by 4 X 15 s trials of CS only presentation (extinction).
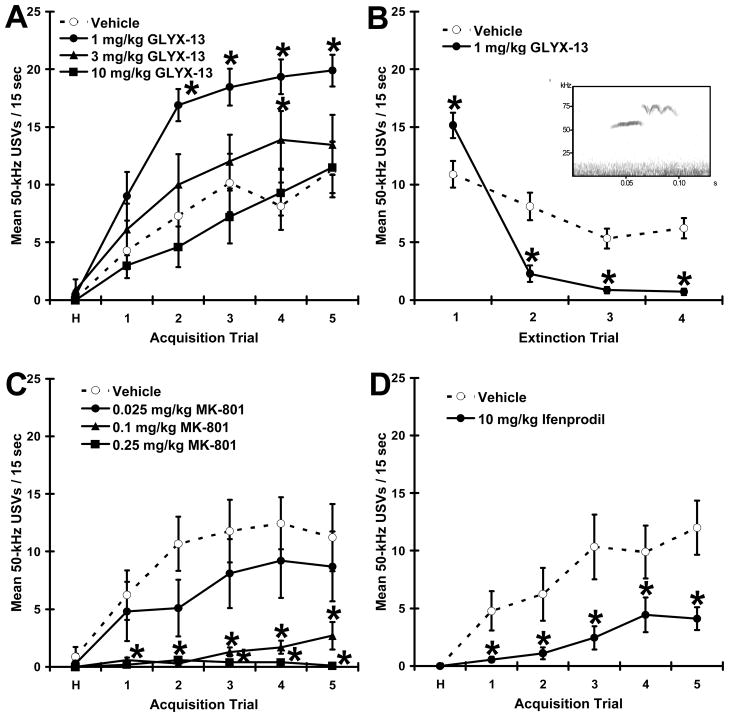
Figure 2. The GluN2B subunit plays a functional role in positive emotional learning.
Mean ± SEM rates of hedonic 50-kHz USVs in response to a conditioned stimulus that predicts heterospecific rough-and-tumble play in adult male rats pretreated with (A-B) the GluN2B preferring glycine site functional partial agonist GLYX-13 (1, 3, 10 mg/kg i.v.), (C) the NMDA receptor antagonist MK-801 (0.025, 0.1, .25 mg / kg i.p.), or (D) the GluN2B selective antagonist ifenprodil (10 mg/kg i.p.). Inset to Figure B depicts a sonogram of a representative hedonic 50-kHz USVs. N = 7-10 per group. * P < .05 Fisher PLSD post test vs. vehicle.
Selectively bred animals
One month old Long Evans rats of both genders selectively bred for low rates of heterospecific play-induced hedonic USVs (Burgdorf et al., 2005, Burgdorf et al., 2009a) were used in this study. Animals were injected subcutaneously with 0, 10, or 50 mg/kg GLYX-13 or 0.9% saline vehicle (n= 9 per group, within subjects design) 20 min before the start of testing in a blind manner. As a comparison, non-selected random line animals (n = 20 both genders) from the S14 generation that were tested for heterospecific play induced USVs as described in (Burgdorf et al., 2009a).
Ultrasonic vocalization recording and analysis
Methods used were identical to (Burgdorf et al., 2008, Burgdorf et al., 2010a). High frequency recordings of ultrasonic vocalizations were captured using a condenser microphone amplified by a bat detector (D980, Pettersson Elektronik, Sweden) and recorded with a Fostex FR2 field recorder (192 kHz sampling rate, 24 bit) onto compact flash cards (SanDisk, USA) as .wav files. Ultrasonic vocalizations were scored manually in a blind manner from sonograms generated by Avisoft SAS Lab Pro (Germany). Hedonic 50-kHz USVs, defined as having a peak frequency of greater than 40-kHz and a bandwidth greater than 18-kHz, were scored (Burgdorf et al., 2008). High inter- and intra-rater reliability for these measures (Pearson’s r > .90) has been established for this method (Burgdorf et al., 2008).
Experiment 3. Effect of medial prefrontal cortex injections of GLYX-13 on hedonic USVs
We tested the ability of MPFC (prelimbic and infralimbic cortex) injections of GLYX-13 to produce hedonic USVs, and the specificity of the effects using both dorsal control injections (primary and secondary motor cortex) and a scrambled peptide control injection into the MPFC.
Male 2–3 month old Sprague Dawley rats were allowed 1 week to recover from surgery before the start of testing. Animals were given injections of GLYX-13 (TPPT; 0.1, 1, 10 μg / side), scrambled peptide (1 μg / side PTPT; Bachem USA), or saline vehicle (0.5 μl / side) across 1 min using a blind between subjects design. Injection cannulae were left in place for an additional 0.5 min after injection. 20 min after injection, animals received 3 min of heterospecific play testing as described above (n = 10–11 per group, between subjects design).
Surgeries
Bilateral 22-gauge guide cannulae (Plastic Products, USA) were stereotaxically implanted into the medial prefrontal cortex (+2.7 mm anterior,± 1.2 mm lateral, 3.1 mm ventral to bregma angled 12° away from the midline) or motor cortex (+2.7 mm anterior,± 1.2 mm lateral, 1.0 mm ventral to bregma angled 12° away from the midline) under isoflurane anesthesia and secured to the skull with jewelers’ screws and dental cement as described in (Burgdorf et al., 2010a). Animals were allowed 1 week to recover from surgery before the start of testing.
Histology
Histology was conducted as described in (Burgdorf et al., 2001). After the completion of behavioral testing, animals were given microinjections of Evans blue (1% in sterile saline; Sigma, USA) to mark the cannulae tips, and brains were frozen on dry ice and stored at −80°C. Brains were sectioned (50 μm) on a cryostat, and cannulae tip locations were localized under light microcopy with the aid of an atlas (Paxinos and Watson, 2007). For medial prefrontal cannulae, all tips were located within the prelimbic or infralimbic cortex 2.2 – 3.2 mm anterior to bregma. For motor cortex cannulae, all tips were located within the primary or secondary motor cortex 2.2 – 3.2 mm anterior to bregma.
Results
Experiment 1: Effect of rough-and-tumble play on NMDA GluN1 and GluN2B mRNA and protein levels
Hedonic Ultrasonic vocalizations
Conspecific play elicited a 2238.3 ± 60.0 (Mean ± SEM) hedonic 50-kHz USVs / 30 min and heterospecific play elicited 1791.4 ± 50.3 (Mean ± SEM) hedonic 50-kHz USVs / 20 min.
Microarray
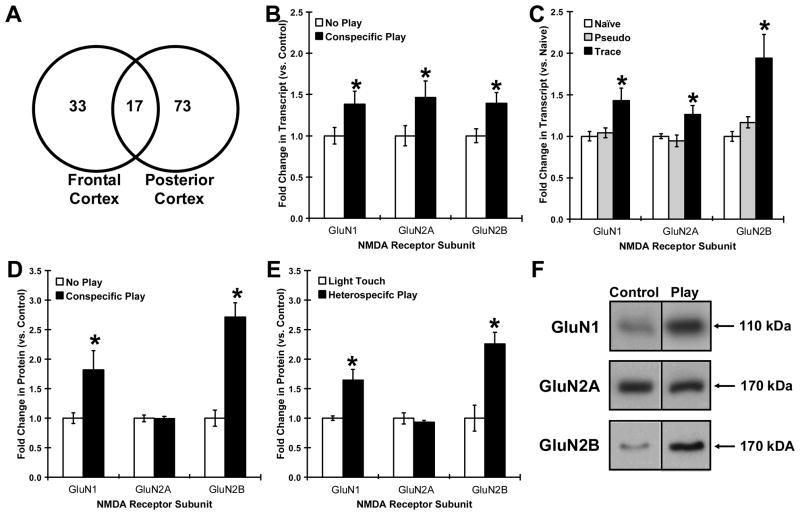
In order to profile gene expression associated with positive affective states associated with hedonic 50-kHz USVs, we examined gene expression changes in the frontal and posterior cortex following conspecific rough-and-tumble play. NMDA receptor subunit genes (GluN1, GluN2A-D, GluN3A-B) were significantly changed in the frontal and posterior cortex 6 hrs after conspecific rough-and-tumble play (1 changed gene – GluN1 / 10 total changed genes) compared to the chip set bias (7 NMDA receptor genes on chip / 1040 total genes on chip), representing a 14.9 fold enrichment (χ2 = 12.8, P < .0005). Similarly, a GoMiner analysis revealed a significant enrichment in the N-methyl-D-aspartate selective glutamate receptor complex (GO ID # 17146) in the co-expressed genes 6 hours after play (P < . 05).
qRT-PCR
As shown in figure 1A-B, conspecific play increased mRNA levels of GluN1, and GluN2B in the frontal and posterior cortex as measured by qRT-PCR as indexed by a significant main effect for play (F(1,16) = 6.5, P < . 05) followed by significant Fisher PLSD post hoc tests comparing play vs. control for GluN1, GluN2A, and GluN2B (all p’s < . 05; Figure 1A). Trace eyeblink conditioning also increased mRNA levels of GluN1, GluN2A and GluN2B in the hippocampus as measured by qRT-PCR as indexed by a significant group main effect (F(2,60) = 11.12, P < .001) followed by significant Fisher PLSD post hoc tests comparing the trace eyeblink group to all other groups (all p’s < .05; Figure 1B). The false discovery rate for each of the significant comparisons was less than 5%.
Figure 1. Both rough-and-tumble play and trace-eyeblink conditioning upregulate NMDA receptors in rats.
(A) Venn diagram of significantly changed genes in the frontal and posterior cortex 6 hrs after play, with NMDA receptors being significantly enriched in the 17 shared genes. Mean ± SEM mRNA levels (top panels) or protein levels (bottom panels) of GluN1, GluN2A and GluN2B NMDA receptor subunits following (B, D) conspecific rough-and-tumble play, (E) heterospecific rough-and-tumble play, or (C) trace eyeblink conditioning. (F) Representative Western analyses depicting GluN1, GluN2A and GluN2B NMDA receptor subunit expression following rough-and-tumble play. N = 6–12 per group. * P < .05 Fisher PLSD post hoc test vs. control.
Western blots
As shown in Figure 1C-D, conspecific play increased GluN1 and GluN2B protein levels in the frontal and posterior cortex as measured by Western blot and indexed by a significant main effect for play (F(1,16) = 42.9, P < . 0001; Figure 1 left panel) followed by significant Fisher PLSD post hoc tests comparing play vs. control for GluN1 and GluN2B (all p’s < . 05) but not GluN2A (P > .05). Heterospecific play also increased GluN1 and GluN2B protein levels in the frontal and posterior cortex as indexed by a significant main effect for play (F(1,16) = 17.7, P < . 001; Figure 1 right panel) followed by significant Fisher PLSD post hoc tests comparing play vs. control for GluN1 and GluN2B (all p’s < . 05). The false discovery rate for each of the significant comparisons was less than 5%. We also measured GluN2C-D and GluN3A-B protein levels in the frontal and posterior cortex by Western blot and found that protein levels for these subunits were not significantly altered in either heterospecific or conspecific play (all P’s > .05, data not shown).
Experiment 2: Effect of NMDA GluN2B modulation on hedonic USVs
As shown in Figure 2A, GLYX-13 (1 & 3 mg/kg i.v.) increased rates of hedonic USVs in response to a conditioned stimulus that predicts rough-and-tumble play across trials (main effect for drug F(3,31) = 5.41, P < .005; main effect for trial (F(5,31) = 75.06, P < .0001; drug X trial interaction (F(15,31) = 3.11, P < .0005); Fisher PLDS post hoc tests 1 or 3 mg/kg vs. vehicle, P < .05), without effecting locomotor behavior as measured by line crosses (P >.05 data not shown). As shown in figure 2B, GLYX-13 (1 mg/kg i.v.) increased the rate of extinction to a CS that no longer predicted rough-and-tumble play across trial blocks as measured by hedonic USVs (main effect for drug F(1,14) = 7.00, P < .05; main effect for trial (F(3,14) = 110.48, P < .001; drug X trial interaction (F(3,14) = 29.53, P < .001), without effecting locomotor behavior as measured by line crosses (P > .05). As shown in figure 2C, MK-801 (0.025, 0.1, .25 mg/kg) decreased rates of positive emotional learning (main effect for drug F(3,35) = 7.91, P < .001; main effect for trial (F(5,35) = 14.3, P < .001; drug X trial interaction (F(15,36) = 3.48, P < .001); Fisher PLDS post hoc tests for each MK-801 dose vs. vehicle, all P’s < .05), and only the 0.25 mg/kg dose significantly altered (increased) locomotor activity (main effect F(3,35) = 6.66, P < . 001; Fishers PLSD post hoc test 0.25 mg/kg dose vs. all other group, all P’s < .05). As shown in figure 2D, ifenprodil reduced rates of positive emotional learning (main effect for drug F(1,16) = 6.88, P < .05; main effect for trial (F(5,16) = 21.45, P < .001; drug X trial interaction (F(5,16) = 4.80, P < .001), without effecting locomotor behavior as measured by line crosses (P > .05).
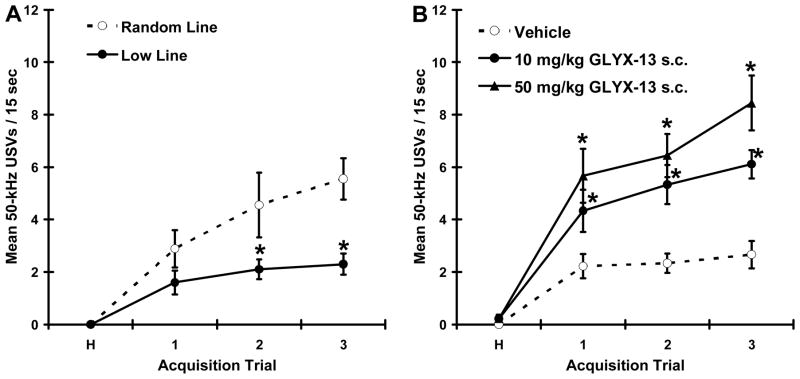
As shown in figure 3A, animals selectively bred for low rates of hedonic 50-kHz USVs show reduced rates of positive emotional learning as compared to wild type control animals (main effect for drug F(1,17) = 9.05, P < .01; main effect for trial (F(3,17) = 19.31, P < .001; drug X trial interaction (F(3,17) = 3.25, P < .05). As shown in figure 3B, GLYX-13 (10 & 50 mg/kg s.c.) increased rates of positive emotional learning in the low line animals (main effect for drug F(2,24) = 13.17, P < .001; main effect for trial (F(3,24) = 70.72, P < .001; drug X trial interaction (F(6,24) = 5.51, P < .001), without affecting locomotor behavior as measured by line crosses (P > .05). Following GLYX-13 (10 & 50 mg/kg s.c.) administration, rates of in low line animals were not significantly different from untreated wild type-animals (all p’s > .05).
Figure 3. Animals selectively bred for low rates of hedonic 50-kHz USVs show deficits in positive emotional learning that are rescued by GLYX-13.
Mean ± SEM rates of hedonic 50-kHz USVs in response to a conditioned stimulus that predicts heterospecific rough-and-tumble play in adolescent rats (A) selectively bred from low rates of 50-kHz USVs or wild type (random line) controls; (B) Low line rats pretreated with GLYX-13 (10, 50 mg/kg i.p.). N = 9–10 per group. * P < .05 Fisher PLSD post test vs. vehicle.
Experiment 3: Effect of medial prefrontal cortex injections of GLYX-13 on hedonic USVs
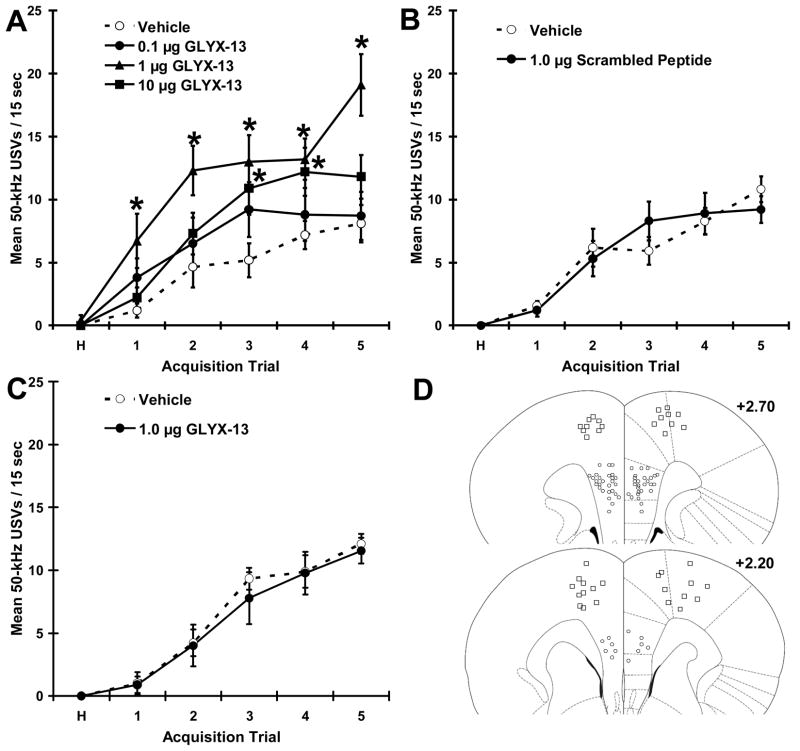
As shown in Figure 4A, MPFC injections of GLYX-13 (1 & 10 μg / side) increased rates of hedonic USVs (main effect for drug F(3,36) = 8.55, P < .001; main effect for trial (F(5,36) = 35.09, P < .001; drug X trial interaction (F(15,36) = 7.22, P < .001); Fisher PLDS post hoc tests 1 or 10 μg vs. vehicle, P < .05), without effecting locomotor activity (P > .05). As shown in Figure 4B-C, neither MPFC injection of a scrambled peptide (1 μg / side; 4B) nor dorsal control injection of GLYX-13 (1 μg / side; 4C) altered rates of positive emotional learning or locomotor behavior (all p’s >.05).
Figure 4. Medial prefrontal cortex injections of GLYX-13 increase positive emotional learning.
Mean ± SEM Rates of Hedonic 50-kHz USVs in response to a conditioned stimulus that predicts heterospecific rough-and-tumble play in adult male rats pretreated with infralimbic / prelimbic cortex injections of (A) GLYX-13 (0.1, 1, 10 μg / side), (B) injection of a scrambled peptide variant of GLYX-13 (1 μg / side), or (C) injection of GLYX-13 (1 μg / side) into the primary or secondary motor cortex. (D) Anatomical location of the cannulae tips in the rat brain using the atlas of (Paxinos and Watson, 2007); medial prefrontal cortex, mostly prelimbic region (● ), Motor cortex (□).N = 9 - 11 per group. * P < .05 Fisher PLSD post test vs. vehicle.
Discussion
It has been well documented that GluN2B containing NMDA receptor play a functional role in spatial / temporal learning in the hippocampus. We now demonstrate that GluN2B-containing NMDAR play a functional role in the expression of positive emotional learning in the medial prefrontal cortex as indexed by hedonic 50-kHz vocalizations. First, activation of GluN2B-containing NMDAR by GLYX-13 increased and inhibition of GluN2B-containing NMDAR by ifenprodil decreased positive emotional learning. Second, GLYX-13 also facilitated extinction of positive emotional learning. Third, GLYX-13 facilitation of positive emotional learning was localized to the infralimbic and prelimbic cortex. Fourth, animals selectively bred for low rates of hedonic could be returned to wild-type levels of positive emotional learning following GLYX-13 treatment. And fifth, rough-and-tumble play increased cortical mRNA and protein levels of the GluN1 and GluN2B subunits of the NMDAR.
Hedonic 50-kHz USVs have been shown to be specific to positive affective states. Positive affective stimuli are specific in their ability to elevate rates of hedonic 50-kHz USVs, whereas negative affective stimuli uniformly reduce rates of hedonic USVs (Burgdorf and Panksepp, 2006). Hedonic 50-kHz USVs are specific to positively valenced incentive states given that 50 kHz USVs are increased during the anticipation of delivered reward, which in humans has been shown to elicit a positive affective state (Knutson et al., 2001); whereas during extinction bursts or “frustrative non-reward” such appetitive behavior decreases rates of 50-kHz calls and increase rates of aversive 20-kHz calls (Burgdorf et al., 2000). Hedonic USVs can be dissociated from locomotor behavior with only 10% of 50-kHz USVs coincident with thoracic compressions associated with locomotor activity (Panksepp and Burgdorf, 2003). 50-kHz USVs that occur during aggressive encounters are primarily the non-hedonic flat 50-kHz USVs variety and the few hedonic 50-kHz USVs that are evident occur before the onset of aggressive behavior (Burgdorf et al., 2008). In contrast, greater than 95% of all 50-kHz USVs that occur during play behavior are hedonic USVs (Burgdorf et al., 2008). Lastly, hedonic USVs can be dissociated from arousal states; highly arousing aversive stimuli decrease rates of 50-kHz calls, whereas highly arousing rewarding stimuli increase rates of 50-kHz calls (Burgdorf and Panksepp, 2006, Burgdorf et al., 2008).
The medial prefrontal cortex has been implicated in positive affective states in humans and hedonic USVs in rats (Burgdorf and Panksepp, 2006; Burgdorf et al., 2007). Deep brain stimulation of the medial prefrontal cortex in depressed patients increases positive affective states and decreases negative affective states (Mayberg et al., 2005), and positive affective states increase neuronal activity in the medial prefrontal cortex as measured by fMRI or PET brain imaging (Damasio et al., 2000, Blood and Zatorre, 2001). In laboratory rats, electrical brain stimulation of the medial prefrontal cortex robustly elicits hedonic USVs and supports self-stimulation (Burgdorf et al., 2007).
Both hippocampus-dependent spatial / temporal learning, and amygdala-dependent fear conditioning are increased by the NMDAR-specific, glycine site partial agonist, D-cycloserine, and blocked by the pan-NMDA receptor antagonist, MK-801 or GluN2B-specific antagonists such as ifenprodil (Baker and Azorlosa, 1996, Thompson and Disterhoft, 1997a, b, Gould et al., 2002, Walker et al., 2002, Sotres-Bayon et al., 2007). Further, transgenic mice that overexpress forebrain GluN2B-containing NMDARs showed increased LTP and in vivo learning, whereas hippocampal knockdown of GluN2B-containing NMDARs showed decreased LTP and in vivo learning (Tang et al., 1999, Clayton et al., 2002). It is unlikely that the reduction in hedonic USVs caused by ifenprodil was caused by its actions on adrenergic receptors. The NMDAR antagonist, MK-801, which does not affect adrenergic receptors, also decreased rates of hedonic USVs comparable to ifenprodil.
GluN2B-containing NMDARs may play a similar role in positive emotional learning in the MPFC as they do in spatial / temporal learning in the hippocampus. GLYX-13 (1 mg/kg i.v.) facilitates both positive emotional learning and facilitates hippocampus-dependent tEBC, Morris water maze learning, and learning in an appetitive food motivation version of the alternating t-maze test (Burgdorf et al., 2009b). Moreover in positive emotional learning and tEBC, both GluN1 and GluN2B subunits of NMDARs were selectively and significantly elevated.
Partial agonists typically show inverted U-shaped dose response curves. As expected, higher doses of GLYX-13 did not consistently facilitate learning. Previous reports with D-cycloserine also show similar inverted U-shaped curves in learning paradigms (Lanthorn, 1994). It should also be noted that the reason that the subcutaneous doses of GLYX-13 required to facilitate positive emotional learning were higher than the intravenous doses is because of the effect of the route of administration on bioavailability of the compound.
Both heterospecific and conspecific play increased cortical GluN1 and GluN2B cortical protein levels to a similar extent and, moreover, both groups elicited nearly maximal rates of hedonic 50-kHz USVs. Thus we suggest that the similarity in protein levels was due to a ceiling effect as reflected by the 50-kHz USVs data.
It is possible that the peripheral injections of the GluN2B-specific antagonist, ifenprodil, inhibited positive emotional learning-associated NMDA receptors outside the medial prefrontal cortex. Thus it would be premature to state that only GluN2B-containing NMDARs located in the MPFC are associated with positive emotional learning. Nevertheless, the significant changes in NR1 and GluN2B mRNA in the MPFC along with the increases in learning seen with direct MPFC injection of GLYX-13 do show that GluN2B-containing NMDARs nevertheless play an important role in positive emotional learning.
NMDA receptors have numerous functions in the PFC that are not related to plasticity (Homayoun and Moghaddam, 2007). Thus we cannot rule out that MPFC NMDARs may facilitate positive emotional learning through modulation of non-synaptic plasticity-dependent mechanisms as well.
GLYX-13 was chosen for the studies reported here for several reasons. GLYX-13 unlike DCS is a GluN2B-preferring, NMDAR glycine site partial agonist (Haring et al., 1991, Thompson et al., 1992, Moskal et al., 2005, Zhang et al., 2008, Burgdorf et al., 2009b). GLYX-13 is unique among NMDAR modulators in its ability to simultaneously facilitate in vitro hippocampal LTP and reduce hippocampal LTD (Zhang et al., 2008). GLYX-13 (1 mg/kg i.v.) also facilitated learning and memory in a series of in vivo hippocampus-dependent learning and memory tasks in both young adult and learning-impaired aging rats (Burgdorf et al., 2009b), and at higher doses (3–10 mg/kg i.v.) produces an antidepressant-like effect in the rat Porsolt swim test (Burgdorf et al., 2010b).
Conclusions
Positive emotional learning in the MPFC, like spatial / temporal learning in the hippocampus, appears to all be mediated in part by GluN2B-containing NMDARs. Thus the mechanisms that regulate positive emotional learning appear to have some mechanistic similarities to those that govern synaptic plasticity in the hippocampus.
Acknowledgments
This research was supported by the Hope for Depression Research Foundation, New York, NY (JB, JP, and JRM), The Ralph and Marian Falk Medical Research Trust, Chicago IL (JRM), and R01NS059879 to CW and R01MH047340 to JFD. We thank Northwestern University Behavioral Phenotyping Core for its assistance. We thank Ms. Mary Schmidt for her expert technical assistance and Dr. J. David Leander for his expert advice and critique of this manuscript.
References
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114:320–327. [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Beinfeld MC, Panksepp J, Moskal JR. Uncovering the molecular basis of positive affect using rough-and-tumble play in rats: a role for insulin-like growth factor I. Neuroscience. 2010a;168:769–777. doi: 10.1016/j.neuroscience.2010.03.045. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122:357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72:167–173. doi: 10.1016/s0031-9384(00)00411-x. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neurosci Biobehav Rev. 2006;30:173–187. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Beinfeld MC, Kroes RA, Moskal JR. Regional brain cholecystokinin changes as a function of rough-and-tumble play behavior in adolescent rats. Peptides. 2006;27:172–177. doi: 10.1016/j.peptides.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Brudzynski SM, Beinfeld MC, Cromwell HC, Kroes RA, Moskal JR. The effects of selective breeding for differential rates of 50-kHz ultrasonic vocalizations on emotional behavior in rats. Dev Psychobiol. 2009a;51:34–46. doi: 10.1002/dev.20343. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Brudzynski SM, Kroes R, Moskal JR. Breeding for 50-kHz positive affective vocalization in rats. Behav Genet. 2005;35:67–72. doi: 10.1007/s10519-004-0856-5. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Moskal JR. Frequency-modulated 50kHz ultrasonic vocalizations a tool for uncovering the molecular substrates of positive affect. Neurosci Biobehav Rev. 2010b doi: 10.1016/j.neubiorev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Westrich L, Sprouse J, Kroes R, Burch R, Moskal J. The antidepressant and anxiolytic properties of GLYX-13: A novel NMDA receptor glycine site functional partial agonist Society for Neuroscience Abstract. 2010c. p. 572.23. [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Weiss C, Matthews E, Disterhoft JF, Stanton PK, Moskal JR. The N-methyl-d-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol Aging. 2009b doi: 10.1016/j.neurobiolaging.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. J Pers Soc Psychol. 2003;84:365–376. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, McCarthy MM, Keith RA. MK-801 disrupts acquisition of contextual fear conditioning but enhances memory consolidation of cued fear conditioning. Behav Pharmacol. 2002;13:287–294. doi: 10.1097/00008877-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Haring R, Stanton PK, Scheideler MA, Moskal JR. Glycine-like modulation of N-methyl-D-aspartate receptors by a monoclonal antibody that enhances long-term potentiation. J Neurochem. 1991;57:323–332. doi: 10.1111/j.1471-4159.1991.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes RA, Panksepp J, Burgdorf J, Otto NJ, Moskal JR. Modeling depression: social dominance-submission gene expression patterns in rat neocortex. Neuroscience. 2006;137:37–49. doi: 10.1016/j.neuroscience.2005.08.076. [DOI] [PubMed] [Google Scholar]
- Lanthorn TH. D-Cycloserine - Agonist Turned Antagonist. Amino Acids. 1994;6:247–260. doi: 10.1007/BF00813745. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: does happiness lead to success? Psychol Bull. 2005;131:803–855. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Moskal JR, Kuo AG, Weiss C, Wood PL, O'Connor Hanson A, Kelso S, Harris RB, Disterhoft JF. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005;49:1077–1087. doi: 10.1016/j.neuropharm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Burgdorf J. "Laughing" rats and the evolutionary antecedents of human joy? Physiol Behav. 2003;79:533–547. doi: 10.1016/s0031-9384(03)00159-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam ; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Potter PE, Aguilar J, Decandia M, Moskal JR. Neuroprotection by a novel NMDAR functional glycine site partial agonist, GLYX-13. Neuroreport. 2009;20:1193–1197. doi: 10.1097/WNR.0b013e32832f5130. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Disterhoft JF. Age- and dose-dependent facilitation of associative eyeblink conditioning by D-cycloserine in rabbits. Behav Neurosci. 1997a;111:1303–1312. doi: 10.1037//0735-7044.111.6.1303. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Disterhoft JF. N-methyl-D-aspartate receptors in associative eyeblink conditioning: both MK-801 and phencyclidine produce task- and dose-dependent impairments. J Pharmacol Exp Ther. 1997b;281:928–940. [PubMed] [Google Scholar]
- Thompson LT, Moskal JR, Disterhoft JF. Hippocampus-dependent learning facilitated by a monoclonal antibody or D-cycloserine. Nature. 1992;359:638–641. doi: 10.1038/359638a0. [DOI] [PubMed] [Google Scholar]
- Van Gelder RN, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci U S A. 1990;87:1663–1667. doi: 10.1073/pnas.87.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav Brain Res. 1999a;99:123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Weiss C, Knuttinen MG, Power JM, Patel RI, O'Connor MS, Disterhoft JF. Trace eyeblink conditioning in the freely moving rat: optimizing the conditioning parameters. Behav Neurosci. 1999b;113:1100–1105. doi: 10.1037//0735-7044.113.5.1100. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Zhang XL, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008;55:1238–1250. doi: 10.1016/j.neuropharm.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]