Abstract
Only a few studies have examined hormones in psychopathy and results have been mixed. It has been suggested that since hormone systems are highly interconnected, it may be important to examine multiple systems simultaneously to gain a clearer picture of how hormones work together to predispose for a certain construct. In the present study, we attempt to clarify the role of the hormones cortisol and testosterone in psychopathy by examining both hormones in a community sample of 178 adults demonstrating a wide range of psychopathy scores. Results showed that psychopathy scores were associated with an increased ratio of testosterone (baseline) to cortisol responsivity to a stressor. Psychopathy was not associated with either of these measures independently, or with baseline cortisol levels. These findings suggest that these highly interconnected hormone systems may work in concert to predispose to psychopathy.
Keywords: psychopathy, cortisol, testosterone, hormone, antisocial, aggression
The hypothalamus -pituitary-adrenal (HPA) axis and the hypothalamus-pituitary-gonadal (HPG) axis are two hormonal axes that work together to maintain an appropriate balance between withdrawing in the presence of fearful or threatening stimuli, and approaching in the presence of rewarding stimuli. Psychopathy is a disorder associated with an apparent imbalance in these processes, as it is characterized by traits such as reduced fearfulness, insensitivity to punishment, reward-seeking, and aggression (Hare, 2003). Only a few studies have examined the relationship between hormone systems and psychopathy and results have been mixed, possibly in part due to the examination of only one hormone at a time. Hormone researchers have begun to emphasize the interconnectedness of hormone systems, and recommend examining multiple systems within a study in order to examine potential interactions between them (Bauer, Quas, & Boyce, 2002; Brown, et al., 2008; Lovallo & Thomas, 2000); several studies have demonstrated the value of this approach (El-Sheikh, Erath, Buckhalt, Granger, & Mize, 2008; Gordis, Granger, Susman, & Trickett, 2006). Thus, the goal of the present study was to simultaneously examine the HPA and HPG hormone systems, via measurements of their end products, cortisol and testosterone, in relation to psychopathy.
The HPA axis is involved in potentiating the state of fear, generating sensitivity to punishment, and inducing withdrawal behavior (Schulkin, Gold, & McEwen, 1998), leading to the hypothesis that this system may be hypoactive in psychopathic individuals. In antisocial groups more generally, low cortisol levels have been observed in aggressive children (McBurnett, Lahey, Rathouz, & Loeber, 2000), adolescents with conduct disorder (Pajer, Gardner, Rubin, Perel, & Neal, 2001) and violent adults (Virkkunen, 1985). However, results from prior studies of psychopathy that measure cortisol are mixed. Holi et al. (2006) found reduced cortisol levels in young adult male psychopathic offenders with a history of violence. Similarly, Cima, Smeets, & Jelicic (2008) found lower average daily cortisol levels in a group of psychopathic offenders. However, others have failed to replicate this finding (van Honk, Schutter, Hermans, & Putman, 2003). A study by O’Leary, Loney, & Eckel (2007) found reduced cortisol responses to a stressor in male undergraduates scoring higher in psychopathy, but no differences in pre-stressor levels of cortisol.
The HPG axis is hypothesized to be associated with psychopathy because its end product testosterone has been associated with approach-related behaviors including reward-seeking (Daitzman & Zuckerman, 1980), dominance (Archer, 2006), and aggression (Dabbs, Jurkovic, & Frady, 1991). Testosterone has been associated with a variety of antisocial behaviors including difficulties on the job, law breaking, marriage failures, drug use, alcohol abuse, and violent behavior (Mazur & Booth, 1998), which are commonly observed in psychopathy. However, only one study has tested the relationship between testosterone and psychopathy in adults. Stalenheim et al. (1998) found testosterone levels to be positively correlated with the impulsive and antisocial behavior aspects of psychopathy, but not with psychopathy as a whole. A study of youth with callous-unemotional traits, which are thought to be similar to psychopathic traits in adulthood, found no difference in testosterone levels in these youth compared to control participants (Loney, Butler, Lima, Counts, & Eckel, 2006).
We sought to test the main effects and interactions between cortisol and testosterone in relation to psychopathy, as well as in relation to the different aspects of psychopathy. Psychopathy has been divided into four facets roughly representing superficial charm, manipulativeness, and deceitfulness (Facet 1 – Interpersonal), reduced guilt and emotional responsiveness (Facet 2 – Affective), impulsivity and stimulation-seeking (Facet 3 – Lifestyle), and antisocial behavior (Facet 4 – Antisocial). These facets have two overarching factors (Factor 1 – Interpersonal-Affective and Factor 2 – Lifestyle-Antisocial).
In addition to exploring the main effects and interactions between cortisol and testosterone, a secondary goal of the study was to test a recent theory put forth that the ratio between testosterone and cortisol may predispose to more severe forms of social aggression that include both instrumental and reactive forms of aggression, as observed in psychopathy (Terburg, Morgan, & Van Honk, 2009). Terburg et al. (2009) base the ratio hypothesis on the Triple Balance Model of Emotion set forth by van Honk & Schutter (2006) which explains the role that cortisol and testosterone may play in the development of psychopathic traits. This model highlights that the HPA and HPG axes counteract each other, and that the relative activity of the two axes can significantly influence brain regions and pathways that have been implicated in psychopathy. The HPA and HPG axes are mutually inhibitory – testosterone inhibits functioning of the HPA axis at the level of the hypothalamus, while cortisol suppresses the activity of the HPG axis at all levels, diminishing the production of testosterone and inhibiting the action of testosterone at target tissues (Johnson, Kamilaris, Chrousos, & Gold, 1992; Tilbrook, Turner, & Clark, 2000). Animal studies have shown that one of the primary targets of testosterone and cortisol is the amygdala (Koolhass, Van den Brink, Roozendaal, & Boorsma, 1990), a brain region that is consistently implicated in psychopathy (Blair, 2007). In the amygdala, cortisol is hypothesized to promote fearfulness and withdrawal behavior (Schulkin, et al., 1998); testosterone has the opposite effect – it serves to promote reward-seeking and approach behavior (Daitzman & Zuckerman, 1980).
If the balance between these two hormones is changed so that there is more testosterone relative to cortisol acting on the amygdala, an individual may become less fearful, and more reward seeking and aggressive (van Honk, Harmon-Jones, Morgan, & Schutter, 2010; van Honk & Schutter, 2006); these traits are associated with Facets 2 (Affective), 3 (Lifestyle), and 4 (Antisocial) of psychopathy, respectively. Furthermore, cortisol and testosterone affect the amount of communication between subcortical regions, such as the amygdala, and cortical regions, such as the orbitofrontal cortex. Cortisol has been found to strengthen the communication between these regions, whereas testosterone has been found to reduce it (Schutter & van Honk, 2005; van Wingen, Mattern, Verkes, Buitelaar, & Fernandez, 2010). Increased testosterone relative to cortisol may reduce the communication between the amygdala and orbitofrontal cortex, meaning that there is less emotional input from the amygdala to guide decision-making in the orbitofrontal cortex. This may result in the key components of Facet 2 of psychopathy – callousness, lack of empathy, and relatedly, increased instrumental aggression (Facet 4). Conversely, the cortical regions that are important in emotion regulation and inhibition are less able to regulate input from sub-cortical regions, including impulsive, reward-seeking, and aggressive urges (i.e. related to Facets 3 and 4). In sum, a high testosterone/cortisol ratio may enhance sensitivity to reward relative to punishment, promote approach rather than avoidance reactions, and reduce the emotional input from the amygdala to the orbitofrontal cortex that is critical for empathy and recognizing cues that a decision may be risky or harmful. It may also impair the ability to regulate emotion and aggression. Together, it is hypothesized that these mechanisms may predispose toward psychopathy (Terburg, et al., 2009; van Honk & Schutter, 2006). However, a full understanding of this relationship remains to be elucidated. Based on the mechanisms described, we hypothesized that the ratio of testosterone to cortisol would be associated with psychopathy, and that the strongest relationships would be with Facets 2, 3, and 4.
Method
Participants
Participants were 178 adults (22 females) recruited from temporary employment agencies in the greater Los Angeles area. Because participation in the study included magnetic resonance brain imaging, participants were excluded if they were under 18 or over 45 years of age; nonfluent in English; claustrophobic; or had a pacemaker, metal implants, or history of epilepsy. Qualified participants were informed of the nature of the study and of the study’s potential risks and benefits. After giving signed, informed consent, participants were individually tested over two days. Prior to beginning data collection, the principal investigator obtained a certificate of confidentiality from the Secretary of Health pursuant to Section 303(a) of Public Health Act 42. Participants were informed that any information they might provide about uninvestigated crimes could not be subpoenaed by any United States federal, state, or local court.
Psychopathy Assessment
Psychopathy was assessed using the PCL-R: 2nd Edition (Hare, 2003), which consists of a semi-structured interview and is supplemented by collateral data. The PCL-R: 2nd Edition consists of 20 items and reflects two overarching factors: interpersonal/affective characteristics (e.g., glibness/superficial charm, pathological lying, shallow affect) and antisocial behavior (e.g., impulsivity, need for stimulation/proneness to boredom, juvenile delinquency; Hare, 2003). Ratings were made by a Ph.D. clinical graduate student who received intensive, systematic training on the administration and scoring of the PCL-R by Dr. Robert D. Hare and Dr. Adelle Forth—including the completion of a series of PCL-R assessments on standardized videotaped case histories of adult male offenders (Pearson r correlations between rater’s and standardized criterion scores: Total PCL-R = .92, Factor 1 = .93, Factor 2 = .91). Raters were also supervised by one of the authors (A.R.) who has substantial prior working/assessment knowledge of the PCL-R. Internal reliability (Cronbach’s alpha) was .87, and the scale had good external validity in relation to Antisocial Personality Disorder symptom count (r = .43, p < .001).
The seven collateral data sources for assessing psychopathy were: (a) information gained from the Interpersonal Measure of Psychopathy (IM-P; Kosson, Steuwerald, Forth, & Kirkhart, 1997), a measure designed to be completed by the interviewer which asks about the participant’s psychopathic interpersonal behaviors that may have occurred during the session1; the IM-P has demonstrated construct validity with the PCL-R in a prison sample, and has been validated for use with nonincarcerated samples (i.e., college students; Kosson et al., 1997); (b) self-reported theft, drug offenses, and violent crime as assessed by an adult extension (Raine, Lencz, Bihrle, LaCasse, & Colletti, 2000) of the National Youth Survey self-report delinquency measure (Elliot, Ageton, Huizinga, Knowles, & Canter, 1983); (c) official state Department of Justice criminal records; (d) professional nationwide criminal and court record database searches; (e) data derived from, and behavioral observations made during, the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1996), and (f) the SCID Axis II Personality Disorders (SCID-II; First, Gibbon, Spitzer, Williams, & Benjamin, 1997). The SCID I and II were administered by a trained clinical Ph.D. student (see Ventura, Liberman, Green, Shaner, & Mintz, 1998). Finally, independent IM-P ratings made by two different laboratory assistants during separate phases of testing provided a seventh source of collateral data2.
Saliva Sample Collection
Baseline
Three resting saliva samples were collected in the morning of two consecutive days between 900 and 1000 h. The samples were obtained in 15-minute intervals. All six samples (three from each day) were assayed for cortisol and the values were averaged; one sample from each morning was assayed for testosterone levels (as in Cohan, Booth, & Granger, 2003) and the values were averaged.
For each sample, participants were required to deposit 6 mL of saliva by passive drool through a short straw into separate collection vials (Granger, et al., 2007). Participants were asked to abstain from exercise, smoking, eating, and consuming caffeinated beverages or alcohol for 1 hour prior to the collection of saliva in all instances. Samples were immediately frozen at -85°Celsius in a Revco upright Elite 13.4 ft3 deep freezer.
Stressor Tasks
In the afternoon (1300 to 1800 h) on one of the testing days, four saliva samples were collected to track the hormone response to two consecutive stressor tasks. The samples were collected in approximately 20-minute intervals, with the first sample representing baseline levels, the second and third samples representing responses to the stressors, and the fourth sample representing a return to baseline levels. Two consecutive stressor tasks were used – one involving an uncontrollable stressor and one involving a socially-relevant stressor. According to a meta-analysis (Dickerson & Kemeny, 2004), psychological stressors that contain uncontrollable elements or socially-relevant elements are found to induce the greatest cortisol stress response. The study was designed to obtain an overall measure of reactivity, rather than separate analyses of the effects of each type of stressor. The first sample was obtained after an 8-minute rest period. Next, the participants performed a countdown task (uncontrollable stressor). In this task, participants were told that they would see numbers counting down from 12 to 0 on a computer monitor situated directly in front of them and that at the end of the countdown they would hear a loud, white noise through headphones. In addition to the anticipated loud noise following the countdown, there were two trials in which the noise was presented unannounced and could occur at any point during the countdown. The loud noise had a frequency of 5000 Hz, a rise and decay time of 0.5 ms, and was presented at 105 dB for a duration of 1s. There were 3 “expected countdown” and 2 “unnanounced” trials in total. The trials were presented in the following order: countdown (41 second ITI), unannounced (46 second ITI), countdown (45 second ITI), unannounced (49 second ITI), and countdown. Both the countdown and the unannounced trials lasted 12 seconds. This task lasted 6 minutes in total. The second saliva sample was collected approximately 20 minutes after the first sample.
The second stressor was a modified version of the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993), which was adapted for this sample by shortening its duration and increasing its relevance to the participants. During this task, the participant was asked to give a speech about the worst thing he/she has ever done. The participant was given two minutes to think about and prepare the speech. In the next two minutes, the participant presented the speech about the worst thing he/she had ever done. The speech was videotaped and a research assistant remained in the room to enhance the stressfulness of the situation. If the participant had difficulty speaking continuously, the research assistant prompted him/her to elaborate and give specific examples to enhance social demands on the participant. The third saliva sample was collected approximately 20-minutes after the collection of the second sample.
A final saliva sample was collected after another 20-minute period. During this time participants were engaged in non-stressful tasks. The timing between sample collection varied slightly between participants, and thus was recorded and included in analyses.
Hormone Data Analysis
Saliva samples were analyzed using commercially available enzyme immunoassay kits without modification to the manufacturers recommended protocols (Salmetrics LLC – State College, PA). Samples were assayed in duplicate and the average of duplicate tests were used in analyses.
For cortisol, average recovery across saliva samples with known cortisol concentrations is 100.8%, and sensitivity of the cortisol kit is 0.003 μg/dL to 3.0 ug/dL. For this study, inter-assay and intra-assay precision (coefficient of variation) were less than 5.0%. For testosterone, average recovery across saliva samples of known concentrations of testosterone is 105.3%, and sensitivity of the kit is 1.0 to 600 pg/mL. Inter-assay and intra-assay precision (coefficient of variation) were, on average, less than 5.0%.
Statistical Analyses
Testosterone scores were not significantly skewed and did not require transformation. Cortisol scores were log-transformed to adjust for skewness. Outliers defined as values more than three standard deviations from the mean group score were removed (Gordis, et al., 2006). Deleted values were interpolated when sufficient remaining data was available. Five male participants were excluded from analyses involving cortisol reactivity due to outliers that required removing.
Cortisol reactivity to the stressors was measured by calculating the area under the curve (AUC) with respect to ground for the four samples obtained during the stressor tasks (Gordis, et al., 2006). The formula is given by:
where ti is the precise interval between sample i and sample i+1 (these times are specific for each subject and each interval) and mi is the level of the hormone for sample i. This analysis resulted in one number representing a general index of cortisol reactivity for each subject. This was used in multiple regression analyses to test for associations with psychopathy scores, controlling for gender and age. The start time of the stressor session was also included as a covariate to account for diurnal variation. Cortisol AUC has been used in the past to represent cortisol reactivity to a stressor. However, it is important to note that the reactivity to the stressor is overlaid on each participant’s normal diurnal decline in cortisol. Thus, the area under the curve represents the combined effect of the response to the stressor, and declining cortisol levels due to the diurnal cycle. In some cases, a small response to the stressor may have been masked by the diurnal change (i.e., their slope may not have dropped as steeply as it might have had the stressor not been present, although the overall distribution appears to decline with time).
To examine the testosterone/cortisol ratio, distributions for baseline testosterone, baseline cortisol, and cortisol reactivity were standardized to t scores (mean 50; SD 10) and individual testosterone/cortisol and testosterone/cortisol reactivity ratio scores were calculated, as in Hermans, Ramsey, & van Honk (2009).
Multiple regression analyses were used to examine main effects of hormone variables (baseline testosterone, baseline cortisol, cortisol reactivity, ratio scores) on total psychopathy scores, controlling for gender and start time of the stressor when necessary. In the event that a hormone variable was significantly associated with the total score, additional regression analyses were performed entering the two psychopathy factors simultaneously as predictors of the variable in order to determine if one factor uniquely contributed to the relationship. If one factor was a unique predictor, the two facets of that factor were then entered into the model, along with the other factor.
Multiple regression was also used to test for interactive effects between baseline cortisol and testosterone, and between baseline testosterone and cortisol reactivity. All variables were standardized prior to entry into the model.
Because our sample was primarily composed of males, and because hormone systems function differently in males and females, we also analyzed the data in the sample of males only (See Appendix). Age was significantly correlated with testosterone in the male sample, and therefore was included in regressions involving testosterone.
Results
Descriptive statistics for the sample are provided in Table 1. Baseline levels of testosterone and cortisol are within range of previously reported studies (Brown, et al., 2008). As expected, there were clear gender differences in baseline testosterone levels (t = 6.57, p < .001) and thus was controlled for in analyses involving testosterone. There were no gender differences in baseline cortisol levels (t = 1.66, p = .25). Age was not correlated with any of the hormone variables (p>.06) and therefore was not used as a covariate. In analyses of cortisol reactivity, we also controlled for the start time of the stressor session in order to account for variation in the diurnal cycle.
Table 1.
Descriptive Information
| Mean (SD) | |
|---|---|
| Age (years) | 36.5 (8.8) |
| Psychopathy total scores | 18.5 (9.0) |
| Baseline Cortisol (ug/dL) | .264 (.20) |
| Cortisol (Stressor session) | |
| Time 1 | .161 (.16) |
| Time 2 | .144 (.13) |
| Time 3 | .143 (.12) |
| Time 4 | .135 (.17) |
| Baseline Testosterone (pg/ml) | 146.6 (65.5) |
Testosterone
When controlling for gender, baseline testosterone was not found to be significantly associated with psychopathy scores (β = .11, p = .22).
Cortisol
In regression analyses, baseline cortisol levels were not significantly associated with total psychopathy scores (β = .06, p = .42).
We proceeded with planned analyses of cortisol reactivity by calculating the area under the curve for each participant (cortisol AUC). When controlling for start time, cortisol AUC was not significantly associated with psychopathy scores (β = -.08, p = .40).
Upon examining the cortisol response during the stressor session, we found that there was not an overall increase in cortisol values in response to the stressors (i.e., Time 2 or Time 3). Upon further examination, we found that only 35% of participants showed an increase in cortisol from Time 1 to Time 2. Of these subjects, the mean increase was 53.1%. Only 34% of participants showed an increase from Time 1 to Time 3. Of these subjects, the mean increase from Time 1 to Time 3 was 77.3%. Because not all participants demonstrated a cortisol response to the stressor task, we decided to perform an additional analysis in which we divided the sample into groups of “Responders” and “Non-responders.” Responders were defined as individuals with an increase in cortisol values of 10% or more at either Time 2 or Time 3, compared to Time 1. Non-responders were defined as participants whose cortisol levels either decreased or stayed the same for Time 2 and Time 3. This resulted in 59 participants being classified as Responders and 77 being classified as Non-responders. We found that non-responders demonstrated marginally higher scores on total psychopathy (t = 1.7, p = .09), as well as on Factor 2 (Lifestyle-Antisocial) (t = 1.8, p = .07) and its Lifestyle Facet 3 (t = 2.0, p = .05), although these did not reach significance. There was no significant difference on the remainder of the factors/facets (all p > .11). We also looked within the Responders to see if there was an association between psychopathy scores and cortisol reactivity. This relationship was not significant (β = -.11, p = .42, N = 59).
Interactions
A multiple regression with psychopathy total scores as the dependent variable and with gender, baseline testosterone, baseline cortisol, and baseline testosterone × baseline cortisol as independent variables revealed no significant interaction between baseline testosterone and cortisol levels (β = .23, p = .52). A multiple regression with psychopathy total scores as the dependent variable and with gender, start time of the stressor, baseline testosterone, cortisol AUC, and baseline testosterone × cortisol AUC as independent variables also revealed no significant interaction between baseline testosterone and cortisol AUC (β = -.12, p = .23).
Ratio
We tested the hypothesis set forth by Terburg et al. (2009) that psychopathy is associated with the ratio of testosterone to cortisol. After controlling for gender, we did not find a significant association between psychopathy and the ratio of baseline testosterone to baseline cortisol (β = .06, p = .46). However, we did find a significant association between psychopathy scores and the ratio of baseline testosterone to cortisol reactivity (AUC) (β = .28, p <.01), controlling for gender and the start time of the stressor. This ratio score accounted for 5% of the variance in psychopathy scores; the variance accounted for by the ratio score, controlling for the covariates, was significant (R2 = .05, F(1, 169) = 7.1, p < .01).
To determine whether one factor of psychopathy contributed uniquely to this relationship, Factors 1 and 2 were entered as predictors, along with gender and the start time of the stressor, and the ratio score was entered as the dependent variable. When entered simultaneously, Factor 2 (Lifestyle-Antisocial) significantly predicted ratio scores, but Factor 1 (Interpersonal-Affective) did not (Table 2). We ran an additional regression analysis replacing Factor 2 scores with Facets 3 (Lifestyle) and 4 (Antisocial), along with Factor 1. Neither facet was a significant predictor, suggesting that the common variance between these facets is associated with the ratio score.
Table 2.
Regression Analyses Demonstrating the Association between Psychopathy Scores and the Ratio between Baseline Testosterone and Cortisol Reactivity
| Testosterone/Cortisol AUC | ||
|---|---|---|
| β | p | |
| Total psychopathy scoresa | .25* | <.01 |
| Entered simultaneouslyb: | ||
| Factor 1 – Interpersonal-Affective | -.11 | .41 |
| Factor 2 – Lifestyle-Antisocial | .32* | .01 |
| Entered simultaneouslyb: | ||
| Factor 1 – Interpersonal-Affective | -.06 | .64 |
| Facet 3 – Lifestyle | .19 | .12 |
| Facet 4 – Antisocial | .11 | .29 |
Summary of estimates from multiple regression models predicting psychopathy from testosterone/cortisol AUC ratio score, gender, and start time of stressor session. Postive beta values represent higher ratio scores (higher testosterone, lower cortisol) in individuals with higher psychopathy scores.
Summary of estimates from multiple regression models predicting Testosterone/Cortisol AUC from gender, start time of stressor, and psychopathy factors.
Additional analyses were conducted to determine whether the significant association between psychopathy and the ratio of testosterone to cortisol reactivity was specific to psychopathy. Controlling for gender and the start time of the stressor, none of the following variables were associated with the ratio score: Antisocial Personality Disorder symptom count (β = .07, p = .56), Interpersonal Measure of Psychopathy score (β = .07, p = .51), or number of self-report violent offenses (β = .02, p = .88).
Finally, in order to accurately interpret the ratio score, we conducted further analyses of the relationship with psychopathy. Since the ratio score is a relative measure of testosterone to cortisol AUC, we wanted to determine whether the relationship with psychopathy was significant at different absolute levels of testosterone or cortisol AUC (e.g., Is the effect driven by individuals with high levels of testosterone who have a high ratio, or is it true across all levels of testosterone?). Therefore, we tested the interaction between baseline testosterone and the ratio score in predicting psychopathy. A multiple regression with psychopathy total scores as the dependent variable and with gender, start time of the stressor, baseline testosterone, the ratio score, and baseline testosterone × the ratio score as independent variables revealed a significant interaction between baseline testosterone and the ratio score (β = .28, p = .003). In contrast, a multiple regression with psychopathy total scores as the dependent variable and with gender, start time of the stressor, cortisol AUC, the ratio score, and cortisol AUC × the ratio score as independent variables revealed no significant interaction between cortisol AUC and the ratio score (β = .10, p = .293). To deconstruct the interaction between testosterone and the ratio score, we used procedures described by Aiken and West (1991) and plotted the slope of the relation between psychopathy and the ratio score at 1 standard deviation above and below the mean on testosterone. We found that at 1 SD above the mean on testosterone, the simple slope of the relation between the ratio score and psychopathy was significant and positive (β = .54, p = .03), whereas at 1 SD below the mean, the relationship was null (β = .10, p = .67). Figure 1 illustrates that individuals with high baseline testosterone who have a high ratio score exhibit relatively higher levels of psychopathic traits than those with a low ratio score. In contrast, psychopathic traits were unrelated to the ratio score in individuals with low levels of testosterone.
Figure 1.
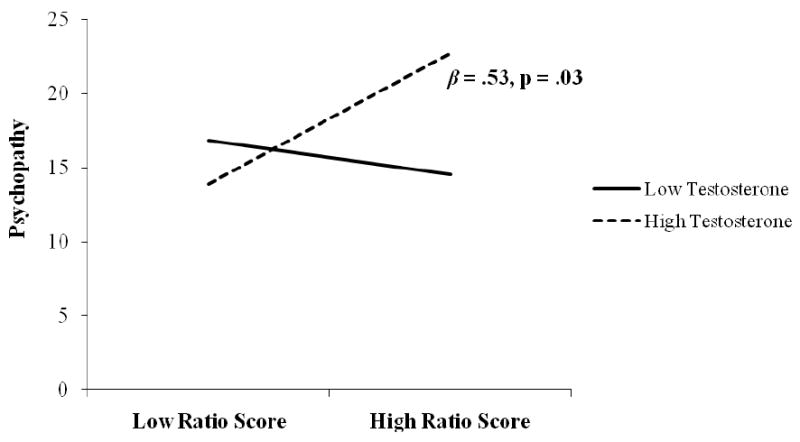
Relation between the ratio of baseline testosterone to cortisol reactivity and psychopathy at high and low values of baseline testosterone.
Correlations
Zero-order correlations between psychopathy, hormone measures, and covariates appear in Table 3. Psychopathy was not significantly associated with morning-time baseline cortisol or testosterone, or the ratio between them. Nor was it associated with cortisol reactivity. However, psychopathy and its two factors were significantly associated with the testosterone/cortisol reactivity ratio. In addition, Facets 2 (Affective) and 3 (Lifestyle) were correlated with the ratio.
Table 3.
Intercorrelations Among All Covariates, Predictors, and Outcome Variables (N =178)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Psychopathy | - | ||||||||||||
| 2. F1 – Interpersonal-Affective | .92** | - | |||||||||||
| 3. F2 – Lifestyle-Antisocial | .94** | .75** | - | ||||||||||
| 4. Facet 1 – Interpersonal | .83** | .91** | .66** | - | |||||||||
| 5. Facet 2 – Affective | .89** | .94** | .75** | .73** | - | ||||||||
| 6. Facet 3 – Lifestyle | .85** | .68** | .90** | .59** | .69** | - | |||||||
| 7. Facet 4 – Antisocial | .76** | .58** | .83** | .55** | .58** | .56** | - | ||||||
| 8. Baseline Testosterone | .10 | .10 | .08 | .03 | .10 | .07 | .00 | - | |||||
| 9. Baseline Cortisol | .05 | .08 | .03 | .08 | .04 | -.01 | -.01 | .50** | - | ||||
| 10. Cortisol AUC | -.07 | -.03 | -.10 | -.04 | -.05 | -.11 | -.11 | .45** | .47** | - | |||
| 11. Ratio Testo/CortBaseline | .09 | .06 | .08 | .01 | .05 | .06 | .04 | .51** | -.47** | -.02 | |||
| 12. Ratio Testo/Cort AUC | .21* | .19* | .20* | .10 | .19* | .17* | .08 | .53** | .02 | -.51** | .52** | - | |
| 13. Sex | -.09 | -.18* | .01 | -.09 | -.10 | .02 | .18* | -.45** | -.09 | -.05 | -.40** | -.43** | - |
| 14. Age | .01 | .00 | -.02 | .01 | -.01 | .03 | -.06 | -.14 | -.15 | -.09 | .05 | -.06 | -.11 |
Male Sample Analyses
Supplementary analyses restricted to the male participants only can be found in the Appendix. Results largely paralleled those from the whole group analyses. Unlike the total sample, the ratio score was not correlated with Factor 1 (Interpersonal-Affective) or either of its facets in the male sample. The ratio score was correlated with Facet 4 (Antisocial) in the male sample, as well as with Facet 3 (Lifestyle), which was also observed in the total sample.
Discussion
In a large sample of adults, no significant relationships were observed between psychopathy and baseline testosterone or cortisol, or cortisol reactivity to a stressor. Furthermore, there were no significant interactions between these variables. Although we did not observe a relationship between psychopathy and the ratio of baseline testosterone to cortisol, predicted by Terburg et al. (2009), we did observe a significant relationship between psychopathy and the ratio of baseline testosterone to cortisol reactivity. Individuals scoring higher in psychopathy had a higher ratio of baseline testosterone to cortisol reactivity; this accounted for 5% of the variance in psychopathic traits. This effect was only true for individuals with high baseline levels of testosterone. These findings highlight the importance of a multi-system approach in hormone research.
The fact that we observed a significant relationship with the ratio score (at high levels of testosterone), but not with levels of the individual hormones or their interactions, may be indicative of the interconnected nature of the hormone systems. The ratio score indicates the level of testosterone relative to cortisol reactivity within an individual. This score could be viewed as a general index of the imbalance between the HPA and HPG axes within that individual. In contrast, the interaction term for cortisol × testosterone treats the hormones as two distinct variables, with the individual’s score on each hormone being relative to the scores of the group. For example, an individual may have high testosterone (relative to the group) and low cortisol reactivity (relative to the group), but the important question seems to be ‘how high is the individual’s testosterone relative to his own cortisol reactivity?’ This makes sense given the high degree of interconnectedness between the HPA and HPG axes. High HPG axis activity relative to HPA axis activity may affect the sensitivity of brain regions such as the amygdala. Both testosterone and cortisol regulate and facilitate neuropeptide gene expression in the amygdala, and their influence on the probability of approach versus withdrawal is in opposing directions (Schulkin, 2003; Szot & Dorsa, 1994) – cortisol facilitates withdrawal and fearfulness, whereas testosterone facilitates approach and reward-seeking. Therefore, the relative contribution of each hormone is important in determining the reactivity of the amygdala to environmental stimuli. Similarly, the HPA and HPG axes act in opposite directions in their influence on the connectivity between subcortical and cortical regions. Increased levels of cortisol have been associated with enhanced functional connectivity between subcortical and cortical regions (Schutter & van Honk, 2005), whereas injections of testosterone have been found to reduce this communication (Schutter & van Honk, 2004; van Wingen, et al., 2010). Therefore, the activity of these two systems relative to each other seems have a significant effect on brain systems that are relevant to psychopathy.
A high ratio of testosterone relative to cortisol reactivity may mean that amygdala functioning is driven more by testosterone than cortisol, so the individual becomes more likely to engage in approach-related or aggressive behavior, is more sensitive to reward, and is less fearful and less sensitive to cues of punishment or threat (Terburg, et al., 2009; van Honk & Schutter, 2006). This may contribute to the fearlessness, reward-seeking, impulsiveness, and poor decision-making observed in psychopathy. Furthermore, the decoupling between subcortical and cortical regions that results from increased testosterone relative to cortisol may have effects in two ways: 1) During decision-making, emotion-related information from the amygdala that signals cues of threat, risk, or harm to others may not be able to reach cortical areas in order to inform the decision. This may result in the callousness, lack of empathy, risk-taking, and instrumental aggression observed in psychopathy. 2) Cortical regions may be less able to send inhibitory signals to subcortical regions, resulting in deficits in emotion regulation and inhibition (van Honk & Schutter, 2006), which contribute to reactive aggression and labile affect observed in psychopathy. Thus, through these processes, a high ratio between testosterone and cortisol reactivity may contribute to a variety of psychopathic traits, including both instrumental and reactive forms of aggression.
An additional finding of the present study was that psychopathy was only related to the ratio score at high levels of testosterone. This may mean that at lower levels of testosterone, the effect of testosterone on the amygdala and its connectivity to cortical regions is minimal, and the ratio of testosterone to cortisol becomes less relevant to behavior. Low testosterone levels have previously been described as a protective factor against antisocial behavior (Farrington & Coid, 2003). In contrast, at higher levels, testosterone may have a pronounced effect on amygdala functioning and connectivity; without sufficient cortisol responding to counterbalance these effects and to promote withdrawal behavior and fearfulness, psychopathic traits may develop.
It is unclear why the ratio involving cortisol reactivity was significant whereas the ratio involving baseline cortisol levels was not – it is unknown whether this discrepancy is a result of measurement factors, or whether there is a neurobiological explanation. With regards to measurement, it has been suggested that the degree of cortisol reactivity to a stressor is a more robust indicator than baseline cortisol of how an individual responds to cues of threat or punishment; baseline cortisol may be a less reliable and valid indicator of stress reactivity, as it is influenced by a multitude of daily living factors that can impact cortisol levels (Loney, et al., 2006). Stress induced changes in cortisol may provide a more precise measure of the functioning of the HPA axis and may be less susceptible to the influence of confounding factors (O’Leary, et al., 2007). In the current study, the correlation between baseline cortisol and cortisol reactivity (AUC) was 0.47, suggesting that the two variables are clearly related, but that having high baseline cortisol levels does not directly translate into increased cortisol reactivity – other factors are involved in this process.
A possible neurobiological explanation is based on the idea that testosterone has more of an influence on cortisol reactivity than on baseline cortisol levels. In animals, castration and androgen replacement studies have found that androgens inhibit stress-stimulated cortisol release, but not baseline cortisol concentrations (Handa, et al., 1994; Papadopoulos & Wardlaw, 2000). Similarly, in humans, testosterone was found to decrease cortisol reactivity (as measured by area under the curve) to stress-stimulation, but not baseline cortisol levels (Rubinow, et al., 2005). Therefore, the association between testosterone and cortisol reactivity may be the most relevant indicator of how the HPA and HPG axes interact. As we see in the present study, not all individuals with high testosterone levels had a high testosterone to cortisol reactivity ratio, indicating that there are individual differences in the degree to which testosterone suppresses the cortisol response. Individuals with high testosterone levels in which testosterone suppresses cortisol reactivity to a greater extent, may have the most pronounced alterations in amygdala functioning. In these individuals, the amygdala may be tuned to the testosterone-driven reward-seeking and approach-related behavior (Daitzman & Zuckerman, 1980), and much less responsive to cues of fear or threat that are facilitated by the HPA axis (Schulkin, et al., 1998), which would predispose for psychopathic traits. Furthermore, the higher levels of testosterone may reduce the connectivity between the amygdala and orbitofrontal cortex, thus impairing decision-making and inhibitory mechanisms (discussed above).
Analyses of the subfactors of psychopathy revealed that Factor 2 (Lifestyle-Antisocial) of psychopathy was a unique predictor of the testosterone-cortisol reactivity ratio. When entered together neither of its subfactors (Lifestyle or Antisocial) were significant, suggesting that it is the common variance that is shared between the two factors that is most associated with the ratio score. The altered imbalance between the HPA and HPG axis that may generally increase the probability of approach over withdrawal behavior and increase sensitivity to reward versus punishment (discussed above) may be associated with a latent factor that contributes to the specific features of Facets 3 and 4. Increased sensitivity to reward versus punishment, as well as an inclination toward approach behavior would likely result in the development of the more specific traits and behavior such as impulsivity, stimulation-seeking, irresponsibility (Facet 3), poor behavioral controls, and aggressive and deviant behavior (Facet 4).
Zero-order correlations also revealed a significant correlation between the ratio score and Facet 2 (Affective), suggesting that this factor may be related despite a lack of significance with the overall Interpersonal – Affective factor. This result may be potentially explained by reduced communication between the amygdala and cortical regions – if emotional input from the amygdala is unable to influence cognitive processes such as decision-making, the result may be that decision-making becomes cold and calculated, and the individual is described as callous and unemotional.
Limitations of this study include the fact that the sample was predominantly male, so findings cannot be generalized to psychopathic women, particularly considering the large gender differences in hormones between males and females. Many participants did not respond to the stressor task, which may be due to the fact that this temporary employment agency is a high-risk sample that attracts disproportionately high numbers of antisocial individuals. We do not have inter-rater reliability information for the PCL-R assessments, which were conducted by three raters. Finally, due to practical considerations, baseline saliva samples were acquired when participants came into the lab, rather than at waking, so it is possible that effects may not have been detected. However, all correlations with baseline cortisol and testosterone were .1 or less, so it is likely that any effects would have been small.
Although the present findings did not support the exact hypothesis of Terburg et al. (2009), the significant relationship between psychopathy and the testosterone/cortisol reactivity ratio provides support for the idea that the HPA and HPG axes may work in concert to predispose toward psychopathic traits, and highlights the importance of a multi-system approach. The interconnected nature of these systems may help to explain the mixed findings in previous studies examining individual hormones, as well as the lack of main effects of individual hormones in the present study. Future research will be necessary to elucidate the mechanisms of hormone action outlined by Terburg et al. (2009) and van Honk & Schutter (2006). Research on hormones may be a key element in aiding our understanding of the how brain abnormalities associated with psychopathy may arise.
Acknowledgments
This study was supported by grants from the National Institute of Mental Health to Dr. Raine (Research Scientist Development Award No. K02 MH01114-01, Independent Scientist Award K02 MH01114-01, and Grant No. 5 R03 MH50940-02).
Appendix
Appendix Table 1.
Descriptive Information (Males Only, n = 156)
| Mean (SD) | |
|---|---|
| Age (years) | 36.8 (8.6) |
| Psychopathy total scores | 18.8 (9.0) |
| Baseline Cortisol (ug/dL) | .216 (.12) |
| Cortisol (Stressor session) | |
| Time 1 | .160 (.16) |
| Time 2 | .145 (.13) |
| Time 3 | .141 (.12) |
| Time 4 | .133 (.16) |
| Baseline Testosterone (pg/ml) | 157.3 (62.0) |
Appendix Table 2.
Regression Values for Males Only (n = 156)
| Psychopathy Total Score | ||
|---|---|---|
| β | p | |
| Baseline Testosterone | .10 | .22a |
| Baseline Cortisol | .06 | .45 |
| Cortisol AUC | -.09 | .34b |
| Ratio Testo/CortBaseline | -.04 | .66a |
| Ratio Testo/Cort AUC | .30 | <.01a, b |
Controlling for age
Controlling for start time of stressor
Appendix Table 3.
Regression Analyses Demonstrating the Association between Psychopathy Scores and the Ratio between Baseline Testosterone and Cortisol Reactivity for Males Only (n = 156)
| Testosterone/Cortisol AUC | ||
|---|---|---|
| β | p | |
| Total psychopathy scoresa | .30 | <.01 |
| Entered simultaneouslyb: | ||
| Factor 1 – Interpersonal-Affective | -.10 | .47 |
| Factor 2 – Lifestyle-Antisocial | .41 | <.01 |
| Entered simultaneouslyb: | ||
| Factor 1 – Interpersonal-Affective | -.06 | .66 |
| Facet 3 – Lifestyle | .25 | .07 |
| Facet 4 – Antisocial | .15 | .19 |
Summary of estimates from multiple regression models predicting psychopathy scores from testosterone/cortisol AUC ratio score, age, and start time of stressor session. Postive beta values represent higher ratio scores (higher testosterone, lower cortisol) in individuals with higher psychopathy scores.
Summary of estimates from multiple regression models predicting Testosterone/Cortisol AUC from age, start time of stressor, and psychopathy factors.
Appendix Table 4.
Intercorrelations Among All Covariates, Predictors, and Outcome Variables (Males Only, n =156)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Psychopathy | - | |||||||||||
| 2. F1 – Interpersonal –Affective | .92** | - | ||||||||||
| 3. F2 – Lifestyle-Antisocial | .94** | .76** | - | |||||||||
| 4. Facet 1 – Interpersonal | .82** | .92** | .65** | - | ||||||||
| 5. Facet 2 – Affective | .89** | .94** | .74** | .74** | - | |||||||
| 6. Facet 3 – Lifestyle | .85** | .68** | .90** | .57** | .69** | - | ||||||
| 7. Facet 4 – Antisocial | .77** | .60** | .83** | .53** | .59** | .53** | - | |||||
| 8. Baseline Testosterone | .07 | .02 | .10 | -.02 | .07 | .09 | .10 | - | ||||
| 9. Baseline Cortisol | .06 | .08 | .04 | .07 | .09 | .06 | .02 | .53** | - | |||
| 10. Cortisol AUC | -.10 | -.06 | -.13 | -.06 | -.06 | -.08 | -.13 | .52** | .49** | - | ||
| 11. Ratio Testo/CortBaseline | .03 | -.04 | .07 | -.06 | .00 | .04 | .11 | .40** | -.54** | -.04 | - | |
| 12. Ratio Testo/Cort AUC | .22* | .13 | .25** | .08 | .18 | .23* | .20* | .41** | -.01 | -.55** | .42** | - |
| 13. Age | .00 | -.03 | -.01 | -.02 | -.02 | .03 | -.03 | -.21** | -.16 | -.13 | .00 | -.12 |
Footnotes
The IM-P asks about nonverbal behavior and interpersonal tendencies that are not directly assessed in the interview itself. The information gained from the IM-P was used as supplementary evidence and was never used as a sole piece of evidence for determining the rating for an item.
Although not empirically tested, the authors of the IM-P state that information gained from the measure after other types of interviews or unstructured professional interactions may also prove useful (D. Kosson, personal communication, April 23, 3009). This information was used a supplementary information and never used alone when determining ratings.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/ABN
References
- Aiken LS, West SG. Testing and Interpreting Interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: advantages of a multisystem approach. Journal of Developmental Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Brown GL, McGarvey EL, Shirtcliff EA, Keller A, Granger DA, Flavin K. Salivary cortisol, dehydroepiandrosterone, and testosterone interrelationships in healthy young males: A pilot study with implications for studies of aggressive behavior. Psychiatry Research. 2008;159:67–76. doi: 10.1016/j.psychres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Cima M, Smeets T, Jelicic M. Self-reported trauma, cortisol levels, and aggression in psychopathic and non-psychoathic prison inmates. Biological Psychiatry. 2008;78:75–86. doi: 10.1016/j.biopsycho.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Cohan CL, Booth A, Granger DA. Gender moderates the relationship between testosterone and marital interactions. Journal of Family Psychology. 2003;17(1):29–40. doi: 10.1037//0893-3200.17.1.29. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Jurkovic GJ, Frady RL. Salivary testosterone and cortisol among late adolescent male offenders. Journal of Abnormal Child Psychology. 1991;19(4):469–478. doi: 10.1007/BF00919089. [DOI] [PubMed] [Google Scholar]
- Daitzman R, Zuckerman M. Disinhibitory sensation seeking, personality and gonadal hormones. Personality & Individual Differences. 1980;1:103–110. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: the moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology. 2008;36:601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- Elliot DS, Ageton SS, Huizinga D, Knowles BA, Canter RJ. The prevalence and incidence of delinquent behavior: 1976-1980. Boulder, CO: Behavioral Research Institute; 1983. [Google Scholar]
- Farrington DP, Coid JW. Early Prevention of Adult Antisocial Behavior. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders: SCID-I Clinician Version. Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to agressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiology & Behavior. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiology & Behavior. 1994;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Hare RD. Hare Psychopathy Checklist-Revised (PCL-R): 2nd Edition. Toronto: Multi-Health Systems, Inc; 2003. [Google Scholar]
- Hermans EJ, Ramsey NF, Van Honk J. Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biological Psychiatry. 2009;63:263–270. doi: 10.1016/j.biopsych.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Holi M, Auvinen-Lintunen L, Lindberg N, Tani P, Virkkunen M. Inverse correlation between severity of psychopathic traits and serum cortisol levels in young adult violent male offenders. Psychopathology. 2006;39:102–104. doi: 10.1159/000091021. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos G, Gold PW. Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neuroscience and Biobehavioral Reviews. 1992;16:115–130. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ -a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Koolhass JM, Van den Brink THC, Roozendaal B, Boorsma F. Medial amygdala and aggressive behavior: Interaction between testosterone and vasopressin. Aggressive Behavior. 1990;16:223–229. [Google Scholar]
- Kosson DS, Steuwerald BL, Forth AE, Kirkhart KJ. A new method for assessing the interpersonal behavior of psychopathic individuals: Preliminary validation studies. Psychological Assessment. 1997;9:89–101. [Google Scholar]
- Loney BR, Butler MA, Lima EN, Counts CA, Eckel LA. The relation between salivary cortisol, callous-unemotional traits, and conduct problems in an adolescent non-referred sample. Journal of Child Psychology and Psychiatry. 2006;47(1):30–36. doi: 10.1111/j.1469-7610.2005.01444.x. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Thomas TL. Stress hormones in psychophysiological research: Emotional, behavioral, and cognitive implications. In: Cacioppo JT, Tassinar LG, Bernston GG, editors. Handbook of Psychophysiology. 2. New York: Cambridge University Press; 2000. pp. 342–367. [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. The Behavioral and Brain Sciences. 1998;21:353–397. [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- O’Leary MM, Loney BR, Eckel LA. Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology. 2007;32(2):183–191. doi: 10.1016/j.psyneuen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry. 2001;58:297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Papadopoulos AD, Wardlaw SL. Testosterone suppresses the response of the hypothalamic-pituitary-adrenal axis to interleukin-6. Neuroimmunomodulation. 2000;8:39–44. doi: 10.1159/000026451. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Roca CA, Schmidt PJ, Danaceau MA, Putnam K, Cizza G, et al. Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology. 2005;30:1906–1912. doi: 10.1038/sj.npp.1300742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J. Allostasis: A neural behavioral perspective. Hormones and Behavior. 2003;43:21–27. doi: 10.1016/s0018-506x(02)00035-1. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Schutter DJLG, van Honk J. Decoupling of midfrontal delta-beta oscillations after testosterone administration. International Journal of Psychophysiology. 2004;53(1):71–73. doi: 10.1016/j.ijpsycho.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Schutter DJLG, van Honk J. Salivary cortisol levels and the coupling of midfrontal delta-beta oscillations. International Journal of Psychophysiology. 2005;55(1):127–129. doi: 10.1016/j.ijpsycho.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Stalenheim EG, Eriksson E, von Knorring L, Wide L. Testosterone as a biological marker in psychopathy and alcoholism. Psychiatry Research. 1998;77:79–88. doi: 10.1016/s0165-1781(97)00143-1. [DOI] [PubMed] [Google Scholar]
- Szot P, Dorsa DM. Expression of cytoplasmic and nuclear vassopressin RNA following castration and testosterone replacement: Evidence for transcriptional regulation. Molecular and Cellular Neurosciences. 1994;5:1–10. doi: 10.1006/mcne.1994.1001. [DOI] [PubMed] [Google Scholar]
- Terburg D, Morgan B, Van Honk J. The testosterone-cortisol ratio: A hormonal marker for proneness to social aggression. International Journal of Law and Psychiatry. 2009;32:216–223. doi: 10.1016/j.ijlp.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI, Clark IJ. Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Reviews of Reproduction. 2000;5:105–113. doi: 10.1530/ror.0.0050105. [DOI] [PubMed] [Google Scholar]
- van Honk J, Harmon-Jones E, Morgan BE, Schutter DJLG. Socially explosive minds: The triple imbalance hypothesis of reactive aggression. Journal of Personality. 2010;78:67–94. doi: 10.1111/j.1467-6494.2009.00609.x. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJLG. Unmasking feigned sanity: A neurobiological model of emotion processing in primary psychopathy. Cognitive Neuropsychiatry. 2006;11(3):285–306. doi: 10.1080/13546800500233728. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJLG, Hermans EJ, Putman P. Low cortisol levels and the balance between punishment sensitivity and reward dependency. Neuroreport. 2003;14(15):1993–1996. doi: 10.1097/00001756-200310270-00023. [DOI] [PubMed] [Google Scholar]
- van Wingen G, Mattern C, Verkes RJ, Buitelaar JK, Fernandez G. Testosterone reduces amygdala--orbitofrontal cortex coupling. Psychoneuroendocrinology. 2010;35:105–113. doi: 10.1016/j.psyneuen.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Virkkunen M. Urinary free cortisol secretion in habitually violent offenders. Acta Psychiatrica Scandinavica. 1985;72:40–44. doi: 10.1111/j.1600-0447.1985.tb02568.x. [DOI] [PubMed] [Google Scholar]


