Abstract
Cancers of the stomach and large intestine (LI) are the second and fourth leading causes of human cancer mortality. A review of the National Toxicology Program (NTP) database and the Carcinogenic Potency Database (CPDB) reveals that chemically induced neoplasms of the gastrointestinal tract (GIT) are relatively common. Within the GIT, epithelial tumors of the forestomach in mice and rats and LI of the rat are most common. Generally, there is a high species concordance for forestomach with at least 26 chemicals inducing tumors in both species. Glandular stomach tumors are rare, and the few reported are usually neuroendocrine tumors (carcinoids) originating from the enterochromaffin-like (ECL) cells. Of 290 carcinogenic agents identified by the NTP, 19 (7%) caused intestinal neoplasia, 14 in the rat and 5 in the mouse. Neoplasms occurred in both males and females, exclusively in the small intestine (SI) of the mouse and in the LI or both SI and LI in the rat. Enteric carcinogens (NTP) frequently induced neoplasms at other alimentary sites (oral cavity, esophagus, and stomach). In conclusion, the most common induced GIT tumors are squamous neoplasms of the forestomach, glandular neoplasms of the stomach are rare, and rats appear more prone to developing LI (colorectal) cancer compared to mice.
Keywords: cancer, gastrointestinal tract, forestomach, stomach, intestine, rat, mouse, rodent, carcinoid, papilloma, carcinoma, adenoma, adenocarcinoma, neuroendocrine tumor, colon, colorectal, carcinogens, genotoxic, non-genotoxic, molecular biology, review, p53, animal model, NTP
Introduction
Cancers of the stomach and large intestine are the second and fourth leading causes of human cancer mortality (Shibuya et al. 2002). Although there are numerous publications on short-term experimental animal models of chemical-induced gastrointestinal carcinogenesis, there is a paucity of literature on chemically induced carcinogenesis of the gastrointestinal tract (GIT) in rodents mimicking lifetime exposure to carcinogens. This overview focuses on the incidences and causes of epithelial gastric and intestinal chemical carcinogenesis in the two major rodent species: rat and mouse. Emphasis is given to the results obtained in chronic (two-year) bioassays listed in the National Toxicology Program (NTP) database and the Carcinogenic Potency Database (CPDB).
Gastric Carcinogenesis
In humans, gastric cancer is the fourth most common cancer and the second leading cause of cancer-related mortality worldwide (Crew and Neugut 2004). In contrast, spontaneous or chemically induced gastric adenocarcinomas similar to that observed in man are extremely rare in rodents, although several experimental animal models exist. Spontaneous and chemically induced gastric tumors in rodents are primarily squamous cell papillomas or squamous cell carcinomas of the forestomach; while chemically induced tumors of the glandular stomach, which are rare, are almost exclusively neuroendocrine tumors (carcinoids) of the enterochromaffin-like cells. The background incidence of gastric neoplasia and the number of agents associated with gastric neoplasia in rats and/or mice are listed in Tables 1 and 2.
Table 1.
Incidence of spontaneous gastric and intestinal neoplasms in control F344/N rats and B6C3F1 mice from recent NTP chronic (two-year) bioassay studies (in percentages).a
| Site and diagnosis | Male rat (1,398)b | Female rat (1,350) | Male mouse (1,449) | Female mouse (1,498) |
|---|---|---|---|---|
| Forestomach | ||||
| Squamous cell papilloma | 0.2 | 0.3 | 1.4 | 1.7 |
| Squamous cell carcinoma | 0 | 0.1 | 0.5 | 0.1 |
| Combined | 0.2 | 0.4 | 1.8 | 1.9 |
| Glandular stomach | ||||
| Adenoma | 0 | 0 | 0 | 0.1 |
| Adenocarcinoma | 0 | 0 | 0 | 0.1 |
| Neuroendocrine tumor | 0 | 0 | 0 | 0 |
| Combined | 0 | 0 | 0 | 0.2 |
| Small intestine | ||||
| Adenoma | 0 | 0 | 0.9 | 0.5 |
| Adenocarcinoma | 0.1 | 0 | 1.6 | 0.3 |
| Combined | 0.1 | 0 | 2.4 | 0.8 |
| Large intestine | ||||
| Adenoma | 0.1 | 0.2 | 0 | 0 |
| Adenocarcinoma | 0.1 | 0.1 | 0.3 | 0 |
| Combined | 0.2c | 0.3c | 0.3d | 0 |
NTP Historical Controls Report (May 2009). There were 49 to 50 animals per group for 27 to 30 studies regardless of the route of administration reported in the past 5 years. The rat strain is F344/N and mouse strain B6C3F1.
Number of animals (n).
All were in colon or rectum.
All 4 were in the cecum.
Table 2.
| Source of data | Target organ | Number and percentage of rodent carcinogens |
|---|---|---|
| NTPa | Stomach | 36/290c (12%) |
| Intestines | 19/290 (7%) | |
| CPDBb (Rat) | Stomach | 88/564d (15%) |
| Small intestine | 29/564 (5%) | |
| Large intestine | 32/564 (6%) | |
| CPDBb (Mouse) | Stomach | 69/442 (16%) |
| Small intestine | 6/442 (1.4%) | |
| Large intestine | 3/442 (1%) |
Out of over 570 chronic studies conducted by the NTP (http://ntp.niehs.nih.gov/).
Data obtained from the Carcinogenic Potency Database (CPDB). The numbers of chemicals that are listed as carcinogenic to the intestines were obtained from a comprehensive table listing frequency of target organs for 564 carcinogens in rats and 442 carcinogens in mice. (http://potency.berkeley.edu/). Many chemicals induce tumors at more than one site, and these are counted at each relevant target site.
Rodent carcinogens were those classified as some or clear evidence of carcinogenic activity or positive in NTP studies.
Rodent carcinogens were identified by CPDB criteria.
Forestomach
Proliferative lesions of the forestomach include hyperplasia, hyperkeratosis, and parakeratosis; and the neoplasms consist of squamous cell papilloma and squamous cell carcinoma (Frantz et al. 1991) arising from and recapitulating the lining epithelium (Figure 1).
Figure 1.

Squamous cell papilloma (top panel) and squamous cell carcinoma in rats with invasion into submucosa and muscularis (bottom panel). H&E.
Forestomach tumors are common and account for the vast majority of rodent gastric neoplasms. They are the sixth most common induced neoplasm in NTP (http://ntp.niehs.nih.gov/) studies, being induced in 35 (12%) of 290 agents found to be rodent carcinogens. The top five most common sites of tumor induction in the rat or mouse in over 570 NTP studies are liver, lung, kidney, mammary gland, and hematopoietic system. According to one estimate, a total of 120 genotoxic and non-genotoxic substances have been identified as carcinogenic to the forestomach in rodents including rats, mice, and/or hamsters (Proctor et al. 2007). The pathogenesis and genetic alterations in rodent forestomach tumors induced by genotoxic and non-genotoxic carcinogens are somewhat different. Genotoxic carcinogens, such as N-methyl-N′-nitro-N-nitrosoguanidine (MNNG), N-methylnitrosourethane (MNUR) and N-methyl-N-nitrosourea (MNU) are considered to interact with DNA in their target organ cells, for example in the forestomach, by alkylating DNA bases or forming DNA adducts, resulting in DNA damage and finally causing genetic alterations that are considered to be irreversible (Hirose et al. 1979, 1989; Ohgaki et al. 1992; Cui et al. 1995). Although morphologic features of forestomach tumors induced by genotoxic and non-genotoxic carcinogens are similar, marked differences exist in alterations of genetic pathways or protein expression. H-ras and p53 gene mutations were observed at high and relatively low frequencies, respectively, in forestomach tumors induced by genotoxic carcinogens, while forestomach tumors due to the non-genotoxic carcinogens had no mutations of the H-ras and p53 genes (Kaneko et al. 2002). Relative overexpression of cyclin D1 and p53 was detected in forestomach tumors induced by the genotoxic carcinogens, while their non-genotoxic counterparts had a tendency to show low expression of those genes (Kaneko et al. 2002).
The mode of action can be different for each forestomach carcinogen such as butylated hydroxyanisole (BHA), caffeic acid (CA), or ethyl acrylate (EA), which tend to act through non-genotoxic mechanisms. For non-genotoxic chemicals, chronic inflammation or local irritation of forestomach mucosa due to physical trauma, or chemical-induced irritation and/or ulceration associated with high-dose regimens, may play a role in continuous induction of cell proliferation and the ultimate development of carcinomas (Ito, Hirose, and Takahashi 1993; Hirose, Takahashi, and Shirai 1995; Rodrigues et al. 1986). The vital role of sustained cell proliferation in the progression of hyperplasia to neoplasia has been well characterized for EA, a non-gentoxic forestomach carcinogen of rats and mice. Carcinogenic doses of EA administered by gavage for three, six, or twelve months caused a sustained increase in forestomach squamous epithelial hyperplasia for as long as exposure to EA continued; however, in a stop-study, hyperplasia regressed, and no neoplasms developed when animals received EA for three or six months and were allowed to recover. In contrast, rats that were treated for twelve months and allowed to recover developed squamous cell carcinomas and/or papillomas in the forestomach (Ghanayem et al. 1993, 1994). This work indicates that cell proliferation, sustained for a sufficient time (twelve months), results in the development of neoplasia despite cessation of chemical administration.
Route of exposure influences forestomach carcinogenesis. Currently (2009), 48 genotoxic and non-genotoxic chemicals have been listed as carcinogenic to the forestomach in rats and/or mice (http://ntp.niehs.nih.gov/) by the NTP. Among these, 43 agents were administered by the oral route (gavage, dosed feed, or dosed water), 4 were administered by inhalation, and 1 was administered topically. In addition to the forestomach, a vast majority (38 chemicals) also induced tumors at other sites. A schematic representation of the two main proposed mechanisms involved in forestomach carcinogenesis is illustrated in Figure 2.
Figure 2.

Schematic representation outlining the two main proposed pathways of forestomach carcinogenesis in rodents. *Salient features of forestomach tumors induced by genotoxic (MNNG) and non-genotoxic (BHA) chemicals are outlined (Kaneko et al. 2002; Kagawa et al. 1993).
Glandular Stomach
The NTP has conducted over 570 two-year rodent bioassay studies with only a few occurrences of neoplasms of the glandular stomach. An adenoma and two adenocarcinomas were observed in male rats administered C.I. Disperse Yellow 3 (NTP, Technical Report no. 222), while two high-dose female rats had carcinomas with clonitralid (NTP, Technical Report no. 091). Likewise, spontaneous gastric adenomas and adenocarcinomas occur very rarely in most strains of mice or rats. There were no glandular stomach tumors in F344 rats (n = 2,800 rats) in a recent (May 2009) compilation of control rat tumor data from the NTP, while only 3 out of 1,498 female B6C3F1 mice had glandular stomach tumors. Spontaneous carcinoids of the glandular stomach have been observed in cotton rats (Sigmodon hispidus) and the African rodent Mastomys natalensis. Carcinoids originate from the enterochromaffin-like neuroendocrine cells (Waldum et al. 1999; Cui et al. 2000; Kumazawa et al. 1989). In conventional laboratory rodents, the background incidence of carcinoids is generally very low, with eight cases of gastric carcinoids reported in NTP studies (Leininger et al. 1999). A recent publication (Tsukamoto, Mizoshita, and Tatematsu 2007) on animal models to study human stomach cancer has an excellent overview on gastric carcinomas.
Chemically induced tumors of the glandular stomach observed in lifetime carcinogenesis bioassays are primarily carcinoids of the enterochromaffin-like cells (ECL cells). Hyperplasia of ECL cells and neuroendocrine tumors are reported for both rats and mice after treatment with a variety of pharmaceuticals such as omeprazole (Greaves 1990; Ekman et al. 1985), ranitidine (Havu et al. 1990), ciprofibrate (Spencer et al. 1989), H2-receptor antagonist SK&F 93479 (Betton et al. 1987, 1988), and cimetidine (Colin-Jones et al. 1985). The mechanism of induction of carcinoids by antisecretory drugs such as omeprazole has been referred to as the “gastrin hypothesis” and is outlined in Figure 3 (Larsson et al. 1988; Håkanson and Sundler 1990). The hypothesis may be outlined as follows: (1) Inhibition of gastric acid secretion leads to elevated antral pH and, secondarily, to release of gastrin from the antral gastrin cells into the blood stream. (2) Gastrin causes both general hypertrophy of the oxyntic mucosa and hyperplasia of the ECL cells in the oxyntic mucosa. These drug-induced effects are reversible and can be prevented by antrectomy, which supports the hypothesis that hypergastrinemia is a causative event in oxyntic mucosal hypertrophy and ECL cell proliferation (Larsson et al. 1986). Gastric ECL hyperplasia and/or carcinoids can be difficult to visualize unless correct staining techniques such as Grimelius and Sevier-Munger silver stains are employed (Carlsson et al. 1990). Hypergastrinemia secondary to inhibition of gastric acid secretion by drugs such as omeprazole is generally associated with a trophic effect on the fundic mucosa resulting in increased stomach weight and increased mucosal thickness (hypertrophy) (White et al. 1998; Rohr and Tuch 1992; Creutzfeldt et al. 1986).
Figure 3.

Schematic representation of the “gastrin hypothesis”: a proposed mechanism for induction of gastric carcinoids in rodents by gastric acid blockade. Adapted from Håkanson and Sundler (1990).
In addition to pharmaceuticals (e.g., omeprazole, ranitidine, and cimetidine), carcinoids were also observed in both rats and mice in an NTP two-year bioassay with methyleugenol (Figure 4), a non-genotoxic chemical (NTP 2000). The cellular target or the mechanism of action for methyleugenol is not known. Short-term (thirty- and ninety-day) toxicity studies conducted with methyeugenol revealed elevated gastric pH and serum gastrin in rats and mice (NTP 2000), similar to those observed with antisecretory pharmaceuticals, suggesting “the gastrin hypothesis” may also be applicable to this chemical (Table 4).
Figure 4.
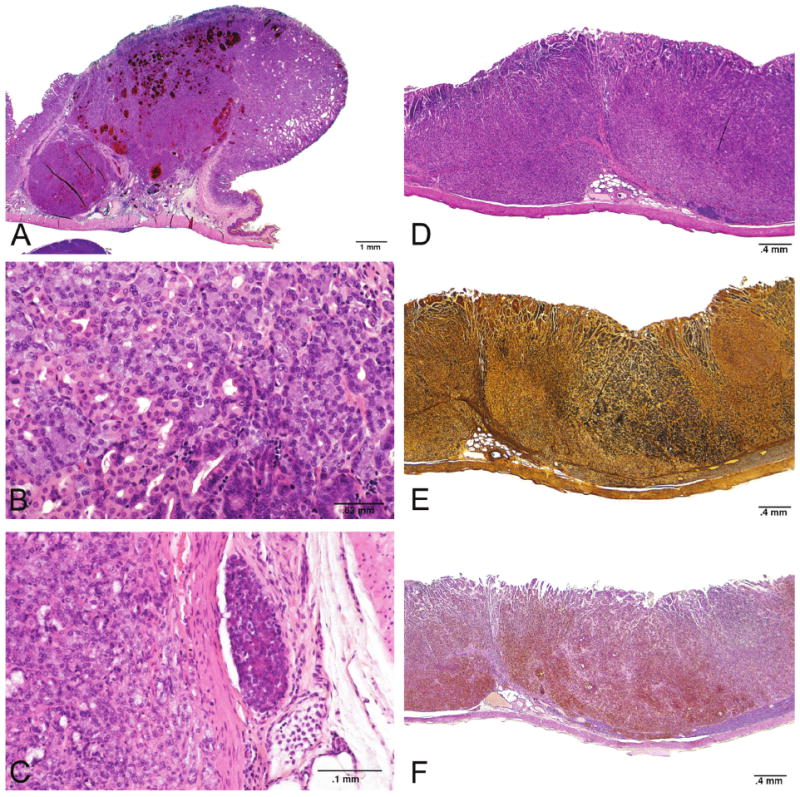
Glandular stomach neuroendocrine tumors (carcinoids) observed in the NTP carcinogenicity study with methyleugenol in rats. Malignant neuroendocrine tumor (A, B, C). Three panels on the right (D, E, F) are serial sections of a malignant tumor stained with H&E, Sevier-Munger, and NSE respectively.
Table 4.
Comparison of serum gastrin, gastric pH, and gastric neuroendocrine cell hyperplasia and neoplasia in female F344 rats administered Methyleugenol.a
| 14-week studyb (female F344/N rats) | 2-year studyc (female F344/N rats) | |||||
|---|---|---|---|---|---|---|
| Methyleugenol dose (mg/kg/day) | Serum gastrin: 30 days (n = 10) | Serum gastrin: 90 days (n = 10) | Glandular stomach pH: 30 days (n = 10) | Glandular stomach pH: 90 days (n = 10) | Glandular stomach: Neuroendocrine cell hyperplasia (n = 50) | Glandular stomach: Benign or malignant neuroendocrine tumor (n = 50) |
| 0 | 62 ± 7 | 41 ± 3 | 2.2 ± 0.1 | 2.2 ± 0.3 | 0 | 0 |
| 37 | 25 ± 4d | 46 ± 3 | 2.4 ± 0.3 | 1.8 ± 0.1 | 5 | 1 |
| 75 | 22 ± 2d | 60 ± 6 | 1.7 ± 0.1 | 2.2 ± 0.2 | 11 | 25 |
| 150 | 57 ± 14 | 88 ± 11d | 1.9 ± 0.2 | 2.0 ± 0.2 | 9 | 34 |
| 300 | 127 ± 23d | 409 ± 68c | 1.6 ± 0.2c | 3.1 ± 0.5c | 3 | 41 |
A simplified table to illustrate the interrelationship between serum gastrin, gastric pH, and neuroendocrine cell hyperplasia or neoplasia of the glandular stomach.
National Toxicology Program—Technical Report 391 (2000).
The 30/90 day investigative study was conducted only in female F344 and male B6C3F1 mice. Data are presented as mean ± standard error.
The 2-year carcinogenesis bioassay was conducted in both genders of F344 rats and B6C3F1 mice. Neuroendocrine tumors were observed in rats of both genders and male mice.
Significantly different from controls.
Intestinal Carcinogenesis
The study of experimental colon carcinogenesis in rodents has had a remarkably long history, dating back almost eighty years (Krebs 1928). Since tumors of the lower bowel are the fourth leading cause of human cancer mortality (Shibuya et al. 2002), there are numerous animal models to study experimental intestinal carcinogenesis. Excellent reviews (Tanaka 2009; Rosenberg, Giardina, and Tanaka 2009; Corpet and Pierre 2005; Taketo and Edelmann 2009) on animal models of intestinal carcinogenesis have been published recently. Published literature on chemically induced intestinal carcinogenesis mimicking lifetime exposure to potential carcinogens is generally very sparse. Proliferative lesions of the intestine include hyperplasia, reactive hyperplasia, focal atypical hyperplasia, and squamous metaplasia; and neoplasms include adenoma and adenocarcinoma (Figure 5) (Whiteley et al. 1996). The objective of this section is to present an overview of intestinal neoplasia in two-year chronic bioassays.
Figure 5.

Large intestinal adenoma (top panel) and adenocarcinoma (bottom panel) observed in the bromodichloromethane NTP carcinogenicity study in rats. H&E.
The spontaneous incidence of tumors of the intestines is generally low (Table 1) in rodents, ranging from 0.1% to 0.3% for the F344/N rat and 0.3% to 2.4% in the male B6C3F1 mouse in NTP studies. A review of the NTP database and the carcinogenic potency project database (CPDB) database (http://potency.berkeley.edu/rev) reveals a significantly higher number of intestinal carcinogens in rats rather than mice (Table 2). In NTP studies, 7% of rodent carcinogens target at least the intestinal tract compared to 12% for forestomach and 57% for liver (primarily mouse liver—data not shown). Furthermore, the numbers of chemicals that are carcinogenic to the large intestine are slightly greater than those that are carcinogenic in the small intestine. Of the 19 agents identified to cause intestinal cancer in NTP studies, none were enteric carcinogens in both the mouse and rat. More enteric carcinogens occurred in the rat (14) than the mouse (5); and of those 14, 8 (57%) caused neoplasms in both the small and large intestine (Table 3). Eighteen of the 19 intestinal carcinogens were by oral route of administration (gavage, feed, or drinking water); and many of the agents, such as 2,2-bis(Bromomethyl)-1,3-propanediol, 2,3-Dibromo-1-propanol, and sodium dichromate dihydrate (VI) induced neoplasms at multiple sites of the alimentary tract including oral cavity, esophagus, and/or stomach.
Table 3.
Number of chemicals associated with intestinal neoplasiaa in NTP studies.
Adenoma or adenocarcinoma.
1-amino-2,4-dibromoanthraquinone; asbestos, chrysoltile; 2,2-bis(bromomethyl)-1,3-propanediol; bromodichloromethane; C.I. acid red 114; C.I. direct blue 15; 2,3-dibromo-1-propanol; 3,3′-dimethoxybenzidine dihydrochloride; 3,3′-dimethylbenzidine dihdrochloride; glycidol; o-nitroanisole; phenazopyridine hydrochloride; 4,4′-thiodianiline; tribromomethane.
Captan; indium phosphide; methylene blue trihydrate; o-nitrotoluene; sodium dichromate dihydrate.
Chemical Structure and Intestinal Carcinogenesis: Nitrotoluenes and Trihalomethanes (THM)
The nitrotoluenes, o-nitrotoluene, and P-nitrotoluenes are structurally related, non-genotoxic chemicals that have been extensively studied by the NTP (Dunnick 1993; Dunnick, Elwell, and Bucher 1994; Dunnick et al. 2003). There was equivocal or no evidence of carcinogenic activity in rats and/or mice administered p-nitrotoluene in feed for two years (NTP 2002; Dunnick et al. 2003). In contrast to p-nitrotoluene, o-nitrotoluene administered in the feed for up to two years caused clear evidence for cancer at multiple sites in both rats and mice including large intestinal (cecal) tumors in mice (Sills et al. 2004). The cecal tumor response observed in B6C3F1 mice with o-nitrotoluene had both morphologic and molecular characteristics of human colon cancer. Morphologically, the tumors formed glands and were lined by tall columnar cytokeratin 20 positive epithelial cells (data not shown). Furthermore, these tumors had beta-catenin and p53 protein expression (Figure 6) as well as cyclin D1 expression with mutation in the Catnb, p53, and K-ras genes (data not shown). The alterations in cancer genes and proteins found in the mouse large intestinal tumors following o-nitrotoluene exposure are also hallmarks of human colon cancer (Sills et al. 2004).
Figure 6.
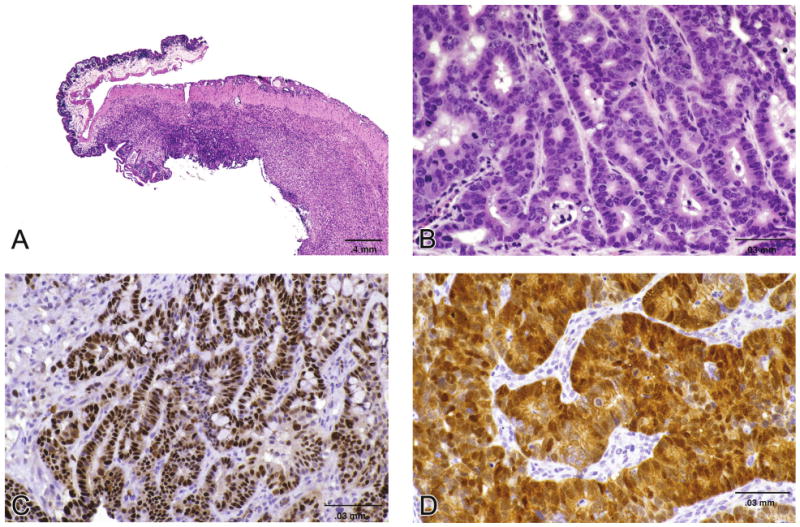
Cecal carcinoma observed in the o-nitrotoluene NTP carcinogenicity study in mice (A & B). Tumors were immunohistochemically stained for p53 (C) and beta catenin (D) (Sills, et al., 2004).
The halomethanes make up one class of the approximately 600 drinking-water disinfection by-products (DBPs) that have been identified. Within the halomethane class are the trihalomethanes (THM) (chloroform, bromoform, bromodichloromethane, and chlorodibromomethane). Although all four structurally related THMs are carcinogenic in rodents, intestinal carcinogenesis is observed only in rats administered bromoform (tribromomethane) or bromodichloromethane by gavage (NTP 1989; George et al. 2002), both of which are genotoxic chemicals. Chemical structures of nitrotoluenes and THM are illustrated in Figure 7.
Figure 7.

Chemical structures of nitrotoluenes and Trihalomethanes (THM) to illustrate that structurally similar chemicals are not site specific carcinogens. o-nitrotoluene, bromodichloromethane, and tribromomethane (left panel) are intestinal carcinogens; whereas p-nitrotoluene, chlorodibromomethane, and chloroform are not intestinal carcinogens.
The profound difference with regard to the carcinogenicity results observed between structurally similar chemicals illustrates the complexity in predicting carcinogenic potential by structure-activity relationship. In the case of nitrotoluenes, the position for substitution on the aromatic ring is important for predicting chemical carcinogenic response in rodents, although not necessarily the target site for chemically induced cancer (Dunnick et al. 2003). The nitrotoluenes (o and p) are both non-genotoxic chemicals.
In conclusion, B6C3F1 mice have a greater incidence of spontaneous neoplasms of the forestomach (up to 1.9%) and small intestine (up to 2.4%) compared to F344/N rats. The most common induced tumors of the rodent GIT are squamous neoplasms of the forestomach in mice, followed by intestinal carcinoma of the colon or rectum in rats. Rats appear more prone to developing intestinal (primarily colorectal) cancer compared to mice, and neuroendocrine tumors (carcinoids) are the most common type of glandular stomach neoplasm.
Acknowledgments
We acknowledge Drs. Bob Maronpot, Robert Sills, and John Peckham for review of the manuscript. In addition, we would like to thank Ms. Maureen Puccini and the entire imaging staff at EPL for helping us with the photomicrographs.
This research was supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Abbreviations
- CPDB
Carcinogenic Potency Database
- NTP
National Toxicology Program
- GIT
gastrointestinal tract
- ECL
enterochromaffin-like cells
- MNNG
N-methyl-N-nitro-N-nitroguanidine
- MNU
N-methyl-N-nitrosourea
- SI
small intestine
- GI
gastrointestinal
- MNUR
N-methylnitrosourethane
- BHA
butylated hydroxyanisole
- CA
caffeic acid
- EA
ethyl acrylate
- DBPs
drinking-water disinfection by-products
- THM
Trihalomethanes
Footnotes
For reprints and permissions queries, please visit SAGE's Web site at http://www.sagepub.com/journalsPermissions.nav.
References
- Betton GR, Dormer CS, Wells T, Pert P, Price CA, Buckley P. Fundic mucosal ECL hyperplasi a and carcinoids in rodents following chronic administration of the histamine H2-receptor antagonist SK&F 93479 and other antisecretory agents. Toxicol Pathol. 1987;15:365. doi: 10.1177/019262338801600222. [DOI] [PubMed] [Google Scholar]
- Betton GR, Dormer CS, Wells T, Pert P, Price CA, Buckley P. Gastric ECL-cell hyperplasia and carcinoids in rodents following chronic administration of H2-antagonists SK&F 93479 and oxmetidine and omeprazole. Toxicol Pathol. 1988;16:2–98. doi: 10.1177/019262338801600222. [DOI] [PubMed] [Google Scholar]
- Carlsson E, Havu N, Mattsson H, Ekman L. Gastrin and gastric enterochromaffin-like cell carcinoids in the rat. Digestion. 1990;47:17–23. doi: 10.1159/000200510. [DOI] [PubMed] [Google Scholar]
- Colin-Jones DG, Langman MJS, Lawson DH, Vessey MP. Postmarketing surveillance of the safety of cimetidine: Mortality during second, third and fourth years of follow-up. Br Med J. 1985;291:1084–88. doi: 10.1136/bmj.291.6502.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur J Cancer. 2005;41:1911–22. doi: 10.1016/j.ejca.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W, Stöckmann F, Conlon JM, Fölsch UR, Bonatz G, Wülfrath M. Effect of short- and long-term feeding of omeprazole on rat gastric endocrine cells. Digestion. 1986;35 1:84–97. doi: 10.1159/000199384. [DOI] [PubMed] [Google Scholar]
- Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450–64. doi: 10.1053/j.seminoncol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Cui G, Qvigstad G, Falkmer S, Sandvik AK, Kawase S, Waldum HL. Spontaneous ECLomas in cotton rats (Sigmodon hispidus): Tumours occurring in hypoacidic/hypergastrinaemic animals with normal parietal cells. Carcinogenesis. 2000;21:23–27. doi: 10.1093/carcin/21.1.23. [DOI] [PubMed] [Google Scholar]
- Cui XS, Torndal UB, Eriksson LC, Moller L. Early formation of DNA adducts compared with tumor formation in a long-term tumor study in rats after administration of 2-nitrofluorene. Carcinogenesis. 1995;16:2135–41. doi: 10.1093/carcin/16.9.2135. [DOI] [PubMed] [Google Scholar]
- Dunnick J. NTP technical report on the toxicity studies of ortho-, meta-, and para-Nitrotoluenes (Cas Nos. 88-72-2, 99-08-1, 99-99-0) administered in dosed feed to F344/N rats and B6C3F1 mice. Toxic Rep Ser Mar. 1993;23:1–E4. [PubMed] [Google Scholar]
- Dunnick JK, Burka LT, Mahler J, Sills R. Carcinogenic potential of o-nitrotuluene and p-nitrotoluene. Toxicology. 2003;183:221–34. doi: 10.1016/s0300-483x(02)00543-7. [DOI] [PubMed] [Google Scholar]
- Dunnick JK, Elwell MR, Bucher JR. Comparative toxicities of o-, m-, and p-nitrotuluene in 13-week feed studies in F344 rats and B6C3F1 mice. Fundam Appl Toxicol. 1994;22:411–21. doi: 10.1006/faat.1994.1047. [DOI] [PubMed] [Google Scholar]
- Ekman L, Hansson E, Havu N, Carlsson E, Lundberg C. Toxicological studies on omeprazole. Scand J Gastroenterol Suppl. 1985;108:53. [PubMed] [Google Scholar]
- Frantz JD, Betton G, Cartwright ME, Crissman JW, Macklin AW, Maronpot RR. Guides for Toxicologic Pathology. STP/ARP/AFIP; Washington, DC: 1991. Proliferative lesions of the non-glandular and glandular stomach in rats (1991). GI-3. [Google Scholar]
- George MH, Olson GR, Doerfler DT, Moore S, Kilburn S, DeAngelo AB. Carcinogenicity of bromodichloromethane administered in drinking water to male F344/N rats and B6C3F1 mice. Int J Toxicol. 2002;21:219–30. doi: 10.1080/10915810290096351. [DOI] [PubMed] [Google Scholar]
- Ghanayem BI, Sanchez IM, Maronpot RR, Elwell MR, Matthews HB. Relationship between the time of sustained ethyl acrylate forestomach hyperplasia and carcinogenicity. Environ Health Perspect. 1993;5:277–79. doi: 10.1289/ehp.93101s5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanayem BI, Sanchez I, Matthews HB, Elwell MR. Demonstration of a temporal relationship between ethyl acrylate-induced forestomach cell proliferation and carcinogenicity. Toxicol Pathol. 1994;22:497–509. doi: 10.1177/019262339402200504. [DOI] [PubMed] [Google Scholar]
- Greaves P. Histopathology of Preclinical Toxicity Studies—Interpretation and Relevance in Drug Safety Evaluation. Elsevier; Amsterdam: 1990. VII. Digestive system1; pp. 321–325. [Google Scholar]
- Håkanson R, Sundler F. Proposed mechanism of induction of gastric carcinoids: The gastrin hypothesis. Eur J Clin Invest. 1990;20(Suppl 1):S65–71. doi: 10.1111/j.1365-2362.1990.tb01780.x. [DOI] [PubMed] [Google Scholar]
- Havu M, Mattson H, Ekman L, Carlsson E. Enterochromafin-like cell carcinoids in the rat gastric mucosa following long-term administration of Ranitidine. Digestion. 1990;45:189–95. doi: 10.1159/000200245. [DOI] [PubMed] [Google Scholar]
- Hirose M, Maekawa A, Kamiya S, Odashima S. Carcinogenic effect of N-ethyl- and N-amyl-N-nitrosourethanes on female Donryu rats. Jpn J Cancer Res (Gann) 1979;70:653–62. [PubMed] [Google Scholar]
- Hirose M, Takahashi S, Shirai T. Characteristics of forestomach carcinogenesis by non-genotoxic phenolic compounds. J Toxicol Pathol. 1995;8:277–84. [Google Scholar]
- Hirose M, Yamaguchi S, Fukushima S, Hasegawa R, Takahashi S, Ito N. Promotion by dihydroxybenzene derivatives of N-methyl-N′-nitro-N- nitrosoguanidine-induced F344 rat forestomach and glandular stomach carcinogenesis. Cancer Res. 1989;49:5143–7. [PubMed] [Google Scholar]
- Ito N, Hirose M, Takahashi S. Cell proliferation and forestomach carcinogenesis. Environ Health Perspect. 1993;101:107–10. doi: 10.1289/ehp.93101s5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa M, Hakoi K, Yamamoto A, Futakuchi M, Hirose M. Comparison of reversibility of rat forestomach lesions induced by genotoxic and non-genotoxic carcinogens. Jpn J Cancer Res. 1993;84:1120–29. doi: 10.1111/j.1349-7006.1993.tb02811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Morimura K, Nishikawa T, Wanibuchi H, Takada N, Osugi H, Kinoshita H, Fukushima S. Different genetic alterations in rat forestomach tumors induced by genotoxic and non-genotoxic carcinogens. Carcinogenesis. 2002;23:1729–35. doi: 10.1093/carcin/23.10.1729. [DOI] [PubMed] [Google Scholar]
- Krebs C. Experimenteller Alkoholkrebs bei weissen Mäusen. Z. Immun Exp Therap. 1928;50:203–18. [Google Scholar]
- Kumazawa H, Takagi H, Sudo K, Nakamura W, Hosoda S. Adenocarcinoma and carcinoid developing spontaneously in the stomach of mutant strains of Mastomys natalensis. Virchows Arch A Pathol Anat Histopathol. 1989;416:141–51. doi: 10.1007/BF01606319. [DOI] [PubMed] [Google Scholar]
- Larsson H, Carlsson E, Mattson H, Lundell L, Sundler F, Sundell G, Wallmark B, Watanabe T, Hakanson R. Plasma gastrin and gastric enterochromaffinlike cell activation and proliferation. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology. 1986;90:391–99. doi: 10.1016/0016-5085(86)90938-8. [DOI] [PubMed] [Google Scholar]
- Larsson H, Håkanson R, Mattsson H, Ryberg B, Sundler F, Carlsson E. Omeprazole: Its influence on gastric acid secretion, gastrin and ECL cells. Toxicol Pathol. 1988;16:267–72. doi: 10.1177/019262338801600220. [DOI] [PubMed] [Google Scholar]
- Leininger JR, Jokinen MP, Dangler CA, Whiteley LO. Oral cavity, esophagus, and stomach. In: Maronpot RR, Boorman GA, Gaul BW, editors. Pathology of the Mouse. Cache River Press; Vienna, IL: 1999. pp. 42–45. [Google Scholar]
- National Toxicology Program. Toxicology and carcinogenesis studies of tribromomethane (bromoform) (CAS no. 75-25-2) in F344/N rats and B6C3F1 mice (gavage studies) Natl Toxicol Program Tech Rep Ser. 1989;350:1–194. [PubMed] [Google Scholar]
- National Toxicology Program. NTP toxicology and carcinogenesis studies of methyleugenol (Cas no. 93-15-2) in F344/N rats and B6C3F1 mice (gavage studies) Natl Toxicol Program Tech Rep Ser. 2000;491:1–412. [PubMed] [Google Scholar]
- National Toxicology Program. Toxicology and carcinogenesis studies of p-nitrotoluene (Cas no. 99-99-0) in F344/N rats and B6C3F(1) mice (feed studies) Natl Toxicol Program Tech Rep Ser. 2002;498:1–277. [PubMed] [Google Scholar]
- Ohgaki H, Hard GC, Hirota N, Maekawa A, Takahashi M, Kleihues P. Selective mutation of codons 204 and 213 of the p53 gene in rats tumors induced by alkylating N-nitroso compounds. Cancer Res. 1992;52:2995–98. [PubMed] [Google Scholar]
- Proctor DM, Gatto NM, Hong SJ, Allamneni KP. Mode-of-action framework for evaluating the relevance of rodent forestomach tumors in cancer risk assessment. Toxicol Sci. 2007;98:313–26. doi: 10.1093/toxsci/kfm075. [DOI] [PubMed] [Google Scholar]
- Rodrigues C, Lok E, Nera E, Iverson F, Page D, Karpinski K, Clayson DB. Short-term effects of various phenols and acids on the Fisher 344 male rat forestomach epithelium. Toxicology. 1986;38:103–17. doi: 10.1016/0300-483x(86)90176-9. [DOI] [PubMed] [Google Scholar]
- Rohr I, Tuck K. Effects of short-term treatment with an H+,K(+)-ATPase inhibitor (B8301-078) on enterochromaffin-like (ECL) cells in rat fundic mucosa. Toxicol Pathol. 1992;20:141–45. doi: 10.1177/019262339202000201. [DOI] [PubMed] [Google Scholar]
- Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30:183–96. doi: 10.1093/carcin/bgn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ. Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer. 2002;26:2–37. doi: 10.1186/1471-2407-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills RC, Hong HL, Flake G, Moomaw C, Clayton N, Boorman GA, Dunnick J, Devereux TR. o-Nitrotoluene-induced large intestinal tumors in B6C3F1 mice model human colon cancer in their molecular pathogenesis. Carcinogenesis. 2004;25:605–12. doi: 10.1093/carcin/bgh044. [DOI] [PubMed] [Google Scholar]
- Spencer AJ, Barbolt TA, Henry DC, Eason CT, Sauerschell RJ, Bonner FW. Gastric morphological changes including carcinoid tumors in animals treated with a potent hypolipidemic agent, ciprofibrate. Toxicol Pathol. 1989;17:1–15. doi: 10.1177/01926233890171P102. [DOI] [PubMed] [Google Scholar]
- Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology. 2009;136:780–98. doi: 10.1053/j.gastro.2008.12.049. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Colorectal carcinogenesis: review of human and experimental animal studies. J Carcinog. 2009;8:5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Mizoshita T, Tatematsu M. Animal models of stomach carcinogenesis. Toxicol Pathol. 2007;35:636–48. doi: 10.1080/01926230701420632. [DOI] [PubMed] [Google Scholar]
- Waldum HL, Rorvik H, Falkmer S, Kawase S. Neuroendocrine (ECL cell) differentiation of spontaneous gastric carcinomas of cotton rats (Sigmodon hispidus) Lab Anim Sci. 1999;49:241–47. [PubMed] [Google Scholar]
- White SL, Smith WC, Fisher LF, Gatlin CL, Hanasono GK, Jordan WH. Quantitation of glandular gastric changes in rats given a proton pump inhibitor for 3 months with emphasis on sampling scheme selection. Toxicol Pathol. 1998;26:403–10. doi: 10.1177/019262339802600315. [DOI] [PubMed] [Google Scholar]
- Whiteley LO, Anver MR, Botts S, Jokinen MP. Guides for Toxicologic Pathology. STP/ARP/AFIP; Washington, DC: 1996. Proliferative lesions of the intestine, salivary glands, oral cavity, and esophagus in rats. GI-1/2/4. [Google Scholar]


