Abstract
Pneumococcal infections have a substantial burden in Turkey, particularly in the elderly (>60 years) and at-risk adults (18–59 years). VCR is low at approximately 2%. The first aim of this study was the evaluation of the burden of pneumococcal infections (pneumonia and bacteremia) from a public payer perspective in elderly and at-risk adults. The second aim was the evaluation of cost effectiveness of implementing a large PPV program in these populations. A decision tree model was employed using demographic and epidemiological inputs obtained from Turkish official sources and international literature. Vaccination was assumed to protect for 5 years with 60% and 50% effectiveness against BPP in elderly and at-risk adults respectively. Vaccination effectiveness of 21% against NBPP was assumed for both populations. Costs input were obtained from a previous study conducted between 2002 and 2008 in a public university hospital in Ankara, Turkey. Univariate sensitivity analyses and Monte-Carlo simulations were performed. The vaccination program was cost saving compared to no vaccination. Pneumococcal vaccination with 60% coverage led to a mean of 4,695 LYG in the elderly and 2,134 LYG in at-risk adults with 40% coverage. Mean incremental savings reached 45.4 million YTL in the elderly and 21.8 million YTL in at-risk adults. This analysis suggests that pneumococcal vaccination of elderly and at-risk adults is associated with a positive return on investment from a public payer perspective and supports the continued recommendation of pneumococcal vaccines, as well as their full funding in Turkey.
Key words: pneumococcal polysaccharide vaccine, pneumococcal disease, cost effectiveness, Turkey
Introduction
Streptococcus pneumoniae infections are a major cause of illness and death with about 1.6 million cases of fatal pneumococcal disease occurring worldwide annually, mostly in infants and the elderly.1 S. pneumoniae infections can be invasive or non-invasive.2 In adults and elderly, non-invasive disease can manifest as pneumonia, whereas bacteremia and meningitis are the most common invasive diseases. NBPP and BPP represent about 90% of all pneumococcal-related outcomes in this population according to Fedson.3 Pneumococcal disease affects all age groups, although the elderly and immunocompromised are at highest risk from infection.4 Other groups of people at increased risk for pneumococcal disease include those with chronic diseases of the heart, lung, liver or kidneys, as well as those with diabetes mellitus, alcoholism or malignancies. In addition, in 2008, the ACIP recommended pneumococcal vaccination of two additional groups, these being asthmatics and smokers.
In Europe and the United States, pneumococcal pneumonia has been reported to be the most common community-acquired bacterial pneumonia, especially in adults, causing approximately 30–50% of CAP requiring hospitalization in adults, and up to 50% of nosocomial pneumonia.4 Furthermore, in Europe and the United States, approximately 1 per 1,000 adults are estimated to be affected by pneumococcal pneumonia each year.1 As a result, S. pneumoniae infections have been identified as an area of public health importance in Europe.5
Pneumococcal resistance to commonly used antimicrobials is a serious and increasing problem worldwide, which complicates the treatment of infection. Effective immunization against S. pneumoniae is the best way to reduce the impact of pneumococcal infections.4 Indeed, a growing number of national and international health bodies now recommend pneumococcal vaccination of elderly and at-risk groups.4 Currently, there are two commercially available vaccines for the prevention of pneumococcal infections: the PCV (7 & 10 & 13) used in infants and young children,6 and PPV23 used in at risk people aged more than 2 years old and the elderly. PPV23 contains purified capsular polysaccharide from each of 23 pneumococcal serotypes, which account for approximately 90% of the types responsible for invasive pneumococcal infections in developed and developing countries.2 PPV23 has been shown to effectively prevent pneumococcal pneumonia with or without bacteremia, and decreased rates of overall pneumonia and of mortality due to pneumonia.7
In Turkey, S. pneumoniae infections incur a substantial medical and economic burden: pneumonia diseases have the second highest average cost of hospitalization (1,479 YTL/€778) following lung cancer (1,978 YTL/€1,041).8 Consequently, the vaccine is available in Turkey from 1994 and vaccination of risk groups including elderly with PPV23 is reimbursed by the government since 2007. However, only 2% of elderly and at-risk adults in Turkey are vaccinated against pneumococcal infections.9 Increasing the knowledge around the benefits of this vaccination is expected to increase the coverage rate.10
This cost-effectiveness study aimed to evaluate the economic burden of pneumococcal infections in Turkey in persons at high risk of pneumococcal infection i.e., at risk adults (18–59 years old) and elderly (≥60 years old). In addition, the study assessed the public health and economic benefits of implementing a routine vaccination program in these two population groups in Turkey. The strategy to vaccinate only at-risk elderly was not evaluated considering the lower cost-effectiveness of a risk-based vaccination program as compared with an aged-based program.11,12
Results
Cost of pneumococcal infections.
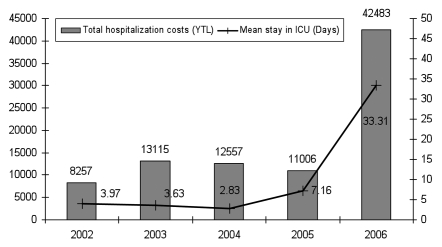
Since data on the cost of pneumococcal infections in Turkey were not available in the literature, a retrospective study was conducted in Hacettepe University Hospital in Ankara in patients more than 18 years old with confirmed S. pneumoniae. In this study, a total of 138 patients were included from 2002 to 2006 with 100 NBPP patients and 38 BPP patients. These five years allowed reaching the minimum of patients requiring in order to have interpretable statistical tests. Patient resources consumed were analyzed from 2002 because the health system and the unit cost of hospital services were changed in 2001. The mean age of patients was 61.0 ± 15.39. Among all people involved, the majority were considered as at-risk for pneumococcal infections, with 87% at risk in 2002 and 100% in 2006. On all cases, 52.3% had chronic lung diseases, 47.7% circulator system diseases and 43.1% cardiac diseases. Some patients had more than one chronic disease. The total hospitalization costs by study year were calculated and a sharp increase in costs was observed between 2006 and the other study years (Fig. 1) due to the higher number of patients with at least one comorbidity, the higher proportion of lung cancer diseases among all admitted cases and the high usage of the intensive care unit in 2006. In the base case, the mean costs of hospitalization from 2002 to 2006 were used, and in the univariate sensitivity analysis the 2006 costs were excluded in order to evaluate the impact of these high costs on the cost-effectiveness ratio. Over the 5 study years, the breakdown of all costs elements for the all study population was 6.89%, 62.95%, 10.08% and 20.08% for the cost of stay, the cost of treatments, the cost of examinations and consultations, and the costs of procedures respectively.
Figure 1.
Distribution of the mean total hospitalization cost (YTL) and mean distribution of stay (days) in intensive care unit by study years (19 NBPP and 8 BPP cases in 2002, 20 NBPP and 7 BPP cases in 2003, 21 NBPP and 5 BPP cases in 2004, 22 NBPP and 8 BPP cases in 2005, 18 NBPP and 10 BPP cases in 2006).
In absence of data, a prospective study was also conducted in Hacettepe University Hospital in Ankara, on patients more than 18 years old with non bacteremic cases. In this study, data on outpatient and inpatient resources used by 45 NBPP patients in May and April 2007 and 85 NBPP patients in November 2007 to April 2008 were collected. The mean age of patients was 65.3 ± 13.53. As in the retrospective study, almost all patients included were at-risk (96.9%). In total, 85% of these patients were hospitalized: 11 patients were not hospitalized and consumed only outpatient resources, 8 patients were hospitalized for <1 day, 3 patients consumed outpatient resources and were hospitalized afterwards for >1 day, and 108 patients were directly hospitalized.
To improve the robustness of the mean cost of hospitalization by increasing the number of patients, the NBPP inpatient costs from the retrospective and prospective datasets were combined. The mean calculated NBPP and BPP costs of each study and the NBPP combined costs, which are used for the cost-effectiveness analysis, are presented in Table 1.
Table 1.
Direct medical inpatient and outpatient costs from the retrospective and prospective studies, and combined values
| No. of cases | Mean cost in YTL* | Standard deviation | |
| DIRECT MEDICAL INPATIENT COSTS (values excluding 2006 data) | |||
| Retrospective study | |||
| NBPP | |||
| At-risk adults | 46 (40) | 16,834 (15,603) | 25,929 |
| Elderly | 54 (42) | 14,639 (7,404) | 28,806 |
| BPP | |||
| At-risk adults | 22 (15) | 18,543(11,444) | 30,768 |
| Elderly | 16 (13) | 21,890(9,415) | 37,960 |
| Prospective study | |||
| NBPP | |||
| At-risk adults | 26 | 4,960 | 10,485 |
| Elderly | 85 | 4,144 | 11,149 |
| Retrospective and Prospective studies: NBPP combined values | |||
| NBPP | |||
| At-risk adults | 72 | 14,535 | 27,748 |
| Elderly | 139 | 12,048 | 25,155 |
| DIRECT MEDICAL OUTPATIENT COSTS, YTL (€)* | |||
| Prospective study | |||
| NBPP without hospitalization | |||
| At-risk adults | 19 for both populations | 381 | 212 |
| Elderly | 329 | 117 | |
| NBPP with hospitalization | |||
| At-risk adults | 3 for both populations | 227 | 197 |
| Elderly | 218 | 190 | |
Values used in the base case are in bold.
The mean exchange rate 2008 was used: 1€ = 1.9 YTL.
Cost effectiveness analyses.
In the base case scenario (as defined in Tables 2 and 3) where a routine PPV23 vaccination program is implemented in Turkey for both at-risk adults (40% coverage rate) and the elderly (60% coverage rate), vaccination markedly reduced the number of episodes of pneumococcal disease (Tables 4 and 5): In the elderly with a cohort of 5,688,237 individuals, the number of NBPP and BPP cases avoided after 5 years due to pneumococcal vaccination was 30,572 and 3,964 respectively; in at-risk adults, with a cohort of 3,754,653 individuals, the number of NBPP and BPP cases avoided after 5 years due to pneumococcal vaccination was 14,581 and 1,575 respectively. The number of LYG by vaccination was 4,695 in the elderly and 2,134 in at-risk adults. The difference between the elderly and at-risk populations was due to the non-similarity of cohort size, VCR and BPP efficacy.
Table 2.
Epidemiological inputs used in the model for elderly and at risk adults
| Item | Pneumococcal infection | Base case | Range | Sources/comments |
| Incidence (per 100,000) | NBPP | 1136 | 728–2092 | Jackson, et al. 2004:46 Value in 65+ on all CAP in US. Using the rate 30–50% of CAP are pneumococcal pneumonia, NBPP incidence was calculated. |
| BPP | 51.5 | 18–85 | WHO Weekly Epidemiologic Record 2008:84 Range of 24–85 in 65+ in industrialized countries. Minimum value of 18 from Kaplan 200247 | |
| Hospitalization rates | NBPP | 19.4% | 15.5–23.3% | For NBPP , value from Turkish MoH records and National Burden of Disease survey31,32 ±20% for the range |
| BPP | 95.5% | 76.4–100% | Vila Corcoles, et al. 2006,7 which reports 1/22 IPD cases not being hospitalised, i.e., around 4.5%. The IPD hospitalization rate reported was therefore 95.5%. ± 20% for the range | |
| Case fatality rate | NBPP | 0.036 | 0.004–0.068 | Jackson, et al. 2004:46 (rate on 65+ in US for all CAP, CAP in outpatient for the minimum of the range, calculated value for the maximum of the range to have a mean at 0.036) |
| BPP | 0.225 | 0.143–0.308 | Middleton, et al. 2008:34 rate between 14.3% for 65–74 yo and 30.8% for 85+ yo (confirmed by WHO WER Oct 2008 saying that BPP CFR may reach 30–40% in elderly and in industrialized countries)4 |
Table 3.
Vaccination inputs used in the model
| Item | Base case | Range | Sources/comments | |
| Vaccine effectiveness against NBPP for both high-risk adults and elderly | 21% | 0–42% | Vila-Corcoles, et al. 2009;25 (42% in 50+ against NBPP ); Vila-Corcoles, et al. 2006;7 (39% in 65+ against NBPP and 21% against all CAP); Huss, et al. 2009;22 (11% in elderly and chronically ill patients against all CAP from 11 RCT but high heterogeneity) | |
| Vaccine effectiveness against BPP | at-risk adults | 50% | 40–60% | Mooney, et al. 2008;42 (61.70% in elderly); WHO WER 2008;4 (50–80% in elderly); Vila-Corcoles, et al. 2009;25 (66% in 50+); Vila-Corcoles, et al. 2010;38 (72% in 60+); Fedson, et al. 2004;24 (50–70% in elderly); Jackson, et al. 2003;43 (44% in 65+); Domingez, et al. 2005;41 (70% in all elderly); Shapiro, et al. 1991;45 (46% to 80% in 65+); Butler, et al. 1993;39 (75% in 65+) ±10 for the range |
| elderly | 60% | 50–70% | ||
| Vaccination coverage rate | at-risk adults | 40% | - | Hypothesis taken regarding an age-based or a risk-based vaccination strategy36,37 |
| elderly | 60% | - | ||
| Vaccination cost for social security (public price + procedure fee) | YTL 30.48 (€16) | - | Companies have to refund 11% and pharmacies have to give 3.5% to social security institution. | |
Table 4.
Base case results of the cost-effectiveness analysis of polysaccharide pneumococcal vaccination in elderly
| Non-vaccinated | Vaccinated | |
| Number of episodes | ||
| NBPP | 293,538 | 262,966 |
| BPP | 13,307 | 9,343 |
| Mortality reduction thanks to vaccination | ||
| Number of LYG | 4,695 | |
| Costs, in million YTL | ||
| Cost of NBPP infections | 713.2 | 640.4 |
| Cost of BPP infections | 255.6 | 178.9 |
| Vaccination | - | 104 |
| Cost reduction thanks to vaccination, in million YTL (million €, 1€ = 1.9 YTL) | ||
| Incremental costs (no vaccination—vaccination) | −45.4 (−23.9) | |
| Cost effectiveness analysis | ||
| ICER (YTL/LYG) | Cost-savings | |
Target coverage rate in elderly assumed to be 60%.
Table 5.
Base case results of the cost-effectiveness analysis of polysaccharide pneumococcal vaccination in at-risk adults
| Non-vaccinated | Vaccinated | |
| Number of episodes | ||
| NBPP | 212,075 | 197,494 |
| BPP | 9,614 | 8,039 |
| Mortality reduction thanks to vaccination | ||
| Number of LYG | 2,134 | |
| Costs, in million YTL | ||
| Cost of NBPP infections | 616.1 | 574.2 |
| Cost of BPP infections | 155.9 | 130.3 |
| Vaccination | - | 45.7 |
| Cost reduction thanks to vaccination, in million YTL (million €, 1€ = 1.9 YTL) | ||
| Incremental costs (no vaccination—vaccination) | −21.8 (−11.5) | |
| Cost effectiveness analysis | ||
| ICER (YTL/LYG) | Cost-savings |
Target coverage rate in at-risk adults assumed to be 40%.
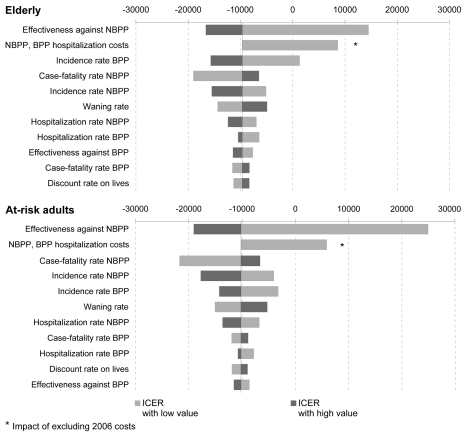
In the elderly simulation, the cost of NBPP was 713.2 million YTL and BPP was 255.6 million YTL in the non-vaccinated cohort, and 640.4 million YTL and 178.9 million YTL in the vaccinated cohort. The cost of the vaccination program was estimated to be approximately 104 million YTL. The incremental cost was therefore around −45.4 million YTL (−€23.7 million): the strategy of “vaccinate all elderly” is likely to be cost saving compared with the non-vaccination strategy. In the at-risk adult simulation, the cost of NBPP was 616.1 million YTL and BPP was 155.9 million YTL in the non-vaccinated cohort and 574.2 million YTL and 130.3 million YTL, respectively, in the vaccinated cohort. The cost of the vaccination program was estimated at around 45.7 million YTL. The incremental cost was therefore approximately −21.8 million YTL (−€11.5 million), and thus, the strategy of ‘vaccinating at-risk adults between 18 and 59 years old’ is likely to be cost saving compared with the non-vaccination strategy. In both populations, the overall cost of pneumococcal infections avoided was greater than the overall cost of a vaccination program. The vaccination program is likely to be cost saving in the case of a routine vaccination in elderly and at-risk adults in Turkey, with higher savings in the elderly group. Sensitivity analyses. In the univariate sensitivity analysis, we evaluated the individual effects of epidemiological and vaccination parameters on the incremental cost-effectiveness ratio (ICER) of the vaccination strategy using the minimum and the maximum of the inputs ranges (Tables 2 and 3). These analyses suggested that the factors having the greatest impact on effectiveness results were vaccine effectiveness against NBPP, NBPP and BPP hospitalization costs, NBPP case-fatality rate, and NBPP and BPP incidence rates. With the more pessimistic values for the vaccine effectiveness against NBPP and hospitalization costs; the ICER become positive but remains far under the treshold of three times the GDP per capita in Turkey (GDP per capita: 10:436 USD in 2008 i.e. around 15,800 YTL at December 2009 exchange rate).13 The influence of costs discounting was also evaluated. However, since the model was run for only 5 years, this parameter had a very limited impact on the incremental cost (Fig. 2).
Figure 2.
Tornado diagram of the univariate sensitivity analysis of the ICE R of the pneumococcal vaccination from a government perspective in elderly and at-risk adults. The X axis represents the absolute change in ICE R compared to baseline.
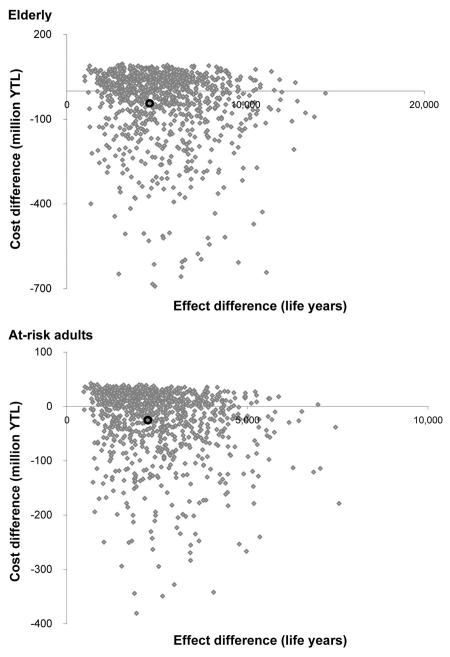
Since the 2006 costs obtained from the retrospective study were higher than expected, we also ran the model using the most conservative cost of illness, i.e., excluding 2006 BPP costs values (11,444 YTL (95% CI, 3,867–19,023) in at-risk adults [n = 15] and 9,415 YTL (95% CI, 3,121–15,707) in elderly [n = 13]) and using the NBPP costs value only from the prospective study. The incremental cost became positive and was estimated at 12.6 million YTL in at-risk adults and 40.5 million YTL in elderly. In this conservative case, ICER was 5,896 YTL/LYG and 8,625 YTL/LYG respectively which is lower than the national GDP per capita in Turkey. Therefore, regarding this ratio with the most pessimistic values of cost of disease, vaccination program can be considered as being very cost-effective. A probabilistic sensitivity analysis of the cost-effectiveness results is shown as a cost-effectiveness plane in Figure 3. Considering the input parameters and their assumed distribution, there is a 95% chance that the ICER that the incremental cost per LYG from the public payer perspective is contained in the interval [-121,288; 27,879] YTL in the elderly and [-125,966; 28,629] YTL in at-risk adults. 99.4% and 99.0% of the simulations in elderly and at-risk adults respectively lie below the willingness to pay threshold of YTL 47,400/LYG (corresponding in 3 times the GDP). It means that, taking into accound the uncertainty in the parameters, there is a probability of almost 100% that the strategy of funding pneumococcal vaccination in elderly and at-risk adults is cost-effective compared with the current no vaccination situation.
Discussion
In the base case simulation, the costs of pneumococcal disease that were avoided by vaccination were greater than the cost of a vaccination program, thus indicating that PPV23 vaccination was cost saving. The results of our analyses are consistent with other analyses performed on pneumococcal polysaccharide vaccines, which demonstrated that a pneumococcal vaccination program is likely to be cost effective if not cost saving.11,14–18 Pneumococcal vaccination has previously been reported to be cost-effective across 10 western European countries, including the prevention of bacteremia alone.19 However, pneumococcal vaccines were found to be greatly underused in the elderly population.17,18 In addition, a cost-effectiveness analysis for IPD in the elderly in England and Wales showed that routine vaccination of all elderly appeared to be cost effective, although the results were dependent on the uncertainties around vaccine effectiveness estimates and the number of hospitalizations and deaths attributable to IPD.11 Moreover, when also a small proportion of NBPP cases are prevented, the cost-effectiveness of pneumococcal vaccination increased markedly compared with preventing BPP alone.14 The present study does have limitations including the large standard deviation associated with the costing data. In the retrospective study during 2006, the inpatient costs were unexpectedly high compared with other years, which would have impacted the mean costs calculated. This could have been due to the intensive-care unit being used more in 2006 than in previous years, the fact that 100% of patients included in the 2006 data were high risk (compared to 87%, 97% and 92% in 2002, 2003 and 2004, and 2005 respectively), and the low sample size. Nevertheless, data found in patient's file were more precise in 2006 than previous year following hospital recommendations which can explain the higher cost but also increase the robustness of these costs. In comparison of IPD costs of several western European countries,15,20 the costs of illness found in our retrospective study are higher. However, they are similar to US cost data.18 2006 costs have been discussed with local hospitalization team and they validated the reliability of them. Therefore they were included in the base case analysis and the mean cost used should accurately represent the situation in clinical practice as they were obtained from resources consumed over 6 years. Univariate sensitivity analyses were performed using the costs excluding 2006 data from the retrospective study to evaluate the impact of cost data on the cost-effectiveness results. With much more lower costs for NBPP and BPP, a PPV23 program was not found to be cost-saving, however it was determined to be a very cost-effective strategy.
A second limitation is that costs of pneumococcal infections were retrieved from a university hospital where the population might not be representative of the general population in this country. However, since this hospital is located in one of the 3 biggest cities in Turkey and most of the complicated cases are referred to this hospital from other cities close to and far from Ankara, this population should be quite representative of the general Turkish population.
Costs are likely to be higher in a teaching hospital than in a public hospital due to the availability of a greater number of procedures and more advanced technology. According to principle of reimbursement system in Turkey, less severely ill patients should go to public hospital. Thus, this study should be repeated in state hospital. However, in such a setting it may be more difficult to obtain reliable and detailed records of costs.
Comprehensive meta-analyses of studies assessing the PPV23 vaccine efficacy have shown a protective effect against IPD and all-cause pneumonia in the elderly population.21,22 At the opposite, the Cochrane meta-analysis concluded that there was not sufficient evidence to support the routine use of PPV23 to prevent pneumonia.21 The lack of a sensitive and specific method for the diagnosis of pneumococcal pneumonia has limited the study of PPV23 and the conclusions of this meta-analysis can be considered a cause for debate.23 Furthermore, an extensive literature review strongly suggested that the published meta-analyses did not contain a sufficient number of person-years of observation to reach a reliable conclusion as to the efficacy of PPV23 in the prevention of pneumonia or death.24 Recently, a matched case-control study demonstrated that the vaccine was effective against IPD, and also prevented non-bacteremic pneumococcal pneumonia.25
In the present cost-effectiveness analysis only NBPP and BPP were considered. As S. pneumoniae can be also responsible for other conditions such as meningitis, which have an important social and economic burden, this calculation would produce a conservative estimate of the cost of pneumococcal infection. Furthermore, outpatient costs after hospitalization were only available for BPP. Inclusion of NBPP's costs would probably have increased the overall cost of pneumococcal infection, and thus, the benefits of vaccination.
It should be noted that although the simulation was for a single dose of vaccine, which is commonly recommended, two doses are sometimes appropriate for some at-risk individuals. In addition, the simulation assumed that an age-based vaccination policy resulted in a higher VCR than one that was risk based. Indeed, an age-based strategy has been shown to be easier to implement than a risk-based strategy for the vaccination program.11,12
Our model did not take into account the protective effect that vaccinating infants with PCV7 has been demonstrated to have adults (i.e., herd immunity). In US, where the coverage rate in children was around 80–90% over the last 6 years, herd immunity has globally led to a 38% decrease in the rate of IPD among elderly.26 However, an increase of the incidence of serotypes not covered by PCV7 in adults and elderly people has been observed, and subsequently, PPV23 is the only protection available against these non-PCV serotypes.27 At present, Turkey has not undertaken universal immunization of infants with PCV7, although it is considering the implementation of such a program. The future effect of a PCV7 program in Turkey will depend on the VCR and serotype prevalence, and would not likely to be observed before the program is well established with a high VCR. However, in terms of the conclusions drawn on cost-effectiveness, PPV immunization should not changed, since its cost-effectiveness has recently been demonstrated by an analysis performed in the US.18 This study showed that vaccination remains economically attractive and cost-effective even if the incidence of pneumococcal disease has decreased among adults. Indeed, the lower IPD incidence in adults simply means that the Cost Effectiveness (CE) ratio must increase. Since the initial CE ratio was very low, even if doubled the cost-effectiveness of PPV23 for elderly and high risk adults would still be highly acceptable. Using the GDP threshold given by the WHO guidelines (strategy not cost-effective if superior to 3 times the GDP per capita i.e., superior to 47,400 YTL for Turkey), we evaluated the value of the IPD incidence where the public funding of PPV23 in elderly and at-risk people were not cost-effective. The IPD incidence should decrease by 60% (21 per 100,000) and 85% (7.9 per 100,000) from the base case values in elderly and in at-risk adults respectively in order to give an ICER not cost-effective that is higher than 47,400 YTL/QALYs.
The current VCR in Turkey of 2% of at-risk adults and elderly is very low despite the reimbursement of PPV23 in these groups since 2007. The reasons for this may implicate factors involving intervention by public health organizations or the perspective of physicians and the general public on pneumococcal vaccination. Low VCR can be explained by the lack of an effective vaccination program or absence of systems for delivering the vaccination, such as in the workplace, nursing homes or healthcare center.28,29 Indeed, in Turkey, every patient should visit pharmacy, buy the vaccine and then come to health care setting to receive prescribed vaccine, knowing that physicians can not offer PPV before the patient's discharge from their clinic. In addition, physicians have a low awareness of the risks of pneumococcal disease and the benefits of vaccination which affects their recommendation of PPV23, health care workers recommendation being the most important driver of the coverage rate's improvement.10 Lastly, other clinical priorities compete against vaccination programs and physicians delegate vaccination issues to the primary care settings. Offering vaccination in different places such as adult vaccination rooms or before discharging patient from hospital seems to increase such a low PPV23 vaccination rates in Turkey. Data from the active promotion of the benefits of pneumococcal vaccination were not included in this study since the results have been difficult to evaluate. However, costs saved by a pneumococcal vaccination program could be reinvested in the education of physicians and the public on the benefits of vaccination. In conclusion, this model suggests that a pneumococcal vaccination program in the elderly and at-risk populations in Turkey would be cost saving. These results are consistent with previous studies conducted with pneumococcal polysaccharide vaccines. Vaccination of the elderly with PPV23 is now publicly funded in Turkey. Nevertheless, the VCR in Turkey has been minimal, and its increase would improve public health. In addition, an awareness campaign to promote the benefits of pneumococcal vaccination in the elderly and at-risk adults should be undertaken in Turkey, targeting selected high-risk populations and the medical community. An appropriate use of the money saved as a result of pneumococcal vaccination would be to promote the benefits of vaccination and thereby raise the VCR.
Methods
Model specification and parameters used for the analysis.
A decision-analytic model was developed to estimate the effectiveness and cost-effectiveness of a catch-up PPV23 vaccination program in the adult population (>18 years old) with the opportunity to focus the analysis on an age-based or a risk-based vaccination program. Two identical hypothetical cohorts that were either vaccinated with the PPV23 or non-vaccinated were followed during a 5-year period (to provide a conservative estimate of the total duration of PPV23 effectiveness) split into 5 periods of 1 year. Each hypothetical cohort, vaccinated or non-vaccinated, was designed to reflect the Turkish elderly population (≥60 years of age) and at-risk adults (18–59 years old). The definition of people considered at high risk of developing pneumococcal disease was taken from the World Health Organization recommendations.4 Infections due to S. pneumoniae were classified as BPP and NBPP, which comprise around 90% of pneumococcal-related outcomes that are preventable by PPV23 vaccination.2
For each cohort, the number of life years experienced and the costs of pneumococcal infections were calculated and compared. To compare the costs and health consequences of vaccination versus non-vaccination, an ICER was estimated as the incremental cost per LYG. Since there are no health utility data available in Turkey, the quality of life gained due to vaccination was not evaluated. As recommended by the WHO health economic guidelines on vaccination, a discounted rate of 3% on costs and lives was applied in the base case analysis. The base case was conducted from a public payer perspective, i.e., all costs collected and used in the model were those borne by the government.
Demographic and epidemiological model inputs.
Representative demographic data on the Turkish population was based on the national statistics data from June 2008.30 It was assumed that 10% of the adult population (18–59 years old) was at risk from pneumococcal infection.31 The all-cause mortality rate was obtained from a local study conducted in 2004.31,32 For at-risk people from 18 to 59 years old, the conservative approach of all-cause mortality rate from the general population was used in the absence of specific data.
International pneumococcal incidence rates and casefatality rates were used as there were no Turkish data available (Table 2).4,33 However, fatality rates from a cost study on pneumococcal infections conducted in Ankara and described below, were consistent with these international data.4,34 Incidence rates and case-fatality rates related to S. pneumococcus were assumed to be similar between both elderly and at-risk adult populations in the absence of specific data. Hospitalization rates were calculated as 19.4% for NBPP, based on the National Burden of Disease Survey performed from March 2002 to December 2004. The total number of hospitalizations related to pneumonia was detected according to the data from different sources especially National Household Survey (12,000 households, sampling determined by TÜIK (Turkish Institute of Statistics) and the World Health Questionnaire utilized),35 and the Verbal Autopsy Survey (60,000 households, cases, hospitalizations and causes of death reported out).31,32 For BPP, the hospitalization rate was higher considering the severity of the disease. Sisk et al. supported a 100% hospitalization rate;17 Robinson et al. a 96%;48 Vila-Corcoles et al. reported 4.5% of IPD cases not hospitalized (1/22 cases) and therefore an hospitalization rate of 95.5%.7 In order to be conservative, 95.5% were used in the base case for BPP (Table 2). This parameter was varied (±20%) in the deterministic sensitivity analysis.
Vaccination model inputs.
A one-dose vaccination program was simulated in both populations. Vaccination coverage was assumed to be 60% in the elderly. In at-risk adults, a lower value was used considering the effect of a risk-based versus an aged-based public vaccination program.36 Indeed, a survey of IPD surveillance systems in the European Union, updated in 2006, demonstrated that coverage rates for at-risk people were: 36.5% (chronic heart disease), 34.7% (diabetes), 22.9% (immunosuppressed), 28.7% (chronic renal disease), 15.9% (sickle cell disease) and 12.6% (chronic respiratory disease).37 In our analysis, vaccination coverage was assumed to be 40% in at-risk adults.
Although multiple studies have been conducted over the past 30 years, the efficacy and effectiveness of PPV23 remain controversial.21,22 Numerous problems contribute to the difficulty of measuring the efficacy and effectiveness of this vaccine, including the low frequency of the most specific outcomes and the inaccuracy of the diagnostic criteria for more common outcomes.4
Pneumococcal vaccine effectiveness against IPD is the most firmly established, however the level of effectiveness depends on risk status and age. Meta-analyses of 10 randomized clinical trials involving 35,483 participants have shown that PPV23 is 74% (95% CI, 54–85) effective against IPD among generally healthy young adults and, to a lesser extent, in the general population of elderly. However, no significant effect of PPV23 vaccination on IPD was seen in adults with chronic diseases.21 Based on observational studies, the October 2008 WHO Weekly Epidemiological Record reported PPV23 to be 50–80% effective in preventing IPD among immunocompetent adults and individuals with various underlying illnesses who were not severely immunosuppressed.4 In our analyses, the values for vaccine effectiveness against IPD were based on multiple observational studies which had the advantage of being conducted in large unselected natural populations.7,25,38–43 One of these recent studies, conducted in Scotland in persons aged ≥65 years, demonstrated a protective effect of PPV23 against IPD of 61.7% (95% CI, 45.1–73.2).42 In Spain, a protective effect of 66% (95% CI, 27–66) against IPD was reported by Vila-Corcoles et al. in persons over 50 years old irrespective of their risk status.25 In addition, the same author reported PPV23 to be 72% (95% CI, 46–95) effective in persons over 60 years old, regardless of risk factor.38 These recent values were in line with the 14-year nationwide surveillance study conducted by the US CDC in the elderly and in persons with underlying chronic disease (effectiveness higher than 65%).39 As a result of these studies, effectiveness against BPP in the base case was assumed to be 60% for all elderly, which is the mean effectiveness of observational studies (Table 3), and 50%, which is the lower bound reported by the WHO for at-risk adults. In the sensitivity analyses, we applied a variation of ±10 onto the base cases which lead to an efficacy range of 50–70% and 40–60% for elderly and at-risk adults respectively.
The evidence for effectiveness of PPV23 against NBPP is less established mainly due to the difficulties in identifying S. pneumoniae as etiological agent of pneumonia. Meta-analysis on randomized clinical trials did not show effectiveness against NBPP or were inconclusive because of the heterogeneity of the results or the lack of power of the study.21,22 However, we considered observational studies to be more likely to estimate real-life effectiveness of the vaccine. For example, the systematic review of observational studies performed by Conaty et al. estimated the combined effectiveness of PPV23 vaccine to be 32% against all pneumonia.40 In addition, two recent clinical studies in Spain, in which bacteria were identified by radiography, sputum culture and Binex antigen test, demonstrated an effectiveness against NBPP of 42% (95% CI, 14–61) in people >50 years old and 39% (95% CI, −6–65) in elderly >65 irrespectively to the risk status.7,25 Consequently in the current analysis, since our populations were aged ≥60 years or between 18–59 years and at-risk, we considered a range of 0–42% effectiveness against NBPP for both population in the sensitivity analyses. The mean value of this range was used for the base case: 21%.
In addition, as reported in various publications, we decided to decrease vaccine effectiveness on BPP and NBPP over time, applying a waning rate of 10% each year in the elderly and at-risk populations.17,18,20,34,44,45 The total duration of effectiveness was fixed conservatively at 5 years.
Vaccination costs included the vaccine price and the procedure fees for vaccine administration were borne by the Turkish social security. According to local regulations, all pharmaceutical companies and pharmacies have to refund 11% and 3.5% respectively of total vaccine price to social security institution. Taking into consideration this local regulation, the total public vaccination cost used was 30.48 YTL (€16, mean exchange rate 2008: 1.9).
All vaccine inputs used in the modelling are presented in Table 3.
Cost of illness data.
Since data on the cost of pneumococcal infections in Turkey were not available in the literature, they were collected from two studies: a retrospective study conducted in Hacettepe University Hospital in Ankara in patients more than 18 years old with confirmed S. pneumoniae and a prospective study, also conducted in Hacettepe University Hospital in Ankara, on patients more than 18 years old with CAP (no bacteremic case). S. pneumoniae were isolated from sputum and blood for the retrospective study and confirmed cases were selected according to Pneumococcal-specific ICD 10-codes (J13 for NBPP and A40.3 for BPP). The risk status of patients were identified using the comorbidities placing them more at risk of getting pneumococcal infections or complications. The comorbidities considered were: chronic cardiac disease, chronic pulmonary disease, diabetes, chronic renal disease, rheumatologic disease, dementia or stroke, malignancy, immunosuppressive disorders and alcoholism. The perspective was from that of the public payers. Direct inpatient costs from both studies were the costs of procedures (e.g., X-ray and surgery), laboratory tests (serology, biochemistry and virology), medical examinations and treatments, as well as the costs associated with the length of stay in the intensive care unit and the general ward. Direct outpatient costs with and without hospitalization were only collected from the prospective study and covered the costs of medical visits, laboratory tests and treatment.
Variables were retrieved from patient files in the retrospective study and face-to-face discussions in the prospective study. Costs were calculated using the Republic of Turkey Ministry of Finance and General Directorate of budget and fiscal control formal health institution price tariff.
All cost of illness inputs used in the modelling are presented in Table 1.
Sensitivity analyses.
Under base-case assumptions, parameter values were varied individually in a one-way sensitivity analysis to identify if those variables with a mean value had a major impact on the cost-effectiveness results. All inputs were tested and their values are shown in Tables 2 and 3. Concerning the discount rate on lives, 0% and 5% were used respectively for the low and high value.
In addition, parameters listed in Tables 2 and 3 (NBPP incidence, BPP incidence, NBPP case fatality rate, BPP case fatality rate, Vaccine effectiveness against NBPP for both high-risk adults and elderly and Vaccine effectiveness against BPP) were varied simultaneously in probabilistic sensitivity analyses, where random draws from each parameter's distribution were performed and the effectiveness and cost-effectiveness calculated. This procedure was repeated 1,000 times. Parameter distributions were chosen based on the parameter type and level of certainty. Those parameters whose distributions were least certain (epidemiological data from international literature) were assigned uniform distributions, where all values in a range are equally likely to be chosen. Parameters whose distributions were most certain (cost data from a local cost of illness study) were assigned log-normal distributions.
Financial support
Sanofi Pasteur funded the cost of illness study.
Figure 3.
The results of the 1,000 Monte Carlo simulations represented on the cost-effectiveness plane for at-risk adults and the elderly. The black cycle represents the mean cost-effectiveness ratio.
Abbreviations
- ACIP
advisory committee of immunization practice
- BPP
bacteremic pneumococcal pneumonia
- CAP
community-acquired pneumonia
- ICER
incremental cost-effectiveness ratio
- IPD
invasive pneumococcal disease
- LYG
life-years gained
- NBPP
non-bacteremic pneumococcal pneumonia
- PPV
polysaccharide pneumococcal vaccination
- PCV
polysaccharide-protein conjugated vaccines
- PPV23
23-valent pneumococcal polysaccharide vaccine
- VCR
vaccine coverage rate
References
- 1.World Health Organization, author. Estimates of disease burden and cost-effectiveness. 2009. [Fev-2009]. http://who.int/immunization_monitoring/burden/estimates_burden/en/index.html.
- 2.Fedson DS, Neuzil KM. Pneumococcal polysaccharide vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. Elsevier Inc; 2004. [Google Scholar]
- 3.Fedson DS. Pneumococcal vaccination for older adults: the first 20 years. Drugs Aging. 1999;15:21–30. doi: 10.2165/00002512-199915001-00003. [DOI] [PubMed] [Google Scholar]
- 4.23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec. 2008;83:373–384. [PubMed] [Google Scholar]
- 5.Cartwright K. Pneumococcal disease in western Europe: burden of disease, antibiotic resistance and management. Eur J Pediatr. 2002;161:188–195. doi: 10.1007/s00431-001-0907-3. [DOI] [PubMed] [Google Scholar]
- 6.Rozenbaum MH, Sanders EA, van Hoek AJ, Jansen AG, van der EA, van den DG, et al. Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. BMJ. 2010;340:2509. doi: 10.1136/bmj.c2509. [DOI] [PubMed] [Google Scholar]
- 7.Vila-Corcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodriguez T, Llor C. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis. 2006;43:860–868. doi: 10.1086/507340. [DOI] [PubMed] [Google Scholar]
- 8.Hacýevliyagil SS, Mutlu LC. [Comparative Analysis of the cost of hospitalization of the patients hospitalized in Pulmonary Diseases Clinic] Tuberk Toraks. 2006;1:11–16. [Google Scholar]
- 9.Biberođlu K. [Importance of adult immunization. Where is adult immunization in Turkey?] Actual Medicine. 2006:18–24. [Google Scholar]
- 10.Blank PR, Schwenkglenks M, Szucs TD. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect. 2009;58:446–458. doi: 10.1016/j.jinf.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Melegaro A, Edmunds WJ. The 23-valent pneumococcal polysaccharide vaccine. Part II. A cost-effectiveness analysis for invasive disease in the elderly in England and Wales. Eur J Epidemiol. 2004;19:365–375. doi: 10.1023/b:ejep.0000024752.48929.bd. [DOI] [PubMed] [Google Scholar]
- 12.Weaver M, Krieger J, Castorina J, Walls M, Ciske S. Cost-effectiveness of combined outreach for the pneumococcal and influenza vaccines. Arch Intern Med. 2001;161:111–120. doi: 10.1001/archinte.161.1.111. [DOI] [PubMed] [Google Scholar]
- 13.TURKSTAT SPO, Annual Programme 2009. It can be reachable at http://www.hazine.gov.tr/irj/go/km/docs/documents/Treasury%20Web/Statistics/Economic%20Indicators/egosterge/Sunumlar/Ekonomi_Sunumu_ENG.pdf.
- 14.Ament A, Fedson DS, Christie P. Pneumococcal vaccination and pneumonia: even a low level of clinical effectiveness is highly cost-effective. Clin Infect Dis. 2001;33:2078–2079. doi: 10.1086/324356. [DOI] [PubMed] [Google Scholar]
- 15.Evers SM, Ament AJ, Colombo GL, Konradsen HB, Reinert RR, Sauerland D, et al. Cost-effectiveness of pneumococcal vaccination for prevention of invasive pneumococcal disease in the elderly: an update for 10 western European countries. Eur J Clin Microbiol Infect Dis. 2007;26:531–540. doi: 10.1007/s10096-007-0327-z. [DOI] [PubMed] [Google Scholar]
- 16.Mangtani P, Roberts JA, Hall AJ, Cutts FT. An economic analysis of a pneumococcal vaccine programme in people aged over 64 years in a developed country setting. Int J Epidemiol. 2005;34:565–574. doi: 10.1093/ije/dyh341. [DOI] [PubMed] [Google Scholar]
- 17.Sisk JE, Moskowitz AJ, Whang W, Lin JD, Fedson DS, McBean AM, et al. Cost-effectiveness of vaccination against pneumococcal bacteremia among elderly people. JAMA. 1997;278:1333–1339. [PubMed] [Google Scholar]
- 18.Smith KJ, Zimmerman RK, Lin CJ, Nowalk MP, Ko FS, McEllistrem MC, Roberts MS. Alternative strategies for adult pneumococcal polysaccharide vaccination: a cost-effectiveness analysis. Vaccine. 2008;26:1420–1431. doi: 10.1016/j.vaccine.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Evers SM, Ament AJ, Colombo GL, Konradsen HB, Reinert RR, Sauerland D, et al. Cost-effectiveness of pneumococcal vaccination for prevention of invasive pneumococcal disease in the elderly: an update for 10 western European countries. Eur J Clin Microbiol Infect Dis. 2007;26:531–540. doi: 10.1007/s10096-007-0327-z. [DOI] [PubMed] [Google Scholar]
- 20.Merito M, Giorgi RP, Mantovani J, Curtale F, Borgia P, Guasticchi G. Cost-effectiveness of vaccinating for invasive pneumococcal disease in the elderly in the Lazio region of Italy. Vaccine. 2007;25:458–465. doi: 10.1016/j.vaccine.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008:422. doi: 10.1002/14651858.CD000422.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vila-Corcoles A. Is the pneumococcal polysaccharide vaccine effective in preventing pneumonia? Lancet Infect Dis. 2008;8:405–406. doi: 10.1016/S1473-3099(08)70134-8. [DOI] [PubMed] [Google Scholar]
- 24.Fedson DS, Liss C. Precise answers to the wrong question: prospective clinical trials and the meta-analyses of pneumococcal vaccine in elderly and high-risk adults. Vaccine. 2004;22:927–946. doi: 10.1016/j.vaccine.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Vila-Corcoles A, Salsench E, Rodriguez-Blanco T, Ochoa-Gondar O, de Diego C, Valdivieso A, et al. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in middle-aged and older adults: a matched case-control study. Vaccine. 2009;27:1504–1510. doi: 10.1016/j.vaccine.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis. 2008;47:1328–1338. doi: 10.1086/592691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ACIP Pneumococcal Vaccines Workgroup, author. Use of pneumococcal polysaccharide vaccine (PPV23) in adults aged >50 years. 2008. Jun 25, [3-5-09]. Available at: http://www cdc gov/vaccines/recs/acip/downloads/mtg-slides-jun08/03-1-pneu pdf.
- 28.Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 29.Whitney CG, Schaffner W, Butler JC. Rethinking recommendations for use of pneumococcal vaccines in adults. Clin Infect Dis. 2001;33:662–675. doi: 10.1086/322676. [DOI] [PubMed] [Google Scholar]
- 30.Turkish Statistical Institute, author. Population Statistics and Projections. 2009. [20-2-09]. Available at http://www.turkstat.gov.tr/VeriBilgi.do?tb_id=39&ust_id=11.
- 31.MoH, author. Turkey burden of disease study. Refik Saydam Hygiene Center Presidency, School of Public Health; 2006. [Google Scholar]
- 32.MoH, author. Turkey burden of disease study. Refik Saydam Hygiene Center Presidency, School of Public Health; 2004. [Google Scholar]
- 33.Jackson ML, Neuzil KM, Thompson WW, Shay DK, Yu O, Hanson CA, Jackson LA. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis. 2004;39:1642–1650. doi: 10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Middleton DB, Lin CJ, Smith KJ, Zimmerman RK, Nowalk MP, Roberts MS, Fox DE. Economic evaluation of standing order programs for pneumococcal vaccination of hospitalized elderly patients. Infect Control Hosp Epidemiol. 2008;29:385–894. doi: 10.1086/587155. [DOI] [PubMed] [Google Scholar]
- 35.National Household Survey, MoH, Refik Saydam Hygiene Center, School of Public Health Ministry of Health Publication No: 701HM-SB-HM-2007/11. Ankara, Turkey: 2003. [Google Scholar]
- 36.Honkanen PO, Keistinen T, Kivela SL. The impact of vaccination strategy and methods of information on influenza and pneumococcal vaccination coverage in the elderly population. Vaccine. 1997;15:317–320. doi: 10.1016/s0264-410x(96)00171-5. [DOI] [PubMed] [Google Scholar]
- 37.Pebody RG, Hellenbrand W, D'Ancona F, Ruutu P. Pneumococcal disease surveillance in Europe. Euro Surveill. 2006;11:171–178. [PubMed] [Google Scholar]
- 38.Vila-Corcoles A, Ochoa-Gondar O, Guzman JA, Rodriguez-Blanco T, Salsench E, Fuentes CM. Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older. BMC Infect Dis. 2010;10:73. doi: 10.1186/1471-2334-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA. 1993;270:1826–1831. [PubMed] [Google Scholar]
- 40.Conaty S, Watson L, Dinnes J, Waugh N. The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials. Vaccine. 2004;22:3214–3224. doi: 10.1016/j.vaccine.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 41.Dominguez A, Salleras L, Fedson DS, Izquierdo C, Ruiz L, Ciruela P, et al. Effectiveness of pneumococcal vaccination for elderly people in Catalonia, Spain: a case-control study. Clin Infect Dis. 2005;40:1250–1257. doi: 10.1086/429236. [DOI] [PubMed] [Google Scholar]
- 42.Mooney JD, Weir A, McMenamin J, Ritchie LD, Macfarlane TV, Simpson CR, et al. The impact and effectiveness of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infect Dis. 2008;8:53. doi: 10.1186/1471-2334-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 44.Postma MJ, Heijnen ML, Jager JC. Cost-effectiveness analysis of pneumococcal vaccination for elderly individuals in The Netherlands. Pharmacoeconomics. 2001;19(2):215–222. doi: 10.2165/00019053-200119020-00008. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 46.Jackson ML, Neuzil KM, Thompson WW, Shay DK, Yu O, Hanson CA, Jackson LA. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis. 2004;39:1642–1650. doi: 10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaplan V, Angus DC, Griffin MF, Clermont G, Scott Watson R, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165:766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 48.Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: Opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285:1729–1735. doi: 10.1001/jama.285.13.1729. [DOI] [PubMed] [Google Scholar]