Abstract
The serotonin system responds to the ovarian steroid steroids, estradiol (E) and progesterone (P), in women and female animal models. In macaques, ovarian steroid administration to ovariectomized (Ovx) individuals improves serotonin neural function through actions on pivotal serotonin-related genes and proteins, such as TPH2 (tryptophan hydroxylase 2), SERT (serotonin reuptake transporter) and the 5HT1A autoreceptor. In addition, ovarian steroid administration reduces gene and protein expression in the caspase-independent pathway and reduces DNA fragmentation in serotonin neurons. This study examines the hypothesis that long-term ovariectomy will lead to a loss of serotonin neurons and compromised gene expression in serotonin neurons. Female Japanese macaques were ovariectomized or tubal-ligated (n=5/group) at 3 years of age and returned to their natal troop. After 3 years, the animals were collected, administered a fenfluramine challenge to determine global serotonin availability and then euthanized. Fev, TPH2, SERT and 5HT1A expression were examined with digoxigenin in situ hybridization (dig-ISH) and quantitative image analysis. Cell number, positive pixel area and average pixel density were determined. In the Ovx group, Fev, TPH2, SERT and 5HT1A showed a significant decease in average and total cell number and positive pixel area. The reduction in Fev-positive neurons suggests that there were fewer serotonin neurons in Ovx animals compared to ovary-intact animals. Compared to ovary-intact animals, SERT also showed a decrease in positive-pixel density. The decrease in TPH2 in the Ovx animals was consistent with earlier results in 5-month Ovx animals, but it may be due to the decrease in cell number rather than a decrease in expression on an individual cell basis. The decrease in SERT and 5HT1A in long-term Ovx differed from previous studies in short-term Ovx. In summary, long-term ovarian steroid loss resulted in fewer serotonin neurons and overall lower Fev, TPH2, SERT and 5HT1A gene expression. This may be due to serotonin cell death or to a negative impact on a long-term developmental process in young female macaques.
Introduction
Optimal function of the serotonin neural system is critical for numerous and wide-ranging outcomes such as mood or affect (Halbreich, 1990), anxiety (Linthorst, 2005, Reimold et al., 2008), cognition (Merens et al., 2008), metabolic syndrome (Muldoon et al., 2004), sleep (Dugovic, 2001), pituitary hormone secretion (Van de Kar et al., 2001) and vulnerability to stress (Bethea et al., 2005a, Maier and Watkins, 2005, Bethea et al., 2008). In women, and female animal models, ovarian steroids support serotonin neural function and loss of ovarian hormones leads to suboptimal serotonin neurotransmission (Halbreich et al., 1995, Bethea et al., 2002, Hiroi et al., 2006, Frey et al., 2008, Bethea et al., 2009).
Women experience ovarian failure and loss of ovarian steroid production around 50 years of age. Thus, with extended life spans, a woman may live 35-40 years without ovarian steroid secretion. After menopause, a significant number of women become more anxious, and less able to cope with stress, leading to new onset of depression (Maki et al., Conde et al., 2006, Heikkinen et al., 2006, Tangen and Mykletun, 2008). The role of ovarian hormones in depression and stress sensitivity is of great interest for women transitioning through menopause.
We have devoted significant effort to understanding the effect of ovarian steroids on the serotonin system in a macaque model of surgical menopause. Ovarian hormone replacement to ovariectomized macaques changed the expression of serotonin related genes and proteins in a manner that would cause an increase in serotonin neurotransmission (Bethea et al., 2002, Smith et al., 2004, Sanchez et al., 2005). Furthermore, we showed that ovarian steroid treatment increased serotonin delivery to the medial basal hypothalamus of ovariectomized macaques (Centeno et al., 2007a). Recently, we found that 1 month of estrogen (E) or estrogen plus progesterone (E+P) administration to ovariectomized macaques decreased gene expression in laser captured serotonin neurons and decreased protein expression in the raphe of pivotal regulatory agents in the caspase-independent apoptosis pathway (Bethea and Reddy, 2008, Tokuyama et al., 2008). Subsequently, we showed that 1 month of E+P treatment significantly decreased DNA fragmentation in serotonin neurons of macaques, as determined with a TUNEL assay (Lima and Bethea, 2009). This observation led us to suggest that in ovariectomized macaques, serotonin neurons are in a vulnerable endangered state (Bethea et al., 2009) and that ovarian steroid administration increased DNA repair. All together, our data indicate that ovarian steroids not only promote serotonin neurotransmission, but also maintain serotonin cell health and viability. Conversely, we speculated that loss of ovarian hormones would decrease serotonin neuron viability, and that over time there could be a loss of serotonin neurons.
The previous studies utilized a macaque model of hormone replacement therapy in which healthy adult monkeys were acquired that had been ovariectomized in other programs. The animals were rested for up to 8 months and then treated for 1 month with placebo, E or E+P. Subcutaneous Silastic capsules that were either empty (control) or filled with crystalline estradiol or progesterone were used for steroid delivery. This model was highly cost effective and it demonstrated robust differences in serotonin gene expression between treatment groups. However, the surgical menopause model has certain limitations in comparison to natural menopause. The animals were young adults and not old; they were pair housed indoors and not in a community, and they were Ovx for a relatively short period of time. In addition, we could not know if hormone therapy in Ovx females produced a state similar to intact cycling females.
Therefore, questions remained regarding the regulation of serotonin in intact monkeys compared to ovariectomized individuals, and whether the length of ovariectomy was an issue. We hypothesized that long-term ovariectomized female macaques may have fewer serotonin neurons and reduced expression of pivotal serotonin related genes compared to ovarian intact females. The genes examined were tryptophan hydroxylase 2 (TPH2) which codes for the rate-limiting enzyme in serotonin synthesis; the serotonin reuptake transporter (SERT) which regulates extracellular serotonin, and the 5HT1A autoreceptor which inhibits serotonin neuron excitation, and Fev, an ETS domain transcription factor that determines whether a neuron is serotonergic, and functions specifically in the differentiation and maintenance of serotonin neurons. Conserved Fev-1 binding sites are present in or near the promoter regions of the human and mouse TPH2, serotonin transporter and 5-HT1A receptor genes (Hendricks et al., 1999, Hendricks et al., 2003).
To test these hypotheses, we utilized Japanese macaques that were born and maintained in a 2-acre outdoor corral at the Oregon National Primate Research Center (ONPRC). Young females were ovariectomized or had their fallopian tubes ligated (tubal ligation) to prevent pregnancy. The animals were released back into the troop for social interaction. At the end of 3 years, global serotonin function was determined with a fenfluramine challenge followed by euthanasia for examination of gene expression. This study was not intended to fully model menopause, but rather to examine the effects of long-term ovariectomy, in a semi-natural, social environment without the confounds of age or high fat diet.
Materials and Methods
The Institutional Animal Care and Use Committee of the Oregon National Primate Research Center (ONPRC) approved this study, which followed the guidelines of the Animal Welfare Act.
Animals
Ten pubertal females 3 years of age, born into a troop of Japanese macaques housed in a naturalistic setting were chosen. At the time, there were 12 matriarchal lineages in the corral. The Japanese macaques go through puberty between 3 and 4 years of age. We have observed 4 year olds get pregnant although it is rare. Other than that, we are unable to determine the stages of puberty, such as Tanner scores in girls.
Five pairs of juvenile females were selected that represented different ranks in the dominance hierarchy. One individual of each pair was ovariectomized (Ovx; reproductive tract remains) and the other individual was sterilized by tubal ligation, but the ovaries remained intact. The surgeries were conducted during the annual round up of all of the animals in the corral. The animals were returned to the troop and allowed to mature for 3 years. Behavioral observations were conducted during their 3 years in the corral. One animal from each treatment group died in the troop during year 1 and was replaced with animal of similar age and lineage. At the end of 3 years the animals were collected for temperament tests, pharmacological challenges and euthanasia. The results of the behavioral observations are under review elsewhere.
Description of the troop
The monkeys used in this study were part of a troop of approximately 300 individuals housed in a two-acre outdoor corral at the Oregon National Primate Research Center (ONPRC). The corral was surrounded by steel walls, and contained several platforms and climbing structures for play and exploration. A 5x5 meter observation tower overlooking the corral allowed for unobtrusive observations. Monkeys were fed commercially available monkey chow twice daily, supplemented by daily fresh produce or grains. Water was available ad libitum. As part of general animal husbandry of ONPRC, all animals were given unique markings on their backs, allowing for individual identification of all members of the troop from the observation tower. Animal care staff walked through the corrals daily to look for sick or injured monkeys. Japanese macaques are seasonal breeders, and at the ONPRC, the birth season was typically between May and August, with the majority of births occurring in June and July. The mating season was typically November through February.
The troop arrived at ONPRC in 1965. The troop composition is relatively stable and the age structure is comparable to that of a natural troop (Maruhashi, 1982, Eaton, 1974, Rostal et al., 1986). Like other macaque species, the hierarchical organization of the troop is along matriarchal lineages. The matriarchal lines and dominance hierarchies within the troop are well documented, and have remained stable for the past 45 years.
Annual round ups
At least once a year, the animals were “rounded up”, or brought from the corral into a capture run (“catch area”) adjacent to the corral. This round up allowed collection of the monkeys for procedures that could not be done in an open field, such as annual veterinary exams, weighing and dye marking. Typically, the monkeys were brought into this area one or two times per year, and so most were acclimated to this procedure. During the round-up, monkeys ran through tunnels into the catch area, where they jumped into transport boxes for transfer to single cages. Annual exams were done on all members of the corral in the fall, after the birthing season, and before the mating season. In addition to the physical examination, animals were also weighed and tested for tuberculosis, and new infants were identified. The animals were typically kept in cages in the catch area for approximately one week.
Surgeries
Subjects were either ovariectomized (Ovx; n=5) or tubal ligated (tubal-ligated or ovary intact; n=5) at puberty (age three), and returned to their semi-free ranging troop, remaining until the age of six. For ovariectomy or tubal ligation, each animal was sedated with ketamine (10 mg/kg) in its cage in the catch area and transported to a surgical suite. Ovariectomy and tubal ligation were performed by the surgical personnel of ONPRC using a laparoscopic approach.
Fenfluramine challenges
All of the behavioral tests were completed by October 2009, and after round-up the animals were put into a large indoor/outdoor pen (approximately 1060 square feet) to reduce the stress of single caging prior to fenfluramine challenge. During this time, one Ovx monkey died from an unusual infection, and the others were moved to standard monkey cages until fenfluramine challenge and euthanasia within one week. Thus, for remaining experiments, there were 4 Ovx animals and 5 tubal-ligated animals.
For the fenfluramine challenge, the animals (one at a time) were lightly sedated with Ketamine in the home cage and transported to a surgical suite. A pediatric catheter was inserted into each femoral vein. The animals were further sedated with IV bolus injections of propofol at 500 ug/kg body weight followed by maintenance IV infusion of propofol at 180 ug/kg/min delivered by Harvard pump. Blood samples were collected every 10 minutes into sample tubes. After one hour of baseline samples, fenfluramine was administered as a bolus IV injection at 5 mg/kg. The fenfluramine was dissolved in saline and filter sterilized prior to injection. Samples were obtained for an additional 2 hours after fenfluramine administration. All blood samples were stored on ice until the end of the protocol. Upon termination of the protocol, the blood samples are centrifuged to remove the serum, which was stored at -20C until assay for prolactin and cortisol in the Endocrine Services Laboratory. The first baseline sample from the intact animals was also assayed for estradiol and progesterone to estimate the stage of their menstrual cycle. The animals were then transported to necropsy without awaking. Adrenal weights were obtained. The brain was obtained for future neuroanatomical assays.
Hormone assays
Prolactin, cortisol, estradiol and progesterone concentrations in serum were performed utilizing an automatic Immulite 2000 platform (Siemens, HealthCare Diagnostics, Deerfield, IL), which has been validated in the Endocrine Technology and Support Laboratory, ONPRC, for macaque serum by comparison with traditional radioimmunoassays. The sensitivity and range of the prolactin assay was 0.5 and 150 ng/ml, respectively. The sensitivity and range of the cortisol assay was 2 and 500 ng/ml, respectively. The sensitivity and range of the estradiol assay was 5 and 4250 pg/ml, respectively. The sensitivity and range of the progesterone assay was 0.035 and 59 ng/ml, respectively. Inter- and intra-assay coefficients of variation were less than 10% for all assays.
Tissue Preparation
The monkeys were euthanized after the fenfluramine challenges according to procedures recommended by the Panel on Euthanasia of the American Veterinary Association. Each animal was sedated with ketamine in the home cage, transported to the necropsy suite, given an overdose of pentobarbital (25 mg/kg, i.v.), and exsanguinated by severance of the descending aorta.
Following euthanasia, the left ventricle of the heart was cannulated and the head of each animal was perfused with 1 liter of saline followed by 7 liters of 4% paraformaldehyde in 3.8% borate, pH 9.5 (both solutions made with DEPC-treated water [0.1% diethyl pyrocarbonate] to minimize RNase contamination). The brains were removed and dissected. Tissue blocks were post-fixed in 4% paraformaldehyde for 3 h, then transferred to 0.02M potassium phosphate-buffered saline (KPBS) containing 10%, followed by 20% glycerol and 2% dimethyl sulfoxide at 4°C for 3 days to cryoprotect the tissue. After infiltration, the block was frozen in isopentene cooled to -55°C, and stored at -80°C until sectioning. Sections (25 μm) were cut on a sliding microtome, mounted on Super Frost plus slides (Fisher Scientific, Pittsburgh, PA), dehydrated under vacuum and then frozen at –80°C until processing for digoxigenin ISH.
Riboprobes
A monkey specific partial cDNA clone of Fev containing 268 bp was contructed and the linearization and digoxigenin incorporation were described previously (Lima et al., 2009). A monkey specific partial cDNA clone of TPH2 containing 251 bp of the 5’ region, which has very little homology to TPH1, was previously constructed (Sanchez et al., 2005). A recombinant subclone of the 5’ cytoplasmic domain of human SERT was previously generated with a 253 bp insert (Pecins-Thompson et al., 1998, Bethea et al., 2005b). The insert was recloned into a pGEM vector for digoxigenin incorporation. A monkey specific partial cDNA clone of the 5HT1A autoreceptor was previously constructed that contained 432 bp extending from base 647 to 1078 of the 5HT1A receptor (Pecins-Thompson and Bethea, 1998, Centeno et al., 2007b). This region corresponds to the area between transmembrane domains V and VI and it has the least sequence homology with other 5HT receptors, the β2-adrenergic and the α1-adrenergic receptors that also share sequence homology with the 5HT1A receptor (Julius, 1991). The insert was recloned into a pGEM vector for digoxigenin incorporation. The cRNAs incorporating digoxigenin were prepared using these cDNAs as templates and SP6 RNA polymerase to drive the transcription reaction (Berg-von der Emde et al., 1995).
In situ hybridization (ISH) assays
A non-isotopic in situ hybridization procedure utilizing digoxigenin-labeled cRNA, described in detail elsewhere, was used to determine if mRNA abundance in serotonin neurons differed between Ovx and tubal-ligated monkeys (Berg-von der Emde et al., 1995, Lima et al., 2009). Following an overnight hybridization at 55-56°C, the slides were washed and processed for digoxigenin detection of each mRNA, as reported (Berg-von der Emde et al., 1995). After developing the digoxigenin/antidigoxigenin-alkaline phosphatase conjugate reaction by an overnight incubation in a 4-nitrobluetetrazolium chloride/5-bromo-4-chloro-3 indoyl-phosphate staining solution, the slides were extensively washed in potassium phosphate-sodium chloride buffer pH 7.4, dehydrated in graded ethanol, dried, and coverslipped for microscopic examination. The sections were matched anatomically. Five levels of the dorsal raphe were analyzed at 250 μm intervals for Fev and 4 levels of the dorsal raphe were analyzed at 250 μm intervals for TPH2 gene expression. Also, 4 levels of the dorsal raphe were analyzed at 500 μm intervals for SERT and 5HT1A gene expresssion.
Densitometric Analysis
Each section was video-captured with the Marianas Stereology Workstation and Slidebook 4.2. A montage of the dorsal raphe was created by Slidebook, which was subjected to further analysis with Image J. For each anatomical level, the largest representation of the dorsal raphe was chosen from amongst all of the animals. A square outline was placed over the chosen dorsal raphe and the exact dimensions were recorded. The same size square was then used for all of the animals at that anatomical level. On each section, the operator outlined (boxed) the dorsal raphe nucleus and the image was segmented into positive (stained) and negative (unstained) pixels. Three measurements were generated from the segmented image. The area covered by the positive pixels was obtained, and called positive pixel area. Then, the number of positive cells was obtained using size cut-off values. In addition, the mean gray level of the positive cells was obtained, which is the average density of the signal in the cells. This then represents the average signal intensity in the positive cells. The same procedure was applied at each anatomical level from the rostral to the caudal extent of the dorsal raphe nucleus.
Statistical Analysis
Fenfluramine results
The serum concentrations of prolactin and cortisol were analyzed with a 2 way ANOVA for repeated measures.
ISH assay results
The positive pixel area, the positive cell number and the mean gray level were (a) averaged across levels and (b) totalled across levels, generating one overall average or total for each animal. The average of the animals in each group was then obtained for statistical analysis. Based upon a previous study in stress-sensitive animals, in which each serotonin-related gene reflected Fev expression (Lima et al., 2009), we predicted that each of the serotonin-related genes would reflect the Fev values. That is, there would be fewer Fev positive cells and lower Fev positive pixel area in the Ovx animals and each of the other genes would be similar. Therefore, each data set was compared with a one-tailed Students t-test. For reference, the two-tailed p-value can be obtained by doubling the one-tail p-value, and all of the differences that were significant with the 1-tailed test were also significant with the 2-tailed test. The variance in the groups reflects the difference between animals. F values were computed to assure the variances were equal. If the variance was unequal, the modified Welsh t-test was applied. This test was used once for the signal intensity in the SERT assay.
All statistical analyses were conducted using the Prism Statistic Program 5.0 (GraphPad, San Diego, CA). A confidence level of p<0.05 was considered significant.
Results
It was not possible to monitor the menstrual cycle in the individual tubal-ligated animals while they were in the corral or group housing; and they were in single cages for too brief a time to determine menstrual cycle stage from the presence of blood in the waste pans. Therefore, we measured estradiol and progesterone in the first sample of the baseline period prior to the fenfluramine challenge. Serum estradiol and progesterone concentrations in the animals at the time of fenfluramine challenge, and prior to euthanasia, are shown in Table 1. We found that serum estradiol and progesterone were very low in the ovariectomized animals. Low levels of estradiol are produced by fat tissue and these concentrations are consistent with previous analyses of serum from ovariectomized monkeys (Bethea et al., 2009). Serum estradiol and progesterone were differentially higher among the intact females, indicating that the intact animals were at various stages of the menstrual cycle, as designated on the table. We could not euthanize the animals at a particular stage of the cycle since this information was not available a priori. We propose that the fenfluramine-induced prolactin secretion and gene expression results reflect the overall average of the ovarian hormones that were present in the animals and reflect the presence or near absence of these steroids for a 3-year period. However, we cannot completely rule out that the possibility that the stage of the menstrual cycle increased the variance in the tubal- ligated ovary intact group.
Table 1.
Estradiol and progesterone concentrations in serum from the female Japanese macaques, 3 hours before euthanasia.
| Treatment Group | Estradiol-17β | Progesterone | Stage |
|---|---|---|---|
| Animal ID | pg/ml | ng/ml | |
| Ovx | |||
| 24018 | 14 | 0.30 | n/a |
| 24020 | 28 | 0.85 | n/a |
| 24028 | 20 | 0.69 | n/a |
| 24029 | 10 | 0.60 | n/a |
| Avg | 18 | 0.61 | |
| Intact | |||
| 24032 | 19 | 0.23 | menstrual |
| 24037 | 64 | 0.12 | early-follicular |
| 24040 | 431 | 1.17 | early luteal |
| 24042 | 18 | 1.20 | late luteal |
| 24049 | 107 | 0.86 | mid-follicular |
| Avg | 127.73 | 0.72 |
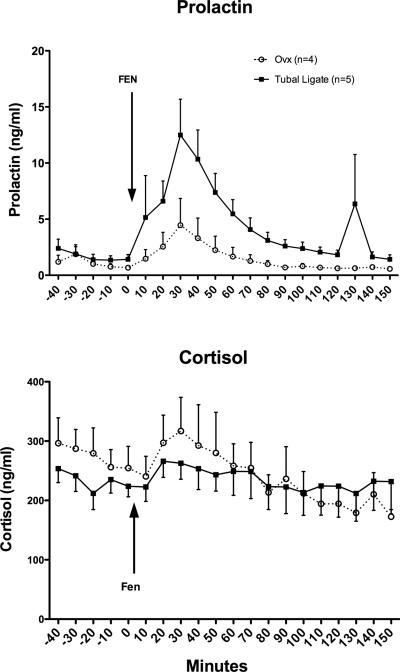
As illustrated in Figure 1, prolactin secretion was significantly elevated in both groups by fenfluramine injection (2-way repeated measures ANOVA p = 0.0001, for time effect). However, prolactin secretion was significantly lower in the Ovx group compared to the tubal-ligated group (2-way repeated measures ANOVA p = 0.011, for treatment effect). Cortisol was also significantly increased in both groups by fenfluramine injection (2-way repeated measures ANOVA p =0.0001, for time effect). However, there was no difference in cortisol secretion between the groups. The matching was highly effective.
Figure 1.
The effect of fenfluramine on prolactin and cortisol secretion in ovariectomized and tubal ligated female Japanese macaques. Top. Prolactin secretion was significantly different across time (2-way repeated measures ANOVA p = 0.0001) indicating that fenfluramine significantly increased prolactin secretion. In addition, there was significant effect of ovariectomy (2-way repeated measures ANOVA p = 0.011) indicating that the ovariectomized females secreted significantly less prolactin in response to fenfluramine than the tubal ligated females. The matching was effective (p = 0.007). However, there was no interaction. Bottom. Cortisol secretion was significantly different across time (2-way repeated measures ANOVA p < 0.0001) indicating that fenfluramine significantly increased cortisol secretion. However, ovariectomy had no effect on cortisol secretion in response to fenfluramine (2-way repeated measures ANOVA p=0.8). The matching was highly effective (p < 0.0001), but there was no interaction.
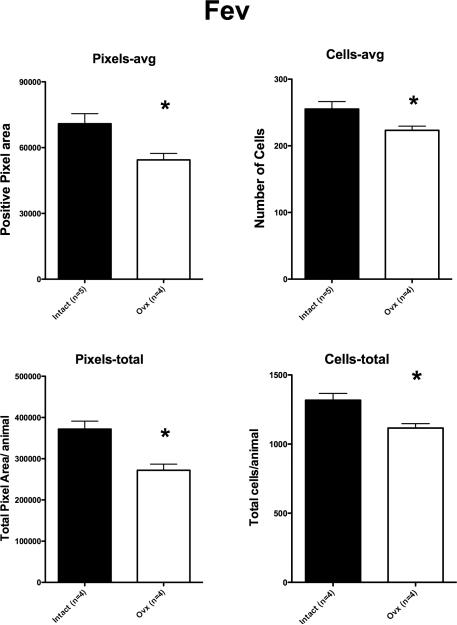
Representative sections from the rostral dorsal raphe processed for Fev digoxigenin-ISH are shown in Figure 2. In these selected images, the Fev ISH signal was lighter in the Ovx animal than in the tubal-ligated animal. Please note that the background intensity is identical. Five levels of the dorsal raphe from each animal were examined with Image J and the results are illustrated in Figure 3. There was a significant reduction in the average (p=0.012) and total (p=0.003) Fev-positive pixel area in the Ovx animals compared to the tubal-ligated animals. In addition, there was a significant reduction in the average number (p=0.027) and total number (p=0.007) of Fev-positive cells in the Ovx animals compared to the tubal-ligated animals. To determine if there was a difference in signal intensity within the cells, we obtained the mean gray value, which is an average of the signal intensity in the positive cells of the section. The intensity of the signal is represented on a scale where 0 equals white and 256 equals black. There was no difference between the groups in the average signal intensity of the Fev-positive cells (Tubal ligated = 63.7±11.0: Ovx= 65.4±4.1). There was no difference in the variances between the groups with any comparison.
Figure 2.
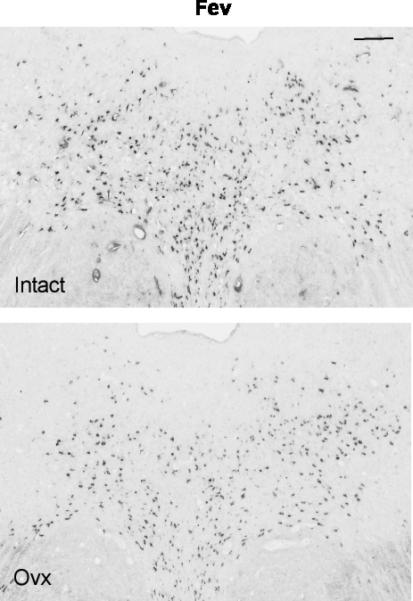
Illustration of Fev expression in the rostral dorsal raphe in a representative monkey from each treatment group. The scale bar equals 100 μm. There is robust expression of Fev in the intact animal, but Fev expression is reduced in the Ovx animal.
Figure 3.
Fev expression parameters for each group (n=4-5/group). There was a significant difference in the average positive pixel area per animal (p=0.012), the average positive cell number per animal (p=0.027), the total positive pixel area per animal (p=0.003) and the total cell number per animal (p=0.007). The average positive pixel area and average cell number were computed by calculating the average of the 5 sections for each animal and then obtaining the averages of the animals in each group. The total positive pixel area and total cell number were computed by obtaining the total of each parameter in the 5 sections from each animal and then obtaining the average±sem of the totals.
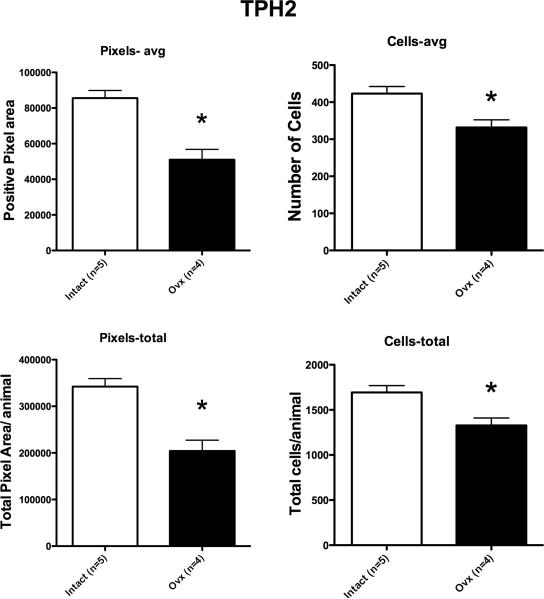
Representative sections from the middle region of the dorsal raphe that were processed for TPH2 digoxigenin-ISH are shown in Figure 4. In the chosen sections, the TPH2 ISH signal is lighter in the Ovx animal compared to the tubal-ligated animal, but the background intensity is identical. Four levels of the dorsal raphe from each animal were examined with Image J and the results are illustrated in Figure 5. There was a significant reduction in the average (p=0.0009) and total (p=0.0009) TPH2-positive pixel area in the Ovx animals compared to the tubal-ligated animals. In addition, there was a significant reduction in the average number (p=0.007) and total number (p=0.007) of TPH2-positive cells in the Ovx animals compared to the tubal-ligated animals. There was no difference between the groups in the average signal intensity of the TPH2 positive cells although it trended lower in the Ovx group (Tubal ligated = 80.7±6.3; Ovx= 72.0±5.1). There was no difference in the variances between the groups in any comparison.
Figure 4.
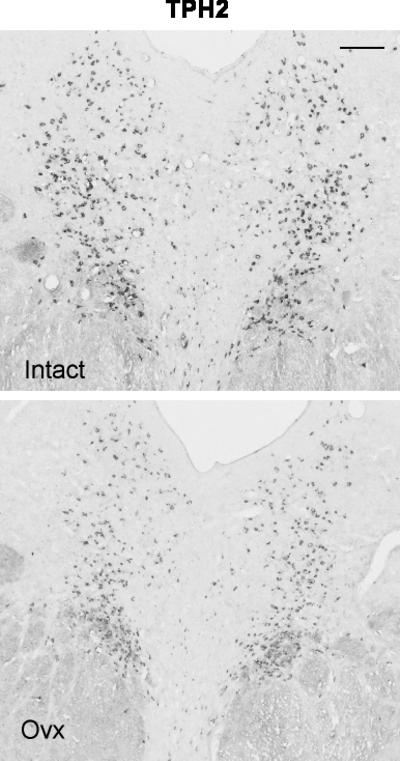
Illustration of TPH2 expression in the rostral dorsal raphe in a representative monkey from each treatment group. The scale bar equals 100 μm. There is robust expression of TPH2 in the intact animal, but TPH2 expression is reduced in the Ovx animal.
Figure 5.
TPH2 expression parameters for each group (n=4-5/group). There was a significant difference in the average positive pixel area per animal (p=0.0009), the average positive cell number per animal (p=0.007), the total positive pixel area per animal (p=0.0009) and the total cell number per animal (p=0.007). The calculation of these parameters is described in the legend to Figure 3.
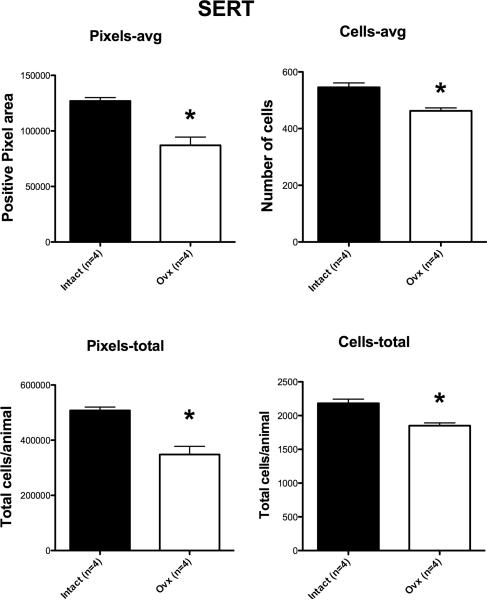
Figure 6 illustrates two sections from the rostral region of the dorsal raphe that were processed for SERT digoxigenin-ISH. The SERT signal appeared lighter in the chosen Ovx animal compared to the chosen tubal-ligated animal and background was identical. One animal in the tubal-ligated group was 2 standard deviations from the mean in all SERT endpoints and was excluded as an outlier (24042). Four levels of the dorsal raphe from each animal were examined with Image J and the results are illustrated in Figure 7. There was a significant reduction in the average (p=0.001) and total (p=0.001) SERT-positive pixel area in the Ovx animals compared to the tubal-ligated animals. There was also a significant reduction in the average (p=0.002) and total (p=0.002) SERT-positive cell number in the Ovx animals compared to the tubal-ligated animals. There were no differences in the variance for the pixel area or cell number comparisons. The Ovx group also had significantly lower average signal intensity in the SERT-positive cells compared to the tubal-ligated group (Tubal ligated = 98.1±4.0;Ovx = 81.58±0.83). However, the variances were unequal, so the modified Welch t-test was performed which allows for unequal variances (1-tail p = 0.014; 2-tail p= 0.028).
Figure 6.
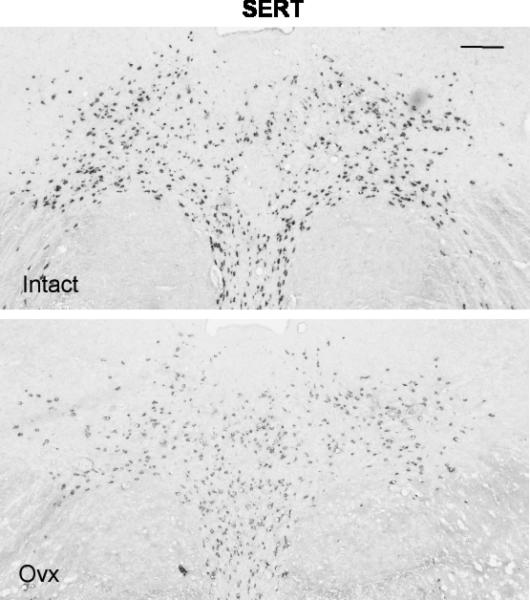
Illustration of SERT expression in the rostral dorsal raphe in a representative monkey from each treatment group. The scale bar equals 100 μm. There is robust expression of SERT in the intact animal, but SERT expression is reduced in the Ovx animal.
Figure 7.
SERT parameters for each group (n=4-5/group). There was a significant difference in the average positive pixel area per animal (p=0.001), the average positive cell number per animal (p=0.002), the total positive pixel area per animal (p=0.001) and the total cell number per animal (p=0.002). The calculation of these parameters is described in the legend to Figure 3.
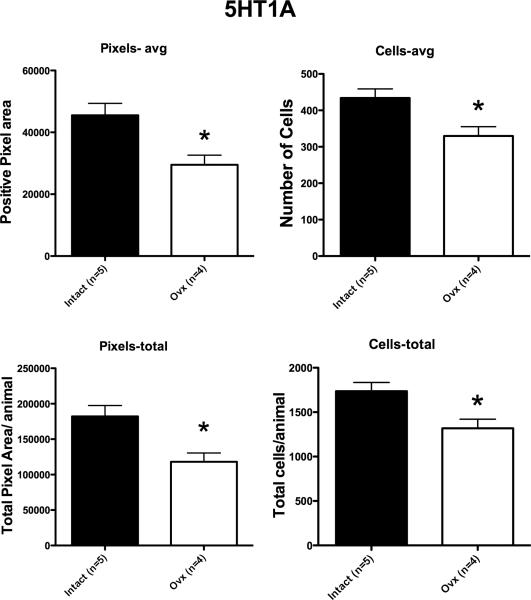
Representative sections from the middle region of the dorsal raphe that were processed for 5HT1A digoxigenin-ISH are shown in Figure 8. The 5HT1A ISH signal appeared lighter in the representative Ovx section compared to the representative section from a tubal-ligated animal, but the background intensity is identical. Four levels of the dorsal raphe from each animal were examined with Image J and the results are illustrated in Figure 9. There was a significant reduction in the average (p=0.008) and total (p=0.008) 5HT1A-positive pixel area in the Ovx animals compared to the tubal-ligated animals. In addition, there was a significant reduction in the average number (p=0.011) and total number (p=0.011) of 5HT1A-positive cells in the Ovx animals compared to the tubal-ligated animals. There was no difference between the groups in the average signal intensity of the 5HT1A-positive cells although it trended lower in the Ovx group (Tubal ligated = 55.6±3.7; Ovx= 50.19±4.4). There was no difference in the variances between the groups with any comparison.
Figure 8.
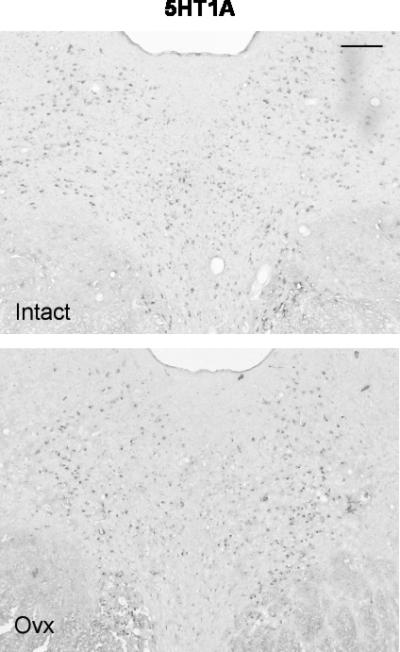
Illustration of 5HT1A expression in the rostral dorsal raphe in a representative monkey from each treatment group. The scale bar equals 100 μm. There is robust expression of 5HT1A in the intact animal, but 5HT1A expression is reduced in the Ovx animal.
Figure 9.
5HT1A parameters for each group (n=4-5/group). There was a significant difference in the average positive pixel area per animal (p=0.008), the average positive cell number per animal (p=0.011), the total positive pixel area per animal (p=0.008) and the total cell number per animal (p=0.011). The calculation of these parameters is described in the legend to Figure 3.
In addition, there was no correlation between estradiol or progesterone or rank at the end of the study with any of the endpoints examined (p's > 0.2). R2 values for TPH2 average pixel area versus E and P equaled 0.316 and 0.4, respectively, and the other genes were similar. R2 values for Fev, TPH2, SERT and 5HT1A average pixel area versus rank ranged from 0.0018 to 0.172.
Discussion
This study demonstrated that long-term loss of ovarian hormones in healthy young adult female monkeys leads to a decrease in global serotonin availability; fewer serotonin neurons, and a reduction in expression of pivotal genes related to serotonin neural function compared to ovarian intact animals. We speculate that the decrease in serotonin neurons and the consequences of such a decrease might be more pronounced if the animals were maintained for longer than one grant cycle. It is also attractive to speculate that if ovariectomy occurred in an older individual, then the consequences might be more severe.
Prolactin release was significantly less in Ovx monkeys in response to the fenfluramine challenge compared to tubal ligated monkeys, suggesting that global serotonin availability and serotonergic function were reduced in these animals. This is consistent with the report that prolactin secretion is significantly greater in Ovx monkeys administered estrogen than placebo (Mook et al., 2005). We have previously reported that stress-sensitive cynomolgus macaques exhibit decreased prolactin secretion in response to fenfluramine; have fewer Fev-positive neurons, and exhibit decreased serotonin-related gene expression compared to stress resilient macaques (Bethea et al., 2005a, Bethea et al., 2005b, Bethea et al., 2008). The Ovx females in this study had the same type of fenfluramine response and the same pattern of serotonin-related gene expression. It is attractive to speculate that the Ovx females in this study are, or would become, stress-sensitive.
However, cortisol release in response to fenfluramine was similar between Ovx and tubal ligated monkeys. In addition, the adrenal/body weight ratio was identical (not shown). Wilson and colleagues also found that estrogen administration had little effect on the cortisol response to fenfluramine (Mook et al., 2005). We previously found that stress-sensitive cynomolgus macaques have a larger caudal PVN with more CRF neurons than stress-resilient counterparts (Centeno et al., 2007c) and that stress-sensitive macaques have greater CRF fiber density amongst the serotonin neurons of the dorsal raphe nucleus (Weissheimer, 2010). However, there was little difference in several responses of the HPA axis in stress-sensitive compared to stress-resilient monkeys (Herod et al., 2011a, Herod et al., 2011b). Previous data suggest that separate CRF pathways impinge upon the HPA and serotonin systems, that ovarian steroids have little effect on the HPA axis and that the HPA axis was functioning in a similar manner in our Ovx and tubal ligated groups. Studies are underway to examine CRF fiber innervation in the dorsal raphe of our Ovx and tubal ligated groups.
The results of this study had several interesting differences from our previous studies in ~5-month (short-term) Ovx rhesus macaques with one month of hormone replacement. In the short-term Ovx rhesus monkeys, we previously found no significant difference in the number of serotonin neurons as detected with serotonin immunocytochemistry (Bethea, 1994). This was important for interpretation of gene expression results on autoradiographs, in that there was no need to normalize the signal for cell number (Bethea et al., 2002). It was also consistent with the short time frame of the ovariectomy. In the long-term Ovx animals, we predicted a decrease in cell number, so it was important to utilize the digoxigenin-ISH assay, which provides a higher degree of cellular resolution than radiolabeled probes. Thus, it was possible to determine positive cell number as well as, positive pixel area. We examined signal density to determine whether gene expression decreased in individual cells or whether a reduction in signal was due to fewer cells. However, this analysis should be viewed with caution. The enzymatic reaction in digoxigenin ISH signal may not be linear, which would call into question the signal density. Unfortunately, we did not have sufficient sections to perform additional assays with radiolabeled probes.
The long-term Ovx animals exhibited a decrease in Fev expression and a decrease in Fev-positive cells, but there was no difference in the average signal intensity in the positive cells between the groups. Fev (called Pet1 in rodents) is an ETS transcription factor that is thought to be the „master gene’ in serotonin neurons. It is necessary for achievement of the serotonin phenotype during embryogenesis and it drives the expression of TPH2, SERT and 5HT1A gene expression (Hendricks et al., 1999, Hendricks et al., 2003). Fev is not only present in early development, but its expression also persists into adulthood and it is necessary for the function of serotonin neurons at different stages of life (Liu et al., 2010). Since Fev determines whether a neuron is serotonergic or not, we reasoned that if Fev is undetectable then it follows that the neuron is not phenotypically serotonergic. The digoxigenin-ISH assay is very sensitive, so we interpreted the reduction in Fev-positive neurons as fewer phenotypically serotonin neurons. The reduction in Fev-positive cell number, while significant, was on the order of 20%. This was not robust, but we hypothesize that serotonin cell number would continue to decline over time and could be exacerbated by age and environmental conditions. The Fev-expression signal in individual cells was variable and there was no statistical difference in the average signal intensity of the FEV-positive cells between the groups. Indeed, there was a grade of signal intensity in positive cells within each section, which could be due to a true grade of Fev expression amongst cells or to differential slicing and orientation of cells presented on the surface and the penetration of the rib probe/digoxigenin antiserum. Thus, the overall reduction in Fev-positive pixel area may derive from the reduction in Fev-positive neurons in the Ovx group.
Whether the animals were still developing their serotonin system at ovariectomy is unknown. When Fev is removed during development, as in the Pet1 knockout mouse, there are abnormal serotonin precursor neurons present (Krueger and Deneris, 2008, Beck et al., 2010). We do not know if adolescent monkeys still have precursor neurons available, which failed to fully differentiate upon ovariectomy. Also, we do not know whether a total shutdown in Fev expression in an adult neuron would cause it to revert to a non-serotonergic phenotype that could later be revived to serotonergic status. However, we should probably not completely disregard these possibilities in the long-term Ovx monkeys.
In the short-term Ovx monkeys with similar serotonin cell number, TPH2 is markedly reduced on autoradiograms in Ovx animals compared to E or E+P- treated animals (Sanchez et al., 2005), indicating that individual cells were transcribing less TPH2. Fev drives TPH2 expression, so the decrease in TPH2-positive cell number in the long-term Ovx monkeys is consistent with the reduction in Fev-positive cells and with our notion that there are fewer serotonin neurons. In addition, the expression of TPH2 also appeared to be lower within individual cells in the long-term Ovx animals in a manner similar to the short-term Ovx animals (Sanchez et al., 2005). However, statistically there was no difference between the groups in the average signal intensity of the TPH2-positive cells. Again, expression in individual cells varies within the section. Hence, the overall reduction in TPH2-positive pixel area probably derives from the reduction in TPH2-positive neurons in the Ovx group. Indeed, if serotonin neurons were lost and cannot be replaced, then administration of ovarian steroids for 1 month to the long-term Ovx animals may increase TPH2 expression in individual cells, but cell number would remain lower than the intact animals. Whether steroid-induced TPH2 expression could compensate for fewer cells may depend on the severity of cell loss, which speculatively may depend on the time passed in the absence of ovarian steroids. Conversely, if there were steroid responsive precursor neurons, which could respond to estrogen with an increase in Fev, then steroid treatment of long-term Ovx animals could also restore serotonin cell number and increase TPH2 expression. We would like to test this hypothesis.
Gene expression of SERT was not consistent with our previous studies of hormone replacement in short-term Ovx monkeys. In the short-term Ovx monkeys with similar serotonin cell number, SERT mRNA was marginally higher in Ovx controls; there was no difference in SERT protein in the cell body region, but lower immunoreactive SERT in axons, compared to steroid treated animals (Pecins-Thompson et al., 1998, Lu et al., 2003, Smith et al., 2004). In the long-term Ovx animals, there was a decrease in SERT cell number, indicating that there were fewer SERT-positive neurons. Fev drives SERT gene expression, which reflects the reduction in Fev-positive cells. In addition, the average signal intensity of the SERT-positive cells was lower in the Ovx animals, although the intensity must be viewed with caution. The reduction in SERT expression in the Ovx animals resembles that of stress-sensitive monkeys (Lima et al., 2009). Examination of SERT binding in the dorsal raphe with PET imaging in anxiety or unipolar and bipolar depression has yielded various results. Different groups have observed an increase, a decrease or no change although different ligands were employed (Bhagwagar et al., 2007, Cannon et al., 2007, Meyer, 2007, Reimold et al., 2008). Our data suggest that different numbers of serotonin neurons and/or Fev expression may also contribute to the differences in the PET imaging results and these values may vary.
Gene expression of 5HT1A was markedly different from the regulation that we previously observed with 1 month of hormone replacement in short-term Ovx monkeys. In short-term Ovx monkeys, the expression of 5HT1A at gene and protein levels was consistently higher than animals with E±P treatment (Pecins-Thompson and Bethea, 1998). Moreover, the binding of the 5HT1A agonist, 8-OH-DPAT and the coupling of the 5HT1A receptor to its G protein transducer was also higher in both the raphe and hypothalamus of short-term Ovx monkeys compared to animals with hormone replacement (Lu and Bethea, 2002). In this study, the tubal-ligated animals had a significantly greater number of 5HT1A-positive cells and higher 5HT1A-positive pixel area than the Ovx animals. While these results reflect Fev expression, they differ markedly from the administration of ovarian steroids to short-term Ovx animals, which reduced 5HT1A expression. However, they closely resemble the reduction of 5HT1A expression in stress-sensitive monkeys compared to stress-resilient monkeys (Lima et al., 2009). There was no significant difference in signal intensity between groups in the 5HT1A-positive cells although the Ovx group tended lower than the tubal-ligated group, and signal intensity was highly variable. Direct comparisons are not possible, but 5HT1A mRNA expression appeared very robust in the short-term Ovx control group in previous studies (Pecins-Thompson and Bethea, 1998) compared to the long-term Ovx group in this study.
Different groups have examined the effect of depression on 5HT1A binding in the dorsal raphe with PET imaging. Increases, decreases or no change have been reported (Drevets et al., 2000, Bhagwagar et al., 2004, Parsey et al., 2006, Drevets et al., 2007, Moses-Kolko et al., 2008, Savitz et al., 2009). Our data suggest that the number of serotonin neurons could affect PET imaging results in the raphe. The longer time frame may have led to a different homeostasis in the expression of this gene in light of the decrease in TPH2 and SERT. For example, 5HT1A expression is reduced in SERT knockout mice (Li et al., 1999), which leads to the possibility that the reduction in SERT may be linked to the reduction in 5HT1A expression in this study. In addition, the 5HT1A promoter has multiple Fev binding elements and 5HT1A receptors are decreased in the raphe of Pet1/Fev knock out mice, indicating that Fev plays a pivotal role in 5HT1A transcription (Jacobsen et al., 2011)
The notion of neuronal health as a dynamic equilibrium that spans a spectrum between resilient and vulnerable neuronal states is accepted. Thus, at any given time, neurons may be very healthy and functioning well including optimal DNA repair, or somewhere on a slippery slope where they are unhealthy and falling farther and farther behind in DNA repair, but not dead (Isacson, 1993). We previously observed greater DNA fragmentation in short-term placebo-treated Ovx monkeys compared to monkeys with 1 month of hormone therapy (Lima and Bethea, 2009). This observation coupled with increases in gene and protein expression in the caspase-independent apoptosis pathway in Ovx controls (Bethea and Reddy, 2008, Tokuyama et al., 2008) suggested that serotonin neurons exist in a vulnerable, endangered state following loss of ovarian steroids. We then hypothesized that with additional time or stress, the cells could die. That hypothesis is supported by the data in this study. Although we cannot maintain young monkeys in a longer protocol, we now hypothesize that the decrease in serotonin neurons would continue over time, and would be exacerbated if older animals were ovariectomized for 3 years. Moreover, if old monkeys were maintained on a typical western diet, which is much higher in fat and sugar than monkey chow, the decrease may be further exacerbated. We hope to test this hypothesis in the future.
These data are consistent with information from numerous laboratories showing that ovarian steroids are neuroprotective (Bourque et al., 2009, De Nicola et al., 2009, Garcia-Segura and Balthazart, 2009, Kipp and Beyer, 2009, Pike et al., 2009, Simpkins et al., 2009, Suzuki et al., 2009). However, animal models that use injury or toxicity to damage neurons are often employed to show that E, P or E+P reduces damage. Our studies in monkeys do not utilize any kind of injury or toxicity. We speculate that normal life stresses and chemicals in the environment are sufficient over a longer life span to endanger serotonin neurons, and that ovarian steroids protect them from accumulated stresses. Global DNA repair is more active in dividing cells than terminally differentiated cells, such as neurons. However, nuclear excision repair is active in neurons and acts on genes utilized for transcription. A decrease in the activity of transcription-coupled DNA repair enzymes would lead to accumulated DNA fragmentation and this can ultimately lead to cell death (Nouspikel and Hanawalt, 2000, 2002, Nouspikel, 2009). In our study of DNA fragmentation in serotonin neurons, it is important to note that one month of hormone replacement in short-term Ovx monkeys eliminated a significant amount of DNA fragmentation (Lima and Bethea, 2009). These data indicate that one action of ovarian steroids in neuroprotection may be via an increase in DNA repair enzymes. Indeed, we have observed that gene expression related to DNA repair was increased by E±P administration to Ovx monkeys in a microarray analysis of laser captured serotonin neurons (unpublished). We need to confirm this observation with qRT-PCR as well as pursue this hypothesis at protein and functional levels. We also need to know if this effect would rescue serotonin neurons after long-term Ovx.
There is an alternate interpretation of these data that was alluded to earlier and requires consideration as well. The developmental pattern of serotonin neurons has not been examined in detail in nonhuman primates and the animals in this study were adolescents when they were ovariectomized. Hence, a possibility exists that the number of serotonin neurons found in the Ovx animals represents the normal number of neurons for a 3-year old monkey and this value did not change between 3 and 6 years of age. Rather, the number of serotonin neurons in the intact animals increased over the 3-year period, and the increase may be due to the increase in ovarian steroids that occurs from 3-6 years of age in female macaques. It is thought that there are serotonin neuron precursors in the midbrain of Pet1 (Fev) knockout mice, but the absence of Fev prevents the neurons from becoming phenotypically serotonergic (Krueger and Deneris, 2008, Beck et al., 2010). We cannot rule out the possibility that there are similar precursors in the midbrain of adolescent macaques, nor the possibility that ovarian steroids induce Fev in those neurons resulting in the appearance of more serotonin neurons as the animal approaches full adulthood and reproductive competence. This alternative interpretation does not take into account the observation of DNA fragmentation in adult Ovx rhesus monkeys, but we cannot rule out an ongoing developmental process in the adolescent intact monkeys in this study, or a contribution of both mechanisms to the final outcome. Unfortunately, it was not feasible to remove 10-12 adult females from our Japanese macaque troop because of their contribution to social stability. We hope to pursue these ideas in older rhesus macaques.
The usual computation of human years from monkey years is 3 to 1, so the Ovx group is somewhat comparable to women after 9 years of steroid deprivation, though significantly younger than menopausal women. Nonetheless, these studies promote further understanding of the effects of long-term ovarian steroid loss in females and suggest that as time after ovariectomy increases, changes may occur in the serotonin system which could be permanent and therefore, not completely amenable to later hormone replacement. If serotonin neurons are lost, and there is no evidence of stem cells in the midbrain, then extremely delayed hormone therapy may not replace those cells. Other neural systems supported by serotonin may not be rescued either. A study of immediate versus delayed hormone therapy in older monkeys on a western diet would be of great interest in this regard.
In conclusion, 3 years of ovariectomy reduced serotonin cell number as well as Fev, TPH2, SERT and 5HT1A gene expression. The reduction in gene expression as determined by positive pixel area reflected the reduction in neuron number and could be a result of fewer serotonin neurons in Ovx animals. To our eyes, there appeared to be a reduction in the signal intensity of each gene in the positive neurons of the Ovx animals. However, the quantification may not be reliable, and only SERT exhibited a significant decrease in positive cell signal intensity. Nonetheless, the serotonin system and the regulation of pivotal genes in the long-term Ovx and intact females were different compared to short-term Ovx plus or minus hormone replacement. Rather, serotonin-related gene expression in the long-term Ovx group resembles that of stress-sensitive monkeys (Lima et al., 2009). The effect of hormone therapy on these parameters in the serotonin system after long-term ovariectomy is unknown.
Highlights.
Three years of ovariectomy in Japanese macaques is approximately equal to 9 years in women.
The prolactin response to fenfluramine was reduced by ovariectomy.
Serotonin neuron number was reduced by ovariectomy.
Fev, TPH2, SERT and 5HT1A expression were reduced by ovariectomy.
Acknowledgements
We are deeply grateful to the technicians of the Division of Animal Resources, including surgery personnel, for their help with all aspects of this study. We especially thank Dr. Kris Coleman, Head of Behavioral Sciences, who provided expert knowledge and advice on the Japanese macaque troop, on Japanese macaque behavior and what it means to other monkeys. We also are especially grateful to Dr. Coleman's assistant, Nicola Roberts, who was intimately involved in monitoring the behavior and well being of the animals in this study. This study was supported by NIH grants MH62677 to CLB and P51-RR000163 for the operation of ONPRC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Beck S, Craige C, Piel D, Krueger K, Deneris E, Calizo L, Crawford L. Annual meeting of the American College of Neuropsychopharmacology. Miami, FL: 2010. Abnormal physiology and morphology of raphe serotonin neurons early in development and in those lacking the Pet-1 transcriptional factor; p. 257. [Google Scholar]
- Berg-von der Emde K, Dees WL, Hiney JK, Hill DF, Dissen GA, Costa ME, Moholt-Siebert M, Ojeda SR. Neurotrophins and the neuroendocrine brain: different neurotrophins sustain anatomically and functionally segregated subsets of hypothalamic dopaminergic neurons. J Neurosci. 1995;15:4223–4237. doi: 10.1523/JNEUROSCI.15-06-04223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL. Regulation of progestin receptors in raphe neurons of steroid-treated monkeys. Neuroendocrinology. 1994;60:50–61. doi: 10.1159/000126719. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol. 2008;38:199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in Neuroendocrinology. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril. 2005a;83:148–155. doi: 10.1016/j.fertnstert.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP. Effect of ovarian hormones on survival genes in laser captured serotonin neurons from macaques. J Neurochem. 2008;105:1129–1143. doi: 10.1111/j.1471-4159.2008.05213.x. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009;30:212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Mirkes SJ, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience. 2005b;132:151–166. doi: 10.1016/j.neuroscience.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Murthy N, Selvaraj S, Hinz R, Taylor M, Fancy S, Grasby P, Cowen P. 5-HTT binding in recovered depressed patients and healthy volunteers: a positron emission tomography study with [11C]DASB. Am J Psychiatry. 2007;164:1858–1865. doi: 10.1176/appi.ajp.2007.06111933. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol. 2009;30:142–157. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Reddy AP, Smith LJ, Sanchez RL, Henderson JA, Salli NC, Hess DJ, Pau FK, Bethea CL. Serotonin in microdialysate from the mediobasal hypothalamus increases after progesterone administration to estrogen primed macaques. Eur J Pharmacol. 2007a;555:67–75. doi: 10.1016/j.ejphar.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic expression of serotonin 1A, 2A and 2C receptors and GAD67 in female cynomolgus monkeys with different sensitivity to stress. Brain Research. 2007b;1142:1–12. doi: 10.1016/j.brainres.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Reddy AP, Cameron JL, Bethea CL. Corticotropin-releasing hormone and pro-opiomelanocortin gene expression in female monkeys with differences in sensitivity to stress. Neuroendocrinology. 2007c;86:277–288. doi: 10.1159/000109877. [DOI] [PubMed] [Google Scholar]
- Conde DM, Pinto-Neto AM, Santos-Sa D, Costa-Paiva L, Martinez EZ. Factors associated with quality of life in a cohort of postmenopausal women. Gynecol Endocrinol. 2006;22:441–446. doi: 10.1080/09513590600890306. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Labombarda F, Deniselle MC, Gonzalez SL, Garay L, Meyer M, Gargiulo G, Guennoun R, Schumacher M. Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front Neuroendocrinol. 2009;30:173–187. doi: 10.1016/j.yfrne.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nucl Med Biol. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C. Role of serotonin in sleep mechanisms. Rev Neurol (Paris) 2001;157:S16–19. [PubMed] [Google Scholar]
- Eaton GG. Male dominance and aggression in Japanese macaque reproduction. Adv Behav Biol. 1974;11:287–297. doi: 10.1007/978-1-4684-3069-1_13. [DOI] [PubMed] [Google Scholar]
- Frey BN, Lord C, Soares CN. Depression during menopausal transition: a review of treatment strategies and pathophysiological correlates. Menopause Int. 2008;14:123–128. doi: 10.1258/mi.2008.008019. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Balthazart J. Steroids and neuroprotection: New advances. Front Neuroendocrinol. 2009;30:v–ix. doi: 10.1016/j.yfrne.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U. Gonadal hormones and antihormones, serotonin and mood. Psychopharmacology Bulletin. 1990;26(3):291–295. [PubMed] [Google Scholar]
- Halbreich U, Rojansky N, Palter S, Tworek H, Hissin P, Wang K. Estrogen augments serotonergic activity in postmenopausal women. Biological Psychiatry. 1995;37:434–441. doi: 10.1016/0006-3223(94)00181-2. [DOI] [PubMed] [Google Scholar]
- Heikkinen J, Vaheri R, Timonen U. A 10-year follow-up of postmenopausal women on long-term continuous combined hormone replacement therapy: Update of safety and quality-of-life findings. J Br Menopause Soc. 2006;12:115–125. doi: 10.1258/136218006778234093. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Herod SM, Dettmer AM, Novak MA, Meyer JS, Cameron JL. Sensitivity to stress-induced reproductive dysfunction is associated with a selective but not a generalized increase in activity of the adrenal axis. Am J Physiol Endocrinol Metab. 2011a;300:E28–36. doi: 10.1152/ajpendo.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am J Physiol Endocrinol Metab. 2011b;300:E19–27. doi: 10.1152/ajpendo.00224.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Isacson O. On neuronal health. Trends Neurosci. 1993;16:306–308. doi: 10.1016/0166-2236(93)90104-t. [DOI] [PubMed] [Google Scholar]
- Jacobsen KX, Czesak M, Deria M, Le Francois B, Albert PR. Region-specific regulation of 5-HT1A receptor expression by Pet-1-dependent mechanisms in vivo. J Neurochem. 2011;116:1066–1076. doi: 10.1111/j.1471-4159.2010.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D. Molecular biology of serotonin receptors. Annual Review of Neuroscience. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- Kipp M, Beyer C. Impact of sex steroids on neuroinflammatory processes and experimental multiple sclerosis. Front Neuroendocrinol. 2009;30:188–200. doi: 10.1016/j.yfrne.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Krueger KC, Deneris ES. Serotonergic transcription of human FEV reveals direct GATA factor interactions and fate of Pet-1-deficient serotonin neuron precursors. J Neurosci. 2008;28:12748–12758. doi: 10.1523/JNEUROSCI.4349-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- Lima FB, Bethea CL. Ovarian steroids decrease DNA fragmentation in the serotonin neurons of non-injured rhesus macaques. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima FB, Centeno ML, Costa ME, Reddy AP, Cameron JL, Bethea CL. Stress sensitive female macaques have decreased fifth Ewing variant (Fev) and serotonin-related gene expression that is not reversed by citalopram. Neuroscience. 2009;164:676–691. doi: 10.1016/j.neuroscience.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst AC. Interactions between corticotropin-releasing hormone and serotonin: implications for the aetiology and treatment of anxiety disorders. Handb Exp Pharmacol. 2005:181–204. doi: 10.1007/3-540-28082-0_7. [DOI] [PubMed] [Google Scholar]
- Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010;13:1190–1198. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding,distribution and function in female macaques. Mol Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maki PM, Freeman EW, Greendale GA, Henderson VW, Newhouse PA, Schmidt PJ, Scott NF, Shively CA. Soares CN Summary of the National Institute on Aging-sponsored conference on depressive symptoms and cognitive complaints in the menopausal transition. Menopause. 17:815–822. doi: 10.1097/gme.0b013e3181d763d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruhashi T. An ecological study of troop fissions of Japanese monkeys (Macaca fuscata yaku) on Yakushima Island, Japan. Primates. 1982;23:317–337. [Google Scholar]
- Merens W, Booij L, Haffmans PM, Van der Does AJ. The effects of experimentally lowered serotonin function on emotional information processing and memory in remitted depressed patients. J Psychopharmacol. 2008 doi: 10.1177/0269881107081531. [DOI] [PubMed] [Google Scholar]
- Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci. 2007;32:86–102. [PMC free article] [PubMed] [Google Scholar]
- Mook D, Felger J, Graves F, Wallen K, Wilson ME. Tamoxifen fails to affect central serotonergic tone but increases indices of anxiety in female rhesus macaques. Psychoneuroendocrinology. 2005;30:273–283. doi: 10.1016/j.psyneuen.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Wisner KL, Price JC, Berga SL, Drevets WC, Hanusa BH, Loucks TL, Meltzer CC. Serotonin 1A receptor reductions in postpartum depression: a positron emission tomography study. Fertil Steril. 2008;89:685–692. doi: 10.1016/j.fertnstert.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon MF, Mackey RH, Williams KV, Korytkowski MT, Flory JD, Manuck SB. Low central nervous system serotonergic responsivity is associated with the metabolic syndrome and physical inactivity. J Clin Endocrinol Metab. 2004;89:266–271. doi: 10.1210/jc.2003-031295. [DOI] [PubMed] [Google Scholar]
- Nouspikel T. DNA repair in mammalian cells : Nucleotide excision repair: variations on versatility. Cell Mol Life Sci. 2009;66:994–1009. doi: 10.1007/s00018-009-8737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouspikel T, Hanawalt PC. Terminally differentiated human neurons repair transcribed genes but display attenuated global DNA repair and modulation of repair gene expression. Mol Cell Biol. 2000;20:1562–1570. doi: 10.1128/mcb.20.5.1562-1570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouspikel T, Hanawalt PC. DNA repair in terminally differentiated cells. DNA Repair (Amst) 2002;1:59–75. doi: 10.1016/s1568-7864(01)00005-2. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of 5HT1A autoreceptor messenger ribonucleic acid expression in the dorsal raphe of rhesus macaques. Neuroscience. 1998;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Mol Brain Research. 1998;53:120–129. doi: 10.1016/s0169-328x(97)00286-6. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold M, Batra A, Knobel A, Smolka MN, Zimmer A, Mann K, Solbach C, Reischl G, Schwarzler F, Grunder G, Machulla HJ, Bares R, Heinz A. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [11C]DASB PET study. Mol Psychiatry. 2008;13:606–613. 557. doi: 10.1038/sj.mp.4002149. [DOI] [PubMed] [Google Scholar]
- Rostal DC, Glick BB, Eaton GG, Resko JA. Seasonality of adult male Japanese macaques (Macaca fuscata): androgens and behavior in a confined troop. Horm Behav. 1986;20:452–462. doi: 10.1016/0018-506x(86)90007-3. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Mol Brain Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Yi KD, Yang SH. Role of protein phosphatases and mitochondria in the neuroprotective effects of estrogens. Front Neuroendocrinol. 2009;30:93–105. doi: 10.1016/j.yfrne.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29:2035–2045. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangen T, Mykletun A. Depression and anxiety through the climacteric period: an epidemiological study (HUNT-II). J Psychosom Obstet Gynaecol. 2008;29:125–131. doi: 10.1080/01674820701733945. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Reddy AP, Bethea CL. Neuroprotective actions of ovarian hormones without insult in the raphe region of rhesus macaques. Neuroscience. 2008;154:720–731. doi: 10.1016/j.neuroscience.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kar LD, Javed A, Zhang Y, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissheimer KV, Herod SM, Cameron JL, Bethea CL. Interactions of corticotropin-releasing factor, urocortin and citalopram in a primate model of stress-induced amenorhea. Neuroendocrinology. 2010;92:224–234. doi: 10.1159/000319257. [DOI] [PMC free article] [PubMed] [Google Scholar]