Abstract
When an artificial biomaterial (e.g., a stent or implantable pump) is exposed to blood, plasma proteins immediately adhere to the surface, creating a new interface between the biomaterial and the blood. The recognition proteins within the complement and contact activation/coagulation cascade systems of the blood will be bound to, or inserted into, this protein film and generate different mediators that will activate polymorphonuclear leukocytes and monocytes, as well as platelets. Under clinical conditions, the ultimate outcome of these processes may be thrombotic and inflammatory reactions, and consequently the composition and conformation of the proteins in the initial layer formed on the surface will to a large extent determine the outcome of a treatment involving the biomaterial, affecting both the functionality of the material and the patient’s life quality. This review presents models of biomaterial-induced activation processes and describes various strategies to attenuate potential adverse reactions by conjugating bioactive molecules to surfaces or by introducing nanostructures.
Keywords: Autoprotection, Biomaterial, Coagulation, Complement, Contact Activation, Inflammation, Innate immunity
1 Introduction
1.1 Hemocompatibility
Medicine today utilizes a wide range of biomaterials, most of which make contact with blood either permanently or transiently. In the United States alone, it is estimated that more than 25 million patients have some kind of implanted device [1]. Even more patients are treated with non-implanted and temporary biomaterials (including 400 million catheters, 25 million renal dialyzers, and more than 2 million stents annually) [2]. In 2007, the combined applications in drug delivery and medical devices have been estimated to generate a market of $200 billion in the US alone [3]. Despite considerable progress in biomaterial engineering and clinical development, many materials and procedures are still associated with undesirable side effects. For example, hemodialysis can contribute to systemic inflammation and accelerated arteriosclerosis, and treatment with stents is associated with thrombosis. These adverse reactions are initiated by contact between the biomaterial and the defense systems in the blood, primarily the cascade systems, which leads to cellular activation. Thus, the elusive goal of blood compatibility has not yet been attained [4].
Under physiologic conditions, direct contact with the blood is restricted to the intact endothelial cell lining of the blood vessels, the only truly hemocompatible surface found in nature. Activation of complement and clotting are triggered by any disruption of this surface or by the introduction of foreign materials, non-blood cells, or microorganisms into the circulation. The plasma cascades comprising the complement, coagulation, contact activation (or kallikrein/kinin), and fibrinolysis systems all act according to similar principles; and interactions between these systems, both direct and indirect, (i.e. cell-mediated) have long been known to occur. Furthermore, there is tight crosstalk between these cascade systems and the platelets and leukocytes during the induction of clotting and inflammation [5–7]. In addition, the endothelial cells play active roles not only in propagating an inflammatory/thrombotic event [6] but also by providing platelet inhibitory compounds [8].
1.2 Complement
The complement system is a primary contributor to the innate immune system of the host, clearing the body of foreign cells and organisms through direct lysis or by recruiting leukocytes that promote phagocytosis. The cascade consists of an intricate network of plasma proteins and cell surface bound receptors and regulators. Its activation involves four steps: i) recognition of non-self surface patterns via initiation of different pathways; the classical and lectin pathways (CP and LP) are induced by antigen-antibody complexes and by certain carbohydrates, respectively, and the alternative pathway (AP), may be triggered directly by foreign surfaces (e.g. by man-made biomaterials); ii) activation of complement component C3 into C3a and opsonizing C3b by two multi-molecular enzyme complexes called C3 convertases; iii) initiation of an amplification loop by the AP, which leads to the vast majority of all C3 activation, because surface-deposited C3b initiates the formation of more AP convertase complexes (C3bBb); iv) generation of convertases that are able to activate component C5 into the potent anaphylatoxin C5a and the fragment C5b, which may induce formation of the terminal complement complexes (TCC or sC5b-9). The anaphylatoxins (C3a and C5a) activate and recruit phagocytes and other immune cells, while target-bound C3 fragments facilitate binding to and activation of the recruited cells [9].
In vivo, the complement system is controlled by several soluble and membrane-bound regulators that protect self-cells against damage caused by autologous complement activation products. Most of the modulators are members of the regulators of complement activation (RCA) superfamily, which act at the level of the convertases. The plasma proteins Factor H (regulator of the AP) and C4b-binding protein (C4BP, regulator of the CP), as well as the cell surface-bound proteins membrane cofactor protein (MCP), decay acceleration factor (DAF), and complement receptor 1 (CR1 or CD35) all belong to this family. Both C4BP and Factor H not only attenuate complement activation in circulation but also recognize specific pattern (e.g., glycosaminoglycans [GAGs] and sialic acid) on host cells and thereby support complement inhibition on surfaces (i.e., self-recognition) [9, 10].
1.3 Crosstalk between blood cascade systems and cells in inflammation
It has been known for several decades that activation products of the contact activation system (Factor XIIa [FXIIa] and kallikrein), as well as thrombin and plasmin, are able to cleave purified complement component or fragments thereof in vitro [11–15]. Recently, these early observations have been confirmed and extended, and FXIa, FXa, and FIXa have been added to the list of proteases that potentially are able to bypass convertases and directly generate C3a and C5a, respectively [16]. In addition, thrombin-mediated generation of C5a has been demonstrated to take place in C3-knockout mice, which cannot form C5 convertases and thus are unable to activate C5 by conventional mechanisms [17].
A reciprocal connection in which complement activation would lead to coagulation activation, has also been described in the case of C5a-mediated upregulation of tissue factor (TF), the potent initiator of the extrinsic pathway (= the TF pathway) of coagulation, on both endothelial cells [18] and circulating polymorphonuclear leukocytes (PMNs) [19]. Furthermore, it has been demonstrated that complement activation occurring in vivo during the hemodialysis of patients with end- stage renal disease leads to the generation of C5a and expression of functionally active TF on PMNs, thereby resulting in a procoagulative state that may contribute to the increased risk of thrombosis in these patients [20].
Platelet activation during thrombotic events is intimately associated with the activation of complement and the contact system, which in turn leads to inflammation. Chondroitin sulfate A (CS-A), released from alpha granules during platelet activation, is a potent mediator of crosstalk between platelets and the complement system. Thrombin receptor activated platelets are stong promotors of inflammation since the released CS-A activates complement in the fluid phase and generates anaphylatoxins that induce leukocyte activation [21–23]. In addition, platelet activation leads to the activation of the contact system enzymes FXIIa and FXIa, which are specifically inhibited by antithrombin (AT) rather than by C1INH, as is the case when contact activation is induced by material surfaces [24, 25].
2 Biomaterials
2.1 Biocompatibility
The term “biocompatibility” refers to the “ability of a material to perform with an appropriate host response in a specific application” [26]. Most biomaterials come in contact with whole blood, either continuously or during implantation. Consequently, they will be exposed to and identified by the recognition molecules of the different cascade systems: C1q, mannose-binding lectin (MBL), and properdin of the complement system; FXII and high molecular weight kininogen (HMWK) of the contact activation system, and FVII and TF of the coagulation system.
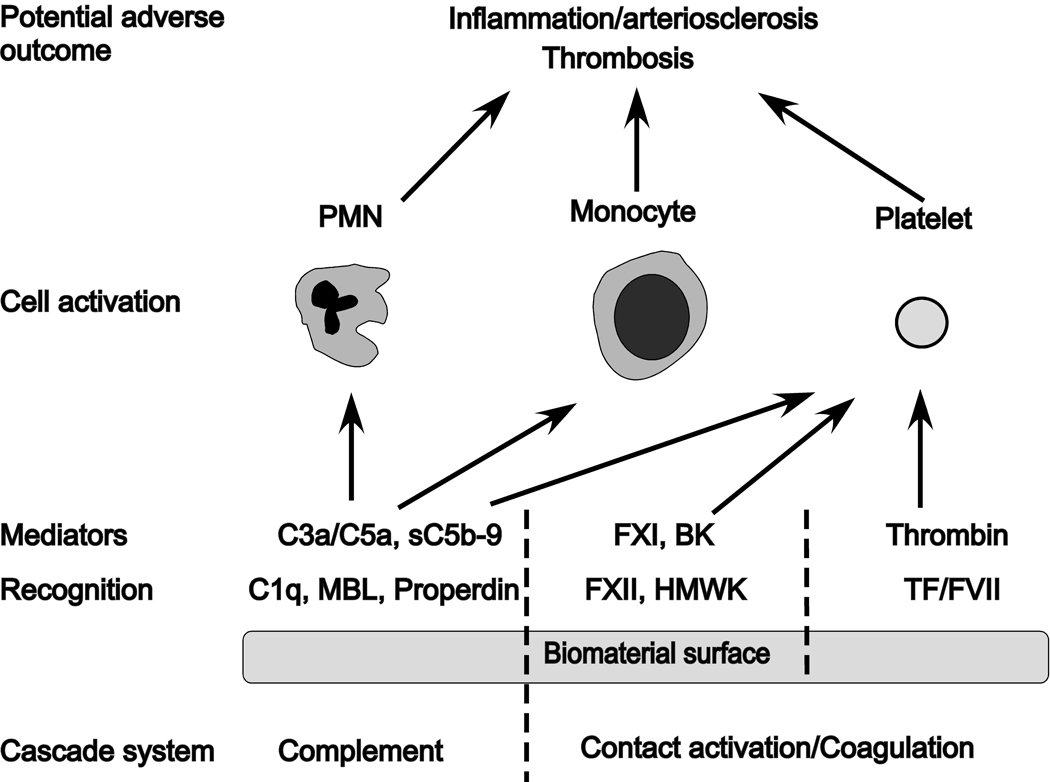
This initial contact leads to the generation of potent mediators: the anaphylatoxins C3a and C5a, and the lytic sC5b-9 complex (complement system), bradykinin (contact activation system), and thrombin (coagulation system). These mediators trigger leukocytes (PMNs and monocytes) and platelets, leading to inflammatory and thrombotic reactions. The processes that may manifest locally and directed against the biomaterial, or in severe cases, systemically and cause whole body inflammation that may be detrimental or even fatal to the patient (Figure 1).
Fig 1.
Innate immunity reactions triggered by the interaction between blood and a biomaterial surface. Recognition molecules of the various cascade systems target non-self structures on the surface: C1q, mannose-binding lectin (MBL), and properdin trigger the complement system generating the anaphylatoxins C3a and C5a as well as the lytic sC5b-9 complex; activation of the contact system is triggered by Factor XII (F XII) and high molecular weight kininogen (HMWK), leading to the activation of FXI and generation of the potent anaphylatoxin bradykinin (BK); binding of FVII and tissue factor (TF) of the coagulation system ultimately leads to the generation of thrombin from prothrombin. Collectively, these mediators generated by the cascade systems trigger the activation of polymorphonuclear leukocytes (PMN), monocytes, and platelets that may lead to inflammatory and thrombotic reactions that can cause serious harm to the biomaterial and/or the patient.
2.2 Yesterday’s biomaterials
Few inventions have shaped medicine in such a dramatic way as biomaterials, dating back to the use of glass eyes [3, 27] and the application of gold in dentistry 2000 years ago [28]. A first revolution in the evolution of biomaterials was triggered by the advent of synthetic polymers in the early 20th century, which allowed reproducible manufacturing of materials with distinct characteristics. While originally adapted for medical applications from other sources (e.g., textiles, commodity plastics), it became clear that such polymers have to be carefully tailored to optimize their performance. Many of these early successes were the result of serendipity rather than design. Engineered implants employing common and material “borrowed” from other fields, developed through collaborations of physicians and engineers, have taken advantage of advances in materials science (albeit from other fields). Commonly used biomaterials from this era include dacron and parachute cloth for vascular implants, titanium alloy for dental and orthopedic implants, cobalt-chromium-molybdinum for orthopedic implants, and ultra-high molecular weight (UHMW) polyethylene bearing surfaces for total joint replacements, heart valves, and pacemakers.
A point of interest is that one of the first clinical complication reported of complement activation on biomaterials surface was made in relation with hemodialysis using cellulose-derivatized membranes. The relatively large areas of membranes caused a massive complement activation leading to increased levels of C3a and C5a in patient blood and granulocyte aggregation [29]. In another early report, other extracorporeal treatments procedures such as nylon fiber filtration leukapheresis, were found to induce profound complement activation [30]. Since then, much more careful selections of blood contacting polymers have been made.
2.3 Today’s biomaterials
Concurrent with the progress occurring in materials science, rapid developments in molecular biology, micro-manufacturing, and nanotechnology have produced a second, ongoing revolution that has introduced biomaterials to hitherto-unimagined fields. Today, we define biomaterials as any “substances other than food and drugs contained in therapeutic or diagnostic systems that are in contact with tissue or biological fluids” [27]. Their application ranges from drug delivery systems and implantable devices (e.g., insulin pumps) to extracorporeal circuits used during cardio-pulmonary bypass surgery [3, 27, 31]. Today’s biomaterials consist of bioengineered implants using bioengineered materials and some modified and new polymeric devices, with few examples on the market but many under development. Cutting-edge examples include tissue-engineered implants designed to re-grow rather than replace tissues, artificial skin, cartilage cell procedures, resorbable bone repair cements, and genetically engineered “biological” components.
Despite this plethora of available biomaterials, their effective use is still challenging because they stimulate both application-directed and potentially adverse reactions by the human body; after all, biomaterials are foreign objects that can induce in vivo defense systems. Given their enormous impact and potential, the urgent need for developing polymers with improved biocompatibility and methods for testing their effects on the body has now been recognized [27]. The elucidation, prevention, and active modulation of adverse reactions mediated by immune and contact-systems is considered a highly important aspect of the development of tomorrow’s biomaterials.
3. A model of complement activation on a biomaterial surface
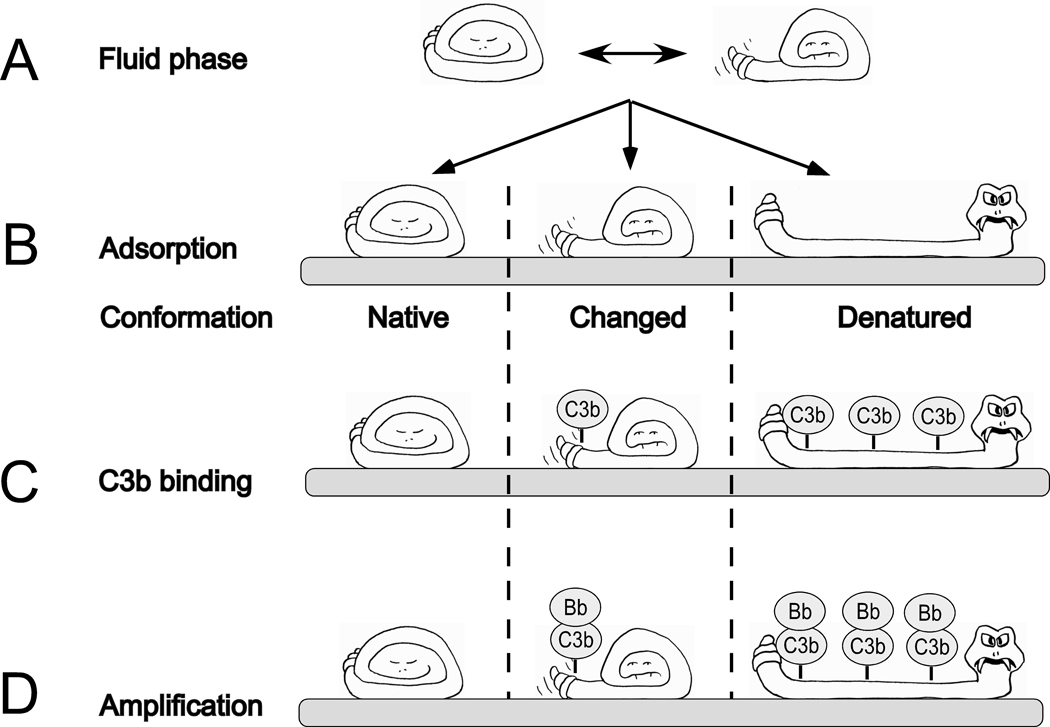
During the inevitable exposure of biomaterials to blood, either transiently during administration or implantation or continuously during their lifetime in the body, their artificial surfaces immediately become covered by a film of plasma proteins, that is essentially a monolayer [32]. This adsorption can be described as a recognition phase, a passive process that may or may not include conformational changes in individual proteins within this layer (Figure 2A,B). Examples of proteins that appear particularly prone to undergo conformational changes upon binding to such surface layers are complement component C3 [33] and IgG [34, 35], both of which can induce activation of the complement system on a biomaterial surface via the AP or CP, respectively. Other examples are adsorbed FXII, which triggers contact activation [36], and fibrinogen [37, 38], which binds to GPIIb/IIIa on platelets, thereby inducing their activation. Deposition of this primary protein layer triggers the activation of the complement, coagulation, and contact systems in ways that are dependent on the composition and conformation of the adsorbed proteins (Figure 1). The recognition molecules of these cascade systems may bind to deposited proteins or become incorporated into the initial protein film. Because of the absence of specific regulators on the biomaterial surface and a lack of self-recognition, activation of each cascade system typically leads to a rapid amplification of the respective response.
Fig 2.
Schematic representation of the relationship between the conformations of plasma proteins adsorbed to a biomaterial surface and their ability to support complement activation. In the fluid phase, plasma proteins oscillate between different conformational forms, native and conformationally changed, which are in equilibrium (A). The proteins may adsorb to artificial surfaces in a native or conformationally changed form, or be subject to denaturation upon binding (B). The adsorbed proteins expose varying numbers of acceptor sites for C3 fragments (C3b) in the various conformational forms (C) and, consequently, the number of formed alternative pathway convertase complexes (C3bBb) shows great variability (D).
Continuous complement activation leads to the generation and accumulating deposition of C3b and its degradation fragments (iC3b, C3dg) on top of the initially adsorbed protein layer and the concomitant release of anaphylatoxins to an extent that is dependent on the conformational exposure of acceptor sites for C3 (Figure 2C) [32]. Enhancement through the AP amplification loop (Figure 2D) may finally lead to a total concealment of the initial protein layer by C3 activation fragments. The generated C3a and C5a anaphylatoxins act as strong chemo-attractants that recruit PMNs and monocytes to the site of biomaterial-induced complement activation. The sequence of events is summarized in Figure 3. In addition, anaphylatoxins exert strong pro-inflammatory effects, which can lead to acute and/or chronic systemic inflammation. Whereas opsonization of foreign surfaces by C3b and iC3b usually facilitates the phagocytic removal of non-self particles via recognition by complement receptors such as CR3 (CD11b/CD18) on activated leukocytes, the large size of biomaterial devices for clinically use often prevents such uptake; consequently, the unresolved activation may lead to a change in the status of recruited immune cells, as has been exemplified by the increased fusion of macrophages to foreign body giant cells [39].
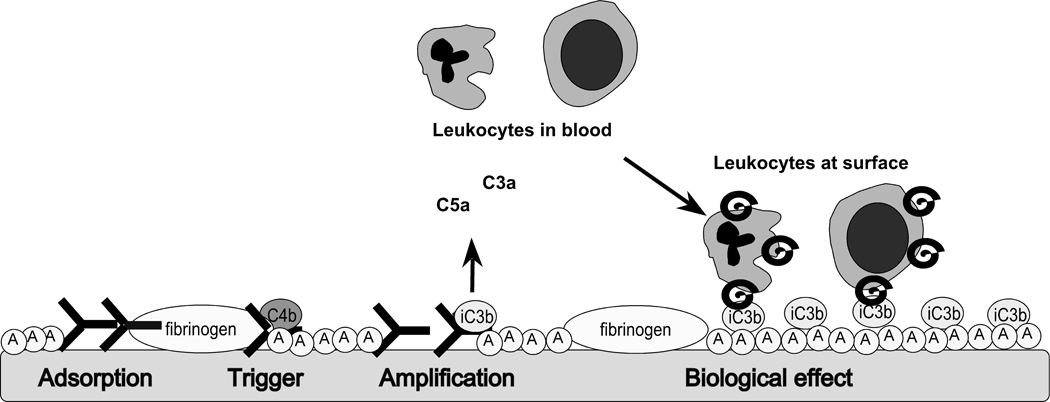
Fig 3.
A model for complement activation on biomaterial surfaces. Upon exposure to blood, the surface rapidly becomes covered by a protein film consisting of proteins from the various cascade systems, as well as immunoglobulins and albumin (encircled A). Complement becomes activated on top of this layer, generating fluid-phase anaphylatoxins (C3a and C5a) that recruit and activate PMNs and monocytes; these leukocytes then bind via CD11b (helix) to C3 fragments (iC3b) deposited on the initial protein layer.
In addition, the activation of the contact activation/coagulation systems ultimately generates thrombin, which is a powerful platelet activator. The thrombin-activated platelets release CS-A, which activates fluid phase complement, further amplifying the ongoing inflammatory reaction [21, 23]. Together with the C5a-mediated induction of TF on PMNs and monocytes [19, 20, 40], these events lead to a marked increase in coagulopathy. Contact system activation also produces the potent vasoactive peptide bradykinin (Figure 1). In summary, the complement-related surface opsonization and release of anaphylatoxins and the subsequent recruitment and activation of leukocyte populations, in combination with the thrombin-mediated activation of platelets, results in inflammatory and thrombotic reactions that can be detrimental to the biomaterial and/or the patient. Thus, the outcome of a medical treatment is to a large extent determined by the composition and conformation of the layer of plasma proteins that is initially formed on the bio-artificial surface.
4. Impact of matrix surfaces on biomaterial-induced complement activation
Solid surface in general such as polymer surfaces, metal surfaces or ceramic surfaces, adsorb blood or tissue proteins as a general phenomenon [41]. Protein adsorption usually takes place as rapid initial adsorption in the first milliseconds until a monomolecular layer of proteins has been formatted. After the formation of a more or less stable monolayer, the adsorption is inhibited. The patterns of individual proteins adsorbed is influenced by the concentration of the proteins in the biological fluids (e.g. blood) as well as the chemistry of the biomaterial surfaces [42]. Activation of the plasma cascade systems, (complement, coagulation/contact activation) is also initiated by direct protein interactions with the biomaterial surfaces, but in contrast to the rapid initial protein adsorption, the effects of the contact activation systems prolongs for a longer time and involve cell engagement at the surface [43].
Factors that affect the amount, composition, and conformation of proteins within the initial layer include the hydrophobicity/hydrophilicity of a surface, as well as its charge and the distribution of charged groups. In general, proteins are more prone to undergo substantial conformational changes when binding to hydrophobic than to hydrophilic surfaces [44]. This higher level of binding then results in a higher packing density of the proteins deposited on hydrophobic surfaces [44]. One protein that has been extensively studied is complement component C3, which is known to undergo profound conformational changes when binding to hydrophobic surfaces, as demonstrated using monoclonal antibodies specific for neo-epitopes in denatured or biologically activated C3 [45, 46]. In more recent studies, it has been demonstrated that complement is more readily activated on hydrophobic compared to hydrophilic surfaces [47, 48].
Numerous reports have demonstrated that surfaces coated with polyethylene glycol (PEG) generally feature low nonspecific protein adsorption, [49, 50] and therefore a decreased activation of the coagulation system and subsequent platelet and cellular adhesion [51]; however, activation of the complement system was still substantial on PEG-coated surfaces [52, 53] (Table 1). The protein-repellent nature of PEG has been attributed to numerous factors, including surface hydrophilicity and steric repulsion [54, 55]. The molecular weight, and surface density of the polymers chains influence the protein binding, and understanding and modifying these factors is the subject of substantial research worldwide [56–61].
Table 1.
Examples of surface modification procedures, effects on the hemocompatibility, and examples of biomedical devices with each surface modification.
| PEG 1 | Low | Low | Varying | Low | Experimental (Drug delivery systems 2) | [49–53] |
| Low energy plasma | Varying | - 3 | Low | - | Experimental | [46, 66, 67] |
| Heparin | Low | Low | Moderate | Low | Perfusion systems (e.g oxygenators), central venous catheters, stents | [68–73] |
| RCA 4 | - | - | Low | - | Stents | [52, 74] |
| Anti-RCA peptide | - | - | Low | - | Experimental | [85, 87] |
| Molecular imprint | - | - | Low | - | Drug eluting contact lenses | [92, 94] |
| Nanostructure | - | - | Low | Low | Dental titanium implants | [98, 105] |
Polyethylene glycol;
Discussed in chapter 6 in this issue of Advanced Drug Delivery Reviews;
Little or no data regarding systematic investigation of these parameters is available;
Regulators of complement activation
Another, well documented, strategy to design inert or “nonfouling” surfaces with low protein binding involves the conjugation of poly(2-methacryloyloxyethyl phosphorylcholine) e.g. [62, 63]. In addition, recently, a surface coated with poly(carboxybetaine acrylamide) was reported to show greatly reduced protein binding when exposed to undiluted human blood serum or plasma [64].
A factor that should be taken into consideration when evaluating the complement-activating potential of an artificial surface is the possibility that complement activation (recognition, convertase assembly, and deposition of C3 fragments) may take place transiently but that these compounds later detach from the surface [43]. This phenomenon has been reported to occur on hydrophilic surfaces [43]. Consequently, such surfaces would appear as low complement activators when the evaluation is restricted to fragments on the material surface, yet may indeed be strong activators regarding fluid-phase activation products such as C3a/C5a and sC5b-9. In contrast, a heavily charged surface can induce substantial complement activation but subsequently adsorb the highly cationic compounds C3a and C5a thereby reducing their levels in the circulation; thus, the activation potential of such a material would be underestimated if these activation markers were to be measured only in the fluid phase. In such a case, a more accurate estimate would be obtained by eluting the anaphylatoxins from the material surface [65]. A comparatively high-level binding of complement-initiators such as IgG and C1q can be counteracted by a simultaneous high-level binding of inhibitors such as C1INH, resulting in a lower activation than on a surface binding identically high amounts of activators but lower amounts of inhibitors [65].
The binding and activating properties of the original surface can also be further modified by low-energy plasma treatment [46, 66]. A recent example is a study by Andersen et al., who demonstrated that the modification of a medical device consisting of silicone rubber with plasma-polymerized vinyl pyrrolidone (ppVP) coating can strongly decrease the surface activation of the blood complement system [67] (Table 1).
5. Controlling biomaterial-induced complement activation
5.1. Active shielding (binding intact biomolecules)
Heparin coatings have been extensively used to render biomaterials blood-compatible, with regard to coagulation, contact system, and complement activation (Figure 4A, left). The accepted hypothesis is that the inhibition is achieved by acquiring regulators such as AT and Factor H through direct binding to heparin coated surfaces [68]. However, the coagulation system is inhibited by heparin at much lower concentrations than is the complement system [69], so the concentration of surface-bound heparin when optimized to inhibit activation of the coagulation system causes insufficient inhibition of complement (Table 1). Complement activation may be further attenuated by higher heparin surface concentrations, but this effect is not the result of increased binding of Factor H; this, it appears that Factor H is not the only, or even the main, regulator of complement on heparin surfaces [70]. Furthermore, since complement activation at surfaces is far from obliterated, it is clear that improved and more specific methods must be developed to inhibit complement activation on biomaterials [70–73]. In addition, heparin is known to interact with a plethora of plasma proteins and may cause undesired effects because of its “broadband” specificity.
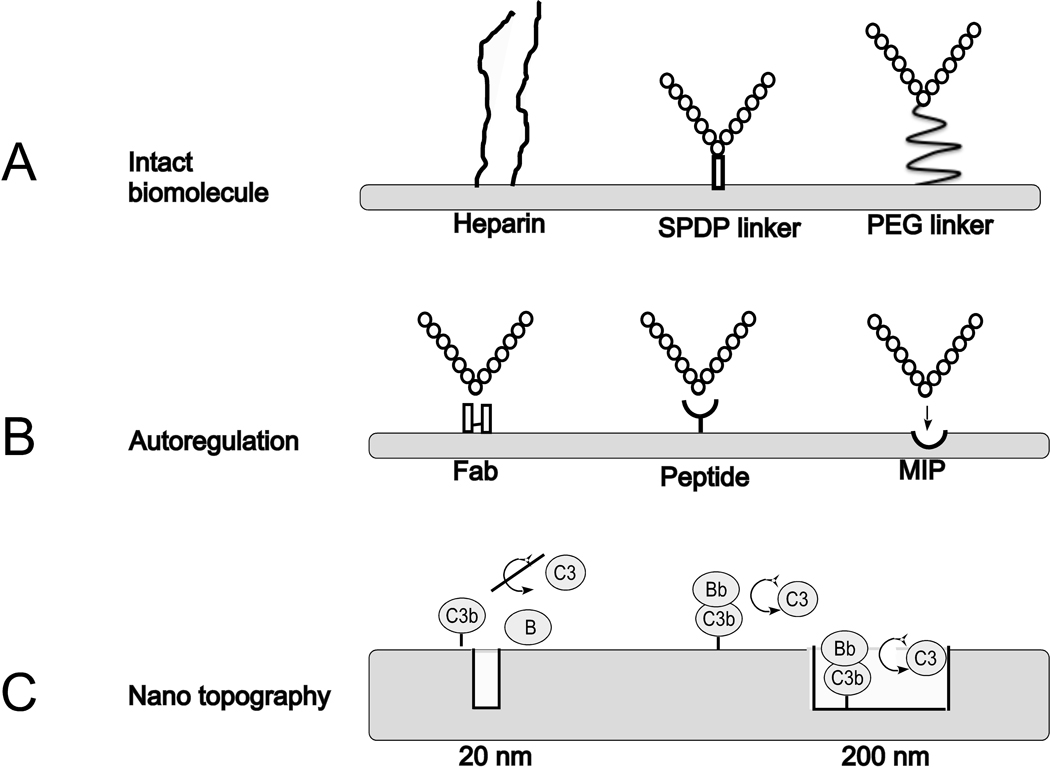
Fig 4.
Examples of surface modification strategies designed to cope with innate immunity-related recognition of biomaterial surfaces. A: Coating with intact biomolecules such as heparin (left) or a regulator of complement activation (RCA) using an N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP) linker (middle) or a polyethylene glycol (PEG) linker (right). B: Inducing affinity for an RCA by conjugating an Fab fragment of an antibody (left) or an RCA-specific synthetic peptide (middle), or by using the molecular imprinting (MIP) technique (right). C: Impact of nanopore structure. C3b and Factor B (B) are excluded from the pores of a nanostructured surface with narrow pores (20 nm; left). Consequently, fewer alternative pathway convertase (C3bBb) complexes are formed. On surfaces with larger pores (200 nm), convertase complexes formation is independent of surface topography (right).
Conjugation of a surface with biologically active, naturally occurring RCAs is a potential approach to lowering the complement activation on the material surfaces. This concept has been tested by covalently binding human purified Factor H to a model biomaterial, polystyrene, using two different linkers, N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP) and PEG (Figure 4A, middle, right). In the case of both surfaces, Factor H, when bound in native form, as determined by quartz crystal balance with dissipation (QCM-D), was able to completely abrogate complement activation upon exposure to human blood [52, 74]. Offering a promising alternative to the use of immunosuppressive and cytotoxic drugs on stents, the Factor H-PEG surface is currently being prepared for human clinical trials to prevent inflammation, coagulation, and vascular tissue damage (Table 1).
5.2. Autoprotection
Surfaces lacking regulators or self-recognition patterns (microbial intruders as well as artificial biomaterials) will likely trigger amplification of complement response. As described in section 5.1, heparin coating of biomaterials with the aim to actively adsorb Factor H and thereby inhibit complement activation show varying results, in some cases insufficient efficacy [68, 70, 71, 75]. A more targeted approach that selectively recruits Factor H is therefore considered important for studying complement-induced effects but may also directly lead to clinically applicable products. An obvious choice would be to utilize antibodies (either intact, or Fab, or single chain fragments) to target surfaces (Figure 4B, left). However, such coatings with large natural structures would be rather complex and costly to produce and may therefore be difficult to translate into clinical applications. Alternative sources of regulator-recruiting entities are therefore desired.
Intriguingly, some human pathogens expose RCA-capturing molecules as part of their immune evasion strategy [76]: for example, M proteins of Streptococcus pyogenes bind C4BP [77–79], Neisseria meningitidis expresses a Factor H-binding protein that mimics glycan patterns [80–82], and staphylococcal proteins (Efb, Sbi) have recently been implicated in the enhancement of Factor H binding [83, 84]. Whereas coating with such capturing intact proteins would appear less feasible because of size and immunogenicity concerns, M protein-derived peptides have recently been shown to recruit C4BP to model polystyrene surfaces and reduce complement activation [85] (Figure 4B, middle).
Despite these promising proof-of-principle studies, those M protein-derived peptides do not lend themselves to cost-effective synthesis because they are still some 50 amino acids long. Also, it is imperative to target the AP amplification loop, preferably by exploiting its main inhibitor Factor H, because this pathway plays a pivotal role in biomaterial-induced complement activation, as illustrated by the fact that it contributes >80% of the C5 activation in a model in which complement activation is initiated by the CP [86]. With the aim of developing a small-molecule, Factor H-specific capturing compound, we recently screened variable cysteine-constrained phage-displayed peptide libraries and identified several small peptides with high Factor H-capturing activity. One of these peptides was found to retain the functional integrity of factor H by capturing at a non-regulatory area of the regulator. Indeed, coating of a model biomaterial (polystyrene) with this peptide recruited high levels of factor H efficiently inhibit complement activation by the AP (Figure 4B, middle) [87]. Such peptides are therefore evaluated and optimized for potential clinical applications (Table 1).
5.3 Molecular imprinting
The use of functional synthetic materials with a predetermined molecular-level structure as biomaterials is a fascinating possibility and offers a potential alternative to approaches based upon immobilized biomolecules, with their inherent instability. Two fundamentally different strategies to achieve this end are the topic of current research: The first involves the use of molecular imprinting techniques (MIPs) for developing autoregulatory biomaterial surfaces that provide a surface pattern capable of selective recruiting RCAs (Figure 4B, right). The second strategy involves the use of nanostructured surfaces with dimensions that produce steric constraints, thereby inhibiting cascade recognition events (see section 5.4 and Figure 4C).
The MIP approach [88] involves the formation of cavities in a synthetic polymer matrix that are structurally and functionally complementary to a template molecule/entity. The ability of MIPs to selectively recognize and bind a template structure in the presence of closely related chemical species has led to attempts to apply them to a wide range of biomedical and biotechnological applications. These antibody combining-site mimics have demonstrated binding affinities and cross-reactivity profiles comparable to their biological counterparts and have even been employed as substitutes for biological antibodies in clinical diagnostic assays [89]. While the molecular imprinting process in many ways parallels the immune system’s production of antibodies, it also exhibits significant contrasting features and offers certain advantages, for example, a lack of hapten-conjugation protocols and situations involving non-immunogenic substances pose no problem. Moreover, materials formed using MIPS are stable under extreme conditions of pH, temperature, and organic solvent exposure, quite unlike their biological counterparts [90]. Accordingly, these materials have great potential for basic research and for the biotechnology and pharmaceutical industries as potential biomaterials. Indeed, recent efforts aimed at developing molecularly imprinted contact lenses as vehicles for the controlled release of medication [91], e.g., beta blockers such as timolol for the treatment of glaucoma, have proven useful [92] (Table 1).
The first reports of imprinted polymer surfaces, being developed as biomaterials, have recently appeared. Generally, the template surfaces are prepared by attaching specific biomolecules of interest to a glass surface (the template surface). The template surface is then placed in contact with a suitably derivatized surface, and a water-soluble monomer mixture, e.g., one based on acrylates or suitable acrylamides [93], is allowed to polymerize between the two surfaces. After separation of the imprinted surface from the template, acceptor sites are revealed on the imprint surface (Figure 4B, right). We have recently demonstrated that prototype imprints of heparin are significantly less prone to activate complement than are control polymer surfaces [94].
5.4 Nanostructure
The synthesis of nanostructured surfaces for applications in tissue engineering and drug delivery is a rapidly emerging field [95–97]. In a recent study, Ferraz et al. demonstrated that complement activation on aluminum surfaces is strictly dependent on pore diameter; they found that activation on surfaces with a pore diameter of 20 nm was significantly lower than on surfaces with 200 nm pores, despite the fact that the total surface of the former material was much greater [98]. In order for complement activation to occur, a number of large proteins need to interact at a surface; that is, the subunits of the AP C3 convertase (C3bBb) need to associate with and cleave nearby C3 molecules. Structures that are too narrow may not allow for such interactions to take place at normal rates (Figure 4C, left). In contrast, on surfaces with more expansive structure, convertase assembly and cleavage will take place both inside and between the pores; in this case, the effect by introducing the nanostructure is to increase the accessible surface area (Figure 4D, right; Table 1).
Similarly, in a recent study, it was demonstrated that synthetic polymers with similar a charge but different pore size differ in terms of their protein binding and complement activation, with lower values being obtained for polymers with small pores than for those with larger ones [65].
5.5. Soluble inhibitors
In addition to modifying biomaterials to shield them from unwanted reactions, it is possible to use soluble complement-targeting drugs to suppress the amplification of the complement response. This approach might be particularly suitable in situations with repetitive yet temporally limited periods of exposure to artificial surfaces (e.g., hemodialysis) or at the initial stages of implantation, to allow better embedding. A variety of complement-targeted drugs are currently on the market or in clinical or pre-clinical trials [99, 100]. The first complement inhibitor to be licenced as an orphan drug, was eculizumab, an humanized anti-C5 antibody which is used for treatment of the rare disorder paroxysmal nocturnal hemoglobinuria [101]. However, in view of the key involvement of the AP in biomaterial-induced complement activation, inhibition at the level of C3 appears to be most promising. RCA-based therapeutics have been of recent interest as a result of the development of chimeras between the regulatory domains of Factor H and the C3d-binding domains of CR2 [102] for use in targeting regulators to sites of activation. However, the cost of such protein therapeutics is rather high, and these chimeras only target the AP amplification step.
In contrast, Compstatin is a peptidic complement inhibitor that interacts with C3/C3b and thereby inhibits the activation of both the CP/LP and AP; the safety of this compound has recently been established in a Phase I clinical trial for age-related macular degeneration, and it has also been successfully used in a variety of disease models [103]. Most importantly, Compstatin analogues have recently been tested in a model of hemodialysis and found to efficiently suppress the filter-induced activation of complement and neutrophils and to reduce the expression of TF [20]. The use of novel Compstatin analogues with increased inhibitory potency for biomaterial-related applications is therefore considered promising [104].
6. Conclusions
Overall, considerably more success has been achieved in reducing the thrombogenicity of bio-artifical surfaces than in controlling complement activation: For example, surfaces coated with different forms of heparin or PEG are associated with low or negligible activation of coagulation and subsequent platelet loss. Thus, there are numerous surfaces that have low thrombogenicity available that still bear substantial complement-activating capacity. Some of these materials will no doubt be suitable starting material for exploring the various modification procedures for disarming complement activation that have been described in Sections 5.2–5.4 and summarized in Table 1. Table 1 also gives examples of surface modification procedures used in different medical devices. The ultimate goal of this approach is to create a hybrid surface that combines the inherent coagulation-inert properties of the original surface with specific complement-autoregulation, thus minimizing the risk of short-circuiting of the systems via platelet derived CS-A, C5a and TF as discussed in Section 3. Such a biomaterial would show superior blood compatibility and fewer detrimental side effects (with regard to both the patient and the biomaterial), as compared to the materials available in the clinic today.
Acknowledgments
This work was supported by grants from the Swedish Research Council (VR) 2009-4675, 2009-4462, from the Swedish Research Council, and Swedish Research Council/SSF/Vinnova contract grant number 60761701, by U.S. National Institutes of Health grants AI068730, AI030040, AI072106, EB3968, and GM062134, and by faculty grants from the Linnæus University. We thank Dr. Deborah McClellan for excellent editorial assistance and Mr Hans Nilsson for preparing the artwork in Figure 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marwick C. Implant recommendations. Jama. 2000;283:869. [PubMed] [Google Scholar]
- 2.Ratner BD. A paradigm shift: biomaterials that heal. Polymer international. 2007;56:1183–1185. [Google Scholar]
- 3.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462:426–432. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratner BD. The catastrophe revisited: Blood compatibility in the 21st Century. Biomaterials. 2007;28:5144–5147. doi: 10.1016/j.biomaterials.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25:5681–5703. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Hamad OA, Bäck J, Nilsson PH, Nilsson B, Ekdahl KN. Platelets, complement, and contact activation: Partners in inflammation and thrombosis. Advances in Experimental Medicine and Biology. 2011 doi: 10.1007/978-1-4614-0106-3_11. in press. [DOI] [PubMed] [Google Scholar]
- 8.Jin RC, Voetsch B, Loscalzo J. Endogenous mechanisms of inhibition of platelet function. Microcirculation. 2005;12:247–258. doi: 10.1080/10739680590925493. [DOI] [PubMed] [Google Scholar]
- 9.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 11.Ghebrehiwet B, Randazzo BP, Dunn JT, Silverberg M, Kaplan AP. Mechanisms of activation of the classical pathway of complement by Hageman factor fragment. J Clin Invest. 1983;71:1450–1456. doi: 10.1172/JCI110898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thoman ML, Meuth JL, Morgan EL, Weigle WO, Hugli TE. C3d-K, a kallikrein cleavage fragment of iC3b is a potent inhibitor of cellular proliferation. J Immunol. 1984;133:2629–2633. [PubMed] [Google Scholar]
- 13.Spath P, Gabl F. Critical role of the conversion of the third complement component C3 (beta 1C/beta 1A) for its immunochemical quantitation. Clin Chim Acta. 1976;73:171–175. doi: 10.1016/0009-8981(76)90319-3. [DOI] [PubMed] [Google Scholar]
- 14.Goldberger G, Thomas ML, Tack BF, Williams J, Colten HR, Abraham GN. NH2-terminal structure and cleavage of guinea pig pro-C3, the precursor of the third complement component. J Biol Chem. 1981;256:12617–12619. [PubMed] [Google Scholar]
- 15.Lachmann PJ, Pangburn MK, Oldroyd RG. Breakdown of C3 after complement activation. Identification of a new fragment C3g, using monoclonal antibodies. J Exp Med. 1982;156:205–216. doi: 10.1084/jem.156.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Bruckner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost. 1997;77:394–398. [PubMed] [Google Scholar]
- 19.Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris JD. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 20.Kourtzelis I, Markiewski MM, Doumas M, Rafail S, Kambas K, Mitroulis I, Panagoutsos S, Passadakis P, Vargemezis V, Magotti P, Qu H, Mollnes TE, Ritis K, Lambris JD. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamad OA, Ekdahl KN, Nilsson PH, Andersson J, Magotti P, Lambris JD, Nilsson B. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J Thromb Haemost. 2008;6:1413–1421. doi: 10.1111/j.1538-7836.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamad OA, Nilsson PH, Lasaosa M, Ricklin D, Lambris JD, Nilsson B, Ekdahl KN. Contribution of chondroitin sulfate A to the binding of complement proteins to activated platelets. PLoS One. 2010;5:e12889. doi: 10.1371/journal.pone.0012889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamad OA, Nilsson PH, Wouters D, Lambris JD, Ekdahl KN, Nilsson B. Complement component C3 binds to activated normal platelets without preceding proteolytic activation and promotes binding to complement receptor 1. J Immunol. 2010;185:2686–2692. doi: 10.4049/jimmunol.0902810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bäck J, Huber-Lang M, Elgue G, Kalbitz M, Sanchez J, Ekdahl KN, Nilsson B. Distinctive regulation of contact activation by antithrombin and C1-inhibitor on activated platelets and material surfaces. Biomaterials. 2009;30:6573–6580. doi: 10.1016/j.biomaterials.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 25.Bäck J, Sanchez J, Elgue G, Ekdahl KN, Nilsson B. Activated human platelets induce factor XIIa-mediated contact activation. Biochem Biophys Res Commun. 2010;391:11–17. doi: 10.1016/j.bbrc.2009.10.123. [DOI] [PubMed] [Google Scholar]
- 26.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263:1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- 28.Donaldson JA. The use of gold in dentistry: an historical overview. Gold Bull. 1980;13:117–124. doi: 10.1007/BF03216551. (114),160-115. [DOI] [PubMed] [Google Scholar]
- 29.Ivanovich P, Chenoweth DE, Schmidt R, Klinkmann H, Boxer LA, Jacob HS, Hammerschmidt DE. Symptoms and activation of granulocytes and complement with two dialysis membranes. Kidney Int. 1983;24:758–763. doi: 10.1038/ki.1983.224. [DOI] [PubMed] [Google Scholar]
- 30.Hammerschmidt DE, Craddock PR, McCullough F, Kronenberg RS, Dalmasso AP, Jacob HS. Complement activation and pulmonary leukotasis during nylon fiber filtration leukapheresis. Blood. 1978;51:721–730. [PubMed] [Google Scholar]
- 31.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 32.Andersson J, Ekdahl KN, Lambris JD, Nilsson B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials. 2005;26:1477–1485. doi: 10.1016/j.biomaterials.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Andersson J, Ekdahl KN, Larsson R, Nilsson UR, Nilsson B. C3 adsorbed to a polymer surface can form an initiating alternative pathway convertase. J Immunol. 2002;168:5786–5791. doi: 10.4049/jimmunol.168.11.5786. [DOI] [PubMed] [Google Scholar]
- 34.Wettero J, Bengtsson T, Tengvall P. C1q-independent activation of neutrophils by immunoglobulin M-coated surfaces. J Biomed Mater Res. 2001;57:550–558. doi: 10.1002/1097-4636(20011215)57:4<550::aid-jbm1201>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 35.Tengvall P, Askendal A, Lundstrom I. Ellipsometric in vitro studies on the activation of complement by human immunoglobulins M and G after adsorption to methylated silicon. Colloids Surf B Biointerfaces. 2001;20:51–62. doi: 10.1016/s0927-7765(00)00174-0. [DOI] [PubMed] [Google Scholar]
- 36.Schousboe I, Nystrøm BT, Hansen GH. Differential binding of factor XII and activated factor XII to soluble and immobilized fibronectin--localization of the Hep-1/Fib-1 binding site for activated factor XII. FEBS J. 2008;275:5161–5172. doi: 10.1111/j.1742-4658.2008.06647.x. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Simonovsky FI, Ratner BD, Horbett TA. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: a comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J Biomed Mater Res A. 2005;74:722–738. doi: 10.1002/jbm.a.30381. [DOI] [PubMed] [Google Scholar]
- 38.Berglin M, Pinori E, Sellborn A, Andersson M, Hulander M, Elwing H. Fibrinogen adsorption and conformational change on model polymers: novel aspects of mutual molecular rearrangement. Langmuir. 2009;25:5602–5608. doi: 10.1021/la803686m. [DOI] [PubMed] [Google Scholar]
- 39.Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater. 2006;1:R1–R9. doi: 10.1088/1748-6041/1/1/R01. [DOI] [PubMed] [Google Scholar]
- 40.Fischer M, Sperling C, Tengvall P, Werner C. The ability of surface characteristics of materials to trigger leukocyte tissue factor expression. Biomaterials. 2010;31:2498–2507. doi: 10.1016/j.biomaterials.2009.12.016. 31. [DOI] [PubMed] [Google Scholar]
- 41.Castner DG, Ratner BD. Biomedical surface science: Foundations to frontiers. Surface Science. 2002;500:28–60. [Google Scholar]
- 42.Norde W. Adsorption of proteins from solutions at the solid-liquid interface. Advances in collodid and interface science. 1986;25:267–340. doi: 10.1016/0001-8686(86)80012-4. [DOI] [PubMed] [Google Scholar]
- 43.Andersson M, Andersson J, Sellborn A, Berglin M, Nilsson B, Elwing H. Quartz crystal microbalance-with dissipation monitoring (QCM-D) for real time measurements of blood coagulation density and immune complement activation on artificial surfaces. Biosens Bioelectron. 2005;21:79–86. doi: 10.1016/j.bios.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Andrade J, Hlady V. Protein Adsorption and Materials Biocompatibility: A Tutorial Review and Suggested Hypotheses. Berlin, Heidelberg: Springer-Verlag; 1986. [Google Scholar]
- 45.Elwing H, Nilsson B, Svensson K-E, Askendal A, Nilsson UR, Lundström I. Conformational Changes of a Model Protein (Complement Factor C3) Adsorbed on Hydrophilic Solid Surfaces. J Coll Interf Sci. 1988;125:139–145. [Google Scholar]
- 46.Ekdahl KN, Nilsson B, Gölander CG, Elwing H, Lassen B, Nilsson UR. Complement activation on radiofrequency plasma modified polystyrene surfaces. J Colloid Interface Sci. 1993;158:121–128. [Google Scholar]
- 47.Sellborn A, Andersson M, Hedlund J, Andersson J, Berglin M, Elwing H. Immune complement activation on polystyrene and silicon dioxide surfaces. Impact of reversible IgG adsorption. Mol Immunol. 2005;42:569–574. doi: 10.1016/j.molimm.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Sperling C, Schweiss RB, Streller U, Werner C. In vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials. 2005;26:6547–6557. doi: 10.1016/j.biomaterials.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 49.Amiji M, Park K. Prevention of protein adsorption and platelet adhesion on surfaces by PEO/PPO/PEO triblock copolymers. Biomaterials. 1992;13:682–692. doi: 10.1016/0142-9612(92)90128-b. [DOI] [PubMed] [Google Scholar]
- 50.Green RJ, Davies MC, Roberts CJ, Tendler SJB. A surface plasmon resonance study of albumin adsorption to PEO–PPO–PEO triblock copolymers. J Biomed Mater Res. 1998;42:165–171. doi: 10.1002/(sici)1097-4636(199811)42:2<165::aid-jbm1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 51.Tan JS, Butterfield DE, Voycheck CL, Caldwell KD, Li JT. Surface modification of nanoparticles by PEO/PPO block copolymers to minimize interactions with blood components and prolong blood circulation in rats. Biomaterials. 1993;14:823–833. doi: 10.1016/0142-9612(93)90004-l. [DOI] [PubMed] [Google Scholar]
- 52.Andersson J, Larsson R, Richter R, Ekdahl KN, Nilsson B. Binding of a model regulator of complement activation (RCA) to a biomaterial surface: surface-bound factor H inhibits complement activation. Biomaterials. 2001;22:2435–2443. doi: 10.1016/s0142-9612(00)00431-2. [DOI] [PubMed] [Google Scholar]
- 53.Arima Y, Toda M, Iwata H. Complement activation on surfaces modified with ethylene glycol units. Biomaterials. 2008;29:551–560. doi: 10.1016/j.biomaterials.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Elbert DL, Hubbell JA. Surface treatments of polymers for biocompatibility. Annu Rev Mater Sci. 1996;26:365–394. [Google Scholar]
- 55.Jeon SI, Lee JH, Andrade JD, DeGennes PG. Protein–surface interactions in the presence of polyethylene oxide. J Colloid Interface Sci. 1991;132:149–158. [Google Scholar]
- 56.Prime KL, Whitesides GM. Adsorption of Proteins onto Surfaces Containing End-Attached Oligo(ethylene oxide): A Model System Using Self-Assembled Monolayers. J Am Chem Soc. 1993;115:10714–10721. [Google Scholar]
- 57.Malmsten M, Emoto K, Van Alstine J. Effect of Chain Density on Inhibition of Protein Adsorption by Poly (ethylene glycol) Based Coatings. J Coll Interf Sci. 1998;202:507–517. [Google Scholar]
- 58.Sofia S, Premnath V, Merrill E. Poly(ethylene oxide) Grafted to Silicon Surfaces: Grafting Density and Protein Adsorption. Macromolecules. 1998;31:5059–5070. doi: 10.1021/ma971016l. [DOI] [PubMed] [Google Scholar]
- 59.Kenausis GL, Vörös J, Elbert DL, Huang N, Hofer R, Ruiz-Taylo L, Textor M, Hubbell JA, Spencer ND. Poly(L-lysine)-g-Poly(ethylene glycol) Layers on Metal Oxide Surfaces: Attachment Mechanism and Effects of Polymer Architecture on Resistance to Protein Adsorption. J Phys Chem B. 2000;104:3298–3309. [Google Scholar]
- 60.Vanderah DJ, Vierling RL, Walker ML. Oligo(ethylene oxide) Self-Assembled Monolayers with Self-Limiting Packing Densities for the Inhibition of Nonspecific Protein Adsorption. Langmuir. 2009;25:5026–5030. doi: 10.1021/la803896a. [DOI] [PubMed] [Google Scholar]
- 61.Murthy R, Shell CE, Grunlan MA. The influence of poly(ethylene oxide) grafting via siloxane tethers on protein adsorption. Biomaterials. 2009;30:2433–2439. doi: 10.1016/j.biomaterials.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 62.Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipid polymers reduce protein adsorption? J Biomed Mater Res. 1998;39:323–330. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 63.Inoue Y, Ishihara K. Reduction of protein adsorption on well-characterized polymer brush layers with varying chemical structures. Colloids Surf B Biointerfaces. 2010;81:350–357. doi: 10.1016/j.colsurfb.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 64.Yang W, Xue H, Li W, Zhang J, Jiang S. Pursuing "zero" protein adsorption of poly(carboxybetaine) from undiluted blood serum and plasma. Langmuir. 2009;25:11911–11916. doi: 10.1021/la9015788. [DOI] [PubMed] [Google Scholar]
- 65.Engberg AE, Rosengren-Holmberg JP, Chen H, Nilsson B, Lambris JD, Nicholls IA, N EK. Blood protein-polymer adsorption: Implications for understanding complement-mediated hemoincompatibility. J Biomed Mater Res A. 2011;94A:74–84. doi: 10.1002/jbm.a.33030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaklamani G, Mehrban N, Chen J, Bowen J, Dong H, Grover L, Stamboulis A. Effect of plasma surface modification on the biocompatibility of UHMWPE. Biomed Mater. 2010;5 doi: 10.1088/1748-6041/5/5/054102. Epub 2010 Sep 2028. [DOI] [PubMed] [Google Scholar]
- 67.Andersen TE, Palarasah Y, Skjødt MO, Ogaki R, Benter M, Alei M, Kolmos HJ, Koch C, Kingshott P. Decreased material-activation of the complement system using low-energy plasma polymerized poly(vinyl pyrrolidone) coatings. Biomaterials. 2011;32:4481–4488. doi: 10.1016/j.biomaterials.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Videm V, Mollnes TE, Bergh K, Fosse E, Mohr B, Hagve TA, Aasen AO, Svennevig JL. Heparin-coated cardiopulmonary bypass equipment. II. Mechanisms for reduced complement activation in vivo. J Thorac Cardiovasc Surg. 1999;117:803–809. doi: 10.1016/S0022-5223(99)70302-8. [DOI] [PubMed] [Google Scholar]
- 69.Gong J, Larsson R, Ekdahl KN, Mollnes TE, Nilsson U, Nilsson B. Tubing loops as a model for cardiopulmonary bypass circuits: both the biomaterial and the blood-gas phase interfaces induce complement activation in an in vitro model. J Clin Immunol. 1996;16:222–229. doi: 10.1007/BF01541228. [DOI] [PubMed] [Google Scholar]
- 70.Andersson J, Sanchez J, Ekdahl KN, Elgue G, Nilsson B, Larsson R. Optimal heparin surface concentration and antithrombin binding capacity as evaluated with human non-anticoagulated blood in vitro. J Biomed Mater Res A. 2003;67:458–466. doi: 10.1002/jbm.a.10104. [DOI] [PubMed] [Google Scholar]
- 71.Lappegard KT, Fung M, Bergseth G, Riesenfeld J, Lambris JD, Videm V, Mollnes TE. Effect of complement inhibition and heparin coating on artificial surface-induced leukocyte and platelet activation. Ann Thorac Surg. 2004;77:932–941. doi: 10.1016/S0003-4975(03)01519-4. [DOI] [PubMed] [Google Scholar]
- 72.Moen O, Høgåsen K, Fosse E, Dregelid E, Brockmeier V, Venge P, Harboe M, Mollnes TE. Attenuation of changes in leukocyte surface markers and complement activation with heparin-coated cardiopulmonary bypass. Ann Thorac Surg. 1997;63:105–111. doi: 10.1016/s0003-4975(96)00743-6. [DOI] [PubMed] [Google Scholar]
- 73.Mollnes TE, Riesenfeld J, Garred P, Nordström E, Høgåsen K, Fosse E, Götze O, Harboe M. A new model for evaluation of biocompatibility: combined determination of neoepitopes in blood and on artificial surfaces demonstrates reduced complement activation by immobilization of heparin. Artif Organs. 1995;19:909–917. doi: 10.1111/j.1525-1594.1995.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 74.Andersson J, Bexborn F, Klinth J, Nilsson B, Ekdahl KN. Surface-attached PEO in the form of activated Pluronic with immobilized factor H reduces both coagulation and complement activation in a whole-blood model. J Biomed Mater Res A. 2006;76:25–34. doi: 10.1002/jbm.a.30377. [DOI] [PubMed] [Google Scholar]
- 75.Baksaas ST, Videm V, Pedersen T, Karlsen H, Mollnes TE, Brosstad F, Svennevig JL. Comparison of three oxygenator-coated and one total-circuit-coated extracorporeal devices. Perfusion. 1999;14:119–127. doi: 10.1177/026765919901400205. [DOI] [PubMed] [Google Scholar]
- 76.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jenkins HT, Mark L, Ball G, Persson J, Lindahl G, Uhrin D, Blom AM, Barlow PN. Human C4b-binding protein, structural basis for interaction with streptococcal M protein, a major bacterial virulence factor. J Biol Chem. 2006;281:3690–3697. doi: 10.1074/jbc.M511563200. [DOI] [PubMed] [Google Scholar]
- 78.Persson J, Beall B, Linse S, Lindahl G. Extreme sequence divergence but conserved ligand-binding specificity in Streptococcus pyogenes M protein. PLoS Pathog. 2006;2:e47. doi: 10.1371/journal.ppat.0020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thern A, Stenberg L, Dahlback B, Lindahl G. Ig-binding surface proteins of Streptococcus pyogenes also bind human C4b-binding protein (C4BP), a regulatory component of the complement system. J Immunol. 1995;154:375–386. [PubMed] [Google Scholar]
- 80.Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, Tang CM. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176:7566–7575. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 82.Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, Lea SM. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009;458:890–893. doi: 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen H, Ricklin D, Hammel M, Garcia BL, McWhorter WJ, Sfyroera G, Wu YQ, Tzekou A, Li S, Geisbrecht BV, Woods VL, Jr, Lambris JD. Allosteric inhibition of complement function by a staphylococcal immune evasion protein. Proc Natl Acad Sci U S A. 2010;107:17621–17626. doi: 10.1073/pnas.1003750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haupt K, Reuter M, van den Elsen J, Burman J, Halbich S, Richter J, Skerka C, Zipfel PF. The Staphylococcus aureus protein Sbi acts as a complement inhibitor and forms a tripartite complex with host complement Factor H and C3b. PLoS Pathog. 2008;4:e1000250. doi: 10.1371/journal.ppat.1000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Engberg AE, Sandholm K, Bexborn F, Persson J, Nilsson B, Lindahl G, Ekdahl KN. Inhibition of complement activation on a model biomaterial surface by streptococcal M protein-derived peptides. Biomaterials. 2009;30:2653–2659. doi: 10.1016/j.biomaterials.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138:439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu YQ, Qu H, Sfyroera G, Tzekou A, Kay BK, Nilsson B, Ekdahl KN, Ricklin D, Lambris JD. Protection of Nonself Surfaces from Complement Attack by Factor H-Binding Peptides: Implications for Therapeutic Medicine. J Immunol. 2011;186:4269–4277. doi: 10.4049/jimmunol.1003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alexander C, Andersson HS, Andersson LI, Ansell RJ, Kirsch N, Nicholls IA, O'Mahony J, Whitcombe MJ. Molecular imprinting science and technology: a survey of the literature for the years up to and including 2003. J Mol Recognit. 2006;19:106–180. doi: 10.1002/jmr.760. [DOI] [PubMed] [Google Scholar]
- 89.Vlatakis G, Andersson LI, Müller R, Mosbach K. Drug assay using antibody mimics made by molecular imprinting. Nature. 1993;361:645–647. doi: 10.1038/361645a0. [DOI] [PubMed] [Google Scholar]
- 90.Svensson J, Nicholls IA. On the thermal and chemical stability of molecularly imprinted polymers. Analytica Chimica Acta. 2001;435:19–24. [Google Scholar]
- 91.Norell MC, Andersson HS, Nicholls IA. Theophylline molecularly imprinted polymer dissociation kinetics: a novel sustained release drug dosage mechanism. J Mol Recognit. 1998;11:98–102. doi: 10.1002/(SICI)1099-1352(199812)11:1/6<98::AID-JMR399>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 92.White CJ, Byrne ME. Molecularly imprinted therapeutic contact lenses. Expert Opin Drug Deliv. 2010;7:765–780. doi: 10.1517/17425241003770098. [DOI] [PubMed] [Google Scholar]
- 93.Piletsky SA, Andersson HS, Nicholls IA. Combined hydrophobic and electrostatic interaction-based recognition in molecularly imprinted polymers. Macromolecules. 1999;32:633–636. [Google Scholar]
- 94.Rosengren-Holmberg JP, Andersson J, Smith JR, Alexander C, Alexander M, Tovar G, Ekdahl KN, Nicholls IA. Attenuation of complement and coagulation activation with heparin molecularly imprinted surfaces. Unpublished results. 2011 doi: 10.1039/c5bm00047e. [DOI] [PubMed] [Google Scholar]
- 95.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 96.Curtis A, Wilkinson C. Nanotechniques and approaches in biotechnology. Trends Biotechnol. 2001;19:97–101. doi: 10.1016/s0167-7799(00)01536-5. [DOI] [PubMed] [Google Scholar]
- 97.Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18:241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferraz N, Karlsson Ott M, Hong J. Time sequence of blood activation by nanoporous alumia: Studies on platelets and complement system. Microsc Res Tech. 2010;73:1101–1109. doi: 10.1002/jemt.20854. [DOI] [PubMed] [Google Scholar]
- 99.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qu H, Ricklin D, Lambris JD. Recent developments in low molecular weight complement inhibitors. Mol Immunol. 2009;47:185–195. doi: 10.1016/j.molimm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schrezenmeier H, Höchsmann B. Eculizumab opens a new era of treatment for paroxysmal nocturnal hemoglobinuria. Expert Rev Hematol. 2009;2:7–16. doi: 10.1586/17474086.2.1.7. [DOI] [PubMed] [Google Scholar]
- 102.Banda NK, Levitt B, Glogowska MJ, Thurman JM, Takahashi K, Stahl GL, Tomlinson S, Arend WP, Holers VM. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. J Immunol. 2009;183:5928–5937. doi: 10.4049/jimmunol.0901826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ricklin D, Lambris JD. Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol. 2008;632:273–292. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qu H, Magotti P, Ricklin D, Wu E, Kourtzelis I, Wu Y, Kaznessis YN, Lambris JD. Novel analogues of the therapeutic complement inhibitor compstatin with significantly improved affinity and potency. Mol Immunol. 2011;48:481–489. doi: 10.1016/j.molimm.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wennberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Impl Res. 2009;20 suppl 4:172–184. doi: 10.1111/j.1600-0501.2009.01775.x. [DOI] [PubMed] [Google Scholar]