Abstract
Background
IMC-A12, a fully human antibody that blocks ligand binding to the Type 1 insulin-like growth factor receptor, and rapamycin, a selective inhibitor of mTORC1 signaling, have both demonstrated significant antitumor activity against PPTP solid tumor models. Here we have evaluated antitumor activity of each agent individually and in combination against nine tumor models.
Procedures
IMC-A12 was administered twice weekly and rapamycin was administered daily for 5 days per week for a planned 4 weeks. The impact of combining IMC-A12 with rapamycin was evaluated using two measures: 1) the “therapeutic enhancement” measure, and 2) a linear regression model for time-to-event to formally evaluate for sub- and supra-additivity for the combination compared to the agents used alone.
Results
Two osteosarcomas, and 1 Ewing sarcoma of the nine xenografts tested showed therapeutic enhancement. The combination effect was most dramatic for EW5 for which PD2 responses of short duration were observed for both single agents and a prolonged PR response was observed for the combination. Both OS-2 and OS-9 showed significantly longer times to progression with the combination compared to either of the single agents, although objective response criteria were not met.
Conclusions
The combination of IMC-A12 with rapamycin was well tolerated, and induced tumor responses that were superior to either single agent alone in several models. These studies confirm reports using other antibodies that inhibit IGF-1 receptor-mediated signaling that indicate enhanced therapeutic effect for this combination, and extend the range of histotypes to encompass additional tumors expressing IGF-1R where this approach may be effective.
Keywords: Preclinical Testing, Developmental Therapeutics, Rapamycin, IMC-A12
Introduction
Rapamycin inhibits the proliferation of many tumor cell lines in vitro including cell lines derived from childhood cancers [1,2], and it showed significant antitumor activity against syngeneic tumor models in the NCI in vivo screening program [3] and against childhood cancer xenografts [2]. In our previous study rapamycin induced significant differences in event free survival (EFS) distribution in 28 of 36 solid tumor xenografts and in 5 of 8 ALL xenografts, and objective responses were observed in several panels [2]. Rapamycin and related mTOR inhibitors have also been shown to have antiangiogenic activity [4].
The rapamycin analogs temsirolimus (CCI-779) and everolimus (RAD001) have been approved for treatment of refractory renal cell carcinoma [5,6], and temsirolimus demonstrates a high response rate against mantle cell lymphoma at relapse [7]. Both temsirolimus and everolimus have completed phase I trials in pediatric patients [8]. While the efficacy of rapamycin or its analogs is being assessed in phase II trials, their integration into current chemotherapy regimens used for treatment of childhood cancers would appear to be a logical progression in their clinical development [9].
Insulin-like growth factor I receptor (IGF1R) has long been recognized as biologically relevant in the pediatric malignancies. Signaling through IGF1R is mediated by IGF-1 and IGF-2. Tissue samples and cell lines derived from both alveolar and embryonal rhabdomyosarcoma consistently over-expression of IGF-2 [10,11] and IGF1R [12]. Overexpression studies in C2C12 myoblasts show that PAX3-FKHR interacts with IGF-2 to play a critical role in the oncogenesis of rhabdomyosarcoma [13]. In Ewing sarcoma cell lines and patient-derived tumors, IGF-1 and IGF1R are consistently expressed, suggesting the potential for autocrine growth stimulation [14]. Mesenchymal cells transformed by EWS-FLI-1 increase IGF-1 secretion and are dependent on IGF1R signaling for growth and survival [15]. In neuroblastoma, primary neuroblastoma tumor specimens express IGF-2 and IGF1R mRNA [16,17], and inhibition of IGF1R blocks the mitogenic effects of IGF-1 and IGF-2 on cultured neuroblastoma cell lines providing further evidence for the role of IGF1R in pediatric solid tumors [18]. The Pediatric Preclinical Testing Program (PPTP) has recently evaluated IGF1R targeted monoclonal antibodies capable of inhibiting the binding of IGF-1 and/or IGF-2. Promising activity has been reported for IGF1R targeted therapies for Ewing sarcoma, rhabdomyosarcoma, osteosarcoma and neuroblastoma xenografts [19–21].
It has been previously reported for Ewing sarcoma, rhabdomyosarcoma and osteosarcoma that inhibition of mTOR may increase the dependency of tumors on IGF signaling [21–24]. Inhibitors of IGF1R act synergistically with rapamycin in sarcoma xenografts by inhibiting hyperphosphorylation of Akt in response to mTOR inhibition [24]. IMC-A12 is a fully human IgG1 monoclonal antibody that specifically blocks IGF1R, and it has completed pediatric phase I testing [25]. The current report includes a thorough evaluation of IMC-A12 in combination with rapamycin in an abbreviated panel of PPTP solid tumor xenografts.
Materials and Methods
In vivo tumor growth inhibition studies
CB17SC scid−/− female mice (Taconic Farms, Germantown, NY) were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described [26–28]. Tumor volumes (cm3) [solid tumor xenografts] or percentages of human CD45-positive [hCD45] cells [ALL xenografts] were determined as previously described [29]. Responses were determined using three activity measures as previously described [29].
The Stage 2 testing plan called for 4 weeks of treatment using the same doses and schedules for rapamycin and IMC-A12 as were used for evaluating their single agent activity: rapamycin by intraperitoneal (IP) administration at 5 mg/kg daily × 5 repeated weekly and IMC-A12 by IP administration at a dose of 1 mg per mouse administered twice weekly. The PPTP study design of limiting treatment to 4 weeks for both IMC-A12 and for rapamycin was utilized to better evaluate whether there was a benefit from use of the combination compared to the agents used alone. Table 1 lists the xenografts for which the PPTP evaluated the combination of IMC-A12 and rapamycin. In the table, rapapmycin (R) and IMC-A12 (IMC) following the xenograft names designate the data for single agent rapamycin treatment and single agent IMC-A12 treatment, respectively. One focus of the combination testing was on rhabdomyosarcoma, as this was the histology for which the most consistent single agent activity for IMC-A12 was observed. Another goal was to develop data for other histologies, particularly where other IGF-1-targeted antibodies have shown activity (e.g. sarcomas) as potent combination activity could promote clinical development of the combination for these diagnoses.
Table I.
Single agent IMC-A12 results for rapamycin and IMC-A12 in tested xenografts1
| Xenograft | Panel | KM Estimate of Median Time to Event | P-value | EFS T/C | Response |
|---|---|---|---|---|---|
| EW5 (R) | Ewing | 26.4 | <0.001 | 4.9 | PD2 |
| EW5 (IMC) | Ewing | 17.6 | <0.001 | 2.6 | PD2 |
| Rh28 (R) | ALV RMS | 18.8 | 0.282 | 1.2 | PD1 |
| Rh28 (IMC) | ALV RMS | > EP | <0.001 | > 2.8 | MCR |
| Rh30 (R) | ALV RMS | 28.9 | <0.001 | 2.4 | PD2 |
| Rh30 (IMC) | ALV RMS | 30.5 | 0.004 | 2.3 | PD2 |
| Rh41 (R) | ALV RMS | 11.7 | <0.001 | 1.7 | PD2 |
| Rh41 (IMC) | ALV RMS | 38.6 | <0.001 | 3.3 | PD2 |
| NB-1643 (R) | Neuroblastoma | 19.7 | 0.028 | 1.3 | PD1 |
| NB-1643 (IMC) | Neuroblastoma | 26.3 | <0.001 | 4.2 | PD2 |
| NB-EBc1 (R) | Neuroblastoma | 24.7 | 0.019 | 2 | PD2 |
| NB-EBc1 (IMC) | Neuroblastoma | 11 | 0.041 | 2 | PD2 |
| OS-2 (R) | Osteosarcoma | > EP | <0.001 | > 2.7 | PD2 |
| OS-2 (IMC) | Osteosarcoma | 34.2 | <0.001 | 2.1 | PD2 |
| OS-9 (R) | Osteosarcoma | > EP | 0.002 | > 1.3 | PD2 |
| OS-9 (IMC) | Osteosarcoma | > EP | <0.001 | > 2.3 | PD2 |
| GBM2 (R) | Glioblastoma | 18.5 | <0.001 | 2.6 | PD2 |
| GBM2 (IMC) | Glioblastoma | 21.7 | <0.001 | 1.4 | PD1 |
In Vivo Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS®, was used to compare event-free survival distributions between treatment and control groups and between combinations and respective single-agent treatment groups. P-values were two-sided.
Therapeutic Enhancement
Therapeutic enhancement represents a therapeutic effect achieved with a tolerated regimen of a combination treatment that exceeds the optimal effect achieved at any tolerated dose of monotherapy associated with the same drugs used in the combination [30]. This definition was operationalized as follows [31]: Briefly, therapeutic enhancement was considered present when the tumor growth delay (T-C) for a combination was greater than the tumor growth delay for both of the single agents tested at their MTD and when the EFS distribution for the combination treatment was significantly better (p<0.01) than the EFS distributions for both of the single agents tested at their MTD. Testing was considered not evaluable for therapeutic enhancement when the agent used alone and in combination produced median EFS beyond the observation period. If a treatment group exhibited excessive toxicity (>25% toxic deaths), therapeutic enhancement was not evaluated.
Model-Based Analysis
A linear regression model for time-to-event (T) was employed, with testing to determine whether the treatment interaction of the two-drug combination is significantly different from 0. Time-to-event is defined as the interval between study initiation and event (4 times initial tumor volume in solid tumor lines). Mice without events at the end of the study period were censored and the corresponding times to event were treated as censored observations in the EFS analysis. The interaction model was as follows: T = a0 + a1*I1 + a2*I2 + a3*I1*I2 in which I1 and I2 are the indicator functions for drug 1 and drug 2, respectively. The coefficients are for the no-treatment control (a0), the two drug effects relative to control (a1 and a2), individually, and the treatment interaction effect of the two drugs relative to control (a3). Whether the value of a3 is significantly greater or less than 0 (p < 0.01) indicates supra-additivity or sub-additivity of the drug combination, respectively. Otherwise, the drug combination was considered additive. In addition, the estimated values for these coefficients are the estimated times (or additional times) to event associated with the corresponding treatment effects. To allow comparison of the a3 values across xenografts, the values were normalized by using the ratio of a3 to the expected additive effect for the combination under the assumption of additivity (i.e., a1 + a2). This “normalized interaction term” can be conceptualized as representing the percentage gain or loss of the expected treatment effect observed for the combination under additivity. If any animals were censored in any treatment group, then the combination was not considered evaluable unless the censoring occurred exclusively in the combination group and the combination effect was supra-additive or unless the censoring occurred exclusively in one or both single agent groups and the combination effect was sub-additive.
Drugs and Formulation
Rapamycin was purchased from LC Laboratories (Woburn, MA). Rapamycin was dissolved in DMSO (5% final concentration) and diluted in 5% Tween 80 in water and administered IP daily × 5 for 4 consecutive weeks at a dose of 5 mg/kg. IMC-A12 was provided by ImClone Systems and was diluted in sterile saline and administered at 1 mg per mouse twice weekly. Treatment was for a planned four consecutive weeks. Female mice were used irrespective of the gender from which the tumor was derived, and ten mice per group were used.
Results
IMC-A12 was tested alone or in combination with rapamycin against nine solid tumor models. The models were selected based on some level of significant growth inhibition to single agent IMC-A12, or rapamycin (Table I). Twelve of 400 mice died during the study (3.0%), with 0 of 100 in the control arm (0%), 2 of 100 (2.0%) in both the IMC-A12 and rapamycin arms, and 8 of 100 in the combination treatment arm (8.0%). As most of the toxicity was seen in a single combination treatment group these may not be related to treatment as toxicity was not observed in repeat testing. A complete summary of results is provided in Supplemental Table I, including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values. Results for each of single agent and for the combination the treatment minus control (T-C) values, the objective response categories (in parentheses), the p-value for the comparison of the combination to each of the single agent treatments, and whether therapeutic enhancement was attained are presented in Table II. NB-1643 testing was repeated to confirm the single agent CR activity for single agent IMC-A12, as this result was better than observed for single agent IMC-A12 during Stage 1 testing in which good growth control in the absence of regression was noted [32].
Table II.
Enhancement of Rapamycin with IMC-A12
| Xenograft | IMC-A12 T-C | RAP T-C | RAP+IMC T-C | P-value [combination vs. RAP] | P-value [combination vs. IMC-A12] | Therapeutic Enhancement |
|---|---|---|---|---|---|---|
|
| ||||||
| EW5 | 12.6 | 10.7 | 60.1 | <0.01 | <0.01 | YES |
| (PD2) | (PD2) | (PR) | ||||
|
| ||||||
| Rh28 | >64.8 | 20.8 | >64.8 | <0.01 | 0.44 | NE |
| (MCR) | (PD2) | (MCR) | ||||
|
| ||||||
| Rh30 | 5.8 | 7.5 | 11.1 | 0.77 | <0.01 | NO |
| (PD2) | (PD2) | (PD2) | ||||
|
| ||||||
| Rh41 | 36.0 | 8.8 | 50.3 | <0.01 | 0.03 | NO |
| (PD2) | (PD2) | (CR) | ||||
|
| ||||||
| GBM2 | 0.1 | 20.6 | 22.4 | 0.84 | <0.01 | NO |
| (PD1) | (PD2) | (PD2) | ||||
|
| ||||||
| NB-EBc1 | 7.2 | 6.6 | 17.2 | 0.04 | 0.07 | NO |
| (PD2) | (PD2) | (PD2) | ||||
|
| ||||||
| NB-1643 | 59.4 | 5.4 | 70.8 | <0.01 | 0.10 | NO |
| (CR) | (PD2) | (CR) | ||||
|
| ||||||
| OS-2 | 26.2 | 21.7 | 38.3 | <0.01 | <0.01 | YES |
| (PD2) | (PD2) | (PD2) | ||||
|
| ||||||
| OS-9 | 14.5 | 15.8 | 31.0 | <0.01 | <0.01 | YES |
| (PD2) | (PD2) | (PD2) | ||||
Three of the eight xenografts tested showed therapeutic enhancement (EW-5, OS-2, and OS-9), while a fourth (Rh41) approached meeting criteria for therapeutic enhancement Figure 1. The combination effect was most dramatic for EW5 for which PD2 responses of short duration were observed for both single agents and a prolonged PR response was observed for the combination. Both OS-2 and OS-9 showed significantly longer times to progression with the combination compared to either of the single agents, although objective response criteria (PR or CR) were not met for either xenograft.
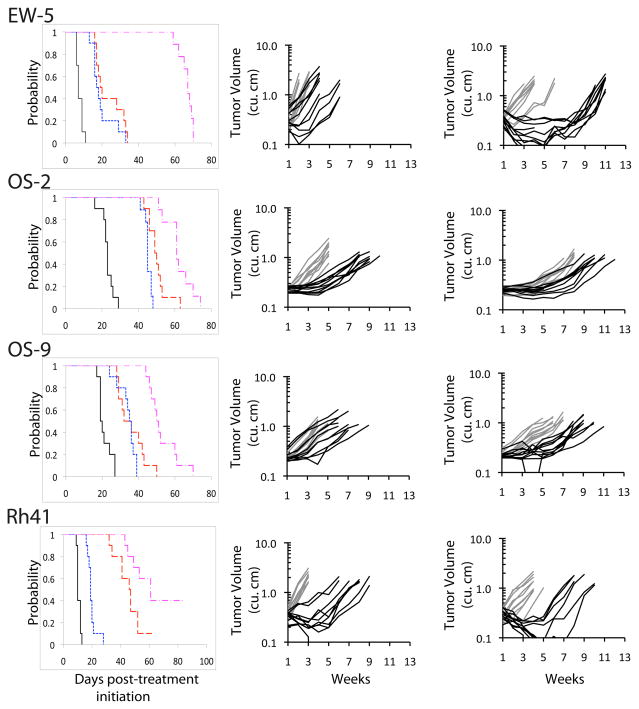
Figure 1.
Responses of sarcoma xenografts to IMC-A12 and rapamycin administered as single agents or in combination. Tumor-bearing mice were treated with IMC-A12 by IP administration at a dose of 1 mg per mouse administered twice weekly, and rapamycin by intraperitoneal (IP) administration at 5 mg/kg daily × 5 repeated weekly for a planned four consecutive weeks.
Left panels: Kaplan-Meier Event-Free Survival estimates for control and treated groups. Control (_____); IMC-A12 (
 ); Rapamycin (
); Rapamycin (
 ); IMC-A12 + rapamycin (
); IMC-A12 + rapamycin (
 ). Center panels: Individual tumor growth curves. Controls (gray), IMC-A12 treated (black). Right panels: Individual tumor growth curves. Rapamycin (gray), IMC-A12 + rapamycin (black).
). Center panels: Individual tumor growth curves. Controls (gray), IMC-A12 treated (black). Right panels: Individual tumor growth curves. Rapamycin (gray), IMC-A12 + rapamycin (black).
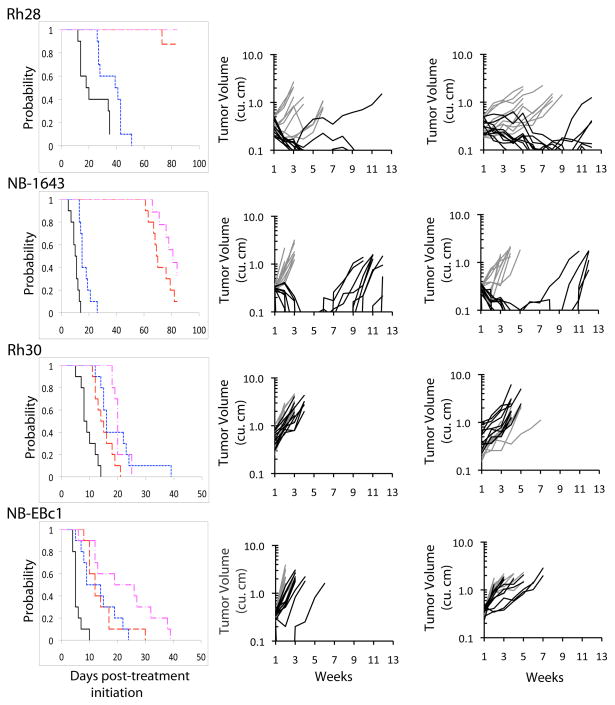
A second pattern of response was noted for Rh28 and NB-1643, for which single agent IMC-A12 was so effective that it was difficult to detect an enhancement in activity for the addition of rapamycin Figure 2. For both of these xenografts, the regression achieved with 4 weeks of treatment was maintained through most or all of the total 12 week monitoring period. A third pattern of response was observed for Rh30, NB-EBc1 and GBM2. Rh30 showed little or no response to either rapamycin or IMC-A12, NB-EBc1 showed limited responses to each agent used alone (Figure 2), and GBM2 showed no response to IMC-A12 and modest growth inhibition to rapamycin. For each of these xenografts, the effect of the combination was unremarkable and not significantly better than the response to single agent rapamycin.
Figure 2.
Responses of sarcoma and neuroblastoma xenografts to IMC-A12 and rapamycin administered as single agents or in combination. Tumor-bearing mice were treated with IMC-A12 by IP administration at a dose of 1 mg per mouse administered twice weekly, and rapamycin by intraperitoneal (IP) administration at 5 mg/kg daily × 5 repeated weekly for a planned four consecutive weeks.
Left panels: Kaplan-Meier Event-Free Survival estimates for control and treated groups. Control (____); IMC-A12 (
 ); Rapamycin (
); Rapamycin (
 ); IMC-A12 + rapamycin (
); IMC-A12 + rapamycin (
 ). Center panels: Individual tumor growth curves. Controls (gray), IMC-A12 treated (black). Right panels: Individual tumor growth curves. Rapamycin (gray), IMC-A12 + rapamycin (black).
). Center panels: Individual tumor growth curves. Controls (gray), IMC-A12 treated (black). Right panels: Individual tumor growth curves. Rapamycin (gray), IMC-A12 + rapamycin (black).
A test of formal supra- or sub-additivity was also performed [9], although censoring of animals not having events limited the ability to apply the method to four of the models. EW5 showed clear evidence of supra-additivity. Of the two osteosarcoma xenografts that showed therapeutic enhancement, OS-2 showed a sub-additive effect for the combination while OS-9 showed an additive effect.
Discussion
The combination of the fully human anti-IGF1R antibody IMC-A12 and the mTOR inhibitor rapamycin has been evaluated in nine well-characterized pediatric solid tumor lines that had previously shown significant tumor growth inhibition to IMC-A12, Therapeutic enhancement is reported for 3 of the xenograft lines: the EW5 Ewing sarcoma line, and the OS9 and OS2 osteosarcoma lines. In a fourth line, the Rh41 alveolar rhabdomyosarcoma line, criteria for therapeutic enhancement were not quite met despite a complete response in the combination group compared to progressive disease in both the IMC-A12 and rapamycin treatment groups. In the Rh28 alveolar rhabdomyosarcoma and NB-1643 neuroblastoma xenograft lines, treatment with IMC-A12 induced a complete response making any assessment of therapeutic enhancement difficult. The combination was well tolerated with little or no added toxicity observed in the combination treatment groups.
Included in the current report is an analysis of both therapeutic enhancement and a linear regression model of time to event to assess additivity. In the 3 lines for which the combination of IMC-A12 and rapamycin demonstrates therapeutic enhancement, linear regression analysis of time to event suggests the combination is supra-additive in EW5 and additive in OS9, but sub-additive in OS2. While the presence of therapeutic enhancement and sub-additivity may seem incompatible initially, these are not necessarily inconsistent with each other. Rather, the effect of the combination is significantly greater than that of either single agent (hence the claim for therapeutic enhancement), but the effect of the combination is not as superior to that obtained with the single agents as would be predicted were formal additivity of the single agent treatment effects observed (hence the sub-additivity for the model-based interaction assessment).
The mechanism of therapeutic enhancement has been extensively studied in previous reports. mTOR kinase is the catalytic component of two distinct but related complexes, MTORC1 and MTORC2. MTORC1, consisting of mTOR, RAPTOR, mLST8 and PRAS40, regulates protein synthesis and degradation. Inhibition of mTOR by rapamycin, may release of Akt from MTORC1-mediated negative feedback [22,23]. The effect of releasing the negative feedback inhibition of Akt by inhibition mTOR is to increase cellular dependence on IGF1R-AKT mediated signaling. Dual inhibition of mTOR and IGF1R has proven in this and other preclinical studies to be more effective than monotherapy in a subset of pediatric solid tumors signaling [21,23,24].
As new agents targeting components of IGF1R and mTOR signaling advance through clinical trials, it will be necessary to solidify an understanding of how best to combine these new agents to enhance efficacy. In targeting a central signaling molecule like mTOR, consideration should be given to the consequences of disengaging the molecule from its negative feed back functions. In the case of mTOR, inhibition by rapamycin may result in compensatory signaling through an alternate pathway that is still capable of maintaining tumor growth. As IMC-A12 as well as other IGF1R inhibitors and the rapamycin analogs advance through clinical trials, combination trials should be considered to thoroughly define the therapeutic potential of the agents. As illustrated by the EW5 xenograft line, the absence of tumor regressing activity of a single targeted agent does not necessarily imply that the agent missed its target or that the target is irrelevant to tumor growth.
Supplementary Material
Acknowledgments
This work was supported by NO1-CM-42216, NO1-CM91001-03, CA21765, and CA108786 from the National Cancer Institute. IMC-A12 was supplied to the PPTP by ImClone Systems. In addition to the authors, this reportrepresents work contributed by the following: Sherry Ansher, Joshua Courtright, Edward Favours, Henry S. Friedman, Debbie Payne-Turner, Charles Stopford, Chandra Tucker, Joe Zeidner, Ellen Zhang, and Jian Zhang. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children’s Hospital.
Footnotes
CONFLICT OF INTEREST STATEMENT: The authors consider that there are no actual or perceived conflicts of interest.
References
- 1.Dilling MB, Dias P, Shapiro DN, et al. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer Res. 1994;54(4):903–907. [PubMed] [Google Scholar]
- 2.Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2008;50(4):799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- 3.Houchens DP, Ovejera AA, Riblet SM, et al. Human brain tumor xenografts in nude mice as a chemotherapy model. Eur J Cancer Clin Oncol. 1983;19(6):799–805. doi: 10.1016/0277-5379(83)90012-3. [DOI] [PubMed] [Google Scholar]
- 4.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 5.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 7.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23(23):5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 8.Fouladi M, Laningham F, Wu J, et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol. 2007;25(30):4806–4812. doi: 10.1200/JCO.2007.11.4017. [DOI] [PubMed] [Google Scholar]
- 9.Houghton PJ, Morton CL, Gorlick R, et al. Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program. Mol Cancer Ther. 2010;9(1):101–112. doi: 10.1158/1535-7163.MCT-09-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun K. A new marker for rhabdomyosarcoma. Insulin-like growth factor II. Lab Invest. 1992;67(5):653–664. [PubMed] [Google Scholar]
- 11.Wan X, Helman LJ. Levels of PTEN protein modulate Akt phosphorylation on serine 473, but not on threonine 308, in IGF-II-overexpressing rhabdomyosarcomas cells. Oncogene. 2003;22(50):8205–8211. doi: 10.1038/sj.onc.1206878. [DOI] [PubMed] [Google Scholar]
- 12.El-Badry OM, Minniti C, Kohn EC, et al. Insulin-like growth factor II acts as an autocrine growth and motility factor in human rhabdomyosarcoma tumors. Cell Growth Differ. 1990;1(7):325–331. [PubMed] [Google Scholar]
- 13.Wang W, Kumar P, Epstein J, et al. Insulin-like growth factor II and PAX3-FKHR cooperate in the oncogenesis of rhabdomyosarcoma. Cancer Res. 1998;58(19):4426–4433. [PubMed] [Google Scholar]
- 14.Yee D, Favoni RE, Lebovic GS, et al. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation. A potential autocrine growth factor. J Clin Invest. 1990;86(6):1806–1814. doi: 10.1172/JCI114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riggi N, Cironi L, Provero P, et al. Development of Ewing’s sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65(24):11459–11468. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan KA, Castle VP, Hanash SM, et al. Insulin-like growth factor II in the pathogenesis of human neuroblastoma. Am J Pathol. 1995;147(6):1790–1798. [PMC free article] [PubMed] [Google Scholar]
- 17.El-Badry OM, Helman LJ, Chatten J, et al. Insulin-like growth factor II-mediated proliferation of human neuroblastoma. J Clin Invest. 1991;87(2):648–657. doi: 10.1172/JCI115042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Badry OM, Romanus JA, Helman LJ, et al. Autonomous growth of a human neuroblastoma cell line is mediated by insulin-like growth factor II. J Clin Invest. 1989;84(3):829–839. doi: 10.1172/JCI114243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houghton PJ, Morton CL, Gorlick R, et al. Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;54(7):921–926. doi: 10.1002/pbc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolb EA, Gorlick R, Houghton PJ, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(6):1190–1197. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- 21.Kolb EA, Kamara D, Zhang W, et al. R1507, a fully human monoclonal antibody targeting IGF-1R, is effective alone and in combination with rapamycin in inhibiting growth of osteosarcoma xenografts. Pediatr Blood Cancer. 2010;55(1):67–75. doi: 10.1002/pbc.22479. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Yan H, Frost P, et al. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4(10):1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 23.Wan X, Harkavy B, Shen N, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 24.Kurmasheva RT, Dudkin L, Billups C, et al. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69(19):7662–7671. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malempati S, Weigel B, Ingle AM, et al. A phase I trial and pharmacokinetic study of IMC-A12 in pediatric patients with relapsed/refractory solid tumors: A Children’s Oncology Group Phase I Consortium study. J Clin Oncol. 2009;27(suppl) abstr 10013. [Google Scholar]
- 26.Friedman HS, Colvin OM, Skapek SX, et al. Experimental chemotherapy of human medulloblastoma cell lines and transplantable xenografts with bifunctional alkylating agents. Cancer Res. 1988;48(15):4189–4195. [PubMed] [Google Scholar]
- 27.Graham C, Tucker C, Creech J, et al. Evaluation of the antitumor efficacy, pharmacokinetics, and pharmacodynamics of the histone deacetylase inhibitor depsipeptide in childhood cancer models in vivo. Clin Cancer Res. 2006;12(1):223–234. doi: 10.1158/1078-0432.CCR-05-1225. [DOI] [PubMed] [Google Scholar]
- 28.Peterson JK, Tucker C, Favours E, et al. In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models. Clin Cancer Res. 2005;11(19 Pt 1):6950–6958. doi: 10.1158/1078-0432.CCR-05-0740. [DOI] [PubMed] [Google Scholar]
- 29.Houghton PJ, Morton CL, Tucker C, et al. The Pediatric Preclinical Testing Program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 30.Rose WC, Wild R. Therapeutic synergy of oral taxane BMS-275183 and cetuximab versus human tumor xenografts. Clin Cancer Res. 2004;10(21):7413–7417. doi: 10.1158/1078-0432.CCR-04-1045. [DOI] [PubMed] [Google Scholar]
- 31.Gorlick R, Kolb EA, Houghton PJ, et al. Initial testing (stage 1) of lapatinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2009;53(4):594–598. doi: 10.1002/pbc.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houghton PJ, Morton CL, Gorlick R, et al. Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;54(7):921–926. doi: 10.1002/pbc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.