Abstract
Recent reports indicate that exposure to some stressors, such as shipping or immune challenge with the bacterial endotoxin, lipopolysaccharide (LPS), during the peripubertal period reduces sexual receptivity in response to ovarian hormones in adulthood. We hypothesized that a peripubertal immune challenge would also disrupt the response of a non-reproductive behavior, anxiety-like behavior, to ovarian hormones in adulthood. Female C57Bl/6 mice were injected with LPS during the peripubertal period and tested for anxiety-like behavior in adulthood, following ovariectomy and ovarian hormone treatment. Treatment with estradiol followed by progesterone reduced anxiety-like behavior in control, but not LPS-treated females. We next determined if the disruptive effect of LPS on adult behavior were limited to the peripubertal period by treating mice with LPS either during this period or in adulthood. LPS treatment during the peripubertal period disrupted the anxiolytic effect of ovarian hormones, whereas treatment in adulthood did not. We further tested if this model of peripubertal immune challenge was applicable to an outbred strain of mice (CD-1). Similar to C57Bl/6 mice, LPS treatment during the peripubertal period, but not later, disrupted the anxiolytic effect of estradiol and progesterone. These data suggest that a peripubertal immune challenge disrupts the regulation of anxiety-like behavior by ovarian hormones in a manner that persists at least for weeks after the termination of the immune challenge.
Keywords: anxiety, estradiol, progesterone, puberty, adolescence, stress, immune challenge, lipopolysaccharide
Introduction
Early life environment can influence brain development resulting in lasting effects on physiology and behavior. Early life environment impacts the development of stress reactivity (Caldji et al., 2000; Papaioannou et al., 2002), hypothalamic-pituitary-gonadal axis regulation (Herrenkohl and Politch, 1978), and the development of reproductive (Herrenkohl, 1986) and anxiety-like (Hava et al., 2006) behaviors. Puberty has recently been recognized as a critical period for the development of hypothalamic-pituitary-adrenal (Romeo et al., 2005; Romeo et al., 2006) and hypothalamic-pituitary-gonadal axis regulation (Meek et al., 1997), as well as the establishment of some reproductive behaviors (Romeo et al., 2002a; Romeo et al., 2002b; Schulz et al., 2004). This suggests that the pubertal environment impacts adult behavior and physiology.
Exposure to stressors during the peripubertal period exerts lasting effects on stress-related behavior and physiology. For instance, stress exposure during the peripubertal period increases the display of anxiety- and depression-like behavior in response to stress in adulthood (Tsoory et al., 2007), as well as interfere with daily rhythms of corticosterone secretion (Paris and Ramaley, 1974). Peripubertal exposure to stressors also has persistent effects on the hypothalamic-pituitary-gonadal axis. For example, exposure to heat, immobilization, or ether stress at this time reduces fertility, possibly due to a reduction in sexual behavior (Paris et al., 1973).
Peripubertal exposure to some stressors can also alter adult behavioral responses to gonadal hormones. Mice shipped from commercial suppliers during the peripubertal period display reduced sexual behavior in response to gonadal hormones in adulthood (Laroche et al., 2009b). Similarly, brief exposure to lipopolysaccharide (LPS), a bacterial endotoxin derived from the cell walls of gram-negative bacteria reduces the sexual behavior of female C57Bl/6 mice following treatment with ovarian hormones in adulthood (Laroche et al., 2009a). This suggests that particular peripubertal stressors cause lasting impairment in the response to ovarian steroids; however, it remains unclear if these stressors can also alter other behaviors influenced by ovarian hormones.
In addition to regulating reproductive physiology and behavior, ovarian hormones also influence many non-reproductive processes, including the expression of anxiety-like behavior. In both rats (Diaz-Veliz et al., 1997a; Diaz-Veliz et al., 2000; Diaz-Veliz et al., 1997b; Diaz-Veliz et al., 1989; Fernandez-Guasti and Picazo, 1997; Marcondes et al., 2001; Marvan et al., 1996; Marvan et al., 1997; Mora et al., 1996a; Picazo and Fernandez-Guasti, 1998), and mice (Galeeva and Tuohimaa, 2001) natural variations in anxiety-like behaviors occur over the course of the estrous cycle, such that females display reduced anxiety-like behavior during proestrus and estrus, when ovarian hormone concentrations are high, compared to diestrus. Estradiol treatment during diestrus reduces anxiety-like behavior to proestrus-like levels in rats (Marcondes et al., 2001). Furthermore, ovariectomy increases anxiety-like behavior in rats (Diaz-Veliz et al., 1989; Pandaranandaka et al., 2008; Picazo et al., 2006) and mice (Ogawa et al., 1998), and hormone replacement reduces these behaviors in ovariectomized female rats (Diaz-Veliz et al., 1997a; Diaz-Veliz et al., 1997b; Diaz-Veliz et al., 1989; Diaz-Veliz et al., 1991; Mora et al., 1996b; Olivera-Lopez et al., 2008; Pandaranandaka et al., 2006; Pandaranandaka et al., 2008). In ovariectomized mice, chronic treatment with low doses of estradiol reduce anxiety-like behavior (Tomihara et al., 2009), while high doses appear to be anxiogenic (Morgan and Pfaff, 2001; Morgan and Pfaff, 2002; Tomihara et al., 2009). In addition, female mice in which either estrogen receptor α (Choleris et al., 2003; Choleris et al., 2006; Ogawa et al., 1998) or estrogen receptor β (Imwalle et al., 2005; Krezel et al., 2001) have been knocked out display more anxiety-like behavior than wild-type females.
Because some peripubertal stressors impair later regulation of sexual receptivity by ovarian hormones in adulthood, we hypothesized that exposure to a peripubertal stressor would also impair the regulation of anxiety-like behavior by ovarian hormones in adulthood. To test this hypothesis, we examined anxiety-like behavior in response to ovarian hormones in C57Bl/6 mice exposed to an immune challenge with LPS during the peripubertal period. We further investigated whether the effects of LPS on adult behavior require LPS exposure to occur during this period. Finally, we examined whether peripubertal LPS treatment has similar effects in a second, outbred strain of mice.
Materials and Methods
Animals
Female C57Bl/6 and CD-1 mice were shipped from Charles River Laboratories (Kingston, NY) at 3 weeks old. Although shipping pubertal mice has been previously shown to reduce adult response to ovarian hormones, shipping at 3 weeks old was reported to have no effect on adult behavioral response to estradiol and progesterone (Laroche et al., 2009b). Mice were housed 3–5 per cage in an all-female colony room with controlled conditions; a consistent temperature (24 ± 2 C) and reversed 14L:10D light cycle (lights out at 1000h) were maintained. A mix of pine shavings and CareFRESH (International Absorbents, Inc., North Vancouver, British Columbia, Canada) bedding was used, and a fresh Nestlet (Ancare Corp., Bellmore, NY) was provided for each cage when bedding was changed. Food (Harlan Teklad 2014, phytoestrogen-reduced diet, Harlan Teklad, Madison, WI) and water were available ad libitum. This research was approved by the University of Massachusetts Institutional Animal Care and Use Committee.
LPS treatment
LPS was injected within one h of the onset of dark phase. Immediately prior to treatment, mice were weighed and marked on the tail with a Sharpie marker to identify individuals. Mice received a single intraperitoneal injection of 1.5 mg/kg LPS (from Escherichia coli serotype O26:B6; # L3755; Sigma Chemical Co., St. Louis, MO) in a volume of 0.12 ml sterile saline/10 g body weight, or an equivalent volume of saline at either 6 or 8 weeks old. This dose of LPS, when administered during the peripubertal period, has been previously demonstrated to reduce sexual receptivity following exposure to estradiol and progesterone (Laroche et al., 2009a). Although this dose is high compared to some previous work, doses in the 1 – 2.5 mg/kg range have been used by others (Eriksson et al., 2000; Quan et al., 1998; Zhou et al., 1999). Furthermore, in this laboratory, this dose of LPS produces only moderate sickness behavior (Table 2), and all animals appear to be fully recovered within 24–48h.
Table 2.
LPS-induced sickness behavior
| Sickness score | Percent showing sickness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp | Treatment | Age | n | 30m | 4h | 24h | 48h | 30m | 4h | 24h | 48h |
| 1 | Saline | 6 wk | 20 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| LPS | 6 wk | 23 | 1.0±0.2* | 1.6±0.1* | 0.1±0.1* | 0.0±0.0 | 56.5* | 91.3* | 13 | 0.0 | |
| 2 | Saline | 6 wk | 16 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| LPS | 6 wk | 17 | 1.9±0.1* | 2.0±0.0* | 0.2±0.1 | 0.0±0.0 | 100.0* | 100.0* | 17.6 | 0.0 | |
| Saline | 8 wk | 16 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| LPS | 8 wk | 16 | 2.0±0.0* | 1.8±0.1* | 0.0±0.0 | 0.0±0.0 | 100.0* | 100.0* | 0.0 | 0.0 | |
| 3 | Saline | 6 wk | 17 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| LPS | 6 wk | 16 | 1.3±0.3* | 2.0±0.0* | 0.7±0.1* | 0.0±0.0 | 75.0* | 100.0* | 69.0* | 0.0 | |
| Saline | 8 wk | 16 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| LPS | 8 wk | 17 | 1.2±0.2* | 1.9±0.1* | 0.8±0.2* | 0.0±0.0 | 76.0* | 100.0* | 42.0* | 0.0 | |
LPS-induced sickness behavior in experiments 1–3. Sickness scores are the number of symptoms shown (mean±S.E.M.).
indicates p<0.05 contrasted to saline control.
Sickness behavior
Sickness behavior was scored 30min, 4h, 24h, and 48h following treatment using a 0–4 scale modified from Gibb et al. (Gibb et al., 2008). Mice were assessed for lethargy, huddling, ptosis and piloerection by an observer blind to treatment group. The number of symptoms displayed is the sickness score. The percentage of mice in each group displaying any symptoms of sickness was also calculated.
Ovariectomy and hormone priming
One week after LPS treatment, mice were ovariectomized under isoflurane anesthesia and allowed to recover for one week prior to behavioral testing. Mice were hormone primed with a subcutaneous injection of estradiol benzoate (EB; 2 μg in 0.1 ml sesame oil) followed 44h later by progesterone (P; 100 μg in 0.1 ml sesame oil containing 5% benzyl alcohol and 15% benzyl benzoate) or equivalent volumes of vehicle alone. Behavioral testing began 4h after the second injection.
Anxiety-like behavior testing
Testing began 2h after the start of the dark phase and was conducted under dim red light. Mice were tested in the light-dark box, followed by the elevated-plus maze, and, finally, the marble burying paradigm. To eliminate the possible confound of repeated hormone exposure, each animal was tested in all three paradigms in a single day with a 2 minute rest period between tests. All testing for each day was completed within 3.5 h. Behavioral tests were scored by an experimenter blind to treatment condition. The light/dark box and elevated plus maze were video recorded and scored using the Observer XT 8.0 software (Noldus Information Technology, Wageningen, The Netherlands). The marble burying test was scored by hand, as it was conducted.
Light/dark box
The light/dark box has been validated as a test of anxiety-like behavior in mice (Costall et al., 1989). A 45 cm long × 20 cm wide × 25 cm high chamber composed of one light and one dark compartment was used. The light compartment (30 cm long) was made of transparent polycarbonate, and the dark compartment (15 cm long) was made of opaque black polycarbonate and covered. The compartments were connected by an opaque black insert with an 8 cm long × 5cm high hole cut in the bottom to allow mice free movement between the sides. A white incandescent light was placed 12 inches from the top of the light compartment. Mice were placed in the light compartment facing away from the entry into the dark chamber and video recorded over a 5 min testing period. Movement between compartments was monitored, and the total time spent in the light compartment was quantified. Mice were considered to have entered a compartment when all four paws were in the compartment.
Elevated plus maze
The elevated plus maze is a validated and widely used test of anxiety-like behavior in mice (Lister, 1987). A standard mouse elevated plus maze constructed of gray non-reflective material with two open and two closed arms was used (Stoelting, Wood Dale, IL). Arms were 5 cm wide × 35 cm long, connected by a 5×5 cm2 center chamber. Closed arms were fitted with 15 cm high walls. The maze was elevated 40 cm off the floor. Mice were placed in the center chamber facing an open arm and video recorded for 5 minutes. Entries onto the open and closed arms and the total time spent on the open and closed arms and in the center chamber were quantified. Mice were considered to have entered an arm or the center chamber when all four paws crossed in that portion of the maze.
Marble burying test
A Plexiglass arena (18 × 38 cm) was filled 5 cm deep with Sani-chip bedding (P.J. Murphy Forest Products, Montville, NJ). Ten marbles (25 mm) were evenly spaced on top of the bedding. Mice were placed in the center of the arena and observed over a 30 minute testing period. The number of marbles covered ¾ or more with bedding was assessed every 5 minutes. The maximum number of marbles buried and the latency to bury 3 marbles were quantified.
Statistical Analyses
Data were analyzed using SPSS Grad Pack 11.5 software (SPSS, Inc., Chicago, IL) and SigmaStat 3.5 (Systat Software, Inc. San Jose, CA). Sickness scores were analyzed using Mann-Whitney Rank Sum tests (experiment 1) or Kruskal-Wallis tests followed by Tukey HSD comparisons (experiments 2 and 3) at each time point. Fisher Exact tests were used to compare the percentages of animals displaying sickness behavior at each time point. Anxiety-like behavior was analyzed by two-way (experiment 1) or three-way (experiments 2 and 3) ANOVAs followed by Tukey HSD Posthoc tests or Bonferroni planned contrasts. Criterion for significance was set at p<0.05.
Experiment 1: Does peripubertal LPS treatment cause lasting changes in regulation of anxiety-like behavior by ovarian hormones?
43 female C57Bl/6 mice were treated with LPS or saline at 6 weeks old. This time point was chosen, because LPS treatment at this time causes maximal reduction in adult sexual receptivity in response to ovarian hormones (Laroche et al., 2009a). Mice were ovariectomized one week after LPS treatment. One week after ovariectomy, mice were injected with EB followed by P (saline, n=10; LPS, n=12) or sesame oil-vehicle at each time (saline, n=10; LPS, n=11) and tested for anxiety-like behavior. Table 1 depicts the experimental timeline, including ages of shipping, treatment, ovariectomy and behavioral testing.
Table 1.
Experimental timelines
| Age | |||||
|---|---|---|---|---|---|
| Exp | Treatment | Shipping | Treatment | OVX | Testing |
| 1 | Saline | 3 wk | 6 wk | 7 wk | 8 wk |
| LPS | 3 wk | 6 wk | 7 wk | 8 wk | |
| 2 | Saline | 3 wk | 6 wk | 7 wk | 8 wk |
| LPS | 3 wk | 6 wk | 7wk | 8 wk | |
| Saline | 3 wk | 8 wk | 9 wk | 10 wk | |
| LPS | 3 wk | 8 wk | 9 wk | 10 wk | |
| 3 | Saline | 3 wk | 6 wk | 7 wk | 8 wk |
| LPS | 3 wk | 6 wk | 7 wk | 8 wk | |
| Saline | 3 wk | 8 wk | 9 wk | 10 wk | |
| LPS | 3 wk | 8 wk | 9 wk | 10 wk | |
Ages of shipping, LPS or vehicle treatment, ovariectomy (OVX) and behavioral testing for each experiment.
Experiment 2: Is the disruptive effect of LPS on later hormone regulation of anxiety-like behavior limited to the peripubertal period?
65 female C57Bl/6 mice were treated with LPS or saline at 6 or 8 weeks old and ovariectomized one week later. Eight weeks was chosen as a negative control because treatment with LPS at this age does not alter later sexual receptivity in response to ovarian hormones (Laroche et al., 2009a). One week after ovariectomy, mice were injected with EB followed by P (6wk saline, n=8; 6wk LPS, n=8; 8wk saline, n=8; 8wk LPS, n=8) or oil-vehicle at each time (6wk saline, n=8; 6wk LPS, n=8; 8wk saline, n=8; 8wk LPS, n=9) and tested for anxiety-like behavior. Table 1 depicts the experimental timeline, including ages of shipping, treatment, ovariectomy and behavioral testing.
Experiment 3: Does peripubertal LPS disrupt ovarian hormone regulation of anxiety-like behavior in an outbred strain of mice?
To rule out the possibility that the disruptive effects of peripubertal LPS on later hormone regulation of behavior are due to a genetic sensitivity specific to the inbred C57Bl/6 strain of mice, we also examined the effects of peripubertal LPS in an outbred strain, CD-1 mice. 66 female CD-1 mice were treated with LPS or saline at 6 or 8 weeks old and ovariectomized one week later. Mice were injected with EB followed by P (6wk saline, n=9; 6 wk LPS, n=8; 8wk saline, n=8; 8wk LPS, n=8) or oil-vehicle at each time (6wk saline, n=8; 6wk LPS, n=8; 8wk saline, n=8; 8wk LPS, n=9) and tested for anxiety-like behavior one week after ovariectomy. Table 1 depicts the experimental timeline, including ages of shipping, treatment, ovariectomy and behavioral testing.
Results
Experiment 1: Does peripubertal LPS treatment cause lasting changes in regulation of anxiety-like behavior by ovarian hormones?
Sickness behavior
As expected, LPS treatment induced sickness behavior (Table 2). Mann-Whitney rank sum tests revealed that LPS-treated mice displayed higher sickness scores than saline-treated mice at 30 min (U=360.0, T=310.0, p<0.001) and 4 hrs (U=57.5, T=612.5, p<0.001) post injection. Fisher Exact tests at each time point indicated that a greater percentage of LPS-treated mice, contrasted to saline-treated mice, displayed sickness behavior at 30 min (p<0.001) and 4 hrs (p<0.001) post-injection.
Light/dark box
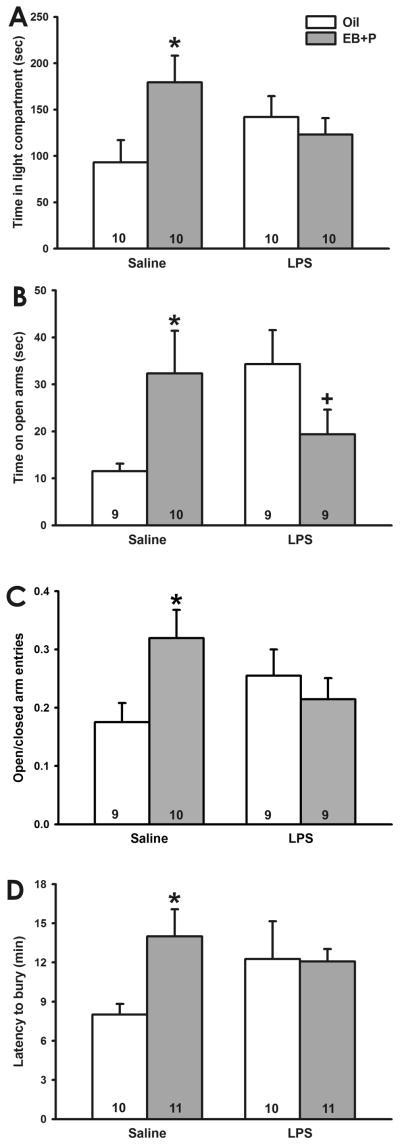
As predicted, LPS treatment altered hormonal regulation of anxiety-like behavior in the light/dark box. Although no main effects of treatment or hormone injections were found, a two-way ANOVA identified a significant treatment x hormone priming interaction effect (F1,37=7.22, p<0.02). Posthoc analyses indicated that, among saline-treated mice, EB followed by P treatment increased the amount of time spent in the light side of the chamber (q=3.98, p<0.01). In contrast, EB followed by P treatment did not alter the amount of time LPS-treated mice spent in the light side of the chamber (Figure 1A).
Fig. 1.
Effect of pubertal LPS on ovarian hormone regulation of anxiety-like behavior. A, Time spent in the light compartment of the light/dark box. (*, p<0.05 vs. oil) EB followed by P increased the amount of time spent in the light compartment by saline-, but not LPS-, treated mice. B, Time spent on the open arms of the elevated plus maze. (+, 0.05<p<0.1 vs. oil) EB followed by P increased the amount of time saline-treated mice spent on the open arms. In contrast, EB followed by P tended to decrease the amount of time LPS-treated mice spent on the open arms. C. Ratio of open/closed arm entries in the elevated plus maze. (*, p<0.05 vs. oil) EB followed by P increased the ratio of open/closed arm entries in saline-, but not LPS-, treated mice. D. Latency to bury marbles in the marble burying test. (*, p<0.05 vs. oil) EB followed by P increased the latency to bury in saline-, but not LPS-, treated mice. (Numbers at base of bars indicates sample size per group.)
Elevated plus maze
LPS treatment disrupted the influence of estradiol and progesterone treatment on anxiety-like behavior in the elevated plus maze. Four mice were excluded from this analysis because they fell from the maze multiple times. These mice belonged to several different treatment groups, suggesting that this was not due to treatment. No main effects of treatment or hormone priming were found on the amount of time spent on the open arms of the maze; however, a two-way ANOVA indicated a significant interaction effect (F1,34=8.29, p<0.01). Posthoc tests indicated that, among saline-treated mice, injection of EB followed by P increased the time spent on the open arms (q=3.11, p<0.05). In contrast, although it did not reach significance, EB followed by P tended to decrease the amount time LPS-treated mice spent on the open arms (q=2.63, p=0.072; Figure 1B). Oil treated mice that had been injected with LPS two weeks earlier spent more time in the open arms than mice injected with saline two weeks earlier (q=3.37, p<0.05).
There was also a significant treatment x hormone priming interaction effect (F1,34=5.25, p<0.05) on the ratio of open/closed arm entries, although no main effects of treatment or hormone priming were found. Post hoc tests indicated that, among saline-treated mice, EB followed by P increased the ratio of open/closed arm entries (q=3.48, p<0.02; Figure 1C). In contrast, EB followed by P did not alter the ratio of open/closed arm entries made by LPS-treated mice. LPS alone did not alter the ratio of open/closed arm entries.
Marble burying
No main effects of treatment or hormone priming were found on the latency to bury 3 marbles. Although it did not reach significance, the interaction between treatment and hormone priming did tend to influence latency to bury 3 marbles (F1,39=3.74, p=0.061). Bonferroni planned comparisons indicated that among saline-treated mice, EB followed by P increased the latency to bury 3 marbles (t=2.41, p<0.03, Bonferroni corrected). In contrast, EB followed by P did not alter the latency to bury 3 marbles in LPS-treated mice (Figure 1D). A two-way ANOVA found no effects of treatment, hormone priming, or the interaction between these factors on the maximum number of marbles buried.
Experiment 2: Is the disruptive effect of LPS on later hormone regulation of anxiety-like behavior limited to the peripubertal period?
Sickness behavior
As expected, LPS induced sickness behavior. Kruskal-Wallis tests indicated that mice treated with LPS displayed higher sickness scores than mice treated with saline at 30m (χ2=63.1, p<0.001), 4h (χ2=61.6, p<0.001), and 24h (χ2=8.7, p<0.05) post-injection. Tukey contrasts indicated that among animals treated at 6 weeks old, LPS-treated mice displayed higher sickness scores than saline-treated mice at 30 min (q=34.08, p<0.001), 4h (q=36.2, p<0.001), and 24h (q=3.19, p<0.05) post-injection. 8 weeks old, LPS-treated mice displayed higher sickness scores than saline-treated mice at 30m (q=56.13, p<0.001) and 4h (q=50.87, p<0.001) post-injection (Table 2). Fisher Exact tests indicated that a greater percentage of LPS-treated than saline-treated mice displayed sickness behavior at 30m (6wk p<0.001; 8wk p<0.001) and 4h (6wk p<0.001; 8wk p<0.001) post-injection, regardless of age of treatment. No age differences in the percentage of animals showing symptoms of sickness were found at any time point.
Light/dark box
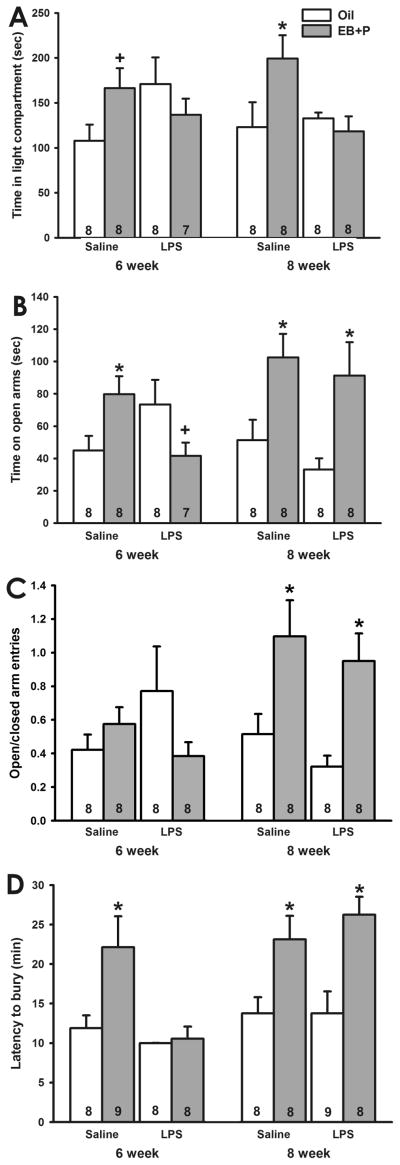
Although no main effects were identified, a three-way ANOVA indicated a treatment x hormone interaction effect (F1,54=10.30, p<0.005) on the amount of time spent in the light compartment. Posthoc analyses indicated that, among saline-treated mice, EB followed by P increased the amount of time mice treated at 8 weeks old spent in the light side of the light/dark box (q=3.96, p<0.01). Although it did not reach statistical significance, EB followed by P also tended to increase the amount of time mice treated with saline at 6 weeks old spent in the light compartment (q=2.59, p=0.077), consistent with the results of Experiment 1. In contrast, EB followed by P did not alter the amount of time LPS-treated animals spent in the light compartment, regardless of age of treatment (Figure 2A). Although it did not reach significance, oil-treated mice that had been injected with LPS at 6 weeks old tended to spend more time in the light compartment than mice treated with saline at 6 weeks (q=2.79, p=0.058). LPS treatment at 8 weeks did not alter the amount of time oil-treated mice spent in the light compartment.
Fig. 2.
Effect of age of treatment on LPS-induced disruption of ovarian hormone regulation of anxiety-like behavior. A, Time spent in the light compartment of the light/dark box. (*, p<0.05 vs. oil; +, 0.05<p<0.1 vs. oil) EB followed by P increased the amount of time saline- treated mice spent in the light compartment. LPS treatment prevented this increase at both ages of treatment. B, Time spent on the open arms of the elevated plus maze. (*, p<0.05 vs. oil; +, 0.05<p<0.1 vs. oil) EB followed by P increased the amount of time saline-treated mice spent on the open arms. LPS treatment at 6 weeks old reversed the direction of EB followed by P’s effect, whereas LPS treatment at 8 weeks old did not alter response to EB followed by P. C. Ratio of open/closed arm entries in the elevated plus maze. (*, p<0.05 vs. oil) EB followed by P increased the ratio of open/closed arm entries in mice treated at 8 weeks old, regardless of LPS treatment. D. Latency to bury marbles in the marble burying test. (*, p<0.05) EB followed by P increased the latency to bury in saline-treated mice. LPS treatment at 6, but not 8, weeks old prevented the EB followed by P-induced increase in the latency to bury. (Numbers at base of bars indicates sample size per group.)
Elevated plus maze
A three-way ANOVA identified a main effect of EB followed by P (F1,54=9.30, p<0.005) and an age x hormone interaction effect (F1,54=8.33, p<0.01) on the amount of time spent on the open arms of the elevated plus maze. Posthoc tests indicated that EB followed by P increased the amount of time mice previously injected with saline spent on the open arms, regardless of age of treatment (6wk, q=3.09, p<0.05; 8wk, q=3.35, p<0.05). EB followed by P also increased the amount of time mice treated with LPS at 8 weeks of spent on the open arms (q=4.06, p<0.01). In contrast, EB followed by P tended to reduce the amount of time mice treated with LPS at 6 weeks spent on the open arms (q=2.83, p=0.055; Figure 2B). Consistent with the light/dark box and Experiment 1, oil-treated mice that were injected with LPS at 6 weeks tended to spend more time than mice treated with saline at 6 weeks on the open arms (q=2.53, p=0.085). LPS treatment at 8 weeks did not alter the amount of time oil-treated mice spent on the open arms.
A main effect of hormone priming (F1,55=5.88, p<0.02) and a hormone x age interaction (F1,55=10.04, p<0.005) also impacted the ratio of open/closed arm entries. Posthoc analyses indicated that EB followed by P increased the ratio of open/closed arm entries made mice treated at 8 weeks, regardless of treatment (saline q=3.41, p<0.05; LPS q=3.81, p<0.02; Figure 2C). In contrast, EB followed by P did not alter the ratio of open/closed arm entries made by mice treated at 6 weeks old, regardless of treatment condition.
Marble burying
Treatment, age and hormone priming all influenced the latency to bury 3 marbles. Main effects of age (F1,56=11.38, p<0.001) and hormone priming (F1,56=22.57, p<0.001) and an age x treatment interaction (F1,56=5.69, p<0.05) were identified. Posthoc analyses indicated that, among saline-treated mice, EB followed by P increased the latency to bury 3 marbles, regardless of age of treatment (6wk q=4.79, p<0.005; 8wk q=3.67, p<0.02). Among LPS-treated animals, EB followed by P increased the latency of mice treated at 8 weeks old (q=4.90, p<0.005) to bury 3 marbles. In contrast, EB followed by P did not alter the latency of mice treated with LPS at 6 weeks old to bury marbles (Figure 2D). LPS treatment at either age did not alter the latency of oil-treated mice to bury 3 marbles.
A three-way ANOVA identified a main effect of hormone priming on the maximum number of marbles buried (F1,56=9.05, p<0.005). Mice primed with EB followed by P buried fewer marbles than those primed with oil. Treatment, age, and interactions of these three factors did not alter the number of marbles buried.
Experiment 3: Does peripuertal LPS disrupt ovarian hormone regulation of anxiety-like behavior in an outbred strain of mice?
Sickness behavior
As expected, Kruskal-Wallis tests indicated that LPS treatment induced sickness behavior at 30 min (χ2=37.3, p<0.001), 4h (χ2=63.3, p<0.001) and 24h (χ2=29.8, p<0.001). Posthoc contrasts indicated that LPS-treated mice, regardless of age of treatment, displayed higher sickness scores than saline-treated mice at 30m (6wk q=11.02, p<0.001; 8wk q=13.13, p<0.001), 4h (6wk q= 17.63, p<0.001; 8wk q=20.00, p<0.001), and 24h (6wk q=7.16, p<0.001; 8wk q=7.50, p<0.001) post-injection (Table 2). Sickness scores did not differ by age at any time point.
Fisher’s Exact tests indicated that a higher percentage of LPS-treated mice than saline-treated mice displayed sickness behavior at 30m (6wk p<0.001; 8wk p<0.001), 4h (6wk p<0.001; 8wk p<0.001), and 24h (6wk p<0.001; 8wk p=0.007) post-injection. Percentages of mice displaying sickness behavior did not differ by age at any time point.
Light/dark box
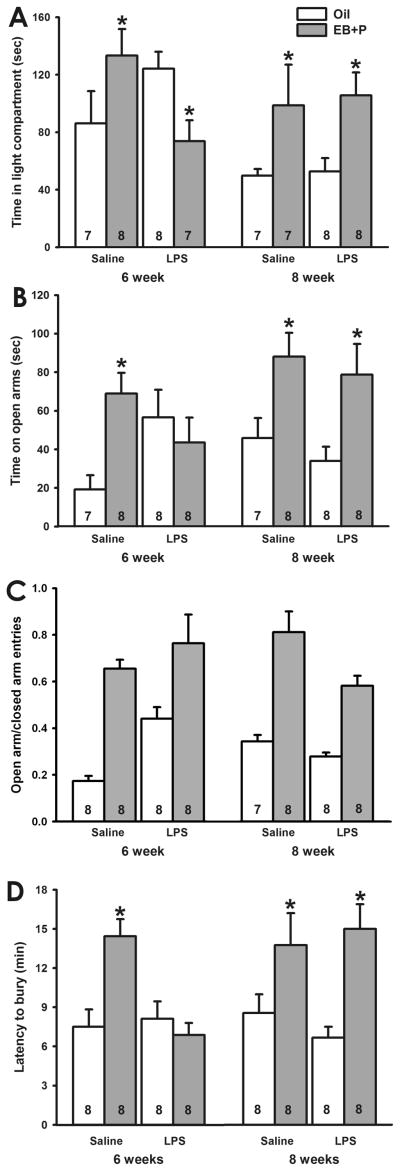
As expected, age, treatment and hormone priming all influenced the time spent on the light side of the light/dark box. A three-way ANOVA identified a main effect of age (F1,52=5.59, p<0.05), an age x hormone interaction effect (F1,52=4.60, p<0.05), and an age x treatment x hormone interaction effect (F1,52=4.96, p<0.05) on time spent in the light side. Posthoc tests indicated that EB followed by P increased the amount of time saline-treated mice spent on the light side of the chamber, regardless of age of treatment (6wk q=3.50, p<0.05; 8wk q=3.23, p<0.05). In mice treated with LPS at 8 weeks old, EB followed by P also increased the amount of time spent in the light compartment (q=3.26, p<0.05); however, in mice treated with LPS at 6 weeks old, EB followed by P reduced the amount of time mice spent in the light compartment (q=2.96, p<0.05; Figure 3A). Figure 3 about here
Fig. 3.
Effect of LPS on ovarian hormone regulation of anxiety-like behavior in CD-1 mice. A, Time spent in the light compartment of the light/dark box. (*, p<0.05 vs. oil) EB followed by P increased the amount of time spent in the light compartment by saline- treated mice, regardless of age of treatment. LPS treatment at 6 weeks old reversed the direction of the effects of EB followed by P, whereas LPS treatment at 8 weeks old did not alter the EB followed by P-induced increase time spent in the light compartment. B, Time spent on the open arms of the elevated plus maze. (*, p<0.05 vs. oil) EB followed by P increased the amount of time saline-treated mice spent on the open arms. LPS treatment at 6 weeks old prevented the EB followed by P-induced increase in time spent on the open arms. LPS treatment at 8 weeks did not alter the effect of EB followed by P. C, Ratio of open/closed arm entries in the elevated plus maze. (*, p<0.05 vs. oil) EB followed by P increased the ratio of open/closed arm entries. D. Latency to bury marbles in the marble burying test. (*, p<0.05) EB followed by P increased the latency to bury in saline-treated mice. LPS treatment at 6 weeks old prevented the EB followed by P-induced increase in latency to bury, whereas LPS treatment at 8 weeks did not alter the effect of EB followed by P. (Numbers at base of bars indicates sample size per group.)
Elevated plus maze
As predicted, age, treatment and hormone priming interacted to alter the amount of time spent on the open arms of the elevated plus maze. A three-way ANOVA identified a main effect of hormone (F1,55=11.42, p<0.005), an age x hormone interaction effect (F1,55=4.78, p<0.05), a treatment x hormone interaction effect (F1,55=6.27, p<0.02), and an age x treatment x hormone interaction effect (F1,55=6.92, p<0.02). Posthoc analyses indicated that EB followed by P increased the amount of time saline-treated mice spent on the open arms, regardless of age of treatment (6wk q=4.97, p<0.002; 8wk q=3.56, p<0.010). EB followed by P also increased the amount of time animals treated at with LPS at 8 weeks old spent on the open arms (q=3.97, p<0.005). In contrast, EB followed by P did not alter the amount of time mice treated at 6 weeks old spent on the open arms (Figure 3B). Consistent with Experiments 1 and 2, oil-treated mice that were injected with LPS at 6 weeks spent more time on the open arms than mice treated with saline at 6 weeks (q=4.27, p<0.01). LPS treatment at 8 weeks did not alter the amount of time oil-treated mice spent on the open arms.
A three-way ANOVA identified a main effect of hormone on the ratio of open/closed arm entries (p<0.001; Figure 4C). Mice primed with EB followed by P displayed more open/closed arm entries than those primed with oil. No effects of age, treatment, or the interactions of these three factors were identified (p>0.05).
Marble burying
As expected, age, treatment, and hormone priming all influence latency to bury marbles. A main effect of hormone (F1,57=20.44, p<0.001) and an age x treatment x hormone interaction effect (F1,57=7.14, p<0.02) were identified. Posthoc test indicated that EB followed by P increased the latency to bury marbles in saline-treated mice, regardless of age of treatment (6wk, q= 5.69, p<0.001; 8wk, q=4.18, p<0.05). EB followed by P also increased the latency to bury of mice treated with LPS at 8 weeks old (q=4.95, p<0.001). In contrast, EB followed by P did not alter the latency to bury of mice treated with LPS at 6 weeks old (Figure 3D).
A three-way ANOVA identified a main effect of age on the number of marbles buried (F1,58=4.09, p<0.05). Mice treated at 6 weeks old buried more marbles than those treated at 8 weeks old. Treatment, hormone priming, and interactions between these three factors did not alter the number of marbles buried.
Discussion
As hypothesized, peripubertal LPS injection disrupted the anxiolytic effects of ovarian hormones two weeks later in C57BL/6 mice. That is, in mice injected with saline during the pubertal period, estradiol and progesterone increased the amounts of time spent in the light side of the light/dark box and on the open arms of the elevated plus maze and the latency to bury marbles. Peripubertal LPS treatment interfered with each of these effects. This is consistent with previous findings suggesting that peripubertal LPS interferes with the display of sexual receptivity in response to ovarian hormones in adulthood (Laroche et al., 2009a). Further, peripubertal LPS disrupted the anxiolytic effects of ovarian hormones in both C57Bl/6 and CD-1 mice, suggesting that this is not due simply to a genetic effect in a single strain.
Interestingly, peripubertal LPS treatment caused a reversal in the direction of ovarian hormone regulation of anxiety-like behavior in some cases. That is, although estradiol and progesterone were anxiolytic in control mice, CD-1 mice treated with LPS during the peripubertal period displayed anxiogenic responses to ovarian hormones in the light/dark box. A similar trend is seen in C57Bl/6 mice in the elevated plus maze. Although it is unclear why peripubertal LPS caused the response to ovarian hormones to switch from anxiolytic to anxiogenic, peripubertal LPS appears to cause a similar reversal of the antidepressive effects of estradiol treatment. In control adult, female mice, estradiol reduced depression-like behavior in the forced swim and tail suspension tests, but estradiol increased depression-like behavior in mice treated with LPS during the peripubertal period (Ismail, DiGloria, Fitzpatrick, Kumlin, Olesen and Blaustein, unpublished data).
The disruptive effects of LPS on later response to ovarian hormones appear to be largely limited to the peripubertal period. This is in agreement with the apparent sensitive period for LPS to alter sexual receptivity in response to ovarian hormones. C57Bl/6 mice exposed to LPS between four and six weeks old display reduced sexual receptivity in response to ovarian steroids in adulthood, but exposure to LPS before or after this timeframe did not alter later response to ovarian hormones (Laroche et al., 2009a). It is unclear why LPS treatment at 8 weeks old interfered with the anxiolytic effect of estradiol and progesterone in the light/dark box in C57Bl/6 mice; this was not the case for the EPM and marble burying task in which LPS injection only at 6 weeks interfered with the later anxiolytic effects of EB followed by P. Although it is possible that this is specific to this cohort of mice, we cannot exclude the possibility that the sensitive period for the disruptive effect of LPS is both task- and strain-dependent. Most importantly, this was not seen in the CD-1 strain of mice.
We believe that the effects of LPS/immune challenge are specifically linked to the hormonal changes accompanying puberty. The sensitive period for LPS treatment to alter later response to ovarian hormones appears to be limited to 4–6 weeks of age (Laroche et al., 2009a). During this sensitive period, mice are unlikely to be sexually mature. Although they achieve vaginal opening around 27 days of age, C57Bl/6 mice in our laboratory still display constant cornified vaginal smears at six weeks old, and they do not begin estrous cycling until 60–65 days old (Ismail and Blaustein, unpublished data), so six weeks of age is still during the peripubertal period. Furthermore, ovariectomy prior to LPS treatment eliminates the lasting effects of immune challenge on response to ovarian hormones (Rappleyea, Ismail and Blaustein, unpublished data), suggesting that sensitivity to these effects of immune challenge is mediated by peripubertal hormonal changes.
In addition to preventing the anxiolytic effects of ovarian hormones, LPS treatment at six weeks, but not at eight weeks, also appeared to reduce baseline anxiety-like behavior in the light/dark box and elevated plus maze. This is consistent with previous research suggesting that adolescent environment, including chronic stress and alcohol exposure, can influence anxiety-like behavior in adulthood (Conrad and Winder, 2010). Additionally, several studies have demonstrated altered anxiety-like behavior in mice exposed to immune stressors during early development, although the direction of the change in anxiety-like behavior remains controversial. For example, some studies suggest that adult female mice exposed to LPS in utero display increased anxiety-like behavior (Hava et al., 2006; Lucchina et al., 2010), while others indicate that prenatal exposure to LPS reduces anxiety-like behavior in adulthood (Wang et al., 2010). The data presented here suggest that the peripubertal period is another developmental period during which basal adult anxiety-like behavior can be influenced by immune challenge. It is unclear why peripubertal LPS reduced baseline anxiety-like behavior in the light/dark box and elevated plus maze, but not the marble burying test; however, this is likely to be related to tests examining different aspects of anxiety-like behavior. The light/dark box and elevated plus maze are tests of non-specific anxiety-like behavior (Bourin, 1997), whereas the marble burying test has recently been discussed as a model of compulsive behavior, such as that observed in obsessive-compulsive disorder (Li et al., 2006).
Peripubertal exposure to LPS interfered with anxiolytic responses to ovarian hormones two weeks after termination of the stressor. Although it is unclear how long beyond this time point the effect on anxiolytic responses persists, the reduction in sexual receptivity following ovarian hormone treatment in mice shipped during the peripubertal period or injected with LPS during this time persisted for at least 11–12 weeks (Laroche et al., 2009a; Laroche et al., 2009b). This suggests that the changes in ovarian hormone regulation of anxiety-like behavior induced by peripubertal LPS treatment may be similarly long-lasting.
It should be noted that, in order to control for the timing of ovariectomy and testing relative to LPS treatment, age at the time of ovariectomy and testing differed by two weeks for mice treated at six versus eight weeks old. Although some evidence suggests that prepubertal ovariectomy can alter later responses to steroid hormones (De Jonge et al., 1988), it is unlikely that ovariectomizing the mice treated at 6 weeks old, late in the peripubertal period, can account for the lasting effect of LPS on later response to hormones. Previous data indicate that females ovariectomized at 7 weeks old show similar levels of sexual receptivity in response to ovarian hormones compared to females ovariectomized at 12 weeks old (Laroche et al., 2009a). Furthermore, peripubertal LPS consistently reduces the response to ovarian hormones, regardless of whether ovariectomy occurs at 7 or 12 weeks old (Laroche et al., 2009a). Similarly, previous data suggest age of testing does not alter levels of sexual receptivity or the effects of peripubertal LPS on these responses to ovarian hormones (Laroche et al., 2009a), suggesting that age at ovariectomy and testing are not likely to confound the data presented here.
The mechanisms by which peripubertal LPS alters later behavioral responses to ovarian hormones remain unclear. LPS induces the central and peripheral synthesis and release of cytokines (Hadid et al., 1999; Singh and Jiang, 2004; Vallieres and Rivest, 1997) and activates the hypothalamic-pituitary-adrenal axis (Spinedi et al., 1997; Spinedi et al., 1992). Many of the stressors that induce similar levels of corticosterone release do not alter behavioral response to ovarian hormones when administered during the peripubertal period (Laroche et al., 2009a). This suggests that acute changes in corticosterone alone cannot account for changes in the behavioral response to ovarian steroids. We cannot rule out acute changes in other components of the hypothalamic-pituitary-adrenal axis or cytokine concentrations as mediating factors.
Because peripubertal LPS treatment disrupts the response to ovarian steroids in many behavioral paradigms, including sexual (Laroche et al., 2009a), anxiety-like, depression-like (Ismail and Blaustein, unpublished data), and cognitive behaviors (Ismail and Blaustein, unpublished data), we believe that the mechanisms involved are specific to response to ovarian hormones, rather than to anxiety-like behavior. These studies do not indicate whether peripubertal LPS treatment is interfering specifically with the effects of estradiol, progesterone, or both. Both hormones influence the expression of anxiety-like behavior in female rodents. Estradiol influences anxiety-like behavior by acting on both estrogen receptor α and estrogen receptor β; anxiolytic effects appear to involve both receptor subtypes (Lund et al., 2005; Osterlund et al., 2005; Spiteri et al., 2010; Tomihara et al., 2008), whereas anxiogenic effects, such as those seen in response to chronic treatment with high doses of estradiol (Morgan and Pfaff, 2001; Morgan and Pfaff, 2002), appear to depend upon estrogen receptor α (Lund et al., 2005). Progesterone has anxiolytic effects in female rodents (Bitran et al., 1995; Mora et al., 1996a; Picazo and Fernandez-Guasti, 1995), but it can also counter the anxiolytic effects of estradiol in ovariectomized females (Diaz-Veliz et al., 1994). Regulation of anxiety-like behavior by progesterone has largely been attributed to its actions on the GABAA receptor, via conversion to allopregnanolone (Bitran et al., 1995); however, recent evidence suggests that nuclear progestin receptors may also mediate the anxiolytic effects of progesterone (Auger and Forbes-Lorman, 2008). This suggests that peripubertal LPS may alter hormonal regulation of anxiety-like behavior by changing steroid hormone receptor expression or neurosteroid synthesis.
The data presented here suggest that a peripubertal immune challenge can have a complex effect on the regulation of anxiety-like behaviors by ovarian hormones. From a practical design perspective, this indicates that the peripubertal environment should be considered when designing and interpreting experiments that address the regulation of behavior by ovarian hormones. More importantly, these data also suggest that the peripubertal period is a critical time for the development of response to ovarian hormones with regard to disorders of mental health.
Research Highlight.
Ovarian hormones are anxiolytic in female mice.
A pubertal immune stressor disrupts the anxiolytic effects of ovarian hormones.
Puberty is a critical time for the development of response to ovarian hormones.
Acknowledgments
This work was supported by NIH grant NS 19327 and an Isis grant from the Society for Women’s Health Research. KMO was supported in part by NIH training grant T32 MH020051.
We would like to thank Sarah Servattalab and Celeste DiGloria for their technical assistance.
Footnotes
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auger CJ, Forbes-Lorman RM. Progestin receptor-mediated reduction of anxiety-like behavior in male rats. PLoS ONE. 2008;3:e3606. doi: 10.1371/journal.pone.0003606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 3.Bourin M. Animal models of anxiety: are they suitable for predicting drug action in humans? Pol J Pharmacol. 1997;49:79–84. [PubMed] [Google Scholar]
- 4.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 5.Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 7.Conrad KL, Winder DG. Altered anxiety-like behavior and long-term potentiation in the bed nucleus of the stria terminalis in adult mice exposed to chronic social isolation, unpredictable stress, and ethanol beginning in adolescence. Alcohol. 2010 doi: 10.1016/j.alcohol.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 9.De Jonge FH, Muntjewerff JW, Louwerse AL, van de Poll NE. Sexual behavior and sexual orientation of the female rat after hormonal treatment during various stages of development. Horm Behav. 1988;22:100–115. doi: 10.1016/0018-506x(88)90034-7. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Veliz G, Alarcon T, Espinoza C, Dussaubat N, Mora S. Ketanserin and anxiety levels: influence of gender, estrous cycle, ovariectomy and ovarian hormones in female rats. Pharmacol Biochem Behav. 1997a;58:637–642. doi: 10.1016/s0091-3057(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Veliz G, Butron S, Benavides MS, Dussaubat N, Mora S. Gender, estrous cycle, ovariectomy, and ovarian hormones influence the effects of diazepam on avoidance conditioning in rats. Pharmacol Biochem Behav. 2000;66:887–892. doi: 10.1016/s0091-3057(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Veliz G, Dussaubat N, Mora S. Ketanserin effects on rat behavioral responses: modifications by the estrous cycle, ovariectomy and estradiol replacement. Pharmacol Biochem Behav. 1997b;57:687–692. doi: 10.1016/s0091-3057(96)00394-2. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Veliz G, Soto V, Dussaubat N, Mora S. Influence of the estrous cycle, ovariectomy and estradiol replacement upon the acquisition of conditioned avoidance responses in rats. Physiol Behav. 1989;46:397–401. doi: 10.1016/0031-9384(89)90010-3. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Veliz G, Urresta F, Dussaubat N, Mora S. Effects of estradiol replacement in ovariectomized rats on conditioned avoidance responses and other behaviors. Physiol Behav. 1991;50:61–65. doi: 10.1016/0031-9384(91)90498-d. [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Veliz G, Urresta F, Dussaubat N, Mora S. Progesterone effects on the acquisition of conditioned avoidance responses an other motoric behaviors in intact and ovariectomized rats. Psychoneuroendocrinology. 1994;19:387–394. doi: 10.1016/0306-4530(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson C, Nobel S, Winblad B, Schultzberg M. Expression of interleukin 1 alpha and beta, and interleukin 1 receptor antagonist mRNA in the rat central nervous system after peripheral administration of lipopolysaccharides. Cytokine. 2000;12:423–431. doi: 10.1006/cyto.1999.0582. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Guasti A, Picazo O. Anxiolytic actions of diazepam, but not of buspirone, are influenced by gender and the endocrine stage. Behav Brain Res. 1997;88:213–218. doi: 10.1016/s0166-4328(97)00047-8. [DOI] [PubMed] [Google Scholar]
- 18.Galeeva A, Tuohimaa P. Analysis of mouse plus-maze behavior modulated by ovarian steroids. Behav Brain Res. 2001;119:41–47. doi: 10.1016/s0166-4328(00)00341-7. [DOI] [PubMed] [Google Scholar]
- 19.Gibb J, Hayley S, Gandhi R, Poulter MO, Anisman H. Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: Circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain Behav Immun. 2008;22:573–589. doi: 10.1016/j.bbi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Hadid R, Spinedi E, Chautard T, Giacomini M, Gaillard RC. Role of several mediators of inflammation on the mouse hypothalamo-pituitary-adrenal axis response during acute endotoxemia. Neuroimmunomodulation. 1999;6:336–343. doi: 10.1159/000026393. [DOI] [PubMed] [Google Scholar]
- 21.Hava G, Vered L, Yael M, Mordechai H, Mahoud H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev Psychobiol. 2006;48:162–168. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- 22.Herrenkohl LR. Prenatal stress disrupts reproductive behavior and physiology in offspring. Ann N Y Acad Sci. 1986;474:120–128. doi: 10.1111/j.1749-6632.1986.tb28003.x. [DOI] [PubMed] [Google Scholar]
- 23.Herrenkohl LR, Politch JA. Effects of prenatal stress on the estrous cycle of female offspring as adults. Experientia. 1978;34:1240–1241. doi: 10.1007/BF01922982. [DOI] [PubMed] [Google Scholar]
- 24.Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laroche J, Gasbarro L, Herman JP, Blaustein JD. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 2009a;150:3717–3725. doi: 10.1210/en.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009b;150:2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Morrow D, Witkin JM. Decreases in nestlet shredding of mice by serotonin uptake inhibitors: comparison with marble burying. Life Sci. 2006;78:1933–1939. doi: 10.1016/j.lfs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 30.Lucchina L, Carola V, Pitossi F, Depino AM. Evaluating the interaction between early postnatal inflammation and maternal care in the programming of adult anxiety and depression-related behaviors. Behav Brain Res. 2010;213:56–65. doi: 10.1016/j.bbr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 32.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 33.Marvan ML, Chavez-Chavez L, Santana S. Clomipramine modifies fluctuations of forced swimming immobility in different phases of the rat estrous cycle. Arch Med Res. 1996;27:83–86. [PubMed] [Google Scholar]
- 34.Marvan ML, Santana S, Chavez CL, Bertran M. Inescapable shocks accentuate fluctuations of forced swimming immobility in different phases of the rat estrous cycle. Arch Med Res. 1997;28:369–372. [PubMed] [Google Scholar]
- 35.Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Horm Behav. 1997;31:75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- 36.Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996a;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 37.Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996b;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 38.Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- 39.Morgan MA, Pfaff DW. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behav Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 41.Olivera-Lopez JI, Molina-Hernandez M, Tellez-Alcantara NP, Jaramillo MT. Estradiol and neuropeptide Y (intra-lateral septal) reduce anxiety-like behavior in two animal models of anxiety. Peptides. 2008;29:1396–1403. doi: 10.1016/j.peptides.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Osterlund MK, Witt MR, Gustafsson JA. Estrogen action in mood and neurodegenerative disorders: estrogenic compounds with selective properties-the next generation of therapeutics. Endocrine. 2005;28:235–242. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- 43.Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiol Behav. 2006;87:828–835. doi: 10.1016/j.physbeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Differential effects of exogenous and endogenous estrogen on anxiety as measured by elevated T-maze in relation to the serotonergic system. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 45.Papaioannou A, Dafni U, Alikaridis F, Bolaris S, Stylianopoulou F. Effects of neonatal handling on basal and stress-induced monoamine levels in the male and female rat brain. Neuroscience. 2002;114:195–206. doi: 10.1016/s0306-4522(02)00129-x. [DOI] [PubMed] [Google Scholar]
- 46.Paris A, Kelly P, Ramaley JA. Effects on short-term stress upon fertility. II After puberty. Fertil Steril. 1973;24:546–552. doi: 10.1016/s0015-0282(16)39796-5. [DOI] [PubMed] [Google Scholar]
- 47.Paris AL, Ramaley JA. Adrenal-gonadal relations and fertility: the effects of repeated stress upon the adrenal rhythm. Neuroendocrinology. 1974;15:126–136. doi: 10.1159/000122301. [DOI] [PubMed] [Google Scholar]
- 48.Picazo O, Estrada-Camarena E, Hernandez-Aragon A. Influence of the post-ovariectomy time frame on the experimental anxiety and the behavioural actions of some anxiolytic agents. Eur J Pharmacol. 2006;530:88–94. doi: 10.1016/j.ejphar.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 49.Picazo O, Fernandez-Guasti A. Anti-anxiety effects of progesterone and some of its reduced metabolites: an evaluation using the burying behavior test. Brain Res. 1995;680:135–141. doi: 10.1016/0006-8993(95)00254-n. [DOI] [PubMed] [Google Scholar]
- 50.Picazo O, Fernandez-Guasti A. Antianxiety effects of diazepam and buspirone in rats under different hormonal status. Proc West Pharmacol Soc. 1998;41:205–206. [PubMed] [Google Scholar]
- 51.Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281–293. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- 52.Romeo RD, Bellani R, McEwen BS. Stress-induced progesterone secretion and progesterone receptor immunoreactivity in the paraventricular nucleus are modulated by pubertal development in male rats. Stress. 2005;8:265–271. doi: 10.1080/10253890500489320. [DOI] [PubMed] [Google Scholar]
- 53.Romeo RD, Karatsoreos IN, McEwen BS. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm Behav. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Romeo RD, Richardson HN, Sisk CL. Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential. Neurosci Biobehav Rev. 2002a;26:381–391. doi: 10.1016/s0149-7634(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 55.Romeo RD, Wagner CK, Jansen HT, Diedrich SL, Sisk CL. Estradiol induces hypothalamic progesterone receptors but does not activate mating behavior in male hamsters (Mesocricetus auratus) before puberty. Behav Neurosci. 2002b;116:198–205. doi: 10.1037//0735-7044.116.2.198. [DOI] [PubMed] [Google Scholar]
- 56.Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Spinedi E, Chisari A, Pralong F, Gaillard RC. Sexual dimorphism in the mouse hypothalamic-pituitary-adrenal axis function after endotoxin and insulin stresses during development. Neuroimmunomodulation. 1997;4:77–83. doi: 10.1159/000097324. [DOI] [PubMed] [Google Scholar]
- 59.Spinedi E, Suescun MO, Hadid R, Daneva T, Gaillard RC. Effects of gonadectomy and sex hormone therapy on the endotoxin-stimulated hypothalamo-pituitary-adrenal axis: evidence for a neuroendocrine-immunological sexual dimorphism. Endocrinology. 1992;131:2430–2436. doi: 10.1210/endo.131.5.1330501. [DOI] [PubMed] [Google Scholar]
- 60.Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Agmo A. The role of the estrogen receptor alpha in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav Brain Res. 2010;210:211–220. doi: 10.1016/j.bbr.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 61.Tomihara K, Soga T, Nomura M, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2008 doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol. 2007;17:245–256. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 63.Vallieres L, Rivest S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1beta. J Neurochem. 1997;69:1668–1683. doi: 10.1046/j.1471-4159.1997.69041668.x. [DOI] [PubMed] [Google Scholar]
- 64.Wang H, Meng XH, Ning H, Zhao XF, Wang Q, Liu P, Zhang H, Zhang C, Chen GH, Xu DX. Age- and gender-dependent impairments of neurobehaviors in mice whose mothers were exposed to lipopolysaccharide during pregnancy. Toxicol Lett. 2010;192:245–251. doi: 10.1016/j.toxlet.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 65.Zhou HR, Harkema JR, Yan D, Pestka JJ. Amplified proinflammatory cytokine expression and toxicity in mice coexposed to lipopolysaccharide and the trichothecene vomitoxin (deoxynivalenol) J Toxicol Environ Health A. 1999;57:115–136. doi: 10.1080/009841099157818. [DOI] [PubMed] [Google Scholar]