Abstract
“We do not grow absolutely, chronologically. We grow sometimes in one dimension, and not in another; unevenly. We grow partially. We are relative. We are mature in one realm, childish in another. The past, present and future mingle and pull us backward, forward, or fix us in the present. We are made up of layers, cells, constellations.”—Anaïs Nin
It has long been recognized that the developing immune system exhibits certain peculiarities when compared to the adult immune system. Nonetheless, many still regard the fetal immune system as simply being an immature version of the adult immune system. Here we discuss historical evidence as well as recent findings, which suggest that the human immune system may develop in distinct layers with specific functions at different stages of development.
Key words: fetal, tolerance, hematopoiesis, immune system, hematopoietic stem cell
A Brief Historical Perspective on the Development of Lymphocytes in Mammals
Over two decades ago, a model was proposed by Leonore and Leonard Herzenberg which suggested that the immune system in mammals did not arise in a linear fashion from immaturity to maturity, as is often believed, but rather in distinct layers which could perform unique functions at different stages of development.1 This model was supported by a series of observations, made first in avian species2,3 and later in the mouse,4–10 that revealed the existence of unique waves of lymphocyte production during fetal and neonatal development.
In the early 1980s, Nicole Le Dourain and colleagues demonstrated in a quail/chick chimeric model that the avian thymus is seeded by three distinct waves of thymocytes, temporally defined during the course of fetal development.2,3 The final wave occurred around hatching time and was observed to almost completely replace the earlier waves of precursors. However, despite the elegant demonstration that distinct layers of thymopoiesis can be observed in this model, the functional significance of these observations remained largely unknown.
The invention of multiparameter flow cytometry facilitated additional examination of the phenotype and function of distinct lymphocyte populations that arose during development.11,12 By comparing surface marker expression, two distinct B cell populations (termed B-1 and B-2 lymphocytes) could be identified based on the expression of CD5 (originally named Ly-1).12 CD5+ B cells, which later became known as B-1 cells, predominated during neonatal life in mice and were primarily localized to the peritoneum.4–6,13 Using adoptive transfer experiments, it was demonstrated that injection of mature B-1 B cells into congenic recipients led to repopulation of the B-1 B cell compartment but not to “conventional” CD5-B cells (B-2 B cells).5 Reciprocally, transfer of adult bone marrow resulted in the generation of B-2 B cells but very few B-1 B cells.5 These observations raised the hypothesis that the immune system was stratified into layers with successive populations of distinct hematopoietic stem cells (HSC), each giving rise to distinct lymphocyte populations at varying stages of development.14 Decades later, it was conclusively shown that a specific B-1 progenitor cell existed primarily during fetal development and was replaced by B-2 progenitor cells after birth, a finding that provided strong evidence in support of the “layered immune system” hypothesis.7
As the early work on B-1 and B-2 lineages of B cells was being reported, work from the laboratories of James Allison and Irving Weissman provided equally compelling evidence supporting the existence of a layered immune system in the mouse with respect to T-cell development.8–10 Again using multiparameter flow cytometry, it became apparent that the first wave of thymocytes that developed in the fetal mouse thymus was considerably different from that which appeared later during adult life.8,9 Thus, the initial wave of thymocytes was almost entirely composed of cells with a γ/δ T-cell receptor (TCR) whereas later waves of thymocytes expressed α/β TCR instead. The first wave of γ/δ T cells also bore a very specific γ/δ TCR (Vγ3/Vδ1), permitting ready identification with Vγ3/Vδ1 TCR antibodies.8 These cells emerged during the initial wave of thymopoiesis and were then almost entirely replaced by a second wave of cells bearing a more diverse array of γ/δ TCRs.8,9 In the days leading up to birth, the γ/δ T cells in the fetal thymus are gradually replaced by α/β T cells, which then predominate throughout adulthood. Following the initial characterization of Vγ3/Vδ1 T cells, it was shown definitively that fetal but not adult HSC could give rise to this population of T cells, again providing evidence for a layered model of T-cell development, akin to that seen for B-1 and B-2 B cells.10
Despite compelling evidence supporting the existence of a layered immune system throughout development, work in this area tapered off following these initial discoveries and was primarily limited to studies specializing on B-1 and B-2 B.cell development. For this reason, the concept of a layered immune system has remained largely unknown by many outside of these specialized fields. In fact, much of the literature continues to invoke a linear model for development in which the early immune system is “immature” and, after antigenic exposure at the time of birth, becomes “mature” (reviewed in ref. 15). In this sense, “maturity” refers to antigen experience and the establishment of immunological “memory” (or the capacity for recall responses) to specific pathogens encountered throughout the lifetime of an organism.
Development of the Human Fetal Adaptive Immune System
Recent work on the development of the human fetal T-cell compartment suggests that, as in murine and avian species, the human adaptive immune system is also built upon distinct layers of hematopoiesis that arise during different stages of ontogeny.16 These layers are comprised of T cells with distinct functions specific to different stages of development, with fetal T cells promoting tolerance and adult T cells being more likely to engage in immunoreactive responses to foreign antigens instead (Fig. 1). Importantly, our findings suggest a model that is consistent with observations that the fetal immune system is compromised in its capacity to generate sterilizing immunity, yet also incorporates the potential for the early fetal immune system to play an active role in establishing tolerance during development. Thus, the concept of a layered immune system is consistent with seemingly incongruent observations regarding reduced immunological function during fetal and neonatal life,15,17 and the capacity for tolerance induction in the developing human fetus.18–20 (Of note, because we have specifically focused on the role of the peripheral immune system in responding to foreign antigens, we have neglected to discuss the critical role that central tolerance through thymic deletion of reactive T cells plays during development. Clearly, there is a large body of evidence demonstrating the crucial role that thymic selection plays in promoting tolerance to both self and foreign antigens throughout all stages of development. A more detailed discussion of the potential for overlapping roles of central and peripheral tolerance as well as specific functions of each process in maintaining fetal tolerance will thus be reserved for a review in a later issue of Chimerism.)
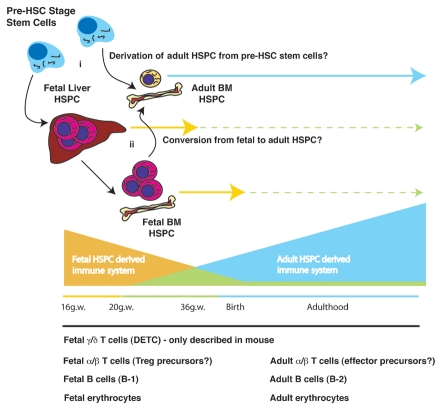
Figure 1.
Model for transition from fetal to adult hematopoiesis. Several potential scenarios could account for the layering of the adaptive immune sysytem during development. Based on the linear decline in the frequency of Treg cells in umbilical cord blood across the third trimester of development, we propose that a shift in HSC identity from fetal to adult occurs at some point during this time. Whether this occurs through (i) the de novo generation of adult HSC from an upstrean progenitor cell or (ii) from a direct conversion of fetal HSC to adult-type HSC remains unknown. Examples of different hematopoietic lineages that have been shown to arise during fetal development and after birth are listed below the figure. HSPC, hematopoietic stem and progenitor cells.
As mentioned above, the concept of “layering” bears particular importance for understanding how immunological tolerance can develop in the fetus. Over 50 years has passed since Billingham, Brent and Medawar's seminal work demonstrating that immunological tolerance could be achieved to a foreign antigen if it was administered prior to “maturation” of the adaptive immune system.21 Decades later, clinical evidence obtained in transplant recipients revealed a similar type of tolerance to non-inherited maternal alloantigens (NIMA).18,19 The presumptive conclusion drawn from these clinical findings was that the fetus had somehow come in contact with maternal cells during development and that this led to the acquisition of tolerance, akin to that seen in experimental tolerance induction in mice. However, and in contrast to laboratory mice, an adaptive immune system develops in many larger mammals (including human beings) at early stages of fetal development.22–27 Thus, the appearance of immunological tolerance cannot be explained by a failure to generate adaptive immunity to foreign antigen, as was initially thought in mice.
Several clues emerged early on that led to the conclusion that the human fetal immune system might function differently from that of the adult. First, several independent laboratories reported that the human fetal immune system appeared to be enriched for CD4+CD25+ regulatory T cells (Treg) at early stages of development, e.g., as early as the 1st trimester (as opposed to after birth in the case of the laboratory mouse).28–30 These cells were nearly identical to those that had been previously described in the adult human with respect to both phenotype and function. In earlier studies by Max Cooper and colleagues, this population had been identified as a group of T cells that may have become spontaneously activated within the peripheral lymphoid organs of the fetus.31 This early report came at a time when CD25 was considered a marker of recent T-cell activation (and not, as we now also know it, a marker of Tregs).32–34 However, using this marker in combination with the transcription factor, Foxp3, it was possible to conclusively show that these fetal CD4+CD25high cells were, in fact, Tregs.35,36 The relative frequency of Tregs in the mid-gestation human fetus was found to be substantially greater than that observed in newborns or in adults.30 Analysis of the relative frequencies of Tregs within the CD4+ T-cell compartment over the course of fetal development demonstrated a linear decrease in this population spanning the third trimester of development and reaching adult levels near birth.37
Another surprising result was that, after depletion of the fetal Treg population from total fetal lymph node or splenocyte preparations, the remaining T cells proliferated readily and produced the inflammatory cytokine, IFNγ.30 While this observation was made in vitro, it corroborated observations made in vivo in the rare inherited disorder, immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX).38,39 IPEX results from mutations in the gene encoding the transcription factor, Foxp3, which is critical for Treg development and function.35 In severe cases, fetal demise can result from a massive multi-organ autoimmune response that originates prior to 30 gestational weeks of fetal development.40 Thus, in the absence of the appropriate development of Tregs in utero, the fetal immune system is fully competent to initiate inflammatory responses that can prove fatal to the developing fetus.
Tolerance to Maternal Alloantigens in the Human Fetus
Rather than attempt to define potential autoantigens that the fetal Treg may recognize, we instead chose to investigate how fetal immunity to NIMA might relate to the apparent tolerogenic properties that are observed in transplantation settings.20 We chose NIMA because of the potential for larger numbers of cells that could respond to a given alloantigen when compared with individual autoantigens.41 Our findings suggested a potential model for how tolerance could be achieved to antigens perceived by the fetal immune system in utero.42 We and others showed that human fetal lymphoid tissues, and some non-lymphoid tissues, contained small but detectable levels of maternal cells.20,43 Fetal immunity to NIMA was found to be reduced compared to fetal T-cell responses to allogeneic cells from unrelated donors. Depletion of fetal Treg from total fetal lymph node cultures resulted in a significant increase in fetal T-cell responses to NIMA, suggesting that fetal Tregs are capable of suppressing fetal anti-maternal T-cell responses. Finally, we found that activation of “naïve” fetal CD4+ T cells with allogeneic cells resulted in a large fraction of the fetal T cells adopting a Treg fate, as revealed by upregulation of Foxp3 and acquisition of suppressor function. The generation of fetal Treg from naïve CD4+ T cells was dependent upon TGFβ signaling and TGFβ family members were found to be enriched in fetal lymphoid tissues compared to adults. Thus, it appears that the fetal lymphoid tissues are specialized to promote Treg cell differentiation through the production of TGFβ, implying that the fetal immune system is prone to tolerance.
Our initial results clearly suggested that a propensity towards immune tolerance could be operational in the developing human fetus. However, there were still several aspects of the fetal immune response that remained unexplained. First, the frequency of fetal T cells that responded to antigen presenting cells from a single allogeneic donor was far greater than that observed in parallel cultures of adult cells. Additionally, many non-responding fetal T cells appeared to die during the short culture periods in vitro (i.e., within 3–5 days). Finally, fetal Tregs were found to be highly sensitive to IL-2 and proliferated extensively in vitro in the absence of TCR stimulation, usually a strict requirement for conventional “adult” Tregs (20 and unpublished observations).
To explore these observations further, we performed a global gene expression analysis of fetal and adult T-cell subpopulations. This analysis demonstrated that there is a substantially different gene expression program in fetal and adult T cells that are otherwise phenotypically identical.16 In addition, a large number of the genes that were found to be differentially expressed by fetal and adult naïve T cells were also differentially expressed by fetal and adult Tregs, suggesting a specific fetal and adult gene signature that is shared by different lymphocyte populations. In all, it appeared from this analysis that fetal and adult T cells could represent distinct cell lineages (see model in Fig. 1).
Given the earlier work in mice and birds from the Herzenbergs, Le Dourain, and Weissman, we decided to test for the presence of a “layered immune system” in humans. To do so, enriched populations of human fetal and adult Lin-CD34+CD38+/− hematopoietic stem and progenitor cells (HSPCs) were injected into the irradiated Thy/Liv implants in SCID-hu mice, a model which had previously been shown to support multilineage human hematopoiesis. Our findings confirmed our initial hypothesis that fetal and adult HSC were capable of generating mature lymphocytes that, while phenotypically similar, exhibited striking differences in gene expression and functional attributes. Importantly, we found that CD3+CD4+ “single positive” thymocytes derived from fetal HSPC were predisposed to generating Tregs both during thymic maturation and following activation by alloantigens in vitro. Thus, we could recapitulate the in vitro observations made concerning fetal and adult peripheral naïve CD4+ T cells.16
These findings offer a revised version of how fetal tolerance can be achieved in human beings. In this new model, we believe that fetal HSC produce lymphocytes that are predisposed to generating tolerance. In conjunction with elevated levels of TGFβ family members in fetal lymphoid tissues, such T-cell maturation promotes an environment that is designed to instill a tolerogenic program for all cells activated in the fetal periphery. Additionally, and through a mechanism that remains undefined, fetal T cells appear to be highly susceptible to activation.16,20 Accordingly, a fetal T cell enters the fetal peripheral tissues ready to respond to antigens and to differentiate into Tregs. In this manner, the initial wave of fetal T cells provides a peripheral Treg repertoire that is specialized to suppress immunity towards the vast array of antigens (both self and non-self) present in the fetal periphery. This adds an additional layer of immunological tolerance to that achieved by central tolerance in the thymus, which is responsible for deleting autoreactive T-cell clones as well as generating Tregs specific for thymically expressed (presumably self ) antigens. These Tregs would then be situated in the peripheral tissues ready to suppress any autoreactive T-cell clones that might escape thymic deletion as additional waves of mature lymphocytes enter the periphery.
Mixed Chimerism and Immunological Tolerance
In retrospect, when revisiting the work of Medawar on induced immunological tolerance in mice, it is important to appreciate the fact that this work finds its roots in earlier observations made by Ray Owen in Freemartin cattle twins.44 Thus, Owen initially noted that evidence of substantial mixed chimerism could be observed in the circulating blood of fraternal (non-identical) cattle twins, suggesting the development of lasting immunological tolerance to foreign antigens in this setting. Later, Medawar would experimentally prove that immunological tolerance could arise in cattle when he failed at devising a strategy to determine whether cattle twins were fraternal or identical through transplantation of skin grafts between siblings.45 Though this work would be much less heralded than the later demonstrations in murine models,21 the early work in cattle may be more reflective of what happens in human beings. Like humans, cattle develop a peripheral adaptive immune system during fetal development.46 Thus, acquired tolerance to blood cells trafficking between genetically distinct cattle twins may not necessarily arise from a failure to generate an adaptive immune response. In subsequent studies, it was noted that skin grafts transplanted between heterozygotic cattle twins were not always tolerated, although they tended to be better accepted than those from unrelated donors.47 Given our current understanding of the fetal immune response, it would be interesting to revisit these observations and determine the role of the peripheral adaptive immune system in the development of tolerance in Freemartin cattle.
As was discussed previously, it is becoming increasingly recognized that mixed chimerism is a common occurrence in nature.48 During pregnancy, there is evidence that cells with multilineage potential passage between the mother and fetus and can engraft a broad array of tissues in each individual, persisting long after birth.43,48–51 Thus, it is interesting to consider whether the existence of chimerism in human pregnancy may reflect an active process, rather than an accidental event that results from a less than perfect barrier between the mother and fetus. Interestingly, there are situations that have been observed in nature, where cells with multilineage potential traffic between genetically disparate organisms in the same species and establish mixed chimerism.52–55
This process, called stem cell parasitism, is commonplace in colonial tunicates (Botryllus schlosseri).52,53 This species of tunicate has evolved an immunological process by which to perceive stem cell parasitism and to discriminate between self and non-self antigens through a mechanism that has been compared to human allorecognition.54,55 Thus, the study of colonial tunicate allorecognition provides some clues about the most primitive immunological recognition system described in nature, a system that appears solely designed to discriminate self and non-self in the setting of mixed chimerism. In this context, it is interesting to speculate how the existence of stem cell parasitism may have influenced the development of the immune system. In fact, it has been suggested that the immune system may have first evolved to perceive stem cell parasitism in colonial organisms.56 Understanding the mechanisms underlying immunological recognition of stem cell parasitism in primitive colonial organisms might provide some insight into the regulation of chimerism in human beings and other viviparous mammals, and the nature of the immune response to chimeric cells.
Layered Immunity and Fetal Tolerance: Impact on Fetal Exposure to Foreign Organisms
One of the most intriguing questions raised by these findings concerns how and when the switch between fetal and adult immune development occurs. The sharp decline in the frequency of peripheral Tregs in human cord blood during the third trimester suggests that the switch in lymphocyte development is likely to occur at this time.37 Perhaps the best evidence for the existence of a switch in T cell lineages during development comes from labeling studies performed in fetal sheep.57–59 Sheep, like humans, produce large numbers of peripheral T cells during fetal development. By examining the incorporation of 3H-thymidine into fetal naïve T cells during fetal development and shortly after birth, it was shown that nearly 75% of fetal naïve T cells are replaced by newly formed T cells at the time immediately following birth in sheep.59 Whether this represents a shift in T-cell production from fetal to adult lineages remains to be determined. However, this pattern of T-cell turnover fits nicely with the layered model for immunological development. A mechanism to ensure that fetal T-cell populations (prone to developing into tolerogenic Tregs) are replaced at a time when the newborn is first exposed to potentially harmful pathogens would likely be required to prevent lethal infections after birth.
This raises a final intriguing question concerning the potential for intrauterine exposure to foreign antigens, including those on infectious agents, during development. The ability of maternal cells to cross through the placenta into the fetus is becoming increasingly accepted as a common occurrence during normal pregnancy, as is the establishment of persistent maternal microchimerism in multiple distinct tissues well into adulthood. If maternal cells can cross the placenta, it seems likely that other foreign antigens (including infectious agents that are much smaller than a cell) will cross the placenta as well. If this is the case, our current model predicts that the fetus will generate Tregs that can specifically shut down immune responses against these organisms, making infection with them even more hazardous and potentially lethal. However, not all pathogens may be equal in this regard. In some cases, it may be beneficial to have a mechanism for dampening immunity to infectious organisms. Viruses like Human Immunodeficiency Virus (HIV) and Hepatitis C (HCV), for instance, can establish chronic infections in humans and it is believed that much of the damage that is caused by these viruses results from persistent inflammation rather than direct cytopathic effects of the virus itself.27 Thus, immunological tolerance to these chronic infections might actually benefit the host. However, this situation may not pertain to all fetal infections. Recent evidence suggests that fetal exposure to Plasmodium falciporum, the causative agent of malaria, leads to increased incidence of adverse effects in the form of reduced immunological control of infections occurring after birth.60 An increase in the frequency of Tregs specific for P. falciparum antigens has been implicated in these outcomes.61 As such, it will be important to consider the nature of different infections when developing strategies for intrauterine vaccinations.
Conclusions and Future Directions
The potential existence of an alternative and tolerogenic immune system that forms during fetal development has implications that extend far beyond those discussed in this brief review. Although we have discussed how tolerance could impact maternal microchimerism in the fetus and adult as well as how intrauterine infections could be impacted by a tolerogenic immune response, we have not begun to consider the impact on neonatal immunity and the development of autoimmune disorders and allergies. Many believe that fetal and neonatal exposure to foreign antigens could have significant effects on the development of these disorders later in life. Whether this is impacted by differences in the contribution of fetal and adult lymphocyte subsets present at birth remains completely unknown. Likewise, we have only focused on a small subset of immune cells in the fetus and adult. Given our findings, coupled with previous findings in other model organisms, it is highly likely that multiple T.and B.cell subsets as well as other hematopoietic lineages (e.g., erythroid and myeloid cells) exhibit a layered pattern of development (see Fig. 1 for examples). As we still do not know the mechanisms underlying the shift from the fetal to adult HSC pool, or the timeframe in which this occurs, it is possible that additional “layers” of immune cells might also exist. We hope that our current findings provide a starting point from which to address these questions and that an increased understanding of the development of the immune system in humans will lead to novel strategies to develop therapeutic interventions for a range of human health concerns.
References
- 1.Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 2.Le Douarin NM, Jotereau FV. Tracing of cells of the avian thymus through embryonic life in interspecific chimeras. J Exp Med. 1975;142:17–40. doi: 10.1084/jem.142.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jotereau FV, Le Douarin NM. Demonstration of a cyclic renewal of the lymphocyte precursor cells in the quail thymus during embryonic and perinatal life. J Immunol. 1982;129:1869–1877. [PubMed] [Google Scholar]
- 4.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalor PA, Stall AM, Adams S, Herzenberg LA. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur J Immunol. 1989;19:501–506. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- 6.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 8.Havran WL, Allison JP. Develomentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 9.Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- 10.Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien YH, Weissman IL. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- 11.Lanier LL, Warner NL, Ledbetter JA, Herzenberg LA. Quantitative immunofluorescent analysis of surface phenotypes of murine B cell lymphomas with monoclonal antibodies. J Immunol. 1981;127:1691–1697. [PubMed] [Google Scholar]
- 12.Hardy RR, Hayakawa K, Haaijman J, Herzenberg LA. B-cell subpopulations identified by two-colour fluorescence analysis. Nature. 1982;297:589–591. doi: 10.1038/297589a0. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa K, Hardy RR, Herzenberg LA. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986;16:450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- 14.Herzenberg LA, Kantor AB, Herzenberg LA. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann NY Acad Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 15.Remington JS, Klein JO, Wilson CB, Baker CJ. Infectious Diseases of the Fetus and Newborn Infant. Philadelphia: Elsevier Saunders; 2006. pp. 11–16. [Google Scholar]
- 16.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaälsson J, Rivera JM, Galkina SA, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nature Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 18.Claas FH, Gijbels Y, van der velden-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815–1817. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- 19.Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, et al. The effect of tolerance to non-inherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657–1664. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 20.Mold JE, Michaälsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 22.Silverstein AM, Lukes RJ. Fetal response to antigenic stimulus. I. Plasmacellular and lymphoid reactions in the human fetus to intrauterine infection. Lab Invest. 1962;11:918–932. [PubMed] [Google Scholar]
- 23.Silverstein AM. Congenital syphilis and the timing of immunogenesis in the human foetus. Nature. 1962;194:196–197. doi: 10.1038/194196a0. [DOI] [PubMed] [Google Scholar]
- 24.Silverstein AM, Uhr JW, Kraner KL, Lukes RJ. Fetal response to antigenic stimulus. II. Antibody production by the fetal lamb. J Exp Med. 1963:799–812. doi: 10.1084/jem.117.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverstein AM, Thorbecke GJ, Kraner KL, Lukes RJ. Fetal response to antigenic stimulus. III. Gamma-globulin production in normal and stimulated fetal lambs. J Immunol. 1963;91:384–395. [PubMed] [Google Scholar]
- 26.Silverstein AM, Prendergast RA, Kraner KL. Fetal response to antigenic stimulus. IV. Rejection of skin homografts by the fetal lamb. J Exp Med. 1964;119:955–964. doi: 10.1084/jem.119.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverstein AM. Ontogeny of the immune response. Science. 1964;144:1423–1428. doi: 10.1126/science.144.3625.1423. [DOI] [PubMed] [Google Scholar]
- 28.Cupedo T, Nagasawa M, Weijer K, Blom B, Spits H. Development and activation of regulatory T cells in the human fetus. Eur J Immunol. 2005;35:383–390. doi: 10.1002/eji.200425763. [DOI] [PubMed] [Google Scholar]
- 29.Darrasse-Jèze G, Marodon G, Salomon BL, Catala M, Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 2005;105:4715–4721. doi: 10.1182/blood-2004-10-4051. [DOI] [PubMed] [Google Scholar]
- 30.Michaälsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 31.Byrne JA, Stankovic AK, Cooper MD. A novel subpopulation of primed T cells in the human fetus. J Immunol. 1994;152:3098–3106. [PubMed] [Google Scholar]
- 32.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains. Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 33.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 36.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Takahata Y, Nomura A, Takada H, Ohga S, Furuno K, Hikino S, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naïve phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32:622–629. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrom is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 39.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 40.Levy-Lahad E, Wildin RS. Neonatal diabetes mellitus, enteropathy, thrombocytopenia and endocrinopathy: further evidence for an X-linked lethal syndrome. J Pediatr. 2001;138:577–580. doi: 10.1067/mpd.2001.111502. [DOI] [PubMed] [Google Scholar]
- 41.Lindahl KF, Wilson DB. Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. J Exp Med. 1977;145:508–522. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burlingham WJ. A lesson in tolerance—Maternal instruction to fetal cells. NEJM. 2009;360:1355–1357. doi: 10.1056/NEJMcibr0810752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonsson AM, Uzunel M, Götherström C, Papdogiannakis N, Westgren M. Maternal microchimerism in human fetal tissues. Am J Obstet Gynecol. 2008;198:1–6. doi: 10.1016/j.ajog.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 44.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 45.Billingham RE, Lampkin GH, Medawar PB, Williams HLL. Tolerance to homografts, twin diagnosis and the freemartin condition in cattle. Heredity. 1952;6:201–212. [Google Scholar]
- 46.Tierney TJ, Simpson-Morgan MW. The profilerative responses of lymphocytes from foetal calves and adult cattle. Vet Immunol Immunopathol. 1997;59:49–64. doi: 10.1016/s0165-2427(97)00057-3. [DOI] [PubMed] [Google Scholar]
- 47.Stone WH, Cragle RG, Swanson EW, Brown DG. Skin grafts: Delayed rejection between pairs of cattle twins showing erythrocyte chimerism. Science. 1965;148:1335–1356. doi: 10.1126/science.148.3675.1335. [DOI] [PubMed] [Google Scholar]
- 48.Nelson JL. Your cells are my cells. Sci Am. 2008;298:64–71. [PubMed] [Google Scholar]
- 49.Evans PC, Lambert N, Maloney S, Furst DE, Moore JM, Nelson JL. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999;93:2033–2037. [PubMed] [Google Scholar]
- 50.Stevens AM, McDonnell WM, Mullarkey ME, Pang JM, Leisenring W, Nelson JL. Liver biopsies from human females contain male hepatocytes in the absence of transplantation. Lab Invest. 2004;84:1603–1609. doi: 10.1038/labinvest.3700193. [DOI] [PubMed] [Google Scholar]
- 51.Adams KM, Nelson JL. Microchimerism: an investigative frontier in autoimmunity and transplantation. JAMA. 2004;291:1127–1131. doi: 10.1001/jama.291.9.1127. [DOI] [PubMed] [Google Scholar]
- 52.Voskoboynik A, Rinkevich B, Weissman IL. Stem cells, chimerism and tolerance: Lessons from mammals and ascidians. In: Rinkevich B, Matranga V, editors. Stem Cells in Marine Organisms. New York: Springer; 2009. pp. 281–308. [Google Scholar]
- 53.Laird DJ, De Tomaso AW, Weissman IL. Stem cells are units of natural selection in a colonial ascidian. Cell. 2005;123:1351–1360. doi: 10.1016/j.cell.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 54.De Tomaso AW, Nyholm SV, Palmeri KJ, Ishizuka KJ, Ludington WB, Mitchel K, Weissman IL. Isolation and characterization of a protochordate histocompatibility locus. Nature. 2005;438:454–459. doi: 10.1038/nature04150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Tomaso AW, Weissman IL. Evolution of a protochordate allorecognition locus. Science. 2004;303:977. doi: 10.1126/science.1094952. [DOI] [PubMed] [Google Scholar]
- 56.De Tomaso AW. Sea squirts and immune tolerance. Dis Model Mech. 2009;2:440–445. doi: 10.1242/dmm.001156. [DOI] [PubMed] [Google Scholar]
- 57.Kimpton WG, Washington EA, Cahill RN. Recirculation of lymphocyte subsets (CD5+, CD4+, CD8+, T19+ and B cells) through fetal lymph nodes. Immunology. 1989;68:575–579. [PMC free article] [PubMed] [Google Scholar]
- 58.Kimpton WG, Washington EA, Cahill RN. Virgin alpha beta and gamma delta T cells recirculate extensively through peripheral tissues and skin during normal development of the fetal immune system. Int Immunol. 1995;7:1567–1577. doi: 10.1093/intimm/7.10.1567. [DOI] [PubMed] [Google Scholar]
- 59.Cahill RN, Kimpton WG, Washington EA, Dudler L, Trnka Z. An immune system switch in T cell lifespan at birth results in extensive loss of naïve fetal T cells during the first week of postnatal life. Int Immunol. 1997;9:1253–1258. doi: 10.1093/intimm/9.9.1253. [DOI] [PubMed] [Google Scholar]
- 60.Malhotra I, Dent A, Mungai P, Wamachi A, Ouma JH, Narum DL, et al. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 2009;6:1000116. doi: 10.1371/journal.pmed.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25 hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. 2011;186:2780–2791. doi: 10.4049/jimmunol.1001188. [DOI] [PubMed] [Google Scholar]