Abstract
Transforming Growth Factor-β (TGFβ) exerts cell type-specific and context-dependent effects. Understanding the intrinsic actions of TGFβ on cancer cells in pancreatic ductal adenocarcinoma (PDAC) is a prerequisite for rationalizing clinical implementation of TGFβ targeted therapies. Since the tumor microenvironment can affect how cancer cells respond to TGFβ, we employed a novel 3-dimensional (3D) culturing system to recapitulate stromal and extracellular matrix interactions. We show here that TGFβ stimulates the growth of several human and murine pancreatic cancer cell lines (PCCs) when embedded in a 3% collagen IV/laminin-rich gelatinous medium (Matrigel™) over a solidified layer of soft agar. Moreover, in this novel 3D model, concomitant treatment with TGFβ1 and epidermal growth factor (EGF) enhanced PCC growth to a greater extent than either growth factor alone and conferred increased chemoresistance to cytotoxic compounds. These cooperative growth-stimulatory effects were blocked by pharmacological inhibition of either the TGFβ type I receptor with SB431542 or the EGF receptor with erlotinib. Co-incubation with SB431542 and erlotinib enhanced the efficacy of gemcitabine and cisplatin in PCCs and in primary cell cultures established from pancreata of genetically-engineered mouse models of PDAC. These findings suggest that concomitant inhibition of TGFβ and EGF signaling may represent an effective therapeutic strategy in PDAC, and that this 3D culturing system could be utilized to test ex vivo the therapeutic response of pancreatic tumor biopsies from PDAC patients, thereby providing a functional assay to facilitate personalized targeted therapies.
Key words: transforming growth factor-β (TGFβ), epidermal growth factor (EGF), pancreatic adenocarcinoma (PDAC), tumor microenvironment, extracellular matrix, Matrigel™, soft agar
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in the United States.1 Late clinical presentation along with rapid tumor growth and early propensity for cancer cells to metastasize preclude successful treatment efforts. Only about 20% of PDAC patients are eligible for surgical resection of the tumor mass, and only about 20% of these patients benefit from this intervention.2 Despite advances in our understanding of the molecular mechanisms contributing to this malignancy, non-surgical therapeutic interventions have yielded little improvement in overall patient survival.3 Gemcitabine (GEM), a nucleoside analog, may prolong life by about 8 weeks, whereas addition of the tyrosine kinase inhibitor erlotinib to GEM as first-line of treatment increases survival by an additional 12 d.4 Thus, new therapeutic approaches and assays for preclinical validation are desperately needed.2
The biological aggressiveness of PDAC is due, in part, to the presence of multiple molecular alterations, including expression of mutated KRAS (95%) and loss of tumor suppressor genes P16/CDKN2A (90%), Tp53 (50–75%) and Smad4/DPC4 (40–55%).5 In addition, pancreatic cancer cells express high levels of the epidermal growth factor receptor (EGFR) and transforming growth factor α (TGFα), as well as other high-affinity tyrosine kinase receptors and their corresponding ligands.6 These cancer cells thrive in a context of marked desmoplasia characterized by activation and proliferation of fibroblasts and pancreatic stellate cells as well as the presence of foci of inflammatory cells.7 These stromal elements respond to cancer cell-secreted growth factors (GFs), including transforming growth factor β (TGFβ). Indeed, cancer cells have been shown to express high levels of all three TGFβ isoforms (TGFβ1, TGFβ2, TGFβ3) and elevated TGFβ immunoreactivity in resected PDACs has been correlated with shorter overall patient survival.8 These in vivo growth promoting effects toward cancer cells have been attributed to the paracrine actions of TGFβs, as underscored by the use of a soluble TGFβ receptor strategy that sequesters cancer-derived TGFβs.9,10 Moreover, TGFβ is a potent activator of pancreatic stellate cells and the resultant reactive stroma produces, stores and releases GFs to the cancer cells.7,11 In addition to their participation in autocrine and paracrine signaling these stromal elements produce a modified extracellular matrix (ECM) that further promotes cancer cell growth and metastasis.11,12
TGFβ effects are cell type-specific and context-dependent. TGFβ suppresses normal epithelial cell growth, stimulates the growth of mesenchymal and endothelial cells, attenuates cancer cell-directed immune mechanisms and supports advanced stage cancer cell growth.13,14 TGFβ signaling is mediated by a network of Smad-dependent and Smad-independent pathways that transduce TGFβ stimuli from the activated heterotetrameric TGFβ type I and type II receptor (TβRI/II) complex.13,14 The frequent mutation of TGFβ signal mediator Smad4 in PDAC lesions suggests a tumor suppressive role of Smad-dependent TGFβ signaling in cancer initiation.5 This notion is supported by enhanced progression of K-Ras-driven mouse models of PDAC with homozygous deletion of either Smad4 or TIIβR genetic locus.15–17
Several approaches for interfering with TGFβ signaling are currently in different stages of pre-clinical and clinical testing, and have potential to yield novel therapeutic strategies in PDAC and other cancer types.13,18,19 However, in vitro studies suggest that pancreatic cancer cell lines (PCCs) are either growth inhibited by or fail to respond to TGFβ. Therefore, blocking TGFβ signaling could be potentially detrimental in PDAC cases in which cancer cell growth is repressed by TGFβs. Given these important clinical implications, we sought to assess the intrinsic response of PCCs to TGFβ and other GFs in a novel 3-dimensional (3D) culture system. This Matrigel™/soft agar-based 3D culture system promotes anchorage-independent growth while concomitantly providing an acellular scaffold composed of collagen and other deposited ECM components, which, in part, recapitulates the tumor microenvironment. We show here that some PCCs of human and mouse origin are growth-stimulated by TGFβ1 in this novel 3D culture system and that this effect is significantly enhanced by EGF. Moreover, the combined presence of EGF and TGFβ1 confers increased resistance to the PCCs against cytotoxic compounds (gemcitabine and cisplatin). Conversely, co-treatment with SB431542 and erlotinib to concomitantly block TGFβ and EGF signaling enhanced the chemosensitivity of cancerous lesions from mouse models of PDAC in ex vivo 3D cultures. These results demonstrate that TGFβ can directly enhance growth of PCCs and support the notion that combination therapy aimed at interfering with EGF and TGFβ may be a valid therapeutic strategy for a subset of PDAC patients.
Results
Optimization of 3D culture system for pancreatic cancer cells.
To study the effects of growth factors on PCCs in a context that recapitulates the tumor microenvironment, we developed a novel modification of the standard Matrigel™-based 3D culture system20,21 that mimics anchorage-independent growth (bottom layer of solidified 1% noble agar), while allowing for interactions with components of the ECM by overlaying with PCCs embedded within a soft layer of 3% growth factor-reduced Matrigel™. PCCs cultured in medium without fetal bovine serum (FBS) or supplemented with 1% FBS formed smaller structures and accordingly the response to GF stimulation was of a lesser magnitude as compared with PCCs in medium with 5% FBS (Fig. S1). The 5% FBS condition was used in all subsequent experiments, since more overt differences could be observed when cells were subjected to different treatments (see below), and since in vivo cancer cells are exposed to the ECM, which is replenished with GFs, cytokines and other nutrients.
ASPC-1, BxPC3, COLO-357 and T3M4 are human PCCs, widely used to study pancreatic cancer biology. These PCCs harbor different mutations in K-Ras, p53, Smad4 and other etiologically relevant genes.22,23 Moreover, under standard culture conditions, COLO-357 and BxPC3 cells are sensitive to TGFβ-mediated growth inhibition whereas ASPC-1 and T3M4 cells are resistant to these growth-modulating effects of TGFβ.24 In addition, we established independent murine cell lines isolated from pancreata of K-Ras-driven mouse models: PK-1 and PK-2 cells from pdx1::cre;K-rasLSL-G1D2/+ animals and RInk-2 cells from pdx1::cre;K-rasLSL-G1D2/+;p16Ink4a/p19Arfflox/flox. In these mouse models, activation of mutated K-RasG12D (excision of Lox-STOP-Lox cassette) and concomitant homozygous deletion of p16Ink4/p19Arf (excision of floxed alleles) is triggered by Cre-mediated recombination within Pdx1 (pancreatic and duodenal homeobox gene)-expressing cells during embryogenesis.5,25 An advantage of these murine PCCs is that they have a well-defined mutation history, largely dependent on the genetically-engineered activation of mutated K-RasG12D and/or loss of tumor suppressor genes.26,27 This panel of human and murine PCCs was used for the studies described below.
TGFβ1 promotes cell growth in 3D culture system and its effect is potentiated by EGF.
To determine how PCCs would integrate potential interactions between EGF and TGFβ1 signals, PCCs were treated 4 days after seeding in 3% Matrigel™/soft agar (hereafter referred to as 3D culture) with TGFβ1 at a final concentration of 100 pM and/or EGF at 1 nM. As expected, the growth of PCCs was stimulated by EGF (Fig. 1A and Fig. S2). TGFβ1 triggered profound morphological changes in TGFβ-responsive COLO-357 and murine PK-1, PK-2 and RInk-2 cells, and dramatically enhanced the growth of murine PCCs (Figs. 1 and 2). These morphological changes initially appeared as budding projections emanating from the epithelial spheroids within 24–36 h of TGFβ1 addition and became distinct protruding finger-like projections after 96 h (Figs. 1B and C; data not shown). By contrast, the growth of ASPC-1, BxPC3 and T3M4 cells was not significantly affected by TGFB-β1 treatment, but a rather minimal inhibitory effect was observed in BxPC-3 and T3M4 cells (Fig. S2). Moreover, the combination of EGF and TGFβ1 exerted dramatic growth stimulatory effects on COLO-357, PK-1, PK-2 and RInk-2 cells, and further enhanced morphological changes (Figs. 1 and 2), whereas TGFβ1 addition did not affect the actions of EGF in ASPC-1, BxPC3 and T3M4 cells (Figs. 1 and Fig. S2). In addition, COLO-357, PK-2, RInk-2 cells exhibited a dose-dependent response to EGF (0.2, 1, 5 nM) and/or TGFβ1 (20, 100, 500 pM) (Fig. S3; data not shown). We determined that 100 pM TGFβ1 and 1 nM EGF exerted sub-maximal growth stimulatory effects in PK-2 cells (Fig. S3). Accordingly, to allow for detection of additional stimulatory or inhibitory effects by other GFs and small molecular weight compounds, we conducted all subsequent experiments using 100 pM TGFβ1 and 1 nM EGF.
Figure 1.
Morphological changes of pancreatic cancer cells in 3D culture upon stimulation with EGF and/or TGFβ1. PCCs were cultured in 3% Matrigel™/soft agar. Four days after seeding, cells were treated with 100 pM TGFβ1 and/or 1 nM EGF as indicated. (A) Images of representative epithelial structures were captured 8 days after treatment. (B) Cells were collected 4 days after treatment, paraffin-embedded, sectioned and stained with H&E to reveal cytological architecture of epithelial structures. (C) Images of representative epithelial structures were captured at different time points after addition of TGFβ1 to establish dynamics of induced morphological changes. Scale bars indicate image magnification in each part.
Figure 2.
Distinct effects of EGF and TGFβ1 on pancreatic cancer cells in 3D and soft agar culture. PCCs were seeded in 3% Matrigel™/soft agar (3D) or in 0.4% soft agar in parallel experiments. (A) Four days after seeding, cells were treated with 100 pM TGFβ1 and/or 1 nM EGF as indicated. Cells were stained with MTT 8 days after treatment and images were captured by highresolution scanning. Scale bars indicate image magnification. (B Colorimetric intensity of MTT signal was quantified; average intensity value and standard deviation are displayed for each sample (n = 3) of a representative experiment. Relative fold change (over +/−2X) and statistical significance of this change with respect to EGF/TGFβ1-treated samples are displayed on top of the sd bars.
Extracellular matrix components are required for growth promoting effects of TGFβ1 alone and in conjunction with EGF.
To determine whether the growth promoting effects of TGFβ1 were due to direct cell-cell interactions or cell interactions with ECM components in Matrigel™, the growth of COLO-357 cells was assayed in parallel in soft agar and in 3D cultures. TGFβ1 decreased the size and number of colonies formed by COLO- 357 cells and antagonized the growth promoting effects of EGF under anchorage-independent growth conditions in soft agar culture (Figs. 2A and Fig. S4). In 3D culture, TGFβ1 exerted growth stimulatory effects on its own and significantly enhanced the growth stimulatory effects of EGF (Fig. 2B). These results suggest that ECM components modulate cellular response to EGF and TGFβ1. Although our murine PCCs formed tumors in athymic nude mice, they did not form measurable colonies in soft agar, which impeded addressing this question directly in these cells.
EGF/TGFβ1 cross-talk is mediated by distinct signaling pathways in murine and human cells.
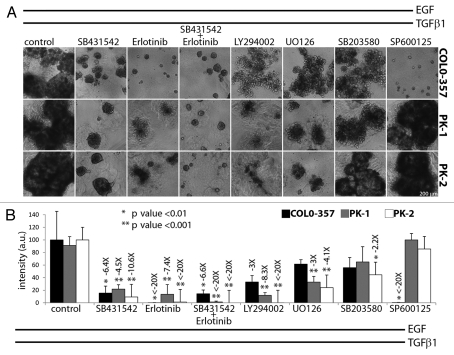
To determine whether the actions of EGF and TGFβ in TGFβ-responsive human and murine PCCs were mediated via the TGFβ type I and II receptor (TβRI/II) complex and/or EGFR, COLO-357, PK-1 and PK-2 cells were treated with EGF and TGFβ1 after 4 days of seeding in 3D culture, in the absence or presence of SB431542 (TβRI inhibitor) and/or erlotinib (EGFR tyrosine kinase inhibitor). Pre-incubation or concomitant addition of SB431542 significantly decreased EGF/TGFβ1-induced growth stimulation in COLO-357, PK-1 and PK-2 cells, whereas SB431542 addition 4 days following incubation with EGF/TGFβ1 halted further growth stimulation, but did not cause regression of already formed epithelial structures (Fig. 3; data not shown). Similarly, concomitant addition of erlotinib significantly decreased EGF/TGFβ1-induced growth stimulation of COLO-357, PK-1 and PK-2 cells (Fig. 3). Incubation with both erlotinib and SB431542 had a more profound inhibitory effect than either inhibitor alone (Figs. 3 and Fig. S5). These results indicate that EGF and TGFβ1 stimuli, transduced via EGFR and TβRI/II signaling pathways, act cooperatively in both human and murine PCCs.
Figure 3.
Cooperative interaction between EGF and TGFβ1 is disrupted by blockade of specific kinase pathways. Cells were seeded in 3% Matrigel™/soft agar and treated after 4 days with EGF/TGFβ1 only (control column) or also with the indicated kinase inhibitors at a final concentration of 100 pM TGFβ1, 1 nM EGF, 20 µM SB431542, 2 µM erlotinib and 10 µM LY294002, UO126, SB203580 or SP600125. (A) Representative images of epithelial structures were captured 4 days after treatment. Scale bars indicate image magnification. (B) Cells were stained with MMT 8 days after treatment. Colorimetric intensity of MTT signal was quantified; average intensity value and standard deviation are displayed for each sample (n = 6) of a representative experiment. Relative fold change (over −2X) and statistical significance of this change with respect to EGF/TGFβ1-treated (control column) samples are displayed on top of the sd bars.
To determine whether cross-talk between TβRI/II and EGFR signaling converged at a specific signaling pathway, PCCs were incubated with EGF/TGFβ1 in the absence or presence of LY294002 (PI3K inhibitor), U0126 (MEK1/2 inhibitor), SB203580 (p38/MAPK inhibitor) or SP600125 (JNK inhibitor). SP600125 completely blocked the stimulatory growth effects of EGF/TGFβ1 on COLO-357 cells, whereas LY294002 had a moderate and U0126 and SB203580 had a mild inhibitory effect (Fig. 3). Conversely, LY294002 almost completely blocked the stimulatory growth effects of EGF/TGFβ1 on PK-1 and PK-2 cells, whereas U0126 had a moderate and SB203580 had a mild inhibitory effect (Fig. 3). By contrast, SP600125 did not inhibit EGF/TGFβ1 actions in PK-1 and PK-2 cells (Fig. 3). Treatment with these kinase inhibitors alone did not alter cell viability at the tested concentrations (Fig. S6). Taken together, these results indicate a predominant requirement for JNK signaling in COLO-357 cells and for PI3K and to a lesser extent MEK1/2 signaling in murine PCCs to mediate the cooperative actions of EGF/TGFβ1.
EGF and TGFβ1 combine to enhance chemoresistance.
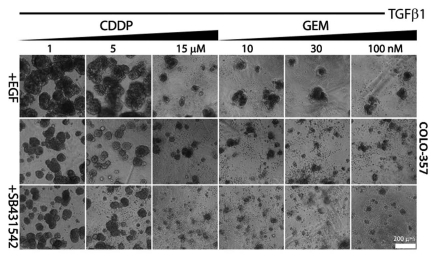
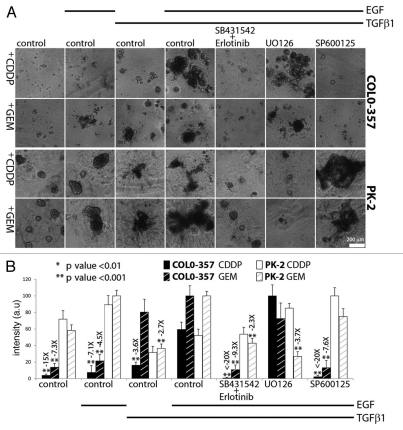
Gemcitabine (GEM), a nucleoside analog, is currently the standard of care in PDAC treatment.3 Cisplatin (CDDP), a DNA-crosslinker agent, has been administered in combination to GEM is some treatment regimens.3 Enhanced chemoresistance against GEM and CDDP has been linked to mesenchymal-like and epithelial-to-mesenchymal transition (EMT) characteristics of several PCCs.28,29 Since the effects of EGF and TGFβ1 in 3D culture correlated with the competence of COLO-357 cells and murine PCCs to undergo EMT as determined by immunostaining with E-cadherin (epithelial marker) and upregulation of intermediate filament vimentin (mesenchymal marker) on standard tissue culture plastic plates (Fig. S7), it was important to determine whether EGF/TGFβ1 co-treatment would enhance the chemoresistance of these PCCs. We first established the killing curve for CDDP and GEM in COLO-357 cells in 3D culture. CDDP exerted a slight cytotoxic effect at a concentration of 5 µM and a maximal effect at 15 µM, whereas a marked cytotoxic effect was already seen with 10 nM GEM (Fig. 4). TGFβ1 attenuated the cytotoxic effects of CDDP and GEM, and this chemoprotective effect was enhanced by EGF/TGFβ1 treatment (Fig. 4). Next, COLO-357 and PK-2 cells, as a representative murine PCC, were incubated for 4 days in 3D culture prior to the addition of vehicle, TGFβ1 or EGF/TGFβ1, with 10 µM CDDP or 10 nM GEM, and small molecule inhibitors that interfere with the tyrosine kinase activity of EGFR or its downstream effector kinases (Fig. 5). CDDP and GEM killed most COLO-357 cells after 4 days of incubation without added GFs, as determined visually by the presence of dispersed single cells or structures composed of few cells (Fig. 5A). Moreover, this cytotoxic effect persisted over the next 4 days, as determined by MTT metabolic staining (Fig. 5B). By contrast, there were larger cellular structures in TGFβ1-treated (>5 cells) and EGF/TGFβ1-treated COLO-357 cells (>20 cells) after 4 and 8 days of incubation (Figs. 4 and 5). The chemoprotective effect of EGF/TGFβ1 was not observed when human PCCs were subjected to these cytotoxic challenges in standard tissue culture (plastic plates) conditions (Fig. S8). PK-2 cells were more chemoresistant to GEM and CDDP than COLO-357 cells, and the enhanced chemoprotection by EGF/TGFβ1 treatment was only evident in the presence of GEM but not CDDP (Fig. 5A). While concomitant treatment with SB431542 and erlotinib blocked the protective effects of EGF/TGFβ1 against GEM and CDDP in both PCCs, SP600125 was markedly effective only in COLO-357 cells and UO126 was only effective in PK-2 cells (Fig. 5). These results reinforce our previous observations (Fig. 3) of a predominant requirement for JNK signaling in COLO-357 cells and for PI3K in PK-2 cells with respect to the cooperative actions of EGF/TGFβ1.
Figure 4.
Dose-response of COLO-357 cells to cytotoxic compounds in 3D culture. COLO-357 cells were cultured in 3% Matrigel™/soft agar. Four days after seeding, cells were incubated with increasing doses of the indicated cytotoxic compounds alone (upper row), or concomitantly with TGFβ1 (middle row) or TGFβ1/EGF (lower row) at a final concentration of 100 pM TGFβ1 and 1 nM EGF. Representative images of epithelial structures (n = 3) were captured 8 days after treatment. Scale bars indicate image magnification.
Figure 5.
Protective effects of EGF/TGFβ1 against cytotoxic compounds. Cells were seeded in 3% Matrigel™/soft agar and treated after 4 days with cytotoxic compounds (CDDP or GEM; column 1) alone or in combination with indicated GF and kinase inhibitorss at a final concentration of 100 pM TGFβ1, 1 nM EGF, 10 µM CDDP, 10 nM GEM, 20 µM SB431542, 2 µM erlotinib, 10 µM UO126 and 10 µM SP600125. (A) Representative images of epithelial structures were captured 4 days after treatment. Scale bars indicate image magnification. (B) Cells were stained with MMT after 8 days of treatment. Colorimetric intensity of MTT signal was quantified; average intensity value and standard deviation are displayed for each sample (n = 6) of this representative experiment. Relative fold change (over −2X) and statistical significance of this change with respect to EGF/TGFβ1-treated with CDDP or GEM (column 3) samples are displayed on top of the sd bars.
Ex vivo culturing of murine pancreatic lesions for assessment of chemosensitivity.
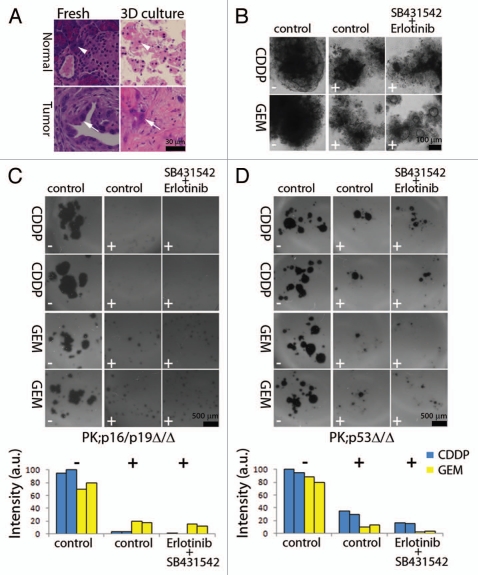
To determine whether this 3D culture system could be used to assess the chemosensitivity of PDAC-derived tissues, we used two well-established genetically-engineered mouse models of PDAC: pdx-1::cre;K-rasLSL-G1D2/+;p53flox/flox (PK;p53Δ/Δ) and pdx-1::cre;K-rasLSL-G1D2/+;p16Ink4a/p19Arfflox/flox (PK;p16/p19Δ/Δ) mice.5,25 Minced pancreatic tissue of about 1–5 mm3 in size were obtained from normal mouse pancreata (sibling non-transgenic controls) and from pancreatic tumors that have arisen in PK;p53Δ/Δ animals. While normal pancreatic tissues underwent severe atrophy, the growth and viability of cancer cells and stroma from the pancreatic tumors was sustained for at least 20 days in 3D culture (Fig. 6A). To assess cell responses to different chemotherapeutic treatments, tumor tissues were digested with collagenase to obtain single cell suspensions for accurate cell counting and equal cell seeding in replicate experimental wells. Treatment with CDDP or GEM reduced the number and size of cellular structures in 3D culture from PK;p53Δ/Δ and PK; p16/p19Δ/Δ animals (Fig. 6B–D). CDDP was more effective at reducing the growth and survival of PK;p16/p19Δ/Δ cells, whereas GEM had a similar cytotoxic effects on both PK;p16/p19Δ/Δ and PK;p53Δ/Δ cells. In all cases, concomitant incubation with erlotinib and SB431542 enhanced the cytotoxic effects of CDDP and GEM (Fig. 6B–D). Taken together, these results suggest that concomitant blockade of EGF and TGFβ signaling attenuates the chemoresistance of murine PCCs.
Figure 6.
Ex vivo culture of pancreatic tissue from mouse models of PDAC. (A) Normal and cancerous pancreatic tissues were dissected and immediately processed for H&E staining (left), or were minced and cultured in 3% Matrigel™/soft agar for 20 days before H&E staining (right). Acinar cells (arrowheads) and cancer cells (arrows) in freshly dissected tissue and ex vivo explants in 3D culture are shown. (B–D) After collagenese digestion, individual cells from cancerous tissues PK;p53Δ/Δ in (A, B and D) and PK;p16/p19Δ/Δ in (C) were plated in 3% Matrigel™ and treated after 4 days with indicated cytotoxic compounds (− denotes absence and + presence of CDDP or GEM) alone or in combination with TβRI and EGFR inhibitors (SB431542 + Erlotinib) at a final concentration of 10 µM CDDP, 10 nM GEM, 20 µM SB431542 and 2 µM erlotinib. (B) Representative images of cellular structures were captured 4 days after treatment. (C and D) Cells were stained with MMT after 8 days of treatment; two representative replicate cultures of PK;p16/p19Δ/Δ and PK;p53Δ/Δ animals are shown. Bar graphs display quantification of MTT signal for each replicate culture. (B and D) display results from independent experiments. Scale bars indicate image magnification in each part.
Discussion
The tumor microenvironment plays an important role in the carcinogenic process. Several 3D culturing systems have been implemented to delineate potential cancer cell-stromal interactions.30 Matrigel™, a collagen IV/laminin-rich extracellular matrix gel, has been used to study cell adhesion, polarity and migration as well as integration of cell signaling.30,31 Matrigel™-based studies conducted mainly in breast cancer cell lines have shown that ECM components upregulate the expression of integrins, EGFR and related receptors (human EGF receptor 2 [HER2] and HER3), thereby activating several signaling pathways. Moreover, the ECM modulates cell responses to anti-HER2 therapy, including the response to a humanized monoclonal anit-HER2 antibody (trastuzumab).32–34
In the present study, we established a 3D culture system in which the anchorage-independent growth of cells in soft agar is modulated by an overlay of 3% Matrigel™ which allows the cells to survive and proliferate in an environment that mimics the ECM. Elimination of the 100% Matrigel™ bed layer used in standard 3D culture20,21 also yields considerable cost savings. Moreover, a metabolic readout assay using MTT staining and a high-throughput 48-well plate format was used to quantitate differences in cell size and viability of epithelial structures (Fig. S5), thereby bypassing the need for sophisticated image analysis instruments.31
We applied this 3D culturing system to study the effects of TGFβ on human and murine PCCs and to assess the consequences of combined EGF and TGFβ signaling. We determined that TGFβ1 exerted growth stimulatory effects on PCCs in our novel 3D culture system (Figs. 1 and 2), whereas growth inhibitory effects were observed with the same PCCs cultured on plastic plates10 and soft agar (Figs. 2 and S4). Moreover, concomitant incubation with EGF/TGFβ1 resulted in a marked increase in cell growth and colony formation (Fig. 2). Several mechanisms may explain the observation that TGFβ1, especially in combination with EGF, increased the growth of PCCs in our 3D culture system: (i) Certain ECM components present in Matrigel™ may modulate TGFβ signal; (ii) Interactions between PCCs, Matrigel™ and soft agar may provide additional pathways for integrating TGFβ signaling in a manner that promotes proliferation; (iii) ECM components present in Matrigel™ may assist in presenting TGFβ to TβRII.
Secondary epithelial cell cultures (less than 20 passages) from mouse models of PDAC (PK-1, PK-2 and RInk-2 cells) were growth stimulated by TGFβ1 in 3D culture and underwent an EMT on plastic plates in response to TGFβ1 (Figs. 1 and 2, S2, S5 and S7; data not shown). By contrast, secondary cultures from PK;Smad4Δ/Δ cells failed to undergo EMT in 2D culture,15,17 were not responsive to TGFβ1 treatment and formed small epithelial structures in 3D culture (our unpublished observations). PK;Smad4Δ/Δ animals exhibit a longer latency than PK;p53Δ/Δ and PK;p16Ink4a/p19ArfΔ/Δ and develop PDAC through different precursor lesions (intraductal papillary mucinous and/or mucinous cystic neoplasms) instead of transitioning via early and advanced pancreatic intraepithelial lesions (PanIN-1/2 to PanIN-3), as observed in other models.15,17 These observations suggest that in murine PCCs, TGFβ1 induces EMT via the canonical TGFβ signaling pathway.
Several studies indicate that TGFβs can act via the Smaddependent canonical pathway, as well as via non-canonical Smadindependent pathways that activate both similar and different downstream signaling pathways, including the PI3K and JNK pathways.13,14,32,35 We determined that, depending on the cell line, both PI3K and JNK pathways were important transducers of the cross-talk between EGF and TGFβ. Thus, in murine PCCs, the marked increase in cell growth engendered by EGF/TGFβ1 was due, in part, to activation of the PI3K pathway, as evidenced by the finding that PI3K inhibition by LY294002 interfered with cooperative growth stimulation of EGF and TGFβ1 (Fig. 3). By contrast, in COLO-357 cells, EGF/TGFβ1 enhanced growth via the JNK pathway, as evidenced by the observation that the JNK inhibitor SP600125 completely blocked the growth stimulatory effects of EGF/TGFβ1 in these cells (Figs. 3 and 5). Interestingly, COLO-357 cells are the only human PCCs in our study with a functional Smad4 gene,36 and the murine PK-1, PK-2 and RInk-2 cells also have a functional Smad4 gene (our unpublished observations). Taken together, our observations suggest that TGFβ1 exerts its growth promoting effects in 3D culture via a Smad4-dependent pathway, whereas the cross-talk between EGF and TGFβ1 which further enhances the growth of these cells appears to be mediated via non-canonical PI3K or JNK signaling cascades. Several targeted approaches have been devised to interfere with TGFβ signaling, and are currently being tested in clinical trials.19 These include TGFβ blocking antibodies, TGFβ antisense RNA molecules, TβRII soluble proteins, small molecule inhibitors of TβRI kinase activity such as SB431542 used in this study.13,18,19 Although it is generally accepted that interfering with TGFβ actions in tumors would decrease proliferation of reactive stroma and could increase tumoricidal immune responses, concerns exist about intrinsic effects of TGFβ on cancer cells depending on the stage of the disease.18,19 Previously, we reported that COLO-357 cells engineered to express a soluble TβRII protein were resistant to TGFβ-mediated growth inhibition in vitro, yet formed smaller tumors in xenograft mouse model.10 Our current results, demonstrating that COLO-357 cells in 3D culture are directly growth-stimulated by TGFβ1, suggest that soluble TβRII could be acting in vivo to suppress the intrinsic ability of PCCs to be growth stimulated by TGFβ, in addition to interfering with the paracrine actions of TGFβs. Moreover, our finding that concomitant inhibition of EGFR and TβRI enhanced the effectiveness of CDDP and GEM in PCCs (Figs. 4 and 5) and in cultures of cells freshly isolated from pancreatic lesions arising in mouse models of PDAC (Fig. 6), support the validity of the concept that targeting TGFβ pathways may have therapeutic benefits for certain PDAC patients.
Resistance to TGFβ-mediated growth inhibition in PCCs arises through a variety of mechanisms, including Smad4 and p15 mutations,37 decreased TβRI expression,24 overexpression of inhibitory Smads 6 and 7,38,39 and rare TβRI or TβRII mutations.40 Ostensibly, TGFβs contribute to enhanced PDAC growth by exerting paracrine effects that lead to aberrant epithelial-mesenchymal interactions, alterations in the ECM, enhanced angiogenesis and suppressed cancer-directed immune mechanisms.41 However, TGFβ1 directly enhances the in vitro invasiveness of COLO-357 cells and increases matrix-metalloproteinase-9 (MMP9) activity in these cells.10 Moreover, a significant proportion of PDACs express high levels of Smad2 and TβRII, and TβRII overexpression is associated with decreased patient survival following PDAC resection and increased expression of PAI-1 and MMP9.42 These observations have suggested that in addition to promoting cancer progression through paracrine mechanisms, TGFβs may act directly on the cancer cells in vivo to enhance their invasive and metastatic potential. Taken together with our current finding that TGFβ1 directly enhances the growth of PCCs in 3D culture, these observations provide a powerful rationale for devising therapeutic strategies aimed at targeting TGFβ pathways in PDAC.
We envision that our novel 3D culture system could be utilized to culture ex vivo pancreatic cell preparations obtained by ultrasound-guided fine needle aspiration, by other types of biopsy or by surgical resection of a pancreatic tumor mass. Such an assay could determine TGFβ and other growth factor dependence of cancer cell growth in a manner that is more reflective of the tumor microenvironment in vivo and assess therapeutic response of combination drug treatments and/or predict chemoresistance to treatment. Moreover, our findings are not necessarily limited to PDAC, but may also be relevant to other cancers. Thus, establishing ex vivo functional subtypes of a variety of solid tumors in different organs could provide a rational and rapid approach for designing personalized therapeutic regimens.
Materials and Methods
Cell lines and treatments.
Human pancreatic cancer cell lines ASPC-1 and BxPC3 were purchased from the American Type Culture Collection, whereas COLO-357 and T3M4 were originally obtained from Dr. Richard S. Metzgar (Duke University). ASPC-1, BxPC3 and T3M4 cells were maintained in RPMI 1640 medium (ThermoScientific HyClone, SH30027-01), whereas COLO-357 cells were maintained in DMEM (ThermoScientific HyClone, SH30243-01). All media were supplemented with 5% Fetal Bovine Serum (FBS; Omega Scientific, Tarzana, CA), 100 units/ml Penicillin and 100 µg/ml Streptomycin (1x Pen/Strep; Mediatech, Manassas, VA). Cells were incubated at 37°C in a 20% O2/5% CO2 humidified atmosphere. The following growth factors and compounds were used in the experiments: EGF (Upstate/Millipore, 01-107), TGFβ1 and erlotinib (Genentech, Inc.), SB431542 and SP600125 (Tocris Biosciences, 1614 and 1496), LY294002 (Calbiochem, 440202), U0126 (Alexis Biochemicals, ALX-270-237), SB203580 (Enzo Life Sciences, ALX-270-179), cis-diamminedichloroplatinum (CDDP; Sigma, P4394) and gemcitabine (GEM; Eli Lily and Company, 0002-7502-01).
3D culture system of pancreatic cancer cell lines.
An equal volume of cell suspensions and ice-chilled 6% Matrigel™ (BD biosciences, 356231) in complete medium (RPMI 1640 supplemented with 5% FBS and 1x Pen/Strep) were mixed and dispensed at 5–15 × 103 cells in 1,000 µL per well, using 12-well plates (Fig. 1B) or 3–10 × 103 cells in 200 µL per well, using 48-well plates (Figs. 1[A–C] and 2–5) on top of a solidified 1% noble agar layer (Difco, 214220). Ice-chilled 3% Matrigel™ in complete medium was used to replenish the medium every 4 days and/or to administer compounds.
Staining of formalin-fixed paraffin-embedded and freshly fixed cells.
Cells cultured in 3% Matrigel™ were collected 4 days after treatments (Fig. 1B) using a 40 µm sieve mesh and chilled PBS to loosen Matrigel™ matrix. Cells were then fixed in 10% formalin for 1 h and coated with melted 1% noble agar. Agar plugs were fixed for up to 4 h in formalin and paraffin-embedded in fully-automated Shandon Pathcenter instrument as described in reference 23. Four µm sections of paraffin-embedded cellular structures were stained with hematoxylin and eosin.
Quantification of epithelial structure viability.
Cells were incubated for 4 h with 2.5 mg/ml of Thiazolyl Blue Tetrazolium Blue (MTT; Sigma, M2128) in PBS at 37°C. The plates containing the cells were then scanned and the resulting images were stored as high resolution (1,200 dpi) grayscale TIFF files. Signal intensity and density were quantified using Image-Pro Plus (Media Cybernetics).
Statistical analysis.
Samples with greater than 2-fold mean difference with respect to reference sample (EGF/TGFβ1-treated cells in Figures 2 and 3; EGF/TGFβ1-treated with CDDP or GEM in Fig. 5) based on continuous values of MTT signal were subjected to Welch's t-test, which corrected for possible unequal variance between samples, to assess statistical significance of these changes. To set a rigorous and reliable cut off to detect growth changes, only comparisons between samples with p values lower than a significance level (α) of 0.01 were reported.
Mouse husbandry and primary cell culture.
The K-rasLSLG1D2/+ (01XJ6-B6.129-Kras2tm4Tyj) and p53flox/flox (FVB.129-Trp53tm1Brn) mice were obtained from the Mouse Models of Human Cancers Consortium (National Cancer Institute, Frederick, MD). The pdx1::cre mice were kindly provided by G. Gu (reviewed in ref. 44) and the p16Ink4a/p19Arfflox/flox and Smad4flox/flox by N. Bardeesy (reviewed in refs. 15 and 45).
Mouse models of PDAC were generated by breeding appropriate founder strains in our animal facility. All studies with mice were approved by Dartmouth Medical School's Institutional Animal Care and Use Committee. Murine pancreata from euthanized animals were mechanically minced and digested with 2 mg/ml of Collagenase Type 4 (Worthington, 4188) for 1 h at 37°C. For ex vivo primary cell culture (Fig. 6), 10,000 cells per well were immediately plated in 3% Matrigel™/soft agar using 48-well plates. For this series of experiments RPMI 1640 medium was supplemented with 20% FBS, 1x Pen/Strep and 25 ng/mL Amphotericin B (MP Biomedicals, LLC, 1672348). For secondary cell cultures from tumor lesions of our mouse models, selection of PK-1 and Rink-1 cancer cells was accomplished by carrying out several rounds of diluted plating in RPMI 1640 medium with a gradual decrease in the FBS concentration from 20–5%, as previously reported in reference 46. PK-2 cells were established after subcutaneous passage of pancreatic tissue from a pdx1::cre;K-rasLSL-G1D2/+ animal into an athymic nude mouse. RInk-2 cells were established after subcutaneous passage of RInk-1 cells (reviewed in ref. 46) into an athymic nude mouse. All experiments with murine PCCs were performed on early passage cells (<20).
Acknowledgments
This work was supported by National Institutes of Health (NIH) and National Cancer Institute (NCI) grants CA-R37-075059 (M.K.) and AACR-PanCAN Laurie and Paul MacCaskill Career Development Award for Pancreatic Cancer Research grant 08-20- 25-SEMP (L.S.).
Supplementary Material
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Philip PA, Mooney M, Jaffe D, Eckhardt G, Moore M, Meropol N, et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol. 2009;27:5660–5669. doi: 10.1200/JCO.2009.21.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preis M, Korc M. Kinase signaling pathways as targets for intervention in pancreatic cancer. Cancer Biol Ther. 2010;9:754–763. doi: 10.4161/cbt.9.10.11534. [DOI] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Maitra A, Hruban RH. Pancreatic Cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer. 2003;2:8. doi: 10.1186/1476-4598-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194:S84–S86. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friess H, Yamanaka Y, Buchler M, Ebert M, Beger HG, Gold LI, et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105:1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 9.Rowland-Goldsmith MA, Maruyama H, Matsuda K, Idezawa T, Ralli M, Ralli S, et al. Soluble type II transforming growth factor-beta receptor attenuates expression of metastasis-associated genes and suppresses pancreatic cancer cell metastasis. Mol Cancer Ther. 2002;1:161–167. [PubMed] [Google Scholar]
- 10.Rowland-Goldsmith MA, Maruyama H, Kusama T, Ralli S, Korc M. Soluble type II transforming growth factor-beta (TGF-beta) receptor inhibits TGF-beta signaling in COLO-357 pancreatic cancer cells in vitro and attenuates tumor formation. Clin Cancer Res. 2001;7:2931–2940. [PubMed] [Google Scholar]
- 11.Vonlaufen A, Phillips PA, Xu Z, Goldstein D, Pirola RC, Wilson JS, et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 2008;68:7707–7710. doi: 10.1158/0008-5472.CAN-08-1132. [DOI] [PubMed] [Google Scholar]
- 12.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 13.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 14.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 15.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11:229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Arteaga CL. Inhibition of TGFbeta signaling in cancer therapy. Curr Opin Genet Dev. 2006;16:30–37. doi: 10.1016/j.gde.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/S1046-2023(03)00032-X. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez-Barrera AM, Menter DG, Abbruzzese JL, Reddy SA. Establishment of three-dimensional cultures of human pancreatic duct epithelial cells. Biochem Biophys Res Commun. 2007;358:698–703. doi: 10.1016/j.bbrc.2007.04.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore PS, Sipos B, Orlandini S, Sorio C, Real FX, Lemoine NR, et al. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- 23.Sipos B, Moser S, Kalthoff H, Torok V, Lohr M, Kloppel G. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Arch. 2003;442:444–452. doi: 10.1007/s00428-003-0784-4. [DOI] [PubMed] [Google Scholar]
- 24.Baldwin RL, Friess H, Yokoyama M, Lopez ME, Kobrin MS, Buchler MW, et al. Attenuated ALK5 receptor expression in human pancreatic cancer: correlation with resistance to growth inhibition. Int J Cancer. 1996;67:283–288. doi: 10.1002/(SICI)1097-0215(19960717)67:23.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 26.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/S1535-6108(03)00309-X. [DOI] [PubMed] [Google Scholar]
- 28.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, et al. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 31.Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol. 2008;130:1105–1118. doi: 10.1007/s00418-008-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang SE, Yu Y, Criswell TL, Debusk LM, Lin PC, Zent R, et al. Oncogenic mutations regulate tumor microenvironment through induction of growth factors and angiogenic mediators. Oncogene. 2010;29:3335–3348. doi: 10.1038/onc.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang SE, Xiang B, Zent R, Quaranta V, Pozzi A, Arteaga CL. Transforming growth factor beta induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 2009;69:475–482. doi: 10.1158/0008-5472.CAN-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat. 2010;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arany PR, Rane SG, Roberts AB. Smad3 deficiency inhibits v-ras-induced transformation by suppression of JNK MAPK signaling and increased farnesyl transferase inhibition. Oncogene. 2008;27:2507–2512. doi: 10.1038/sj.onc.1210889. [DOI] [PubMed] [Google Scholar]
- 36.Kleeff J, Wildi S, Friess H, Korc M. Ligand induced upregulation of the type II transforming growth factor (TGFbeta) receptor enhances TGFbeta responsiveness in COLO-357 cells. Pancreas. 1999;18:364–370. doi: 10.1097/00006676-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Villanueva A, Garcia C, Paules AB, Vicente M, Megias M, Reyes G, et al. Disruption of the antiproliferative TGF-beta signaling pathways in human pancreatic cancer cells. Oncogene. 1998;17:1969–1978. doi: 10.1038/sj.onc.1202118. [DOI] [PubMed] [Google Scholar]
- 38.Kleeff J, Maruyama H, Friess H, Buchler MW, Falb D, Korc M. Smad6 suppresses TGFbeta-induced growth inhibition in COLO-357 pancreatic cancer cells and is overexpressed in pancreatic cancer. Biochem Biophys Res Commun. 1999;255:268–273. doi: 10.1006/bbrc.1999.0171. [DOI] [PubMed] [Google Scholar]
- 39.Kleeff J, Ishiwata T, Maruyama H, Friess H, Truong P, Buchler MW, et al. The TGFbeta signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene. 1999;18:5363–5372. doi: 10.1038/sj.onc.1202909. [DOI] [PubMed] [Google Scholar]
- 40.Goggins M, Shekher M, Turnacioglu K, Yeo CJ, Hruban RH, Kern SE. Genetic alterations of the transforming growth factor beta receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res. 1998;58:5329–5332. [PubMed] [Google Scholar]
- 41.Korc M. Aberrant Transforming Growth Factor-beta. Signaling in Human Pancreatic Cancer: Translational Implications. In: Jakowlew SB, editor. Cancer Drug Discovery and Development: Transforming Growth Factor-β in Cancer Therapy: Cancer Treatment and Therapy. Vol. 2. Totowa, NJ: Humana Press Inc.; 2008. pp. 523–535. [Google Scholar]
- 42.Wagner M, Kleeff J, Friess H, Buchler MW, Korc M. Enhanced expression of the type II transforming growth factor-beta receptor is associated with decreased survival in human pancreatic cancer. Pancreas. 1999;19:370–376. doi: 10.1097/00006676-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Sempere LF, Preis M, Yezefski T, Ouyang H, Suriawinata AA, Silahtaroglu A, et al. Fluorescence-based codetection with protein markers reveals distinct cellular compartments for altered MicroRNA expression in solid tumors. Clin Cancer Res. 2010;16:4246–4255. doi: 10.1158/1078-0432.CCR-10-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35–43. doi: 10.1016/S0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 45.Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci USA. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009;69:422–430. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.