Abstract
Background
Rotaviral gastroenteritis (RVGE) is the leading cause of severe diarrhea in children under five years of age worldwide. This comprehensive review aims to estimate the burden of RVGE among children in Central and Eastern Europe.
Results
This literature search captured 38 studies pertaining to RVGE infection in the region. Among children under 15 years of age, RVGE accounted for between 22.0% and 55.3% of all cases of acute gastroenteritis per year. For most countries RVGE was most common in the winter months, although it was reported year round in Bulgaria. Geographical comparison of genotyping data revealed that three genotype combinations, G1P[8], G4P[8] and G2P[4] were present in all countries for which full genotyping data was available. Genotype predominance varied on a season to season basis within each country. Only limited data was available for healthcare resource utilization and economic burden for this region.
Methods
An extensive search of the biomedical literature (1999–2009) was conducted in major databases. Studies pertaining to the epidemiology and burden of rotavirus in Central and eastern Europe were captured and data from each country was systematically extracted and compared.
Conclusions
RVGE is a common disease associated with significant morbidity and mortality. While three genotype combinations currently predominate in the region, the dominance of a certain serotype can change dramatically from year to year and from country to country. A vaccination program with broad serotype coverage may help to decrease the burden of RVGE in Central and Eastern Europe.
Key words: rotaviral gastroenteritis, burden of illness, Central and Eastern Europe
Introduction
Rotavirus is the leading cause of severe dehydrating diarrhea in children under five years of age worldwide.1 While there are seven sero-groups of rotavirus (groups A–G), only three groups (groups A–C) affect humans. Group A rotaviral infections are the most common, causing diarrheal disease worldwide.2 Group A rotaviruses are further classified into G and P-types, determined by the two outer layer viral proteins, VP7 and VP4, respectively. Protection from rotaviral infection and disease is believed to be type specific as VP7 and VP4 proteins elicit neutralizing antibody responses.3 Since rotaviruses are ubiquitous in the animal kingdom; interspecies transmission and the exchange of genetic material between animal and human strains through re-assortment can lead to the emergence of novel rotavirus strains of epidemiological significance.3 Globally, viruses carrying either G1, G3, G4, G9, in association with P[8], and G2 in association with P[4], are the most common causes of rotavirus disease in humans. G12 is also recognized as an emergent serotype, that may become important in human disease.4 The surveillance methods employed to identify serotypes can vary from country to country, which can have an impact on the detection of emerging serotypes. Hence, the development of surveillance networks such as the EuroRotaNet, which now covers many Central and eastern European countries, is an important improvement in the reporting of rotaviral serotypes.5
Rotavirus infections rates vary seasonally with the majority of cases in temperate climates occurring in the winter months between November and February.2,6 In tropical and developing countries this seasonality is less marked, and infections occur year-round.7 The incidence of infection with particular rotavirus genotypes varies between geographical areas during a rotavirus season and from one season to the next.8
Transmitted by the fecal-oral route, rotavirus infects cells that line the small intestine producing an enterotoxin (NSP4), that induces gastroenteritis.2 Symptoms include a profuse watery diarrhea, vomiting, abdominal pain and possibly fever and severe cases may lead to death, mainly through acute dehydration.2 Rotavirus affects 95% of all children by the age of 5 years.7 Infection rates for rotavirus are highest in the under 5-year old age group and decrease progressively towards adulthood as immunity acquired in childhood protects most adults.7
Often, children suffering from rotavirus gastroenteritis (RVGE) require only outpatient care (medical consultation) however, in the presence of dehydration, emergency care or hospitalization and intravenous hydration are necessary. Each year, worldwide, rotavirus causes approximately 111 million episodes of gastroenteritis that require only home care without GP consultation, 25 million that result in clinic visits and 2 million hospitalization.9 By age 5, 4 out of 5 children will have an episode of rotavirus gastroenteritis, and while not every rotavirus infection (including the first infection) is symptomatic, 1 in 5 will visit a clinic and 1 in 65 will be hospitalized.9 The World Health Organization (WHO) estimates that 527,000 children under the age of five years die of rotavirus induced disease each year,10 with children in the poorest countries accounting for 82% of rotavirus deaths.9 Rotavirus gastroenteritis imposes a heavy economic burden, by incurring direct (consultation, emergency, hospitalization, medication) and indirect costs (parent work days lost, baby-sitting, additional diapers).11–13
The purpose of this study was to conduct a comprehensive review of the published literature on the burden of rotavirus acute gastroenteritis in the pediatric population in Central and eastern Europe.
Results
Studies included in this review.
As shown in Figure 1, this literature search recovered 38 citations which contain relevant data pertaining to acute gastroenteritis associated with rotavirus infection. An overview of the evidence available for each country and the source of that evidence is presented in Table 1. Data was extracted on the following topics: incidence of RVGE (n = 2 studies), proportion of RVGE among acute gastroenteritis cases (n = 17), seasonality (n = 9), serotype prevalence and distribution (n = 23), disease severity (n = 1), mortality (n = 2), healthcare resource utilization (n = 5) and costs (n = 1). Although, initially we limited our search to rotaviral infection in children under five, many of the studies were carried out in pediatric populations that included older patients (up to 16 or in one study 18 years of age).14–19 However, rotaviral infection has the most impact on the pediatric population under 5 years of age.7
Figure 1.
Flow chart representing the selection of relevant citations.
Table 1.
Literature capture and data sources
| Country | Epidemiology | Genotype combination data | Morbidity and mortality | Disease burden | |||||
| Incidence | Proportion RVGE | Seasonality | Disease severity | Mortality | Resource utilization | Costs | |||
| Multicountry* | 20† | 16‡ | 35§ | 47‖ | 20† | ||||
| Albania | 20† | 30, 31 | 30, 34, 35§ | ||||||
| Belarus | 20† | 20 | |||||||
| Bosnia/Herzegovina | 32 | 32 | |||||||
| Bulgaria | 33 | 33 | 33, 35§ | 47‖ | |||||
| Croatia | 35§ | ||||||||
| Czech Republic | 16‡ | 35§, 36 | 47‖ | 16‡ | |||||
| Hungary | 15, 16‡, 21 | 55, 21,14, 37–41, 56 | 47‖ | 15, 16‡ | |||||
| Moldova | 47‖ | ||||||||
| Poland | 16‡, 17, 22–26 | 22, 17, 25, 26 | 26 | 47‖ | 16‡, 26 | 26 | |||
| Romania | 16‡, 27 | 47‖ | 16‡, 20† | ||||||
| Russia | 19, 28 | 19 | 19, 42 | 47‖ | |||||
| Slovakia | 18, 20† | 57 | 47‖ | 18, 20† | |||||
| Slovenia | 20† | 16‡ | 5, 35§, 43, 44, 45, 46 | 20, 47‖ | 16‡, 20† | ||||
| Slovenia and Hungary | 5 | ||||||||
| Ukraine, Georgia and Tajikistan | 29 | 29 | 29 | ||||||
| Ukraine | 47‖ | ||||||||
Study numbers given in this table correspond to the reference list for this publication.
Where data was available for individual countries within a multicountry study, it has been extracted. Therefore, the relevant reference has been added to individual countries above.
Twenty-three countries in the eastern part of the WHO European region.
Forty-nine countries in the WHO European region.
Czech Republic, Slovenia, Croatia, Albania and Bulgaria.
World Health Organization, all countries.
Epidemiology of RVGE.
Incidence of RVGE. Only two studies contained incidence data,18,20 although one of these studies presented community incidence of rotavirus for several countries.20 The survey of rotavirus surveillance and disease burden in countries in the WHO's eastern European Region by Williams et al. described the community incidence of rotavirus in children under five years old.20 Data was available for Albania, Belarus, Serbia, Slovakia and Slovenia.20 The community incidence of rotavirus was 12.3 per 1,000 children under five years of age in Slovenia, 4.2 per 1,000 children under six years of age in Belarus, 0.17 per 1,000 children under five years of age in Slovakia, and 0.11 per 1,000 children under five years of age in Serbia.20 Differences in surveillance programs and detection techniques may account for the large variations seen between countries.20
The second study from Slovakia examined the age related yearly incidence of rotavirus cases (both nosocomial and community acquired) requiring hospital admission in patients up to the age of 18 years.18 The total annual incidence was reported to be 2.6 per 1,000 children, with over 50% of infections affecting patients under 2 years of age.18
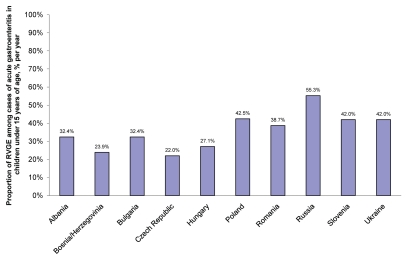
Proportion and seasonality of RVGE. The mean proportion of RVGE among cases of acute gastroenteritis for children under 15 years of age ranged from 22.0% per year to 55.3% per year (Fig. 2).15–17,19,21–33 Among the countries with the lowest mean proportions of RVGE were the Czech Republic, Bosnia/Herzegovina and Hungary (22.0%, 23.9% and 27.1%, respectively).16,32 The country with the highest mean proportion of RVGE was Russia (55.3%).19,28 The remaining countries report a percentage of rotaviral infection of between 32% and 43%.15–17,19,21–31,33
Figure 2.
Mean overall percentage of rotavirus infections among reported acute gastroenteritis cases by country.
A number of countries reported seasonality data (Table 1). For most countries in Central and eastern Europe the season for RVGE is in the winter months from November to May with a peak between March and May.19,22,25,26,29,32 However, a study from Bulgaria reported year-round circulation of rotaviruses in the country, which is unusual for countries with temperate climate. However, a peak of infection was observed between January until March.33
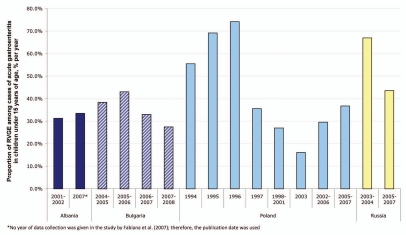
Variation in proportion of RVGE over time. Examining changes in rotavirus infection over time was possible for four countries, Albania, Bulgaria, Russia and Poland (Fig. 3).16,17,19,22–26,28,30,31,33 Because rotaviral infection rates can vary both over time and geographically within the same country, the dates of data collection and the location of study were noted. Both studies from Albania were based in Tirana, in the center of the country;30,31 the proportion of rotaviral infection among cases of acute gastroenteritis remained relatively constant at 31.3% in 2001–2002,30 and 33.5% in 2007.31
Figure 3.
Changes in the percentage of rotaviral infections among reported acute gastroenteritis cases over time.
A four-year survey of rotaviral infection from nine different centers around Bulgaria reported that rotaviral infection rates fell from 43.1% in 2005–2006 to 27.5% in 2007–2008.33 In Russia, the proportion of rotavirus infections among gastroenteritis cases appeared to have declined from 67.0% in 2003–2004 to 43.6% 2005–2007.19,28 However, the first study covering 2005–2007 collected data from eight Russian cities—six in eastern Russia and two in western Russia—while the second, from 2003–2004, originated from a single city in western Russia. In Poland, rates declined sharply from a peak in 1996 of 74.2% to around 30% of gastroenteritis cases between 2002 and 2007.16,17,22–26 Most of the studies in Poland collected data from the eastern Central part of the country and covered data from 1998–2007.17,22–24 Earlier studies collected data from three West Coast cities in 1997,25 and from three major cities in different regions of Poland from 1994 to 1995.26
Rotavirus genotype combinations.
In all, 23 studies from 10 countries contained some genotyping data, however, complete genotyping (G/P) data were only available in 18 studies from eight countries (Albania,30,34,35 Bulgaria,33,35 Croatia,35 Czech Republic,35,36 Hungary,37–41 Russia,19,42 Slovenia,5,35,43–46 and the Ukraine29 [along with Georgia and Tajikistan]).
Distribution of genotype combinations. The geographical distribution of genotype combinations throughout Central and eastern Europe was examined by capturing data from the years for which genotyping data was available for many countries. Thus data from 2005/06 is presented for seven countries (Albania,35 Bulgaria,33,35 Croatia,35 the Czech Republic,35 Hungary,37 Russia42 and Slovenia5,35) and data from 2007/08 is presented for five countries (Bulgaria,33 Hungary,38 Russia,42 Slovenia5 and Ukraine29 [along with Georgia and Tajikistan]). Data from Podkolzin et al. 2009 conducted in Russia was not used because the study only examined a limited number of genotypes (G1–4, P[4] and P[8]) by polymerase chain reaction.19 Data from the study by Novikova, where the genotype data were more comprehensive, were used instead.42
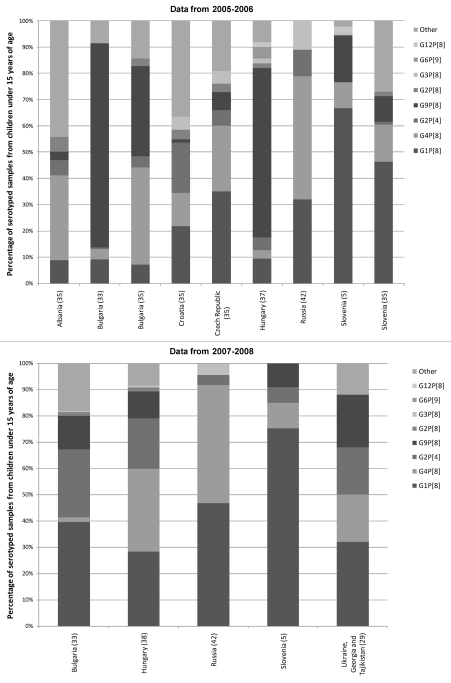
Three genotype combinations were present in all seven countries for which complete genotyping data were available from 2005/06 (G1P[8], G4P[8] and G2P[4]) (Fig. 4). In 2005/06, genotype combination G1P[8] was the most common in three countries, including Croatia35 (21.8%) the Czech Republic35 (35.1%) and Slovenia33,35 (two studies reporting 66.7% and 46.3%). Levels of G1P[8] were the lowest in Albania35 (8.9%) and Bulgaria33,35 (two studies reporting 7.1% and 9.1%). G4P[8] was the most common genotype combination reported in Albania (32.30% of genotyped samples) and Russia42 (47%), and in one of the studies from Bulgaria (37.1%).35 In the second study from Bulgaria G4P[8] only represented 4% of samples serotyped.33 While one study reported data from the genotyping of 912 fecal samples from nine hospitals throughout the country,33 the other reported data from 71 samples genotyped by the National Enterovirus Laboratory, in Sofia. The distribution of sampling was not reported for these 71 samples. G2P[4], was also present in all seven countries in 2005/06, ranged in prevalence from 0.6% in a study from Bulgaria, to 19.2% in Croatia. G9P[8] was present in all countries except Russia.42 This genotype combination was the most prevalent in one study from Bulgaria33 (77.7%) and in Hungary37 (64.5%) (Fig. 4).
Figure 4.
Distribution of rotavirus genotype combinations in the Central and Eastern Europe by country from 2005/06 and 2007/08.
In 2007/08, (G1P[8], G4P[8] and G2P[4]) were also present in all five countries for which data was available (Fig. 4). G1P[8] was the most prevalent in four of those countries (Bulgaria [39.5%],33 Russia [47%],42 Slovenia [75.4%],5 and Ukraine [32%]).29 In Hungary,38 G4P[8] was the most prevalent (31.5%) and also reached high prevalence in Russia (45%).42 G2P[4] was prevalent at between 4% (in Russia),42 and 25.8% (in Bulgaria).33 G9P[8] was present in all countries except Russia at between 9.0% and 20% of genotyped samples.
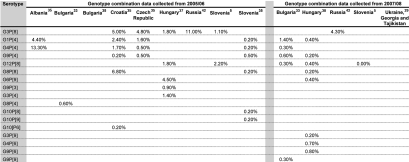
G2P[8] was present in six countries (Albania, Bulgaria, Croatia, the Czech Republic, Hungary and Slovenia) in 2005/06 and in two countries (Bulgaria and Hungary) in 2007/08. In all cases prevalence was below 6% (Fig. 4 and Table 2). Uncommon genotype combinations (G3P[8], G1P[4], G4P[4], G9P[4], G12P[8], G8P[8], G6P[9], G9P[3], G3P[4], G8P[4] G10P[8], G10P[9] and G10[P6]) were also detected in the Central and eastern European region in 2005/06, each of these serotypes was present in 5 countries or fewer and accounted for 14% or less of the total serotypes. In 2007/08, many of the same genotypes were present (G3P[8], G1P[4], G4P[4], G9P[4], G12P[8], G8P[8], G6P[9] and G9P[3]), but only in two countries or fewer and at a prevalence of less than 5%. In addition, in 2007/08 G3P[9] G4P[6], G9P[6] and G9P[9] were found in one country each at a prevalence of less than 1% No uncommon genotype combinations were detected in Ukraine (Table 2).
Table 2.
Uncommon genotypes from central and eastern europe from 2005/06 and 2007/08
 |
The proportion of uncommon genotype, mixed genotypes and non- or partially-typable genotypes were pooled in the “Other” category in Figure 4. Mixed genotypes were detected in most countries in 2005/06 and in two countries in 2007/08. When reported, the proportion of mixed genotypes reported ranged from 0.5% to 12.7% of the total rotavirus positive samples in 2005/06 and from 1.2% to 10.2% in 2007/08. Non-typable and partially typed genotypes accounted for 0.6% to 13.7% of all genotypes in both time periods, in studies where these were reported.
Evolution of genotype combinations over time. Information concerning genotype distribution over time for samples with G and P data available from children was studied for five countries, Albania,30,34,35 Bulgaria,33,35 Hungary,37–39 Russia19,42 and Slovenia.5,35,43
In Albania, changes in genotypes over time were reported in three studies, which were all set in the same city.30,34,35 Levels of the dominant genotype combination G9P[8] rose from 44% in 2000–2001 to 72.1% in 2001 to 2002 before falling to only 3.2% in 2005–2006. During the same time period, G3P[8] declined from 33% of the total in 2000–2001 to 0% in 2001–2002, while G4P[8] rose from 8.8% in 2001–2002 to 32.3% in 2005–2006.30,34,35
In Bulgaria, changes in genotypes over time were reported in two studies, both studies covered the whole country.33,35 G4P[8] was the dominant genotype in 2004–2005 before levels decreased to less than 5% after 2006.33,35 G9P[8] peaked in 2005–2006 at between 34.3% and 77.7%—depending on the study—before declining to 12.8% in 2007–2008.33,35 G9P[8] was largely replaced by G1P[8] (39.5% in 2006–2007) and G2P[4] (41.6% in 2006–2007 and 25.8% in 2007–2008).33,35
In Hungary, G1P[8] was reported to have declined over time from a high of 66% in 2001 to 2002, to a low of 9.5% in 2005–2006 in two studies set in the Central Hospital for Infectious diseases in Budapest.37,39 A study from different regions of Hungary reported that G1P[8] rebounded slightly to 28.3% in 2007.38 The G1P[8] genotype combination was largely replaced by G9P[8] which increased from 11% in 2001 to 2002 to 47% of typed samples in 2002–2003, 33% in 2004–2005 and to a high of 65% in 2005–2006. In 2003–2004, G9P[8] levels were lower at 20%, while the most common genotype combination was G4P[8] (46%).37,39 By 2007, G9P[8] declined to 10% and the most common genotype combinations were G4P[8] (31%), G1P[8] as already stated and G2P[4] (19%).38
A Russian study collected data from 1997–2005, from Nizhni Novgorod and Dzerzhinsk. Over the entire study period, the dominant genotype was G1P[8] at 77%, whilst in 2004–2005 levels of G1P[8] were at 25% and levels of G2P[4] and G3P[8] had increased to 37% and 22%, respectively.42
In Slovenia, three studies provided data from 2004 to 2008.5,35,43 Two of the studies involved centers throughout the country,5,35 while the third included the two major hospitals in the country in Ljubljana and Maribor.43 G1P[8] remained the most common genotype combination throughout the period covered by these studies. The maximum reported level was 75% in 2007–2008 and ranged from 46% to 60% over the previous three years. The levels of other genotype combinations (G4P[8], G9P[8] and G2P[4]) largely remained under 20% and fluctuated from year to year.
Emerging rotavirus genotypes in Central and Eastern Europe. This literature search captured five studies from three countries (Albania, Hungary and Slovenia) that examined emergent rotavirus genotype combinations in those countries.34,40,41,44–46 During an outbreak of rotavirus gastroenteritis which occurred in the winter of 2000, Villena et al. 2003 reported the emergence of multiple rotavirus genotypes. The most common emergent genotype in the outbreak was G9P[8], but G2P[6], G2P[8] and G1P[6] were also detected.34 A study based at a Budapest Hospital described the emergence of G12 strains in Hungary. G12 strains represented 6.9% of all typable strains collected among children hospitalized with rotavirus gastroenteritis in 2005.40 A second Hungarian study reported the emergence of G9P[6] in an Hungarian sentinel hospital in 2007. Apparently imported from India, this was the first report of this genotype in the region.41
A prospective survey based in a university medical center in Slovenia reported the emergence of the G9P[8] genotype in the winter of 2001–2002.44 Prevalence of this genotype reached 28% in patients hospitalized for a severe rotavirus outbreak in that season.44 A later study from the same university hospital described the first detection of a Group C rotavirus in Slovenia in three pediatric cases.45 While the vast majority of rotaviral infections are due to Group A, Group C rotaviruses are associated with sporadic gastroenteritis and outbreaks of diarrhea in children and adults worldwide.45 Finally, a rotavirus surveillance study undertaken in Slovenia from December 2005 to March 2006 detected G8P[8] and G12P[8] rotaviruses.46 This was the first report of G12 rotavirus and the first report of G8 in combination with P[8] in Slovenia.46
Morbidity and mortality due to rotavirus acute gastroenteritis.
Disease severity. Only one retrospective hospital-based study from Poland reported disease severity measures.26 In this study, 61.6% of cases younger than five years had severe rotavirus disease, with a mean score of 14.97 on the Vesikari scale (p < 0.001 versus non-RVGE). No studies were available from the other Central and eastern European studies.
Mortality. Two studies were available that reported rotavirus mortality in Central and eastern Europe.20,47 A regional survey carried out in 2006 among 23 national public health bodies reported mortality data from Belarus, Croatia, Serbia and Montenegro and Slovenia.20 Belarus, Croatia, Serbia, Montenegro and Slovenia reported zero mortality due to RVGE in children under five years, while Slovakia reported a case fatality rate of 0.5 per 1,000 cases of AGE (from 1954 to 2005) in children under five years.20 Additionally, the WHO report, from 2004, estimated the number of deaths and mortality rates in all the countries of interest in this study.47 In Central and eastern Europe, rotavirus fatalities ranged from <10 to 229 (Russia) per year.47 Annual mortality rates per 100,000 were highest in Albania (16 per 100,000). The mortality rate in other countries (Ukraine, Bulgaria, Russia, Romania, Moldova, Macedonia, Serbia and Montenegro) ranged from 2 to 7 per 100,000.47 For Croatia, the Czech Republic, Estonia, Hungary, Latvia, Lithuania and Poland there were <1 death per 100,000 population per year.47 The average annual mortality rate for the region is 2 per 100,000 children under 5 years calculated from UNICEF data.48 This excludes Moldova and Serbia/Montenegro where pediatric population estimates are not available.
Healthcare resource utilization and economic burden.
Hospital admissions. A systematic literature review of studies published between 1997 and 2004 estimated hospital admission rates for RVGE among children of various ages presenting with acute gastroenteritis in the WHO European region. The median percentage of acute gastroenteritis hospital admissions attributable to RVGE were available from eight Central and eastern European countries and ranged from 12% to 42% (Albania: 12%, Bosnia and Herzegovina: 24%, Czech Republic: 22%, Estonia: 26%; Hungary: 24%; Poland: 38.3%, Romania: 25% and Slovenia: 42%).16 In addition, a multi-country surveillance study reported an annual hospital admission rate for RVGE of 2.5 per 1,000 in Albania, 0.13 per 1,000 in Slovakia and 2.8 per 1,000 in Slovenia.20
Intravenous rehydration. Information on the need for intravenous rehydration was available only from one Slovakian retrospective study reporting on the duration of intravenous treatment among children with community-acquired or nosocomial RVGE.18 This study, involving 40,583 children of all ages (<19 years), reported on average 2.11 days of intravenous treatment (of which 0.82 days in the intensive care unit) and a hospital stay of 4.74 days.18
Duration of hospital stay. An analysis of hospital admissions in Hungary assessing mean duration of hospital stay for allcause (rotavirus or non-rotavirus) gastroenteritis by age, reported shorter hospital stay among older children (6.6 days for children 3–4 years versus 8.3 days for those <1 year old).15 A retrospective study of nosocomial rotavirus in three pediatric hospitals in Poland showed reduced hospital stay duration in 1996 (9.52 ± 9.77 days) compared to the previous 2-year period (1994–1996: 12.25 ± 13.81 days).26 Among children admitted for other causes, a nosocomial rotavirus infection—which accounted for 39% of infections in this study between 1994 and 1996 —prolonged the hospital stay by an additional 5.2 days per episode.26
Economic burden associated with RVGE. Only one study from Poland contained cost information for rotavirus infection.26 In Poland, the cost of hospitalization per RVGE episode was estimated at 2009 US $687.78 for community-acquired rotavirus.26 No other cost information from Central and eastern Europe was found.
Discussion
This analysis of the currently available literature on rotavirus burden in Central and eastern Europe revealed that, among the pediatric population, rotaviral infection accounts for between 22% and 55% of acute gastroenteritis cases.16,19,28 Prevalence of RVGE varied from country to country. In the Czech Republic and Bosnia/Herzegovina RVGE accounted for less than 25 percent of cases of acute gastroenteritis, whilst in Russia it accounted for over 50 percent of cases.16,19,28 Overall, in the Central and eastern European region rotaviral infections were responsible for 38.1% of acute gastroenteritis cases, rates similar to those reported for western Europe and Asia.49,50
For those countries for which community incidence data were available from a surveillance study (Albania, Belarus, Serbia, Slovakia and Slovenia), the incidence of rotaviral infection ranged from 0.11 to 12.3 per 1,000 children under five years of age.20 Differences in surveillance programs and detection techniques may account for the large variations seen between countries in this study.20 Rotaviral infections in most countries peaked in the winter months; however, a study from Bulgaria reported the year round circulation of rotavirus.33
Due to the lack of contemporaneous studies from different regions of each country geographical trends or differences in the proportion of RVGE among acute gastroenteritis cases are difficult to determine. However, the proportion of acute gastroenteritis cases due to rotaviral infection appeared to be declining in three out of the four countries. (In Bulgaria between 2005/6 and 2007/8, in Russia between 2003/4 and 2005/7 and in Poland between 1996 and 2002/7) for which data were available.
Several studies (24 studies) included information about rotaviral genotype distribution and predominance. The most commonly isolated genotype combinations in the Central and eastern European region were G1P[8], G4P[8] and G2P[4], according to the studies from 2005/06 and 2007/08. G9P[8] and was also commonin the region. Many other genotype combinations were present at levels under 14% of the total. The proportion of mixed genotypes reported from 2005/06 and 2007/08 ranged from 1.2% to 12.7% of the total rotavirus positive samples. Non-typable and partially typed genotypes accounted for between 0.6% and 13.7% of all genotypes where these were reported. Differences in the proportion of non-typable and partially typed samples are most likely due to differences in study design and setting and laboratory practice from country to country.
Data available did not allow us to distinguish any trends in genotype distribution within the region. Serotype predominance appears to change on a season to season basis within each country and may even differ from region to region within the same country. The data available is not extensive enough to support a detectable trend in genotype distribution within any of the countries examined. Emerging rotavirus genotypes were reported for three countries in Central and eastern Europe. In Albania, during an outbreak of RVGE in the winter of 2000, G9P[8] emerged as the dominant strain.34 G9P[8] was also detected for the first time in a university medical centre in Slovenia in the winter of 2001–2002.44 In Hungary in 2007 the emergence of G9P[6] a strain imported from India, was reported for the first time in the region.41 The emergence of G12 strains in Hungary and Slovenia was reported in 2005.40,46 The first description of G12 rotaviruses dates back to the 1990s, when two unusual human strains (L26, L27) from the Philippines were characterized serologically and by nucleotide sequencing as G12P[4]. Beginning in 2002, reports of the detection and increased presence of G12 strains have appeared in Asia (Thailand, India, Korea, Japan, Bangladesh, Nepal and Saudi Arabia) and the Americas (the United States, Argentina and Brazil).40
Finally, the first detection of a Group C rotavirus was reported in a Slovenian study from 2006, in three pediatric cases.45 While the vast majority of rotaviral infections are due to group A, group C rotaviruses are associated with sporadic gastroenteritis and outbreaks of diarrhea in children and adults worldwide.45
Rotavirus fatalities were relatively low across the region. Annual mortality rates per 100,000 were highest in Albania (16) and ranged between 2 and 7 per 100,000 in Serbia and Montenegro, Macedonia, Moldova Romania, Russia, Bulgaria and Ukraine.20,47 In the remaining countries the mortality rate was less than 1 per 100,000 per year. Comparison with a global literature review of mortality due to rotavirus shows that all of the countries in Central and eastern Europe have similar mortality rates than those reported for the highest income countries (reported as <100 deaths per 100,000 population per year) according to the World Bank Income Groups (GNP per capital: low [<US $756], low-middle [US $756–$2,995], high-middle [US $2,996–$9,265], high [>US $9,265]).9
Only limited data were available for healthcare resource utilization and economic burden for this region. Hospital admissions rates in Central and eastern Europe were similar to figures reported in the RVGE Epidemiology and Viral types in Europe Accounting for Losses in public health and society (REVEAL) study, which was based in western Europe.51 Hospital admissions rates are also similar to those reported in the Middle East (Khoury et al. 2010 submitted). Data on the use of intravenous fluids in the region was limited to a single study from Slovenia which reported on average 2.11 days of intravenous treatment (of which 0.82 days in the intensive care unit).18 Overall, the duration of hospital stay ranged between 4.74 days to 9.5 days with older children tending to be discharged sooner.15,26 This is slightly longer than the hospital stays reported for western European countries in the REVEAL study, which reported and minimum of 2.5 days in Sweden and a maximum of 5 days in Germany.52 Rotavirus cost information was very limited, only a study from Poland was captured; in this study the cost of hospitalization per RVGE episode was estimated at 2008 US $690.2 for community-acquired rotavirus.26 By comparison, in the REVEAL study direct medical costs per hospitalization episode ranged from 2008 US $1,949 (UK) to 2008 US $2,398 (Sweden).52
Results should be considered in light of study limitations. Most recent available data were considered to describe and compare genotype distribution across countries. However, the only available data were not necessarily recent and did not correspond to the same time frame in all countries. In addition, comparability of data is affected by variations in study setting and design. Finally, the lack of available information on the burden of RVGE in terms of mortality, morbidity and economic burden restricted the evaluation of the burden for the region.
Methods
Literature search strategy.
To identify and retrieve articles pertaining to the impact of rotavirus infection on the pediatric population under 5 years of age in Central and eastern Europe, an extensive literature search was conducted in the following databases: National Library of Medicine's Pubmed, the Center for Disease Control (CDC) rotavirus global surveillance53 and the WHO rotavirus surveillance.13 The search, limited to human subjects and articles published in the last ten years, covered the following Central and eastern European countries: Albania, Belarus, Bosnia/Herzegovina, Bulgaria, Croatia, Czech Republic, Estonia, Hungary, Latvia, Lithuania, Macedonia, Moldova, Montenegro, Poland, Romania, Russia, Serbia, Slovakia, Slovenia and Ukraine. English language articles were primarily reviewed along with promising articles in other languages. Records were searched manually to identify the most relevant studies pertaining to the object of this document. Bibliographies of retrieved articles were screened to identify additional sources of information. Search terms included: rotavirus, outcome, mortality, death, incidence, prevalence, serotype, strain, cost, economic, burden and resource use.
Data extraction and analysis.
For data on the proportion of RVGE among cases of acute gastroenteritis, an average of all studies and ranges across studies was calculated and presented by country. Variation over time in the proportion of RVGE was also reported as was data on infection seasonality. Data was extracted for the distribution of rotavirus serotypes with the proportion of mixed, non-typable and rare genotype combinations noted. The geographical distribution of genotype combinations throughout Central and eastern Europe was examined by capturing data from the years for which genotyping data was available for many countries (2005/06 and 2007/08). Data from Podkolzin et al. 2009 was not used since the study only examined a limited range of genotypes (G1–4, P[4] and P[8]) by polymerase chain reaction.19
For health outcomes of RVGE, data extraction was limited to disease severity measures using the 20-point Vesikari scoring system and the severity and proportion of patients suffering from dehydration due to RVGE. The Vesikari scale is based on the duration and intensity of diarrhea and vomiting, intensity of fever and dehydration and need for treatment and hospitalization. 54 A Vesikari score >11 is indicative of severe disease.54
Healthcare resource utilization data was collected and the following parameters were used for comparison across countries: hospital admission rates, need for intravenous rehydration and duration of hospital stay. Cost-of-illness data included direct medical costs, out-of-pocket expenditures and indirect costs attributed to lost productivity by parents of children suffering from rotavirus gastroenteritis. Costs are reported in 2009 US dollars.
Conclusion
In conclusion, data on the burden of RVGE in terms of mortality, morbidity and economic burden is limited for this region. However, the available data does show that RVGE is a common disease in Central and Eastern Europe affecting the pediatric population. While two or three genotype combinations currently predominate in the region, analysis of the evolution of different genotypes over time shows that the dominance of a certain genotype can change dramatically from year to year and from country to country. Vaccination programs may help to reduce the infection rates of this disease; a vaccine with broad serotype coverage would be needed to decrease the burden of RVGE in Central and Eastern Europe.
Acknowledgements
This study was made possible by a grant from Merck Sharp and Dohme Corp.
Abbreviations
- CDC
Center for Disease Control
- RVGE
rotaviral gastroenteritis
- WHO
World Health Organizationy
Conflict of Interest
Isla Ogilvie, Hanane Khoury and Mirielle Goetghebeur have no conflicts of interest. Antoine El Khoury is an employee of Merck Sharp & Dohme Corp., and holds stock from the same Company.
References
- 1.Widdowson MA, Steele D, Vojdani J, Wecker J, Parashar UD. Global rotavirus surveillance: preparing for the introduction of rotavirus vaccines. J Infect Dis. 2009;200:1–8. doi: 10.1086/605061. [DOI] [PubMed] [Google Scholar]
- 2.Diggle L. Rotavirus diarrhoea and future prospects for prevention. Br J Nurs. 2007;16:970–974. doi: 10.12968/bjon.2007.16.16.27074. [DOI] [PubMed] [Google Scholar]
- 3.Iturriza-Gomara M, Kang G, Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol. 2004;31:259–265. doi: 10.1016/j.jcv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Matthijnssens J, Rahman M, Ciarlet M, et al. Emerging Human Rotavirus Genotypes. In: Palumbo E, Kirkwood C, editors. Viruses in the Environment. Kerala, India: Research Signpost; 2009. pp. 171–219. [Google Scholar]
- 5.Iturriza-Gomara M, Dallman T, Banyai K, Bottiger B, Buesa J, Diedrich S, et al. Rotavirus surveillance in europe 2005–2008: web-enabled reporting and realtime analysis of genotyping and epidemiological data. J Infect Dis. 2009;200:215–221. doi: 10.1086/605049. [DOI] [PubMed] [Google Scholar]
- 6.Gleizes O, Desselberger U, Tatochenko V, Rodrigo C, Salman N, Mezner Z, et al. Nosocomial rotavirus infection in European countries: a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis J. 2006;25:12–21. doi: 10.1097/01.inf.0000197563.03895.91. [DOI] [PubMed] [Google Scholar]
- 7.Parashar UD, Bresee JS, Gentsch JR, Glass RI. Rotavirus. Emerg Infect Dis. 1998;4:561–570. doi: 10.3201/eid0404.980406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iturriza-Gomara M, Green J, Brown DW, Ramsay M, Desselberger U, Gray JJ. Molecular epidemiology of human group A rotavirus infections in the United Kingdom between 1995 and 1998. J Clin Microbiol. 2000;38:4394–4401. doi: 10.1128/jcm.38.12.4394-4401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200:9–15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- 11.Grimwood K, Lambert SB. Rotavirus vaccines: opportunities and challenges. Hum Vaccin. 2009;5:57–69. doi: 10.4161/hv.5.2.6924. [DOI] [PubMed] [Google Scholar]
- 12.Soriano-Gabarro M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J. 2006;25:7–11. doi: 10.1097/01.inf.0000197622.98559.01. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization, author. External review of burden of disease attributable to rotavirus 2005. [2 Nov 2009]. http://www.who.int/immunization_monitoring/burden/Rota_virus_Q5_mortality_estimates_external_review_report_2006_may.pdf.
- 14.Banyai K, Sas Y, Varga L, Szucs G. Survey of rotavirus infection in a Hungarian paediatric hospital. A short communication. Acta Microbiol Immunol Hung. 2004;51:431–435. doi: 10.1556/AMicr.51.2004.4.3. [DOI] [PubMed] [Google Scholar]
- 15.Szucs G, Uj M, Mihaly I, Deak J. Burden of human rotavirus-associated hospitalizations in three geographic regions of Hungary. Acta Paediatr Suppl. 1999;88:61–65. doi: 10.1111/j.1651-2227.1999.tb14328.x. [DOI] [PubMed] [Google Scholar]
- 16.Williams CJ, Lobanov A, Pebody RG. Estimated mortality and hospital admission due to rotavirus infection in the WHO European region. Epidemiol Infect. 2009;137:607–616. doi: 10.1017/S0950268808001714. [DOI] [PubMed] [Google Scholar]
- 17.Jackowska T, Klyszewska M, Zakrzewski M, Pawlik K. [Epidemiological trend in rotavirus infections in children hospitalized in the Department of Paediatrics at Warsaw Bielany Hospital 2005–2007] Med Wieku Rozwoj. 2008;12:685–691. [PubMed] [Google Scholar]
- 18.Stefkovicova M, Simurka P, Jurackova L, Hudeckova H, Mad'ar R. Nosocomial rotaviral gastroenteritis in paediatric departments. Cent Eur J Public Health. 2008;16:12–16. doi: 10.21101/cejph.a3453. [DOI] [PubMed] [Google Scholar]
- 19.Podkolzin AT, Fenske EB, Abramycheva NY, Shipulin GA, Sagalova OI, Mazepa VN, et al. Hospital-based surveillance of rotavirus and other viral agents of diarrhea in children and adults in Russia, 2005–2007. J Infect Dis. 2009;200:228–233. doi: 10.1086/605054. [DOI] [PubMed] [Google Scholar]
- 20.Williams CJ, Gray J, Pebody RG, Lobanov A. Survey of rotavirus surveillance, laboratory capacity and disease burden in the eastern part of the WHO European Region. Euro Surveill. 2008:13. doi: 10.2807/ese.13.34.18959-en. [DOI] [PubMed] [Google Scholar]
- 21.Banyai K, Jiang B, Bogdan A, Horvath B, Jakab F, Meleg E, et al. Prevalence and molecular characterization of human group C rotaviruses in Hungary. J Clin Virol. 2006;37:317–322. doi: 10.1016/j.jcv.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Patrzalek M, Patrzalek MP. [The cases of rotaviral diarrhea from Kielce and Kielce district, hospitalized in Kielce Voivodeship Children Hospital in 2002–2006] Przegl Epidemiol. 2008;62:557–563. [PubMed] [Google Scholar]
- 23.Sulik A, Oldak E, Rozkiewicz D, Skorochodzki J, Kurzatkowska B. [Prospective study of rotaviral infections in children hospitalized at the Clinic of Pediatric Infectious Diseases in Bialystok in 2003] Przegl Epidemiol. 2004;58:475–481. [PubMed] [Google Scholar]
- 24.Zurawska-Olszewska J, Krzeslowska I, Dlugosz G, Krasiukianis A. [Etiology of acute diarrheas in children from the Lodz region. I. Occurrence of etiologic agents] Med Dosw Mikrobiol. 2002;54:129–136. [PubMed] [Google Scholar]
- 25.Rytlewska M, Bako W, Ratajczak B, Marek A, Gwizdek A, Czarnecka-Rudnik D, et al. Epidemiological and clinical characteristics of rotaviral diarrhoea in children from Gdansk, Gdynia and Sopot. Med Sci Monit. 2000;6:117–122. [PubMed] [Google Scholar]
- 26.Mrukowicz JZ, Krobicka B, Duplaga M, Kowalska-Duplaga K, Domanski J, Szajewska H, et al. Epidemiology and impact of rotavirus diarrhoea in Poland. Acta Paediatr Suppl. 1999;88:53–60. doi: 10.1111/j.1651-2227.1999.tb14327.x. [DOI] [PubMed] [Google Scholar]
- 27.Mihalache D, Fintinaru R, Iacob M, Simonca C. [Clinical study of acute diarrhea caused by rotavirus] Rev Med Chir Soc Med Nat Iasi. 2005;109:488–491. [PubMed] [Google Scholar]
- 28.Phan TG, Yagyu F, Kozlov V, Kozlov A, Okitsu S, Muller WE, et al. Viral gastroenteritis and genetic characterization of recombinant norovirus circulating in eastern Russia. Clin Lab. 2006;52:247–253. [PubMed] [Google Scholar]
- 29.Mirzayeva R, Cortese MM, Mosina L, Biellik R, Lobanov A, Chernyshova L, et al. Rotavirus burden among children in the newly independent states of the former union of soviet socialist republics: literature review and first-year results from the rotavirus surveillance network. J Infect Dis. 2009;200:203–214. doi: 10.1086/605041. [DOI] [PubMed] [Google Scholar]
- 30.Annarita P, Grassi T, Donia D, De Donno A, Idolo A, Alfio C, et al. Detection and molecular characterization of human rotaviruses isolated in Italy and Albania. J Med Virol. 2010;82:510–518. doi: 10.1002/jmv.21700. [DOI] [PubMed] [Google Scholar]
- 31.Fabiana A, Donia D, Gabrieli R, Petrinca AR, Cenko F, Bebeci D, et al. Influence of enteric viruses on gastroenteritis in Albania: epidemiological and molecular analysis. J Med Virol. 2007;79:1844–1849. doi: 10.1002/jmv.21001. [DOI] [PubMed] [Google Scholar]
- 32.Ahmetagic S, Jusufovic E, Petrovic J, Stojic V, Delibegovic Z. Acute infectious diarrhea in children. Med Arh. 2003;57:87–92. [PubMed] [Google Scholar]
- 33.Mladenova Z, Korsun N, Geonova T, Iturriza-Gomara M. Molecular epidemiology of rotaviruses in Bulgaria: annual shift of the predominant genotype. Eur J Clin Microbiol Infect Dis. 2010;29:555–562. doi: 10.1007/s10096-010-0895-1. [DOI] [PubMed] [Google Scholar]
- 34.Villena C, Gabrieli R, Pinto RM, Guix S, Donia D, Buonomo E, et al. A large infantile gastroenteritis outbreak in Albania caused by multiple emerging rotavirus genotypes. Epidemiol Infect. 2003;131:1105–1110. doi: 10.1017/s0950268803001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tcheremenskaia O, Marucci G, De Petris S, Ruggeri FM, Dovecar D, Sternak SL, et al. Molecular epidemiology of rotavirus in Central and Southeastern Europe. J Clin Microbiol. 2007;45:2197–2204. doi: 10.1128/JCM.00484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pazdiora P, Svecova M. G-serotypes of group A rotaviruses in Pilsen region (Czechia) Folia Microbiol (Praha) 2006;51:133–135. doi: 10.1007/BF02932168. [DOI] [PubMed] [Google Scholar]
- 37.Banyai K, Bogdan A, Domonkos G, Kisfali P, Molnar P, Toth A, et al. Genetic diversity and zoonotic potential of human rotavirus strains 2003–2006, Hungary. J Med Virol. 2009;81:362–370. doi: 10.1002/jmv.21375. [DOI] [PubMed] [Google Scholar]
- 38.Laszlo B, Czellar E, Deak J, Juhasz A, Kovacs J, Konya J, et al. [Post vaccination rotavirus surveillance in Hungary, in 2007] Orv Hetil. 2009;150:1443–1450. doi: 10.1556/OH.2009.28690. [DOI] [PubMed] [Google Scholar]
- 39.Banyai K, Gentsch JR, Schipp R, Jakab F, Meleg E, Mihaly I, et al. Dominating prevalence of P[8],G1 and P[8],G9 rotavirus strains among children admitted to hospital between 2000 and 2003 in Budapest, Hungary. J Med Virol. 2005;76:414–423. doi: 10.1002/jmv.20372. [DOI] [PubMed] [Google Scholar]
- 40.Banyai K, Bogdan A, Kisfali P, Molnar P, Mihaly I, Melegh B, et al. Emergence of serotype G12 rotaviruses, Hungary. Emerg Infect Dis. 2007;13:916–919. doi: 10.3201/eid1306.061181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laszlo B, Nyul Z, Kisfali P, Deak J, Kovacs J, Konya J, et al. First detection of P[6],G9 rotaviruses in Hungary—an imported strain from India? J Travel Med. 2009;16:141–143. doi: 10.1111/j.1708-8305.2008.00293.x. [DOI] [PubMed] [Google Scholar]
- 42.Novikova NA, Fedorova OF, Epifanova NV, Chuprova AB. [G[P] type profiles of group A human rotavirus and their distribution in Nizhni Novgorod and Dzerzhinsk in 1997–2005] Vopr Virusol. 2007;52:19–23. [PubMed] [Google Scholar]
- 43.Steyer A, Poljsak-Prijatelj M, Barlic-Maganja D, Marin J. Human, porcine and bovine rotaviruses in Slovenia: evidence of interspecies transmission and genome reassortment. J Gen Virol. 2008;89:1690–1698. doi: 10.1099/vir.0.2008/001206-0. [DOI] [PubMed] [Google Scholar]
- 44.Steyer A, Poljsak-Prijatelj M, Barlic-Maganja D, Bufon T, Marin J. The emergence of rotavirus genotype G9 in hospitalised children in Slovenia. J Clin Virol. 2005;33:7–11. doi: 10.1016/j.jcv.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 45.Steyer A, Poljsak-Prijatelj M, Bufon T, Sedmak M, Vidmar L, Mijovski JZ, et al. First detection of group C rotavirus in patients with gastroenteritis in Slovenia. J Med Virol. 2006;78:1250–1255. doi: 10.1002/jmv.20687. [DOI] [PubMed] [Google Scholar]
- 46.Steyer A, Poljsak-Prijatelj M, Bufon TL, Marcun-Varda N, Marin J. Rotavirus genotypes in Slovenia: unexpected detection of G8P[8] and G12P[8] genotypes. J Med Virol. 2007;79:626–632. doi: 10.1002/jmv.20811. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization, author. Child rotavirus deaths 2004. [2 Nov 2009]. http://www.who.int/immunization_monitoring/burden/rotavirus_estimates/en/index.html.
- 48.UNICEF, author. Information by country and programme—Demographic indicators 2004. [11 Dec 2009]. http://www.unicef.org/infobycountry/index.html.
- 49.Forster J, Guarino A, Parez N, Moraga F, Roman E, Mory O, et al. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics. 2009;123:393–400. doi: 10.1542/peds.2008-2088. [DOI] [PubMed] [Google Scholar]
- 50.Bresee J, Fang ZY, Wang B, Nelson EA, Tam J, Soenarto Y, et al. First report from the Asian Rotavirus Surveillance Network. Emerg Infect Dis. 2004;10:988–995. doi: 10.3201/eid1006.030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giaquinto C, Van Damme P, Huet F, Gothefors L, Maxwell M, Todd P, et al. Clinical consequences of rotavirus acute gastroenteritis in Europe 2004–2005: the REVEAL study. J Infect Dis. 2007;195:26–35. doi: 10.1086/516717. [DOI] [PubMed] [Google Scholar]
- 52.Giaquinto C, Van Damme P, Huet F, Gothefors L, Van der WM. Costs of community-acquired pediatric rotavirus gastroenteritis in 7 European countries: the REVEAL Study. J Infect Dis. 2007;195:36–44. doi: 10.1086/516716. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention, author. Global rotavirus surveillance 2010. [12 May 2010]. http://www.cdc.gov/rotavirus/global_surveillance/surveillance.htm.
- 54.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 55.Banyai K, Gentsch JR, Glass RI, Uj M, Mihaly I, Szucs G. Eight-year survey of human rotavirus strains demonstrates circulation of unusual G and P types in Hungary. J Clin Microbiol. 2004;42:393–397. doi: 10.1128/JCM.42.1.393-397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banyai K, Gentsch JR, Glass RI, Szucs G. Detection of human rotavirus serotype G6 in Hungary. Epidemiol Infect. 2003;130:107–112. doi: 10.1017/s0950268802007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tietzova J, Petrovicova A. Serotypic characterization of group A rotaviruses associated with children's diarrhea in Slovakia. Folia Microbiol (Praha) 2000;45:183–186. doi: 10.1007/BF02817421. [DOI] [PubMed] [Google Scholar]