Abstract
Approximately 60,000 patients in the US are waiting for a kidney transplant due to genetic, immunologic and environmentally caused kidney failure. Adult human renal stem cells could offer opportunities for autologous transplant and repair of damaged organs. Current data suggest that there are multiple progenitor types in the kidney with distinct localizations. In the present study, we characterize cells derived from human kidney papilla and show their capacity for tubulogenesis. In situ, nestin+ and CD133/1+ cells were found extensively intercalated between tubular epithelia in the loops of Henle of renal papilla, but not of the cortex. Populations of primary cells from the renal cortex and renal papilla were isolated by enzymatic digestion from human kidneys unsuited for transplant and immuno-enriched for CD133/1+ cells. Isolated CD133/1+ papillary cells were positive for nestin, as well as several human embryonic stem cell markers (SSEA4, Nanog, SOX2, and OCT4/POU5F1) and could be triggered to adopt tubular epithelial and neuronal like phenotypes. Isolated papillary cells exhibited morphologic plasticity upon modulation of culture conditions and inhibition of asymmetric cell division. Labeled papillary cells readily associated with cortical tubular epithelia in co-culture and 3-dimensional collagen gel cultures. Heterologous organ culture demonstrated that CD133/1+ progenitors from the papilla and cortex, became integrated into developing kidney tubules. Tubular epithelia did not participate in tubulogenesis. Human renal papilla harbor cells with the hallmarks of adult kidney stem/progenitor cells that can be amplified and phenotypically modulated in culture while retaining the capacity to form new kidney tubules.
Keywords: kidney disease, ADPKD, regenerative medicine, renopoietic, mesenchymal stem cell, Tamm-Horsfall/uromodulin, metanephric organ culture, xanthosine
1.1 INTRODUCTION
Many renal disorders, including common genetic diseases such as autosomal dominant polycystic kidney disease, as well as acute and chronic kidney disease, renal cell carcinoma, and glomerulonephritis, can all lead to severe kidney damage or loss. Over 2 million patients world-wide are currently estimated to suffer from end-stage renal disease. Despite the advances in kidney transplantation, a significant shortage of donor organs severely limits treatment and requires many patients to remain on dialysis for extended periods. The quest for alternate organ restoration methods has resulted in rapid progression of new approaches, such as therapeutic cloning and embryonic/adult stem cell therapy over the past decade (reviewed in [1–5].
The pluripotency of embryonic stem cells presents one possible avenue for the replacement of damaged kidney tissue. There are several reports demonstrating the priming and programming of embryonic stem cells to express renal cell lineage markers and their ability to incorporate into developing kidney tubules [6–11]. However, the functional incorporation of embryonic stem cells into adult kidney tubules has not yet been described [4]. In addition, the transplantation of embryonic stem cells includes numerous potential complications including immune rejection, teratomas and other cancers [5, 12]. The problems associated with the use of embryonic stem cells and the potential benefits of autologous transplantation motivates the characterization of adult renal stem cell populations.
Adult stem cells have been identified in numerous organs including the adult brain, bone marrow, skin and gut, where they can be recruited for organ repair after injury [13–15]. Adult stem cells in kidney have only been identified in the last several years [5, 16–18]. Progenitor-like cells involved in recovery from renal injury in an animal model were first identified in 2003 [19], and subsequently populations of rodent adult kidney stem cells derived from rat and mouse renal papilla were isolated and characterized [16]. Adult rodent stem cells were identified in the interstitium and collecting ducts of rat kidney papilla on the basis of their low cell division rate using bromodeoxyuridine (BrdU) [16, 20, 21]. Proliferation of the BrdU label-retaining cells was confined to the upper papilla at the medullary boundary and the cells were found to exhibit enhanced proliferation and migration into the medulla and cortex in response to ischemic injury, supporting their involvement in organ repair [1, 21]. Low abundance, CD133+ cells isolated from the cortex of human kidneys were able to differentiate into proximal and distal tubular epithelia, as well as endothelia in vitro [22]. The human cortical CD133+ cells were shown to reconstitute renal tubules in SCID mice, however, their regenerative capacity was limited to conditions of renal injury. Another reported source for endogenous replacement of damaged or lost renal tubular epithelia are surviving tubular epithelial cells themselves [23]. Both glomerular and tubular epithelial cells can regress to an embryonic mesenchymal phenotype and can either stimulate the regenerative potential of neighboring surviving cells or themselves replace the damaged cells [18, 19, 24–26]. A functional role of these cells in organ repair is based on their ability to migrate, proliferate and produce growth and trophic factors. Finally, although the role of mesenchymal stem cells in renal regeneration has been debated over the years, the use of a ‘relay culture’ system (a two-step whole embryo followed by organ culture procedure) enabled bone marrow derived mesenchymal cells to participate in kidney organogenesis [27–29]. A resident population of mesenchymal cells lacking CD133 in the Bowman’s capsule is responsible for endothelial and podocyte regeneration [20, 26]. Thus, adult renal stem cells exhibit the capacity to give rise to multiple cell types and represent a critical first step in developing tissue replacement strategies. Nevertheless, there still remain numerous open questions regarding the characteristics that define such adult renal stem cells in humans. In particular, a more comprehensive characterization of stem cell niches in human kidney, an analysis of the phenotypic markers expressed by stem cells from different regions of the kidney and an accounting of their precise differentiation potential are still needed.
In the present study we have undertaken a comparative analysis of CD133/1+ cells in the human kidney papilla versus the cortex. We identify a unique dually CD133+ and nestin+ renal progenitor population that also expresses numerous embryonic stem cell markers embedded in the papillary loops of Henle. The CD133/1+ papillary cells exhibit significant plasticity in culture and are functionally distinct from tubular epithelia in their ability to integrate into developing kidney tubules.
1.2 MATERIALS and METHODS
1.2.1 Isolation and culture of human kidney cells
Tissue considered unsuitable for transplant was obtained through the National Disease Research Interchange (NDRI) and the protocol is judged exempt by the institutional HRRC. Cortical and normal tubular epithelial cells from the renal cortex were isolated by bisecting the kidney cutting pieces of cortex into 1 mm cubes and performing enzymatic digestion (collagenase, hyaluronidase, DNase l, soybean trypsin inhibitor cocktail in DMEM/F-12 media for 1h at 37°C with regular vortexing) as previously described [30, 31]. The average cell yield we aimed for was 5-T75 cm2 flasks, but one can be as high as 10-T125 cm2 flasks if the complete cortex is carefully dissected. After several days in culture, dead cells were removed and new medium was added. The isolated cell mixture was used for purification of CD133+ cells or snap frozen. Cortical tubular epithelial cells were enriched after one passage and were routinely cultured in REGM containing low serum, 10 ng/ml epidermal growth factor, 1 ml/l epinephrine, 5 mg/ml insulin, 6.5 pg/ml triiodothyronine, and 10 mg/ml transferrin or DMEM/F12 containing 10% FBS for up to 4 passages.
Renal papillary cells were isolated from three different human kidneys from anonymous donors through the NDRI, designated (59M04, 67F05, 42M06) and found to give consistent results in the assays. Cells were obtained by bisecting the kidney and scooping out the renal papilla. Papillary cells were disaggregated using a cocktail of enzymes (including collagenase, hyaluronidase, DNAase I, soybean trypsin inhibitor) in DME/F12 medium. Digestion was performed at 37°C for 60 min with vigorous intermittent vortexing. Following digestion, the cells were collected by centrifugation and resuspended in Renal Epithelial Cell Growth Medium (REGM CC-3191; Clonetics, Lonza Walkersville, MD) containing low serum, and 10 ng/ml epidermal growth factor, 1 ml/l epinephrine, 5 mg/ml insulin, 6.5 pg/ml triiodothyronine, and 10 mg/ml transferrin. One confluent T 25 cm2 flask of cells was routinely obtained from the first plating. The cells were expanded or directly frozen and stored. We did not observe a loss in cell viability or differentiation potential caused by freeze thawing. The cells were readily cultured in excess of the 4 passages that is normally the in vitro limit for cortical tubular epithelial cells.
Individual frozen aliquots of these cells were thawed, used for further purification of CD133/1+ cells and cultured under various conditions to study phenotypic properties. For phenotypic plasticity experiments, papillary cells were cultured in REGM with the above supplements or in DMEM/F12 with or without 10% fetal calf serum in 2D cell culture conditions, on plastic (solid) or 0.4 µm pore filter (permeable) supports (Falcon BectonDickinson) or in 3D collagen gel culture. Epidermal growth factor was added at 20 ng/ml to serum-free medium. Immortalized human renal cortical tubular epithelial cells (RCTE) were cultured as described [32].
1.2.2 Colony formation assay with xanthosine and cytotoxicity measurements
To promote clonal expansion through suppression of asymmetric cell division [33, 34], cells were plated at 100 cells per well of a 6-well plate and cultured in normal growth medium supplemented with 0, 200 µM or 400 µM xanthosine. After one week cells were fixed and stained with crystal violet to reveal colonies of cells.
For cytotoxicity measurements, primary cortical epithelia and renal papillary cells were plated at 0.5×104 cells per well (96-well plate) and allowed to adhere overnight. The following day the media was removed and replaced with fresh media containing xanthosine (200 µM or 400 µM) or no drug. After 48 h the samples were analyzed for lactate dehydrogenase (LDH) release (Roche #04744926001). As a control, cells grown under identical conditions were lysed to determine the total LDH in the sample. LDH release was measured colorimetrically at absorbance of 490 nm. Samples from three different patients (42M06, 59M04 and 67F05) were used and all samples were treated and assayed in triplicate.
1.2.3 Purification of CD133/1+ cells from papilla and cortex
To purify the isolated cortical and papillary cells, CD133/1+ cells were enriched by positive selection using magnetic cell separation methods under sterile conditions. To do so, cells from one T75 cm2 flask were released using cold PBS containing 5 mM EDTA and passed through a 30 µm nylon mesh to obtain single cell suspension. Cells were washed with PBS, collected by low speed centrifugation and resuspended in 300 µl PBS containing 0.5% BSA and 2 mM EDTA for 108 cells. An FcR blocking solution (100 µl) was added to the cells to reduce non-specific binding to the beads. Chilled cells in blocking solution were then incubated on ice with 100 µl AC133-coated magnetic microbeads from Miltenyi Biotech (130-050-801) for 30 min in a refrigerator without mixing. Note: the CD133/1 epitope recognized by AC133 mAb is unique to stem cells and present on renal progenitors [35–37]. Cell-microbead mixture was washed with 1–2 ml buffer and collected by centrifugation at 300 g for 10 min. The supernatant was removed and cell microbead mix resuspended in 500 µl PBS containing 0.5% BSA and 2 mM EDTA. Microbead-coated cells were separated from unlabeled cells by sequential passage over two MACS® magnetic columns as per manufacturer’s instructions. After the second magnetic column purification, the labeled bound cells were released by removing the column from the magnet, rinsing and collecting cells by centrifugation. The papillary samples contained an obvious cell pellet, while the cortical sample pellet was not visible, though cells were present. This observation is consistent with the reported low abundance of CD133+ cells in the human kidney cortex and in glomeruli [22, 38]. The magnetic beads are degradable and non-toxic. Therefore, the isolated cells were directly cultured in REGM following isolation and subsequently frozen. The purity of the cells following isolation was confirmed by co-immunostaining for both nestin (using rabbit pAb from Chemicon) and CD133 (using mAb CD133/2-PE from Miltenyi Biotec).
1.2.4 Quantitative real time polymerase chain reaction (PCR)
Primary cortical epithelia and papillary cells (initial isolates) from three separate human kidneys (42M06, 59M04, 67F05) were cultured to near confluence in T75 cm2 flasks. Purified CD133/1+ papillary cells from one patient (42M06) were similarly cultured. RNA was purified using AllPrep DNA/RNA/protein purification kit according to manufacturer’s instructions (Qiagen Valencia, CA). Quality controls were performed with Nanodrop spectrophotometer and Agilent Bioanalyzer. Taqman primers from Life Technologies Applied Biosystems (Foster City, CA) were purchased and used to measure gene expression (Nanog: Hs02387400_g1; SOX2/SRYbox-2: Hs01053049_s1; OCT4/POU5F1: Hs00999632_gH; GAPDH: Hs03929097_g1; PP1A: Hs99999904_m1). Quantitative real time PCR assays were conducted in 384 well plates, for a maximum of 40 cycles. Ct values for all genes were normalized to GAPDH Ct values of the same RNA sample. Average GAPDH Ct values for each RNA sample were used to derive a scaling factor for normalization of the results derived from individual RNA samples. Fold change in papillary gene expression for each patient sample was calculated as a function of the gene expression in cortical epithelia of the corresponding patient. Statistical significance was evaluated using unpaired or independent, one-tailed T-test.
1.2.5 3D Collagen gel culture
Primary kidney epithelial cells from the cortex and CFDA-labeled papillary cells were mixed with collagen I gel (prepared according to manufacturer’s instructions, Chemicon, Temecula, CA) either individually or as mixed populations and plated in 24-well cell culture dishes. After 10 days of collagen gel culture, samples were processed for immunofluorescence staining.
1.2.6 Co-culture with rodent neural stem cells
Purified CD133/1+ renal papillary cells were co-cultured with neural stem/progenitor cells (NSPCs) from E14 mouse telencephalon of the C57BL/6-TgN (ACTbEGFP) strain (The Jackson Laboratory), which express EGFP under a β-actin promoter [39]. Renal papillary cells or cortical tubular epithelia were plated on porous cell culture inserts (0.4 µm pores) and NSPCs were plated in the culture well beneath the filter (see Fig. 2A, cartoon). EGFP-expressing NSPCs were thus easily distinguished from human kidney cells, which allowed a visual control in the event of any contamination between the co-cultured cell types. Cultures were grown in serum-free neural stem cell medium supplemented with 10 ng/ml EGF and 10ng/ml bFGF (see detailed medium composition in [39]), in the presence or absence of NSPCs. Following 3–4 days, renal papillary cells, but not the epithelia were observed to dramatically change their shape and formed clusters of cells that could be observed by light microscopy. For analysis the cells on filters were fixed and immunostained for β-catenin, CD133 and nestin and imaged by fluorescence or confocal microscopy.
1.2.7 Metanephric organ culture
Care and euthanasia of mice conformed to Institutional Animal Care Guidelines. Pregnancy was dated using presence of vaginal plug as embryonic day (E) 0.5. Embryonic mouse kidney rudiments micro-dissected at day E12.5 post-fertilization were cultured on clear Transwell filters (0.4 µm pores), floating on DMEM/Ham’s F12 medium supplemented with 10% FBS, 2 mM Lglutamine, 1 µM dexamethasone, and antibiotics. Culture rudiments were incubated at 37 °C in a humidified, 5% CO2/95% ambient air atmosphere for 72 h with media changes every 24 h.
Timed pregnant FVB mice from Charles River were sacrificed on E12.5. Isolated metanephric organs consisting of both ureteric bud and undifferentiated mesenchyme were placed in a well of a 24-well plate containing culture media on ice until all kidneys were ready for injection. CD133/1+ cells isolated from both the human renal cortex and papilla and non-purified human renal cortical cells were labeled with CFDA and injected into day E12.5 embryonic mouse kidneys using capillary pipets with 40 µm tips (1500 cells per injection site/three injection sites per kidney). Sham injections were performed to analyze metanephric organ integrity. After three days in culture kidneys were fixed with 4% paraformaldehyde and stained with rhodamine-labeled peanut agglutinin to identify kidney tubules (Vector Laboratories, Burlingame, CA) overnight at 4°C in 1% BSA, 1% Triton-X 100 in 1XPBS. The human origin of cells was confirmed by anti-human HLA class I and anti-human CD133 staining (rabbit pAb). Images were collected using an Olympus FV1000 microscopy customized for two-photon microscopy, with a 60X, NA 1.2 water immersion objective (Indiana Center for Biological Microscopy, Indianapolis, IN) or a Zeiss LSM-510 Meta Confocal/Multiphoton Microscope. Images were rendered using Voxx2 (Indiana Center for Biological Microscopy, Indianapolis, IN) [40].
1.2.8 Immunofluorescence staining and quantification
Cells grown on glass coverslips or filters were immunostained as described [31, 41]. Fluorescent lectins for identifying tubule segments were as follows: Dolichos biflorus (DBA) to identify distal and collecting duct and Arachis hypogaea/peanut agglutinin lectin (PNA) to label distal convoluted tubule and intercalated cells of the collecting duct [42, 42, 43]. Antibodies used were as follows: CD133 (rabbit pAb ab16518, Abcam Ltd., Cambridge, MA; Miltenyi Biotec mouse mAb AC133 and 293C3 recognizing the CD133/1 and CD133/2 epitopes, respectively); nestin (mouse mAb, BD Biosciences, San Jose, CA; rabbit pAb, Chemicon/Millipore, Billerica, MA); beta III tubulin (affinity purified rabbit pAb, Covance Research Products, Inc., Denver, PA); β-catenin (mouse mAb Transduction Laboratories/BD Biosciences, Lexington, KY), N-cadherin (mouse mAb from Transduction Laboratories/BD Biosciences; rabbit pAb from Calbiochem, La Jolla, CA) and E-cadherin (mouse mAb from Transduction Laboratories/BD Biosciences; rabbit pAb Santa Cruz, Santa Cruz, CA); pan-cadherin (mouse mAb, Sigma Chemical Co., St. Louis, MO; rabbit pAb Cell Signaling, Danvers, MA); occludin (rabbit pAb, Zymed-Invitrogen, Carlsbad, CA); Tamm-Horsfall antigen (rabbit pAb, Biomedical Technologies, Inc., Stoughton, MA); human embryonic stem cell markers SSEA4, TRA1-60, TRA-1-81, OCT4A, SOX2, and Nanog (Stemlite™ Pluripotency Kit, Cell Signaling Technology, Danvers, MA). All fluorescent secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA).
For co-culture experiments, isolated papillary cells were loaded with green fluorescent 5-(and-6)-carboxyfluorescein diacetate (CFDA, Molecular Probes-Invitrogen, Carlsbad, CA) and then mixed with cortical kidney epithelial cells. After three days in culture, the cells were stained on ice with monomeric perfringolysin O (binds cholesterol [44, 45]) to outline all cell membranes and subsequently fixed.
Collagen gel cultures were washed with PBS to remove growth medium and fixed with 3% paraformaldehyde and processed for immunofluorescence as previously described [46].
Tissue samples for immunostaining were isolated from the kidney cortex, medulla and papilla, snap frozen in OCT and cryosectioned (70 nm). Cryosections (0.5–2 µm thick) were fixed and immunostained as described [41].
All images were acquired on a Zeiss LSM 510 confocal microscope housed in the UNM Fluorescence Microscopy Shared Resource. Quantification of staining was performed on z-stacks taken at 0.35 µm intervals using Image J software.
1.2.9 Light and electron microscopy of human kidney tissue sections
Samples for electron microscopy were isolated from human kidney cortex, medulla and papilla (kidneys received from NDRI) and processed for Epon embedment according to standard procedures and examined on a Hitachi electron microscope at 80 kV and images acquired using a 1 megapixel digital camera.
A thin slice from the normal pole of a surgically removed human kidney was immersion fixed with 4% paraformaldehyde in phosphate buffer, cryoprotected in 2.3 M sucrose and frozen with liquid nitrogen. Semi-thin sections, 0.5–2 µm (Ultracut UCT with the FCS cryo attachment, Leica, Bannockburn, Il) were collected onto glass slides for immunogold labeling followed by silver enhancement. Sections on the glass slides were rinsed with TBS, then incubated in glycine to remove aldehydes for 15 min, rinsed, then blocked for 1 h (5% BSA, 0.1% cold water fish gelatin in TBS), rinsed again, then the primary antibody (CD-133/1, 1:50) for 1 h at room temperature. The sections were thoroughly rinsed prior to labeling with the secondary antibody conjugated to 10 nm colloidal gold (Goat anti rabbit 1:40, Aurion, Hatfield, PA) for 1 hour at room temperature. The sections were then silver enhanced (Aurion, Hatfield, PA) for 25 minutes and viewed with a light microscope.
1.3 RESULTS
1.3.1 Cells expressing stem cell markers are intercalated between tubular epithelia exclusively in loop of Henle segments deep within renal papilla
We assessed two stem cell markers, CD133/1 and nestin, which are both associated with developing kidney to identify and localize stem cells within the human renal papilla and cortex. CD133 (prominin-1) was originally identified in neuroepithelial stem cells as a pentaspanning, cell surface glycoprotein [47]. CD133 functions in plasma membrane protrusion and membrane domain organization required for cell differentiation [48], but it is its association (particularly of the CD133/1 glycosylation epitope) with primitive hematopoietic stem and developing epithelium that has prompted its wide use to identify stem/progenitor cell populations. In the kidney CD133+ cells have been isolated from rodent papilla, human kidney cortex and glomeruli [16, 22, 38]. Nestin is an intermediate filament protein that is frequently co-expressed with CD133 and is a hallmark of normal and cancer stem cells of diverse lineages [49–53]. During kidney development, nestin is prominent in endothelial precursors and mesonephric mesenchyme, which gives rise to tubular epithelia, but in adult kidney expression is reported to be confined to podocytes [49, 54]. We therefore stained human tissue sections taken from various parts of the kidney, including the cortex, medulla and papilla, for both nestin and CD133. (N.B. We have taken care to test multiple CD133 antibodies throughout our study, in consideration of reports that CD133/1 and CD133/2 epitopes are preferentially expressed in progenitor cells [35, 37]).
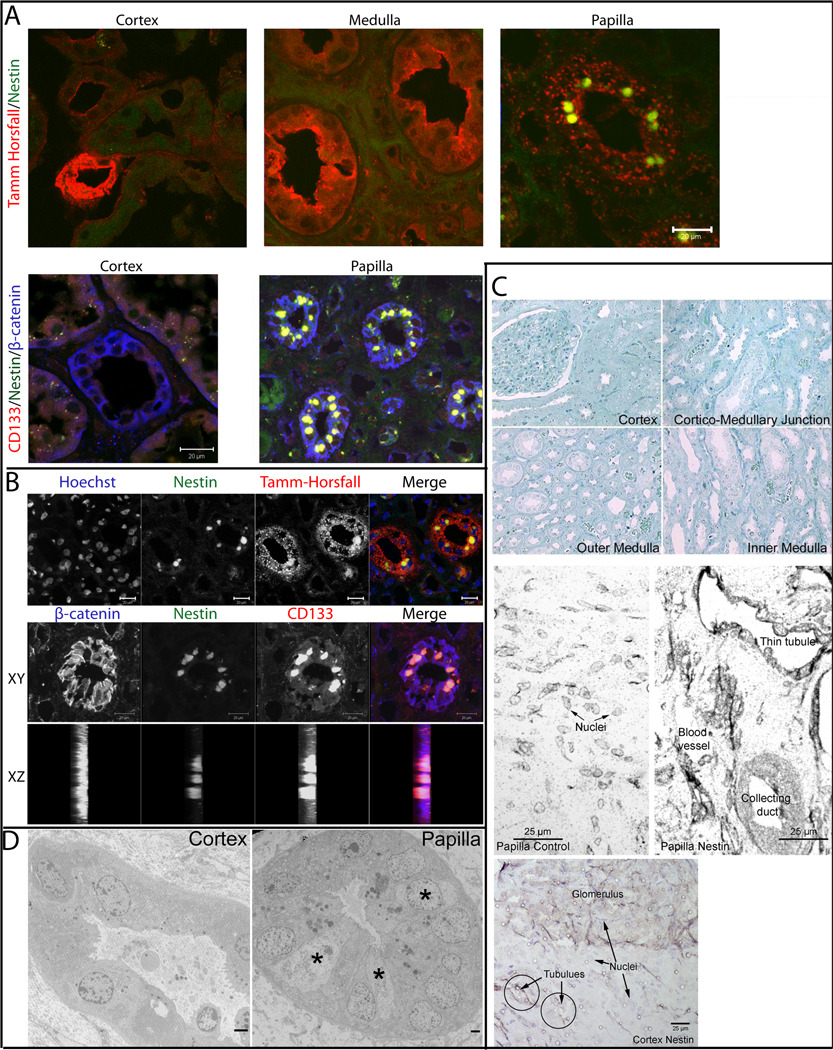
Staining for β-catenin, Tamm-Horsfall antigen and various lectins served as markers to discern the identity of individual tubule segments. Strikingly, cells expressing both nestin and CD133/1 were significantly enriched in the papillary loops of Henle (identified by the presence of Tamm-Horsfall antigen), while the cortical and medullary loops of Henle and tubules were devoid of such CD133+ and nestin+ cells (Fig. 1A). Detailed analysis using Hoechst staining to identify nuclei and confocal x-z and stacked x-y images showed the staining was associated with small cells that were fully integrated between the tubular epithelia (Fig. 1B). These data suggest that the papilla may serve as a reservoir for cells expressing the stem cell markers nestin and CD133/1.
Figure 1. Cells expressing stem cell markers are intercalated between tubular epithelia exclusively in loop of Henle segments deep within renal papilla.
(A) Cryosections from the cortex, medulla and papilla derived from a normal human kidney were fixed and immunostained for markers as indicated on each panel. (Upper panel) Samples labeled with antibodies specific for Tamm Horsfall (red) and nestin (green). (Lower panel) Samples labeled with antibodies specific for CD133 (red), nestin (green), and β-catenin (blue). Scale bars 20 µm. (B) Cryosections from human kidney papilla were fixed and immunostained for Hoechst, nestin, and Tamm-Horsfall or β-catenin, nestin, and CD133. A single tubule was imaged at 0.4 µm intervals and the composite projections viewed in the x-y and x-z directions are shown. Scale bars 20 µm. (C) Human kidney samples from distinct kidney regions were cryosectioned and methylene blue labeled (top four panels) to evaluate morphology or immunogold labeled with silver enhancement (middle papilla control and papilla nestin panels). Papillary section immunogold labeled for nestin (papilla nestin) shows strong staining on collecting duct, thin tubules and blood vessels. The papilla control section was labeled with a control antibody, wherein only counterstained nuclei are evident. Bottom panel shows cortical section immunostained for nestin, circles demark tubules devoid of nestin, while glomerulus in top half of panel is distinctly positive. Magnification 20×. Scale bars 25 µm. (D) Ultrastructural analyses of Epon embedded human kidney samples identify a morphologically distinct cell type (*) in papillary tubules (right) that is absent from cortical tubules (left). Magnification 3,000–3500×. Scale bars 2 µm.
1.3.2 Unique cell type embedded within papillary renal tubules revealed by ultrastructural analysis
The morphological integrity of the kidney regions (cortex, and medulla and into the papilla) showed good preservation without evidence of any pathology in the normal kidneys used for further study (Fig. 1C, top four panels). Thin cryosections from these regions were immunogold-stained (with silver enhancement) for nestin and compared to control antibody labeled sections. Shown are nestin+ kidney tubule epithelia in the papilla, especially thin tubules, while vasa recta endothelia were negative (Fig. 1C, papilla nestin). The nestin label is seen throughout the cytoplasm and counterstained nuclei are only weakly evident. In a parallel sample labeled with a control antibody, only the counterstained nuclei were evident (Fig. 1C, papilla control). In the renal cortex, tubules were devoid of nestin, in contrast to nestin+ glomeruli (Fig. 1C, bottom panel). Nestin expression in glomeruli is in agreement with previous reports [54–56].
Electron microscopic analysis of Epon embedded kidney tissue identified a population of morphologically distinct cells with a large nucleus and a thin rim of cytoplasm (Fig. 1D, right panel marked with asterisks). Based on the facts that the cells have few microvilli, are not especially cuboidal and exhibit a highly interdigitated association with the underlying matrix, they appear to be distinct from collecting duct. These cells were not seen in cortical tubules (Fig. 1D, left panel).
1.3.3 Isolation of human kidney cells from papilla with the hallmarks of renal progenitor cells
Human primary papillary cells were immunopurified using magnetic beads coated with AC133 mAb that is specific for progenitor cells expressing the CD133/1 epitope [35–37]. The immunopurified cells were analyzed for the expression of further stem cell markers by quantitative real time PCR and immunostaining.
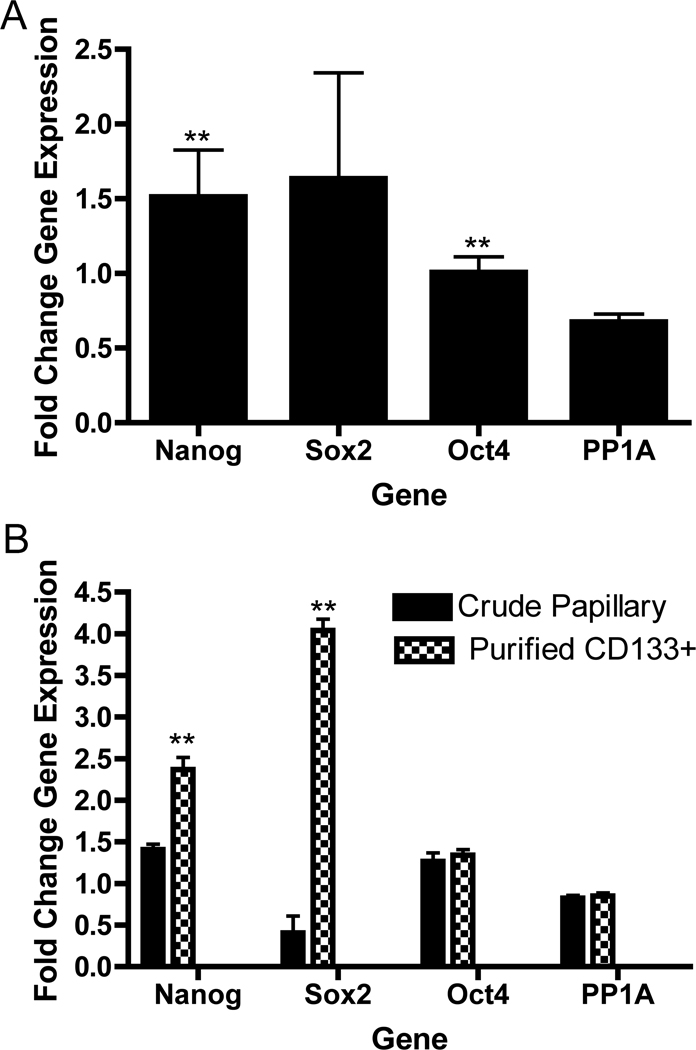
Primary cortical and papillary cells isolated from three different patients were analyzed for the expression of embryonic stem cell transcription factors (Nanog, SOX2/SRY-box-2, and OCT4/POU5F1) that are frequently used as measures of pluripotency and also shown to be associated with adult mesenchymal stem cells [57–61]. Nanog and OCT4 exhibited increased expression in papillary cell fractions (non-purified) that were statistically significantly different from the expression in cortical epithelia and the housekeeping gene protein phosphatase 1A (PP1A) (Fig. 2A). Nanog and SOX2 were significantly co-purified in cells selected for CD133/1+ relative to the non-purified papillary fraction from the same patient (Fig. 2B).
Figure 2. Stem cell markers are enriched in CD133/1+ cells purified from the renal papilla.
RNA was prepared from three different patients in matched sets of cortical epithelia and mixed population papillary cells (42M06, 59M04, 67F05). For comparison, RNA was also from purified CD133/1+ cells isolated from 42M06. Quantitative real time PCR was performed using Taqman primers (Applied Biosystems) to measure gene expression. Plotted are the mean fold changes in gene expression ± SEM comparing papillary cells to cortical epithelia. All values were normalized to GAPDH expression. Protein phosphatase 1A (PP1A) serves as an independent control for consistency of housekeeping gene expression across samples. N=3. (A) Mixed papillary cells show 1.5 fold greater expression of Nanog and SOX2 over cortical epithelia. Asterisks (**) indicate statistically significant differences based on independent, one tailed T-test comparison of stem cell markers vs. PP1A housekeeping gene expression: Nanog p=0.07; OCT4/POU5F1 p=0.03; SOX2 p=0.12. (B) CD133+ cells show significant enrichment in Nanog and SOX2 gene expression, but not of OCT4 relative to initial papillary fraction. Asterisks (**) indicate statistically significant differences based on unpaired, one tailed T-test comparison of each stem cell marker in crude papillary cells vs. same marker in purified CD133/1+ papillary cells from single patient: Nanog p=0.002, OCT4/POU5F1 p=0.289, SOX2 p=0.0003, PP1A p=0.313.
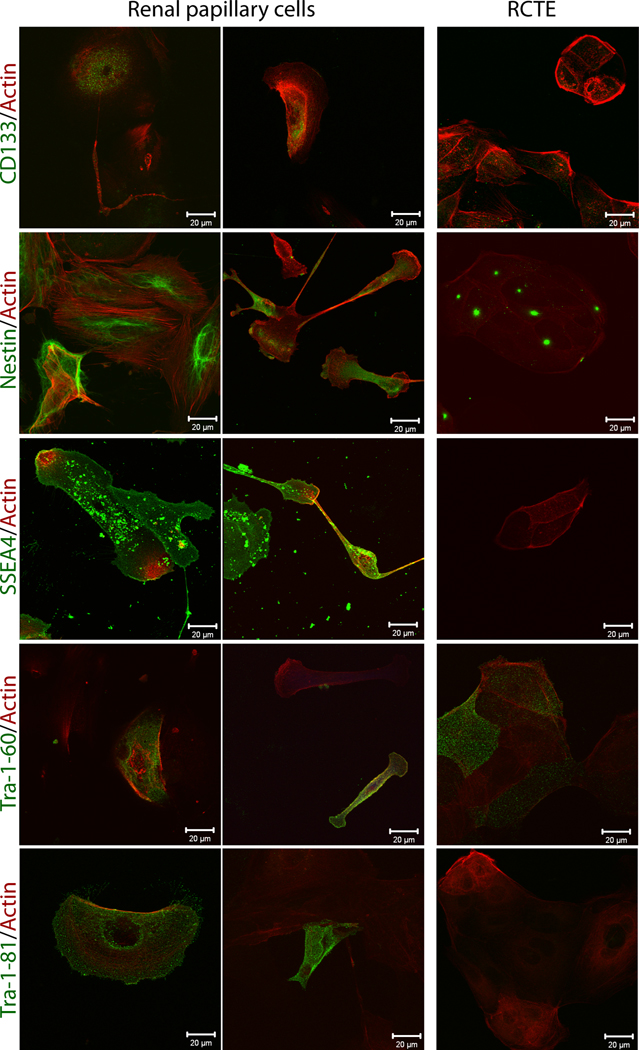
Immunostaining of the CD133/1+ papillary cells revealed nestin filaments throughout the cytoplasm (Fig. 3). CD133 was absent in immortalized RCTE cells even at subconfluence, though nestin showed a specific localization to centrosomes in these proliferating epithelia. The centrosomal localization of nestin will be interesting to follow-up in light of the facts that intermediate filament proteins are intimately associated with microtubules, BBS proteins are associated with ciliopathies and centrosomes; and together with centrosomes may play a role in the development of cell polarity and ciliopathies [62–67]. The immunopurified papillary cells robustly expressed Stage Specific Embryonic Antigen-4 (SSEA4), a human plasma membrane glycosphingolipid [68], which could not be detected in renal tubular epithelial cells used as a differentiated cell type control (RCTE) (Fig. 3). Two other embryonic stem cell surface markers, TRA-1-81 and TRA-1-60, which are expressed in fetal and adult kidney were also analyzed [69, 70]. TRA-1-81 was expressed only on occasional purified CD133/1+ and unsorted papillary cells, but not in RCTE cells (Fig. 3). TRA1-60 was observed on a small subset of both papillary cells and differentiated RCTE cells (Fig. 3). The data are consistent with a recent report showing that in adult kidney Tra-1-60 is most abundant in thin limb of the loop of Henle and papillary collecting ducts [70]. Lectin staining of papillary cells found them negative for PNA and <9% positive for DBA. Because the CD133/1+ papillary cells were nearly all positive for nestin (expression restricted to metanephric mesenchyme, [54]), and only occasional cells were Tra-1-60 positive, it is likely that the cells are of mesenchymal rather than of collecting duct/ureteric bud origin.
Figure 3. Purified CD133/1+ cells isolated from human kidney papilla express mesenchymal and embryonic stem cell markers.
Renal CD133/1+ papillary cells and immortalized renal tubular epithelia (RCTE) grown on glass coverslips for 24 h were fixed and immunostained for CD133/1, nestin, SSEA4, TRA-1-60 and TRA-1-81 (all in green). Rhodamine phalloidin (red) staining was used as a general marker to identify all cells in the cultures. Scale bars 20 µm.
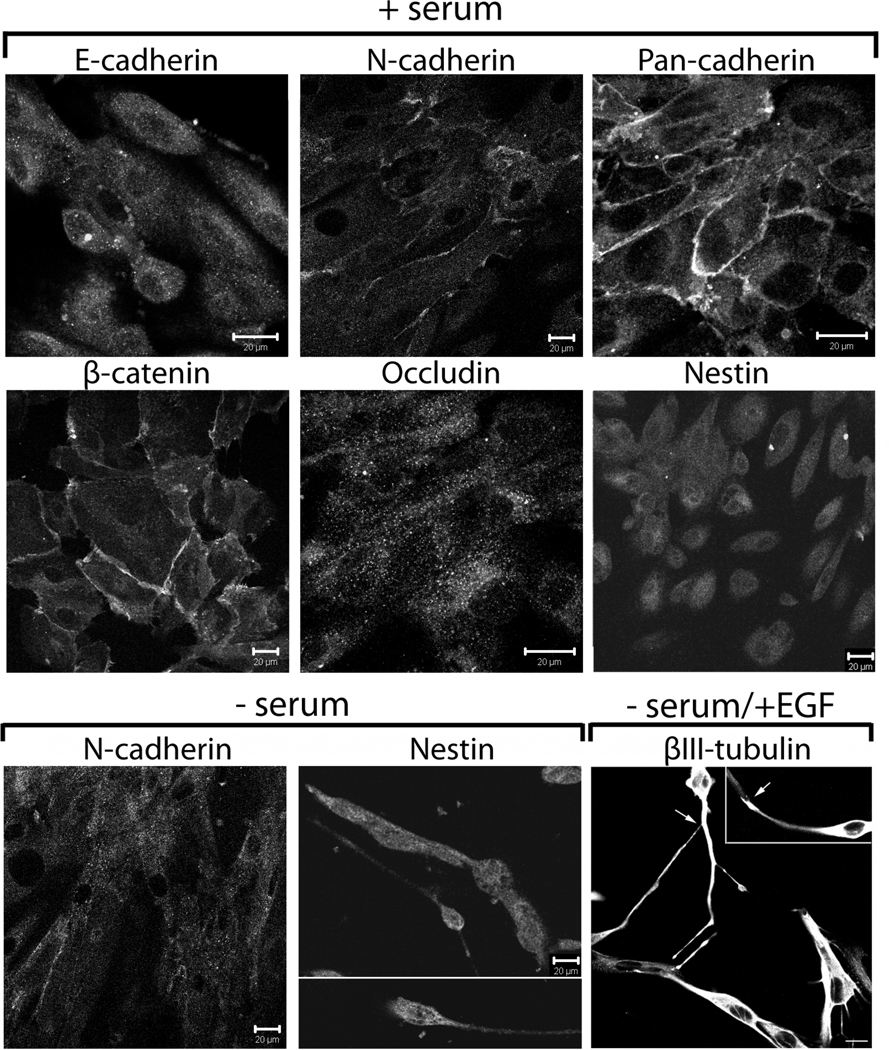
The in vitro growth properties and plasticity of the isolated cells was also examined. The isolated cells developed an immature epithelial-like phenotype and their shape was similar to the shape of primary human kidney epithelial cells when cultured for 3–4 days on plastic dishes with serum (Fig. 4, + serum). The cells lacked tight junctions (no occludin at the lateral membranes), but formed adherens junctions composed of the mesenchymal adhesion molecule N-cadherin seen weakly at the lateral membranes with β-catenin. Based on the strong staining of the cell membranes with a pan-cadherin antibody, there is most likely another cadherin that contributes to papillary cell-cell adhesion. Staining for cadherin-6 (K-cadherin) and adhesion molecule N-cam did not detect either protein at the cell-cell adherens junctions, suggesting these cadherins are not involved in papillary cell adhesion (not shown). The cells remained slightly positive for the neural stem cell marker nestin (Fig. 4, + serum), though its expression was highly sensitive to changes in culture conditions. Overall the traits of the renal papillary cells are quite distinct from those we have described for mature primary human renal tubular epithelial cells, which robustly express E-cadherin and tight junctions [31, 41]. Characterization of the cortical epithelia from the patients used in the present study by lectin staining showed 25% were of distal tubule and collecting duct origin, while the majority were of proximal tubule origin in agreement with our previous work [71, 72].
Figure 4. Papillary cells exhibit phenotypic plasticity upon modulation of culture conditions.
Human papillary cells were grown on glass coverslips for 3–4 d in the presence (+) or absence (−) of serum or in serum-free medium + EGF as indicated. Fixed cells were immunostained with antibodies against cell junction proteins (adherens junctions: E-cadherin, N-cadherin, pan-cadherin and β-catenin; tight junctions: occludin), the neural stem cell marker nestin, or beta III tubulin, a specific marker for immature neurons. Scale bars 20 µm.
The phenotype of rodent papillary cells was sensitive to the content of serum or growth factors in the media. Similar to cells isolated from rodent papilla [16], the human papillary cells exhibited dramatic changes in cell shape when grown in serum-free medium. Following 3–4 days in serum-free media the cells became highly elongated and spindle-shaped (Fig. 4, − serum). The expression of N-cadherin decreased, whereas expression of nestin increased and the cells developed a neuronal-like shape. Nestin expression was highest when cells were less differentiated in serum-free medium and decreased with time in culture or in the presence of serum. When grown in serum-free medium supplemented with EGF the cells became positive for beta III tubulin (specific marker for immature neurons) and appeared to ‘synapse’ with each other (Fig. 4, − serum/+ EGF).
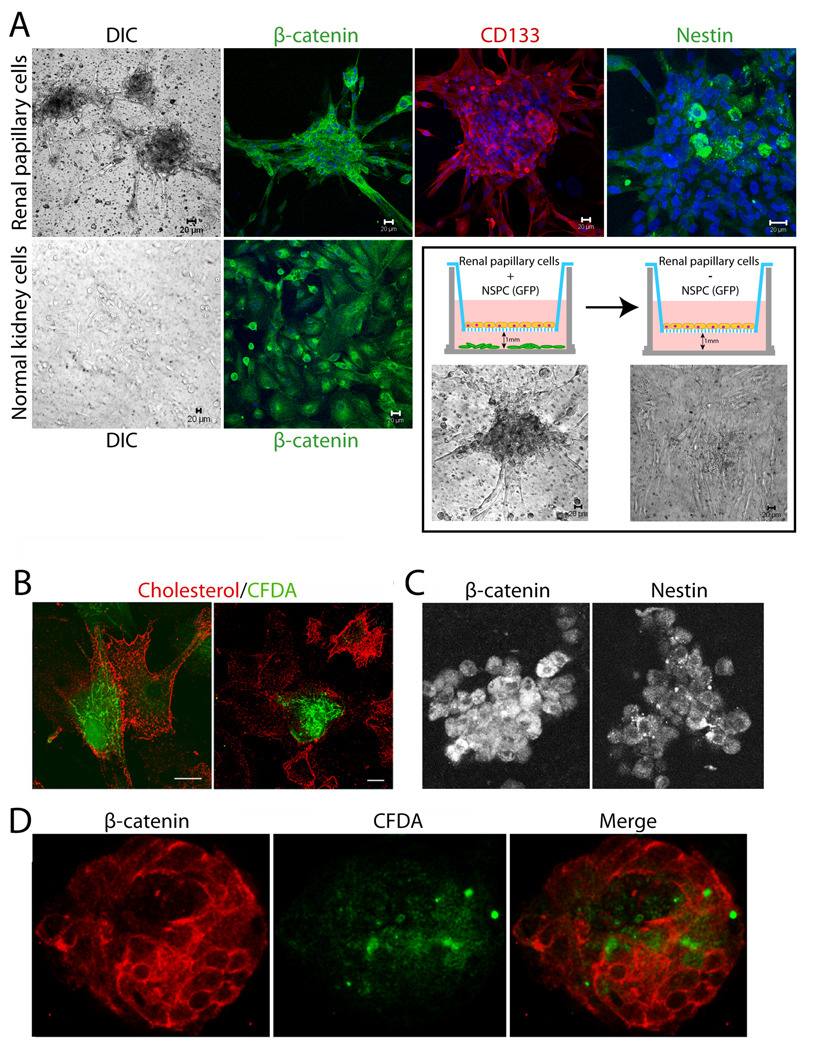
Human papillary cells were also cultured on filters in the presence of diffusible factors produced by rodent neural stem/progenitor cells (NSPCs) present in the well under the filters but not in direct contact with the human papillary cells (Fig. 5A, cartoon). In the presence of EGFP-NSPCs, the papillary cells formed spheroid structures that were positive for intracellular β-catenin, plasma membrane localized CD133 and intracellular nestin in the core of the spheres (Fig. 5A, top row). Cortical tubular epithelia cultured in the presence of the NSPCs on the other hand, did not undergo these dramatic shape changes and expressed β-catenin at the lateral membranes and intracellularly (Fig. 5A, bottom row, left two panels). Withdrawal of NSPCs, by transfer of the filters with the papillary cell spheroids to culture plates containing kidney cell growth medium alone, resulted in a reversal of cell phenotype to a flattened and disperse morphology within just 24 h (Fig. 5A, bottom row, boxed panels). Analogous shape changes, as well as the formation of aggregates/spheres were described for the rodent papillary cells under serum-free conditions [16].
Figure 5. Human papillary cells associate with cortical tubular epithelia and undergo significant morphogenesis in co-culture systems.
(A) CD133/1+ papillary cells were grown in the presence or absence of GFP-neural stem/progenitor cells for 3–4 d, in defined serum-free neural stem cell medium as illustrated in the cartoon. Top row left to right shows human papillary cells cultured on filters above GFP-neural progenitors: DIC image of spheroids, β-catenin (green, single slice), CD133 (red, 3D reconstruction of complete Z-stack) and nestin (green, single slice) stained spheroids. Bottom row left two panels show primary normal human cortical tubular epithelia cultured on filters above GFP-neural progenitors: DIC image, β-catenin (green). Nuclei stained with Hoechst are in blue. DIC images within the outlined box (below cartoons) show flattening of papillary spheroids when filter cultures are transferred to wells lacking neural stem cells for 24 h. (B–D) Papillary cells loaded with CFDA fluorescent dye (green) readily form contacts with primary normal human cortical tubular cells in co-culture experiments on plastic dishes. Staining for cholesterol using perfringolysin O (red) was used to identify all cells in the culture. (B) Primary tubular epithelia (red) and papillary cells (green and red) interact closely on plastic dishes irrespective of whether tubular epithelia are from the same or a different patient origin as papillary cells. (C) Papillary cells grown 10 d in collagen gel cultures stained for nestin and β-catenin. (D) Primary tubular epithelia (red) and CD133+ papillary cells (green and red) grown in collagen gel co-cultures interact and form tubulospherical structures with hollow cores. See supplemental movie 1 to view entire Z-stack.
Hepatic stem cells have been reported to undergo clonal expansion upon suppression of asymmetric division with xanthosine in vitro [33] and label-retaining mammary stem cells in vivo were found to undergo proliferation and expansion upon xanthosine treatment in vivo [34]. Therefore, we tested the response of renal papillary cells and cortical tubular epithelia to culture in the presence of varying doses of xanthosine. Cells were plated under conditions to detect clonal expansion of single cells as described in 1.2.1 and colonies were detected by crystal violet and cells in the colonies imaged using light microscopy (Supplemental Fig. A.1). Papillary cells responded positively to increasing doses of xanthosine up to 400 µM with increasing numbers of colonies formed. In contrast tubular epithelia readily formed colonies in the absence of xanthosine and colony formation decreased precipitously in the presence of 400 µM xanthosine. Examination of the papillary cell morphology showed the proliferation and expansion of loosely associated cells in the presence of increasing doses of xanthosine. Tubular epithelia on the other hand lost cell-cell contacts, stopped dividing became larger, flattened and vacuolated and appeared on the verge of cell death in 400 µM xanthosine. Xanthosine was not cytotoxic to either papillary or cortical epithelia from three different patients based on a lactate dehydrogenase release assay and 48 h of treatment (Supplemental Fig. A.1 graph).
The cumulative data suggest that human renal papilla harbor cells with adult renal stem cell-like properties in terms of stem cell marker expression and morphologic plasticity that is modulated by culture conditions.
1.3.4 Papillary cells form close contacts with cortical tubular epithelia and participate in in vitro renal tubulogenesis in 3D collagen gel co-cultures
The potential for interactions between cells from kidney papilla and normal cortical kidney epithelial cells was also evaluated. The isolated papillary cells were first loaded with green fluorescent dye (CFDA) and then co-cultured with cortical kidney epithelial cells (primarily of proximal tubule origin based on lectin staining) on plastic dishes or in three-dimensional (3D) collagen gel cultures (Fig. 5B–D). After three days on plastic dishes, all cells were visualized with monomeric perfringolysin O (binds cholesterol) to outline cell membranes (Fig. 5B). Papillary cells were consistently seen incorporated within mixed population cell monolayers and made contacts with the cortical epithelial cells irrespective of whether the cells were derived from the same patient as the papillary cells or from a different patient.
The final outcome of complex events associated with cell differentiation is a functional three-dimensional organization of cells into structures and organs. Extracellular matrix gels used in 3D cultures provide a model that mimics the “in vivo” environment in which cells grow and differentiate. Epithelial cells plated in collagen gels migrate, proliferate, differentiate and eventually form hollow spheres [73]. Primary kidney epithelial cells from the cortex and cells isolated from human papilla were grown individually or co-cultured in 3D collagen I gels (Fig. 5C–D). Even after 10 days in collagen gels, human papillary cells remained as aggregates of loosely attached round cells, positive for nestin and intracellular β-catenin when cultured alone (Fig. 5C). But when co-cultured with primary kidney epithelial cells, CFDA-labeled papillary cells readily incorporated into hollow spheres with budding tubules and intact adherens junctions shown by the honey-comb-like β-catenin staining of epithelia in the structure (Fig. 5D and Supplemental movie 1).
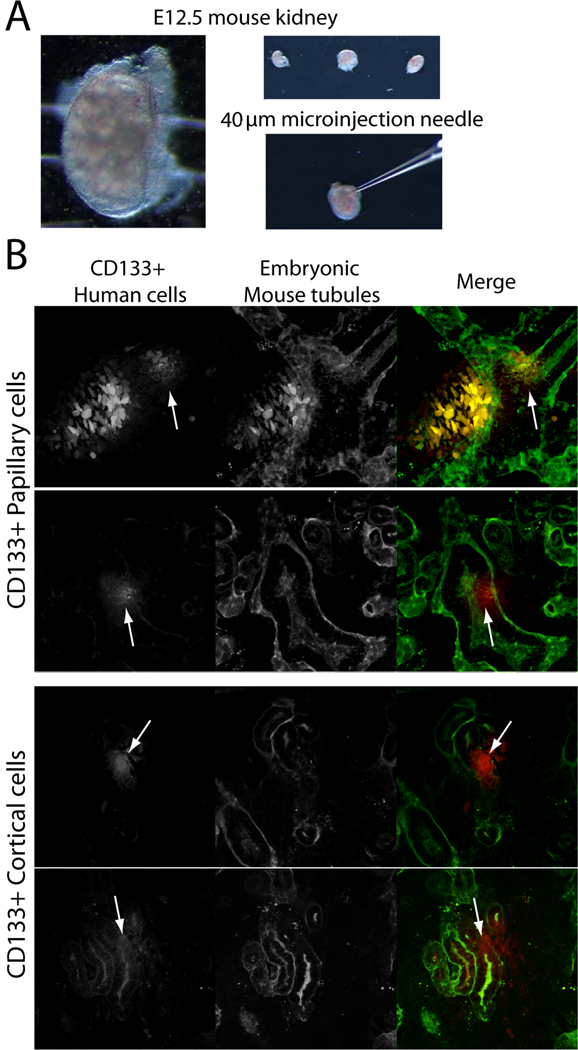
1.3.5 Purified human CD133/1+ papillary and cortical cells, but not tubular epithelia, integrate with developing mouse tubules in heterotypic, metanephric organ culture
CD133/1+ cells were immunopurified from isolated cortical or renal papillary fractions as described in Methods 1.2.1. The immunopurified cells were expanded in culture without passage and individually microinjected into multiple, isolated E12.5 developing mouse kidneys (Fig. 6A). Cortical tubular epithelia and sham injected kidneys were used as controls. The papillary CD133/1+ cells readily underwent tubulogenesis and were seen intercalated in the lectin-stained tubules of the developing mouse kidney in all three kidneys examined with multiple injection sites/kidney (Fig. 6B top two panels, human cells red, mouse tubules green, arrows denote tubule embedded human cells). CD133/1+ cells from the cortex were also competent to integrate into the developing tubules (Fig. 6B, lower two panels, arrows denote tubule embedded human cells). The integration within tubules can be most clearly seen by stepping through sequential optical sections of the whole mount kidneys, which is simulated in Supplemental movie 2. Non-sorted cortical cells consisting largely of tubular epithelia did not integrate into tubules and were seen primarily in the interstitium (Supplemental Fig. A.2). An orthogonal xz view (bottom panel of Supplemental Fig. A.2A) shows that the human CFDA labeled cells (red) can be seen to clearly lie outside the wall of the mouse kidney tubule (green). Sham injected kidneys underwent normal tubular development suggesting that injection did not alter tubulogenesis (Supplemental Fig. A.2B). To further confirm the identity of the CFDA-labeled cells as being of human origin, we also stained for HLA class I and CD133 (Supplemental Fig. A.3).
Figure 6. Human CD133/1+ adult renal stem cells incorporate within tubules of metanephric organ cultures.
(A) Kidneys were harvested from E12.5 FVB/n mice and (B) injected with 1500cells/site CD133/1+ human kidney cells from the papilla or cortex using capillary pipets with 40 µm tips. Three individual kidneys were injected with each cell type. (B) Human cells were prelabeled with fluorescent CFDA to allow tracking (CFDA, pseudocolored red). Kidney cultures were incubated in DMEM/F12 for three days post-injection, fixed, stained with fluorescent peanut agglutinin (pseudocolored green) and imaged with a two-photon microscope. Arrows denote cells integrated into tubules. See supplemental movie 2 for view through entire Z-stack.
1.4 DISCUSSION
We identify a previously uncharacterized population of cells expressing stem cell markers that are prevalent within the papilla of human kidneys where they localize to Tamm-Horsfall positive loops of Henle and distal tubules. The two key stem cell markers, CD133 and nestin used for initial characterizations are both established markers of kidney progenitors both in development and adult tissues. However, rare CD133+ cells (~1% of population) have thus far only been fractionated from human kidney cortex and glomeruli, and nestin expression has been previously reported only in adult human podocytes [22, 38, 54, 55]. Interestingly, nestin is reexpressed during tubulointerstitial and ischemic injury and leads to cell migration toward the cortex [53, 56, 74]. Increased nestin expression is also critical for mesangial cell proliferation and the production of new podocytes [75]. What is perhaps most notable about the human papillary progenitor cells that are dually CD133+ and nestin+ is that they are extensively intercalated in tubules where they reside between tubular epithelial cells. In contrast, the CD133+ cells identified in the human cortex were found in the interstitium and adjacent to glomeruli, and in rodent models CD133+ cells were dispersed throughout the papillary interstitium and in collecting ducts [16, 21]. In transgenic mice expressing GFP-nestin, the nestin+ cells were also highly enriched in the papilla, but were localized outside the tubules [56], suggesting there may be important differences between rodent models and human kidney that need to be further analyzed.
The exquisite tubular restriction of human papillary cells expressing stem cell markers is interesting in light of the facts that papilla are relatively hypoxic and the extension of loop of Henle segments is a late developmental event, which is regulated by EGF [16, 76]. Human papillary cells in culture were highly responsive to EGF, a growth factor that also plays a critical role in vivo in the regulation of loop of Henle growth and differentiation during development [77, 78]. Selective ablation of cells in the thick ascending loops of Henle using a novel transgenic mouse model resulted in severe acute kidney injury demonstrating the importance of this kidney segment [79]. During ischemic injury induced by cross-clamping, distal segments normally support proximal repair, which has thus far been ascribed only to the production of cytokines and Tamm-Horsfall protein produced uniquely by cells of the thick ascending loop of Henle [80, 81]. In light of our findings, it may be that human loop of Henle segments in the papilla serve an additional role as a niche for harboring renal progenitor cells into adulthood by protecting them from oxidative injury, yet positioning them where they can proliferate and differentiate to give rise to renal tubular epithelia in the event of injury or disease.
One hallmark of adult stem/progenitor cells is their capacity to respond to injury and an innate phenotypic plasticity. Rodent papillary cells were previously shown to become activated and migrate out of papilla in an ischemic injury model [16]. The kidneys used for our studies were deemed unsuited for transplant due to signs of ischemic injury possibly due to the manner of death of the donors; all died of cerebral injury or hemorrhage. Thus, it is conceivable that the resident stem cells were activated in vivo, which facilitated their identification and characterization. Isolated human papillary cells exhibited significant phenotypic and morphologic plasticity in 2D and 3D culture. The isolated cells extensively expressed the stem cell markers nestin, CD133 and SSEA4 ex vivo, though expression was replaced by neural or epithelial cell lineage markers when the cells were triggered to differentiate in serum free medium or with the addition of growth factors. The human cells could be clonally expanded in xanthosine, which has been previously shown to induce proliferation of both hepatic and mammary stem cells in vitro and in vivo [33, 34]. The ability of the human CD133+ papillary cells to form spheroid structures is a noteworthy property shared with the rodent stem cells isolated from renal papilla of adult rats and mice [16]. ‘Nephrospheres’ expressing multiple renal transcription factors are formed by rodent embryonic stem cells and suggests that such structures may be a common hallmark for phenotyping renal stem cells as it is for neural stem cells [82, 83]. Finally, the capacity of the papillary cells to coordinately form hollow spheres and new tubules in 3D collagen gel cultures and heterologous metanephric organ culture further illustrates their differentiation potential. The data also demonstrate that heterologous metanephric organ culture can serve as a surrogate system for evaluating the differentiation and proliferative potential of the human cells. Coupling such heterologous culture with new methods for dissociating embryonic kidneys and preserving tubulogenesis following cell reaggregation offer exciting opportunities for more rapid assessment of stem cell properties, small volume assays and high throughput screening [84, 85].
The utility of adult renal progenitor cells for organ repair and replacement especially in the case of genetic and chronic kidney diseases must be considered in the context of parallel advances in regenerative medicine. In early experiments bovine fibroblasts subjected to nuclear transplantation were seeded onto polymer tubes ending in dialysis bags and implanted into cows for three months [2, 86]. This renal device was shown to allow renal cell differentiation complete with glomerulus, tubular segments and vascularization and to produce a yellow urine-like fluid. More recent studies used a decellularized rat kidney as a scaffold and demonstrated that mouse embryonic stem cells effectively reformed glomeruli, tubules and vasculature on this template [87]. There has also been progress in developing technologies that promote embryonic and adult mesenchymal stem cells to form kidneys de novo in specialized culture systems or in vivo [88, 89]. Thus, there are increasing opportunities to test isolated embryonic and adult renal progenitor cells for their capacity to reconstitute kidney function.
In sum, we provide evidence for a renal progenitor population in human renal papillary loops of Henle with the capacity to differentiate into neural- and epithelial-like lineages and to undergo tubulogenesis. The cells may serve in kidney repair or could be useful in combination with organ replacement strategies using artificial templates or specialized culture systems that are in development in other laboratories.
Supplementary Material
Cells were plated at 100 cells per well of a 6-well plate and cultured in normal growth medium supplemented with 200 µM or 400 µM xanthosine. After one week cells were fixed and stained with crystal violet. Top panels show low magnification of entire well. Bottom panels show cell morphology and density. Graph shows results of lactate dehydrogenase release assays conducted to measure the cytoxicity of xanthosine on cortical and papillary cells from three different patients. Detergent lysed cells served as a positive control.
(A) E12.5 FVB/n mouse kidneys were injected with 1500cells/site non-immunopurified human cortical tubular epithelial cells using capillary pipets with 40 µm tips. Human cells were prelabeled with fluorescent CFDA to allow tracking (pseudocolored red). Kidney cultures were incubated in DMEM/F12 for 3 d post-injection, fixed, stained with fluorescent peanut agglutinin (pseudocolored green) and imaged with a two-photon microscope. Two different metanephric organ cultures are shown. Bottom panel shows XZ projection of second panel. (B) Sham injected E12.5 metanephric mouse kidney cultured for 3 d and labeled with peanut agglutinin (green), as expected no signal was detected in CFDA channel, showing specificity of label for injected human cells.
Collagen gel cultures were fixed after 10 d and processed as detailed in Methods 1.2.3. Tubular epithelia were identified using β-catenin (red) and papillary cells were identified using CFDA (green). A complete Z-stack image (74 µm) of a single sphere was acquired on a Zeiss LSM 510 at 1 µm intervals. The images were processed and movie generated using Voxx2. Movie shows entire stack (rotation) followed by front-to-back and back-to-front pass-through, and ends with single slice views through the stack. Note the hollow core developing in upper left quadrant and the intercalation of the green CFDA-positive papillary cells within the red cortical tubular epithelial network.
CD133+ papillary cells were microinjected into E12.5 mouse kidneys and cultured for 3 d before fixation and staining as detailed in Methods 1.2.5. The figure and movie show sequential optical slices through the whole mounted metanephric kidney imaged on a Zeiss LSM-510 Meta Confocal/Multiphoton Microscope. A complete Z-stack image (35 µm) of a single sphere was acquired on a Zeiss LSM 510 at 0.29 µm intervals. The images were processed and movie generated using Voxx2. Movie shows single slice views through the entire z-stack. Developing mouse kidney tubules labeled with peanut agglutinin are seen in white. Human CFDA and CD133+ (upper panels and movie) or HLA-1+ cells (lower panel) are seen in the merge as green or blue and individual channels are shown in white.
ACKNOWLEDGEMENTS
Human kidneys not suited for transplant were obtained through the National Disease Research Interchange (NDRI, Philadelphia, PA). We are indebted to the anonymous patients and their families who donated tissue through the NDRI to make this research possible. National Kidney Foundation Fellowships to TR and HHW supported this work. UNM Research Allocations Committee Award C-2274–R, The University of New Mexico Clinical and Translational Science Center DHHS/NIH/NCRR 1UL1RR031977-01 pilot funds, and NIDDK R01DK50141 to AWN; NCI 2R01CA058443 and 5R01CA105257 to SN. Confocal images in this paper were generated in the University of New Mexico Fluorescence Microscopy Shared Resource, supported as detailed: http://hsc.unm.edu/crtc/microscopy/acknowledgement.shtml. Nucleic acid analyses and quantitative real time PCR used facilities and services provided by the Keck-UNM Genomics Resource, a facility supported by a grant from the WM Keck Foundation as well as the State of New Mexico and the UNM Cancer Research and Treatment Center. We express our gratitude to Ms. Jamie Padilla for expert technical assistance with quantitative real time PCR assays. Multi-photon imaging was performed at the Indiana Center for Biological Microscopy in the Indiana University School of Medicine, Division of Nephrology supported by a George M. O’Brien Center award from NIDDK 5P30DK079312. We thank Ms. Caroline Miller at the Indiana University School of Medicine Electron Microscopy Center (generously supported by the Polycystic Kidney Disease Research Foundation) for performing immunogold labeling of kidney sections. We are grateful to Dr. George Tanner and Dr. Sherry Clendenon from Indiana University School of Medicine for assistance with kidney microinjection techniques.
ABBREVIATIONS
- 2D
two-dimensional
- 3D
three-dimensional
- ADPKD
autosomal dominant polycystic kidney disease
- BrdU
bromodeoxyuridine
- CD133
or prominin 1
- CFDA
5-(and-6)-carboxyfluorescein diacetate
- DBA
Dolichos biflorus
- E
embryonic day
- EGF
epidermal growth factor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HLA I
human leukocyte antigen I
- LDH
lactate dehydrogenase
- mAb
monoclonal antibody
- NDRI
National Disease Research Interchange
- NSPC
neural stem/progenitor cell
- OCT4/POU5F1
octamer-binding transcription factor 4/ POU domain, class 5, transcription factor 1
- pAb
polyclonal antibody
- PCR
polymerase chain reaction
- PNA
Arachis hypogaea or peanut agglutinin lectin
- PP1A
protein phosphatase 1
- RCTE
renal cortical tubular epithelial cells
- REGM
renal epithelial growth medium
- SOX2
SRY-box containing gene 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Heather H. Ward, Email: hhward@unm.edu.
Elsa Romero, Email: eromero@salud.unm.edu.
Angela Welford, Email: awelford@salud.unm.edu.
Gavin Pickett, Email: ggpickett@salud.unm.edu.
Robert Bacallao, Email: rbacalla@iupui.edu.
Vincent H. Gattone, II, Email: vgattone@iupui.edu.
Scott A. Ness, Email: ness@unm.edu.
Angela Wandinger-Ness, Email: wness@unm.edu.
Tamara Roitbak, Email: troitbak@salud.unm.edu.
REFERENCES
- 1.Al-Awqati Q, Oliver JA. The kidney papilla is a stem cells niche. Stem Cell Rev. 2006;2:181–184. doi: 10.1007/s12015-006-0046-3. [DOI] [PubMed] [Google Scholar]
- 2.Atala A. Tissue engineering for the replacement of organ function in the genitourinary system. Am. J. Transplant. 2004;4(Suppl 6):58–73. doi: 10.1111/j.1600-6135.2004.0346.x. [DOI] [PubMed] [Google Scholar]
- 3.Benigni A, Morigi M, Remuzzi G. Kidney regeneration. Lancet. 2010;375:1310–1317. doi: 10.1016/S0140-6736(10)60237-1. [DOI] [PubMed] [Google Scholar]
- 4.Guo JK, Cantley LG. Cellular maintenance and repair of the kidney. Annu. Rev. Physiol. 2010;72:357–376. doi: 10.1146/annurev.physiol.010908.163245. [DOI] [PubMed] [Google Scholar]
- 5.Ricardo SD, Deane JA. Adult stem cells in renal injury and repair. Nephrology (Carlton) 2005;10:276–282. doi: 10.1111/j.1440-1797.2005.00373.x. [DOI] [PubMed] [Google Scholar]
- 6.Bruce SJ, Rea RW, Steptoe AL, Busslinger M, Bertram JF, Perkins AC. In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation. 2007;75:337–349. doi: 10.1111/j.1432-0436.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J. Am. Soc. Nephrol. 2005;16:3527–3534. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Tanaka H, Kuwana H, Inoshita S, Teraoka H, Sasaki S, Terada Y. Wnt4-transformed mouse embryonic stem cells differentiate into renal tubular cells. Biochem. Biophys. Res. Commun. 2005;336:585–595. doi: 10.1016/j.bbrc.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 9.Steenhard BM, Isom KS, Cazcarro P, Dunmore JH, Godwin AR, St John PL, Abrahamson DR. Integration of embryonic stem cells in metanephric kidney organ culture. J. Am. Soc. Nephrol. 2005;16:1623–1631. doi: 10.1681/ASN.2004070584. [DOI] [PubMed] [Google Scholar]
- 10.Kramer J, Steinhoff J, Klinger M, Fricke L, Rohwedel J. Cells differentiated from mouse embryonic stem cells via embryoid bodies express renal marker molecules. Differentiation. 2006;74:91–104. doi: 10.1111/j.1432-0436.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- 11.Vigneau C, Polgar K, Striker G, Elliott J, Hyink D, Weber O, Fehling HJ, Keller G, Burrow C, Wilson P. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J. Am. Soc. Nephrol. 2007;18:1709–1720. doi: 10.1681/ASN.2006101078. [DOI] [PubMed] [Google Scholar]
- 12.Teramoto K, Hara Y, Kumashiro Y, Chinzei R, Tanaka Y, Shimizu-Saito K, Asahina K, Teraoka H, Arii S. Teratoma formation and hepatocyte differentiation in mouse liver transplanted with mouse embryonic stem cell-derived embryoid bodies. Transplant Proc. 2005;37:285–286. doi: 10.1016/j.transproceed.2004.12.120. [DOI] [PubMed] [Google Scholar]
- 13.Nardi NB. All the adult stem cells, where do they all come from? An external source for organ-specific stem cell pools. Med. Hypotheses. 2005;64:811–817. doi: 10.1016/j.mehy.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Leedham SJ, Brittan M, McDonald SA, Wright NA. Intestinal stem cells. J. Cell. Mol. Med. 2005;9:11–24. doi: 10.1111/j.1582-4934.2005.tb00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin F. Stem cells in kidney regeneration following acute renal injury. Pediatr. Res. 2006;59:74R–78R. doi: 10.1203/01.pdr.0000205156.85990.12. [DOI] [PubMed] [Google Scholar]
- 16.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J. Clin. Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anglani F, Forino M, Del Prete D, Tosetto E, Torregrossa R, D'Angelo A. In search of adult renal stem cells. J. Cell. Mol. Med. 2004;8:474–487. doi: 10.1111/j.1582-4934.2004.tb00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbattista MR, Schena FP. Stem cells and kidney diseases. Minerva Med. 2004;95:411–418. [PubMed] [Google Scholar]
- 19.Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J. Am. Soc. Nephrol. 2003;14:3138–3146. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- 20.Bruno S, Bussolati B, Grange C, Collino F, di Cantogno LV, Herrera MB, Biancone L, Tetta C, Segoloni G, Camussi G. Isolation and characterization of resident mesenchymal stem cells in human glomeruli. Stem Cells Dev. 2009;18:867–880. doi: 10.1089/scd.2008.0320. [DOI] [PubMed] [Google Scholar]
- 21.Oliver JA, Klinakis A, Cheema FH, Friedlander J, Sampogna RV, Martens TP, Liu C, Efstratiadis A, Al-Awqati Q. Proliferation and migration of label-retaining cells of the kidney papilla. J. Am. Soc. Nephrol. 2009;20:2315–2327. doi: 10.1681/ASN.2008111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 2003;14(Suppl 1):S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 24.Abbate M, Brown D, Bonventre JV. Expression of NCAM recapitulates tubulogenic development in kidneys recovering from acute ischemia. Am. J. Physiol. 1999;277:F454–F463. doi: 10.1152/ajprenal.1999.277.3.F454. [DOI] [PubMed] [Google Scholar]
- 25.Villanueva S, Cespedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 26.Romagnani P. Toward the identification of a "renopoietic system"? Stem Cells. 2009;27:2247–2253. doi: 10.1002/stem.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoo T, Ohashi T, Shen JS, Sakurai K, Miyazaki Y, Utsunomiya Y, Takahashi M, Terada Y, Eto Y, Kawamura T, Osumi N, Hosoya T. Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3296–3300. doi: 10.1073/pnas.0406878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoo T, Fukui A, Ohashi T, Miyazaki Y, Utsunomiya Y, Kawamura T, Hosoya T, Okabe M, Kobayashi E. Xenobiotic kidney organogenesis from human mesenchymal stem cells using a growing rodent embryo. J. Am. Soc. Nephrol. 2006;17:1026–1034. doi: 10.1681/ASN.2005101043. [DOI] [PubMed] [Google Scholar]
- 29.Yokoo T, Kawamura T. Xenobiotic kidney organogenesis: a new avenue for renal transplantation. J. Nephrol. 2009;22:312–317. [PubMed] [Google Scholar]
- 30.Carone FA, Nakamura S, Schumacher BS, Punyarit P, Bauer KD. Cyst-derived cells do not exhibit accelerated growth or features of transformed cells in vitro. Kidney Int. 1989;35:1351–1357. doi: 10.1038/ki.1989.134. [DOI] [PubMed] [Google Scholar]
- 31.Silberberg M, Charron AJ, Bacallao R, Wandinger-Ness A. Mispolarization of desmosomal proteins and altered intercellular adhesion in autosomal dominant polycystic kidney disease. Am. J. Physiol. Renal. Physiol. 2005;288:F1153–F1163. doi: 10.1152/ajprenal.00008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loghman-Adham M, Nauli SM, Soto CE, Kariuki B, Zhou J. Immortalized epithelial cells from human autosomal dominant polycystic kidney cysts. Am. J. Physiol. Renal Physiol. 2003;285:F397–F412. doi: 10.1152/ajprenal.00310.2002. [DOI] [PubMed] [Google Scholar]
- 33.Lee HS, Crane GG, Merok JR, Tunstead JR, Hatch NL, Panchalingam K, Powers MJ, Griffith LG, Sherley JL. Clonal expansion of adult rat hepatic stem cell lines by suppression of asymmetric cell kinetics (SACK) Biotechnol. Bioeng. 2003;83:760–771. doi: 10.1002/bit.10727. [DOI] [PubMed] [Google Scholar]
- 34.Capuco AV, Evock-Clover CM, Minuti A, Wood DL. In vivo expansion of the mammary stem/ progenitor cell population by xanthosine infusion. Exp. Biol. Med. (Maywood) 2009;234:475–482. doi: 10.3181/0811-RM-320. [DOI] [PubMed] [Google Scholar]
- 35.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 36.Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, Zeilstra J, Pals ST, Mehmet H, Stassi G, Medema JP. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res. 2010;70:719–729. doi: 10.1158/0008-5472.CAN-09-1820. [DOI] [PubMed] [Google Scholar]
- 37.Angelotti ML, Lazzeri E, Lasagni L, Romagnani P. Only anti-CD133 antibodies recognizing the CD133/1 or the CD133/2 epitopes can identify human renal progenitors. Kidney Int. 2010;78:620–621. doi: 10.1038/ki.2010.243. author reply 621. [DOI] [PubMed] [Google Scholar]
- 38.Sagrinati C, Mazzinghi B, Netti GS, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of 30 multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J. Am. Soc. Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 39.Roitbak T, Li L, Cunningham LA. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling. J. Cereb. Blood Flow Metab. 2008;28:1530–1542. doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clendenon JL, Phillips CL, Sandoval RM, Fang S, Dunn KW. Voxx: a PC-based, near real-time volume rendering system for biological microscopy. Am. J. Physiol. Cell Physiol. 2002;282:C213–C218. doi: 10.1152/ajpcell.2002.282.1.C213. [DOI] [PubMed] [Google Scholar]
- 41.Roitbak T, Ward CJ, Harris PC, Bacallao R, Ness SA, Wandinger-Ness A. A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol. Biol. Cell. 2004;15:1334–1346. doi: 10.1091/mbc.E03-05-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Neil RG, Hayhurst RA. Functional differentiation of cell types of cortical collecting duct. Am J Physiol. 1985;248:F449–F453. doi: 10.1152/ajprenal.1985.248.3.F449. [DOI] [PubMed] [Google Scholar]
- 43.Silva FG, Nadasdy T, Laszik Z. Immunohistochemical and lectin dissection of the human nephron in health and disease. Arch Pathol Lab Med. 1993;117:1233–1239. [PubMed] [Google Scholar]
- 44.Ramachandran R, Heuck AP, Tweten RK, Johnson AE. Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin. Nat. Struct. Biol. 2002;9:823–827. doi: 10.1038/nsb855. [DOI] [PubMed] [Google Scholar]
- 45.Roitbak T, Surviladze Z, Tikkanen R, Wandinger-Ness A. A polycystin multiprotein complex constitutes a cholesterol-containing signalling microdomain in human kidney epithelia. Biochem. J. 2005;392:29–38. doi: 10.1042/BJ20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollack AL, Runyan RB, Mostov KE. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev. Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- 47.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corbeil D, Roper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- 49.Frojdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE. The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation. 1997;61:243–249. doi: 10.1046/j.1432-0436.1997.6140243.x. [DOI] [PubMed] [Google Scholar]
- 50.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 51.Wroblewski J, Engstrom M, Edwall-Arvidsson C, Sjoberg G, Sejersen T, Lendahl U. Distribution of nestin in the developing mouse limb bud in vivo and in micro-mass cultures of cells isolated from limb buds. Differentiation. 1997;61:151–159. doi: 10.1046/j.1432-0436.1997.6130151.x. [DOI] [PubMed] [Google Scholar]
- 52.Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371–1378. doi: 10.4161/cc.7.10.5954. [DOI] [PubMed] [Google Scholar]
- 53.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2009;20:2593–2603. doi: 10.1681/ASN.2009020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Boyle S, Zhao M, Su W, Takahashi K, Davis L, Decaestecker M, Takahashi T, Breyer MD, Hao CM. Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J. Am. Soc. Nephrol. 2006 17;:1283–1291. doi: 10.1681/ASN.2005101032. [DOI] [PubMed] [Google Scholar]
- 55.Bertelli E, Regoli M, Fonzi L, Occhini R, Mannucci S, Ermini L, Toti P. Nestin expression in adult and developing human kidney. J. Histochem. Cytochem. 2007;55:411–421. doi: 10.1369/jhc.6A7058.2007. [DOI] [PubMed] [Google Scholar]
- 56.Patschan D, Michurina T, Shi HK, Dolff S, Brodsky SV, Vasilieva T, Cohen-Gould L, Winaver J, Chander PN, Enikolopov G, Goligorsky MS. Normal distribution and medullary-to-cortical shift of Nestin-expressing cells in acute renal ischemia. Kidney Int. 2007;71:744–754. doi: 10.1038/sj.ki.5002102. [DOI] [PubMed] [Google Scholar]
- 57.Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009;136:2311–2322. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karantzali E, Lekakis V, Ioannou M, Hadjimichael C, Papamatheakis J, Kretsovali A. Sall1 regulates embryonic stem cell differentiation in association with Nanog. J Biol Chem. 2010 doi: 10.1074/jbc.M110.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karaoz E, Okcu A, Gacar G, Saglam U, Yuruker S, Kenar H. A comprehensive characterization study of human bone marrow MSCs with an emphasis on molecular and ultrastructural properties. J Cell Physiol. 2010 doi: 10.1002/jcp.22468. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, Muceniece R, Ancans J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009;5:378–386. doi: 10.1007/s12015-009-9094-9. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 62.Buendia B, Antony C, Verde F, Bornens M, Karsenti E. A centrosomal antigen localized on intermediate filaments and mitotic spindle poles. J. Cell Sci. 1990;97:259–271. doi: 10.1242/jcs.97.2.259. [DOI] [PubMed] [Google Scholar]
- 63.Salas PJ. Insoluble gamma-tubulin-containing structures are anchored to the apical network of intermediate filaments in polarized CACO-2 epithelial cells. J. Cell Biol. 1999;146:645–658. doi: 10.1083/jcb.146.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oriolo AS, Wald FA, Ramsauer VP, Salas PJ. Intermediate filaments: a role in epithelial polarity. Exp. Cell Res. 2007;313:2255–2264. doi: 10.1016/j.yexcr.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liovic M, D'Alessandro M, Tomic-Canic M, Bolshakov VN, Coats SE, Lane EB. Severe keratin 5 and 14 mutations induce down-regulation of junction proteins in keratinocytes. Exp. Cell Res. 2009;315:2995–3003. doi: 10.1016/j.yexcr.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 66.Hodges ME, Scheumann N, Wickstead B, Langdale JA, Gull K. Reconstructing the evolutionary history of the centriole from protein components. J. Cell Sci. 2010;123:1407–1413. doi: 10.1242/jcs.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oeffner F, Moch C, Neundorf A, Hofmann J, Koch M, Grzeschik KH. Novel interaction partners of Bardet-Biedl syndrome proteins. Cell Motil. Cytoskeleton. 2008;65:143–155. doi: 10.1002/cm.20250. [DOI] [PubMed] [Google Scholar]
- 68.Henderson JK, Draper JS, Baillie HS, Fishel S, Thomson JA, Moore H, Andrews PW. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells. 2002;20:329–337. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- 69.Gubhaju L, Laslett A, Bertram JF, Zulli A, Black MJ. Immunohistochemical localisation of TRA-1-60, TRA-1-81, GCTM-2 and podocalyxin in the developing baboon kidney. Histochem. Cell Biol. 2008;129:651–657. doi: 10.1007/s00418-008-0398-7. [DOI] [PubMed] [Google Scholar]
- 70.Fesenko I, Franklin D, Garnett P, Bass P, Campbell S, Hardyman M, Wilson D, Hanley N, Collins J. Stem cell marker TRA-1-60 is expressed in foetal and adult kidney and upregulated in tubulo-interstitial disease. Histochem Cell Biol. 2010;134:355–369. doi: 10.1007/s00418-010-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu C, Rossetti S, Jiang L, Harris PC, Brown-Glaberman U, Wandinger-Ness A, Bacallao R, Alper SL. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am J Physiol Renal Physiol. 2007;292:F930–F945. doi: 10.1152/ajprenal.00285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2009;296:F1464–F1476. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zegers MM, O'Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 74.Tomioka M, Hiromura K, Sakairi T, Takeuchi S, Maeshima A, Kaneko Y, Kuroiwa T, Takeuchi T, Nojima Y. Nestin is a novel marker for renal tubulointerstitial injury in immunoglobulin A nephropathy. Nephrology (Carlton) 2010;15:568–574. doi: 10.1111/j.1440-1797.2010.01342.x. [DOI] [PubMed] [Google Scholar]
- 75.Daniel C, Albrecht H, Ludke A, Hugo C. Nestin expression in repopulating mesangial cells promotes their proliferation. Lab. Invest. 2008;88:387–397. doi: 10.1038/labinvest.2008.5. [DOI] [PubMed] [Google Scholar]
- 76.Kim J, Lee GS, Tisher CC, Madsen KM. Role of apoptosis in development of the ascending thin limb of the loop of Henle in rat kidney. Am. J. Physiol. 1996;271:F831–F845. doi: 10.1152/ajprenal.1996.271.4.F831. [DOI] [PubMed] [Google Scholar]
- 77.Lee SH, Jung JY, Han KH, Yang CW, Choi KB, Kim J. Effect of epidermal growth factor on the developing rat renal papilla. Am. J. Nephrol. 2004;24:212–220. doi: 10.1159/000077275. [DOI] [PubMed] [Google Scholar]
- 78.Jung JY, Song JH, Li C, Yang CW, Kang TC, Won MH, Jeong YG, Han KH, Choi KB, Lee SH, Kim J. Expression of epidermal growth factor in the developing rat kidney. Am. J. Physiol. Renal Physiol. 2005;288:F227–F235. doi: 10.1152/ajprenal.00058.2004. [DOI] [PubMed] [Google Scholar]
- 79.Srichai MB, Hao C, Davis L, Golovin A, Zhao M, Moeckel G, Dunn S, Bulus N, Harris RC, Zent R, Breyer MD. Apoptosis of the thick ascending limb results in acute kidney injury. J. Am. Soc. Nephrol. 2008;19:1538–1546. doi: 10.1681/ASN.2007101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am. J. Physiol. Renal Physiol. 2008;295:F534–F544. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gobe GC, Johnson DW. Distal tubular epithelial cells of the kidney: Potential support for proximal tubular cell survival after renal injury. Int. J. Biochem. Cell Biol. 2007;39:1551–1561. doi: 10.1016/j.biocel.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 82.Osafune K, Takasato M, Kispert A, Asashima M, Nishinakamura R. Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development. 2006;133:151–161. doi: 10.1242/dev.02174. [DOI] [PubMed] [Google Scholar]
- 83.Lusis M, Li J, Ineson J, Christensen ME, Rice A, Little MH. Isolation of clonogenic, long-term self renewing embryonic renal stem cells. Stem Cell Res. 2010;5:23–39. doi: 10.1016/j.scr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Sebinger DD, Unbekandt M, Ganeva VV, Ofenbauer A, Werner C, Davies JA. A novel, low-volume method for organ culture of embryonic kidneys that allows development of cortico-medullary anatomical organization. PLoS One. 2010;5:e10550. doi: 10.1371/journal.pone.0010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Unbekandt M, Davies JA. Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int. 2010;77:407–416. doi: 10.1038/ki.2009.482. [DOI] [PubMed] [Google Scholar]
- 86.Lanza RP, Chung HY, Yoo JJ, Wettstein PJ, Blackwell C, Borson N, Hofmeister E, Schuch G, Soker S, Moraes CT, West MD, Atala A. Generation of histocompatible tissues using nuclear transplantation. Nat. Biotechnol. 2002;20:689–696. doi: 10.1038/nbt703. [DOI] [PubMed] [Google Scholar]
- 87.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J. Am. Soc. Nephrol. 2009;20:2338–2347. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Osafune K. In vitro regeneration of kidney from pluripotent stem cells. Exp Cell Res. 2010;316:2571–2577. doi: 10.1016/j.yexcr.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 89.Yokoo T, Kawamura T, Kobayashi E. Kidney organogenesis and regeneration: a new era in the treatment of chronic renal failure? Clin. Exp. Nephrol. 2008;12:326–331. doi: 10.1007/s10157-008-0062-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells were plated at 100 cells per well of a 6-well plate and cultured in normal growth medium supplemented with 200 µM or 400 µM xanthosine. After one week cells were fixed and stained with crystal violet. Top panels show low magnification of entire well. Bottom panels show cell morphology and density. Graph shows results of lactate dehydrogenase release assays conducted to measure the cytoxicity of xanthosine on cortical and papillary cells from three different patients. Detergent lysed cells served as a positive control.
(A) E12.5 FVB/n mouse kidneys were injected with 1500cells/site non-immunopurified human cortical tubular epithelial cells using capillary pipets with 40 µm tips. Human cells were prelabeled with fluorescent CFDA to allow tracking (pseudocolored red). Kidney cultures were incubated in DMEM/F12 for 3 d post-injection, fixed, stained with fluorescent peanut agglutinin (pseudocolored green) and imaged with a two-photon microscope. Two different metanephric organ cultures are shown. Bottom panel shows XZ projection of second panel. (B) Sham injected E12.5 metanephric mouse kidney cultured for 3 d and labeled with peanut agglutinin (green), as expected no signal was detected in CFDA channel, showing specificity of label for injected human cells.
Collagen gel cultures were fixed after 10 d and processed as detailed in Methods 1.2.3. Tubular epithelia were identified using β-catenin (red) and papillary cells were identified using CFDA (green). A complete Z-stack image (74 µm) of a single sphere was acquired on a Zeiss LSM 510 at 1 µm intervals. The images were processed and movie generated using Voxx2. Movie shows entire stack (rotation) followed by front-to-back and back-to-front pass-through, and ends with single slice views through the stack. Note the hollow core developing in upper left quadrant and the intercalation of the green CFDA-positive papillary cells within the red cortical tubular epithelial network.
CD133+ papillary cells were microinjected into E12.5 mouse kidneys and cultured for 3 d before fixation and staining as detailed in Methods 1.2.5. The figure and movie show sequential optical slices through the whole mounted metanephric kidney imaged on a Zeiss LSM-510 Meta Confocal/Multiphoton Microscope. A complete Z-stack image (35 µm) of a single sphere was acquired on a Zeiss LSM 510 at 0.29 µm intervals. The images were processed and movie generated using Voxx2. Movie shows single slice views through the entire z-stack. Developing mouse kidney tubules labeled with peanut agglutinin are seen in white. Human CFDA and CD133+ (upper panels and movie) or HLA-1+ cells (lower panel) are seen in the merge as green or blue and individual channels are shown in white.