Abstract
A 7-year-old captive female prothonotary warbler (Protonotaria citrea) died following chronic feather and weight loss. At necropsy, the right eye had a 2 × 2 × 1 mm corneal plaque of inspissated yellow-tan material and edema of the lower eyelid. Microscopically, both eyes exhibited diffuse, severe pyogranulomatous endophthalmitis with retinal necrosis and detachment. Numerous intralesional branching, gram-positive, beaded, filamentous bacteria formed a thick mat attached to the retinal pigmented epithelium and extending into the pecten. Bacteria were strongly acid-fast positive by Fite’s stain but only occasionally acid-fast positive by Ziehl-Neelsen staining, a characteristic consistent with a Nocardia spp. Infected regions demonstrated positive in situ hybridization reactivity with a probe complementary to the 16S rRNA gene of Nocardia spp. There was no evidence of primary bacterial infection in the other organs examined.
Keywords: Acid-fast stain, avian, eye, Fite’s acid-fast stain, Nocardia, Passeriformes, prothonotary warbler, Protonotaria citrea, pyogranulomatous endophthalmitis
Nocardial endophthalmitis is rare in both people and animals. The disease usually occurs in conjunction with immune suppression or underlying disease in human beings.10 In animals, Nocardia is typically aspirated, leading to a granulomatous pneumonia with subsequent hematogenous dissemination.2,6,9,11 Nocardial infection may follow immune suppression caused by chronic disease in exotic species3,7,12 or be associated with environmental stressors in wildlife.5,16 There is one previous report of nocardial pectenitis in a rainbow lorikeet.13 This report describes a diffuse, extensive endophthalmitis involving the retina and choroid as well as the pecten.
A 7-year-old female prothonotary warbler (Protonotaria citrea) died in an aviary at the North Carolina Zoological Park a week after developing right ocular swelling and discharge and 5 months after the onset of progressive, generalized feather loss. The bird had been captured and placed into the collection when it was approximately 1 year of age. Previously, fecal samples occasionally contained coccidial oocysts (some identified as Isospora spp.), and the bird was treated for this infection. The bird was not observed to molt until approximately 6 years of age, when generalized feather loss that was most evident over the breast (estimated 80% of feathers) was noted. After 2 months with no improvement of the feather loss, a clinical diagnosis of cutaneous mycotic infection involving the feathers was made, and treatment with itraconazole was initiated. A month later, the bird developed a 3-mm hard mass that covered the right eye, interpreted as inspissated exudate, a swollen lower eyelid, and epiphora. A mildly elevated titer for Aspergillus spp. (antigen 1.0, antibody 1.9, University of Miami) was detected. There was a profound leukocytosis (33,000/10 µl) comprised of 61% heterophils and 39% lymphocytes. A fecal check was negative for acid-fast organisms but positive for budding yeast and large numbers of coccidia oocysts. Despite treatment with enrofloxacin, sulfadimethoxine, and nystatin, the bird was found dead 6 days later.
At necropsy, the bird was in poor body condition and weighed 12.5 g; this represents a 30% decrease in body weight over a 2-month period. The ventriculus contained scant dark brown, mucoid digesta. Ovaries were smooth and regular, with no evidence of follicular development. Each ocular globe measured 4 × 3 × 3 mm. On cross-section, both eyes had total anterior and posterior synechia, with the lens resting on the posterior surface of the cornea. The vitreal chambers were filled with inspissated material, and each had a detached retina. Tissues were placed in 10% neutral buffered formalin for fixation, paraffin embedded, sectioned at 5 µm, and stained with hematoxylin and eosin.
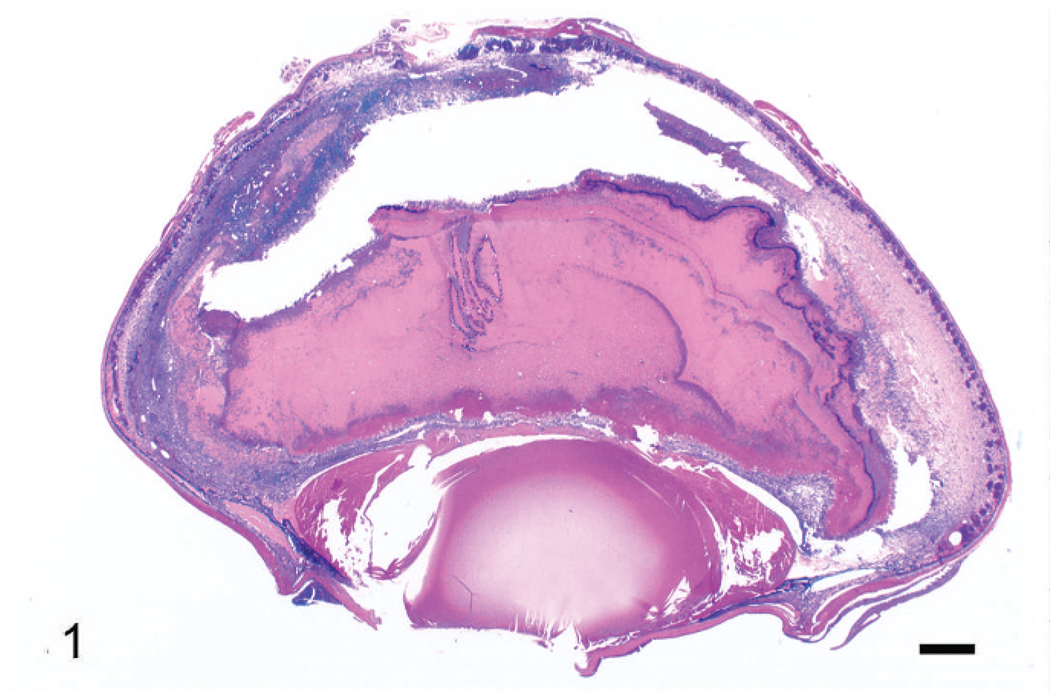
Microscopically, both eyes exhibited diffuse, severe pyogranulomatous nocardial endophthalmitis, with retinal necrosis and detachment, and posterior and anterior synechia. The retina was detached, and there was full-thickness retinal necrosis. The retina lay within a lake of amorphous, homogenous, eosinophilic material containing scattered heterophils, lymphocytes, macrophages, nuclear debris, and laminated aggregations of filamentous bacteria. The choroid was markedly expanded by an exudate of macrophages, lymphocytes, and plasma cells (Fig. 1), and there was segmental hypertrophy of the choroidal pigmented epithelium, evidence of antemortem retinal detachment. A similar inflammatory infiltrate was present in the iris of both eyes and pecten of the left eye (Fig. 2; the pecten was not present in sections of the right eye). Immediately posterior to the necrotic retinal pigmented epithelium, there was a laminar zone of filamentous bacteria with an underlying concentric accumulation of numerous foamy macrophages and multinucleated giant cells. In the right eye, a cataract, composed of multiple swollen lens fibers and large round to oval (morgagnian) globules of degenerated lens protein, was present along the caudolateral aspect of the lens. In the left eye, a segment of retina was viable, and the lens was normal. In both eyes there was diffuse, mild to moderate hypertrophy of the corneal epithelium.
Fig. 1.
Right eye. Note retinal detachment, filling of subretinal space and vitreal chambers with amorphous eosinophilic material containing a cellular infiltrate and necrotic debris, and anterior (partially caused by collapse of the anterior chamber) and posterior synechiae. Wright’s stain. Bar = 500 µm.
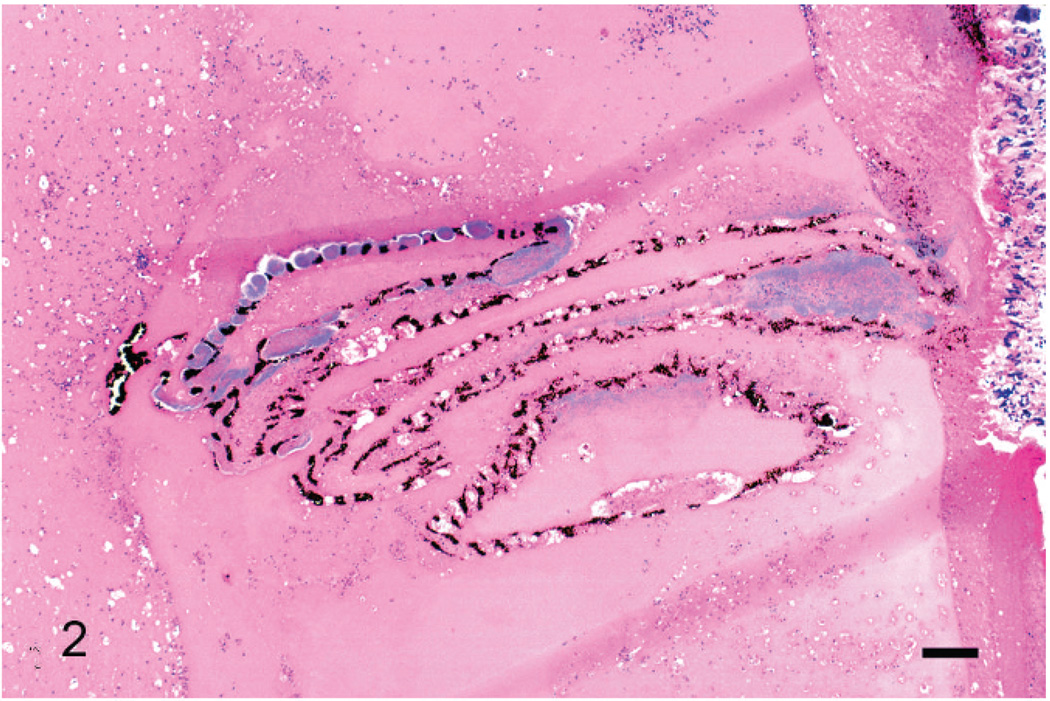
Fig. 2.
Left eye, pecten. Aggregates of bacteria are associated with the pecten, which rests in an amorphous lake of eosinophilic material containing numerous inflammatory cells. Wright’s stain. Bar = 200 µm.
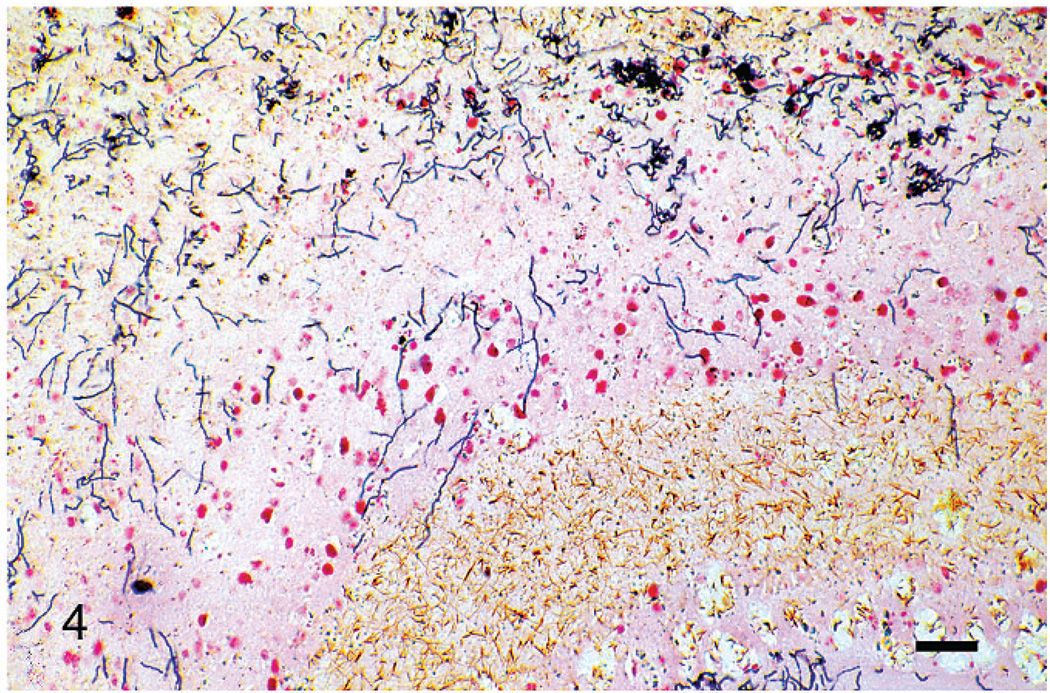
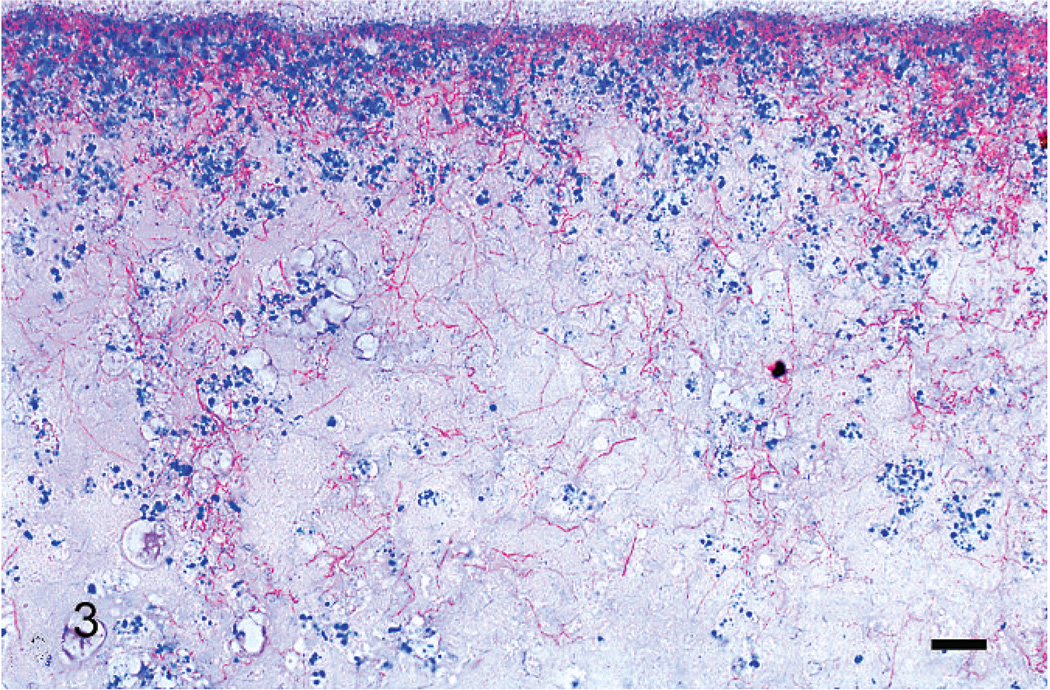
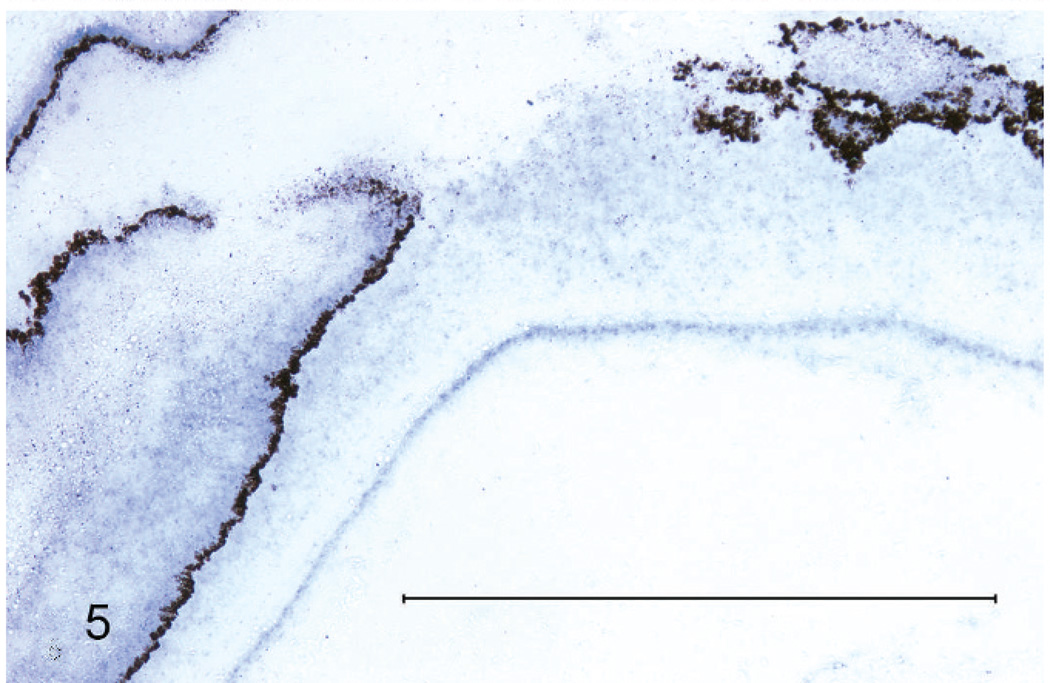
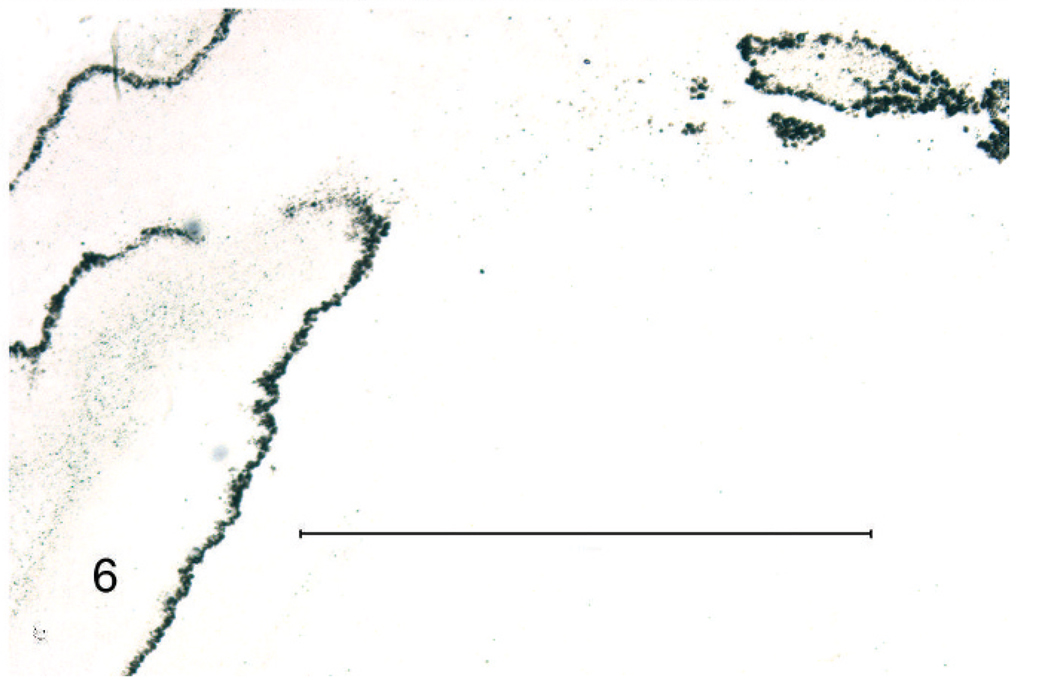
Gram stains of both eyes revealed numerous, long, beaded, filamentous, interlacing, gram-positive bacteria that measured 0.5–1 µm in width and branched at approximately 45-degree angles. Bacteria were arranged in a thick mat posterior to the necrotic retinal pigmented epithelium, within the homogenous lakes of eosinophilic material both anterior and posterior to the detached retina, and penetrating the pectenate vasculature (Fig. 4). The filamentous bacteria were strongly acid-fast positive by Fite’s stain (Fig. 3) and occasionally acid-fast positive by Ziehl-Neelsen stain, a characteristic typical of Nocardia and not Actinomyces spp.8,14,15 Moderate, diffuse in situ hybridization reactivity with an antisense probe complementary to a portion of the nocardial 16S rRNA gene (B77) was found in the appropriate locations. No reactivity was observed with the sense probe (B68) (Figs. 5, 6).
Fig. 4.
Right eye, retina. Retinal pigmented epithelium is sloughed, and there are long, beaded, filamentous, gram-positive bacteria that measure 0.5–1 µm in width and branch at approximately 45-degree angles. Gram’s stain. Bar = 30 µm.
Fig. 3.
Right eye, retina. Inflammatory infiltrate, necrotic debris, and mats of interlacing, acid-fast, branching, beaded, bacterial filaments are attached to the retina. Fite’s acid-fast stain. Bar = 50 µm.
Fig. 5.
Right eye. Diffuse reactivity along anterior and posterior surface of sloughed retinal pigmented epithelium; in situ hybridization with 16S rRNA antisense (positive) probe for Nocardia spp. Bar = 1 mm.
Fig. 6.
Compare with Fig. 5. No reactivity with in situ hybridization sense probe (negative control). Bar = 1 mm.
Additional microscopic findings included multifocal, mild, lymphoplasmacytic and occasionally heterophilic ingluvitis and enteritis; mild enteric protozoal (coccidial) infection; multifocal, random, moderate, vacuolation of individual cardiac myocytes; and multifocal pulmonary congestion and hemorrhage in the main airways. In addition, a large pulmonary vessel contained an aneurysmal-type defect in the wall characterized by focal marked thinning of the wall with associated extensive acute hemorrhage in the adjacent, surrounding tissues. In the liver, there were mild infiltrates of heterophils and macrophages in the portal tracts and occasional foci of perivascular extramedullary granulopoiesis. The following organs were examined and found to contain no significant microscopic lesions: esophagus, ventriculus, pancreas, ovary, kidney, skin, skeletal muscle, brown fat, and brain. No bacteria were identified in sections of liver or lung, despite the use of special stains.
This case illustrates the importance of the avian pecten and choroid vasculature in endophthalmitis. Within the avian eye, blood vessels are only present in the choroid and pecten. In this case, hematogenous dissemination was likely, given that infection was bilateral and subretinal, with extension into the choroid and pecten. In human beings and dogs, the most common form of nocardiosis is pulmonary. Less commonly, infection follows traumatic percutaneous abrasions.15 The agent localizes in subcutaneous tissues, bones and various organs, with frequent central nervous system involvement through hematogenous dissemination. Between 20% and 30% of people with nocardiosis exhibit symptoms of nervous system involvement.1 In this case, with the exception of multifocal portal infiltrates of inflammatory cells in the liver, interpreted as nonspecific reactive hepatitis, evidence of infection in other organ systems was absent. There is a single case report of chronic sinusitis in an Amazon parrot (Amazona autumnalis autumnalis) caused by infection with Nocardia asteroides.3 Therefore, the upper respiratory tract should not be excluded as a possible site of primary infection; however, it was not examined in this case.
The natural habitat of a prothonotary warbler is bottomland forest or mangrove swamp where they feed on invertebrates. Because of the retinal necrosis and detachment, the bird would have had impaired vision, which likely contributed to an inability to forage for food as well as increased susceptibility to trauma. The scattered vacuolation in the myocardium may have been caused by glycogen accumulation related to malnourishment. The most likely immediate cause of death was rupture of the pulmonary aneurysm, but alternative causes include septic shock and cardiovascular collapse. The single mildly elevated titer for Aspergillus spp., coupled with a lack of histologic evidence of fungal infection, is not considered significant and may indicate previous exposure. The few protozoal organisms found in the intestine were consistent with coccidia but unlikely to have been clinically significant. The advancing age of this bird (7 years) may have contributed to decreased immunocompetence, allowing an opportunistic infection to develop. This case illustrates that nocardial endophthalmitis in birds can occur without evidence of pulmonary infection or penetrating wound and without detection of an underlying immunosuppressive disease. Nocardial endophthalmitis in birds may be more common than is currently recognized.
Acknowledgements
We would like to thank Sandra Horton and Monica Mattmuller (NCSU) for preparing histologic sections and performing special stains.
Contributor Information
T. L. Reynolds, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD
H. J. Barnes, Department of Population Health and Pathobiology, North Carolina State University College of Veterinary Medicine, Raleigh, NC
B. Wolfe, Hanes Veterinary Medical Center, North Carolina Zoological Park, Asheboro, NC
L. Lu, Medical School/RFU, North Chicago, IL
D. M. Camp, William Beaumont Hospital Research Institute, Royal Oak, MI
D. E. Malarkey, Cellular and Molecular Pathology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, NC
References
- 1.Acha PN, Szyfres P. Washington, DC: Pan American Health Organization, Regional Office of the World Health Organization; Zoonoses and Communicable Diseases Common to Man and Animals. 2003;vol. 1:196–197.
- 2.Bacciarini LN, Posthaus H, Pagan O, Miserex R. Nocardia nova causing pulmonary nocardiosis of black crakes (Limmocorax flavirostra) Vet Pathol. 1999;36:345–347. doi: 10.1354/vp.36-4-345. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner R, Hoop RK, Widmer R. Atypical nocardiosis in a red-lored Amazon parrot (Amazona autumnalis autumnalis) J Assoc Avian Vet. 1994;8:125–127. [Google Scholar]
- 4.Chapman G, Beaman BL, Loeffler DA, Camp DM, Domino EF, Dickson DW, Ellis WG, Chen I, Bachus SE, LeWitt PA. In situ hybridization for detection of nocardial 16S rRNA: reactivity within intracellular inclusions in experimentally infected cynomolgus monkeys—and in Lewy body-containing human brain specimens. Exp Neurol. 2003;184:715–725. doi: 10.1016/S0014-4886(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JE. Post-mortem findings in East African birds of prey. J Wildl Dis. 1973;9:368–375. doi: 10.7589/0090-3558-9.4.368. [DOI] [PubMed] [Google Scholar]
- 6.Darzi MM, Mir MS, Nashiruddullah N, Kamil SA. Nocardiosis in domestic pigeons (Columba livia) Vet Rec. 2006;158:834–836. doi: 10.1136/vr.158.24.834. [DOI] [PubMed] [Google Scholar]
- 7.Ehrsam H, Hauser B. Nocardiosis in blue-winged royal parakeets (Alisterus ambioninensis hypophonius) Schweiz Arch Tierheilkd. 1979;121:195–200. [PubMed] [Google Scholar]
- 8.Gillespie JH, Timoney JF. Hagan and Bruner’s Infectious Diseases of Domestic Animals. 7th ed. Ithaca, NY: Comstock Publishing Associates, Cornell University Press; 1981. [Google Scholar]
- 9.Iyer PK, Rao AT, Acharjyo LN, Sahu S, Mishara SK. Systemic nocardiosis in a hill mynah (Gracula religiosa). A pathological study. Mycopathol Mycol Appl. 1972;15(48):223–226. doi: 10.1007/BF02063061. [DOI] [PubMed] [Google Scholar]
- 10.Knouse MC, Lorber B. Early diagnosis of Nocardia Asteriodes endophthalmitis by retinal biopsy: case report and review. Rev Infect Dis. 1990;12:393–398. doi: 10.1093/clinids/12.3.393. [DOI] [PubMed] [Google Scholar]
- 11.Long P, Choi G, Silberman M. Nocardiosis in two Pesquet’s parrots (Psittrichas fulgidus) Avian Dis. 1983;27:855–859. [PubMed] [Google Scholar]
- 12.Parnell MJ, Hubbard GB, Fletcher KC, Schmidt RE. Nocardia asteriodes infection in a purple throated sunbird (Nectarinia sperapa) Vet Pathol. 1983;20:497–500. doi: 10.1177/030098588302000414. [DOI] [PubMed] [Google Scholar]
- 13.Raidal S. Bilateral necrotizing pectinitis causing blindness in a rainbow lorikeet (Trichoglossus haernatodus) Avian Pathol. 1997;26:871–876. doi: 10.1080/03079459708419261. [DOI] [PubMed] [Google Scholar]
- 14.Saubolle MA. Aerobic Actinomycetes. In: McClatchey KD, editor. Clinical Laboratory Medicine. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; pp. 1201–1220. [Google Scholar]
- 15.Saubolle MA, Sussland D. Nocardiosis: Review of clinical and laboratory experience. J Clin Microbiol. 2003;41:4497–4501. doi: 10.1128/JCM.41.10.4497-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sileo L, Sievert PR, Samuel MD. Causes of mortality of albatross at Midway Atoll. J Wildl Dis. 1990;26:329–338. doi: 10.7589/0090-3558-26.3.329. [DOI] [PubMed] [Google Scholar]