Abstract
BACKGROUND
Buprenorphine (BUP) is under investigation as a medication therapy for opioid-dependent pregnant women. We investigated BUP and metabolite disposition in urine from women maintained on BUP during the second and third trimesters of pregnancy and postpartum.
METHODS
We measured BUP, norbuprenorphine (NBUP), buprenorphine glucuronide (BUP-Gluc), and NBUP-Gluc concentrations in 515 urine specimens collected thrice weekly from 9 women during pregnancy and postpartum. Specimens were analyzed using a fully validated liquid chromatography-mass spectrometry method with limits of quantification of 5 µg/L for BUP and BUP-Gluc and 25 µg/L for NBUP and its conjugated metabolite. We examined ratios of metabolites across trimesters and postpartum to identify possible changes in metabolism during pregnancy.
RESULTS
NBUP-Gluc was the primary metabolite identified in urine and exceeded BUP-Gluc concentrations in 99% of specimens. Whereas BUP-Gluc was identified in more specimens than NBUP, NBUP exceeded BUP-Gluc concentrations in 77.9% of specimens that contained both analytes. Among all participants, the mean BUP-Gluc:NBUP-Gluc ratio was significantly higher in the second trimester compared to the third trimester, and there were significant intrasubject differences between trimesters in 71% of participants. In 3 women, the percent daily dose excreted was higher during pregnancy than postpregnancy, consistent with other data indicating increased renal elimination of drugs during pregnancy.
CONCLUSIONS
These data are the first to evaluate urinary disposition of BUP and metabolites in a cohort of pregnant women. Variable BUP excretion during pregnancy may indicate metabolic changes requiring dose adjustment during later stages of gestation.
Buprenorphine (BUP)5 is a semisynthetic thebaine derivative with partial agonist activity at the μ-opioid receptor. Although BUP is well absorbed in the gastrointestinal tract, bioavailability is estimated at 15% via oral administration owing to high first-pass metabolism (1). Sublingual (SL) bioavailability is 30%–55% (2). BUP undergoes N-dealkylation in the liver, forming norbuprenorphine (NBUP); BUP and NBUP also are subject to oxidation, forming hydroxylated metabolites. In addition, phase 2 metabolism produces glucuronide conjugates of BUP, NBUP, and hydroxylated analogs. The majority of BUP is excreted in the feces (3), with 10%–30% of a dose excreted in urine, primarily as conjugated metabolites (3, 4). NBUP is an active metabolite with approximately 25% of BUP’s intrinsic analgesic effect; however, it is unable to cross the blood–brain barrier, yielding an apparent analgesic effect of only 2% (5). Despite NBUP’s inability to penetrate the blood–brain barrier at therapeutic doses of BUP, significant respiratory depression occurred in rats, possibly by acting directly on μ-receptors in the lung (6).
Urine monitoring for compliance with BUP maintenance and detection of nonprescribed use (7–11) requires specific assays with low detection limits. Gas chromatography (12–15) and liquid chromatography (16–20) methods are available. Our laboratory recently developed and validated an LC-MS method for simultaneously quantifying BUP, NBUP, buprenorphine glucuronide (BUP-Gluc), and NBUP-Gluc in urine (21).
Several factors can modify a drug’s pharmacokinetic profile during different stages of pregnancy, impacting bioavailability and clearance. Maternal cardiac output increases up to 30%, blood volume up to 50%, and total body water by an average of 8 L (22–24). In addition, decreases in serum albumin concentrations and increases in steroids and placental hormones occupying binding sites lead to reduced drug–protein binding (22, 24). Changes in metabolic enzyme expression also can affect urinary excretion of BUP and metabolites. The activity of CYP3A4, the primary enzyme responsible for BUP N-dealkylation to NBUP, and several uridine diphosphate glucuronosyltransferase (UGT) isoenzymes increase during pregnancy (25).
The objective of this study was to prospectively investigate urinary BUP and metabolites in women undergoing supervised BUP maintenance therapy during the second and third trimesters of pregnancy and post-partum, addressing several unanswered research questions such as intra- and intersubject variations in BUP urinary excretion and changes in total excretion during a 24-h period. Answers to these questions can serve as an evidence-based framework for treatment providers and policymakers to determine the best dosing approaches for pregnant opioid-dependent patients treated with BUP.
Materials and Methods
CHEMICALS AND REAGENTS
BUP, NBUP, BUP-Gluc, NBUP-Gluc, NBUP-d3, and BUP-d4 for calibrators and internal standards were purchased from Cerilliant; BUP for control preparation from Lipomed; and BUP-Gluc and NBUP-Gluc controls from ElSohly Laboratories. A separate NBUP manufacturer was unavailable; controls were made from a different lot of Cerilliant stock when possible, and on a different day than calibrators. Reagent-grade formic and phosphoric acids were from Sigma Chemicals and JT Baker, respectively. All solvents were HPLC grade.
STUDY PARTICIPANTS AND SPECIMEN COLLECTION
Participants were women delivering a child while enrolled in a randomized, double-blind, double-dummy, flexible dosing, parallel group controlled trial evaluating safety and efficacy of BUP compared to methadone for opioid dependence treatment during pregnancy (26, 27). The Johns Hopkins Bayview Medical Center and National Institute on Drug Abuse Institutional Review Boards approved the study; women provided written informed consent to participate. Inclusion criteria were age 21–40 years, 16–30 weeks’ gestation, current Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) opioid-dependence diagnosis, maintenance pharmacotherapy request, recent self-reported opioid use in more than 4 of 7 days, and an opiate-positive urine specimen. Exclusion criteria were undocumented methadone-positive urine, current DSM-IV alcohol abuse or dependence, self-reported benzodiazepine use >7 times monthly or once weekly, currently taking another Axis I disorder medication, serious concurrent illness, previous preterm labor diagnosis, fetal malformation evidence, or HIV or sickle-cell trait positive. Nine women met the inclusion and exclusion criteria, were randomized to BUP, and completed the study.
DOSING AND URINE COLLECTION
On each dosing day, the women provided a urine specimen before receiving 12 SL tablets followed by 40 mL liquid under observation in the clinic. Participants assigned to active BUP received 12 SL tablets in a combination of 2-mg BUP HCl (Subutex™; Reckitt Benckiser Pharmaceuticals Inc.; total BUP dose: 4–24 mg) and matching placebo tablets (Reckitt Benckiser Pharmaceuticals Inc.), followed by 40 mL placebo cherry-flavored liquid. Details of the preparation of the medicine have been published (26, 27).
Mean doses in the second and third trimester and postpartum were calculated based on gestational age at delivery. Missed doses were recorded as zero, and the mean dose was calculated by dividing total dose received by days enrolled in the study during the specified time period. The postpartum period included doses received from the day of delivery through the last dose received.
Thrice-weekly urine specimens (n = 515) were collected a minimum of 16 h after the last dose, from study enrollment through discharge 4–10 weeks after delivery. Specimens were stored at −20 °C for several years before analysis. Three women also participated in 2–4 pharmacokinetic sessions, with individual collection of all urine specimens excreted for 24 h.
URINE ANALYSIS
We analyzed specimens using a fully validated LCMS method for BUP, NBUP, BUP-Gluc, and NBUP-Gluc in urine (21). Briefly, analytes were extracted from 200 µL urine, isolated and concentrated by solid-phase extraction, and quantified on an LCQ Deca XPPlus Ion-Trap Mass Spectrometer with electrospray ionization. Two scan events were performed for each analyte for quantification and identification purposes. The quantification scan event was selected ion monitoring of the precursor ion without fragmentation. Precursor ions were 468, 414, 644, and 590 for BUP, NBUP, BUP-Gluc, and NBUP-Gluc, respectively. BUP and NBUP were identified by the presence of 4 characteristic (396, 414, 426, and 450 for BUP; 340, 364, 382, and 396 for NBUP) ions from MH+ fragmentation. We identified glucuronidated analytes by fragmenting MH+, cleaving the glucuronide moiety, and further fragmenting the surviving molecule and monitoring 2 characteristic ions. Qualification ions were 396 and 414 for BUP-Gluc and 340 and 396 for NBUP-Gluc. Limits of quantification (LOQs), as empirically determined by analysis of samples with decreasing concentrations of analyte, were 25 µg/L for NBUP-Gluc and NBUP and 5 µg/L for BUP-Gluc and BUP, with linearity to 1000 µg/L for all analytes. The LOQ was defined as the lowest concentration with acceptable chromatography, presence of all qualifier ions with signal-to-noise ratios of at least 10 for all ions, retention time within 0.2 min of the average calibrator retention time, and acceptable accuracy with concentrations within 20% of target. We measured urine creatinine concentrations using the Axiom Diagnostic Test True™ Creatinine Assay (Axion Diagnostics) on a Hitachi P-Module analyzer using manufacturer-recommended instrument parameters.
DATA ANALYSIS
We performed statistical analyses using SPSS 13.0 for Windows (SPSS, Inc.). We evaluated correlations between BUP dose and creatinine-corrected analyte concentrations using Spearman correlation because normality of BUP dose could not be assumed. One-way ANOVA with post-hoc tests examined the relationship between BUP dose and trimester. Analyte ratios were compared among participants and trimesters with 2-way ANOVA including Scheffé’s post-hoc test. Intra-subject variation in ratios between trimesters was evaluated by nonparametric Kruskal–Wallis tests with Dunn multiple comparison test for posthoc analysis. Because SPSS does not perform post-hoc analysis for nonparametric comparisons, we used GraphPad Prism 3.0 (GraphPad Software Inc.) for these analyses. The amount of analyte excreted in each urine specimen collected over 24 h was calculated by multiplying the concentration by specimen volume. The percent dose excreted was calculated by converting total metabolite excreted to BUP equivalents, summing the metabolites and parent drug, and dividing by the dose administered. P values at or below 0.05 were considered statistically significant.
Results
PARTICIPANT DEMOGRAPHICS AND DOSING INFORMATION
The 9 BUP-maintained women (8 African American, 1 white) gave birth to 10 infants. The women were 22–32 years of age [mean (SD) 30.0 (1.1) years] with an estimated gestational age at admission of 22.8 (1.2) weeks (range 18–26 weeks). All participants met criteria for current opioid-dependence based on DSM-IV.
Mean daily doses throughout the study and in each trimester can be found in Table 1. The mean BUP dose significantly increased from the second to the third trimester, and was again higher in the postpartum period (P ≤ 0.001 for all comparisons). Two of the 9 participants had no significant changes in mean dose throughout the study.
Table 1.
Maternal buprenorphine dosing.a
| Participant | Dose at delivery, mg/day |
Average daily dose, mg/day | P < 0.05 | |||
|---|---|---|---|---|---|---|
| Overall | Second trimester |
Third trimester |
Postpartum | |||
| A | 20 | 16.5 (4.7) | 12.8 (1.8) | 16.2 (4.9) | 19.3 (3.8) | 2nd trimester < 3rd < postpartum |
| B | 16 | 14.9 (3.6) | 11.6 (1.2) | 14.8 (1.9) | 15.7 (5.1) | 2nd trimester < 3rd and postpartum |
| C | 24 | 19.0 (4.0) | 15.2 (2.5) | 21.0 (2.4) | 24.0 (0.0) | 2nd trimester < 3rd < postpartum |
| D | 18 | 16.5 (3.0) | 13.1 (1.5) | 16.9 (2.4) | 17.5 (3.0) | 2nd trimester < 3rd < postpartum |
| E | 24 | 19.2 (6.5) | 14.1 (6.6) | 21.8 (4.5) | 23.5 (2.4) | 2nd trimester < 3rd and postpartum |
| F | 16 | 11.2 (7.0) | 11.7 (6.2) | 10.4 (7.6) | 13.5 (6.0) | 2nd trimester < 3rd and postpartum |
| G | 18 | 16.3 (2.3) | 13.0 (2.4) | 17.1 (1.0) | 18.0 (0.0) | NS |
| H | 18 | 13.4 (6.7) | 10.9 (5.3) | 13.3 (7.0) | 16.2 (6.6) | 2nd trimester < 3rd < postpartum |
| I | 14 | 13.4 (2.2) | 12.9 (1.0) | 13.7 (2.1) | 12.8 (2.9) | NS |
| All | 15.4 (5.5) | 12.8 (4.8) | 15.8 (5.5) | 17.4 (5.1) | 2nd trimester < 3rd < postpartum | |
Data are mean (SD). NS, not significant.
URINARY BUP AND METABOLITE DISPOSITION
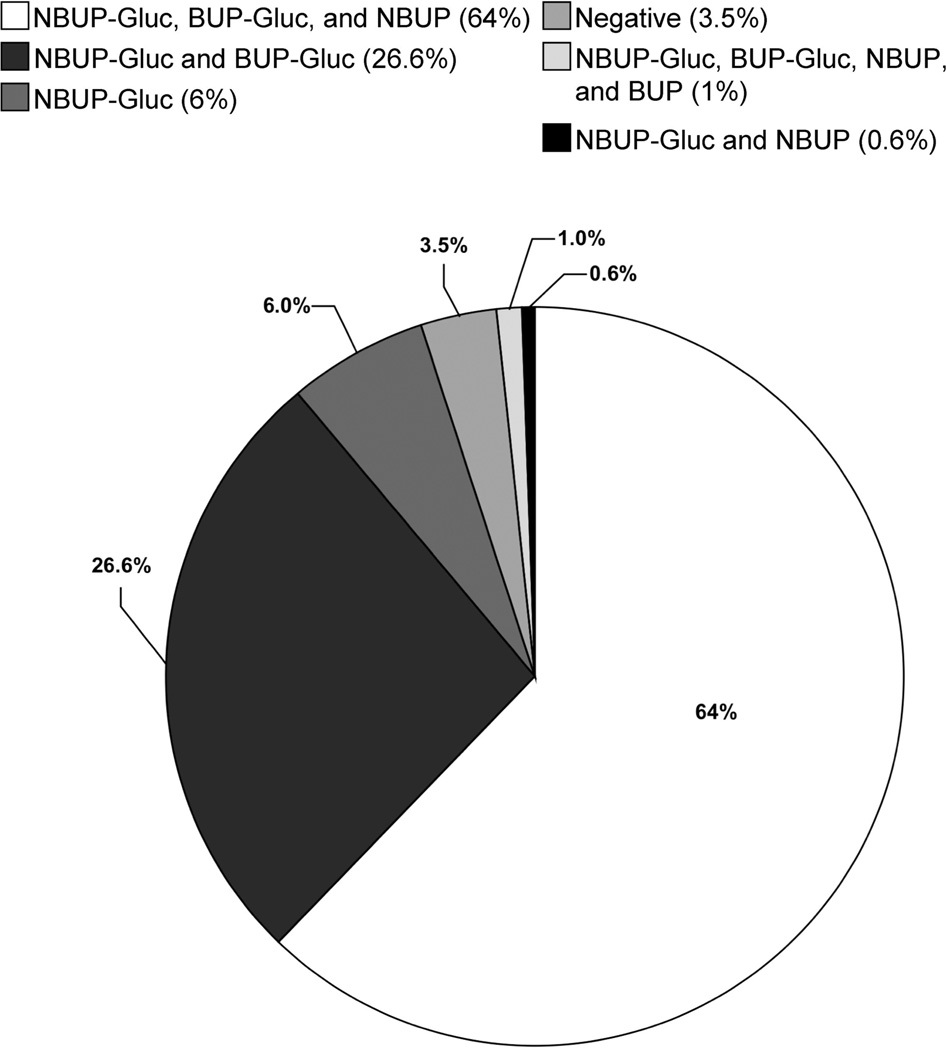
The frequency of BUP, NBUP, BUP-Gluc, and NBUP-Gluc detection in all thrice-weekly urine specimens and those individually collected for 24 h after dosing (n = 667) was 1.2%, 63.9%, 89.9%, and 96.5% of urine specimens, respectively. The distribution of individual analytes in urine specimens is depicted in Fig. 1. Six of 9 specimens that contained BUP were from participant D, and 53% of 19 negative specimens occurred within the first week of dosing or within 3 days of a series of 3 or more missed doses.
Fig. 1.
Disetribution of BUP, NBUP, BUP-Gluc, and NBUP-Gluc in 667 urine specimens collected from 9 women during pregnancy.
Creatinine-corrected urinary BUP, NBUP, BUP-Gluc, and NBUP-Gluc concentrations from the thrice-weekly urine specimens (n = 515) are summarized in Table 2. NBUP-Gluc concentrations always exceeded NBUP concentrations and were generally (99%) greater than BUP-Gluc concentrations. Participant C had 6 specimens, collected over a 2-week period (12–24 days in treatment), with BUP-Gluc concentrations greater than NBUP-Gluc.
Table 2.
Values for 515 urine specimens collected from 9 treatment patients and analyzed by liquid chromatography–tandem mass spectrometry.
| Participant (samples) | BUP | NBUP | BUP-Gluc | NBUP-Gluc |
|---|---|---|---|---|
| A (n = 41) | ||||
| Median, ng/mg creatininea | —b | 100.6 | 57.6 | 1048.6 |
| Interquartile range | — | 70.7–131.7 | 34.9–79.4 | 670.4–1327.2 |
| Range | — | 39.4–627.8 | 22.6–317.0 | 326.0–4394.7 |
| % Positivec | 0 | 75.6 | 90.2 | 95.1 |
| B (n = 67) | ||||
| Median, ng/mg creatinine | — | 109.7 | 32.9 | 259.3 |
| Interquartile range | — | 56.7–200.7 | 20.9–74.2 | 159.3–699.1 |
| Range | — | 36.5–1125.5 | 6.0–671.2 | 58.8–4494.3 |
| % Positive | 0 | 28.4 | 74.6 | 89.6 |
| C (n = 57) | ||||
| Median, ng/mg creatinine | — | 91.1 | 148.2 | 1116.1 |
| Interquartile range | — | 72.1–202.4 | 93.8–360.4 | 481.6–2199.5 |
| Range | — | 38.9–1319.5 | 5.8–8902.2 | 51.8–19 720.5 |
| % Positive | 0 | 50.9 | 93 | 94.7 |
| D (n = 63) | ||||
| Median, ng/mg creatinine | 11.1 | 209.4 | 64.9 | 825.8 |
| Interquartile range | 7.5–24.6 | 104.1–311.1 | 37.5–147.4 | 425.9–1521.4 |
| Range | 4.8–56.9 | 42.0–3655.8 | 9.9–2813.9 | 82.0–20 130.2 |
| % Positive | 6.3 | 87.3 | 96.8 | 100 |
| E (n = 58) | ||||
| Median, ng/mg creatinine | 96.9 | 88.2 | 55.4 | 547.5 |
| Interquartile range | — | 54.0–176.1 | 29.7–118.6 | 306.3–1008.0 |
| Range | 96.9–96.9 | 12.0–916.2 | 3.6–901.4 | 21.8–10 294.5 |
| % Positive | 1.7 | 79.3 | 91.4 | 96.6 |
| F (n = 62) | ||||
| Median, ng/mg creatinine | — | 45.6 | 22.3 | 231.7 |
| Interquartile range | — | 27.6–132.0 | 12.0–62.8 | 118.5–427.5 |
| Range | — | 10.0–436.3 | 4.7–330.3 | 28.6–3306.9 |
| % Positive | 0 | 48.4 | 87.1 | 100 |
| G (n = 48) | ||||
| Median, ng/mg creatinine | 11.0 | 70.1 | 58.8 | 598.7 |
| Interquartile range | — | 39.8–96.9 | 44.5–113.5 | 335.4–901.9 |
| Range | 11.0–11.0 | 20.6–205.0 | 5.2–375.7 | 70.4–2298.8 |
| % Positive | 2.1 | 89.6 | 97.9 | 97.9 |
| H (n = 78) | ||||
| Median, ng/mg creatinine | — | 80.9 | 35.5 | 389.8 |
| Interquartile range | — | 51.5–131.8 | 18.5–61.7 | 219.2–675.3 |
| Range | — | 21.8–450.5 | 3.9–276.8 | 38.1–2385.3 |
| % Positive | 0 | 78.2 | 87.2 | 96.2 |
| I (n = 41) | ||||
| Median, ng/mg creatinine | — | 133.6 | 72.1 | 966.4 |
| Interquartile range | — | 65.5–201.6 | 45.9–193.3 | 590.9–2265.2 |
| Range | — | 25.5–945.1 | 5.8–1478.4 | 90.5–9764.1 |
| % Positive | 0 | 36.6 | 97.6 | 100 |
| All (n = 515) | ||||
| Median, ng/mg creatinine | 12.4 | 91.1 | 54.2 | 561.4 |
| Interquartile range | 9.1–46.1 | 53.6–171.8 | 27.0–113.1 | 257.0–1130.3 |
| Range | 4.8–96.9 | 10.0–3655.8 | 3.6–8902.2 | 21.8–20 130.2 |
| % Positive | 1.2 | 63.9 | 89.9 | 96.5 |
Median of positive specimens. A positive specimen was defined as having concentrations greater than or equal to the method LOQ of 5 µxg/L for BUP and BUP-Gluc and 25 µg/L for NBUP and NBUP-Gluc.
—, below LOQ.
Number of positive specimens/total number of specimens (n) * 100.
We compared c BUP-Gluc NBUP-Gluc and NBUP: NBUP-Gluc ratios within and between participants and trimesters (Table 3). Because of large variation in the number of measurements, nonparametric evaluations examined intrasubject variation in analyte ratios for participants with 5 or more ratios per period. Five of 7 participants had statistically significant differences (P < 0.05) in BUP-Gluc:NBUP-Gluc median ratios between trimesters, but post-hoc analysis revealed no pattern of differences. The NBUP:NBUP-Gluc median ratios in the second and third trimesters were significantly lower (P < 0.001 and P < 0.05, respectively) than the postpartum median ratio for participant H, and for participant E the second-trimester median ratio was significantly less than the third-trimester median (P < 0.001), whereas the third-trimester median was significantly lower than postpartum (P < 0.01).
Table 3.
Second trimester, third trimester, and postpartum BUP-Gluc:NBUP-Gluc and NBUP:NBUP-Gluc ratios in urine specimens containing both analytes.
| Participant | BUP-Gluc:NBUP-Gluc | NBUP:NBUP-Gluc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | RSD,a % | n | Mean (SD) | Median | Range | RSD, % | |
| Ab | ||||||||||
| Second trimester | 6 | 0.05 (0.01) | 0.05 | 0.04–0.07 | 20.8 | 4 | 0.08 (0.03) | 0.08 | 0.06–0.12 | 31.1 |
| Third trimester | 21 | 0.07 (0.02) | 0.06 | 0.04–0.12 | 26.8 | 17 | 0.10 (0.05) | 0.08 | 0.05–0.20 | 51.1 |
| Postpartum | 10 | 0.05 (0.01) | 0.05 | 0.03–0.07 | 20.2 | 10 | 0.14 (0.07) | 0.12 | 0.09–0.34 | 52.3 |
| Bc | ||||||||||
| Second trimester | 2 | 0.08 (0.03) | 0.08 | 0.07–0.10 | 31.0 | 1 | 0.15 | 0.15 | 0.15 | — |
| Third trimester | 29 | 0.10 (0.04) | 0.08 | 0.05–0.27 | 43.4 | 9 | 0.18 (0.05) | 0.19 | 0.06–0.25 | 30.8 |
| Postpartum | 19 | 0.17 (0.06) | 0.17 | 0.05–0.27 | 36.3 | 8 | 0.37 (0.19) | 0.31 | 0.18–0.77 | 51.8 |
| Cb | ||||||||||
| Second trimester | 20 | 0.63 (0.63) | 0.28 | 0.06–1.88 | 99.5 | 5 | 0.07 (0.02) | 0.08 | 0.04–0.10 | 33.4 |
| Third trimester | 24 | 0.09 (0.03) | 0.08 | 0.03–0.17 | 34.7 | 19 | 0.06 (0.02) | 0.05 | 0.02–0.12 | 37.0 |
| Postpartum | 9 | 0.10 (0.05) | 0.09 | 0.06–0.21 | 50.3 | 2 | 0.08 (0.01) | 0.08 | 0.07–0.08 | 10.7 |
| Db,d | ||||||||||
| Second trimester | 11 | 0.15 (0.11) | 0.12 | 0.07–0.46 | 71.8 | 7 | 0.18 (0.04 | 0.17 | 0.15–0.24 | 20.1 |
| Third trimester | 21 | 0.08 (0.03) | 0.07 | 0.03–0.13 | 36.3 | 18 | 0.23 (0.07) | 0.21 | 0.13–0.39 | 32.2 |
| Postpartum | 29 | 0.10 (0.05) | 0.09 | 0.04–0.20 | 44.4 | 29 | 0.25 (0.11) | 0.25 | 0.08–0.50 | 42.6 |
| Eb,d | ||||||||||
| Second trimester | 19 | 0.09 (0.03) | 0.08 | 0.04–0.17 | 36.8 | 17 | 0.17 (0.06) | 0.16 | 0.11–0.30 | 34.6 |
| Third trimester | 24 | 0.11 (0.04) | 0.10 | 0.07–0.26 | 38.4 | 20 | 0.12 (0.03) | 0.11 | 0.08–0.20 | 29.6 |
| Postpartum | 10 | 0.11 (0.04) | 0.12 | 0.04–0.17 | 35.4 | 9 | 0.24 (0.11) | 0.22 | 0.16–0.51 | 44.5 |
| Fb | ||||||||||
| Second trimester | 22 | 0.10 (0.06) | 0.09 | 0.03–0.29 | 53.6 | 16 | 0.11 (0.05) | 0.09 | 0.05–0.24 | 45.3 |
| Third trimester | 27 | 0.09 (0.03) | 0.09 | 0.05–0.20 | 36.0 | 13 | 0.13 (0.05) | 0.14 | 0.03–0.19 | 39.9 |
| Postpartum | 5 | 0.08 (0.03) | 0.09 | 0.04–0.11 | 31.9 | 1 | 0.14 | 0.14 | 0.14 | — |
| Gb | ||||||||||
| Second trimester | 11 | 0.11 (0.04) | 0.10 | 0.06–0.22 | 36.1 | 11 | 0.13 (0.05) | 0.14 | 0.05–0.22 | 39.6 |
| Third trimester | 28 | 0.12 (0.04) | 0.11 | 0.07–0.23 | 32.4 | 28 | 0.10 (0.06) | 0.09 | 0.04–0.27 | 53.8 |
| Postpartum | 8 | 0.08 (0.03) | 0.07 | 0.06–0.14 | 32.3 | 4 | 0.09 (0.01) | 0.09 | 0.08–0.11 | 13.9 |
| Hb,d | ||||||||||
| Second trimester | 19 | 0.09 (0.03) | 0.08 | 0.04–0.14 | 31.5 | 16 | 0.16 (0.04) | 0.17 | 0.10–0.27 | 24.2 |
| Third trimester | 33 | 0.08 (0.02) | 0.08 | 0.04–0.15 | 29.6 | 30 | 0.18 (0.05) | 0.19 | 0.08–0.32 | 29.2 |
| Postpartum | 16 | 0.12 (0.05) | 0.11 | 0.06–0.25 | 43.7 | 14 | 0.30 (0.12) | 0.33 | 0.14–0.59 | 39.5 |
| Ic | ||||||||||
| Second trimester | 3 | 0.08 (0.03) | 0.07 | 0.06–0.12 | 41.2 | 3 | 0.09 (0.03) | 0.10 | 0.05–0.11 | 38.6 |
| Third trimester | 36 | 0.08 (0.02) | 0.08 | 0.03–0.15 | 28.9 | 12 | 0.08 (0.04) | 0.09 | 0.03–0.14 | 43.1 |
| Postpartum | 1 | 0.07 | 0.07 | 0.07 | — | 0 | — | — | — | — |
| All | ||||||||||
| Second trimester | 113 | 0.19 (0.33) | 0.10 | 0.03–1.88 | 171.8 | 80 | 0.14 (0.06) | 0.14 | 0.04–0.30 | 40.8 |
| Third trimester | 243 | 0.09 (0.04) | 0.08 | 0.03–0.27 | 39.2 | 166 | 0.13 (0.07) | 0.13 | 0.02–0.39 | 54.0 |
| Postpartum | 107 | 0.11 (0.05) | 0.09 | 0.03–0.27 | 50.0 | 77 | 0.25 (0.14) | 0.21 | 0.07–0.77 | 55.5 |
RSD, relative standard deviation.
Intrasubject variation in BUP-Gluc:NBUP-Gluc ratios across trimesters evaluated with Kruskal–Wallis tests with Dunn multiple comparison test as a post-hoc analysis. At P < 0.05, the following statistically significant differences were identified: participants A and G, third trimester > postpartum; participants C and D, second trimester < third trimester; participant B, second trimester < postpartum; participant H, third trimester < postpartum.
Intrasubject variation not evaluated for BUP-Gluc:NBUP-Gluc or NBUP:NBUP-Gluc because n < 5 for 1 or more time periods.
Intrasubject variation in NBUP:NBUP-Gluc ratios across trimesters evaluated with Kruskal–Wallis tests with Dunn multiple comparison test as a post-hoc analysis. At P < 0.05, the following statistically significant differences were identified: participant E, second trimester < third trimester and third trimester < postpartum; participant H, second and third trimesters < postpartum.
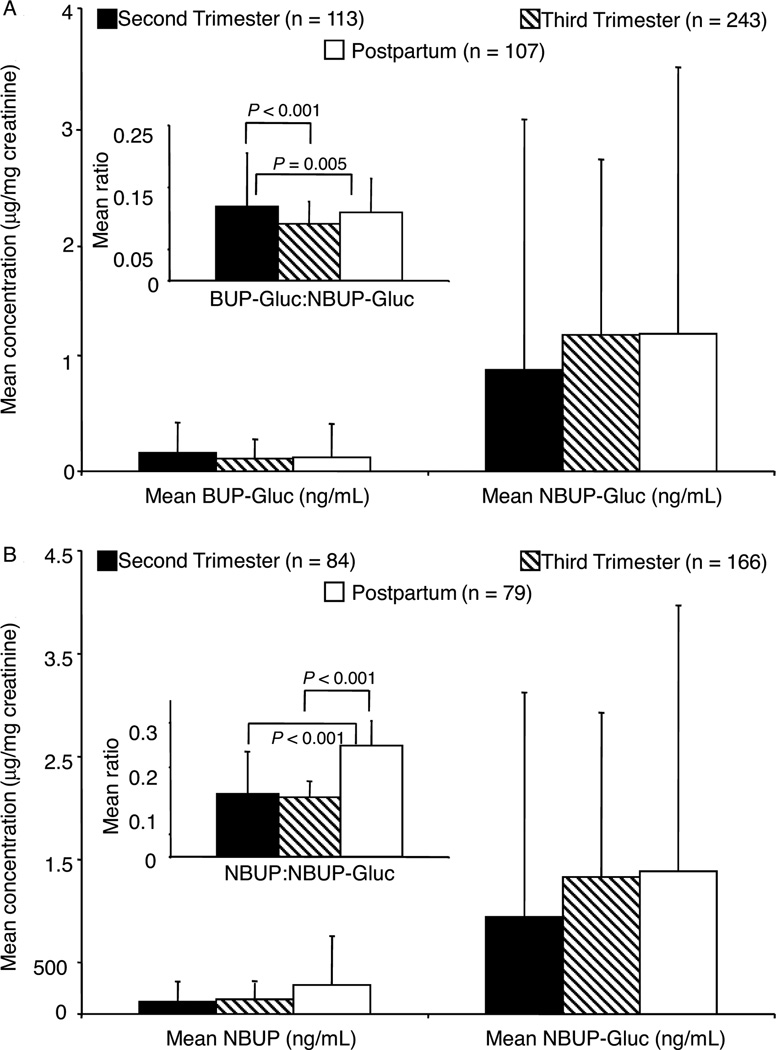
We examined intersubject variation using 2-way ANOVA with participant and trimester as factors. We used Scheffé’s post-hoc test to identify where differences occurred. The BUP-Gluc:NBUP-Gluc ratio was significantly higher for participant C than all other participants (P ≤ 0.009 for all comparisons), including 6 specimens with BUP-Gluc exceeding NBUP-Gluc concentrations. Also, there were statistically significant variations in the mean NBUP:NBUP-Gluc ratio between many participants (P < 0.001 for all comparisons). Statistically significant differences were observed in both ratios across trimesters (Fig. 2). Mean BUP-Gluc:NBUP-Gluc ratio was significantly higher in the second trimester compared to the third trimester with and without inclusion of the 6 specimens with BUP-Gluc greater than NBUP-Gluc concentrations. The second-trimester ratio also was higher than the third-trimester ratio when these 6 specimens were excluded, but there was no longer a statistically significant difference between the second-trimester and postpartum ratios. Postpartum NBUP:NBUP-Gluc was higher than the second- and third-trimester ratios.
Fig. 2.
(A), Mean (SD) BUP-Gluc and NBUP-Gluc creatinine-corrected concentrations.
(B), Mean (SD) NBUP and NBUP-Gluc concentrations and ratios (insets) in urine specimens from all participants containing both analytes during the second and third trimesters and postpartum.
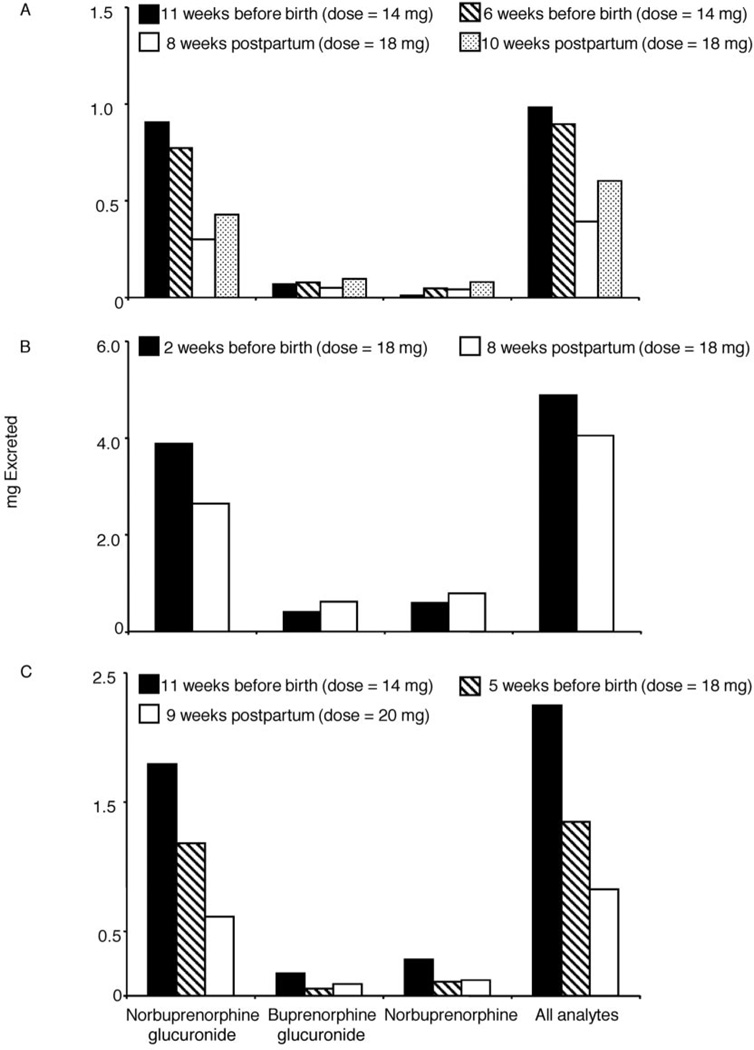
We examined 24-h BUP and metabolite urinary excretion in 3 participants who completed 2–4 inpatient sessions with individual collection of all urine voids. Fig. 3 displays gestational age at the time of participation and total analyte excreted during the 24-h period for each participant. In all participants, cumulative BUP and metabolite excretion was higher in sessions before birth compared to postpartum sessions.
Fig. 3.
Cumulative excretion of NBUP, BUP-Gluc, NBUP-Gluc, and total metabolites for participant B (panel A), participant D (panel B), and participant H (panel C) over 24 h at various time points in pregnancy.
Discussion
BUP is increasingly used as an alternative to methadone for opioid dependence treatment in nonpregnant populations, while researchers continue gathering BUP safety and efficacy data in maternal–neonatal dyads.
BUP, NBUP, and conjugated metabolite excretion has been investigated by GC-MS analysis of urine specimens from a healthy male volunteer receiving subcutaneous (1 and 2 mg), sublingual (2 and 4 mg), and oral (20 and 40 mg) doses of BUP (3). Analyzing specimens with and without enzymatic hydrolysis quantified free and total BUP and NBUP concentrations. Free BUP was never detected in urine at the method LOQ of 10 µg/L; NBUP excretion generally exceeded BUP-Gluc. After sublingual administration, BUP-Gluc excretion equaled or exceeded NBUP-Gluc. In another study, total BUP concentrations (1007–3316 ng/mg creatinine) exceeded total NBUP (636–6990 ng/mg creatinine) in 60% of participants (n = 5) with a long history of BUP abuse (28). Conversely, Kronstrand et al. (18) reported that 94% of patients (n = 16) undergoing BUP maintenance therapy had creatinine-corrected total NBUP concentrations greater than BUP concentrations. This is in agreement with other data reporting higher NBUP-Gluc concentrations than BUP-Gluc after sublingual doses (17, 29, 30). Total NBUP concentrations exceeded total BUP in the current study as well. Only 8 urine specimens had quantifiable BUP, with concentrations (not creatinine corrected) ranging from 5.5 to 12.1 µg/L [mean 7.0 (2.8) µg/L]. Liu et al. (29) reported urine BUP concentrations of 8.2–30.5 µg/L with 100% of patients (n = 11) treated with BUP (dose not reported) having quantifiable results (LOQ 0.5 µg/ L). Huang et al. (17) observed much lower concentrations with mean urine BUP concentration of 0.94 (0.67) µg/L in patients maintained on 16 mg/day BUP for at least 21 days. Mean concentrations of NBUP, BUP-Gluc, and NBUP-Gluc were 112 (58), 94.9 (27), and 663 (219) µg/L, respectively. Relative SDs of creatinine-corrected urine concentrations in the current study ranged from 62% to 275% across all participants and analytes. Within-subject analyte concentrations had similar variation; percentage difference between lowest and highest %CV was <35% for 7 of 9 participants. NBUP varied the least in 8 of 9 participants; in the remaining participant, it was the most variable analyte. Seven of 9 participants had the greatest variability in BUP-Gluc concentrations.
The BUP dose was flexible, with increases or decreases made through clinical decisions based on compliance in taking medication, participant requests, urine toxicology, and participant self-reports of opioid withdrawal symptoms or craving. An algorithm converting initial oral morphine dose to an equivalent BUP dose determined the initial BUP dose. Dose changes were made no more often than every 2 weeks unless clinically indicated. Increases in mean BUP dose during pregnancy and into the postpartum period are suggestive of metabolic changes and/or other physiological changes over time. After delivery, other factors, such as changes in motivation to abstain from drugs, also may have influenced dosage changes. Dosing always occurred after specimen collection, with a minimum of 16 h between last dose and urine collection, minimizing the effects of dosing time on metabolite ratios. Although BUP dose was positively correlated to creatinine-corrected NBUP (P < 0.001, R = 0.197), BUP-Gluc (P = 0.002, R = 0.143), and NBUP-Gluc (P = 0.001, R = 0.182) concentrations across the study, low correlation coefficients indicate that prediction of metabolite concentrations in urine based on BUP dose, or vice versa, would be unreliable.
Stability of glucuronidated metabolites during storage must be considered when interpreting urine data. In general, ester glucuronides can be hydrolyzed under alkaline or acidic conditions or by enzymatic processes and are most stable at temperatures just above freezing (31). Skopp and Pötsch (32) investigated the stability of glucuronidated 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid in authentic human urine specimens and reported the analyte to be highly labile at storage temperatures above 4 °C. Degradation of glucuronide analytes increased free drug concentrations. During method validation of our assay, stability of fortified controls at room temperature for 16 h, 4 °C for 72 h, and after 3 freeze-thaw challenges was substantiated as >80% (21). Additionally, free BUP was rarely detected, indicating that BUP-Gluc was stable during long-term storage.
Supplemental Fig. 1, which accompanies the online version of this article at http://www.clinchem.org/content/vol55/issue6, depicts intra- and intersubject variation in BUP-Gluc:NBUP-Gluc and NBUP: NBUP-Gluc ratios. There were statistically significant differences (P < 0.05) in median BUP-Gluc:NBUP-Gluc ratios in 71% of participants who had at least 5 measurements in each time period; however, there was no pattern to the differences observed. For example, 3 participants had higher third-trimester than postpartum median ratios, whereas median ratios in 2 participants were lower. Fewer participants had sufficient positive specimens for both analytes to compare NBUP/NBUP-Gluc. Two of 3 participants had lower ratios before birth than after. To the best of our knowledge, these are the first urine BUP metabolite ratio data available in pregnant women. Variability in the data limits drawing conclusions about intrasubject ratio variation between the second and third trimesters and postpartum.
The ratios of the BUP metabolites provide insight on possible intersubject variations in BUP metabolism and other changes that may occur during pregnancy. The mean BUP-Gluc:NBUP-Gluc ratio was significantly higher for participant C than all other women (P ≤ 0.009 for all comparisons). Participant C had 6 urine specimens collected during a 2-week period with BUP-Gluc concentrations higher than NBUP-Gluc 12–24 days after beginning treatment. CYP3A4, the enzyme primarily responsible for the metabolism of BUP to NBUP, is inhibited by a host of prescription drugs, over-the-counter medications, and food products, such as grapefruit juice (33). There is no direct evidence that these CYP3A4 inhibitors influence BUP pharmacokinetics, however, and in vivo and in vitro data suggest that BUP metabolism can be modulated by inhibitors such as HIV protease inhibitors and azole antifungal medications (34). Medical records revealed no unusual prescription medication for participant C during this 2-week time period, but it is possible she took an over-the-counter medication, vitamin, or other supplement that inhibited CYP3A4. If this enzyme was inhibited, less NBUP would be produced, leading to a decrease in NBUP-Gluc metabolite. At the same time, more BUP would be available for glucuronidation. Other factors that could influence BUP metabolism include genetic polymorphisms and environmental factors that could induce or inhibit CYP3A4 and UGT expression. Metabolic changes during pregnancy can also affect metabolism of BUP to NBUP and conjugation of parent drug and the dealkylated metabolite. CYP3A4 activity has been shown to increase during pregnancy (25). Additionally, increased clearance of lamotrigine-2-N-glucuronide and morphine glucuronide in pregnant vs nonpregnant women has been reported (35, 36).
This study is the first that we are aware of to examine possible changes in BUP metabolism during pregnancy. Including all participants, the mean BUP-Gluc: NBUP-Gluc ratio was significantly higher in the second compared to the third trimester (P < 0.001) and postpartum (P = 0.005), even after exclusion of the 6 specimens that had higher BUP-Gluc than NBUP-Gluc. Examining creatinine-corrected concentrations revealed that NBUP-Gluc was lower in the second trimester, with nearly equivalent average concentrations in the third trimester and postpartum, although not significantly different (Fig. 2). Less variation was seen in mean BUP-Gluc concentrations. NBUP concentrations increased in the third trimester and postpartum, though not significantly. The increase in NBUP-Gluc and NBUP concentrations observed in this study could reflect an increase in phase 1 (BUP to NBUP) and phase 2 (NBUP to NBUP-Gluc and BUP to BUP-Gluc) metabolic enzymes during pregnancy. In addition to metabolic changes, differences in metabolite ratios could be caused by changes in protein binding or active excretion processes induced by pregnancy.
Total BUP and metabolite 24-h excretion was previously evaluated in 5 nonpregnant patients treated with 16 mg/day BUP for at least 21 days. Mean 24 h recovery of metabolites in urine was 0.014% (0.011%), 1.89% (1.1%), 1.01% (0.42%), and 7.76% (3.51%) of dose for BUP, NBUP, BUP-Gluc, and NBUP-Gluc, respectively. When a similar calculation was performed with the current data collected from 3 pregnant women, excretion ranged from 0.0% to 5.0%, 0.2% to 2.4%, 1.3% to 17.1%, and 1.8% to −22.5% for NBUP, BUP-Gluc, NBUP-Gluc, and all analytes, respectively. In 3 pregnant women, the percent of daily dose excreted was higher during pregnancy than postpartum, consistent with data for other drugs that indicate enhanced renal elimination of drugs during pregnancy (25, 37).
There were several limitations to this study. Women were admitted at varying points in pregnancy and released anywhere from 4 to 10 weeks after delivery. These variations may influence differences seen in maternal dosing during the second and third trimester and postpartum. Additionally, only a limited number of women participated in the 24-h inpatient stays to evaluate changes in total excretion, and no woman participated during her second trimester.
Despite these limitations, this research provided the first opportunity to evaluate urinary disposition of BUP and metabolites in a cohort of pregnant women. These data offer a detailed overview of urinary disposition of BUP during pregnancy. Differences between participants and changes in metabolite ratios throughout pregnancy were evaluated. Additionally, total excretion during 24 h time frames at different points in pregnancy and postpartum provided valuable information on changes in renal clearance of BUP that may occur in pregnant women. BUP excretion changes during gestation suggest metabolic changes that might aid clinicians in adjusting BUP dose to appropriately treat women as pregnancy progresses. Ultimately, data on BUP disposition in pregnancy provides an evidence-based framework for treatment providers and policymakers to determine the best dosing approaches for pregnant opioid-dependent patients treated with BUP.
Acknowledgments
Research Funding: Intramural Research Program, NIH, National Institute on Drug Abuse.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
Nonstandard abbreviations: BUP, buprenorphine; SL, sublingual; NBUP, norbuprenorphine; Gluc, glucuronide; UGT, uridine diphosphate glucuronosyltransferase; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition; LOQ, limit of quantification.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: R.E. Johnson, Clinical, Scientific and Regulatory Affairs, Reckitt Benckiser Pharmaceuticals Inc.
Consultant or Advisory Role: None declared.
Stock Ownership: R.E. Johnson, Reckitt Benckiser Pharmaceuticals Inc.
Honoraria: None declared.
Expert Testimony: None declared.
References
- 1.Bullingham R, McQuay H, Porter E, Allen M, Moore R. Sublingual buprenorphine used postoperatively: ten hour plasma drug concentration analysis. Br J Clin Pharmacol. 1982;13:665–673. doi: 10.1111/j.1365-2125.1982.tb01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marquet P. Pharmacology of high-dose buprenorphine. In: Kintz P, Marquet P, editors. Buprenorphine therapy of opiate addiction. Totowa (NJ): Humana Press; 2002. pp. 1–11. [Google Scholar]
- 3.Cone E, Gorodetzky C, Yousefnejad D, Buchwald W, Johnson R. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 12:577–581. [PubMed] [Google Scholar]
- 4.Walter DS, Inturrisi CE. Absorption, distribution, metabolism, and excretion of buprenorphine in animals and humans. In: Cowan A, Lewis JW, editors. Buprenorphine: combatting drug abuse with a unique opioid. New York: Wiley-Liss; 1995. pp. 113–135. [Google Scholar]
- 5.Ohtani M, Kotaki H, Sawada Y, Iga T. Comparative analysis of buprenorphine- and norbuprenorphine-induced analgesic effects based on pharmacokinetic-pharmacodynamic modeling. J Pharmacol Exp Ther. 1995;272:505–510. [PubMed] [Google Scholar]
- 6.Ohtani M, Kotaki H, Nishitateno K, Sawada Y, Iga T. Kinetics of respiratory depression in rats induced by buprenorphine and its metabolite, norbuprenorphine. J Pharmacol Exp Ther. 1997;281:428–433. [PubMed] [Google Scholar]
- 7.Jenkinson R, Clark N, Fry C, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addiction. 2005;100:197–205. doi: 10.1111/j.1360-0443.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- 8.Vidal-Trecan G, Varescon I, Nabet N, Boissonnas A. Intravenous use of prescribed sublingual buprenorphine tablets by drug users receiving maintenance therapy in France. Drug Alcohol Depend. 2003;69:175–181. doi: 10.1016/s0376-8716(02)00312-5. [DOI] [PubMed] [Google Scholar]
- 9.Obadia Y, Perrin V, Feroni I, Vlahov D, Moatti J. Injecting misuse of buprenorphine among French drug users. Addiction. 2001;96:267–272. doi: 10.1046/j.1360-0443.2001.96226710.x. [DOI] [PubMed] [Google Scholar]
- 10.National Drug Intelligence Center. Intelligence bulletin: Buprenorphine: potential for abuse. US Department of Justice; 2004. Sep, Product no. 2004-L0424-013. [Google Scholar]
- 11.Aalto M, Halme J, Visapää J, Salaspuro M. Buprenorphine misuse in Finland. Subst Use Misuse. 2007;42:1027–1028. doi: 10.1080/10826080701434857. [DOI] [PubMed] [Google Scholar]
- 12.Blom Y, Bondesson U, Anggård E. Analysis of buprenorphine and its N-dealkylated metabolite in plasma and urine by selected-ion monitoring. J Chromatogr. 1985;338:89–98. doi: 10.1016/0378-4347(85)80073-6. [DOI] [PubMed] [Google Scholar]
- 13.George S, George C, Chauhan M. The development and application of a rapid gas chromatography-mass spectrometry method to monitor buprenorphine withdrawal protocols. Forensic Sci Int. 2004;143:121–125. doi: 10.1016/j.forsciint.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Lisi A, Kazlauskas R, Trout G. Gas chromatographic-mass spectrometric quantitation of urinary buprenorphine and norbuprenor-phine after derivatization by direct extractive alkylation. J Chromatogr B Biomed Sci Appl. 1997;692:67–77. doi: 10.1016/s0378-4347(96)00496-3. [DOI] [PubMed] [Google Scholar]
- 15.Cone E, Gorodetzky C, Yousefnejad D, Darwin W. 63Ni electron-capture gas chromatographic assay for buprenorphine and metabolites in human urine and feces. J Chromatogr. 1985;337:291–300. doi: 10.1016/0378-4347(85)80042-6. [DOI] [PubMed] [Google Scholar]
- 16.Debrabandere L, Van Boven M, Daenens P. High-performance liquid chromatography with electrochemical detection of buprenorphine and its major metabolite in urine. J Chromatogr. 1991;564:557–566. doi: 10.1016/0378-4347(91)80524-g. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Moody D, McCance-Katz E. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-electrospray ionization-tandem mass spectrometry. Ther Drug Monit. 2006;28:245–251. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- 18.Kronstrand R, Seldén T, Josefsson M. Analysis of buprenorphine, norbuprenorphine, and their glucuronides in urine by liquid chromatographymass spectrometry. J Anal Toxicol. 2003;27:464–470. doi: 10.1093/jat/27.7.464. [DOI] [PubMed] [Google Scholar]
- 19.Tracqui A, Kintz P, Mangin P. HPLC/MS determination of buprenorphine and norbuprenorphine in biological fluids and hair samples. J Forensic Sci. 1997;42:111–114. [PubMed] [Google Scholar]
- 20.Favretto D, Frison G, Vogliardi S, Ferrara S. Potentials of ion trap collisional spectrometry for liquid chromatography/electrospray ionization tandem mass spectrometry determination of buprenorphine and nor-buprenorphine in urine, blood and hair samples. Rapid Commun Mass Spectrom. 2006;20:1257–1265. doi: 10.1002/rcm.2444. [DOI] [PubMed] [Google Scholar]
- 21.Kacinko S, Concheiro-Guisan M, Shakleya D, Huestis M. Development and validation of a liquid chromatography-tandem mass spectrometry assay for the simultaneous quantification of buprenorphine, norbuprenorphine, and metabolites in human urine. Anal Bioanal Chem. 2008;392:903–911. doi: 10.1007/s00216-008-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koren G. Changes in drug disposition in pregnancy and their clinical implications. In: Koren G, editor. Maternal-fetal toxicology: a clinician’s guide. 3rd ed. New York: Marcel Dekker; 2001. pp. 3–13. [Google Scholar]
- 23.Morgan D. Drug disposition in mother and foetus. Clin Exp Pharmacol Physiol. 1997;24:869–873. doi: 10.1111/j.1440-1681.1997.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 24.Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997;33:328–343. doi: 10.2165/00003088-199733050-00002. [DOI] [PubMed] [Google Scholar]
- 25.Anderson G. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 26.Jones H, Johnson R, Jasinski D, O’Grady K, Chis-holm C, Choo R, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Jones H, Johnson R, Jasinski D, Milio L. Randomized controlled study transitioning opioid-dependent pregnant women from short-acting morphine to buprenorphine or methadone. Drug Alcohol Depend. 2005;78:33–38. doi: 10.1016/j.drugalcdep.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Vincent F, Bessard J, Vacheron J, Mallaret M, Bessard G. Determination of buprenorphine and norbuprenorphine in urine and hair by gas chromatography-mass spectrometry. J Anal Toxicol. 1999;23:270–279. doi: 10.1093/jat/23.4.270. [DOI] [PubMed] [Google Scholar]
- 29.Liu A, Lin T, Su L, Fuh M. Online solid-phase extraction liquid chromatography-electrospray-tandem mass spectrometry analysis of buprenor-phine and three metabolites in human urine. Talanta. 2008;75:198–204. doi: 10.1016/j.talanta.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Hegstad S, Khiabani H, Øiestad E, Berg T, Christophersen A. Rapid quantification of buprenorphine-glucuronide and norbuprenorphine-glucuronide in human urine by LC-MS-MS. J Anal Toxicol. 2007;31:214–219. doi: 10.1093/jat/31.4.214. [DOI] [PubMed] [Google Scholar]
- 31.Dell D. Labile Metabolites. Chromatographia. 2004;59:S138–0. [Google Scholar]
- 32.Skopp G, Pötsch L. Stability of 11-nor-delta(9)-carboxy-tetrahydrocannabinol glucuronide in plasma and urine assessed by liquid chromatography-tandem mass spectrometry. Clin Chem. 2002;48:301–306. [PubMed] [Google Scholar]
- 33.Parkinson A. Biotransformation of xenobiotics. In: Klaassen CD, editor. Casarett and Doull’s toxicology: the basic science of poisons. 6th ed. New York: McGraw-Hill Medical; 2001. pp. 133–224. [Google Scholar]
- 34.Elkader A, Sproule B. Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet. 2005;44:661–680. doi: 10.2165/00003088-200544070-00001. [DOI] [PubMed] [Google Scholar]
- 35.Ohman I, Luef G, Tomson T. Effects of pregnancy and contraception on lamotrigine disposition: new insights through analysis of lamotrigine metabolites. Seizure. 2008;17:199–202. doi: 10.1016/j.seizure.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Gerdin E, Salmonson T, Lindberg B, Rane A. Maternal kinetics of morphine during labour. J Perinat Med. 1990;18:479–487. doi: 10.1515/jpme.1990.18.6.479. [DOI] [PubMed] [Google Scholar]
- 37.Loebstein R, Koren G. Clinical relevance of therapeutic drug monitoring during pregnancy. Ther Drug Monit. 2002;24:15–22. doi: 10.1097/00007691-200202000-00003. [DOI] [PubMed] [Google Scholar]