Abstract
Background
Margin status is one of the strongest prognosticators after resection of pancreatic ductal adenocarcinoma (PDAC). The clinical significance of pancreatic intraepithelial neoplasia (PanIN) at a surgical margin has not been established.
Methods
A total of 208 patients who underwent R0 resection for PDAC between 2004 and 2008 were selected. Intraoperative frozen section slides containing the final pancreatic parenchymal transection margin were evaluated for presence or absence, number, and grade of PanINs. Data were compared to clinicopathologic factors, including patient survival.
Results
PanIN lesions were present in margins in 107 of 208 patients (51.4%). Median number of PanINs per pancreatic resection margin was 1 (range, 1–11). A total of 72 patients had PanIN-1 (34.6%), 44 had PanIN-2 (21.1%), and 16 had PanIN-3 (7.2%) at their margin. Overall median survival was 17.9 (95% confidence interval, 14–21.9) months. Neither the presence nor absence of PanIN nor histological grade had any significant correlation with important clinicopathologic characteristics. There were no significant survival differences between patients with or without PanIN lesions at the resection margin or among patients with PanIN-3 (carcinoma in situ) versus lower PanIN grades. However, patients with R1 resection had a significantly worse outcome compared with patients without invasive cancer at a margin irrespective of the presence of PanIN (P = 0.02).
Conclusions
The presence of PanINs at a resection margin does not affect survival in patients who undergo R0 resection for PDAC. These results have significant clinical implications for surgeons, because no additional resection seems to be indicated when intraoperative frozen sections reveal even high-grade PanIN lesions.
Pancreatic ductal adenocarcinoma (PDAC) has a dismal prognosis. The American Cancer Society estimated that in 2010 approximately 43,140 individuals were diagnosed with pancreatic cancer in the United States and that 36,800 died from it.1 These numbers underscore the extremely poor prognosis for patients with this devastating disease. Almost 80% of all patients have distant metastases (M1) at the time of diagnosis or an advanced primary tumor that is no longer resectable.2 Modern strategies of chemo- or radiotherapy are essentially ineffective to improve the dismal course of disease.
Cure from PDAC is rarely achieved. A complete resection of the cancer, whenever feasible, may improve the patient‘s prognosis. Numerous studies have investigated whether the presence of clear resection margins (R0 resection) influences outcome compared with resection with invasive cancer left at a margin (R1 and R2 resections) with ambiguous results.3–17 While R2 resections can usually be avoided with careful preoperative imaging and planning, microscopic margin involvement (R1 resection) still occurs with relatively high frequency.13,16
The significance of intraoperative frozen section consultation has been an issue of considerable debate, but no international consensus exists on the optimal procedures to assess margins. We and other centers utilize intraoperative frozen-section consultation to adapt the extent of resection to achieve margins free of invasive carcinoma whenever reasonably possible. This approach reduces the risk that the final margins will be positive on permanent sections. The most appropriate approach to the pancreatic parenchymal resection margin when it harbors a non-invasive precursor lesion, is however, not clear. In particular, a consensus has not been achieved on how best to handle pancreatic intraepithelial neoplasia high-grade (PanIN-3; also known as (a.k.a.) carcinoma in situ) when it is present at the pancreatic parenchymal resection margin (the “ pancreatic neck margin”). In such instances, both pathologists and surgeons need to weigh the iatrogenic risks of resecting additional pancreatic parenchyma, such as endocrine insufficiency versus the incremental survival benefit that such additional resections may afford. There is, however, very little evidence-based medicine to determine the impact of leaving a PanIN-3 behind at a resection margin. Indeed, the 7th edition of the American Joint Committee on Cancer (AJCC) staging manual is vague on this issue, classing an R1 resection ambiguously as “microscopic residual tumor.”18 As a result, some centers in Europe have interpreted PanIN-3 at a margin to be an R1 resection, whereas most centers in the United States would reserve that designation for residual invasive carcinoma.
We designed the present study to assess the impact, if any, of PanIN lesions at a surgical margin after curative resection (R0) for PDAC. We did not identify any statistically significant impact of PanINs in the final resection margins on overall survival, including the presence of PanIN-3. Our findings, if validated in larger studies, should provide the basis for consensus in intraoperative decision making, when surgeons and pathologists are faced with a PanIN at a resection margin.
PATIENTS AND METHODS
This study was approved as human subjects exempt by the Johns Hopkins Institutional Review Board. Our prospectively maintained Surgical Pathology Database was queried for patients who underwent R0 resection for PDAC from January 1, 2004 to December 31, 2008, at The John Hopkins Hospital (JHH). R-status was defined following the AJCC guidelines (R0: no residual tumor; R1: microscopic residual tumor; R2: macroscopic residual tumor) with R1 reserved for cases with microscopic residual invasive carcinoma.18 Cases with PanIN only at a margin were considered R0 resections for purposes of this study. We identified 208 consecutive patients with R0 resections and from whom the frozen transection margin slides were available for microscopic reevaluation. For survival calculations, we identified another 141 patients with available survival data treated within the same period of time who had a R1 resection after partial pancreatectomy for PDAC. Patients who underwent total pancreatectomy or cases with pathologic diagnosis other than PDAC or those cancers observed in association with an IPMN or MCN were excluded.
PanINs were defined using standard nomenclature as described below.19 The presence or absence of PanIN lesions at the transection margin were compared with a variety of clinicopathological parameters, including patient age, patient gender, surgical therapy, tumor size, tumor histology, lymph node status, and vascular, perineural, and lymphatic invasion. Patients with PDAC within the pancreatic head, uncinate process, or neck most often underwent pancreaticoduodenectomy with preservation of the pylorus. PDACs located more distally in the pancreatic body or in the tail of the pancreas were resected by a distal pancreatectomy, usually including splenectomy. A lymphadenectomy was undertaken in all patients. A lymph node was regarded as positive whenever it contained carcinoma, including cases in which the invasive cancer directly extended into a lymph node. If the primary tumor proved to infiltrate an en bloc resection was attempted. Data on possible radiation/chemotherapy regimens and resulting response were not collected for our study. Overall survival was the primary endpoint and determined as the time from the cancer-directed operation until death or last patient contact.
Frozen-Section Margin Analysis
Frozen sections of the pancreatic parenchymal transection margin (the “pancreatic neck margin”) prepared at the time of surgery were stained by hematoxylin and eosin staining (H&E staining). Subsequently, resection margins were formalin fixed and paraffin embedded for permanent section evaluation. These permanent sections were the slides retrospectively evaluated in our study. Histopathology review was undertaken by three experts in pancreatic pathology (RHH, AM, SMH). The entire margin was reanalyzed for the presence or absence of an invasive carcinoma. In addition, these sections were also evaluated for the number and type of PanIN lesions. PanINs were classified in accordance to the international consensus guidelines.19 Briefly, PanIN-1A demonstrate a flat epithelium with columnar cells and uniform basally located nuclei; PanIN-1B lesions exhibit a more papillary growth pattern and are otherwise identical to PanIN-1A lesions. PanIN-2 lesions are more architecturally complex and also demonstrate greater nuclear abnormalities than do PanIN-1 lesions. PanIN-3 lesions (a.k.a. carcinoma in situ) have substantial architectural, cytological, and nuclear atypia. PanIN-1A and PanIN-1B lesions were grouped together as PanIN-1 in our study.20
Statistics
Statistical computations were performed by using SPSS® version 17 (SPSS Inc., Chicago, IL). We compared mean values by Student’s t test or simple ANOVA. Continuous variables, including age of patients and tumor size, are presented as median with interquartile range (IQR) and 95% confidence interval (CI). Association between categorical variables were compared using χ2 or Fisher‘s exact tests. We used the Kaplan–Meier method to calculate survival curves compared by the log-rank test. Patients who died ≤30 days postoperatively were excluded from outcome analyses. A P value <0.05 was considered statistically significant.
RESULTS
Patients and Treatment
The clinicopathologic, tumor-related, and surgery-related data are presented in Table 1. Information is presented for all patients and stratified by the presence or absence of PanIN in the final resection margin. Our study included 106 men (51%) and 102 women (49%) with a median age of 66 (IQR, 59–74) years; 179 patients were Caucasian (86.1%). All of the resection margins were confirmed to be free of invasive carcinoma (R0). The primary PDAC was predominantly located in the pancreatic head in 181 patients (87%), and these patients underwent a pylorus-preserving (n = 124; 59.6%) or a classic pancreaticoduodenectomy (n = 57; 27.4%). A distal pancreatectomy was undertaken in 27 patients (13%) whose cancers arose in the neck, body, or tail of the pancreas. The median follow-up for all R0 resected patients of our study was 17.9 (range, 1.6–64.5) months. By the time of most recent data accrual, 77 patients (37%) were still alive with a median postoperative survival time of 10 (range, 1–27) months after their primary tumor resection. The remaining 131 patients (63%) were followed until their death.
TABLE 1.
Demographic, clinicopathological, and treatment characteristics of 208 patients who underwent R0 resection for PDAC; stratified based on the presence or absence of PanIN in the transection margin
| All patients (N = 208) (%) |
Normal margin (n = 101; 48.6%) (%) |
PanIN at margin (n = 107; 51.4%) (%) |
P value | |
|---|---|---|---|---|
| Median age (years) [IQR] | 66 (59–74) | 65 (57–73) | 68 (60–74) | 0.21 |
| Race | ||||
| Caucasian | 179 (86.1) | 86 (85.1) | 93 (86.9) | 0.43 |
| Gender | ||||
| Male | 106 (51) | 57 (56.4) | 49 (45.8) | 0.13 |
| Female | 102 (49) | 44 (43.6) | 58 (54.2) | |
| Surgery | ||||
| Pylorus preserving Whipple | 124 (59.6) | 59 (58.4) | 65 (60.8) | 0.47 |
| Classic Whipple | 57 (27.4) | 31 (30.7) | 26 (24.3) | |
| Distal pancreatectomy | 27 (13) | 11 (10.9) | 16 (15) | |
| Median size (cm) [IQR] | 3 (2.5–4) | 3 (2–4) | 3 (2.5–4) | 0.21 |
| T classification | ||||
| T1 | 12 (6.9) | 6 (7) | 6 (6.9) | 0.28 |
| T2 | 33 (19.1) | 11 (12.8) | 22 (25.3) | |
| T3 | 125 (72.3) | 67 (77.9) | 58 (66.7) | |
| T4 | 3 (1.7) | 2 (2.3) | 1 (1.1) | |
| Lymph node metastases | ||||
| N0 | 50 (24) | 23 (22.8) | 27 (25.2) | 0.87 |
| N1a | 32 (15.4) | 15 (14.9) | 17 (15.9) | |
| N1b | 126 (60.6) | 63 (62.4) | 63 (58.9) | |
| Histologic grade | ||||
| G1 | 9 (4.3) | 5 (5) | 4 (3.7) | 0.58 |
| G2 | 117 (56.3) | 59 (58.4) | 58 (54.2) | |
| G3 | 81 (38.9) | 36 (35.6) | 45 (42.1) | |
| G4 | 1 (0.5) | 1 (1) | 0 (0) | |
| Venous invasion | ||||
| Present | 99 (47.6) | 47 (46.5) | 52 (48.6) | 0.94 |
| Lymphatic invasion | ||||
| Present | 35 (16.8) | 15 (14.9) | 20 (18.7) | 0.76 |
| Perineural invasion | ||||
| Present | 187 (89.9) | 92 (91.1) | 95 (88.8) | 0.85 |
Pathological Data
The median maximum tumor diameter was 3 (IQR, 2.5–4) cm. Most patients had a T3 lesion (n = 125; 72.3%), followed by T2 (n = 33; 19.1%), and T1 tumors (n = 12; 6.9%); only three patients (1.7%) had a T4 lesion. These latter patients were included because they had negative final resection margins. Fifty patients (24%) were pN0. In our series, 158 patients (76%) were pN1 (at least 1 lymph node metastasis). Histologically, most carcinomas were moderately differentiated (G2: n = 117; 56.3%). Eighty-one lesions were poorly differentiated (G3; 38.9%), whereas nine were well-differentiated (G1; 4.3%) and one lesion proved to be undifferentiated (G4; 0.5%). Perineural invasion was identified in 187 patients (89.9%). Microscopic invasion of lymph vessels and blood vessels was observed in 35 patients (16.8%) and 99 patients (47.6%), respectively. The presence or absence of any PanIN lesion at the resection margin had no significant impact in association with a variety of clinicopathological parameters, including the patient’s age, gender, surgical therapy, tumor size, histology, lymph node metastasis, patients‘ survival, and vascular, perineural, and lymphatic invasion (all P > 0.05).
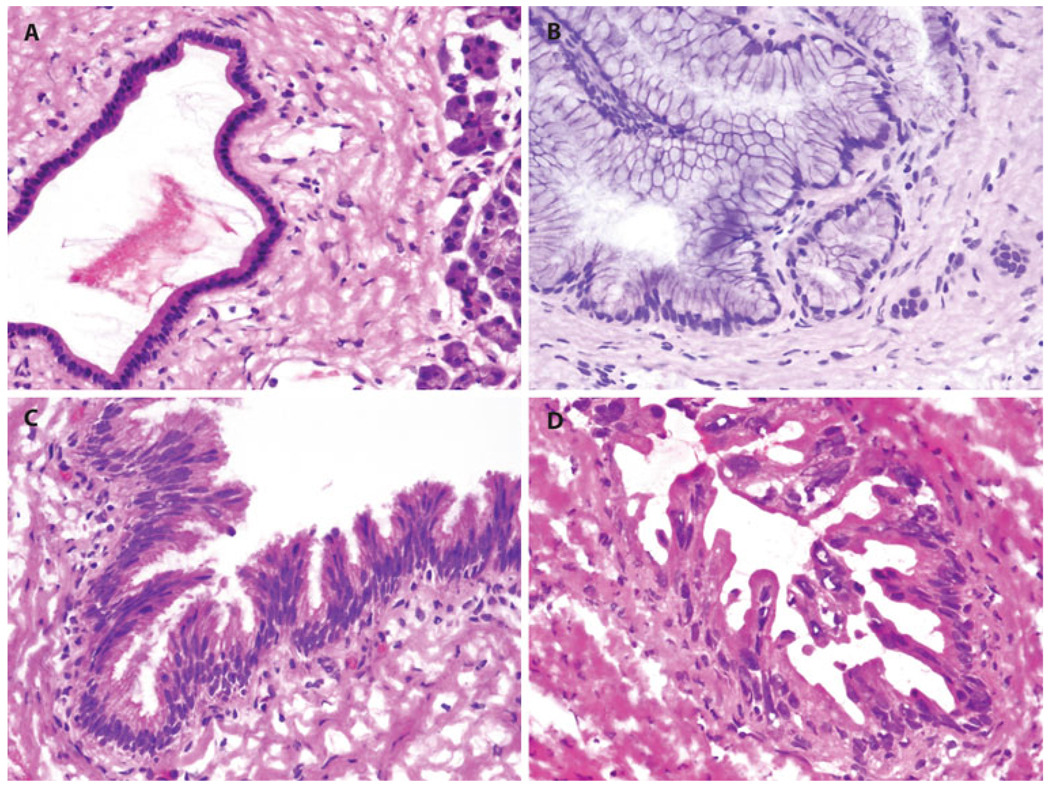
Transection Margin Analysis
The status of PanINs at the pancreatic parenchymal transection margin is presented in Table 2, and representative margins are shown in Fig. 1. PanIN lesions were identified at the margin in 107 patients (51.4%). In those a median of 1 PanIN (range, 1–11 PanINs) was identified at margin. The remaining 101 patients (48.6%) did not have any PanINs in their final pancreatic parenchymal transection margin. Most frequently PanIN-1 lesions were observed (n = 72; 34.6%), followed by PanIN-2 (n = 44; 21.1%) and PanIN-3 lesions (n = 16; 7.2%).
TABLE 2.
PanIN status in transection margins analyzed
| N (%) | |
|---|---|
| PanIN-positive margins | 107 (51.4) |
| No. of PanINs at margin | 1 (1–11) |
| Median (range) | |
| Margin positive for | |
| PanIN-1 | 72 (34.6) |
| PanIN-2 | 44 (21.1) |
| PanIN-3 | 16 (7.2) |
FIG. 1.
Representative images of frozen resection margins with a normal pancreatic duct, b PanIN-1, c PanIN-2, and d PanIN-3
Patients’ Overall Survival According to Presence and Grade of PanIN at the Margin
We compared patient overall survival with respect to presence or absence of PanIN in the pancreatic resection margin. The median overall survival for R0 patients in whom we detected any grade of PanIN in their resection margin was 17.7 months (n = 101; 95% CI, 12.7–22.6 months; 1-year overall survival, 65.4%; 3-year overall survival, 16.2%), whereas it was 18.5 months for R0 patients without any PanIN lesions at the margin (n = 107; 95% CI, 12.9–24 months; 1-year overall survival, 67.9%; 3-year overall survival, 15.2%). There was no significant difference in overall survival for patients with R0 resections relative to the presence or absence of any grade of PanIN in resection margin (log-rank test, P = 0.88; Fig. 2).
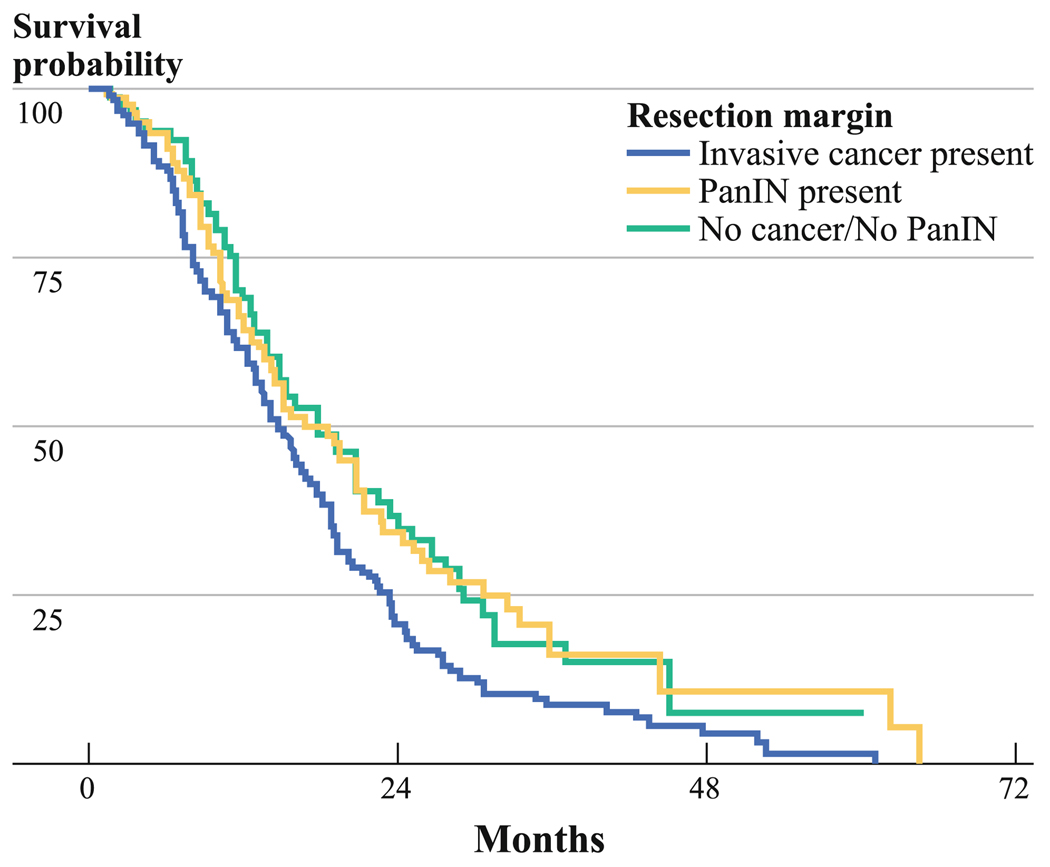
FIG. 2.
Kaplan–Meier survival analysis comparing patients with and without any grade of PanIN, and infiltrating cancers (R1) in the final pancreatic resection margin. The median survival for patients with any grade of PanIN on resection margin (n = 101) was 17.7 months (1-year survival, 65.4%; 3-year survival, 16.2%), whereas it was 18.5 months for those without any grade of PanIN (n = 107; 1-year survival, 67.9%; 3-year survival, 15.2%). There was no significant difference on patients’ survival according to presence or absence of any grade of PanIN on resection margin (log-rank test, P = 0.88). However, the overall survival in patients with PanINs (P = 0.02) and patients without PanIN/cancer (P = 0.02) was significantly better than in a group of 141 patients with infiltrating cancers at margin
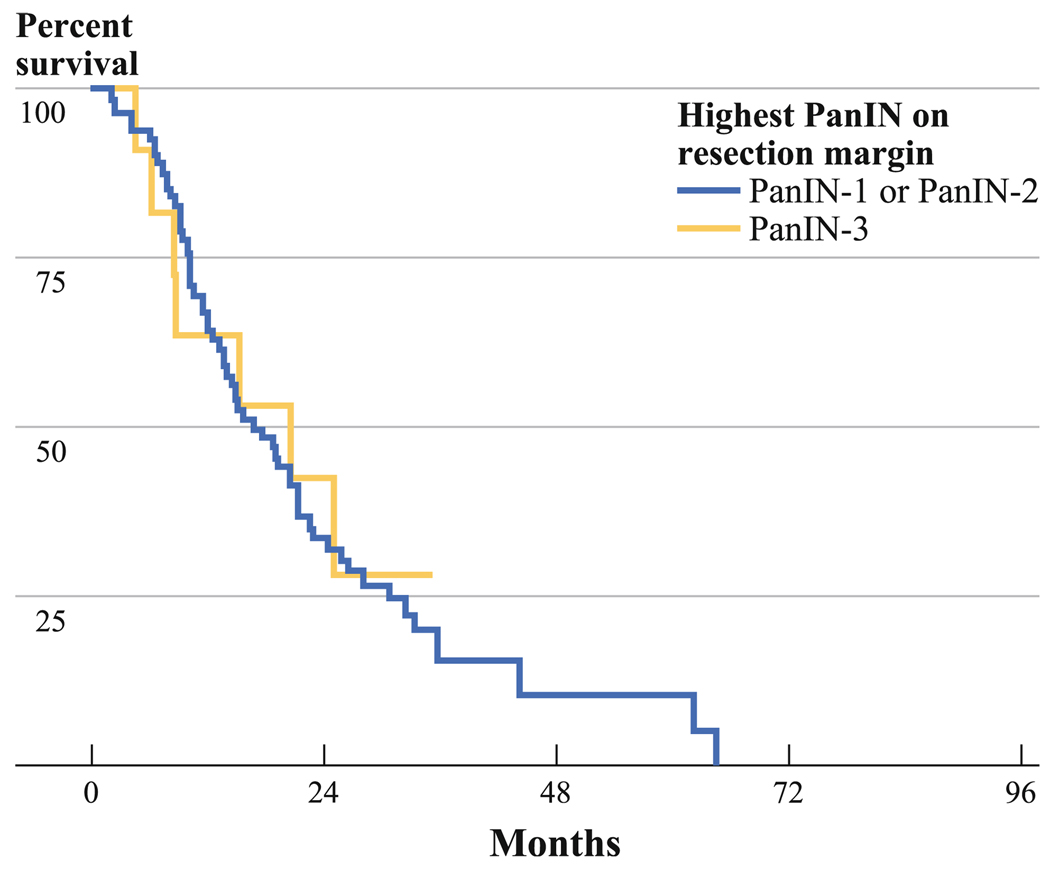
Additionally, we determined if there is overall survival difference between R0 patients with PanIN-3 (n = 15) versus those with PanIN-1 or PanIN-2 (n = 92) as highest grade of PanIN on resection margin. The median overall survival for R0 patients with PanIN-1 or PanIN-2 as highest grade on resection margin was 16.7 months (95% CI, 11.9–21.6 months; 1-year survival, 65.6%; 3-year survival, 10.5%), whereas it was 20.7 months for those with PanIN-3 (95% CI, 3.5–37.9 months, 1-year survival 53%, 3-year survival, 28.3%) as highest grade on resection margin. This difference was not statistically significant (log-rank test, P = 0.87; Fig. 3).
FIG. 3.
Kaplan–Meier survival analysis comparing patients with PanIN-3 versus PanIN-1 or PanIN-2 as the highest grade of PanIN on the final pancreatic resection margin. The median survival for patients with PanIN-3 on resection margin (n = 15) was 20.7 months (1-year survival, 53%; 3-year survival, 28.3%), whereas it was 16.7 months for those with PanIN-1 or PanIN-2 as the highest grade of PanIN on resection margin (n = 92, 1-year survival, 65.6%; 3-year survival, 10.5%). These differences are not statistically significant (log-rank test, P = 0.87)
Finally, we compared the survival of patients with microscopic invasive carcinoma at a resection margin (R1 resections) to the patients with R0 resections. The median overall survival for R1 patients was 14.6 months (n = 141; 95% CI, 12.5–16.7 months; 1-year overall survival, 61.1%; 3-year overall survival, 8.8%). As shown in Fig. 2, patients with R1 resections (microscopic invasive carcinoma at a margin) had a significantly worse survival than did patients with PanIN only (P = 0.02) and patients with no lesion at their margins (P = 0.02).
DISCUSSION
As observed in the present study, numerous, but not all, studies have shown that the presence of microscopic invasive carcinoma at a resection margin (R1) reduces survival after the surgical resection of pancreatic cancer. 3–17,21 The value of the intraoperative frozen-section consultation is an issue of ongoing debate. One major advantage of this procedure is that the surgeon may immediately adapt the extent of resection to the frozen-section margin status, thereby potentially avoiding leaving behind invasive carcinoma at a margin.
Whether noninvasive precursor lesions left in vivo at a margin after the surgical resection of an invasive carcinoma confers an adverse impact on patient outcome is not clear. In particular, PanIN-3 lesions (i.e., carcinoma in situ) are believed to be the immediate precursors to an invasive adenocarcinoma.19,20 Whether further resection of pancreatic parenchyma for a PanIN-3 at a margin is indicated is unknown. The risks and complications from resecting additional pancreatic parenchyma have to be balanced with the risk of the residual disease progressing to invasive cancer.
Our study showed that a majority of patients who undergo surgical resection of an invasive pancreatic cancer have PanIN lesions in their final frozen section margins. Despite investigations that show that the presence of PanIN increases with age we did not find any such correlation.22 Given the multifocal nature of PanIN lesions within cancerous pancreata, most of the lesions at the margin presumably represent independent noninvasive neoplasms. We were not able to demonstrate a statistically significant prognostic impact of PanINs at the margin, including that of high-grade PanIN-3 lesions. This suggests that the patient’s invasive carcinoma is the primary driver of prognosis. Considering the rapid pace at which most invasive pancreatic cancers progress, it is likely that any residual PanINs do not have time to progress to invasive cancer.23 Our results are to a certain extent in contrast to a study by Kim et al., who found that even microscopically tumor-negative margins in PDAC may harbor KRAS mutations and that the presence of mutant KRAS at a margin has adverse prognostic impact in the respective patient.17
Because the AJCC staging manual is vague on the definition of an R1 resection, ambiguously defining R1 as “microscopic residual tumor,” we also compared the survival of patients with PanIN or no lesion at a margin, with a separate group of patients with microscopic residual invasive carcinoma at a final margin.18 We found that patients with microscopic residual invasive carcinoma had a significantly worse (P = 0.02) outcome, suggesting that the designation R1 should be reserved for only cases with residual microscopic invasive carcinoma.
The present study had some limitations, such as the retrospective nature, the relatively small number of patients with PanIN-3 lesion at a margin, and the fact that not all of the patients received the same adjuvant treatment. Although two-thirds of the patients in this study were followed until death, some were lost to follow-up and data on the patterns of recurrence were limited on others. This largely reflects that our institution is a tertiary referral center and postoperative treatment and follow-up often is performed at other hospitals. Hence, no reasonable conclusions could be made about important issues, such as the impact of PanINs at margin on recurrence pattern. In addition, even though our sample size was fairly large (N = 208 patients), we cannot rule out a type II statistical error in our finding that there is no difference in survival between patients with PanIN versus no PanIN at a margin.
CONCLUSIONS
The prognostic significance of PanIN has not been extensively studied. In our study of 208 patients, the presence of PanINs in pancreatic resection margins was not associated with survival in patients who underwent complete resection (R0) for PDAC. These results suggest that no additional resection might be necessary if intraoperative frozen sectioning reveals only PanIN lesions at the margin. Prospective randomized trials, including larger cohorts applying systematic pathologic sampling and assessment standards, may in the future determine the most appropriate margin clearance in PDAC.
ACKNOWLEDGMENT
Hanno Matthaei was supported by a grant from the Mildred-Scheel-Stiftung, Deutsche Krebshilfe, Bonn, Germany. This work was supported by the Michael Rolfe Foundation and P50CA062924.
Footnotes
DISCLOSURE All authors declare no commercial interest.
REFERENCES
- 1.Society AC. [Accessed 21 Dec 2010]; Available at: http://www.cancer.org/cancer/pancreaticcancer/overviewguide/pancreatic-cancer-overview-key-statistics. [Google Scholar]
- 2.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillhoff M, Yates R, Wall K, et al. Intraoperative assessment of pancreatic neck margin at the time of pancreaticoduodenectomy increases likelihood of margin-negative resection in patients with pancreatic cancer. J Gastrointest Surg. 2009;13(5):825–830. doi: 10.1007/s11605-009-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt CM, Glant J, Winter JM, et al. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery. 2007;142(4):572–578. doi: 10.1016/j.surg.2007.07.016. discussion 78–80. [DOI] [PubMed] [Google Scholar]
- 5.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91(5):586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234(6):758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 8.Willett CG, Lewandrowski K, Warshaw AL, Efird J, Compton CC. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg. 1993;217(2):144–148. doi: 10.1097/00000658-199302000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamieson NB, Foulis AK, Oien KA, et al. Positive mobilization margins alone do not influence survival following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2010;251(6):1003–1010. doi: 10.1097/SLA.0b013e3181d77369. [DOI] [PubMed] [Google Scholar]
- 10.Howard TJ, Krug JE, Yu J, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10(10):1338–1345. doi: 10.1016/j.gassur.2006.09.008. discussion 45-6. [DOI] [PubMed] [Google Scholar]
- 11.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 12.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 13.Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15(6):1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 14.Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141(5):610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246(1):52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10(9):1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210-1. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Reber HA, Dry SM, et al. Unfavourable prognosis associated with K-ras gene mutation in pancreatic cancer surgical margins. Gut. 2006;55(11):1598–1605. doi: 10.1136/gut.2005.083063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. New York: Springer; 2010. [Google Scholar]
- 19.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28(8):977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 20.Matthaei H, Maitra A. Precursor lesions of pancreatic cancer. In: Fitzgerald RC, editor. Pre-invasive disease: pathogenesis and clinical management. New York: Springer; 2011. pp. 395–420. [Google Scholar]
- 21.Mayo SC, Austin DF, Sheppard BC, Mori M, Shipley DK, Billingsley KG. Adjuvant therapy and survival after resection of pancreatic adenocarcinoma: a population-based analysis. Cancer. 2010;116(12):2932–2940. doi: 10.1002/cncr.25082. [DOI] [PubMed] [Google Scholar]
- 22.Kozuka S, Sassa R, Taki T, et al. Relation of pancreatic duct hyperplasia to carcinoma. Cancer. 1979;43(4):1418–1428. doi: 10.1002/1097-0142(197904)43:4<1418::aid-cncr2820430431>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]