Abstract
Death-associated protein kinase (DAPk) was recently suggested by sequence homology to be a member of the ROCO family of proteins. Here, we show that DAPk has a functional ROC (Ras of complex proteins) domain that mediates homo-oligomerization and GTP binding through a defined P-loop motif. Upon binding to GTP, the ROC domain negatively regulates the catalytic activity of DAPk and its cellular effects. Mechanistically, GTP binding enhances an inhibitory autophosphorylation at a distal site that suppresses kinase activity. This study presents a new mechanism of intramolecular signal transduction, by which GTP binding operates in cis to affect the catalytic activity of a distal domain in the protein.
Keywords: GTP-binding proteins, DAP-kinase, ROCO family, LRRK2
Introduction
Death-associated protein kinase (DAPk) is a multidomain Ca2+/calmodulin (CaM)-activated Ser/Thr kinase that participates in various programmed cell death pathways, including apoptosis and autophagy. DAPk functions as a tumour suppressor and has a central role in neuronal cell death (Bialik & Kimchi, 2004). DAPk can be activated by two complementary mechanisms that release the autoinhibition of its CaM-binding domain. The first involves dephosphorylation of an inhibitory autophosphorylation at Ser 308 within the CaM-binding domain (Shohat et al, 2001). The PP2A phosphatase has been shown to dephosphorylate this residue, thereby activating kinase activity (Gozuacik et al, 2008; Guenebeaud et al, 2010; Widau et al, 2010). The second mechanism involves the binding of CaM to the CaM-binding domain, which results in the removal of this inhibitory domain from the catalytic cleft and prevents further Ser 308 autophosphorylation (Cohen et al, 1997; Shohat et al, 2001; de Diego et al, 2010).
In addition to the amino-terminal catalytic domain, which is immediately followed by the CaM regulatory domain, DAPk has several protein–protein interaction modules, including ankyrin repeats and a carboxy-terminal death domain (Bialik & Kimchi, 2006). A putative P-loop motif (amino acids 695–702), overlapping with a region responsible for cytoskeletal localization, has been identified (Cohen et al, 1997), but not yet characterized. Within this region (amino acids 667–1,288), DAPk shares weak yet significant sequence homology with a recently described protein family, the ROCO proteins (Marin et al, 2008; Klein et al, 2009; see also Fig 1F). The ROCO family consists of a group of multidomain proteins, mostly kinases, that are characterized by the presence of two domains that always appear in tandem: the ROC (Ras of complex proteins) domain—a guanosine triphosphate (GTP)ase domain similar to Ras and other small G-proteins—and, immediately downstream, the COR (C-terminal of ROC) domain. ROCO proteins are found in both prokaryotes and eukaryotes. The most well-known is human leucine repeat-rich kinase 2 (LRRK2), a kinase implicated in Parkinson disease (Bosgraaf & Van Haastert, 2003; Marin et al, 2008). The ROC domain of LRRK2 has been extensively studied to determine the functional effects of GTPase activity on the biochemical properties of the kinase (Guo et al, 2007; Ito et al, 2007; Deng et al, 2008; Greggio et al, 2008). GTP binding to the ROC domain activates kinase activity, and several Parkinson-disease mutations that interfere with GTP hydrolysis lead to a hyperactivated kinase. The ROC domain also mediates homodimerization of LRRK2 and heterodimerization with other kinases including DAPk (Klein et al, 2009). Interestingly, the COR domain has been shown to contribute to dimerization of the ROC domain in a prokaryotic LRRK2 homologue (Gotthardt et al, 2008). In addition, Parkinson-disease-associated mutations within the COR domain of LRRK2 were found to affect GTPase activity (Marin et al, 2008; Daniels et al, 2011). The importance of the presence of the proposed ROC–COR domains in DAPk awaits functional confirmation by biochemical and cellular studies.
Figure 1.
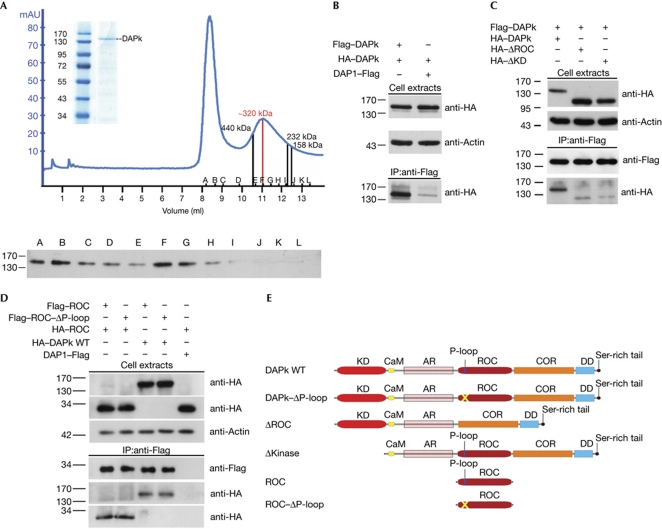
DAPk oligomerizes in cells. (A) Flag–DAPk was immunoprecipitated from HEK293T cells with anti-Flag beads and eluted by competition with Flag peptide. An aliquot of the immunopurified protein was analysed by SDS–polyacrylamide gel electrophoresis stained with GelCode Blue (inset), and the remainder was loaded onto a Superdex200 size-exclusion column. The resulting chromatogram, representing ultraviolet absorbance at 280 nm, is shown. Sizes were determined by the elution profile of known molecular-weight standards. Selected fractions (A–L) were collected and analysed by western blot with Flag antibodies to confirm the presence of DAPk. (B) HEK293T cells were transfected with HA–DAPk and either Flag–DAPk or DAP1–Flag, which was used as a negative control, for 24 h. Cell lysates were immunoprecipitated with Flag antibodies and subjected to western blotting with HA antibodies. Actin was used as a loading control. (C) Cells were co-transfected with Flag–DAPk and either HA–DAPk (WT), HA–ΔROC or HA–ΔKD. Immunoprecipitation and western blot analyses were performed as in B. (D) HEK293T cells were co-transfected with HA–DAPk or HA–ROC along with either Flag–ROC WT or deleted of P-loop (Flag–ROC–ΔP-loop). Immunoprecipitation was performed as in B. (E) Schematic representation of DAPk constructs. AR, ankyrin repeats; CaM, Ca2+/calmodulin-binding region; COR, C-terminal of ROC; DAPk, death-associated protein kinase; DD, death domain; HA, haemagglutinin; IP, immunoprecipitation; KD, kinase domain; ROC, Ras of complex proteins; WT, wild type.
Here, we show that DAPk dimerizes through its ROC and kinase domains. Furthermore, our data indicate that it binds to GTP through the P-loop motif in the ROC domain, and that the ROC–COR fragment shows intrinsic GTPase activity. Upon GTP binding, the ROC domain negatively regulates DAPk catalytic activity and suppresses DAPk-induced cellular effects. Signalling through GTP-binding occurs in cis by increasing Ser 308 inhibitory autophosphorylation in a PP2A-independent manner, thus providing a molecular mechanism for GTP-mediated intramolecular signalling affecting a distal domain of the protein.
Results
DAPk homodimerizes via its ROC and kinase domains
As some members of the ROCO family—including LRRK1 and LRRK2—form dimers (Greggio et al, 2008; Klein et al, 2009), the ability of DAPk to homodimerize was assessed. The nature of DAPk complexes in native conditions was examined by size-exclusion chromatography of immunopurified Flag–DAPk, followed by western blotting analysis of the relevant fractions with Flag antibodies. Flag–DAPk eluted from the Superdex200 column in a defined peak corresponding to approximately 320 kDa (Fig 1A), consistent with dimers of the 160 kDa protein. Notably, the absence of a peak at 160 kDa excluded the existence of monomers under native conditions, and the high purification profile of the input protein (Fig 1A, inset) makes it unlikely that this peak represents complexes of DAPk with other proteins. The first peak, which elutes close to the void volume, might correspond to aggregates of DAPk formed during purification.
Next, the regions in DAPk essential for homodimerization were mapped. HEK293T cells were co-transfected with two DAPk constructs tagged with Flag and haemagglutinin (HA), respectively, followed by immunoprecipitation analyses. Western blot analysis showed the presence of HA–DAPk in the anti-Flag immune complexes only when the two DAPk constructs were co-expressed, and not when DAP1–Flag, used as a negative control, was co-expressed with HA–DAPk (Fig 1B). The contribution of the ROC and catalytic domains to the homodimerization was then assessed. The choice of the catalytic domain was prompted by previous studies showing that it tends to form dimers in vitro (Zimmermann et al, 2010) and to mediate heterodimerization with the highly homologous kinase domain of ZIPk in cells (Shani et al, 2004). Deletion of either the ROC (amino acids 667–954) or kinase (amino acids 1–275) domains reduced the interaction with full-length DAPk (Fig 1C). Thus, both the ROC and kinase domains mediate homodimerization of DAPk. In addition, Flag-tagged ROC domain alone specifically immunoprecipitated both full-length HA–DAPk and HA–ROC (Fig 1D). The P-loop motif was not necessary for ROC/DAPk or ROC/ROC interaction in cells, as both HA–ROC and full-length DAPk co-immunoprecipitated with Flag–ROC deleted of the P-loop motif (ΔP-loop, amino acids 695–702) to the same extent that they did with wild-type ROC (Fig 1D). Together, these experiments indicate that the ROC domain is one of at least two domains essential for DAPk homodimerization.
GTP binding attenuates enzymatic activity of DAPk
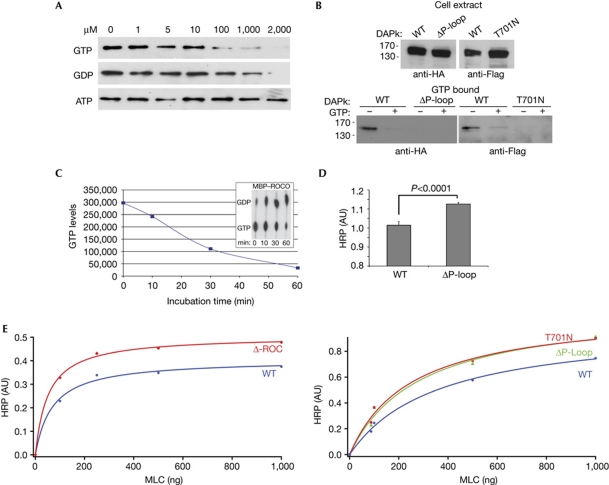
To determine whether the P-loop of DAPk can bind to GTP, lysates from HEK293T cells overexpressing DAPk wild type were incubated with GTP-conjugated agarose beads. Western blots of bound proteins showed that DAPk wild type bound specifically to GTP, as competition with increasing concentrations of free GTP or guanosine diphosphate (GDP), but not adenosine triphosphate (ATP), reduced the interaction (Fig 2A). Furthermore, higher concentrations of GDP were required for titration of DAPk from the GTP-conjugated agarose beads, implying that DAPk has a higher affinity for GTP than GDP. As expected, deletion of the P-loop abrogated interaction with the GTP beads, as did mutation of a single residue within the P-loop, Thr 701 to Asn (T701N), the equivalent of the T1348N mutation in LRRK2 that abolishes nucleotide binding (Ito et al, 2007; Fig 2B). Thus, DAPk is a GTP-binding protein, and the P-loop is required for this interaction.
Figure 2.
GTP binds to the P-loop and attenuates DAPk catalytic activity. (A) Cell lysates expressing Flag–DAPk WT were incubated with GTP-agarose beads. After incubation in the presence of increasing concentrations of free GTP, GDP or ATP, bound proteins were subjected to western blot analysis with Flag antibody. (B) Cell lysates expressing HA–DAPk WT or ΔP-loop (left) or Flag–DAPk WT or T701N (right) were incubated with GTP-agarose beads. After incubation in the presence or absence of free GTP (2 mM), bound proteins were subjected to western blot analysis with HA or Flag antibodies. (C) MBP–ROCO was assayed for GTPase activity in the presence of [α-32P]GTP. Aliquots representing the indicated time points were resolved by thin layer chromatography (inset). The graph represents the loss of [α-32P]GTP along the course of the reaction. (D,E) The catalytic activity of DAPk was assessed by an ELISA assay optimized to detect the phosphorylation of the DAPk substrate MLC on Ser 19. (D) Mean activity±s.d. (n=4) of DAPk WT and ΔP-loop towards non-limiting amounts of MLC (1 μg) on incubation for 3 min, P<0.0001, Student's t-test. (E) The catalytic activity of ΔROC (left) or ΔP-loop and T701N mutant (right) were compared with that of DAPk WT in a Michaelis–Menten plot and the curves were fitted using the method of the least squares. ATP, adenosine triphosphate; AU, arbitrary units; DAPk, death-associated protein kinase; HA, haemagglutinin; HRP, horseradish peroxidase; MBP, maltose-binding protein; ROC, Ras of complex proteins; WT, wild type.
Next, GTP hydrolysis assays were performed to test whether DAPk has intrinsic GTPase activity. Recombinant ROC–COR domains, fused to maltose-binding protein (MBP–ROCO), were produced in bacteria to achieve the high concentrations required for in vitro assessment of intrinsic GTPase activity (supplementary Fig S1A online). MBP–ROCO could bind to GTP-conjugated agarose beads (supplementary Fig S1B online), consistent with previous reports showing that the ROC–COR fragment of the prokaryotic LRRK2 homologue retains the correct fold and biochemical function (Gotthardt et al, 2008). Next, GTPase assays were performed with MBP–ROCO yielding clear time-dependent GTP hydrolysis (Fig 2C). Notably, MBP that was fused to the non-relevant protein AcPS, used as a negative control, did not induce detectable GTP hydrolysis; H-Ras was used as a positive control in these assays (supplementary Fig S1C online). These experiments indicate that DAPk is a GTP-binding protein with intrinsic GTPase activity.
The importance of the ROC domain, and in particular its GTP-binding ability, for the enzymatic activity of DAPk was assessed by in vitro kinase assays using the myosin II regulatory light chain (MLC) substrate. The activity of wild-type DAPk was compared with that of DAPk ΔP-loop under non-limiting MLC concentrations; enhanced catalytic activity of the mutant, compared with the wild-type protein, was observed (Fig 2D). Michaelis–Menten kinetics of DAPk wild type and either ΔROC, ΔP-loop or T701N indicated that all three mutants showed increased kinase activity, indicated by a higher Vmax (Fig 2E). Thus, the ROC domain, specifically its GTP-binding activity, presumably functions to negatively regulate DAPk catalytic activity.
GTP-binding promotes in cis autophosphorylation
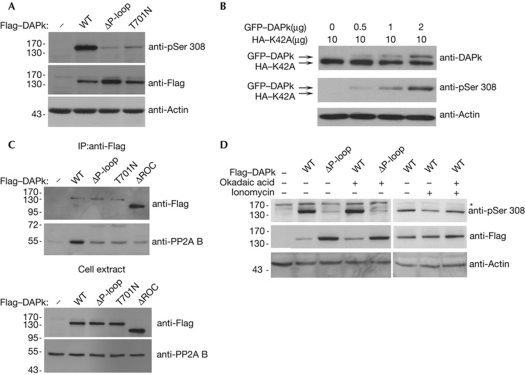
To investigate the mechanism by which GTP binding to the P-loop reduces DAPk catalytic activity, the phosphorylation state of Ser 308, which is inversely linked to kinase activity, was compared between DAPk wild type, ΔP-loop and the T701N mutant, using phosphoSer 308 antibodies. Interestingly, Ser 308 phosphorylation was strongly inhibited in the two GTP-binding-deficient mutants, in comparison to the wild-type protein (Fig 3A). This suggests that GTP binding imposes its inhibitory effect on catalytic activity through regulation of the phosphorylation state of Ser 308.
Figure 3.
GTP binding to the P-loop promotes in cis Ser 308 negative autophosphorylation. (A) HEK293T cells transfected with Flag–DAPk WT, ΔP-loop or T701N mutants were analysed by western blot with phosphoSer 308(pSer 308)-specific and Flag antibodies. Actin was used as a loading control. (B) HEK293T cells were co-transfected with catalytically inactive DAPk (HA–K42A) and increasing amounts of GFP–DAPk, and analysed by western blot with antibodies against total DAPk and pSer 308. Actin was used as a loading control. (C) Flag–DAPk WT or the indicated mutants were immunoprecipitated from HEK293T cells with Flag antibodies and blotted for Flag and co-precipitating endogenous PP2A B’ subunit. (D) HEK293T cells were co-transfected with Flag–DAPk WT or ΔP-loop mutant, 24 h post-transfection cells were treated with 100% ethanol or 100 nM okadaic acid for 50 min. Where indicated, 10 min after okadaic-acid addition, cells were treated with 10 μM ionomycin for the remaining time. DAPk proteins were analysed by western blot with Flag and pSer 308 antibodies. Actin was used as a loading control. DAPk, death-associated protein kinase; GFP, green fluorescent protein; HA, haemagglutinin; IP, immunoprecipitation; ROC, Ras of complex proteins; WT, wild type.
To determine whether this occurs by the action of one DAPk molecule on its DAPk partner within the homodimer (in trans) or intramolecularly (in cis), HEK293T cells were co-transfected with catalytically inactive HA–DAPk (K42A) and increasing amounts of active green fluorescent protein (GFP)–DAPk. Cell extracts were then analysed by western blot for total DAPk and phosphorylated DAPk, and HA–DAPk K42A and GFP–DAPk were distinguished on the basis of the difference in molecular weight. Increasing phosphorylation on Ser 308 was observed in wild-type GFP–DAPk, corresponding to increasing quantities of transfected plasmid. By contrast, HA–DAPk K42A did not show any phosphorylation at Ser 308 when overexpressed alone or when co-expressed with GFP–DAPk (Fig 3B). Although Ser 308 phosphorylation of GFP–DAPk does not distinguish between inter- and intramolecular activity, the only way to achieve Ser 308 phosphorylation in the catalytically silent mutant is by an in trans mechanism in which the GFP–DAPk subunit within the heterodimer phosphorylates the mutant subunit. Hence, the lack of Ser 308 phosphorylation in HA–DAPk K42A excludes the possibility that a trans-phosphorylation event occurs and supports the hypothesis that autophosphorylation occurs in cis only.
The steady-state level of phosphoSer 308 is controlled by an equilibrium between autophosphorylation and PP2A-mediated dephosphorylation. PP2A is recruited by its regulatory B’ subunit to a region of DAPk that overlaps with the ROC and COR domains (Gozuacik et al, 2008; Widau et al, 2010). Therefore, the reduced phosphorylation at Ser 308 in the GTP-binding-deficient mutants could reflect either: increased recruitment of the B’ subunit to DAPk (supplementary Fig S2 online, model A); enhanced access of a constitutively bound PP2A to the Ser 308 phosphorylation site due to conformational changes (supplementary Fig S2 online, model B); or reduced autophosphorylation at Ser 308 (supplementary Fig S2 online, model C). To distinguish between these models, co-immunoprecipitation experiments of endogenous PP2A B’ subunit with Flag-tagged DAPk wild type or the two GTP-binding-deficient mutants, ΔP-loop and T701N, were performed (Fig 3C). Binding of the B’ subunit to either GTP-binding-deficient mutant did not increase, and even decreased in comparison to wild-type DAPk, thus excluding the first model. In fact, the ROC domain provides a main PP2A docking site, as binding of the B’ subunit to DAPk ΔROC was dramatically reduced compared to wild-type DAPk, despite its greater abundance (Fig 3C). Furthermore, treatment with the PP2A inhibitor okadaic acid for 50 min—or 2 and 3 h—failed to restore Ser 308 phosphorylation levels of ΔP-loop (Fig 3D and data not shown). As a control, okadaic-acid treatment prevented ionomycin-induced reduction in Ser 308 phosphorylation of wild-type DAPk, which was previously shown to depend on PP2A activity (Gozuacik et al, 2008; Fig 3D). This result excludes the second model and supports a PP2A-independent model, in which GTP mediates regulation of the phosphorylation event itself through a possible conformational change in the catalytic domain that favors accessibility of Ser 308 to the catalytic cleft (model C in supplementary Fig S2 online).
P-loop deletion enhances the cellular activity of DAPk
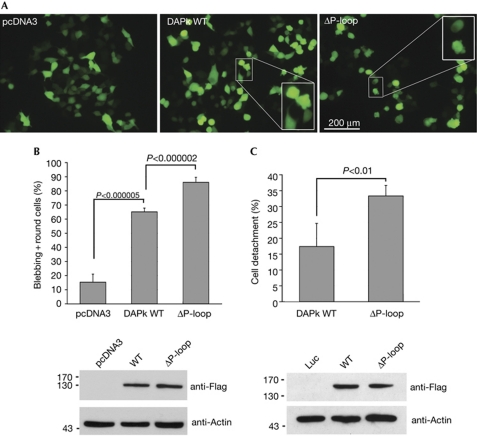
The importance of GTP binding for the functional activity of DAPk within cells was then assessed by transfection of HEK293T cells with either DAPk wild type or ΔP-loop mutant, followed by two assays that quantify the outcome of DAPk effects. These are the induction of membrane blebbing and cell detachment, which have been shown to involve kinase-dependent functions of DAPk: activation of myosin through phosphorylation of MLC and inhibition of integrin function, respectively (Bialik & Kimchi, 2006). Cells transfected with the ΔP-loop mutant showed more membrane blebbing and cell rounding than cells that overexpressed wild-type DAPk (Fig 4A,B). Similarly, ΔP-loop led to increased cell detachment in comparison to the wild-type protein (Fig 4C). Thus, the GTP status of the ROC domain affects cellular functions of DAPK, and consistent with the increased kinase activity observed in vitro, deletion of the P-loop enhanced kinase-dependent cellular effects.
Figure 4.
GTP binding to the P-loop attenuates functional activity of DAPk. (A) Representative fluorescent images of HEK293T cells co-transfected with GFP and either DAPk WT, ΔP-loop or empty vector (pcDNA3). Insets show enlargements of boxed regions. (B) Quantitation of the extent of membrane blebbing and cell rounding seen in A. Each of the three DNA plasmids was transfected independently into three plates. A total of 300 cells were counted separately in each plate. Data represent mean±s.d. calculated from triplicates of 300 scored cells, P-values are calculated from Student's t-test. (C) HEK293T cells were co-transfected with GFP and either DAPk WT, ΔP-loop or luciferase (Luc), as a control, and the extent of cell detachment 24 h post-transfection, as a percentage of luciferase-transfected cells, was quantified. Data represent mean±s.d. (n=3), P-values are calculated from Student's t-test. Western blots in B and C show expression of DAPk constructs. All experiments were repeated at least three times with similar results. DAPk, death-associated protein kinase; GFP, green fluorescent protein; WT, wild type.
Discussion
Our study shows the functional significance of the ROC–COR domains of DAPk, and proves that it is a bona fide G-protein. Consistent with previous results (Klein et al, 2009), we show that DAPk forms dimers and not monomers under native conditions, and demonstrate that both the ROC and kinase domains are crucial for the self-association of the full-length DAPk protein. A basic loop in the catalytic domain, conserved in all members of the DAPk family, might contribute to self-assembly through the kinase domains, as it has also been shown to mediate heterodimerization between DAPk and ZIPk, and homodimerization of DAPk (Shani et al, 2004; Zimmermann et al, 2010). In addition, the ROC fragment alone can bind to full-length DAPk in cells, thus confirming the contribution of at least two regions to homodimerization. This is similar to LRRK2, in which ROC–COR domains are not the exclusive interacting modules (Greggio et al, 2008; Klein et al, 2009). Interestingly, despite the presence of DAPk dimers, autophosphorylation of Ser 308 is an in cis GTP-mediated intramolecular event. Deletion of the ROC domain produces a more-active DAPk, indicating that dimerization of this region is not a prerequisite for catalytic activity. Yet, dimerization might be crucial for GTPase activity, and in this manner indirectly regulate kinase activity, as has been shown for LRRK2 and other ROCO proteins (Deng et al, 2008; Gotthardt et al, 2008; Greggio et al, 2008; Daniels et al, 2011).
DAPk preferentially binds to GTP over GDP through the P-loop in the ROC domain and, the ROC–COR fragment has intrinsic GTPase activity. Moreover, GTP binding negatively affects the catalytic activity of DAPk and, most importantly, its function in cells. The effect on DAPk cellular activity is more pronounced, probably reflecting additional levels of regulation that synergize and/or amplify the GTP-induced mechanism within the cell. Our results suggest that the GTP-bound ROC–COR domains maintain DAPk in a less-active state at basal conditions. Furthermore, GTP binding and hydrolysis might be regulated within the cell by the activity of a putative guanine nucleotide exchange factor (GEF) and/or GTPase-activator protein (GAP). Future investigation is required to identify such regulators and to determine whether GTP hydrolysis is triggered in response to stress signals to activate DAPk in vivo.
At the mechanistic level, GTP-binding promotes Ser 308 autophosphorylation within the distal CaM-binding domain. This does not result from decreased recruitment or activation of the PP2A phosphatase, but directly from increased autophosphorylation at this site. As this autophosphorylation is inhibitory, the outcome is reduced catalytic activity. By extrapolation of the effects of the P-loop mutants to the actual GTP/GDP state of the ROC–COR domains in cells, we propose that DAPk is activated by GTP hydrolysis, in contrast to most G-proteins, in which the GTP-bound state is the ‘on’ state. For example, GTP binding to the ROC domain of LRRK2 has been proposed to function as a timer controlling the kinase activity of protein, with the GTP-bound form representing the active state (Guo et al, 2007). This might be explained by the fact that the two kinases differ in the overall structure and organization of their extracatalytic domains, as well as the nature of their catalytic domains. Therefore, after GTP hydrolysis, the consequence of a conformational change for catalytic activity might be different in each case. Together, the data presented here indicate a new mechanism for DAPk regulation, which can fine-tune the activity of this important kinase by controlling a regulatory autophosphorylation in a GTP-binding-dependent manner.
Methods
Most of the methods used are described in the supplementary information online.
Kinase assays. Maxisorp multi-well polystyrene plates (Nunc, Langenselbold, Germany) were incubated at 4 °C overnight with the indicated amounts of MLC, and kinase reactions were performed on the plate. Briefly, kinase buffer (50 mM Hepes, pH 7.5, 20 mM MgCl2, 0.1 mg/ml BSA, 50 mM β-glycerophosphate, 50 μM ATP) containing various concentrations of enzyme was added to the wells. The reactions were stopped at specific time points by addition of 25 mM EDTA. Standard ELISA detection was performed using rabbit antiphospho MLC (Ser 19) polyclonal antibody (Cell Signaling, Danvers, MA, USA) followed by horseradish peroxidase-conjugated goat antirabbit IgG (Jackson ImmunoResearch, West Grove, PA, USA). Where indicated, the horseradish peroxidase read outs were plotted using the Michaelis–Menten equation and the curves were fit by the least squares method.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank J. Hirsch (Tel Aviv University, Israel) for advice and reagents. This work was supported by a grant from Merck-Serono. A.K. is the incumbent of the Helena Rubinstein Chair of Cancer Research.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bialik S, Kimchi A (2004) DAP-kinase as a target for drug design in cancer and diseases associated with accelerated cell death. Semin Cancer Biol 14: 283–294 [DOI] [PubMed] [Google Scholar]
- Bialik S, Kimchi A (2006) The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem 75: 189–210 [DOI] [PubMed] [Google Scholar]
- Bosgraaf L, Van Haastert PJ (2003) Roc, a Ras/GTPase domain in complex proteins. Biochim Biophys Acta 1643: 5–10 [DOI] [PubMed] [Google Scholar]
- Cohen O, Feinstein E, Kimchi A (1997) DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J 16: 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels V et al. (2011) Insight into the mode of action of the LRRK2 Y1699C pathogenic mutant. J Neurochem 116: 304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Diego I, Kuper J, Bakalova N, Kursula P, Wilmanns M (2010) Molecular basis of the death-associated protein kinase-calcium/calmodulin regulator complex. Sci Signal 3: ra6. [DOI] [PubMed] [Google Scholar]
- Deng J, Lewis PA, Greggio E, Sluch E, Beilina A, Cookson MR (2008) Structure of the ROC domain from the Parkinson's disease-associated leucine-rich repeat kinase 2 reveals a dimeric GTPase. Proc Natl Acad Sci USA 105: 1499–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthardt K, Weyand M, Kortholt A, Van Haastert PJM, Wittinghofer A (2008) Structure of the Roc–COR domain tandem of C. tepidum, a prokaryotic homologue of the human LRRK2 Parkinson kinase. EMBO J 27: 2239–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozuacik D, Bialik S, Raveh T, Mitou G, Shohat G, Sabanay H, Mizushima N, Yoshimori T, Kimchi A (2008) DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ 15: 1875–1886 [DOI] [PubMed] [Google Scholar]
- Greggio E et al. (2008) The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem 283: 16906–16914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenebeaud C et al. (2010) The dependence receptor UNC5H2/B triggers apoptosis via PP2A-mediated dephosphorylation of DAP kinase. Mol Cell 40: 863–876 [DOI] [PubMed] [Google Scholar]
- Guo L, Gandhi PN, Wang W, Petersen RB, Wilson-Delfosse AL, Chen SG (2007) The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp Cell Res 313: 3658–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito G, Okai T, Fujino G, Takeda K, Ichijo H, Katada T, Iwatsubo T (2007) GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson's disease. Biochemistry 46: 1380–1388 [DOI] [PubMed] [Google Scholar]
- Klein CL, Rovelli G, Springer W, Schall C, Gasser T, Kahle PJ (2009) Homo- and heterodimerization of ROCO kinases: LRRK2 kinase inhibition by the LRRK2 ROCO fragment. J Neurochem 111: 703–715 [DOI] [PubMed] [Google Scholar]
- Marin I, van Egmond WN, van Haastert PJ (2008) The Roco protein family: a functional perspective. FASEB J 22: 3103–3110 [DOI] [PubMed] [Google Scholar]
- Shani G, Marash L, Gozuacik D, Bialik S, Teitelbaum L, Shohat G, Kimchi A (2004) Death-associated protein kinase phosphorylates ZIP kinase, forming a unique kinase hierarchy to activate its cell death functions. Mol Cell Biol 24: 8611–8626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat G, Spivak-Kroizman T, Cohen O, Bialik S, Shani G, Berrisi H, Eisenstein M, Kimchi A (2001) The pro-apoptotic function of death-associated protein kinase is controlled by a unique inhibitory autophosphorylation-based mechanism. J Biol Chem 276: 47460–47467 [DOI] [PubMed] [Google Scholar]
- Widau RC, Jin Y, Dixon SA, Wadzinski BE, Gallagher PJ (2010) Protein phosphatase 2A (PP2A) holoenzymes regulate death-associated protein kinase (DAPK) in ceramide-induced anoikis. J Biol Chem 285: 13827–13838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M et al. (2010) Homodimerization of the death-associated protein kinase catalytic domain: development of a new small molecule fluorescent reporter. PLoS ONE 5: e14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.