Abstract
To study the mechanism of centrosome duplication in cycling cells, we established a novel system of multiple centrosome formation in two types of cells: CHO cells treated with a cdk1 inhibitor (RO3306) and DT40 cells, in which Cyclin-dependent kinases (Cdks) were knocked out by chemical genetics. Cdk1-inactivated cells initiated DNA replication and centrosome duplication at the onset of S phase. They became arrested at the end of G2, but the centrosome cycle continued to produce supernumerary centrioles/centrosomes without DNA endoreplication in those cells. Centrosomes were amplified in a highly synchronous and reproducible manner: all of them were located next to the nucleus and spread widely apart from each other with several μm in distance. Double knockout of Cdk1 and Cdk2 caused cell cycle arrest at G1/S and centrosomes were no longer duplicated. However, cells continued to grow and increased their volume over 10-fold during 48 hr of culture. Centrosome components, including γ-tubulin and Cep135, were synthesized and accumulated during the arrest, allowing rapid centrosome multiplication upon recovery from the cell cycle arrest or expression of exogenous Plk4 in G1/S cells. Thus centrosome amplification results from the discoordination of the centrosome cycle from the progression of other cell cycle events, which is controlled by different levels of Cdk activities.
Keywords: Centrosomes, cyclin-dependent kinases, chemical genetics, RO3306, DT40 cells
INTRODUCTION
In animal cells, microtubules are organized from the centrosome, which is composed of a pair of centrioles and a surrounding amorphous cloud of pericentriolar material. During M phase, a centrosome localizes at each spindle pole to assemble the mitotic spindle and provide the orientation for chromosome movement at anaphase. To ensure high fidelity of chromosome segregation, it is essential to establish spindle bipolarity during M phase by producing exactly two centrosomes. The centrosome number is dependent primarily on the number of centrioles. To maintain the precise number of centrosomes, therefore, the cycle of centriole duplication must be tightly coupled with the cell cycle through coordination with other events of the cell cycle [Kuriyama and Borisy, 1981]. When the cell cycle progression is perturbed, cells are likely to produce abnormal numbers of centrioles/centrosomes. Balczon et al. [1995] first demonstrated that some mammalian cells, including CHO cells, undergo repeated cycles of centrosome duplication during S phase arrest with hydroxyurea. This results in the formation of multiple centrosomes, and now is widely used as an assay for identification of the molecular components important for centrosome duplication [for example, see Meraldi et al., 1999]. Similar centrosome amplification has been reported in mammalian cells during the extended duration of G2 phase caused by DNA damage [Dodson et al., 2004].
The cell cycle is controlled by complexes of cyclins and Cyclin-dependent kinases (Cdks): Cdk2/cyclin E regulates the initiation of DNA replication at G1/S, while entry into mitosis is triggered by Cdk1/cyclin B. Although requirement of Cdk2 in centrosome duplication at the onset of S phase has been well-established in both somatic and embryonic cells [Meraldi et al., 1999; Matsumoto et al., 1999; Hinchcliffe et al., 1999; Lacey et al., 1999], a role of Cdk1 in centrosome assembly remains elusive. In Drosophila wing disc cells, Cdk1 inactivation results in abnormal centriole elongation and centrosome duplication [Vidwans et al., 2003]. When Cdk1 is depleted from human fibrosarcoma cells (HT2-19) carrying a single, conditionally-active cdk1 allele, G2/M-arrested cells continue to multiply both DNA and centrosomes [Laronne et al., 2003]. Using DT40 chicken lymphocytes expressing an analog-sensitive Cdk1 mutant, Hochegger et al. [2007] also demonstrated multiple centrosome formation by Cdk1 inactivation. Unlike fibrosarcoma cells, however, DT40 cells do not undergo DNA endoreplication during G2/M arrest, and the process of centrosome amplification has not yet been analyzed in detail. Most recently, Loncarek et al. [2010] have shown that Cdk1 inhibition allows mammalian cells to reduplicate new centrioles onto the preexisting mother centrioles during the G2/M arrest. For better understanding of the Cdk1 function, it is necessary to compare and evaluate cells with inactivated Cdk1 by different treatments.
In contrast to embryonic cells that originate as large cells containing enough materials for construction of 1,000–2,000 centrosomes [Gard et al., 1990], somatic cells must double their cellular constituents during each cell cycle. To produce supernumerary centrosomes by progression of the centrosome cycle, somatic cells must continue their growth cycle during the arrest of cell division. In fact, when cyclin D1, a key regulator of physiological cell proliferation, is overexpressed in liver cells, multiple centrosomes are formed [Nelsen et al., 2005]. However, little attention has been paid to the relation of centrosome formation and cell growth. To study the control of centriole/centrosome formation by Cdks during the cell cycle and growth, we directly compared mammalian and DT40 cells where Cdks were suppressed by either a small chemical inhibitor (RO3306) [Vassilev et al., 2006; Loncarek et al., 2010] or chemical genetics [Stockwell 2000; Hochegger et al., 2007]. Both types of cells arrested at G2/M induced supernumerary centrosomes organized in a unique pattern distinctive from any other cells with multiple centrosomes reported previously. Centrosome amplification results from uncoupling of the centrosome cycle from other cell cycle events, providing a clear connection between centrosome assembly and cell growth/protein synthesis.
RESULTS
Formation of multiple centrosomes in Cdk1-inactivated CHO and DT40 cells
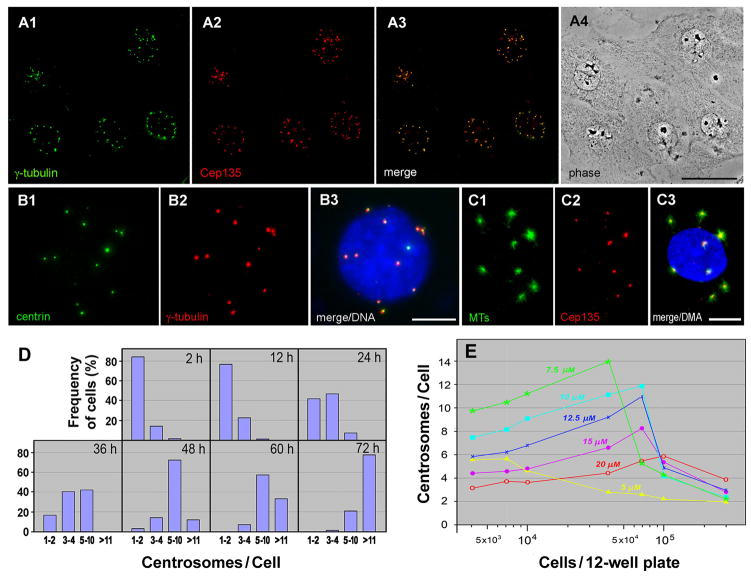
A chemical inhibitor, RO3306 (RO), targets the activity of cyclin-dependent kinases, in particular Cdk1 [Vassilev et al., 2006]. When CHO cells were treated with the drug, the cell cycle was arrested and cell division did not occur. However, cells continued to duplicate centrosomes inducing supernumerary centrosomes (Fig. 1A). Multiple centrosomes were probed for centriole/centrosome markers tested thus far (γ-tubulin, Cep135, centrin in Fig. 1A–C; pericentrin, cenexin, PCM1, Cep170, Nek2, hPOC5, CPAP, ninein, and C-Nap1, not shown), and capable of initiating microtubule polymerization (Fig. 1C). The centrosomes showed unique distribution: they were located next to the nucleus and spread widely apart from each other with several μm in distance. This made it easy to count the precise number of centrosomes, showing that cells amplified centrosome in a highly uniform manner: ~70% of cells induced 5–10 and >11 centrosomes after 48 and 72 hr of drug treatment (Fig. 1D).
Figure 1.
Formation of multiple centrosomes in Cdk1-inactivated CHO cells. A–C: Double fluorescence staining of cells with anti-γ-tubulin and anti-Cep135 antibodies (A1, A2), and GFP-centrin and anti-γ-tubulin antibody (B1, B2). In C, cells were immunostained with anti-α-tubulin and anti-Cep135 antibodies after brief recovery from nocodazole treatment to repolymerize short microtubules onto centrosomes. Cells were treated with 10 μM RO for 48 hr before fixation. Merged images are shown with (B3, C3) and without DAPI-staining (A3), and A4 is a phase image of CHO cells. Bars, 50 μm (A) and 10 μm (B, C). D: Frequency histograms of cells with 1–2, 3–4, 5–10, and over 11 centrosomes after treatment with 10 μM RO for different periods of time. E: Multiple centrosome formation in CHO cells depends on both seeded cell densities and RO concentrations. After plating various numbers of cells in 12-well plates, cells were treated for 48 hr with RO at different concentrations.
Centrosome formation depended on both the concentration of RO and the seeded cell densities (Fig. 1E). The highest number of centrosomes was detected in cells plated at a density of 4–7 × 104 per 12-well plate and treated with 7.5 to 10 μM of RO. When plated at higher cell densities, cell cycle arrest was incomplete and duplicated chromosomes eventually were segregated by the completion of cell division. On the other hand, higher concentrations of the drug caused not only a complete arrest of the cell cycle, but they also blocked the process of centrosome assembly. The most significant effect of RO on centrosome amplification was observed in CHO cells. It was, however, almost negligible in other types of mammalian cells tested thus far, including HeLa, U2OS, HCT116, and hTERT-RPE1 cells, where centrosomes were never spread apart as in CHO cells during extended periods of RO treatment (~ 96 hrs).
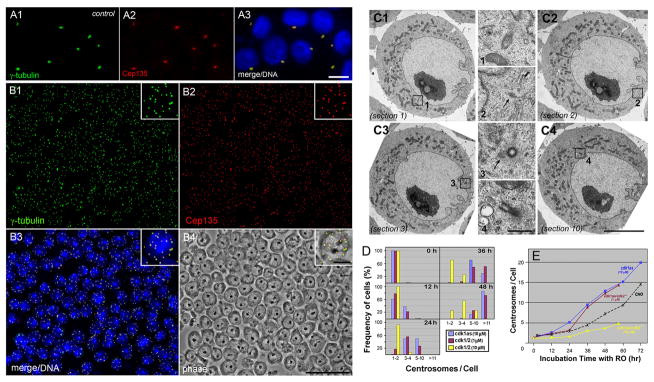
To confirm the effect of Cdk1 on centrosomes, we employed chemical genetics to alter the Cdk1 function in DT40 chicken lymphocytes by addition of an exogenous ligand [Stockwell, 2000]. Cdk1as is a Cdk1 knockout DT40 cell line expressing a mutant Cdk1 sensitive to inhibition by an ATP analog (1NM-PP1; PP1) [Hochegger et al., 2007]. Like wild type DT40 cells, Cdk1as cells typically include one or closely associated two centrosomes in the absence of PP1 (Fig. 2A). However, when cells were treated with 10 μM PP1, the Cdk1 kinase activity was selectively blocked, resulting in the formation of supernumerary centrosomes (Fig. 2B). As we observed in CHO cells, multiple centrosomes in DT40 cells were spread widely apart and emerged in a highly synchronous manner. Fig. 2C shows electron micrographs of a cdk1as cell containing multiple centrioles at different perinuclear locations (outlined areas numbered 1–4): 4–5 centrioles/centriole-like structures were detected in ten serial ultrathin sections (total ~1 μm thickness). By fluorescence microscopy, we counted over 11 centrosomes in nearly 90% of cells after 48 hr of culture at 37°C (Fig. 2D, blue columns). The time course analysis of centrosome amplification shows that cdk1as and CHO cells, both are dividing every ~12 hr at 37°C under normal culturing conditions, steadily increased the centrosome number after 12–24 hr lag time. Cdk1as cells multiplied centrosomes faster than CHO cells (Fig. 2E, blue vs. black dotted lines). Finally, the formation of multiple centrosomes was efficiently blocked by treatment with microtubule drugs, including nocodazole and taxol (Fig. S1).
Figure 2.
Formation of multiple centrosomes in DT40 cells. A, B: Immunostaining of cdk1as cells with anti-γ-tubulin (A1, B1) and anti-Cep135 (A2, B2) antibodies. Cells were treated with (B) and without (A) 10 μM PP1 for 48 hr. Bars, 10 μm (A and inlets of B) and 50 μm (B). C: Electron micrographs of PP1-treated cdk1as cells containing multiple centrioles. Four out of ten serial thin sections are shown in C1 to C4. 4–5 centrioles/centriole-like structures are detected in total ~1 μm thickness of sections as outlined in C1 to C4. Each structure is seen at a higher magnification in middle columns numbered 1 to 4. Note that centrioles at the juxanuclear reposition are spread widely apart from each other. Arrows indicate a position of centriole-like structures. Bars, 5 μm (C1 to C4) and 0.5 μm (1 to 4). D: Frequency histograms of cdk1as (blue) and cdk1/2 (cdk1as/cdk2−/−; brown and yellow) cells containing 1–2, 3–4, 5–10, and over 11 centrosomes after treatment with 1 or 10 μM PP1 for different periods of time. E: Time course analysis of centrosome amplification in CHO and DT40 cells. After incubation of CHO cells with 10 μM RO (black dots), cdk1as cells with 10 μM PP1 (blue), and cdk1as/cdk2−/− cells with either 1 μM (brown) or 10 μM (yellow) PP1 for various times, cells were fixed and double stained with γ-tubulin and Cep135 antibodies to count the number of centrosomes.
Centrosome duplication in cells arrested at G2/M, but not G1/S, without DNA endoreplication
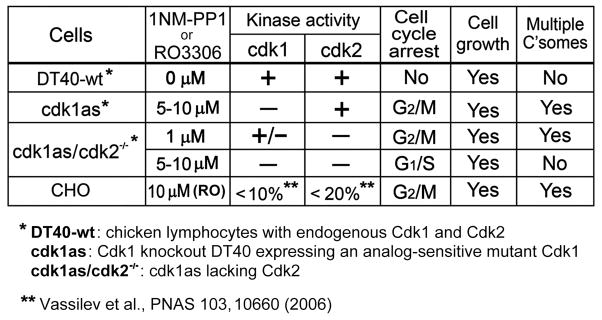
It is well-established that centrosome duplication requires Cdk2 rather than Cdk1. To further study the effect of Cdks on centrosomes, we analyzed cdk1as/cdk2−/− cells, which were generated by disruption of the Cdk2 gene in cdk1as cells [Hochegger et al., 2007]. Treatment of cells with 5–10 μM PP1 caused inactivation of the mutant Cdk1 in a Cdk2-null background, thus both Cdk1 and Cdk2 were inactivated in these cells [Hochegger et al., 2007]. Under these conditions, cells were no longer able to support centrosome amplification (yellow columns and line in Fig. 2D and 2E). However, when incubated with lower concentrations of PP1 (~1 μM), cells expressed a partial cdk1 activity [Hochegger et al., 2007], resulting in centrosome multiplication with kinetics similar to that of cdk1as cells treated with 10 μM PP1 (brown columns and line in Fig. 2D and 2E). Therefore, the Cdk2 activity in Cdk1 knockout cells and a partial Cdk1 activity in Cdk2 knockout cells are required for assembly of supernumerary centrosomes during the cell cycle arrest, which is summarized in Fig. 3.
Figure 3.
Summary of the progression of cell cycle events and centrosome amplification in Cdk-inactivated DT40 and CHO cells.
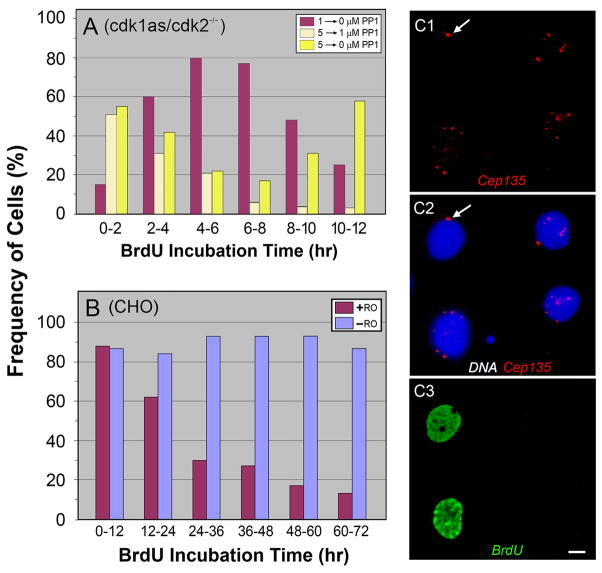
It has been previously shown that RO-treated mammalian cells and PP1-treated cdk1as cells become arrested at G2/M [Vassilev et al., 2006; Hochegger et al., 2007]. We could also detect 80–90% of CHO (Fig. S2A) and over 90% of cdk1as cells (Fig. S2B and C) entering M phase within 30 min after removal of the drugs. A similar change in the mitotic index was noted in cdk1as/cdk2−/− cells upon release from the cell cycle arrest by treatment with 1 μM PP1 (Fig. S2C). To identify the cell cycle stage at which cdk1as/cdk2−/− cells were arrested by 5–10 μM PP1, we measured DNA synthesis by incorporating BrdU into cells. In Fig. 4A, cdk1as/cdk2−/− cells were first treated with 1 or 5μM PP1 for 12–24 hr, and then shifted to different drug concentrations at time zero for monitoring their ability to synthesize DNA every 2 hr. Cells arrested at G2/M by 1 μM PP1 show a peak of DNA synthesis at 4–8 hr after removal of the drug (Fig. 4A, brown columns). This is a striking difference from cells incubated with 5 → 0 μM PP1 (yellow columns), which initiated BrdU incorporation right after removal of the drug, indicating that these cells had been attested at the border of G1 and S and/or S phase. There was a second peak of DNA synthesis at 10–12 hr in the 5 → 0 μM cells, which was not detected in 5 → 1 μM PP1 cells (Fig. 4A, light yellow columns). Apparently, these latter cells became arrested again at the end of G2 after completion of DNA synthesis. It should be pointed out that the G1/S arrest was maintained in cdk1as/cdk2−/−cells treated with 5–10 μM for longer periods of time (> 48 hr; data not shown). Taken together, these results indicate that multiple centrosomes were induced in cells arrested at G2/M, but not G1/S phase (Fig. 3).
Figure 4.
DNA synthesis in DT40 (A) and CHO (B) cells measured by BrdU incorporation. A: Cdk1as/cdk2−/− cells were treated with 1 or 5 μM PP1 for 12–24 hr. At time zero, the drug concentration was shifted 1→0 (brown), 5→1 (light yellow), and 5→0 μM (yellow) PP1, and then BrdU incorporation was measured every 2 hr. Cells treated with 1 and 5 μM PP1 had peaks of BrdU incorporation at 4–8 and 0–2 hr after removal of the drug, suggesting that the cells were arrested at G2/M and G1/S, respectively. Note that a second peak detected in cdk1as/cdk2−/− (5 → 0 μM) is absent in cells treated with (5 →1 μM) PP1. B: Mitotic CHO cells were plated in the presence or absence of 10 μM RO and incubated with BrdU for 12 hr to determine their ability to undergo DNA replication. C: Immunofluorescence staining of CHO cells with anti-Cep135 (C2) and anti-BrdU (C3) antibodies. C2 is a merged image of Cep135 and DAPI staining. Harvested mitotic cells were cultured for 12 hr in the presence of 10 μM RO, and then BrdU was added for an additional 36 hr of culture. Two cells negative for BrdU incorporation into nuclei induced multiple centrosomes, indicating that G2 arrested cells duplicate centrosomes without DNA endoreplication. Arrows indicate a position of non-amplified, clustering centrosomes. Bar, 10 μm.
Cdk1as cells do not endoreplicate DNA during the extended period of G2 arrest [Hochegger et al., 2007]. This was confirmed by BrdU incorporation in cdk1as and cdk1as/cdk2−/− cells during treatment with 10 μM and 1 μM PP up to 60 hr, respectively (data not shown). On the other hand, Cdk1-inactivated human fibrosarcoma cells are capable of undergoing multiple rounds of DNA replication during the G2/M arrest [Laronne et al., 2003]. To determine whether the difference is due to species-specificity (avian vs. mammalian), we determined DNA replication in RO-treated CHO cells (Fig. 4B). Harvested mitotic cells were plated in the presence or absence of RO, then BrdU incorporation was monitored every 12 hr, during which the vast majority of control cells replicate DNA. Because RO does not inhibit DNA synthesis, ~90% of the drug-treated cells replicated DNA during the period of first 12 hr incubation. Afterwards, the number of RO-treated cells positive for BrdU staining gradually decreased. Thus, unlike human fibrosarcoma cells, Cdk1-inactivated CHO cells do not endoreplicate DNA during the G2/M arrest. This was further confirmed by flow cytometric measurements, indicating that CHO cells maintained DNA profiles typical for the G2-M phase during 48 hr incubation with RO (not shown).
To obtain more direct evidence, we visualized centrosomes in BrdU-treated cells (Fig. 4C). After the incubation of harvested mitotic cells with RO for 12 hr, BrdU was added to CHO cells for an additional 36 hr culture with RO. In Fig. 4C3, the nuclei of two cells were positive for BrdU incorporation; apparently they may have not completed DNA replication during the 12 hr culture. The other two cells did not incorporate BrdU, but they produced multiple centrosomes (Fig. 4C1), implying that DNA endoreplication is not required for centrosome amplification. It is worth mentioning that, while almost all, if not all, of the cells with BrdU-negative nuclei induced multiple centrosomes during the 48 h incubation with RO, some cells positive for DNA replication were unable to overduplicate centrosomes (arrows). This is probably due to incomplete arrest of the cell cycle at the end of G2 phase.
Growth of Cdk-inactivated cells as measured by increase of the cell volume
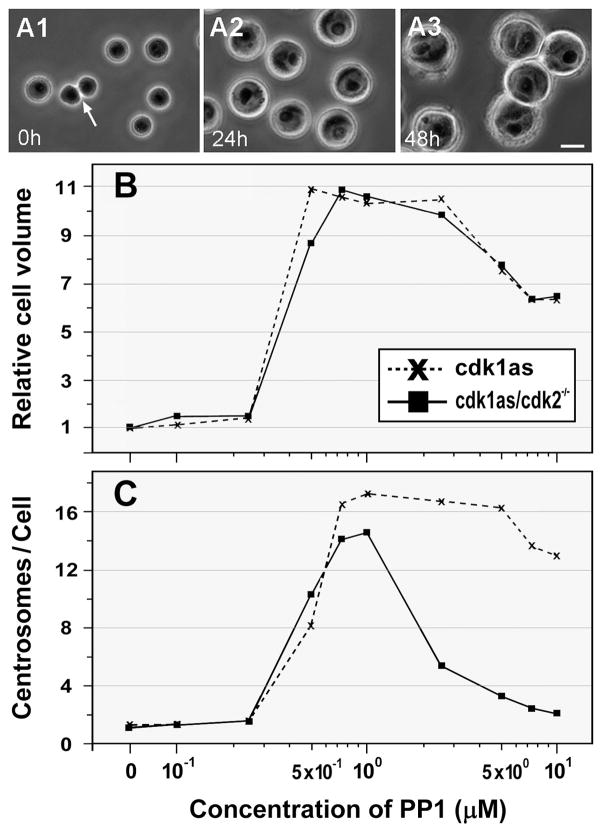
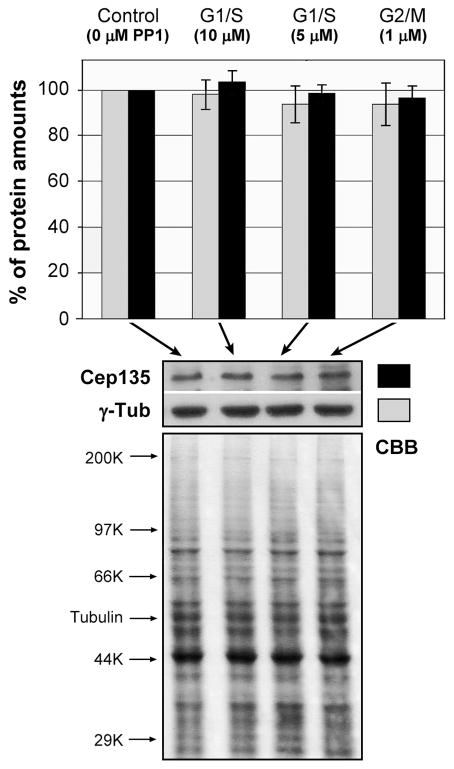
Spherical DT40 cells are typically measured ~10 μm in diameter. During 10 μM PP1 treatment, cdk1as cells increased in size in a highly synchronous manner (Fig. 5A). Fig. 5B shows the change in the volume of both cdk1as and cdl1as/cdk2−/− cells during 48 hr of treatment with various concentrations of PP1. Although no significant difference was noted below a concentration of 0.25 μM PP1, incubation with ~0.5 μM drug resulted in an abrupt increase in the volume of cdk1as cells up to 10-fold; thereafter the cell size gradually declined on treatment with drug concentrations greater than 2.5 μM. Cdk1as/cdk2−/− cells also showed almost identical patterns of the increase in cell mass or size, suggesting that regardless of the stage of cell cycle arrest (G1/S or G2/M), Cdk-inactivated cells continued to grow by synthesizing macromolecules (Fig. 3). Indeed, quantitative immunoblotting analysis shows that cdk1as/cdk2−/− cells treated with 5 and 10 μM (G1/S arrest) and 1 μM PP1 (G2/M arrest) contained almost identical amounts of Cep135 and γ-tubulin to control cells (Fig. 6).
Figure 5.
Analysis of cell size and numbers of centrosomes in DT40 cells with inactivated Cdks. A: Phase-contrast microscopy of cdk1as cells treated with 10 μM PP1 for 0 (A1), 24 (A2), and 48 (A3) hr. An arrow indicates two daughter cells formed after cell division. Bar, 10 μm. B: Relative cell volume of cdk1as and cdk1as/cdk2−/− cells after incubation with various concentrations of PP1 for 48 hr. C: The average number of centrosomes induced in cdk1as and cdk1as/cdk2−/− cells after treatment for 48 hr with various concentrations of PP1. Centrosome overduplication is blocked in cdk1as/cdk2−/− cells treated with over 1 μM PP1.
Figure 6.
Quantification of γ-tubulin (grey) and Cep135 (black) in cdk1as/cdk2−/− cells arrested at G1/S and G2/M phase by treatment with 1–10 μM PP1 for 48 hr. Protein amounts loaded on each well were measured by quantifying both Commassie (CBB) stained whole cell lysates and immunoblotted α-tubulin using NIH ImageJ software programs. Experiments were repeated at least four times to calculate the average numbers.
We next counted the number of centrosomes induced in PP1-treated cells (Fig. 5C). Over 15 centrosomes were formed in cdk1as and cdk1as/cdk2−/− cells after 48 hr of incubation with 0.5–1.0 μM PP1. Cdk1as cells maintained the highest number of centrosomes after treatment up to ~5 μM of the drug, showing that centrosomes are multiplied with kinetics similar to those of cell volume/growth. In contrast, the centrosome numbers sharply declined in double knockout cells treated with over 2.5 μM of PP1. Thus, in Cdk1/Cdk2 double knockout cells, the centrosome cycle stops, while the growth cycle measured by cell volume continues (Fig. 3). It is reasonable to suggest that cell growth is prerequisite for centrosome duplication. Indeed, suppression of cyclin D1, a key regulator of cell growth, interfered with centrosome amplification in CHO cells during 48 hr of culture with RO (Fig. S3).
Rapid centrosome assembly upon release of the G1/S arrest
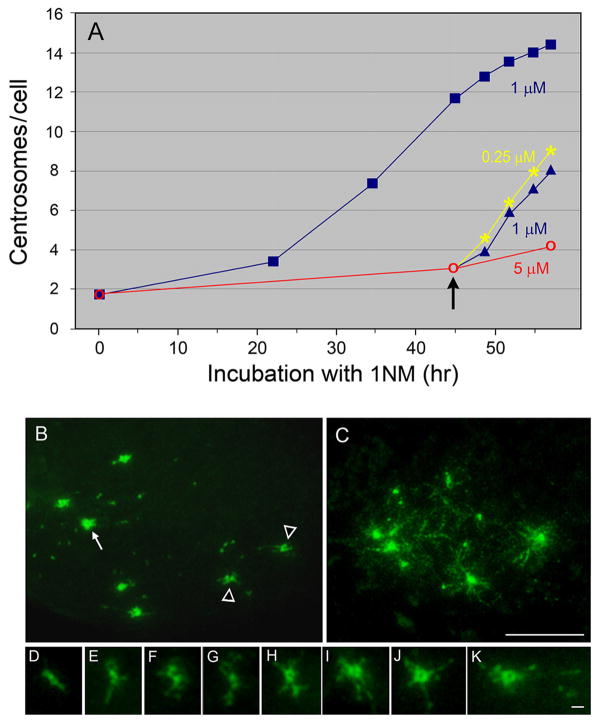
Despite growing in size, cdk1as/cdk2−/− cells are unable to amplify centrosomes during G1 arrest. To understand the relationship between centrosome amplification and cell growth, we counted the centrosome number in cdk1as/cdk2−/− cells cultured under various drug treatments (Fig. 7A). Cells treated with 5 μM PP1 for 45 hr were arrested at G1 and produced an average of ~3.1 centrosomes. By lowering the drug concentration to either 0.25 μM or 1 μM, the Cdk1 activity was partially restored, allowing cells to initiate DNA replication (not shown). During the subsequent 12 hr incubation, these cells assembled significantly more centrosomes (~5 to 6) than cells continuously cultured in the same medium containing either 1 μM or 5 μM PP1 (~1 to 2). These results further support that, although unable to assemble centrosomes, cells arrested at G1/S continue to accumulate centrosomal components to induce rapid centrosome assembly upon recovery from the arrest.
Figure 7.
Acceleration of centrosome assembly in cdk1as/cdk2−/− cells during recovery from G1 arrest. A: The average numbers of centrosomes induced in cdk1as/cdk2−/− cells treated with different concentrations of PP1. After 45 hr of culture in a medium containing 5 μM PP1 (red), cells were shifted to either 0.25 (yellow) or 1 μM PP1 (blue triangles) (arrow). During an additional ~12 hr culture, these cells induced more centrosomes than cells cultured continuously with 1 and 5 μM PP1 (blue squares). B–K: Immunofluorescence staining of cdk1as/cdk2−/− cells undergoing rapid centrosome assembly. γ-Tubulin staining reveals the presence of numerous small spots scattered throughout the cytoplasm (B). A ring-like centriole/centrosome (arrow, corresponding to H–K at a high magnification) and precursor-like centrosomal structures (arrowheads, corresponding to D–G at a higher magnification) are seen. In C–K, thin fiber-like amorphous materials associate with the centrosomes (C, H to K) and precursor-like structures (D–G). Bars, 10 μm (B–C) and 1μm (D–K).
Cells undergoing active centrosome formation frequently revealed the presence of unusual structures (Fig. 7B). While some centrosomes showed a typical ring-like structure by γ-tubulin staining (Fig. 7H–K, corresponding to an arrow in 7B), others resembled a simple bar or horseshoe-like shape (Fig. D–G, corresponding to arrowheads in 7B), which may represent precursors of the centrioles/centrosomes. In addition, numerous γ-tubulin-containing spots were scattered in the cytoplasm (Fig. 7B). The size of these spots was variable, but they were much smaller than centrioles/centrosomes. Equally prominent were fine fibers, often in a punctate pattern, emanating from the center where the centrosome was present (Fig. 7C). As a result, centrosomes/putative precursor-like structures became surrounded by fibrous amorphous materials (Fig. 7C–K).
Induction of multiple centrosomes in G1-arrested cells by Plk4 expression
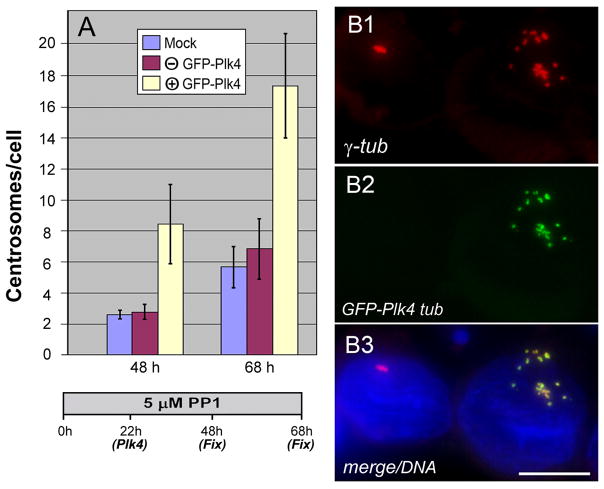
Centriole biogenesis is triggered by a signal(s) mediated by Zyg1/Plk4 kinases in a wide range of species [O’Connell et al., 2001; Habendanck et al., 2005; Bettencourt-Dias et al., 2005]. To determine whether Plk4 is able to induce centrosome amplification in G1 cells, we introduced exogenous chicken Plk4 into G1/S-arrested cells. In Fig. 8A, cdk1as/cdk2−/− cells were preincubated with 5 μM PP1 for 22 hr before introduction of Plk4 by transfection, and further cultured with 5 μM PP1 prior to fixation at 48 and 68 hr. G1 cells expressing GFP-Plk4 induced 3–4 times more centrosomes than non-expressing cells on same coverslips (Fig. 8A, B), indicating that Plk4 initiated assembly of centrosomes in G1 cells where the centrosomal replication machinery was still off. It is noteworthy that, unlike centrosomes amplified in cells arrested at G2, multiple centrosomes in Plk4-expressing cells showed a limited degree of dispersion (Fig. 8B).
Figure 8.
Centrosome amplification in G1/S-arrested cells by expression of exogenous Plk4. A: Histograms of the average numbers of centrosomes counted in mock transfected (blue), and transfected cells expressing (yellow) and non-expressing (brown) GFP-tagged chicken Plk4 during PP1 treatment. B: Cells expressing and non-expressing GFP-Plk4 (B2) were stained with γ-tubulin antibody (B1). After treatment with 5 μM PP1 for 22 hr, cells were transfected with GFP-Plk4 and further cultured prior to fixation at 48 and 68 hr. A merged image with DAPI-staining is shown in B3. Only cells expressing GFP-Plk4 amplified centrosomes. Bar, 10 μm.
DISCUSSION
This study describes the formation of multiple centrosomes in CHO and DT40 cells with inactivated Cdks. Inactivation of Cdk1 by either a chemical inhibitor or chemical genetics causes the cell cycle arrest at G2/M, but active Cdk2 allowed cells to proceed the centrosome cycle and the growth cycle, resulting in the production of multiple centrosomes (Fig. 3). When Cdk1 and Cdk2 were simultaneously knocked out, neither DNA synthesis nor centrosome duplication took place. However, cells were still able to grow to continue synthesis of cellular components, including those molecules necessary for centrosome assembly (Fig. 3). By treatment of cdk1as/cdk2−/− cells with 1 μM PP1, the Cdk1 activity was partially restored [Hochegger et al., 2007], which was sufficient to compensate for the Cdk2 function to initiate DNA replication and centrosome duplication (Fig. 3). In RO-treated CHO cells arrested at G2/M, centrosome amplification was less efficient at higher cell densities and with lower concentrations of the drug (Fig. 1E). Because these cells eventually entered M phase and completed cell division, the inactivation of cdk1 was apparently insufficient to completely arrest the cells at the end of G2. Fewer centrosomes were also observed in CHO cells with increasing drug concentrations, which was likely due to the inhibition of not only Cdk1 but also Cdk2. This is in good agreement with the previous report [Vassilev et al., 2006] that Cdk2 is a target of RO with ~1/10 of the sensitivity of Cdk1
Cdk-inactivated DT40 and CHO cells revealed unique properties of multiple centrosomes. First, cells increased the number of centrosomes in a highly synchronous and reproducible manner (Figs. 1D and 2C). All centrosomes were located next to the nucleus and widely-separated from each other (Figs. 1A–C and 2B). No such centrosomal distribution has ever been reported in cells with supernumerary centrosomes induced by other treatments, such as hydroxyurea [Balczon et al., 1995; Kuriyama et al., 2007], DNA damage [Dodson et al., 2004], and Plk4 overexpression [Habedanck et al., 2005; Bettencourt-Dias et al., 2005]. We also observed Plk4-induced multiple centrosomes showing a limited degree of dispersion in G1/S-arrested DT40 cells (Fig. 8B). It is thus highly likely that centrosomal dispersion occurs in a cell cycle-dependent manner. Centrosomes/mitotic spindle poles separates at the onset of M phase, which is triggered by Cdk1-cyclin B1 activity [Blangy et al., 1995]. In CHO and DT40 cells, however, Cdk1 inactivation may be insufficient to completely block centrosome separation, whereas other mitotic events, such as chromosome condensation and nuclear envelope breakdown, are efficiently suppressed. If cells failed to multiply centrosomes, these centrosomes were generally close to each other (arrows in Fig. 4C). It would be interesting to determine whether centrosome separation is essential for amplification of centrosomes in Cdk1-inactivated cells.
The most striking effect of RO on centrosome multiplication in cultured mammalian cells was observed in CHO cells. Other cells tested thus far showed only a limited degree of centrosome amplification under various conditions of drug treatment. It has been shown that CHO cells are one of only a few mammalian cell lines capable of inducing multiple centrosomes by treatment with hydroxyurea [Balczon et al., 1995]. Thus the specificity of RO effects on mammalian cells may be in part due to the unique properties of the centrosome in CHO cells. Loncarek et al. [2010] have recently reported centriole reduplication in RO-treated human cells. There are, however, several critical differences between CHO and human cells. First, the centrosome amplification in CHO cells was much more robust than human cells, which was evident even when we compared the number of centrosomes labeled with γ-tubulin antibody in CHO cells with that of centrioles revealed by GFP tagged centrin in human cells (Fig. 1 vs. Loncarek, et al., 2010, Fig. 1). In addition, unlike CHO/DT40 cells, human cells appear not to induce centrosomes that spread widely apart. These results may indicate that the mechanism controlling multiple centrosome formation does not entirely overlap between CHO and human cells. It remains to be determined whether CHO and DT40 cells undergo de novo assembly of centrioles/centrosomes to amplify centrosomes during Cdk inactivation.
Cdk1-inactivated human fibrosarcoma cells, but not CHO and DT40 cells, undergo multiple rounds of DNA endoreplication during centrosome duplication. This may be attributed to the difference of Cdk activity necessary to block cell division, but not the progression of centrosome cycle and cell growth among different types of cells. The incomplete inactivation of Cdk1 in fibrosarcoma cells leaves residual kinase activity, which was sufficient in allowing them to enter M phase. However, the APC/C activity detected in these cells [Laronne et al., 2003] became prematurely activated to degrading geminin and ultimately leading to origin licensing and DNA endoreplication. Thus, the residual Cdk1 activity remaining in fibrosarcoma cells may be higher than in CHO/DT40 cells, or fibrosarcoma cells may require a level of the Cdk1 activity higher than CHO/DT40 cells in order to suppress the APC/C activity.
The number of centrosomes steadily increased in Cdk-inactivated cells during drug treatment. Although the timing of centrosome duplication in CHO cells was slower than DT40 cells, RO-treatment allowed CHO cells to amplify centrosomes in a more quick and synchronous manner than hydroxyurea treatment (7.0 and 9.4 centrosomes after 60 hr incubation with hydroxyurea and RO, respectively) [Balczon et al., 1995; Kuriyama et al., 2007]. Centrosome components, such as γ-tubulin and Cep135, are synthesized and accumulated during the growth of G1/S-arrested cells (Fig. 6), which facilitates rapid centrosome assembly upon release of the cell cycle arrest (Fig. 7). Because the kinetics of centrosome multiplication was almost parallel to the increase of cell volume (Fig. 5), it is likely that RO-treated CHO cells grow and increase their size more rapidly and synchronously than cells blocked by hydroxyurea. These results are consistent with the recent report [Collins et al., 2010] that multiple centrosomes emerged quickly in hydroxyurea-treated CHO if cells had accumulated centrosome components by pretreatment with colcemid to block centrosome assembly.
G1/S-arrested cells continuously grow and accumulate centrosome proteins in an almost identical manner to G2/M cells undergoing active centrosome assembly. Nonetheless, G1/S cells were unable to assemble centrosomes, suggesting that some activities or component(s) necessary for initiation of centrosome assembly had been missing in these cells. We demonstrated that Plk4 is one of the candidate molecules (Fig. 8). Plk4 is targeted for degradation by SCF/Slimb [Cunha-Ferreira et al., 2009; Rogers et al., 2009], and Fode et al. [1996] has provided evidence that Plk4 transcripts are lost in mid-G1. In addition, the kinase is inherently unstable through the mechanism of autoregulation [Holland et al., 2010]. It is thus likely that exogenous Plk4 expressed in cdk1as/cdk2−/− cells at G1 might have been partially degraded. This could be a reason why the average number of centrosomes amplified in Plk4-expressed cells was slightly smaller than in cells released from the G1 arrest (Figs. 7A and 8A). Because cdk1as/cdk2−/− cells are able to produce and store enough materials for construction of multiple centrosomes, it would be useful to develop an in vitro system of centrosome assembly by supplementing DT40 cell extracts with exogenous Plk4 proteins.
CONCLUSIONS
A novel system of multiple centrosome formation was developed in CHO and DT40 cells by inactivation of Cdk1 or Cdk2. Cells with inactivated Cdk1 were arrested at G2/S, but the centrosome cycle continued to produce supernumerary centrioles/centrosomes in these cells. Similar centrosome amplification was induced in Cdk2-inactivated DT40 cells, in which a partial Cdk1 activity was expressed, suggesting that Cdk1 and Cdk2 share an essential function of driving the centrosome cycle in a cell cycle-independent manner. When both Cdk1 and Cdk2 were inactivated, the cell cycle stopped at G1/S and centrosomes were no longer duplicated. However, cells continued to grow and accumulate molecules necessary to assemble new centrosomes. Thus robust centrosome amplification was induced upon release of the cell cycle arrest, or by expression of exogenous Plk4, one of the initiators of centriole biogenesis, in G1/S arrested cells.
MATERIALS AND METHODS
Cell culture, synchronization, and drug treatment
Parental CHO and CHO cells expressing GFP-tagged centrin2 [Kuriyama et al., 2007] were cultured in Ham’s F-10 medium containing 10% fetal bovine serum (FBS). Mitotic synchronization was done as reported previously [Ohta et al., 2002]. For induction of multiple centrosomes in CHO cells, cells were plated at a density of 4–7 × 104 per 12-well plate and incubated with 10 μM RO3306 (RO; Alexis Biochemicals). To avoid inhibition of cell division by the drug, RO was added to synchronized mitotic cell populations at ~1 hr after completion of cell division. Chicken DT40 mutants (cdk1as), in which endogenous Cdk1 was replaced by an ATP analog-sensitive mutant and cdk1as lacking Cdk2 (cdk1as/cdk2−/−) were established as described previously [Hochegger et al., 2007]. Cells were cultured in RPMI1640 containing 10% FBS, 1% chicken serum, and 50 nM β-mercaptoethanol in a 5% CO2 incubator at 37°C, and treated with an ATP analog, 1NM-PP1 (PP1; Cellular Genomics), at the final concentrations indicated in each experiment.
Transfection
Chicken cDNAs were synthesized from isolated DT40 mRNA by RT-PCR, and then used as a template for amplification of a Plk4-coding sequence with primers designed from the predict Gallus gallus Plk4 mRNA sequence (Accession number XR_026997.1). Plk4 DNA was amplified and ligated into pEGFP-C1 vector (Clontech). Transfection was done by Amaxa nuclear transfection (Lonza) according to the manufacturer’s protocols. Briefly, 5×106 cells were suspended in 100 μl of a Kit T solution and then mixed with 5 μg of plasmid DNA resuspended in 100 μl of the same solution. After electroporation using the B023 program, cells were immediately recovered in 10 ml of a warm culture medium containing PP1.
Immunofluorescence staining and measurement of centrosomes/cell volume
CHO cells cultured on coverslips were fixed with methanol at −20°C. DT40 cells in a suspension culture (~4×104 per 0.2 ml) were applied to a single cytofunnel (Thermo Shandon) and cytocentrifuged onto a glass slide at 2,000 rpm for 10 min in a Shandon Cytospin 4. Glass slides were fixed by dipping into cold methanol as above. After rehydration in PBS containing 0.05% Tween-20, cells were immunostained for 30 min at 37°C using following primary antibodies: mouse monoclonal anti-γ-tubulin (Sigma-Aldrich), rabbit anti-Cep135 [Ohta et al., 2002], and monoclonal anti-α-tubulin (Sigma) antibodies. A mixture of fluorescein-conjugated anti-mouse IgG plus IgM and Texas red-conjugated anti-rabbit IgG antibodies (Jackson ImmunoResearch) was used as secondary antibodies.
Microscopic observations were carried out using a Nikon Eclipse microscope with a 100X oil immersion objective (N.A. 1.4) and a Photometrics CoolSNAP camera. 0.1–0.2 μm image slices were merged and further deconvoluted using the SlideBook 4.1 program. For statistical analysis of centrosome numbers, 100–500 cells were examined in each sample of γ-tubulin staining and experiments were repeated at least 3 times. For determination of DT40 cell volumes, images of spherical cells in dishes were acquired by phase-contrast microscopy and the radius of each cell was measured using Adobe Photoshop Measure Tool programs to calculate the volume (4/3 × πr3). 100–200 cells were examined in each sample to calculate the mean volume of cells.
Quantification of centrosome proteins
Cdk1as/cdk2−/− cells were treated with 0, 1, 5, and 10 μM PP1 for 48 hr and washed with PBS three times before extraction with SDS sample buffer. After boiling for 15 min, proteins were separated on 7.5% SDS-PAGE and transferred to nitrocellulose membranes. γ-Tubulin and Cep135 bands were visualized by blotting with primary antibodies and HRP-conjugated anti-mouse (Jackson ImmunoResearch) and anti-rabbit (Pierce Scientific) secondary antibodies using commercially available HRP substrates (Luminate Western Substrates, Millipore). The amounts of centrosome proteins and gel loading standard proteins (immunoblotted α-tubulin and Commassie-stained proteins of whole cell lysates) were quantified using NIH ImageJ software programs. Experiments were repeated at least four times to calculate the average number of protein amounts.
Measurement of DNA synthesis
CHO and DT40 cells were incubated with 10–20 μM BrdU for the indicated period of time before fixation with methanol. After rehydration, coverslips were rinsed with distilled water, and then soaked in 2M HCl for 30 min before immunostaining with monoclonal anti-BrdU antibodies (BD Biosciences). BrdU incorporation was determined in 100–200 cells to construct one histogram.
Electron microscopy
Cdk1as cells treated with 10 μM PP1 for 48 hr were fixed with 2.5% glutaraldehyde in 100 mM Pipes at pH 7.0, 1 mM EGTA, 1 mM MgCl2 for 30 min at room temperature. After washing with PBS, the cells were postfixed in 1% OsO4 for 30 min, and then block-stained in 2% uranyl acetate for 1 h. The samples were dehydrated, infiltrated, and embedded in Embed 812 plastic as described previously [Ohta et al., 2002]. Serial thin sections of specimens were cut with a diamond knife and stained with uranyl acetate/lead citrate for examination with a Philips CM12 Transmission Electron Microscope.
Supplementary Material
Acknowledgments
We thank Suanur Cureoglu and Cassandra Kowalski for technical assistance, and Gib Ahlstrand for electron microscopic assistance . This work was supported by grants from NIH (R.K.), Wellcome Trust (H.H.) and Science Foundation Ireland (C.G.M.). E.S. is supported by a CRUK Project Grant.
Abbreviations
- RO
RO3306
- Cdks
Cyclin-dependent kinases
- PP1
1NM-PP1
- Plk4
polo-like kinase 4
References
- Balczon R, Bao L, Zimmer WE, Brown K, Zinkowski RP, Brinkley BR. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J Cell Biol. 1995;130:105–115. doi: 10.1083/jcb.130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Blangy A, Lane HA, d'Hérin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Collins ES, Homick JE, Durcan TM, Collins NS, Archer W, Karanjeet KB, Vaughan KT, Hinchcliffe EH. Centrosome biogenesis continues in the absence of microtubules during prolonged S-phase arrest. J Cell Physiol. 2010;225:454–465. doi: 10.1002/jcp.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Ferreira I, Rodrigues-Martins A, Bento I, Riparbelli M, Zhang W, Laue E, Callaini G, Glove DM, Bettencourt-Dias M. The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr Biol. 2009;19:1–7. doi: 10.1016/j.cub.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Dodson H, Bourke E, Jeffers LJ, Vagnarelli P, Sonoda E, Takeda S, Earnshaw WC, Merdes A, Morrison C. Centrosome amplification induced by DNA damage occurs during a prolonged G2 phase and involves ATM. EMBO J. 2004;23:3864–3873. doi: 10.1038/sj.emboj.7600393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fode C, Binkert C, Dennis JW. Constitutive expression of murine Sak-a suppresses cell growth and induces multinucleation. Mol Cell Bio. 1996;16:4665–4672. doi: 10.1128/mcb.16.9.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DL, Hafezi S, Zhang T, Doxsey SJ. Centrosome duplication continues in cycloheximide-treated Xenopus blastulae in the absence of detectable cell cycle. J Cell Biol. 1990;110:2033–2042. doi: 10.1083/jcb.110.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habendanck R, Stierhof Y-D, Wilkinson CJ, Nigg EA. The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- Holland AJ, Lan W, Niessen S, Hoover H, Cleveland DW. Polo-like kinase 4 activity limits centrosome overduplication by autoregulating its own stability. J Cell Biol. 2010;188:191–198. doi: 10.1083/jcb.200911102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochegger H, Dejsuphong D, Sonoda E, Saberi A, Rajendra E, Kirk J, Hunt T, Takeda S. An essential role for Cdk1 in S phase control is revealed via chemical genetics in vertebrate cells. J Cell Biol. 2007;178:257–268. doi: 10.1083/jcb.200702034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Borisy GG. Centriole cycle in Chinese hamster ovary cells as determined by whole mount electron microscopy. J Cell Biol. 1981;91:814–821. doi: 10.1083/jcb.91.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Terada Y, Lee KS, Wang CL. Centrosome replication in hydroxyurea-arrested CHO cells expressing GFP-tagged centrin2. J Cell Sci. 2007;120:2444–2453. doi: 10.1242/jcs.008938. [DOI] [PubMed] [Google Scholar]
- Lacey KR, Jackson PK, Stearns T. Cyclin-dependent kinase control of centrosome duplication. Proc Natl Acad Sci USA. 1999;96:2817–2822. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronne A, Rotkopf S, Hellman A, Gruenbaum Y, Porter ACG, Brandeis M. Synchronization of interphase events depends neither on mitosis nor on cdk1. Mol Biol Cell. 2003;14:3730–3740. doi: 10.1091/mbc.E02-12-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncarek J, Hergert P, Khodjakov A. Centriole reduplication during prolonged interphase requires procentriole maturation governed by Plk1. Curr Biol. 2010;20:1277–1282. doi: 10.1016/j.cub.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Hayashi K, Nishida E. Cyclin-dependent kinase 2 (cdk2) is required for centrosome duplication in mammalian cells. Curr Biol. 1999;9:429–432. doi: 10.1016/s0960-9822(99)80191-2. [DOI] [PubMed] [Google Scholar]
- Meraldi P, Lukas J, Fry AM, Bartek J, Nigg EA. Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat Cell Biol. 1999;1:88–93. doi: 10.1038/10054. [DOI] [PubMed] [Google Scholar]
- Nelsen CJ, Kuriyama R, Hirsch B, Negron VC, Lingle WL, Goggin MM, Stanley MW, Albrecht JH. Short term cyclin D1 overexpression induces centrosome amplification, mitotic spindle abnormalities, and aneuplidy. J Biol Chem. 2005;280:768–776. doi: 10.1074/jbc.M407105200. [DOI] [PubMed] [Google Scholar]
- O'Connell KF, Caron C, Kopish KR, Hurd DD, Kemphues KJ, White JG. The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell. 2001;105:547–558. doi: 10.1016/s0092-8674(01)00338-5. [DOI] [PubMed] [Google Scholar]
- Ohta T, Essner R, Ryu JH, Palazzo RE, Uetake Y, Kuriyama R. Characterization of Cep135, a novel coiled-coil centrosomal protein involved in microtubule organization in mammalian cells. J Cell Biol. 2002;156:87–99. doi: 10.1083/jcb.200108088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GC, Rusan NM, Roberts DM, Peifer M, Rogers SL. The SCF Slimb ubiquitin ligase regulates Plk4/Sak levels to block centriole reduplication. J Cell Biol. 2009;184:225–239. doi: 10.1083/jcb.200808049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell BR. Chemical genetics: ligand-based discovery of gene function. Nat Rev Genet. 2000;1:116–125. doi: 10.1038/35038557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC, Chen L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci USA. 2006;103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidwans SJ, Wong ML, O’Farrell PH. Anomalous centriole configurations are detected in Drosophila wing disc cells upon Cdk1 inactivation. J Cell Sci. 2003;116:137–143. doi: 10.1242/jcs.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.