Abstract
The activation of innate and adaptive immunity is always balanced by inhibitory signalling mechanisms to maintain tissue integrity. We have identified the E3 ligase c-Cbl––known for its roles in regulating lymphocyte signalling––as a modulator of dendritic cell activation. In c-Cbl-deficient dendritic cells, Toll-like receptor-induced expression of proinflammatory factors, such as interleukin-12, is increased, correlating with a greater potency of dendritic-cell-based vaccines against established tumours. This proinflammatory phenotype is accompanied by an increase in nuclear factor (NF)-κB activity. In addition, c-Cbl deficiency reduces both p50 and p105 levels, which have been shown to modulate the stimulatory function of NF-κB. Our data indicate that c-Cbl has a crucial, RING-domain-dependent role in regulating dendritic cell maturation, probably by facilitating the regulatory function of p105 and/or p50.
Keywords: c-Cbl, dendritic cell, E3 ubiquitin ligase, p105, IL12p70
Introduction
In mammalian cells, Toll-like receptor (TLR) family members recognize both pathogen-derived molecules and endogenous stress factors (Akira et al, 2006; Beutler, 2009). Although distinct TLR expression patterns exist, several ligands elicit strong immune responses—in probably all leukocyte subsets—including so-called ‘professional’ antigen-presenting cells, such as dendritic cells. To avoid excessive cell activation with potentially harmful sequelae such as autoimmunity or sepsis, TLR activation is modulated (Liew et al, 2005). Accordingly, targeting individual homeostatic regulators, such as SOCS1 or A20, has been shown to augment dendritic cell vaccine efficacy in preclinical models (Shen et al, 2004; Song et al, 2008).
Mammalian Cbl proteins, including c-Cbl, Cbl-b and Cbl-c, are RING-domain-containing E3 ubiquitin ligases. Among them, c-Cbl and Cbl-b are implicated in the regulation of immune responses in several physiological settings (Schmidt & Dikic, 2005; Dale et al, 2009). For example, c-Cbl is an important negative regulator in T-cell receptor signalling, and blocking its RING function results in both altered thymic selection and heightened T-cell signalling (Thien et al, 2005; Huang & Gu, 2008). Given its broad function in immunity, we posited that c-Cbl might also regulate dendritic cell function.
The transcription factor nuclear factor (NF)-κB typically refers to a heterodimer comprising a Rel subfamily subunit and a transactivation-domain-lacking p50/NF-κB1 or p52/NF-κB2 subunit (Ghosh et al, 1998). Due to the lack of a transactivation domain, p50 homodimers are generally observed as transcriptional repressors (Plaksin et al, 1993; Ledebur & Parks, 1995). It has been previously reported that p50 homodimers negatively regulate proinflammatory responses when they are associated with Bcl3 (Bohuslav et al, 1998; Carmody et al, 2007). By contrast, the p50 precursor, p105, can also function as an inhibitor of κB proteins (IκB) through its carboxy-terminal ankyrin repeats, and the removal of its IκB-like domain results in severe deregulation of immune responses (Ishikawa et al, 1998). Therefore, maintenance of the protein levels of both p50 and p105 is essential to the regulation of immunity.
Here, we show that c-Cbl stabilizes p50 and p105 proteins in dendritic cells. Thus, c-Cbl deficiency potentiates higher levels of TLR-stimulated proinflammatory cytokines in dendritic cells, including interleukin (IL)-12p70 (IL-12). The upregulation of cytokines is also accompanied by enhanced recruitment of NF-κB, including c-Rel-p50, to NF-κB sites, suggesting a causal relationship. By reconstituting c-Cbl-deficient dendritic cells with different c-Cbl mutants, we observed that p105 stabilization, as well as p50 accumulation, is dependent on the RING domain function of c-Cbl. Importantly, ectopic expression of p50 in c-Cbl-deficient dendritic cells selectively suppresses cytokines that are upregulated on c-Cbl ablation, implying that c-Cbl might inhibit proinflammatory cytokines through p50 accumulation. Overall, we identify c-Cbl as a crucial E3 ubiquitin ligase for TLR-induced accumulation of p50 and show that c-Cbl might do so through p105 stabilization.
Results and Discussion
Cbl-KO dendritic cells enhance proinflammatory cytokines
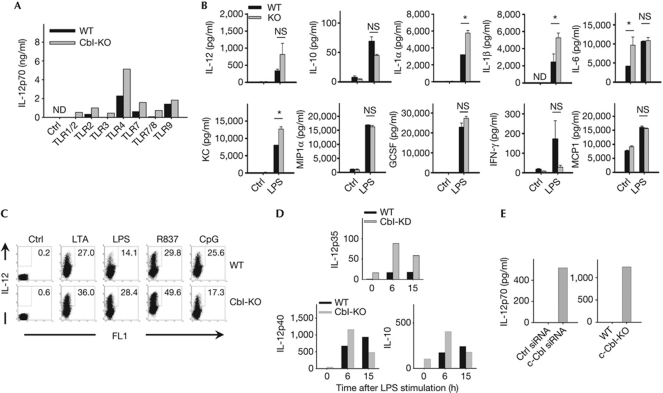
High-level IL-12 secretion is considered to be a characteristic of fully mature dendritic cells and part of the crucial ‘third signal’ affecting the outcome of dendritic cell/T-cell interactions (Curtsinger et al, 1999, 2003). Initial tests showed that c-Cbl-knockout (Cbl-KO) bone-marrow-derived dendritic cells (BMDCs) secrete more IL-12 than wild-type dendritic cells following engagement by several TLRs, excluding TLR9 (Fig 1A). Of note, this phenotype was limited to BMDCs derived from mature female mice (⩾6 to 7 weeks), implying a possible effect of oestrogen on p50 levels (Dai et al, 2007). Concurrent quantification of several additional proinflammatory cytokines and chemokines showed that lipopolysaccharide (LPS)-stimulated Cbl-KO dendritic cells produced significantly higher levels of IL-1α, IL-1β and CXCL1/KC (Fig 1B). Moreover, increased intracellular IL-12p40 staining in Cbl-KO relative to wild-type BMDCs was observed following ligation of several, but not all, TLRs (Fig 1C; supplementary Fig S1A online). Interestingly, both the response to CpG stimulation and macropinocytosis were insensitive to c-Cbl levels (Fig 1A; supplementary Fig S1B online, respectively). Elevated IL-12 production was detected as early as 8 h post stimulation, regardless of the LPS concentration used, and peaked at 16 h (supplementary Fig S1C,D online). Thus, c-Cbl deficiency in BMDCs enhances the secretion of proinflammatory cytokines following engagement of various TLR ligands.
Figure 1.
Cytokine secretion patterns of c-Cbl-knockout dendritic cells. (A) Wild-type and Cbl-KO BMDCs were stimulated with the indicated TLR agonists (Ctrl, dimethylsulphoxide; TLR1/2, Pam3CSK4; TLR2, LTA; TLR3, poly(I:C); TLR4, LPS; TLR7, R837; TLR7/8, CL075; TLR9, CpG) overnight and the supernatants were collected for IL-12p70 measurement by ELISA. (B) Supernatants of cultured BMDCs with indicated genotypes were collected after 24 h of LPS stimulation for quantification of the indicated cytokines and chemokines by LINCOplex assay. Data were pooled from 3–4 pairs of WT and Cbl-KO samples (data represent average±s.e.). (C) WT and Cbl-KO BMDCs were stimulated with the indicated TLR agonists overnight and subjected to intracellular staining of IL-12p40. Cells were treated with brefeldin A and analysed on a BD LSRII cytometer. (D) WT and c-Cbl-deficient BMDCs were stimulated with LPS (1 μg/ml) at the indicated time points before preparation of total RNA. The relative amounts of IL-12p35, p40 and IL-10 were quantified by quantitative real-time polymerase chain reaction with predesigned primers. (E) WT BMDCs nucleofected with the indicated siRNAs (left panel) or paired WT and Cbl-KO BMDCs (right panel) were cultured overnight. Supernatants were collected for analysis of IL-12p70 production by ELISA. All data (except in B) are representative of at least three experiments with consistent results. Cbl-KO, c-Cbl-knockout; ELISA, enzyme-linked immunosorbent assay; G-CSF, granulocyte colony-stimulating factor; IL, interleukin; IFN, interferon; KD, knockdown; LPS, lipopolysaccharide; ND, not determined; NS, not significant; siRNA, small-interfering RNA; TLR, Toll-like receptor; WT, wild type.
To clarify the role of c-Cbl, we investigated cytokine changes at the transcriptional level. Both semi-quantitative reverse-transcription and quantitative real-time PCR was used to quantify LPS-induced IL-12p40 (IL-12b), IL-12p35 (IL-12a) and IL-10 (IL-10) messenger RNA (mRNA) in dendritic cells. c-Cbl deficiency was associated with prolonged, LPS-induced upregulation of IL-12p35 mRNA, but not IL-12p40 (Fig 1D; supplementary Fig S1E online). At the same time, both IL-10 protein and mRNA levels were insensitive to c-Cbl ablation (Fig 1B,D, respectively). In addition, low levels of mRNA for both IL-12p35 and IL-12p40 genes, as well as IL-12p70 protein, were also present in c-Cbl-deficient dendritic cells before stimulation (Fig 1D,E, respectively). Therefore, c-Cbl deficiency not only upregulates expression of TLR-induced proinflammatory cytokines, but also raises their basal expression under steady-state conditions.
Phenotypic comparisons between WT and Cbl-KO DCs
One caveat to results from c-Cbl-KO dendritic cells is that prolonged c-Cbl deficiency could have pleiotropic effects on dendritic cell development or precursor levels (Rathinam et al, 2008). However, by day 6 of in vitro culture in granulocyte–macrophage colony-stimulating factor/IL4-supplemented medium, we consistently generated a typical level of 50 million BMDCs per mouse, which were approximately 50% CD11c+, regardless of c-Cbl expression (supplementary Fig S1F,G online). In addition, surface expression of activation markers, CD40, major histocompatibility complex class I/II, CD80 and CD86, were indistinguishable between wild-type and Cbl-KO BMDCs (supplementary Fig S1H online), as was their induction following LPS stimulation (supplementary Fig S1I online). Therefore, the phenotypic differences we detected between wild-type and Cbl-KO dendritic cells in vitro only affected some aspects of dendritic-cell activation, including cytokine production.
Cbl-KO BMDCs have enhanced pro-TH1 immunogenicity
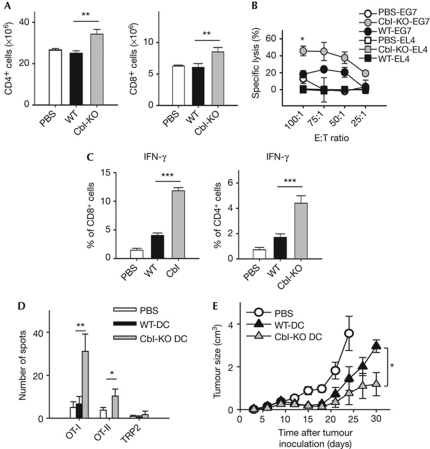
The increased cytokine production by Cbl-KO dendritic cells indicated that they might have enhanced immunogenic properties in vivo. To test this idea, we measured the immunogenicity of c-Cbl-KO compared with wild-type BMDCs pulsed with full-length ovalbumin (Ova) protein. After vaccination, total CD4+ and CD8+ splenocyte numbers were significantly higher when Cbl-KO dendritic cells were used, compared with wild-type dendritic cells (Fig 2A). However, when dendritic cells were stimulated with LPS alone or LPS and CD40L, and mixed in vitro with ovalbumin-specific TCR transgenic, OT-I (Kb-restricted) or OT-II (I-Ab-restricted) splenocytes, proliferation rates of antigen-specific T cells were indistinguishable between wild-type and Cbl-KO dendritic cells (supplementary Fig S2A,B online). One possible reason for the discrepancy between in vivo and in vitro proliferation assays is that in vivo-generated IL-12 might stimulate CD8+ T-cell proliferation only indirectly, as in the presence of primed CD4+ T helper (TH) cells, as suggested previously (Haring et al, 2006).
Figure 2.
c-Cbl-knockout BMDCs have enhanced pro-Th1 immunogenicity in vivo. (A) Naive, 5–6-week-old mice were subjected to footpad vaccination with PBS (n=5), WT (n=12) or Cbl-KO BMDCs (n=12, 2 × 106 cell/mouse) for 2 weeks before being killed. BMDCs were treated with Ova and stimulated with LPS (1 μg/ml) before vaccination. Splenocytes were counted, and percentages of CD8+ cells were quantified by FACS analysis (n=3; **P<0.01). (B) Splenocytes (effector cells) isolated from mice described in (A) were cultured with either the parental EL4 or Ova-expressing E.G7-Ova cells (target cells) at the indicated ratios. Target cells were pre-labelled with 51Cr for quantification of lysis caused by cell-mediated cytotoxicity. Percentage lysis was calculated by normalization against samples lysed with Triton X-100. Data were in triplicate and representative of samples derived from six pairs of WT and Cbl-KO mice (for control group, n=3; *P<0.05). (C) Splenocytes isolated from vaccinated mice described in (A) were stimulated with either OT-I (left panel, n=6) or OT-II peptide (right panel, n=6) overnight before intracellular staining of murine IFN-γ (***P<0.001). (D) Splenocytes from vaccinated mice of the groups described in (A) were subjected to ELISpot analysis for quantification of IFN-γ+ cells 5 weeks post-vaccination (n=9; *P<0.05). Cells were stimulated with indicated peptides overnight before assay. (E) E.G7-Ova tumour-bearing mice that were 5–6 weeks old were vaccinated with the indicated dendritic cell vaccines as described in (A), and subjected to tumour measurements every 3 days (n=5). Before vaccination, BMDCs were treated as described in (A). *P<0.05. Mice bearing tumours larger than 3 cm3 were killed. All data represent mean±s.e. Cbl-KO, c-Cbl-knockout; DC, dendritic cell; IFN, interferon; LPS, lipopolysaccharide; Ova, ovalbumin; TRP2, tyrosinase-related protein 2; WT, wild type.
We next investigated which T-cell lineages were differentially activated in spleens following vaccination. Splenocytes from Cbl-KO dendritic-cell-primed animals showed significantly higher levels of peptide-specific cytotoxicity (Fig 2B), plus interferon (IFN)-γ-secreting (OT-I or OT-II) T cells, consistent with a pro-TH1 lineage bias (Fig 2C). Even 5 weeks after vaccination, Cbl-KO dendritic-cell-vaccinated mice still harboured significant levels of IFN-γ-producing T cells, whereas control groups returned to baseline levels (Fig 2D). By contrast, IL-4 levels (TH2) produced by antigen-specific CD4+ splenocytes were comparable (supplementary Fig S2C online). In addition, serum samples collected at 2–4 weeks post-vaccination all revealed significantly higher levels of Ova-specific, TH1-driven IgG2a secretion from Cbl-KO dendritic-cell-primed mice (right panel, supplementary Fig S2D online). However, the levels of TH2-driven IgG1 secretion from both wild-type and Cbl-KO dendritic-cell-primed mice were comparable, consistent with the result of IL-4 secretion (left panel, supplementary Fig S2D online). Overall, these experiments indicate that Cbl-deficient dendritic cells are more potent inducers of TH1 polarization than wild-type dendritic cells.
Cbl-KO DCs function as a potent tumour vaccine
To evaluate better the clinical relevance of Cbl-KO dendritic cells, we tested their anti-tumour efficacy against preestablished E.G7-Ova lymphomas. Wild-type mice were challenged with tumour cells 3 days before vaccination (day 0). By day 10, all mice had palpable tumours (supplementary Fig S2E online). However, 1 month after tumour inoculation, mice vaccinated with Cbl-KO dendritic cells had significantly smaller tumours, as opposed to control groups (Fig 2E). By day 60, the remaining survivor showed complete resistance to secondary tumour challenge, accompanied by further expansion of Ova-specific T cells (supplementary Fig S2F online). Importantly, the effects of c-Cbl haploinsufficiency could be noted in tumour-size analysis (supplementary Fig S3A online), highlighting the dose-dependent effect of c-Cbl expression on dendritic cell function. In addition, major histocompatibility complex tetramer staining further showed that the level of circulating Ova-specific CD8+ T cells was significantly higher following Cbl-KO vaccination, compared with wild-type DCs (supplementary Fig S3B online). Furthermore, the level of vaccinated dendritic cells that migrated to draining lymph nodes remained essentially unaltered on c-Cbl ablation, reducing the probability that elevated migration is the main cause for the observed differences (supplementary Fig S3C online). Thus, c-Cbl deficiency seems to improve the efficacy of dendritic-cell-based anti-tumour vaccines.
c-Cbl deficiency reduces p105 and p50 in BMDCs
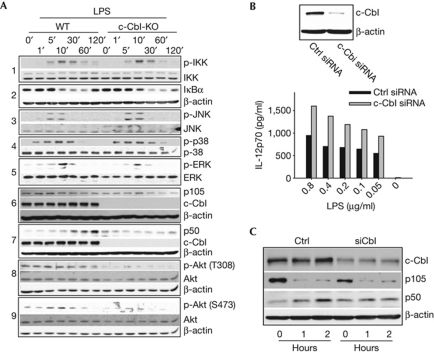
To define the molecular mechanism of c-Cbl function, we analysed several signalling pathways associated with TLRs (Fig 3A). As an initial screen, we examined the phosphorylation of several key signalling molecules implicated in cytokine induction. However, at several time points post-LPS addition, we failed to observe significant differences in the phosphorylation of IKKα/β, Jun N-terminal kinase, p38 and extracellular signal-regulated kinase (ERK), or degradation of IκB protein between wild-type and Cbl-KO dendritic cells (Fig 3A, panels 1, 2, 3, 4, and 5, respectively).
Figure 3.
c-Cbl deficiency reduces both p105 and p50 levels in BMDCs. (A) WT or Cbl-KO BMDCs were stimulated with LPS (1 μg/ml) and lysed at the indicated time points before western blot analysis. All inputs were normalized on the basis of protein assay results. (B) Upper panel: BMDCs were nucleofected with scrambled (Ctrl) or c-Cbl siRNA, and the amount of endogenous c-Cbl expression was quantified by western blot analysis. Control or Cbl-KD BMDCs were stimulated with the titrated amounts of LPS 8–10 h after nucleofection, and the supernatants were collected after overnight stimulation for IL12p70 quantification by ELISA (lower panel). (C) Control or Cbl-KD BMDCs were stimulated with LPS at the indicated time points before preparation for western blot analysis. Data are representative of at least three independent experiments with consistent results. Cbl-KO, c-Cbl-knockout; ELISA, enzyme-linked immunosorbent assay; ERK, extracellular signal-regulated kinase; IκB, inhibitor of κB protein; IL, interleukin; JNK, Jun N-terminal kinase; KD, knockdown; LPS, lipopolysaccharide; siRNA, small-interfering RNA; WT, wild type.
It has been shown previously that LPS-mediated ERK induction was dependent on the p105-associated Ser/Thr kinase, Tpl2/Cot (Dumitru et al, 2000), which, in turn, is activated on release by IKKβ-initiated degradation of p105 (Waterfield et al, 2003). We found that LPS treatment led to protein degradation of the long form (p58) of Tpl2, whereas the short form (p52) remained unchanged (supplementary Fig S4A online; Waterfield et al, 2003). However, we did not observe any effect of c-Cbl on either Tpl2 isoform (supplementary Fig S4A online), reducing the likelihood of a key role for Tpl2 in c-Cbl function. Interestingly, whereas LPS-induced p105 protein degradation was consistently observed in both wild-type and Cbl-KO dendritic cells, total p105 level was lower in Cbl-KO dendritic cells (Fig 3A, panel 6; supplementary Fig S4B online). This finding could not explain why we did not concurrently observe alteration of the TLR-induced Tpl2–ERK pathway. An alternative explanation is that p105 might exist in separate pools and c-Cbl-associated p105 molecules might be distinct from Tpl2-bound p105. For example, Belich et al (1999) have reported that a C-terminal region encompassing the ankyrin repeats of p105 is crucial for Tpl2 binding, implying that its association with Tpl2 or NF-κB dimers is mutually exclusive. Therefore, it is possible that distinct pools of p105 might sequester Tpl2 and NF-κB dimers independently, with c-Cbl mainly stabilizing p105 molecules with IκB-like function.
Levels of p50 are reported to increase after TLR engagement (Donald et al, 1995), which is consistent with our observation in wild-type dendritic cells (Fig 3A, panel 7). However, Cbl-KO dendritic cells failed to show LPS-induced p50 accumulation, which remained relatively constant (Fig 3A, panel 7). We also found that p50 induction under these conditions depends on proteasome function, consistent with the notion of p105 processing into p50 (supplementary Fig S4C online). Thus, c-Cbl ablation could abrogate LPS-induced p105 processing into p50 in dendritic cells. As c-Cbl has been shown to bind to PI3Kp85α in many physiological settings, we also quantified Akt phosphorylation (Meng & Lowell, 1998; Hunter et al, 1999). Indeed, Akt phosphorylation at both Thr 308 and Ser 473 was greatly reduced in c-Cbl-KO dendritic cells following LPS stimulation (Fig 3A, panels 8 and 9, respectively), implying that c-Cbl might be involved in activating the phosphatidylinositol 3-kinase (PI3K)–Akt pathway downstream of TLRs. Thus, both Akt activation and p105/p50 levels are greatly reduced in Cbl-KO dendritic cells following LPS stimulation.
To rule out the possibility of developmental defects, we suppressed endogenous c-Cbl expression in wild-type BMDCs by using RNA interference. The efficacy of c-Cbl small-interfering RNA knockdown was >70% by densitometry (Fig 3B, upper panel). c-Cbl knockdown increased IL-12 production by dendritic cells stimulated with various doses of LPS (Fig 3B, lower panel). Compared with controls, c-Cbl-knockdown BMDCs (Cbl-KD dendritic cells) secreted more IL-1α, IL-1β and KC, but not MCP1 (monocyte chemotactic protein 1), MIP1α (macrophage inflammatory protein 1α) or IL-6, similarly to the cytokine secretion pattern of Cbl-KO dendritic cells (Fig 1A; supplementary Fig S4D online respectively). Conversely, complementation of Cbl-KO dendritic cells with wild-type c-Cbl suppressed IL-12 production (supplementary Fig S4E online). Finally, LPS-mediated p50 accumulation was suppressed in Cbl-KD dendritic cells (Fig 3C). Altogether, the proinflammatory phenotype observed in Cbl-KO dendritic cells seems to be cell autonomous and secondary to c-Cbl deficiency.
c-Cbl inhibits stimulatory NF-κB heterodimers
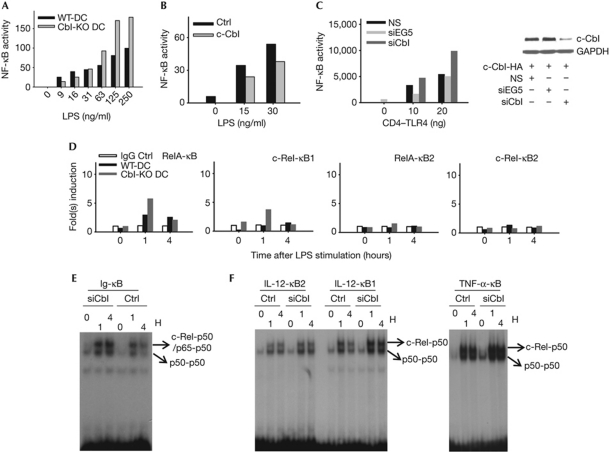
The production of IL-12 by dendritic cells is tightly regulated by NF-κB (Grumont et al, 2001). To test whether c-Cbl regulates IL-12 secretion by NF-κB suppression, we quantified endogenous NF-κB activity. As shown in Fig 4A, Cbl-KO dendritic cells reflected a roughly twofold increase in endogenous NF-κB, but not AP1 (supplementary Fig S4F online), activity in response to different doses of LPS. Furthermore, the increased NF-κB activity in Cbl-KO dendritic cells could be reduced by overexpressing wild-type c-Cbl (Fig 4B). This dose-dependent c-Cbl effect on NF-κB activity was recapitulated in 293T cells activated by a constitutively active CD4–TLR4 fusion protein (Fig 4C; Zhang et al, 2004). It is noteworthy that the upregulation of LPS-induced NF-κB activity in Cbl-KO dendritic cells is not secondary to enhanced nuclear translocation (supplementary Fig S4G,H online). Consistently, LPS-induced recruitment of c-Rel to the proximal NF-κB site of the IL12p35 promoter, as quantified by a chromatin immunoprecipitation assay, was higher on c-Cbl ablation (Fig 4D). Moreover, electrophoretic mobility shift assay (EMSA) suggests enhanced binding of both p50 homo- and heterodimers to different NF-κB probes in c-Cbl-deficient dendritic cells (Fig 4E,F, supplementary Fig S4I,J online). Thus, our data indicate that c-Cbl negatively regulates the transcription of proinflammatory cytokines, possibly through the regulation of various NF-κB complexes and their recruitment to target gene promoters.
Figure 4.
c-Cbl inhibits the expression of proinflammatory cytokines by attenuating the recruitment of stimulatory NF-κB heterodimers. (A) WT and Cbl-KO BMDCs were nucleofected with normalized amounts of NF-κB-SEAP reporter and incubated for 8–10 h. Subsequently, cells were stimulated with titrated LPS overnight. Supernatants were collected and prepared for SEAP assay. (B) Cbl-KO BMDCs were nucleofected with NF-κB-SEAP reporter plus either control vector or WT c-Cbl construct before stimulation with titrated LPS before SEAP assay, as described in (A). (C) 293T cells were transduced with the indicated lentivirus (pGIPZ, NS) and the transductants were selected for the GFP+ population in the presence of puromycin (5 μg/ml). After 2 weeks, normalized numbers of transductants were seeded onto a 12-well plate before transfection with the WT c-Cbl and CD4–TLR4 constructs. Supernatants were collected and prepared for SEAP assay, as described in (A,B). On collection of supernatants, cells were prepared for western blot analysis (right panel). (D) Paired WT and Cbl-KO BMDCs were stimulated with LPS at the indicated time points before fixation for chromatin immunoprecipitation. A previously reported NF-κB site located at the distal end of the murine IL-12p35 promoter was used as negative control (κB2). The proximal NF-κB site derived from the same promoter (κB1) was examined for specific binding of the indicated NF-κB family members. (E,F) Nuclear lysates were prepared and electrophoretic mobility shift assay was performed by using the indicated probes. Data are representative of at least two independent experiments with consistent results. Cbl-KO, c-Cbl-knockout; DC, dendritic cell; GFP, green fluorescent protein; HA, haemagglutinin; IgG, immunoglobulin G; IL, interleukin; LPS, lipopolysaccharide; NF-κB, nuclear factor-κB; NS, non-silencing; siRNA, small-interfering RNA; TLR, Toll-like receptor; TNF, tumour-necrosis factor; WT, wild type.
Interestingly, c-Cbl deficiency not only upregulates cytokine secretion in response to various stimuli, but also derepresses the resting state expression of several cytokines, including IL6 (Fig 1B). This deregulation of proinflammatory cytokines also corresponds to an altered state of NF-κB complexes under steady-state conditions (Fig 4E,F). In addition, the enhanced binding of all p50 dimers observed in Cbl-KD dendritic cells might result from reduced levels of p105/IκBγ (Ishikawa et al, 1998). Therefore, it is possible that in c-Cbl-deficient dendritic cells, reduced p105/IκBγ level partly phenocopies NF-κB1ΔC macrophages, including the deregulation of not only c-Rel/RelA-p50 heterodimers, but also p50 homodimers.
The RING domain of c-Cbl is required for IL-12 inhibition
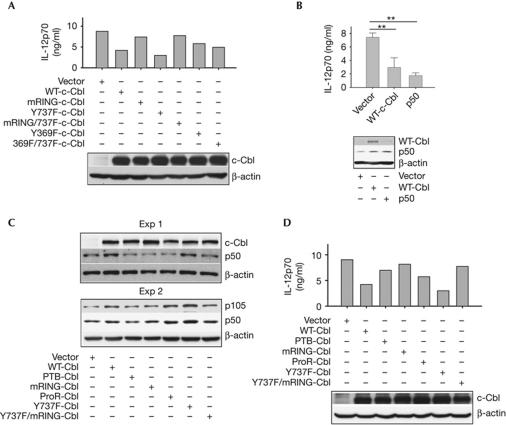
To help determine the mechanism behind the regulation of IL-12 by c-Cbl, we created several c-Cbl mutants containing inactivating mutations within the phosphotyrosine binding (PTB), RING and proline-rich domains, plus Tyr 369, and/or Tyr 737, which is implicated in PI3K binding. These c-Cbl mutants were overexpressed in Cbl-KO dendritic cells before LPS stimulation, and IL-12 protein level was assayed. As the data indicate, both the mutant (m)RING and the mRING/Y737F double mutants were devoid of the inhibitory effect of wild-type c-Cbl on IL-12 (Fig 5A), although both mutants could still coimmunoprecipitate with PI3Kp85α when phosphorylated by c-Src (supplementary Fig S5A online). It is noteworthy that coimmunoprecipitation of c-Cbl and PI3Kp85α correlated with c-Cbl tyrosine phosphorylation levels induced by TRAF6-activated (Wong et al, 1999) or constitutively active c-Src (supplementary Fig S5B online). In addition, the two Y369F-containing c-Cbl mutants (Y369F and Y369F/Y737F double mutant) showed a partial blockade of inhibition (Fig 5A), consistent with its previously reported role in RING domain function (Kassenbrock & Anderson, 2004). By contrast, the Y737F single mutant, which does not bind to PI3Kp85α in the current setting (supplementary Fig S5A online), retained the inhibitory function of c-Cbl, as the observed reduction in IL-12 level was not a by-product of increased cell death (supplementary Fig S5C online). In conclusion, our data indicate that the RING domain function of c-Cbl, but not its Tyr 737 residue, is required for it to inhibit of IL-12 production in dendritic cells.
Figure 5.
The RING domain of c-Cbl is required for IL-12 inhibition, probably through p50 accumulation. (A,B,D) Cbl-KO BMDCs were nucleofected with the indicated constructs before stimulation with LPS overnight. IL-12p70 levels in the supernatants were quantified by enzyme-linked immunosorbent assay. Western blot analysis was used to assess the expression of nucleofected proteins. Data in (B) represent mean±s.d. (n=3; **P<0.01 by one-way analysis of variance). (C) Cbl-KO BMDCs were nucleofected with the indicated constructs before stimulation with LPS for 2 h, followed by western blot analysis of both p105 and p50 expression. Data are representative of at least three independent experiments with consistent results. IL, interleukin; LPS, lipopolysaccharide; KO, knockout; proR, proline-rich domain; WT, wild type.
There are several descriptions of the regulatory function of p50 homodimers on proinflammatory cytokines (Bohuslav et al, 1998; Udalova et al, 2000), which might repress gene transcription through either competitive binding at NF-κB sites (Ledebur & Parks, 1995) or the recruitment of histone-modifying factors such as histone deacetylase 1 (HDAC1; Elsharkawy et al, 2010). Thus, the effects of p50 on IL-6, tumour-necrosis factor (TNF)-α and IL-12 protein expression following LPS stimulation were further tested using p50 overexpression in Cbl-KO dendritic cells (Fig 5B; supplementary Fig S5D online). We found that overexpression of p50, but not p65, recapitulates the effect of wild-type c-Cbl on IL-12p70 and TNF-α, but not IL-6 induction (supplementary Fig S5D online). Consistently, inhibition of LPS-induced TNF-α by adding back wild-type c-Cbl in Cbl-KO dendritic cells echoes that caused by c-Cbl ablation in wild-type dendritic cells (supplementary Fig S5D,E online, respectively). Importantly, these results indicate that c-Cbl might inhibit cytokine expression by inducing Akt-independent p50 accumulation (left panel, supplementary Fig S5F online). This idea was further supported by the strong correlation between the ability of several c-Cbl mutants to induce p50 and their inhibitory effect on IL-12 levels (Fig 5C,D, respectively). In addition, blockade of proteasomal function did not increase p50 level in Cbl-KO dendritic cells following LPS stimulation, refuting the possibility of a direct role of c-Cbl in p50 protein stability (supplementary Fig S5G online). By contrast, c-Cbl-mediated p50 accumulation following TLR engagement does not seem to result from enhanced p105 transcription, because ablation of c-Cbl does not lead to downregulation of p105 mRNA levels (supplementary Fig S5H online). In conclusion, it seems that c-Cbl regulates p50 levels in a RING-domain-dependent manner, by stabilizing p105 protein. Inhibition of c-Cbl can lead to improved dendritic-cell-based vaccines.
Methods
The supplementary experimental procedures online contain details of BMDC preparation, western blot analysis, description of reagents, constructions of plasmids, c-Cbl mutants, NF-κB nuclear translocation assay, in vivo migration assay, in vitro T-cell proliferation assay, cytotoxic T-lymphocyte assay, macropinocytosis assay, IFN-γ ELISpot assays, chromatin immunoprecipitation assay, quantitative reverse transcription polymerase chain reaction, semi-quantitative real-time polymerase chain reaction, lentivirus preparation and quantification of sera antibodies.
Mice and cells. c-Cbl-deficient mice were obtained from Taconic Farms (Hudson, NY, USA) with permission from Hua Gu (Columbia University, NY, USA). Immature BMDCs were differentiated as described previously (Inaba et al, 1992), with some modifications (Lutz et al, 1999). Briefly, bone marrow cells were collected from tibias and femurs of both wild-type and Cbl-KO mice and subsequently cultured in complete RPMI 1640 (with 10% fetal bovine serum and antibiotics) supplemented with murine granulocyte–macrophage colony-stimulating factor (20 ng/ml, Invitrogen, Carlsbad, CA, USA) and IL-4 (10 ng/ml, eBioscience, San Diego, CA, USA) for 6 days before collection for experiments. Mice used for bone marrow preparations were always female, aged between 7 and 10 weeks.
In vivo tumour study. Female 5–7-week-old C57BL/6 mice were inoculated subcutaneously with Ova-expressing E.G7-Ova cells (5 × 105) 3 days before rear footpad vaccination with 2 × 106 CD11c-enriched BMDCs. E.G7-Ova cells were maintained under continuous G418 selection (0.4 mg/ml). Before vaccination, Cbl-KO or wild-type BMDCs were pulsed with Ova protein (50 μg/ml; Worthington Biochemical Corp., Lakewood, NJ, USA) and stimulated with LPS plus CD40 ligand (R&D Systems Inc., Minneapolis, MN, USA) in vitro. Tumour sizes were calculated every 3 days as w2 × l × 0.52. All experimental procedures were conducted according to protocols approved by the Baylor Institutional Animal Care and Use Committee.
Western blots. Cells were lysed with a Brij97-based lysis buffer containing protease inhibitor cocktail (Sigma-Aldrich Inc., St Louis, MO, USA) before SDS–PAGE, as described previously (Zhang et al, 1998). For enhanced chemiluminescence, SuperSignal western blotting substrate was used (Thermo Fisher Scientific Inc., Huntsville, AL, USA). Images were created by using an X-ray film or a 4,000 MM Gel Documenting System (Kodak, Rochester, NY, USA).
Electrophoretic mobility shift assay. EMSA was conducted as described previously (Plevy et al, 1997). Briefly, nuclear extracts from control or Cbl-KD dendritic cells were prepared and lysates were subjected to EMSA with the following 32P-labelled κB oligonucleotide probes: Ig-κB, 5′-CAACGGCAGGGGAATTCCCCTCTCCTT-3′ (Chang et al, 2009); IL-12-κB1, 5′-GATCGTCCTGGGAAAGTCCTGCCGGATC-3′; IL-12-κB2, 5′-GATCCCACTGGGAATCCCTTCAGCCGATC-3′ (Grumont et al, 2001); TNF-α-κB, 5′-GATCCACAGGGGGCTTTCCCTCCA-3′ (Baer et al, 1998). As controls, non-labelled, mutant probes were used (mQ: 5′-GATCCACAGGTTGCTTTCCCTCCA-3′). We used the 3′–5′ Klenow fragment of DNA polymerase I to label the annealed probes with pre-designed sticky ends (New England Biolabs, Ipswich, MA, USA). For the supershift experiments, the indicated antibodies were added to the binding mixture 15 min before probes were included (detailed information of antibodies used for supershift are included in the supplementary experimental procedures online).
Quantification of secreted proteins. Enzyme-linked immunosorbent assays (ELISAs) were conducted according to the manufacturer's instructions (BD OptEIA ELISA Kit, BD Biosciences Inc., San Jose, CA, USA). For simultaneous quantification of several cytokines/chemokines, a LINCOplex Cytokine kit was used according to the manufacturer's protocol (Millipore, Billerica, MA, USA).
Other assays. Secreted alkaline phosphatase (SEAP) reporter assays were conducted as described previously (Hanks et al, 2005). For BMDCs, constructs were delivered by nucleofection as described in the manufacturer's manuals (Mouse DC Nucleofector Kit, Lonza, Basel, Switzerland). The transfection efficiency was consistently more than 50%, as validated by green fluorescent protein expression. For flow cytometry, fluorophore-conjugated antibodies were used according to the manufacturer's protocols. For intracellular cytokine staining, cells were treated with 1 μg/ml brefeldin A (Sigma-Aldrich) for 6 h before staining. Subsequently, staining was performed using a Fix/Permeabilization kit (BD Biosciences). Data were collected with a BD LSRII flow cytometer (BD Biosciences) and analysed with WinMDI software (Joe Trotter, The Scripps Institute, CA, USA).
Statistical analysis. Statistical significance was determined by using Student's t-test between highlighted groups unless indicated otherwise, and a 95% confidence was taken as P<0.05.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank J. Wang and T.-H. Tan for sharing reagents; A. Rice and C. Rooney for valuable discussions and critical reading of the manuscript; members of the Spencer laboratory for technical support and assistance. We also acknowledge the joint participation by Diana Helis Henry Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine. This work was also supported by grants from the National Institutes of Health (RO1-CA120411 and T32-AI07495 (S.-H.C.)).
Author Contributions: S.-H.C. designed all experimental strategies except for Fig 2B, contributed all figures, and wrote the manuscript. R.T.W. contributed Fig 4D,F. P.S. contributed Fig 1D and supplementary Fig S3C online. N.L. contributed Fig 2B and supplementary Fig S2D online. H.H. and S.-C.S. contributed supplementary Figs S1E and S4E online. M.S. contributed the in vivo animal studies and ELISpot assay. J.M.L. critiqued and helped to edit this manuscript. D.M.S. proposed and supervised the c-Cbl project, critiqued the data and edited the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124: 783–801 [DOI] [PubMed] [Google Scholar]
- Baer M, Dillner A, Schwartz RC, Sedon C, Nedospasov S, Johnson PF (1998) Tumor necrosis factor alpha transcription in macrophages is attenuated by an autocrine factor that preferentially induces NF-kappaB p50. Mol Cell Biol 18: 5678–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belich MP, Salmeron A, Johnston LH, Ley SC (1999) TPL-2 kinase regulates the proteolysis of the NF-κB-inhibitory protein NF-κB1 p105. Nature 397: 363–368 [DOI] [PubMed] [Google Scholar]
- Beutler BA (2009) TLRs and innate immunity. Blood 113: 1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohuslav J, Kravchenko VV, Parry GC, Erlich JH, Gerondakis S, Mackman N, Ulevitch RJ (1998) Regulation of an essential innate immune response by the p50 subunit of NF-κB. J Clin Invest 102: 1645–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH (2007) Negative regulation of toll-like receptor signaling by NF-κB p50 ubiquitination blockade. Science 317: 675–678 [DOI] [PubMed] [Google Scholar]
- Chang M, Jin W, Sun SC (2009) Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat Immunol 10: 1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF (1999) Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol 162: 3256–3262 [PubMed] [Google Scholar]
- Curtsinger JM, Lins DC, Mescher MF (2003) Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med 197: 1141–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai R, Phillips RA, Ahmed SA (2007) Despite inhibition of nuclear localization of NF-κB p65, c-Rel, and RelB, 17-β estradiol up-regulates NF-κB signaling in mouse splenocytes: the potential role of Bcl-3. J Immunol 179: 1776–1783 [DOI] [PubMed] [Google Scholar]
- Dale BM, Traum D, Erdjument-Bromage H, Tempst P, Greenberg S (2009) Phagocytosis in macrophages lacking Cbl reveals an unsuspected role for Fc gamma receptor signaling and actin assembly in target binding. J Immunol 182: 5654–5662 [DOI] [PubMed] [Google Scholar]
- Donald R, Ballard DW, Hawiger J (1995) Proteolytic processing of NF-κB/I κB in human monocytes. ATP-dependent induction by pro-inflammatory mediators. J Biol Chem 270: 9–12 [DOI] [PubMed] [Google Scholar]
- Dumitru CD et al. (2000) TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103: 1071–1083 [DOI] [PubMed] [Google Scholar]
- Elsharkawy AM, Oakley F, Lin F, Packham G, Mann DA, Mann J (2010) The NF-κB p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes. J Hepatol 53: 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB (1998) NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16: 225–260 [DOI] [PubMed] [Google Scholar]
- Grumont R, Hochrein H, O’Keeffe M, Gugasyan R, White C, Caminschi I, Cook W, Gerondakis S (2001) c-Rel regulates interleukin 12 p70 expression in CD8(+) dendritic cells by specifically inducing p35 gene transcription. J Exp Med 194: 1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks BA, Jiang J, Singh RA, Song W, Barry M, Huls MH, Slawin KM, Spencer DM (2005) Re-engineered CD40 receptor enables potent pharmacological activation of dendritic-cell cancer vaccines in vivo. Nat Med 11: 130–137 [DOI] [PubMed] [Google Scholar]
- Haring JS, Badovinac VP, Harty JT (2006) Inflaming the CD8+ T cell response. Immunity 25: 19–29 [DOI] [PubMed] [Google Scholar]
- Huang F, Gu H (2008) Negative regulation of lymphocyte development and function by the Cbl family of proteins. Immunol Rev 224: 229–238 [DOI] [PubMed] [Google Scholar]
- Hunter S, Burton EA, Wu SC, Anderson SM (1999) Fyn associates with Cbl and phosphorylates tyrosine 731 in Cbl, a binding site for phosphatidylinositol 3-kinase. J Biol Chem 274: 2097–2106 [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176: 1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Claudio E, Dambach D, Raventos-Suarez C, Ryan C, Bravo R (1998) Chronic inflammation and susceptibility to bacterial infections in mice lacking the polypeptide (p)105 precursor (NF-κB1) but expressing p50. J Exp Med 187: 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassenbrock CK, Anderson SM (2004) Regulation of ubiquitin protein ligase activity in c-Cbl by phosphorylation-induced conformational change and constitutive activation by tyrosine to glutamate point mutations. J Biol Chem 279: 28017–28027 [DOI] [PubMed] [Google Scholar]
- Ledebur HC, Parks TP (1995) Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-κB site and p65 homodimers. J Biol Chem 270: 933–943 [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O’Neill LA (2005) Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5: 446–458 [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223: 77–92 [DOI] [PubMed] [Google Scholar]
- Meng F, Lowell CA (1998) A beta 1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J 17: 4391–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaksin D, Baeuerle PA, Eisenbach L (1993) KBF1 (p50 NF-κB homodimer) acts as a repressor of H-2Kb gene expression in metastatic tumor cells. J Exp Med 177: 1651–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevy SE, Gemberling JH, Hsu S, Dorner AJ, Smale ST (1997) Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol Cell Biol 17: 4572–4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam C, Thien CB, Langdon WY, Gu H, Flavell RA (2008) The E3 ubiquitin ligase c-Cbl restricts development and functions of hematopoietic stem cells. Genes Dev 22: 992–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MH, Dikic I (2005) The Cbl interactome and its functions. Nat Rev Mol Cell Biol 6: 907–918 [DOI] [PubMed] [Google Scholar]
- Shen L, Evel-Kabler K, Strube R, Chen SY (2004) Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol 22: 1546–1553 [DOI] [PubMed] [Google Scholar]
- Song XT, Evel-Kabler K, Shen L, Rollins L, Huang XF, Chen SY (2008) A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat Med 14: 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thien CB, Blystad FD, Zhan Y, Lew AM, Voigt V, Andoniou CE, Langdon WY (2005) Loss of c-Cbl RING finger function results in high-intensity TCR signaling and thymic deletion. EMBO J 24: 3807–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udalova IA, Richardson A, Denys A, Smith C, Ackerman H, Foxwell B, Kwiatkowski D (2000) Functional consequences of a polymorphism affecting NF-κB p50-p50 binding to the TNF promoter region. Mol Cell Biol 20: 9113–9119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield MR, Zhang M, Norman LP, Sun SC (2003) NF-κB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the Tpl2 kinase. Mol Cell 11: 685–694 [DOI] [PubMed] [Google Scholar]
- Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, Choi Y (1999) TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell 4: 1041–1049 [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S (2004) A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303: 1522–1526 [DOI] [PubMed] [Google Scholar]
- Zhang J, Salojin K, Gao JX, Cameron M, Geisler C, Delovitch TL (1998) TCR alpha beta chains associate with the plasma membrane independently of CD3 and TCR zeta chains in murine primary T cells. J Immunol 161: 2930–2937 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.