Abstract
In aging men, the prostate gland becomes hyperproliferative and displays a propensity toward carcinoma. Although this hyperproliferative process has been proposed to represent an inappropriate reactivation of an embryonic differentiation program, the regulatory genes responsible for normal prostate development and function are largely undefined. Here we show that the murine Nkx3.1 homeobox gene is the earliest known marker of prostate epithelium during embryogenesis and is subsequently expressed at all stages of prostate differentiation in vivo as well as in tissue recombinants. A null mutation for Nkx3.1 obtained by targeted gene disruption results in defects in prostate ductal morphogenesis and secretory protein production. Notably, Nkx3.1 mutant mice display prostatic epithelial hyperplasia and dysplasia that increases in severity with age. This epithelial hyperplasia and dysplasia also occurs in heterozygous mice, indicating haploinsufficiency for this phenotype. Because human NKX3.1 is known to map to a prostate cancer hot spot, we propose that NKX3.1 is a prostate-specific tumor suppressor gene and that loss of a single allele may predispose to prostate carcinogenesis. The Nkx3.1 mutant mice provide a unique animal model for examining the relationship between normal prostate differentiation and early stages of prostate carcinogenesis.
Keywords: prostate, bulbourethral gland, organogenesis, hyperplasia/dysplasia, haploinsufficiency, tumor suppressor gene
The prostate gland is of paramount importance for human disease due to the increasing incidence of benign prostatic hyperplasia and prostate carcinoma in aging men. Prostate carcinoma now represents the second leading cause of cancer death in American men (Coffey 1992; Landis et al. 1998). Nonetheless, little is known about the molecular factors that contribute to the onset or progression of prostate cancer. A primary impediment for identifying relevant molecular factors has been the paucity of information regarding the mechanisms of normal prostate growth and differentiation. Few regulatory genes are known to be expressed specifically during prostate development or to be required for prostate function.
The prostate is a ductal gland situated at the base of the bladder that contributes secretory proteins to the seminal fluid. At maturity, the prostate is comprised of tall columnar epithelium surrounded by smooth muscle stroma (Cunha et al. 1987; Cunha 1994). Signaling interactions between epithelium and mesenchyme are required for normal prostate growth and differentiation while deranged interactions may contribute to the inappropriate reactivation of cellular proliferation that occurs during aging (McNeal 1978; Hayward et al. 1996). During embryogenesis, inductive signals from the urogenital sinus mesenchyme induce the adjacent epithelium to form prostatic buds (Cunha et al. 1987; Cunha 1994). Postnatally, reciprocal interactions between epithelium and stroma (mesenchyme) are also required for ductal morphogenesis and prostate maturation (Donjacour and Cunha 1988). At all stages of prostate development as well as maturity, these tissue interactions require functional androgen receptors, initially in the mesenchyme and subsequently in the epithelium (Cunha et al. 1987; Cunha 1994). Although it is known that reciprocal signaling interactions are responsible for prostate formation and function, the relevant molecular factors are largely undefined.
Among the few regulatory genes known to be expressed in the prostate, the NKX3.1 homeobox gene is of particular interest because it maps to the minimal region of human chromosome 8p21 (He et al. 1997; Voeller et al. 1997) that undergoes loss of heterozygosity in 60%–80% of prostate tumors (Bergerheim et al. 1991; Bova et al. 1993; Trapman et al. 1994; Cher et al. 1996; Vocke et al. 1996). In this study we investigate the expression and function of murine Nkx3.1 (Bieberich et al. 1996; Sciavolino et al. 1997) in the developing and mature prostate. We show that Nkx3.1 expression during embryogenesis appears to demarcate prospective prostate epithelium prior to prostate formation and continues to mark prostate epithelium during neonatal development, as well as in tissue recombinants. Furthermore, Nkx3.1 is required for prostate function, as null mutants generated by gene targeting display defects in ductal morphogenesis and secretory protein production. Finally, Nkx3.1 regulates prostate epithelial proliferation, as its loss results in epithelial hyperplasia and dysplasia that increases in severity with age, modeling a preneoplastic condition. Taken together, our results link the regulatory actions of Nkx3.1 in normal prostate development and function with its potential role in prostate carcinogenesis.
Results
Restricted expression of Nkx3.1 in adult prostate and bulbourethral glands
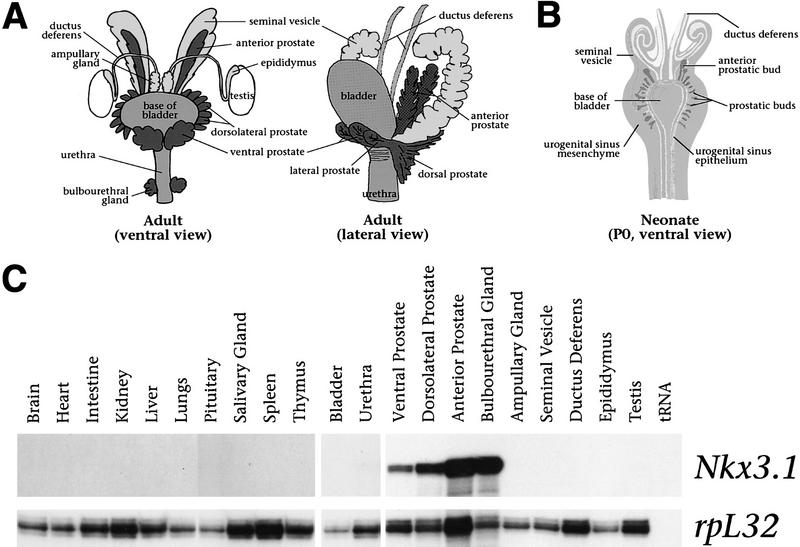
In rodents, the prostate gland consists of three lobes, the anterior prostate (AP; also known as the coagulating gland), the dorsolateral prostate (DLP), and the ventral prostate (VP) (Fig. 1A). These lobes are arranged circumferentially around the urethra and display characteristic patterns of ductal branching and protein secretion (Cunha et al. 1987). In contrast, the adult human prostate lacks discernible lobular organization and, instead, completely envelops the urethra at the base of the bladder (Cunha et al. 1987). The prostatic lobes and bulbourethral gland (BUG; also known as Cowper’s gland in humans) arise from the endodermally derived urogenital sinus epithelium, whereas other ductal tissues of the male urogenital system arise from the mesodermally derived Wolffian ducts (Fig. 1A,B; Cunha et al. 1987).
Figure 1.
Prostate-specific expression of Nkx3.1 in adult male mice. (A) Diagram of the male urogenital system in adult mice, showing the embryological relationships of the tissues (adapted from Cunha et al. 1987; Podlasek et al. 1997). The anterior, dorsolateral, and ventral prostatic lobes, as well as the BUGs (dark gray) are ductal derivatives of the urogenital sinus; the bladder and urethra (medium gray) are its nonductal derivatives. The seminal vesicles, ductus deferens, epididymides, and ampullary glands (light gray) are derived from the Wolffian duct, and the testes (white) from the genital ridge. In the ventral view, only the base of the bladder is shown for clarity. (B) Diagram of the male urogenital system in a newborn mouse [postnatal day 0 (P0; adapted from Cunha et al. 1987)]. By 17.5 dpc, the prostatic buds (dark gray) arise as outbuddings of the urogenital sinus epithelium (white) into the surrounding mesenchyme (medium gray). Also shown are the Wolffianduct-derived seminal vesicles and ductus deferens (light gray). (C) Ribonuclease protection analysis using total RNA (20 μg) from the indicated tissues of adult (8-week) male mice, using a Nkx3.1 antisense riboprobe. The rpL32 riboprobe serves as an internal control for RNA loading.
We examined the distribution of Nkx3.1 transcripts in the adult mouse, with particular emphasis on male urogenital tissues. We found by ribonuclease protection analysis that Nkx3.1 expression was highly restricted to the three prostatic lobes and the BUG (Fig. 1C). In contrast, Nkx3.1 transcripts were not detectable in the seminal vesicle, ampullary gland, ductus deferens, or epididymus, which are derivatives of the Wolffian duct, or in the bladder and urethra, which are nonductal derivatives of the primitive urogenital sinus. Quantitation of Nkx3.1 transcripts demonstrated highest levels in the BUG (normalized to 100%), followed in order by the AP (47%), DLP (26%), and the VP (9%). No expression was detected in other tissues examined, consistent with previous studies (Bieberich et al. 1996; Sciavolino et al. 1997). Thus, these data demonstrate that adult expression of Nkx3.1 is restricted to ductal derivatives of the urogenital sinus.
Nkx3.1 expression defines early stages of prostate development
Given the highly restricted expression of Nkx3.1 in the AP and BUG, we investigated its expression during late stages of embryogenesis, when these tissues arise from the urogenital sinus. We have examined the pattern of Nkx3.1 expression by section in situ hybridization in male mouse embryos from 14.5 through 17.5 days postcoitum (dpc), prior to and during formation of the prostate gland and the BUG (Fig. 2A–M). Our results demonstrate that Nkx3.1 is the earliest known molecular marker of the prostate epithelium and define initial steps in prostate formation.
Figure 2.
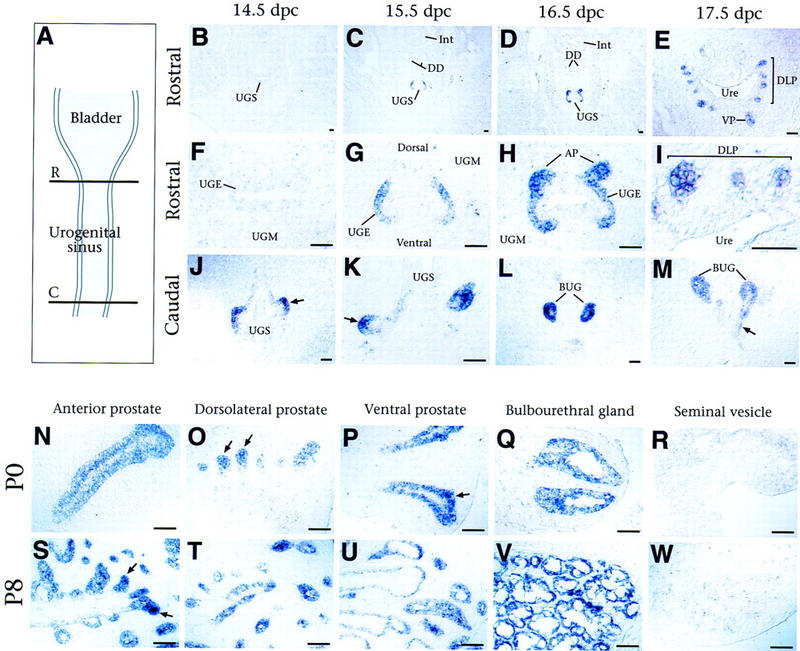
Expression of Nkx3.1 in embryonic and neonatal prostate. (A) Diagram showing transverse planes of section through the urogenital sinus, shown in panels B–M. The rostral region (R) corresponds to the location of the prospective prostatic buds; the caudal region (C) corresponds to the prospective bulbourethral glands. (B–I) In situ hybridization analysis of Nkx3.1 expression in transverse sections through the rostral male urogenital sinus, shown at low (B–E) and high (F–I) power. (B,F) No expression is detected at 14.5 dpc. (C,G) At 15.5 dpc, Nkx3.1 expression is restricted to the lateral urogenital sinus epithelium (UGE) and is excluded from the dorsal and ventral sides (forming the parentheses pattern). (D,H) Nkx3.1 expression continues in the lateral UGE, with elevated expression in the emerging anterior prostatic buds. (E,I) At 17.5 dpc, expression is restricted to the newly formed dorsolateral and ventral prostatic buds and is not found in the prospective urethral epithelium. (J–M) Nkx3.1 expression in transverse sections through the caudal male urogenital sinus. (J) Expression at 14.5 dpc is found in bilateral outpouchings (arrow) from the UGE. (K–M) At 15.5–17.5 dpc, expression is found in the nascent BUGs and the ducts (arrow in M) that join them to the prospective urethra. (N–W) Nkx3.1 expression in isolated tissues from male mice at P0 and P8; staining is more intense at the ends of the outgrowing prostatic ducts (arrows in O, P, and S). (AP) Anterior prostate; (BUG) bulbourethral gland; (C) caudal; (DD) ductus deferens; (DLP) dorsolateral prostate; (Int) large intestine; (R) rostral; (UGE) urogenital sinus epithelium; (UGM) urogenital sinus mesenchyme; (UGS) urogenital sinus; (Ure) urethra; (VP) ventral prostate. Scale bar, 50 μm.
During mid-gestation, the primitive urogenital sinus originates from the terminal hindgut through the division of the cloaca by the urorectal septum. The terminal regions of the primitive urogenital sinus form the urinary bladder and the penile urethra. The prostate gland and the BUG are formed from the intermediate region, which we refer to as the urogenital sinus. The prostatic lobes arise from the rostral urogenital sinus at ∼17.5 dpc, whereas the BUG arise from its caudal end at ∼14.5 dpc.
In the rostral urogenital sinus, Nkx3.1 expression is first detected at 15.5 dpc, in a characteristic ‘parentheses’ pattern that encompasses the lateral aspects of the urogenital sinus epithelium and is excluded from its dorsal and ventral sides (Fig. 2B,C,F,G). Although the urogenital sinus epithelium is multilayered at this stage, Nkx3.1 is only expressed in the basal layer and not in the suprabasal layers (Fig. 2G). At 16.5 dpc, this parentheses pattern of expression becomes more intense at its dorsal boundaries, where the buds of the anterior prostate emerge (Fig. 2D,H). At 17.5 dpc, Nkx3.1 expression becomes restricted to the epithelium of the outgrowing ventral, dorsolateral, and anterior prostatic buds and is excluded from the prospective urethral epithelium (Fig. 2E,I). Thus, Nkx3.1 expression appears to demarcate regions where prostatic buds will arise from the urogenital sinus epithelium.
At the caudal end of the urogenital sinus, Nkx3.1 is expressed at high levels in the epithelial buds of the BUGs (Fig. 2J–M). At 14.5 and 15.5 dpc, this expression was detected in bilateral outpouchings of the urogenital sinus epithelium into the surrounding mesenchyme (Fig. 2J,K). At 16.5 and 17.5 dpc, Nkx3.1 continues to be expressed at high levels in the nascent BUGs, as well as in the epithelial ducts that join the glands to the prospective urethra (Fig. 2L,M).
Nkx3.1 expression is highly restricted within the embryonic male urogenital system to the rostral and caudal ends of the urogenital sinus epithelium; transcripts were not detected at any stage in the bladder or in Wolffian duct derivatives. Furthermore, this expression pattern is male-specific, as Nkx3.1 transcripts were not detected in female urogenital sinus at any stage (data not shown). However, Nkx3.1 expression is found in several nonsexually dimorphic tissues at earlier developmental stages (Sciavolino et al. 1997; Kos et al. 1998; Treier et al. 1998).
In rodents, the prostatic epithelial buds undergo extensive ductal outgrowth and branching during the first 3 weeks of postnatal development. Nkx3.1 expression persists at high levels in the epithelium of all three prostatic lobes at postnatal day (P) 0, 8, and 18 (Fig. 2N–P, S–U; data not shown). Notably, expression appears highest toward the distal ends of the outgrowing ducts, corresponding to regions of active morphogenesis (arrows in Figure 2O,P,S). During this postnatal period, the BUGs also undergo extensive epithelial ductal branching within a capsular stromal layer (Cooke et al. 1987a,b). Nkx3.1 expression continues in the epithelium of the BUGs, although it appears uniform in level throughout the ducts (Fig. 2Q,V). As is the case for embryonic development, Nkx3.1 expression is not found in other tissues of the male urogenital system (Fig. 2R,W; data not shown). Thus, Nkx3.1 is a specific marker for ductal outgrowth and morphogenesis during postnatal growth of the prostate.
Nkx3.1 marks prostate epithelium in tissue recombinants
To further examine the relationship of Nkx3.1 expression to prostate formation, we utilized a tissue recombination system (Fig. 3A). The epithelial–mesenchymal interactions required for prostate formation can be effectively recapitulated in tissue recombinants, such that appropriate combinations will give rise to prostate, identified by ductal histology and the production of characteristic secretory proteins, where different combinations will give rise to bladder or other tissues (Cunha et al. 1987; Cunha 1994). In particular, several nonprostatic epithelia (such as bladder epithelium) will form prostate when combined with the appropriate mesenchyme (such as urogenital sinus mesenchyme).
Figure 3.
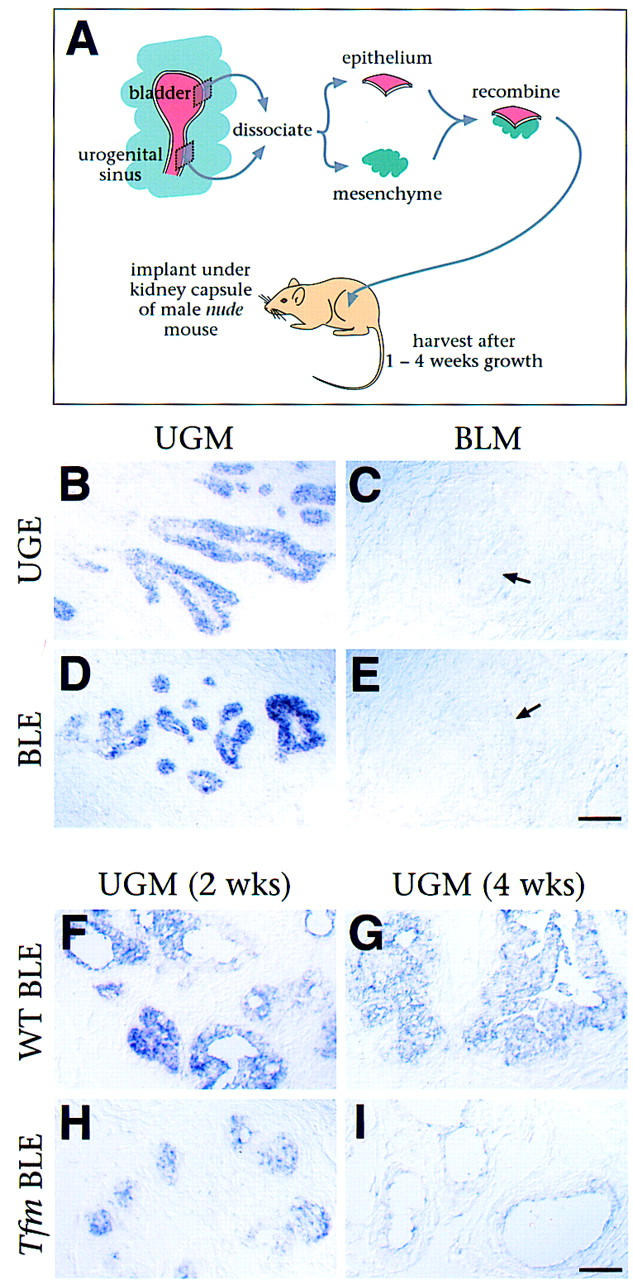
Nkx3.1 marks prostate differentiation in tissue recombinants. (A) Design of the tissue recombination assay. Recombinants of urogenital sinus mesenchyme (UGM) with either urogenital sinus epithelium (UGE) or bladder epithelium (BLE) form prostate; recombinants of bladder mesenchyme (BLM) with either epithelium form bladder. (B–E) In situ hybridization analysis of Nkx3.1 expression in tissue recombinants harvested at 1 week. Expression is found in recombinants that form prostate (UGM + UGE and UGM + BLE) but not in those that form bladder (BLM + UGE and BLM + BLE). Arrows in C and E indicate bladder-like structures that do not express Nkx3.1. (F–I) Nkx3.1 expression in tissue recombinants of UGM with wild-type BLE (WT BLE) vs. UGM with BLE from Tfm mice (Tfm BLE), at 2 and 4 weeks of growth. (B–I) Scale bars, 50 μm.
To ask whether Nkx3.1 is expressed during the acquisition of prostate identity by epithelial tissues that do not form prostate in vivo, we performed tissue recombinations with epithelial and mesenchymal components from embryonic urogenital sinus and neonatal bladder (Fig. 3B–E). Nkx3.1 expression was only detected in tissue recombinants containing urogenital sinus mesenchyme, which induces prostate formation, but not in the tissue recombinants prepared with bladder mesenchyme, which induces bladder. Nkx3.1 was expressed at early stages of prostate formation in the tissue recombinants, when the prostatic ducts have just begun to form. Importantly, Nkx3.1 expression was induced in bladder epithelium combined with urogenital sinus mesenchyme (Fig. 3D). Conversely, expression was not detectable in tissue recombinants of urogenital sinus epithelium with bladder mesenchyme (Fig. 3C), indicating that expression was lost in response to inappropriate mesenchyme. Thus, Nkx3.1 is an early and specific marker of prostate identity in tissue recombinants.
The time course of recombinant growth parallels aspects of prostate development in vivo, as tissue recombinants grown for an extended period resemble mature prostate and produce secretory proteins (Donjacour and Cunha 1993). This maturation process requires androgen receptor signaling in the epithelium (Donjacour and Cunha 1993), as shown using Testicular feminization (Tfm) mutant mice, which lack functional androgen receptors (Lyons and Hawkes 1970). Tissue recombinants prepared with Tfm epithelium initially form prostatic-like ducts, but subsequently fail to mature and express secretory proteins. Consequently, we examined the relationship of Nkx3.1 expression and androgen receptor signaling, using prostatic tissue recombinants with normal (UGM + WT BLE) or defective (UGM + Tfm BLE) epithelial androgen receptor signaling (Fig. 3F–I). At early stages of growth (1 and 2 weeks), Nkx3.1 expression was found in both UGM + WT BLE and UGM + Tfm BLE tissue recombinants (Fig. 3F,H; data not shown), although at lower levels in the latter. At 4 weeks of growth, however, Nkx3.1 expression was greatly reduced or eliminated in UGM + Tfm BLE tissue recombinants (Fig. 3G,I). These findings indicate that epithelial androgen receptors are required for maintenance of Nkx3.1 expression and suggest that Nkx3.1 expression is associated with mature functional prostate.
Targeted disruption of Nkx3.1 results in a defect in prostate ductal morphogenesis
To examine the function of Nkx3.1, we performed targeted gene disruption via homologous recombination in embryonic stem (ES) cells. We constructed a positive–negative replacement vector that would delete the homeodomain and carboxy-terminal protein sequences, and thus should generate a null mutation (Fig. 4A). Following germ-line transmission of the targeted allele, we intercrossed heterozygous animals to recover viable and healthy homozygous adults that lack Nkx3.1 expression (Fig. 4B–E). Although Nkx3.1 homozygotes are fertile, homozygous males have difficulty forming copulatory plugs with advancing age (R. Bhatia-Gaur, C.Abate-Shen, and M.M. Shen, unpubl.)
Figure 4.
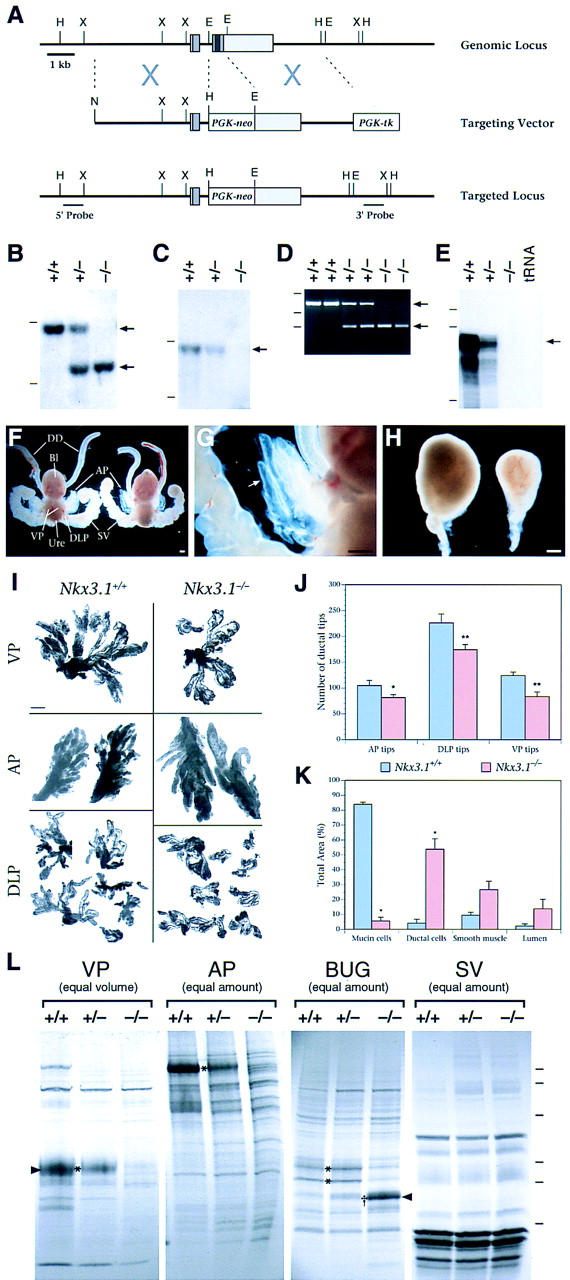
Analysis of Nkx3.1 mutant mice. (A–E) Targeted disruption of Nkx3.1. (A) Strategy for gene disruption. The Nkx3.1 locus comprises two exons (gray boxes), with the coding region (medium gray) contained in both exons and the homeobox in the second exon (dark gray). Homologous recombination with the targeting vector deletes most of the coding region, including the homeobox. The positions of the 5′- and 3′-flanking probes used for Southern blot analysis are shown. (E) EcoRI; (H) HindIII; (N) NotI; (X) XbaI. (B) Southern blot analysis of genomic DNA using the 5′- flanking probe, showing recovery of wild-type (+/+), heterozygous (+/−), and homozygous (−/−) adult mice. This probe detects a 9-kb HindIII wild-type fragment and a 6-kb fragment from the targeted allele (arrows). (C) Southern blot analysis using an internal probe containing the homeobox, confirming its deletion in Nkx3.1 homozygotes. This probe detects a 9-kb HindIII wild-type fragment (arrow) and does not hybridize to the targeted allele. Dashes in B and C indicate positions of markers at 10 and 5 kb. (D) PCR analysis of genomic DNA from wild-type, heterozygous, and homozygous adult mice. Primers (described in Materials and Methods) amplify a 707-bp fragment from wild-type genomic DNA and a 232-bp fragment from the targeted allele (arrows). Dashes indicate positions of markers at 1018, 506, and 220 bp. (E) Ribonuclease protection analysis of total RNA from the APs of 8-week-old mice, using an Nkx3.1 antisense riboprobe corresponding to the homeobox. Dashes indicate positions of markers at 220, 201, and 154 nucleotides. (F–H) Morphology of male urogenital tissues from wild-type and Nkx3.1 mutant littermates. (F) Urogenital systems from wild-type (left) and Nkx3.1 homozygote (right) at 8 weeks of age, showing positions of prostatic lobes (AP, DLP, VP), bladder (Bl), ductus deferens (DD), urethra (Ure), and seminal vesicles (SV). (G) Higher-power view of the mutant anterior prostate shown in E, with semitransparent ducts (arrow). (H) BUGs from wild-type (left) and Nkx3.1 homozygote (right) at 6 weeks of age. Scale bars in F–H, 0.5 mm. (I) Microdissected prostatic lobes from wild-type and Nkx3.1 homozygous mice at 12 weeks of age. Scale bar, 1.0 mm. (J) Quantitation of ductal tips, analyzed as in H. The mean number of ductal tips was significantly smaller in each of the mutant prostatic lobes, at P < 0.1 (*) or P < 0.05 (**). (K) Quantitation of the histological composition of the wild-type and Nkx3.1 mutant BUGs. The total area analyzed was 6.1 × 107 μm2 for wild-type glands and 2.5 × 107 μm2 for mutant glands; significant differences from the wild-type (P < 0.05) are indicated (*). In J and K, error bars represent standard error of the mean (s.e.m.). (L) Analysis of secretory proteins from VP and AP prostatic lobes, BUG, and SV. Protein secretions were collected from tissues of 8-week-old male mice and resolved on a 10%–20% SDS–polyacrylamide gradient gel. (Equal volume) Lanes contain 4 μl of secretory material; (equal amount) lanes contain 10 μg of total protein. Asterisks (*) indicate proteins that are decreased in −/− mice; (†) a protein increased in homozygotes. Arrowheads indicate the protein bands analyzed by microsequencing. Dashes at right mark the positions of molecular mass standards at 102, 81, 46.9, 32.7, 30.2, and 24 kD.
Analysis of homozygous mutant adult males revealed that their urogenital systems were complete, but displayed morphological defects in the prostate gland and the BUG (Fig. 4F–H). Although all three prostatic lobes were present in the homozygous males, the number of prostatic ducts appeared fewer than in wild type. Quantitative analysis of ductal tip number in adult prostatic lobes demonstrated a significant reduction to 60%–75% of wild type (Fig. 4I,J). Moreover, this reduction in ductal tip number is evident as early as 10–11 days of age (data not shown), when ductal branching is nearly complete, but pubertal growth has not yet begun (Sugimura et al. 1986). In contrast, the overall sizes and wet weights of the prostatic lobes in the homozygotes were similar to wild type (data not shown). Because there is reduced ductal branching without an accompanying decrease in overall size, these data indicate reduced ductal complexity in Nkx3.1 mutant prostates.
Nkx3.1 mutant mice display altered production of prostatic secretory proteins
During adult life, the primary function of the prostate is to contribute secretory proteins to the seminal fluid. In our analysis, we observed that the anterior prostate of Nkx3.1 homozygotes frequently displayed a transparent appearance (Fig. 4G), suggesting defects in protein secretion relative to the wild-type gland, which is typically opaque. Consequently, we examined production of prostatic secretory proteins from wild-type, heterozygous, and homozygous mutant mice by SDS–polyacrylamide gel electrophoresis (Fig. 4L).
We found that several major prostatic secretory proteins were greatly reduced or eliminated in homozygous Nkx3.1 males (Fig. 4L, asterisks); no differences were observed in seminal vesicles used as a negative control. We routinely observed that the prostatic lobes of homozygotes contained significantly less secretory material by volume and concentration than wild-type littermate controls; for example, the total protein concentration of ventral prostate secretions in homozygotes was 2.6-fold reduced relative to wild type (n = 6). To determine the identity of a major altered protein band, we performed microsequencing on a protein that is abundant in wild-type ventral prostate secretions but reduced or eliminated in Nkx3.1 heterozygous and homozygous ventral prostate secretions (Fig. 4L; VP band marked with arrowhead). Sequence analyses revealed that this protein corresponds to the prostatic spermine-binding protein (SBP) precursor (R. Bhatia-Gaur, W. Lane, and C. Abate-Shen, unpubl.), which is the major secretory component of the ventral prostate (Mills et al. 1987). These findings demonstrate a profound defect in the production of specific prostatic secretory proteins in Nkx3.1 mutant mice.
The BUG of Nkx3.1 mutants displays altered cellular differentiation
In Nkx3.1 mutant males, the BUGs displayed a marked reduction in overall size and cellular composition relative to wild-type controls (Fig. 4H,K; Fig. 5A–D). In particular, these glands were dramatically reduced in wet weight compared to wild type [14.4 ± 2.4 mg (n = 10) vs. 32.2 ± 2.1 mg (n = 6)]. Furthermore, whereas the wild-type (and heterozygous) BUGs are primarily composed of mucin-producing cells, the homozygous mutant glands show a dramatic loss of these cells, and are instead composed primarily of ductal cells (Fig. 5A–D). Quantitative analysis demonstrated a 15-fold reduction of mucin cells in the homozygote relative to the wild type, and a corresponding 11-fold increase in ductal cells (Fig. 4K). The abundant ductal cells in Nkx3.1 mutants resemble a minor constituent of the wild-type BUG that is primarily found near the neck of the gland (A.A. Donjacour, R.D. Cardiff, G.R. Cunha, C. Abate-Shen, and M.M. Shen, unpubl.).
Figure 5.
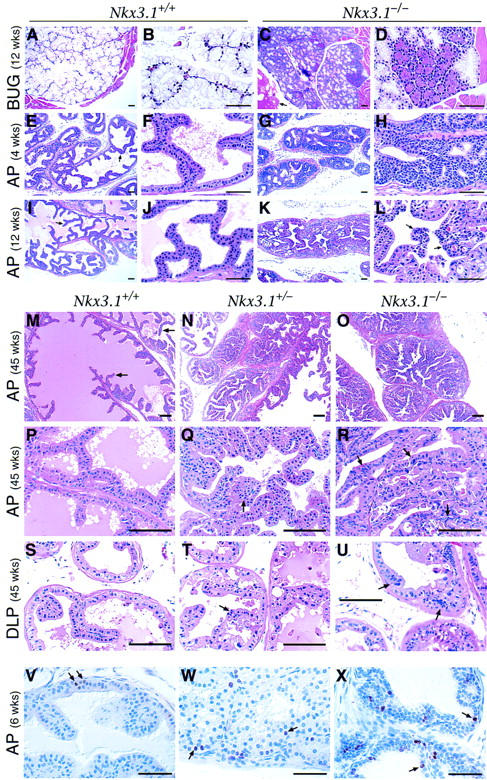
Histology of Nkx3.1 mutant mice. (A–U) H&E staining of paraffin sections of BUG, AP, and DLP in wild-type (Nkx3.1+/+), heterozygous (Nkx3.1+/−), and homozygous (Nkx3.1−/−) mice at 4, 12, and 45 weeks of age. (A–D) At 12 weeks of age, the wild-type BUG (A,B) contains differentiated mucin-producing cells; while the homozygous gland (C,D) largely contains cells with ductal morphology. (E–H) At 4 weeks of age, the wild-type anterior prostate (E,F) contains immature columnar epithelial cells arranged in characteristic papillary tufts (arrow); the homozygous anterior prostate (G,H) contains a multilayered hyperplastic epithelium, with little lumenal space. (I–L) At 12 weeks of age, the wild-type anterior prostate (I,J) contains differentiated columnar epithelial cells with lumenal spaces filled with secretions (lightly staining eosinophilic material). The homozygous AP (K,L) contains hyperplastic epithelium with mildly dysplastic regions (arrows), and little secretory material. (M–R) At 45 weeks of age, the wild-type AP (M,P) contains tall columnar epithelium arranged in papillary tufts (arrow), the heterozygous AP (N,Q) contains hyperplastic epithelium with mildly dysplastic regions (arrow) and reduced lumenal space and secretory protein, and the homozygous AP (O,R) contains severely hyperplastic epithelium and regions of dysplasia (arrows). (S–U) At 45 weeks of age, the wild-type DLP (S) contains columnar epithelium and lumenal secretions, the heterozygous DLP (T) contains areas of mild dysplasia (arrow), and the homozygous DLP (U) contains severely dysplastic epithelium (arrows). (V–X) Ki67 immunoreactivity in the anterior prostates of wild-type (V), heterozygous (W), and homozygous (X) Nkx3.1 mice at 6 weeks of age. Arrows indicate Ki67-labeled nuclei. In total, 55 Ki67-labeled nuclei were observed out of 3767 total nuclei (1.5%) in wild type; 207 of 2991 (6.9%) in heterozygotes; and 315 of 3573 (8.8%) in homozygotes. (A–L and V–X) Scale bars, 50 μm; (M–U) scale bars, 100 μm.
Secretory protein production was also significantly altered in the Nkx3.1 homozygous BUG. In particular, we observed a novel protein species in the secretions from mutant glands (Fig. 4L, dagger), as well as reduced levels of wild-type secretory proteins (Fig. 4L, asterisks). Microsequence analysis of this novel secretory protein (R. Bhatia-Gaur, W. Lane, and C. Abate-Shen, unpubl.) revealed that it corresponds to p20, an abundant component of salivary gland secretion that is related to the rat common salivary protein 1 (CSP1) (Girard et al. 1993; Bekhor et al. 1994). Taken together, these observations demonstrate that Nkx3.1 is essential for the appropriate differentiation and secretory function of the BUG, and suggest that its loss converts a mucin-producing tissue into a ductal tissue.
Nkx3.1 homozygous and heterozygous mice display prostatic epithelial hyperplasia and dysplasia
The most notable phenotype of the Nkx3.1 mutant prostatic lobes is the histological appearance of epithelial hyperplasia and dysplasia, which becomes increasingly severe with advancing age. In wild-type adult mice, the prostate contains a simple tall columnar epithelium, with each prostatic lobe displaying a characteristic histological appearance. In particular, the epithelium of the anterior prostate forms distinct papillary tufts that are apparent by 4 weeks of age (during puberty) and continue to form throughout adult life (Fig. 5E,F,I,J,M,P). In contrast, as early as 4 weeks of age, the anterior prostate of homozygous Nkx3.1 mutants contains a multilayered hyperplastic epithelium with relatively normal nuclear morphology (Fig. 5G,H). By 12 weeks of age, the anterior prostate epithelium of homozygotes also contains dysplastic regions of epithelium showing variation in nuclear size and shape as well as abnormal mitotic figures, with a corresponding loss of lumenal space and secretory material (Fig. 5K,L). This hyperplastic growth may account for why prostatic lobes of Nkx3.1 mutants have a reduced number of ducts yet are not reduced in wet weight (Fig. 4I,J; data not shown).
At 1 year of age, which represents the oldest mice analyzed to date, the anterior prostate of homozygotes displays extensive hyperplastic epithelium with focal areas that are severely dysplastic (Fig. 5O,R), although no overt tumors have yet been observed. Notably, a similar but less severe hyperplastic and dysplastic epithelium is observed in heterozygous Nkx3.1 mutants, indicating haploinsufficiency for this phenotype (Fig. 5N,Q). Furthermore, at 1 year of age, the dorsolateral prostate of homozygotes displays mild hyperplasia and severe dysplasia (Fig. 5U); the heterozygous dorsolateral prostates are also affected, though less severely (Fig. 5T). Interestingly, no histopathological defect has yet been observed in the ventral prostate (data not shown). Analysis of cellular proliferation using an anti-Ki67 antibody in an experimental cohort at 6 weeks of age demonstrated a 5.8-fold increase in proliferating cells in the homozygous anterior prostate and a 4.5-fold increase in the heterozygous, as compared with wild type (Fig. 5V–X). These data demonstrate epithelial hyperproliferation in Nkx3.1 homozygotes and heterozygotes, indicating that the observed cytological and morphological changes model a preneoplastic condition.
Discussion
Our analysis of Nkx3.1 provides a molecular link between the mechanisms that control normal prostate differentiation and those that lead to deregulated epithelial proliferation during prostate carcinogenesis. Thus, we have shown that Nkx3.1 is essential for normal morphogenesis and function of the prostate, whereas its inactivation leads to prostatic epithelial hyperplasia and dysplasia that model a preneoplastic condition (Fig. 6). Taken together with the observation that human NKX3.1 maps to the minimal region of chromosome 8p21 that undergoes loss of heterozygosity in prostate tumors (He et al. 1997; Voeller et al. 1997), we propose that NKX3.1 maintains the differentiated state of normal prostate, whereas its loss represents a predisposing event for prostate carcinogenesis.
Figure 6.
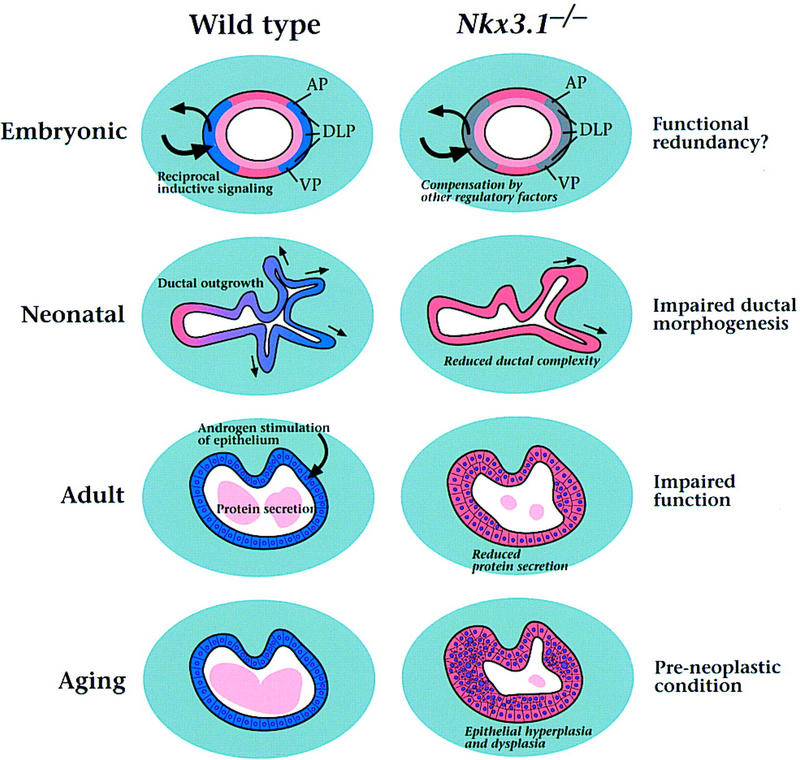
Model for Nkx3.1 activities in prostate development, maturation, and carcinogenesis. The model is described in the text; expression of Nkx3.1 is shown in blue.
Nkx3.1 expression defines early events in prostate formation
Little is known about the early events of prostate formation and the molecular pathways involved in this process. Until now, it has been presumed that signals from the urogenital sinus mesenchyme are solely responsible for inducing the epithelium to form prostatic buds. However, we have found that Nkx3.1 expression marks prospective prostate epithelium 2 days prior to the appearance of prostatic buds, suggesting that the urogenital sinus epithelium has a differential capacity to respond to mesenchymal signals before overt morphogenesis occurs. In particular, the parentheses expression pattern of Nkx3.1 defines zones of urogenital sinus epithelium, such that the dorsal boundaries correspond to the prospective anterior prostate, the intermediate regions to the dorsolateral prostate, and the ventral boundaries to the ventral prostate (Fig. 2, cf. G with H and E; Fig. 6). Thus, we speculate that Nkx3.1 expression reveals a prepatterning of the urogenital sinus epithelium into distinct prostatic and nonprostatic regions.
Although Nkx3.1 is the earliest known differentiation marker of the prostate epithelium, it must cooperate with other regulatory genes, as its loss of function does not result in complete failure of prostate formation (Fig. 6). Among other putative transcription factors, posterior members of the HoxD cluster are known to be expressed in adult prostate and are required for correct prostate morphogenesis (Oefelein et al. 1996; Podlasek et al. 1997). Among secreted signaling molecules, Sonic hedgehog (Shh) is known to regulate Nkx3.1 expression during somite formation (Kos et al. 1998). In preliminary studies, we have observed Shh expression in urogenital sinus epithelium prior to prostatic bud formation (J. Bush, C. Abate-Shen and M.M. Shen, unpubl.). Our description of Nkx3.1 expression provides a foundation for future studies to identify other regulatory components responsible for prostate formation.
Roles for Nkx3.1 in prostate differentiation and function
Nkx3.1 expression is associated with all aspects of embryonic prostate development, neonatal differentiation, and adult function (Fig. 6). In many respects, the expression pattern of Nkx3.1 and the phenotype of mutant mice are analogous to those of other vertebrate Nkx homeobox genes. For example, Nkx2.5 is expressed in precardiac mesoderm and in the developing heart, and null mutation results in defects in cardiac looping morphogenesis and myogenesis (Lints et al. 1993; Lyons et al. 1995). Similarly, Nkx2.1 is expressed during lung development, and targeted disruption leads to severe defects in bronchial branching (Kimura et al. 1996). These Nkx genes are expressed in highly restricted patterns during early stages of tissue specification and subsequent morphogenesis, as is observed for Nkx3.1 expression in prospective as well as differentiating prostate epithelium. Furthermore, mutations in Nkx genes result in defects in morphogenesis as well as in cellular differentiation, analogous to the defects in ductal branching and protein secretion found in Nkx3.1 mutants. Thus, like other Nkx homeobox genes, Nkx3.1 plays an essential role in organogenesis.
In addition to its role in prostate development, Nkx3.1 has a distinct and unique function in the BUG, as Nkx3.1 mutants display a dramatic loss of mucin-producing cells and a corresponding increase of ductal cells. Despite their similar embryological origins, the prostate gland and BUG are morphologically, histologically, and functionally distinct. Whereas the prostatic lobes are comprised of tall columnar epithelium surrounded by smooth muscle stroma, the BUG primarily consists of mucin-producing cells within a skeletal muscle capsule. Notably, the epithelium of the prostate, but not that of the BUG, is highly susceptible to hyperplastic growth and carcinogenesis. Accordingly, loss of Nkx3.1 function results in a profound alteration in cellular composition but does not lead to hyperplastic growth of the bulbourethral epithelium.
Prostate organogenesis is intimately associated with a requirement for androgen signaling from the earliest stages of prostate formation through mature function. During embryogenesis, mesenchymal androgen receptors are required for prostate formation (Cunha et al. 1987), whereas during adulthood, epithelial androgen receptors are required for secretory protein production (Donjacour and Cunha 1993). Our results indicate that androgen receptor signaling in the prostate epithelium is not required for the initiation of Nkx3.1 expression, as its expression precedes the appearance of functional epithelial androgen receptors (Takeda and Chang 1991). However, the absence of Nkx3.1 expression in the female urogenital system implies that mesenchymal androgen receptors are indirectly required for initiation of its expression. Furthermore, maintained expression of Nkx3.1 requires androgen receptor signaling, as shown in vivo and in cultured cells (Bieberich et al. 1996; He et al. 1997; Sciavolino et al. 1997; Prescott et al. 1998). Consistent with these observations, Nkx3.1 is expressed at early, but not later, stages in tissue recombinants lacking epithelial androgen receptors (UGM + Tfm BLE). These tissue recombinants do not produce secretory proteins, further underscoring the relationship between Nkx3.1 expression and secretory protein production. Because Nkx3.1 encodes a putative transcription factor, it may regulate the expression of specific secretory proteins in response to androgen receptor signaling.
Potential role for Nkx3.1 in prostate carcinogenesis
In addition to its chromosomal localization to a prostate cancer hot spot, several lines of evidence implicate NKX3.1 as a candidate prostate tumor suppressor gene. Notably, we have shown that Nkx3.1 mutant mice display epithelial hyperplasia and dysplasia, modeling a preneoplastic condition (Fig. 6). This epithelial hyperplasia and dysplasia mimic the time course of prostate cancer progression in human patients, which occurs as a consequence of aging. Furthermore, we have observed that overexpression of human or murine NKX3.1 suppresses growth and tumorigenicity of prostate carcinoma cells in culture (R. Bhatia-Gaur, M. Kim, M.M. Shen, and C. Abate-Shen, unpubl.). At present, there is no evidence for mutations of the NKX3.1 coding region in human prostate tumors (Voeller et al. 1997). However, our analysis of Nkx3.1 heterozygous mice demonstrates haploinsufficiency for the epithelial hyperplasia and dysplasia phenotype. Therefore, loss of a single NKX3.1 allele may be sufficient to promote prostate carcinogenesis in humans. Haploinsufficiency of other tumor suppressor genes has been implicated in cancer progression (Fero et al. 1998). Because candidate tumor suppressor genes are often not mutated in prostate tumor specimens, haploinsufficiency may be of general significance in prostate cancer.
Although many homeobox genes have been implicated in carcinogenesis, Nkx3.1 is unusual in that it is a candidate tumor suppressor gene, rather than an oncogene. We propose that loss of human NKX3.1 is an early event in prostate carcinogenesis that results in a preneoplastic condition, whereas subsequent genetic events promote progression to overt carcinoma. Candidate genetic events that may act in concert with loss of NKX3.1 include loss of MXI1 and/or PTEN, as the corresponding mutant mice display prostatic epithelial hyperplasia and dysplasia, with no overt neoplastic transformation (Di Crisofano et al. 1998; Schreiber-Agus et al. 1998). Thus, the Nkx3.1 mutant mice should serve as an excellent model for recapitulating the molecular events of prostate cancer initiation and for defining downstream genetic events in prostate cancer progression.
Materials and methods
Expression analysis
Ribonuclease protection analyses were performed on total RNA isolated from individually dissected prostatic lobes or other tissues from 8-week-old male virgin Swiss–Webster mice (Taconic), as described (Shen and Leder 1992). The antisense riboprobes correspond to a 286 bp cDNA fragment spanning exons 1 and 2 (Fig. 1C) or a 187-bp fragment from exon 2 that includes the homeobox (Fig. 4E). Quantitation was performed using a PhosphorImager (Molecular Dynamics), and the Nkx3.1 signal was normalized to the L32 ribosomal protein internal control probe (Shen and Leder 1992). Note that the previously reported expression of Nkx3.1 in seminal vesicle (Sciavolino et al. 1997) was likely due to contamination by anterior prostate. For section in situ hybridization, mouse embryos were obtained at 14.5–17.5 dpc (where day 0.5 is defined as noon of the day of the copulatory plug) and sexed by PCR using primers for the Sry gene (Hogan et al. 1994). Neonatal prostatic lobes and other urogenital tissues were dissected individually at P0, P8, and P18. In situ hybridization was carried out as described (Sciavolino et al. 1997), with at least two and usually four specimens from each stage, using a digoxigenin-labeled riboprobe corresponding to a 1-kb EcoRI fragment of the Nkx3.1 cDNA.
For tissue recombination studies, rat urogenital sinus mesenchyme (17.5 dpc) and mouse urogenital sinus mesenchyme and epithelium (15.5 dpc) were obtained as described (Cunha and Donjacour 1987; Higgins et al. 1989). Bladder mesenchyme and epithelium were obtained (Cunha and Donjacour 1987) from adult or P0 wild-type mice, or from homozygous Tfm mice (Lyons and Hawkes 1970). Tissue recombinants were grafted into adult male nude mouse hosts for 1, 2, or 4 weeks (Cunha and Donjacour 1987). Upon harvesting, tissues were processed for in situ hybridization and histology.
Gene targeting
Nkx3.1 genomic clones were isolated from a λFIXII library constructed from 129Sv/J genomic DNA (Stratagene). The targeting vector was constructed in pPNT (Tybulewicz et al. 1991), using a 4.1-kb EcoRI fragment as the 3′ flank, and a 4.5-kb NotI–EcoRI fragment as the 5′ flank. The linearized construct was electroporated into CJ7 ES cells (Swiatek and Gridley 1993), and targeted clones were obtained at a frequency of 4% (3/85). Chimeric males obtained following blastocyst injection were bred with C57Bl/6J females (Jackson Laboratories), and germ-line transmission was obtained from a single targeted ES clone. The targeted allele has been maintained on a hybrid 129/SvImJ and C57Bl/6J strain background, as well as on an inbred 129/SvImJ background. Results shown were obtained using mice in the hybrid background; the prostate phenotype appears similar in the 129/SvImJ inbred background (R. Bhatia-Gaur, C. Abate-Shen, and M.M. Shen, unpubl.).
Genotyping of the Nkx3.1 mutant mice was performed by Southern blot analysis and PCR. The sequence of the primers used for PCR analysis were 5′-GTCTTGGAGAAGAACTCACCATTG-3′ (wild-type Nkx3.1 forward), 5′-TTCCACATACACTTCATTCTCAGT-3′ (mutated forward), and 5′-GCCAACCTGCCTCAATCACTAAGG-3′ (wild-type and mutated reverse).
Analysis of the Nkx3.1 mutant phenotype
Analyses were performed using virgin male mice from P0 through 12 months of age; experimental cohorts were wild-type, heterozygous, and homozygous littermates (Table 1). For analysis of wet weights and ductal tips, male reproductive organs were dissected and bilateral organ pairs weighed (Sugimura et al. 1986; Donjacour et al. 1998). The gross morphology and wet weights of the epididymides, ductus deferens, ampullary glands, seminal vesicles, preputial glands, and testes of adult homozygous mutants were identical to those of wild type (data not shown). Prostatic ductal tips were traced and counted from digitized images. Organ weights and ductal tips were compared by Student’s t-test. To determine the proportion of cell types in the BUG, random images were captured from hematoxylin-and-eosin (H&E) stained sections, and areas were calculated using NIH Image software. We note that the lack of morphological or histological (see below) defects in the testes or in androgen-dependent tissues, such as the ductus deferens and seminal vesicle, indicates that the reduced number of prostatic ductal tips is not due to decreased androgen levels; however, a very subtle defect in androgen production cannot be excluded.
Table 1.
Number of mice analyzed
| Method
|
Age
|
+/+
|
+/−
|
−/−
|
|---|---|---|---|---|
| Prostate ductal tip counting | 1–2 days | 7 | 6 | 9 |
| 10–12 days | 2 | 5 | 7 | |
| 12 weeks | 6 | 16 | 10 | |
| BUG composition | 12 weeks | 5 | 0 | 5 |
| Protein secretion | 8 weeks | 4 | 1 | 4 |
| 12 weeks | 1 | 1 | 1 | |
| 20 weeks | 1 | 0 | 1 | |
| 11–12 months | 2 | 2 | 2 | |
| Histological analysis | 8 days | 1 | 1 | 1 |
| 3–4 weeks | 2 | 3 | 2 | |
| 8 weeks | 6 | 6 | 6 | |
| 12 weeks | 4 | 6 | 4 | |
| 5 months | 1 | 2 | 2 | |
| 11–12 months | 4 | 4 | 4 |
For analysis of secretory proteins from dissected AP, BUG, and seminal vesicle, secretions were collected in PBS containing 1 mm PMSF by gentle squeezing (Donjacour and Cunha 1993). Dorsolateral and ventral prostate secretions were recovered by scoring of the ducts, followed by centrifugation in PBS with 1 mm PMSF. Secretory proteins were resolved on 10%–20% gradient SDS–polyacrylamide gels (Bio-Rad), followed by visualization with Coomassie Brilliant blue. For protein sequence analysis, individual protein bands were isolated from SDS-Polyacrylamide gels, and analysis performed at the Harvard Microchemistry Facility by microcapillary reverse-phase HPLC tandem mass spectrometry (μLC/MS/MS) on a Finnigan LCQ quadrupole ion trap mass spectrometer.
For histological analysis, dissected tissues were fixed in OmniFix 2000 (Aaron Medical Industries, St. Petersburg, FL), and processed for H&E staining. For all cohorts, the prostatic lobes, seminal vesicles, ductus deferens, epididymides, and testes were examined. For one cohort (8 weeks of age), the lungs, brain, liver, kidney, heart, salivary glands, and intestines were also examined and found to have normal histology (data not shown). The primary histological analysis was performed on a nonblinded basis (by R.D. Cardiff); one of us (M.M. Shen) independently reviewed the histological data on a blinded basis, reaching similar conclusions. Cellular proliferation was analyzed in mice at 6 and 20 weeks of age by immunohistochemical staining of formalin-fixed tissues using a rabbit polyclonal anti-Ki67 antibody (Novocastra Laboratories). Ki67-labeled nuclei were quantitated by counting ∼3000 hematoxylin-stained nuclei from high-power microscopic fields.
Acknowledgments
We thank Whitney Banach and Sandy Price for assistance with animal husbandry, Judy Walls for histology, Yu-Ting Yan for advice on design of the gene targeting vector, and Lu Yang for advice on in situ hybridization. Protein microsequencing was performed by William S. Lane and colleagues at the Microchemistry Facility at Harvard University. We also thank Andy McMahon, Frank Rauscher III, Danny Reinberg, Nicole Schreiber-Agus, and Cliff Tabin for comments on the manuscript, and members of the Abate-Shen, Shen, and Cunha laboratories for helpful discussions. This work was supported by National Institutes of Health grant CA76501 to C.A.-S.; U.S. Army Prostate Cancer Research Program grant DAMD17-98-1-8532 to M.M.S.; NIH grants DK52721, CA59831, DK51101, DK51397, DK45861, CA64872, DK52708, and DK47517 to G.R.C.; NIH grants NS36437 and HD34883 to T.G.; NIH grant CA34196 to the Jackson Laboratory; and NIH training grant T32-MH019957 to R.B.-G.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL abate@mbcl.rutgers.edu, mshen@cabm.rutgers.edu; FAX (732) 235-4850.
References
- Bekhor I, Wen Y, Shi S, Hsieh CH, Denny PA, Denny PC. cDNA cloning, sequencing and in situ localization of a transcript specific to both sublingual demilune cells and parotid intercalated duct cells in mouse salivary glands. Arch Oral Biol. 1994;39:1011–1022. doi: 10.1016/0003-9969(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Bergerheim USR, Kunimi K, Collins VP, Ekman P. Deletion mapping of chromosomes 8, 10, and 16 in human prostatic carcinoma. Genes Chromosomes Cancer. 1991;3:215–220. doi: 10.1002/gcc.2870030308. [DOI] [PubMed] [Google Scholar]
- Bieberich CJ, Fujita K, He WW, Jay G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J Biol Chem. 1996;271:31779–31782. doi: 10.1074/jbc.271.50.31779. [DOI] [PubMed] [Google Scholar]
- Bova GS, Carter BS, Bussemakers MJG, Emi M, Fujiwara Y, Kyprianou N, Jacobs SC, Robinson JC, Epstein JI, Walsh PC, Isaacs WB. Homozygous deletion and frequent allelic loss of chromosome 8p22 loci in human prostate cancer. Cancer Res. 1993;53:3869–3873. [PubMed] [Google Scholar]
- Cher ML, Bova GS, Moore DH, Small EJ, Carroll PR, Pin SS, Epstein JI, Isaacs WB, Jensen RH. Genetic alterations in untreated metastases and androgen-independent prostate cancer detected by comparative genomic hybridization and allelotyping. Cancer Res. 1996;56:3091–3102. [PubMed] [Google Scholar]
- Coffey DS. Prostate cancer: An overview of an increasing dilemma. Cancer. 1992;71:880–886. doi: 10.1002/1097-0142(19930201)71:3+<880::aid-cncr2820711403>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Young PF, Cunha GR. Androgen dependence of growth and epithelial morphogenesis in neonatal mouse bulbourethral glands. Endocrinology. 1987a;121:2153–2160. doi: 10.1210/endo-121-6-2153. [DOI] [PubMed] [Google Scholar]
- ————— A new model system for studying androgen-induced growth and morphogenesis in vitro: The bulbourethral gland. Endocrinology. 1987b;121:2161–2170. doi: 10.1210/endo-121-6-2161. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer. 1994;74:1030–1044. doi: 10.1002/1097-0142(19940801)74:3+<1030::aid-cncr2820741510>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Donjacour A. Mesenchymal-epithelial interactions: Technical considerations. Prog Clin Biol Res. 1987;239:273–282. [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- Di Crisofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR. The effect of androgen deprivation on branching morphogenesis in the mouse prostate. Dev Biol. 1988;128:1–14. doi: 10.1016/0012-1606(88)90260-6. [DOI] [PubMed] [Google Scholar]
- ————— Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology. 1993;132:2342–2350. doi: 10.1210/endo.132.6.7684975. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Thomson AA, Cunha GR. Enlargement of the ampullary gland and seminal vesicle but not the prostate in int2/Fgf-3 transgenic mice. Differentiation. 1998;62:227–237. doi: 10.1046/j.1432-0436.1998.6250227.x. [DOI] [PubMed] [Google Scholar]
- Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard LR, Castle AM, Hand AR, Castle JD, Mirels L. Characterization of common salivary protein 1, a product of rat submandibular, sublingual, and parotid glands. J Biol Chem. 1993;268:26592–26601. [PubMed] [Google Scholar]
- Hayward SW, Cunha GR, Dahiya R. Normal development and carcinogenesis of the prostate. A unifying hypothesis. Ann NY Acad Sci. 1996;784:50–62. doi: 10.1111/j.1749-6632.1996.tb16227.x. [DOI] [PubMed] [Google Scholar]
- He WW, Sciavolino PJ, Wing J, Augustus M, Hudson P, Meissner PS, Curtis RT, Shell BK, Bostwick DG, Tindall DJ, Gelmann EP, Abate-Shen C, Carter KC. A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer. Genomics. 1997;43:69–77. doi: 10.1006/geno.1997.4715. [DOI] [PubMed] [Google Scholar]
- Higgins SJ, Young P, Brody JR, Cunha GR. Induction of functional cytodifferentiation in the epithelium of tissue recombinants. I. Homotypic seminal vesicle recombinants. Development. 1989;106:219–234. doi: 10.1242/dev.106.2.219. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: Thyroid-specific enhancer-binding protein is essential for the organogenesis of the Thyroid, lung, ventral forebrain, and pituitary. Genes & Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kos L, Chiang C, Mahon KA. Mediolateral patterning of somites: Multiple axial signals, including Sonic hedgehog, regulate Nkx-3.1 expression. Mech Dev. 1998;70:25–34. doi: 10.1016/s0925-4773(97)00168-8. [DOI] [PubMed] [Google Scholar]
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics. CA– Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: A novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:419–431. doi: 10.1242/dev.119.2.419. [DOI] [PubMed] [Google Scholar]
- Lyons I, M. PL, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes & Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- Lyons MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- McNeal JE. Evolution of benign prostatic enlargement. Invest Urol. 1978;15:340–345. [PubMed] [Google Scholar]
- Mills JS, Needham M, Thompson TC, Parker MG. Androgen-regulated expression of secretory protein synthesis in mouse ventral prostate. Mol Cell Endocrinol. 1987;53:111–118. doi: 10.1016/0303-7207(87)90197-3. [DOI] [PubMed] [Google Scholar]
- Oefelein M, Chin-Chance C, Bushman W. Expression of the homeotic gene Hox-d13 in the developing and adult mouse prostate. J Urol. 1996;155:342–346. [PubMed] [Google Scholar]
- Podlasek CA, Duboule D, Bushman W. Male accessory sex organ morphogenesis is altered by loss of function of Hoxd-13. Dev Dyn. 1997;208:454–465. doi: 10.1002/(SICI)1097-0177(199704)208:4<454::AID-AJA2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Prescott JL, Blok L, Tindall DJ. Isolation and androgen regulation of the human homeobox cDNA, NKX3.1. Prostate. 1998;35:71–80. doi: 10.1002/(sici)1097-0045(19980401)35:1<71::aid-pros10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Schreiber-Agus N, Meng Y, Hoang T, Hou H, Jr, Chen K, Greenberg R, Cordon-Cardo C, Lee HW, DePinho RA. Role of Mxi1 in ageing organ systems and the regulation of normal and neoplastic growth. Nature. 1998;393:483–487. doi: 10.1038/31008. [DOI] [PubMed] [Google Scholar]
- Sciavolino PJ, Abrams EW, Yang L, Austenberg LP, Shen MM, Abate-Shen C. Tissue-specific expression of murine Nkx3.1 in the male urogenital system. Dev Dyn. 1997;209:127–138. doi: 10.1002/(SICI)1097-0177(199705)209:1<127::AID-AJA12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Shen MM, Leder P. Leukemia inhibitory factor is expressed by the preimplantation uterus and selectively blocks primitive ectoderm formation in vitro. Proc Natl Acad Sci. 1992;89:8240–8244. doi: 10.1073/pnas.89.17.8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- Swiatek PJ, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox-20. Genes & Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- Takeda H, Chang C. Immunohistochemical and in-situ hybridization analysis of androgen receptor expression during the development of the mouse prostate gland. J Endocrinol. 1991;129:83–89. doi: 10.1677/joe.0.1290083. [DOI] [PubMed] [Google Scholar]
- Trapman J, Sleddens HF, van der Weiden MM, Dinjens WJ, Konig JJ, Schroder FH, Faber PW, Bosman FT. Loss of heterozygosity of chromosome 8 microsatellite loci implicates a candidate tumor suppressor gene between the loci D8S87 and D8S133 in human prostate cancer. Cancer Res. 1994;54:6061–6064. [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes & Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Vocke CD, Pozzatti RO, Bostwick DG, Florence CD, Jennings SB, Strup SE, Duray PH, Liotta LA, Emmert-Bucke MR, Lineham WM. Analysis of 99 microdissected prostate carcinomas reveals a high frequency of allelic loss on chromosome 8p12-21. Cancer Res. 1996;56:2411–2416. [PubMed] [Google Scholar]
- Voeller HJ, Augustus M, Madike V, Bova GS, Carter KC, Gelmann EP. Coding region of NKX3.1, a prostate-specific homeobox gene on 8p21, is not mutated in human prostate cancers. Cancer Res. 1997;57:4455–4459. [PubMed] [Google Scholar]