Abstract
The suggested role of cellular prion protein (PrPC) in mediating the toxic effects of oligomeric amyloid β peptide (Aβ) in Alzheimer disease (AD) is controversial. To address the hypothesis that variable PrPC expression is involved in AD pathogenesis, we analyzed PrPC expression in the frontal and temporal cortices and hippocampus of individuals with no cognitive impairment (NCI), amnestic mild cognitive impairment (aMCI), mild AD (mAD) and AD. We found that PrPC expression in all brain regions was not significantly altered among the various patient groups. In addition, PrPC levels in all groups did not correlate with expression of methionine (M) or valine (V) at codon 129 of the PrP gene, a polymorphism that has been linked in some studies to increased risk for AD, and which occurs in close proximity to the proposed binding region for the oligomeric Aβ peptide. Our results indicate that, if PrPC is involved in mediating the toxic effects of the oligomeric Aβ peptide, these effects occur independently of steady state levels of PrP or the codon 129 polymorphism.
Key words: prion protein, PrP codon 129 polymorphism, Alzheimer disease, oligomeric Aβ, Alzheimer precursor protein
Introduction
For many years, the overlapping clinical, pathological and biochemical characteristics of Alzheimer and prion diseases suggested a shared pathogenic mechanism. The prion protein (PrP) and the Aβ peptide, derived from the amyloid precursor protein (APP), both undergo structural transitions associated with a gain of toxic function leading to neurodegeneration.1 APP and PrP are directly or indirectly associated on the cell surface,2 and there are similarities in the post-translational processing of both proteins. Analogous to the α-secretase cleavage of APP, PrPC undergoes proteolytic cleavage at amino acids 110/111 to produce a 17-kDa carboxyl-terminal fragment referred to as C13 by the possible action of TNFα-converting enzyme (TACE) or members of the ADAM (α disintegrin and metalloprotease) family. PrP cleavage following residue 89 results in the formation of an approximately 21 kDa carboxy-terminal C2 fragment, which, in infected brains, is resistant to protease digestion and appears to be facilitated by calpain.4 These two cleavage sites flank the amino acid charge cluster in the central domain where oligomeric Aβ has been proposed to bind PrPC.5 Both proteins contain conserved histidine metal-binding domains, GxxxG transmembrane recognition motifs and histidine-based high-affinity metal-binding sites, which favor the binding of transition metals. Related to this, oxidative stress has been implicated in AD6 and prion disease.7
There is now convincing evidence that amplification of Aβ aggregates occurs by a prion-like mechanism.8–10 Moreover, prion infection is accelerated and formic acid-extractable Aβ1-42 peptide levels are higher, in transgenic mice expressing mutant APP (Tg2576 mice) compared to non-transgenic controls,11 suggesting that cross-seeding of the two abnormally conformed proteins may occur. In line with this observation, the brains of diseased scrapie-infected wild type mice also contain increased levels of the Aβ1-42 peptide.12 Conformational templating also appears to occur in other protein misfolding diseases13 involving tau,14 α-synuclein15 and polyglutamine proteins.16
Although soluble oligomeric forms of the Aβ peptide, derived from APP, are proposed as key mediators of synaptic and cognitive dysfunction in AD, the mechanisms by which these events occur remain unclear. Several recent studies suggest a direct mechanistic link between PrP and the Aβ peptide in AD pathogenesis. The studies of Strittmatter and co-workers implicated PrPC as a major receptor for synthetic soluble Aβ1-42 oligomers, and indicated that PrPC mediates the deleterious effects of oligomeric Aβ1-42 on synaptic function.5 Soluble Aβ1-42 oligomer binding was shown to occur at amino-acid residues 95–110 of PrP, a region close to the PRNP methionine (M)/valine (V) codon 129 polymorphism, which has numerous important influences on human prion diseases, and is also implicated as a risk factor for early-onset AD.17,18 In subsequent studies, this group demonstrated a requirement for PrP expression for axonal degeneration, loss of synaptic markers, early death and learning and memory deficits in AD transgenic mice.19
While the foregoing findings implicate PrP as a potential therapeutic target in AD, other studies have been less supportive of this concept. In independent transgenic mouse studies, ablation or overexpression of PrPC had no effect on impairment of hippocampal synaptic plasticity.20 Moreover, while the interaction of Aβ1-42 oligomers with PrPC was confirmed in separate studies, both PrP-expressing and PrP knockout mice were equally impaired in hippocampal dependent behavioral tests following intracerebroventricular injections of synthetic Aβ1-42 oligomers.21 Finally, other studies in which overexpression of PrP in vitro was shown to negatively regulate β-secretase12 would appear to be consistent with a model in which high levels of PrP result in low levels of Aβ1-42 oligomers.
Here we performed an extensive analysis of PrP levels in AD and pre-AD patients to address the hypothesis that variable PrPC expression is involved in AD pathogenesis.
Results and Discussion
Table 1 summarizes patient characteristics by diagnostic group. Included in our studies were 13 individuals with no cognitive impairment (NCI), 7 patients with amnestic mild cognitive impairment (aMCI), 6 with mild AD (mAD) and 11 with AD. Braak and CERAD scores were determined for all samples (Tables 2 and 3). Age, post-mortem interval (PMI) and brain weight were similar among the various groups (Table 1). Differences in mini-mental state examination (MMSE) scores were highly significant between groups [F(3,33) = 88.853, p < 0.0001]. Post hoc comparisons using the student Newman-Keuls test showed statistically significant differences between NCI and aMCI (p < 0.05), between aMCI and mAD (p < 0.05), and between mAD and AD groups (p < 0.05). All dementia groups showed lower MMSE scores than NCI. Both AD groups showed lower scores than aMCI patients, while the AD group showed lower scores than mAD (Table 1).
Table 1.
General demographics of subjects
| NCI | aMCI | mAD | AD | ANOVA | |
| Group size | 13 | 7 | 6 | 11 | |
| Male | 3 | 4 | 3 | 5 | |
| Female | 10 | 3 | 3 | 6 | |
| Mean age ± SD | 87.4 ± 7.6 | 91.1 ± 5.0 | 85.5 ± 7.2 | 83.9 ± 8.7 | F = 1.416 |
| Range | 70–101 | 84–99 | 77–94 | 68–99 | p > 0.1 |
| PMI (hours) | |||||
| Mean ± SD | 3.0 ± 0.8 | 2.9 ± 0.7 | 3.2 ± 0.9 | 3.5 ± 0.9 | F = 0.988 |
| Range | 2.3–5 | 2–3.5 | 2–4.5 | 2–5 | p > 0.1 |
| Brain weight (g) | F = 0.232 | ||||
| Mean ± SD | 1177 ± 166 | 1181 ± 155 | 1170 ± 57 | 1137 ± 110 | p > 0.1 |
| MMSE Mean ± SD | 28.7 ± 1.7 | 24.7 ± 2.4a | 22.2 ± 1.7ab | 13.4 ± 3.4abc | F = 88.853 |
| Range | 26–30 | 20–27 | 20–25 | 7–19 | p < 0.0001 |
p < 0.05 compared to NCI.
p < 0.05 compared to aMCI.
p < 0.05 compared to mAD.
Table 2.
Braak scores by clinical diagnosis
| Group (N) | 0–II | III–IV | V–VI |
| NCI (13) | 7 | 5 | 1 |
| aMCI (7) | 2 | 3 | 2 |
| mAD (6) | 1 | 2 | 2 |
| AD (11) | 1 | 0 | 10 |
Table 3.
CERAD classification by clinical diagnosis
| Group (N) | None | Possible | Probable | Definite |
| NCI (13) | 9 | 0 | 3 | 1 |
| aMCI (7) | 0 | 2 | 3 | 2 |
| mAD (6) | 1 | 0 | 1 | 4 |
| AD (11) | 0 | 0 | 2 | 9 |
In addition to its important influence on human prion diseases,22 the methionine (M)/valine (V) polymorphism at codon 129 of the prion protein gene (PRNP) has also been implicated as a risk factor for early-onset AD,17,18 although its influence as a risk factor for AD has been challenged.23 Analysis of this polymorphism is also important given its close proximity to the proposed region of Aβ1-42 oligomer binding.5 We analyzed codon 129 genotypes by treating polymerase chain reaction (PCR) amplified patient genomic DNA samples with NspI or MaeII restriction endonucleases (Fig. 1A and B) to distinguish PRNP coding sequence restriction fragment length polymorphisms (RFLPs) produced by the M and V codons. The PRNP M129 and V129 allele frequencies were approximately 77 and 23% respectively in the NCI group compared to 71 and 29% in the cognitively impaired groups. Valine homozygosity was not observed in the NCI group (Table 4). A previous association of 129V homozygosity and increased risk of early-onset AD was reported in a Dutch population.24 The mean age in each AD group in the current study is over 80 (Table 1). The period of follow up for patients was up to 14 years with a mean of 8 years approximately, raising the possibility that some cases may have been younger onset. Nonetheless, patient numbers in our study are too small to draw definitive conclusions.
Figure 1.
Representative restriction fragment length polymorphism (RFLP) analyses. Lanes 1 and 2, brain extracts from Tg(HuPrP-M129)6816+/− mice expressing human PrP-M129; lanes 3 and 4, brain extracts from and Tg(HuPrP-V129)7823+/− mice expressing human PrP-V129; lanes 5 and 6, equal mixture of brain extracts from transgenic mice expressing human PrP-M129 and transgenic mice expressing human PrP-V129; lanes 7–16, five representative human samples. Odd numbers, undigested PCR samples; even numbers, digested PCR samples. (A) PCR amplified samples treated with restriction enzyme NspI, which cleaves PRNP encoding methionine at codon 129. Methionine carriers produce a 363 bp fragment; valine carriers produce a 438 bp fragment. (B) PCR samples treated with MaeII, which cleaves PRNP encoding valine at codon 129. Valine carriers produce a 359 bp fragment.
Table 4.
Codon 129 PRNP allele frequency and genotype of subjects
| NCI | aMCI | mAD | AD | |||||
| N | % | N | % | N | % | N | % | |
| Allele | ||||||||
| M | 20 | 77 | 10 | 71 | 8 | 67 | 16 | 73 |
| V | 6 | 23 | 4 | 29 | 4 | 33 | 6 | 27 |
| Total | 26 | 14 | 12 | 22 | ||||
| Genotype | ||||||||
| MM | 7 | 54 | 4 | 57 | 3 | 50 | 6 | 55 |
| VV | 0 | 0 | 1 | 14 | 1 | 17 | 1 | 9 |
| MV | 6 | 46 | 2 | 29 | 2 | 33 | 4 | 36 |
| Total | 13 | 7 | 6 | 11 | ||||
To examine a possible relationship between cognitive decline and PrPC expression, homogenates were prepared from frozen samples of hippocampus, superior frontal cortex (BA9) and superior-middle temporal cortex (BA21-22) of NCI, aMCI, mAD and AD patients, and immunoblots of samples containing equivalent total protein levels were probed with either anti-PrP monoclonal antibodies (mAb) 6H4 or 3F4. Levels of PrP in these preparations were determined by densitometric analysis of western blots using actin levels as an internal control in each sample. While we observed a tendency of diminished PrP levels in rostral compared to caudal areas when immunblots were probed with mAb 6H4, regional differences were not significant, and rostrocaudal decreases in PrPC were not confirmed when the experiment was repeated using mAb 3F4 (Fig. 2).
Figure 2.
Rostrocaudal analysis of PrPC levels in NCI and AD patients. For all samples, levels of total PrPC and actin were measured by densitometric scanning of western blots. Each PrP value was normalized to its actin value. Normalized PrPC levels were compared among the frontal (F) and temporal (T) cortices and hippocampus (H) in NCI and AD groups. Error bars represent standard deviations from the means.
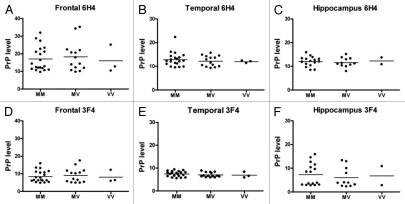
Western blot analysis using both mAb 6H4 and 3F4 showed that expression of PrPC was not significantly altered among NCI compared to aMCI, mAD or AD in either the frontal and temporal cortices or the hippocampus (Fig. 3). Levels of unglycosylated PrP were not higher in AD compared to control cases, although there was a tendency for higher levels of aglycosyl PrPC levels in the temporal cortex of aMCI patients compared to other study groups, and when measured with mAb 3F4 levels were significantly higher in temporal cortex of aMCI compared to NCI and AD patients (Fig. 4). PrPC expression levels were also independent of PRNP codon 129 genotype (Fig. 5).
Figure 3.
Levels of PrPC in different brain regions of patients with differing levels of cognitive impairment. Representative western blot analyses of brain homogenates from NCI, aMCI, mAD and AD patients are shown. Anti-PrP 6H4 (A–C) and 3F4 (D–F) antibodies were used to compare the levels of PrPC among the groups. Actin levels are shown below the 6H4 and 3F4 immunoblots. NCI: lanes 1–4. aMCI: 5–8. mAD: 9–12. AD: 13–16. Three brain regions including the frontal (A, D, G and J), temporal (B, E, H and K) cortices and hippocampus (C, F, I and L) were analyzed. Molecular markers indicated are 53, 36, 28 and 19 kDa. Graphs in (G–L) include analyses of all samples from each group. For each sample, levels of total PrPC were measured by densitometry. For each sample, levels of actin were also assessed. Each PrP value was normalized to its actin value. Means and standard deviations were calculated for each patient group and expressed as the percent value relative to the NCI group.
Figure 4.
Unaltered levels of unglycosylated PrPC in AD patients compared to NCI individuals. Levels of unglycosylated PrPC (∼molecular weight 25–30 kDa) were measured by densitometric analysis of western blots. Means and standard deviations were calculated for samples from each patient group. y-axis values are arbitrary densitometric units. Asterisks indicate p > 0.05.
Figure 5.
Levels of PrPC do not correlate with human PrP 129 polymorphism in AD patients. Levels of PrPC were measured by densitometry. For each sample, levels of actin were also assessed. Each PrP value was normalized to its actin value. These values were compared among individuals with various PRNP genotypes (M/M, M/V, V/V). (A–C) represent the data analyzed with 6H4; (D–F) represent the data analyzed with 3F4. Three brain regions including the frontal (A and D) and temporal (B and E) cortices and hippocampus (C and F) were analyzed.
Our results appear to be in general disagreement with the central topic of recently published studies by Velayos and co-workers29 which indicated a tendency for lower steady state PrPC levels in the brains of AD patients compared to controls,29 especially in the hippocampus, an outcome apparently inconsistent with previous reports of increased PrPC immunoreactivity in the temporal cortex, hippocampus (CA2) and subiculum in AD patients compared to controls.30 Although not discussed in the context of their data, the report of Velayos and co-workers was significant in light of recent studies implicating PrPC as a major receptor that mediates the deleterious effects of oligomeric Aβ1-42 on synaptic function.5 Here we observed no differences in total PrPC levels in AD compared to NCI controls, nor did we observe a graded decrease in PrP levels with increasing cognitive impairment. The minor observed differences between PrPC levels probed with mAb 6H4 and mAb 3F4 could be related to the sites of binding of these mAbs to PrP. The epitope for mAb 6H4 has been mapped to the sequence DYEDRYYRE, corresponding to PrP residues 144–152,25 while the epitope for 3F4 is located in the KTNMKHM, corresponding to residues 106–112.26–28 Thus, while mAb 6H4 can detect the proteolytically processed sub-fragment of PrPC, referred to as C1,3 mAb 3F4 cannot. Because di-glycosylated C1 fragments overlap with mono- and non-glycosylated forms of full-length PrPC, this could potentially affect quantification of PrPC levels and explain the discrepancy between the two mAbs.
What could explain the discrepancies between our study and the previous report? Velayos and coworkers studied three patients with AD. No clinical information was provided, except that one AD patient also had Down syndrome. Western blotting of PrPC was recorded in three control human cases and two AD patients. In the present study, we analyzed PrPC expression in a larger cohort comprised of 37 individuals including 11 AD cases, six mAD, seven aMCI patients and 13 non-demented controls. All patients were well characterized by diagnostic group, and all samples had short post-mortem intervals (Tables 1–3). Our analyses also included actin as an internal loading control for all samples. In addition to mAb 6H4, which was also used in the previous study, we used a second thoroughly characterized mAb (3F4) with reactivity against human PrPC. The high numbers of well-characterized patients, and the rigorous analysis of regional PrPC expression allow for meaningful associations (or lack of association in this case) to be made between levels of PrP and the presence of AD.
Velayos and coworkers also reported a shift in the profile of PrP glycosylation, with the unglycosylated form predominating in AD patients compared to controls. While we were also unable to confirm the finding of increased levels of aglycosyl PrPC in AD or mAD patients, we did observe significantly higher levels of unglycosylated PrP in the temporal cortex of aMCI patients compared to NCI and AD (but not mAD) patients. Whether this change corresponds to a specific role for PrP expressing cells in this critical brain region at early stages of the development of AD, prior to the onset of significant neurodegeneration and neuronal loss, remains to be determined.
In summary, we conclude that, if PrPC is involved in mediating the toxic effects of oligomeric Aβ, then this occurs by a mechanism that does not involve modulation of steady state levels of PrP. This would appear to be an important insight given recent associations of PrP in AD pathogenesis that implicate PrP as a potential therapeutic target.
Materials and Methods
Patients.
Frozen samples of hippocampus, superior frontal cortex (BA9) and superior-middle temporal cortex (BA21-22) were obtained from the University of Kentucky Rapid Autopsy Program of the Alzheimer Disease Clinical Center (UKADC). Tissue samples from 37 individuals with a mean age of 86.7 ± 7.6 years (Table 1) were examined. These individuals were part of a longitudinal clinical-pathologic study of aging and AD at the UKADC.31,32 The Human Investigations Committee at the University of Kentucky College of Medicine approved the studies. Individuals included in these studies agreed to annual clinical evaluation and brain donation at the time of death. For all subjects, cognitive test scores were available within the last year of life; the average interval from last evaluation to time of death was 7.0 ± 3.6 months, with no differences among the three diagnostic groups (p < 0.1). Subjects were categorized as NCI (n = 13), aMCI (n = 7), mAD (n = 6) or AD (n = 11),33 based on cognitive testing prior to death. The NCI subjects were without a history of dementia or other neurological disorders. Standard criteria for exclusion were the presence of (1) significant cerebral stroke regardless of antemortem date, (2) large cortical infarcts identified in the postmortem neuropathologic evaluation, (3) significant trauma within 12 months before autopsy, (4) individuals on a respirator longer than 12 h before death, (5) individuals in coma longer than 12 h immediately before death, (6) individuals currently undergoing radiation therapy for CNS tumor or (7) individuals with Lewy bodies.
Details of the UKADC have been published elsewhere in reference 32. All subjects have detailed mental status testing annually, and have neurologic and physical examinations annually. Subjects had been followed for 1–14 years (median 8.2 years). Once a subject transitioned to having aMCI or mAD, they received the mental status test battery and neurologic evaluation every 6 to 9 months. The 7 subjects with aMCI, 6 with mAD and 11 with AD were initially normal on enrollment into the longitudinal study and later developed aMCI and AD during follow-up. All aMCI subjects were amnestic without multi domain involvement. The diagnosis of aMCI, mAD, AD and NCI were defined by consensus conference. Histological examination of NCI subjects showed only age-related changes and Braak stage score of 0–II, meeting the NIA-RI low-likelihood criteria for the histopathologic diagnosis of AD. The clinical criteria for diagnosis of aMCI included (1) memory complaints, (2) intact activities of daily living, (3) objective memory impairment for age and education, (4) failure to meet criteria for dementia and (5) a clinical dementia rating (CDR) scale score of 0.5. The Braak stage scores had a range of I–V. Clinical progression to AD was diagnostically characterized by (1) a decline in cognitive functions from a previous higher level, (2) decline in one or more areas of cognition in addition to memory, (3) impaired activities of daily living, (4) a CDR score between 0.5–1 and (5) a clinical evaluation that excludes other causes of dementia. The criteria for mAD subjects included the above clinical progression plus a histopathologic diagnosis that included a Braak stage score of II–VI. For an AD categorization, subjects demonstrated a more progressive intellectual decline as described above, a MMSE less than that of the mAD cohort and Braak scores of II–VI. None of the mAD subjects were considered to be at the end-stage of the disease progression.
Transgenic mice.
Tg(HuPrP-M129)6816+/− mice and Tg(HuPrP-V129)7823+/− mice express human PrP encoding either M or V at codon 129, referred to as HuPrP-M129 and HuPrP-V129, respectively.34 Transgenic lines were maintained by breeding with Prnp0/0 mice maintained on an FVB background (FVB/Prnp0/0) and transgenic offspring were identified by tail biopsy and extraction of genomic DNA using a Beckman Biomek FX robotics station followed by PCR screening for the presence of the transgene. Approximately 1 cm of tail tissue was digested overnight at 55°C with proteinase K (0.5 mg/ml final concentration) in 50 mM Tris pH 8.0, 100 mM EDTA, 0.5% SDS, the DNA extracted with phenol and chloroform and concentrated by ethanol precipitation.
Analysis of the PRNP codon 129 polymorphism.
Genomic DNA was extracted from brain homogenates of the 37 individuals. The sense primer (5′-ATG GCG AAC CTT GGC TGC TGG ATG C-3′) and antisense primer (5′-GTG GTT GTG GTG ACC GTG TGC TGC TTG AT-3′) were used to amplify the PRNP cording sequence using PCR. The PRNP codon 129 polymorphism was assessed by digestion of the amplicons with restriction endonucleases NspI (New England Biolabs. Inc.,) and MaeII (HpyCH4IV, New England Biolabs. Inc.,), and the digested products were analyzed on 1.2% agarose gels.
Western blotting.
10% brain homogenates were prepared in sterile phosphate buffered saline (PBS) lacking Ca2+ and Mg2+ ions. The concentration of a total protein in each sample was determined by bicinchoninic acid assay and standardized for each lane (5 µg per lane). Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride Immobilon-FL membranes (Millipore). The transferred membrane was blocked with 5% non-fat milk in 0.5% Tween-20 in Tris-buffered saline (TBST) and immunoprobed with mouse monoclonal antibody anti-PrP 6H4 (Prionics) or 3F4 (Covance) followed by horseradish peroxidase-conjugated anti-mouse secondary antibody. Proteins were visualized using ECL Plus (GE Healthcare) in an FLA-5000 scanner (Fujifilm Life Science). Anti-actin (Pan) Ab-5 monoclonal antibody (NeoMarkers) was used as an internal control.
Statistical analysis.
Statistical analysis of western blot data from the frontal and temporal cortices and hippocampus of NCI, aMCI, mAD and AD was performed using a one-way ANOVA for each region separately. When appropriate, differences between groups were probed using a Newman-Keuls post hoc test. Each histogram of PrPC in western blots was read by MultiGauge (Fujifilm Life Science) and the values were standardized against the histogram value of actin. All data were analyzed with GraphPad Prism 4 and values were expressed as mean standard deviation. Differences with p < 0.05 was considered to be a significant and indicated with asterisks.
Acknowledgments
This study was supported by grants 5P30 AG028383 and 1P01AI077774-015261 to GCT from the National Institute on Aging (NIA), and the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH); AG027219 to SWS from the NIA, NIH, and by the Sanders Brown Center on Aging, University of Kentucky Medical Center, Lexington, KY.
Abbreviations
- PrP
prion protein
- PrPC
cellular prion protein
- PrPSc
scrapie form of the prion protein
- Aβ
amyloid β peptide
- AD
Alzheimer disease
- NCI
no cognitive impairment
- aMCI
amnestic mild cognitive impairment
- mAD
mild AD
- M
methionine
- V
valine
- APP
amyloid precursor protein
- kDa
kilodalton
- TACE
TNFα-converting enzyme
- ADAM
αdisintegrin and metalloprotease
- PRNP
gene encoding human prion protein
- CERAD
consortium to establish a registry for Alzheimer disease
- MMSE
mini-mental state examination
- PCR
polymerase chain reaction
- RFLP
restriction fragment length polymorphism
- mAb
monoclonal antibody
- PMI
post mortem interval
References
- 1.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt-Ulms G, Hansen K, Liu J, Cowdrey C, Yang J, DeArmond SJ, et al. Time-controlled transcardiac perfusion cross-linking for the study of protein interactions in complex tissues. Nat Biotechnol. 2004;22:724–731. doi: 10.1038/nbt969. [DOI] [PubMed] [Google Scholar]
- 3.Chen SG, Teplow DB, Parchi P, Teller JK, Gambetti P, Autilio-Gambetti L. Truncated forms of the human prion protein in normal brain and in prion diseases. J Biol Chem. 1995;270:19173–19180. doi: 10.1074/jbc.270.32.19173. [DOI] [PubMed] [Google Scholar]
- 4.Yadavalli R, Guttmann RP, Seward T, Centers AP, Williamson RA, Telling GC. Calpain-dependent endoproteolytic cleavage of PrPSc modulates scrapie prion propagation. J Biol Chem. 2004;279:21948–21956. doi: 10.1074/jbc.M400793200. [DOI] [PubMed] [Google Scholar]
- 5.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins RN, Harper CG, Stokes GB, Masters CL. Increased cerebral glucose-6-phosphate dehydrogenase activity in Alzheimer's disease may reflect oxidative stress. J Neurochem. 1986;46:1042–1045. doi: 10.1111/j.1471-4159.1986.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 7.Brazier MW, Lewis V, Ciccotosto GD, Klug GM, Lawson VA, Cappai R, et al. Correlative studies support lipid peroxidation is linked to PrP(res) propagation as an early primary pathogenic event in prion disease. Brain Res Bull. 2006;68:346–354. doi: 10.1016/j.brainresbull.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, Yan ZX, et al. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci USA. 2009;106:12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 10.Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baier M, Apelt J, Riemer C, Gultner S, Schwarz A, Bamme T, et al. Prion infection of mice transgenic for human APPSwe: increased accumulation of cortical formic acid extractable Abeta(1-42) and rapid scrapie disease development. Int J Dev Neurosci. 2008;26:821–824. doi: 10.1016/j.ijdevneu.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, Manson JC, et al. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer's amyloid precursor protein. Proc Natl Acad Sci USA. 2007;104:11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Bo R, Scarlato M, Ghezzi S, Martinelli-Boneschi F, Fenoglio C, Galimberti G, et al. Is M129V of PRNP gene associated with Alzheimer's disease? A case-control study and a meta-analysis. Neurobiol Aging. 2006;27:7701–7705. doi: 10.1016/j.neurobiolaging.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Riemenschneider M, Klopp N, Xiang W, Wagenpfeil S, Vollmert C, Muller U, et al. Prion protein codon 129 polymorphism and risk of Alzheimer disease. Neurology. 2004;63:364–366. doi: 10.1212/01.wnl.0000130198.72589.69. [DOI] [PubMed] [Google Scholar]
- 19.Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calella AM, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, et al. Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol Med. 2010;2:306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, et al. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci USA. 107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wadsworth JD, Asante EA, Desbruslais M, Linehan JM, Joiner S, Gowland I, et al. Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science. 2004;306:1793–1796. doi: 10.1126/science.1103932. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Rowland LP, Mitsumoto H, Przedborski S, Bird TD, Schellenberg GD, et al. Prion protein codon 129 genotype prevalence is altered in primary progressive aphasia. Ann Neurol. 2005;58:858–864. doi: 10.1002/ana.20646. [DOI] [PubMed] [Google Scholar]
- 24.Dermaut B, Croes EA, Rademakers R, Van den Broeck M, Cruts M, Hofman A, et al. PRNP Val129 homozygosity increases risk for early-onset Alzheimer's disease. Ann Neurol. 2003;53:409–412. doi: 10.1002/ana.10507. [DOI] [PubMed] [Google Scholar]
- 25.Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, et al. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature. 1997;389:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- 26.Rubenstein R, Kascsak RJ, Papini M, Kascsak R, Carp RI, LaFauci G, et al. Immune surveillance and antigen conformation determines humoral immune response to the prion protein immunogen. J Neurovirol. 1999;5:401–413. doi: 10.3109/13550289909029481. [DOI] [PubMed] [Google Scholar]
- 27.Lund C, Olsen CM, Tveit H, Tranulis MA. Characterization of the prion protein 3F4 epitope and its use as a molecular tag. J Neurosci Methods. 2007;165:183–190. doi: 10.1016/j.jneumeth.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Zou WQ, Langeveld J, Xiao X, Chen S, McGeer PL, Yuan J, et al. PrP conformational transitions alter species preference of a PrP-specific antibody. J Biol Chem. 2010;285:13874–13884. doi: 10.1074/jbc.M109.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velayos JL, Irujo A, Cuadrado-Tejedor M, Paternain B, Moleres FJ, Ferrer V. The cellular prion protein and its role in Alzheimer disease. Prion. 2009;3:110–117. doi: 10.4161/pri.3.2.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voigtlander T, Kloppel S, Birner P, Jarius C, Flicker H, Verghese-Nikolakaki S, et al. Marked increase of neuronal prion protein immunoreactivity in Alzheimer's disease and human prion diseases. Acta Neuropathol. 2001;101:417–423. doi: 10.1007/s004010100405. [DOI] [PubMed] [Google Scholar]
- 31.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 33.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 34.Kurt TD, Telling GC, Zabel MD, Hoover EA. Trans-species amplification of PrP(CWD) and correlation with rigid loop 170N. Virology. 2009;387:235–243. doi: 10.1016/j.virol.2009.02.025. [DOI] [PubMed] [Google Scholar]