Abstract
Like other forms of emotion, anxiety has been theoretically linked to preparation for action. Worry is a type of anticipatory anxiety and the hallmark of generalized anxiety disorder. Research has shown that worry is associated with vigilance to threat cues and increased muscle tension, which may in part be explained by motor facilitation that accompanies preparation for action. This study assessed corticospinal motor responses during worry using transcranial magnetic stimulation (TMS). Participants received TMS during a worry induction, during motor imagery, and during mental arithmetic, while electromyography and force were measured. TMS over the primary motor cortex elicited larger corticospinal motor responses during worry than mental arithmetic and smaller responses than motor imagery of maximum voluntary contraction of targeted muscles. These findings suggest that the association between worry and motor preparation cannot be explained by high cognitive load and provide further support for theoretical accounts emphasizing the role of action preparation in anxiety.
Keywords: anxiety, worry, motor function, transcranial magnetic stimulation, generalized anxiety disorder
INTRODUCTION
Worry, a type of future-oriented anxiety, is the hallmark of generalized anxiety disorder [GAD; American Psychiatric Association, 2000] and frequently occurs in individuals with anxious personality traits [Eysenck and Van Berkum, 1992] and mood disorders [Riskind and Williams, 2005]. Individuals suffering from GAD are hypersensitive to threat cues in the environment. They have faster motoric responses (button presses) to spatial locations associated with previously shown threat words [MacLeod et al., 1986; Mogg et al., 1992]. The faster responses may in part be explained by motor facilitation, consistent with reports of greater resting electromyographic (EMG) activity in GAD patients than non-anxious controls [Hoehn-Saric et al., 1997] and the inclusion of muscle tension in the DSM-IV criteria for GAD [American Psychiatric Association, 2000].
Emotional states, including anxiety and worry [Borkovec and Inz, 1990; Metzger et al., 1990], have been theoretically and empirically linked to action preparation [Barlow, 2000, 2002; Ekman and Davidson, 1994; Frijda, 1986; Hajcak et al., 2007; Izard, 1994]. A promising tool for assessing action preparation is transcranial magnetic stimulation (TMS). Applying TMS over primary motor cortex (M1) results in corticopsinal motor responses that can be measured in multiple ways including single motor unit activity recorded intramuscularly; motor evoked potentials (MEPs) derived from surface EMG; and force, speed, and direction of movement. Earlier work has shown that MEP amplitudes are increased if TMS is delivered during or immediately after the voluntary contraction of muscles [Hess et al., 1986, 1987; Kischka et al., 1993] and during motor imagery [Fadiga et al., 1999; Hashimoto and Rothwell, 1999; Kosslyn et al., 2001; Li et al., 2004; Rossi et al., 1998; Rossini et al., 1999]. TMS over M1 results in single motor unit activity and MEPs in targeted muscle groups even without voluntary motor behavior [Bawa and Lemon, 1993].
There is a growing literature on the use of TMS in research on emotion. A recent study found that TMS over M1 resulted in greater MEP amplitudes in target muscles while participants viewed unpleasant and pleasant pictures compared to neutral pictures [Hajcak et al., 2007], consistent with conceptualizations of emotion that highlight preparation for action. Additional studies using pictures reported effects for supplementary motor area stimulation before motor cortex stimulation [Oliveri et al., 2003] and for music presented simultaneously with the pictures [Baumgartner et al., 2007]. Other studies investigating emotion with different stimuli, tasks, and physiological recording procedures reported a range of findings for lateralization of TMS stimulation [Lo and Fook-Chong, 2004; Lo et al., 2003; Molnar-Szakacs et al., 2005; Tormos et al., 1997], which was not examined in this study or in the above-mentioned studies on affective processing [Baumgartner et al., 2007; Hajcak et al., 2007; Oliveri et al., 2003].
This study builds on this earlier research by investigating the impact of worry on corticospinal motor responses to TMS over primary motor cortex. Corticospinal motor responses were indexed using MEP amplitude in forearm EMG and force production by the index finger, widely used in the respective literatures on TMS [e.g., Bawa and Lemon, 1993; Reynolds and Ashby, 1999] and motor processing [Latash et al., 2003; Li et al., 2004; see also, Oathes and Ray, 2006; Todorov, 2000]. These MEP and force measures were obtained during a worry induction, mental arithmetic, motor imagery, and resting baseline. Mental arithmetic was selected as a control task for the worry induction because both are cognitively demanding and do not explicitly target motor processes. We hypothesized that TMS over M1 would result in larger corticospinal motor responses during a worry induction than resting baseline or a mental arithmetic task in an unselected sample of research volunteers. On the basis of earlier TMS studies investigating motor imagery [e.g., Kosslyn et al., 2001; Li et al., 2004], TMS during the worry induction was expected to generate smaller corticospinal motor responses than during motor imagery of pressing a sensor with maximal effort using the specific muscles innervated by the TMS pulses.
METHOD
PARTICIPANTS
Participants were drawn from introductory psychology courses for class credit. Each individual gave informed consent using a form approved by the Pennsylvania State University Office for Research Protections (Institutional Review Board). The 21 participants were self-identified right-handed males who ranged in age from 18 to 38 years (M=20.33, SD=4.22). The web sign up for the experiment also requested that individuals only participate if they did not suffer from a heart condition, epilepsy, or another neurological disorder and that they did not have medical implants of any kind (standard counter-indicators for magnetic stimulation research). No participants were excluded based on these criteria.
APPARATUS AND PHYSIOLOGICAL RECORDING
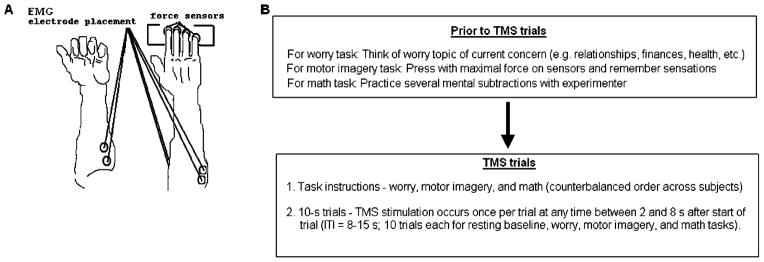
Four unidirectional piezoelectric force sensors (208A03; PCB Piezotronics, Inc., Depew, NY), one for each finger medial to the thumb of the right hand, were used for force measurement. Digitized values were amplified by AC/DC conditioners (PCB Piezotronics) (M482M66) and displayed on a 17-in computer screen. Sensors were laterally spaced 30 mm apart and adjusted in the forward–backward direction within 60 mm to optimally fit each individual’s hand. Bipolar EMG recordings were taken from pairs of 1-cm diameter disposable Ag-AgCl surface electrodes placed over the bellies of the extrinsic flexor and extensor muscles of the right forearm with a distance between the electrode pairs of 3 cm (see Fig. 1A). Pairs of EMG electrodes were also placed over the bellies of the left forearm flexor muscles, with the signal displayed on the screen for the purpose of monitoring subject adherence to task instructions but not recorded for subsequent analysis. EMG signals were amplified, and a bandpass filter (10–500 Hz) was applied.
Figure 1.
(A) Electrodes were placed on the bellies of both forearm flexors and on right arm extensor muscles in pairs. Force sensors were placed under each finger of the right hand and recorded from the right index finger. (B) Experiment timeline. Complete instructions for each task provided in the Appendix. EMG, electromyography; TMS, transcranial magnetic stimulation; ITI, intertrial interval.
Focal magnetic stimuli were delivered using a Magstim 200 stimulator (Magstim Co., Woburn, MA) that delivers monophasic pulses with a maximum field strength of 2.2T. The intersection of the two 70-mm coils (figure-8 shaped) was placed tangential to the surface of the scalp with the handle pointing backward and at a 45° angle from the midline to optimally stimulate corticospinal pathways trans-synaptically via interneurons whose orientations are parallel to the surface of the cortex and thus congruent with the induced magnetic field. The left motor cortex position was used that most reliably produced a right forearm flexor MEP and force response in the sensor underlying the right index finger. At rest, forearm flexor MEPs and index finger force production covaried reliably following stimulations of the primary motor cortex in the locations chosen for each participant, thus allowing the use of a combination of the two measures to determine motor thresholds. Motor threshold was operationalized as the stimulation level that elicited a raw EMG response of at least 50 μV for at least five of ten consecutive trials [e.g., Baumgartner et al., 2007; Li et al., 2004; Rossini et al., 1994; Sparing et al., 2007] and measurable force production in the index finger (approximately 0.1 N). This procedure optimized overlap between index finger force and the corresponding isometric muscle contraction in the forearm flexors. Stimulations for the experimental session were set to 140% of motor threshold determined using this method, consistent with published guidelines for TMS [Rossini et al., 1994] and with other reports [Baumgartner et al., 2007; Li et al., 2004; Sparing et al., 2007]. A Gateway 450 MHz computer (Irvine, CA) was used for data acquisition and processing with signals sampled at 1 KHz by a 16-bit A/D board running LabView software (National Instruments, Austin, TX).
PROCEDURE AND EXPERIMENTAL PARADIGM
During the TMS session, participants sat with both arms lying out symmetrically on the testing table with respect to the body midline. The upper arms were at approximately 45° of abduction in the frontal and sagittal planes with elbow joints fixed at about 135°. Metacarpophalangeal joints were flexed at about 20° and interphalangeal joints were slightly flexed such that the hand formed a dome. Foam blocks, sized to fit comfortably under the palm, helped maintain a constant and comfortable configuration of the hand and fingers. The forearm was secured in position to an underlying board on the test table with Velcro straps at the wrist and upper forearm.
While electrodes were being affixed to their arms, participants were asked to think of a worry topic “of current concern” that could be used later in the experiment. Topics most often chosen by these participants included concerns with relationships, finances, career goals, and academic performance, consistent with other research on worry [e.g., Roemer et al., 1997; Vasey and Borkovec, 1992]. Participants were then asked to use their index finger to press the sensor with maximal force and were told that they would be asked to imagine the same button press later in the experiment (see Fig. 1B).
For the experiment, a baseline rest period always preceded the three tasks—worry induction, mental arithmetic, and motor imagery—which were presented in one of three pseudo-randomized orders (math–worry– motor imagery; worry–motor imagery–math; motor imagery–math–worry). For the worry induction, participants were asked to worry “as intensely as you can, in the way that you usually worry” about the topic that they had self-selected [as in Borkovec and Inz, 1990; Oathes et al., under review] at the beginning of the TMS session. For mental arithmetic, participants were asked to mentally subtract 7 in succession starting with the number 1,320. They were informed that they would have to report the last number they attained at the end of this task to the experimenters. For the motor imagery task, participants were instructed to “imagine pressing as hard as you can with your index finger” on the force sensor under that finger (see Appendix).
TMS pulses were delivered once per 10-sec trial at a random interval between 2 and 8 sec after the trial was initiated, and each task had 10 trials (see Fig. 1B). Trials were initiated manually by the experimenter, yielding intertrial intervals of 8–15 sec. Provided in full in the Appendix, verbal instructions for each task were provided before the first trial (see Fig. 1B), with abbreviated instructions as a reminder after the fifth trial of each task: “worry, worry” for the worry task, “keep going” for the math task, and “imagine pressing” for the motor imagery task. Participants were instructed to reengage in the tasks if they were distracted by the TMS pulses. For the duration of the experiment, participants were requested to refrain from actually pressing the sensors or tensing muscles in any part of their bodies. We monitored right forearm and left forearm flexor EMG and right index finger force to ensure that participants were following instructions to relax their muscles during TMS trials. Each participant had one to three trials with measurable motor responses not induced by the TMS pulse. These trials were discarded and replaced by additional trials after participants were reminded to keep their muscles relaxed.
DATA ANALYSIS
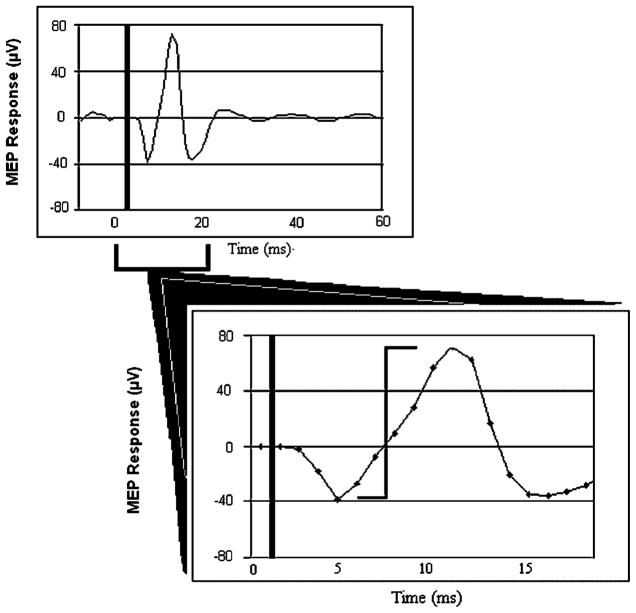
Data were processed off-line using analysis programs created in MatLab (Mathworks, Natick, MA). A second-order, zero-lag Butterworth low-pass filter at 100 Hz [Li et al., 2004] was applied to the right forearm EMG data and resulted in smaller EMG amplitudes compared to the raw signal displayed in Figure 2. To derive MEP and force values, we determined when rectified MEP amplitude of the right forearm flexor EMG exceeded a critical threshold of 2 SD above the whole-trial average following the TMS pulse. A 500-ms window was time-locked to each critical threshold (starting 100 ms before and extending to 400 ms after the time point at which the threshold was exceeded). Mean activity during these time windows was determined for rectified MEP amplitude of the right forearm flexor EMG and for force recorded from the right index finger. We also calculated peak-to-peak amplitude for the MEPs in non-rectified EMG (see Fig. 2) and for index finger force. For these mean and peak metrics of MEP amplitude and force, statistical analyses were conducted on averages across the 10 trials of each task obtained for each subject.
Figure 2.
A typical motor evoked potential (MEP) to transcranial magnetic stimulation measured in forearm electromyography. Approximate time of transcranial magnetic stimulation pulse is represented by thick vertical line at beginning of trial. Second thinner vertical line indicates peak-to-peak measurement for MEP amplitude. μV, microvolts.
An omnibus multivariate analysis of variance with Wilks’ λ [Keselman, 1998] was used to test Task (resting baseline, math, worry, motor imagery) and Metric (mean force, peak force, mean MEP amplitude, peak MEP amplitude) effects. Significant effects were followed up using univariate analysis of variance and paired-sample t tests (two tailed). Effect sizes were calculated for pair-wise contrasts comparing tasks [Cohen, 1988]. The order in which the tasks were presented did not affect motor responding to TMS, as indicated by the absence of significant main or interaction effects for Order when it was included as a factor in the above analyses.
RESULTS
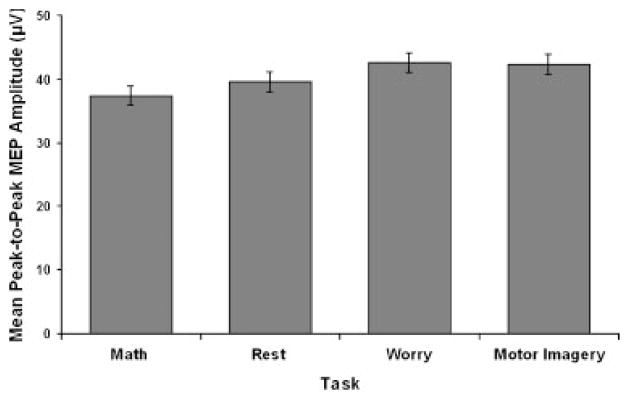
The omnibus multivariate analysis of variance revealed a Task main effect, F(12, 151)=2.25, P=0.01, and no Metric main effect or interaction. A post hoc directional analysis (math, rest, worry, motor imagery) indicated a significant linear trend for peak MEP amplitude, F(1, 20)=5.45, P=0.03 (see Fig. 3). As predicted, TMS-induced larger MEP amplitudes for worry than math, t(20)=2.15, P=0.04, d=0.36 (see Table 1). A similar pattern of larger peak MEP amplitude for motor imagery than math was also observed, t(20)=1.97, P=.06, d=0.41. The linear trend was not significant for the other three metrics. However, additional analyses indicated a consistent pattern of results across all four metrics, including a Task main effect for mean MEP amplitude, F(3, 60)=3.14, P<.05. Motor imagery was associated with larger mean MEP amplitude than worry, t(20)=–2.07, P=.05, d=–0.45, and rest, t(20)=–2.16, P=.04, d=–0.40 (see Table 1). For peak force, motor imagery was also associated with a greater motor response than rest, t(20)=–2.15, P=.04, d=–0.45, and worry showed the same pattern of larger peak force than rest, t(20)=–1.96, P=0.06, d=–0.24 (see Table 1). No effects were observed for mean force.
Figure 3.
Peak flexor motor evoked potential (MEP) amplitude in forearm electromyography as a function of task (Math, Rest, Worry, Motor Imagery). Error bars are 1 SE around the mean after correction for inter-subject variability [Loftus and Masson, 1994].
TABLE 1.
Mean (SD) for EMG flexor motor evoked potential and finger force responses
| Metric | Task
|
|||
|---|---|---|---|---|
| Math | Rest | Worry | Motor imagery | |
| Peak MEP (μV) | 37.4 (13.3)a,* | 39.6 (14.7) | 42.6 (15.4)a | 42.4 (10.5)* |
| Mean MEP (μV) | 15.6 (7.8) | 14.7 (6.5)a | 14.3 (6.3)b | 17.7 (8.4)a,b |
| Peak force (N) | 0.07 (0.10) | 0.05 (0.04)a,* | 0.06 (0.05)* | 0.08 (0.08)a |
| Mean force (N) | 0.05 (0.08) | 0.04 (0.03) | 0.04 (0.02) | 0.05 (0.04) |
Within a row, matched superscript letters indicate significant difference at P<0.05, and asterisk indicates significant difference at P=0.06.
EMG, electromyography; MEP, motor evoked potential; μV, microvolts; N, newtons.
DISCUSSION
It is widely accepted that preparation for action is a central function of emotion and anxiety [Barlow, 2000, 2002; Ekman and Davidson, 1994; Frijda, 1986; Izard, 1994], despite limited data owing to the difficulty of empirically addressing the issue. TMS was employed in this study to investigate the role of motor preparation in worry. During the worry induction, TMS over the primary motor cortex elicited corticospinal motor responses that were measured with forearm EMG and index finger force. We found that worry was associated with larger corticospinal motor responses than a mental arithmetic task with high cognitive load, providing support for the role of action preparation in worry.
Inferred on the basis of TMS-induced motor responses, corticospinal motor excitability is a commonly used term in the TMS literature to describe motor outputs evoked by magnetic stimulation initiated at the cortex and sampled in muscle groups targeted by the stimulation site [Abbruzzese and Trompetto, 2002]. Our operational definition of action preparation was the TMS-evoked response in the corticospinal motor track, consistent with other findings for tasks not requiring voluntary motor behavior [Hajcak et al., 2007; Jeannerod, 2001; Oliveri et al., 2003]. One possibility is that worry includes motor imagery that accounts for this action preparation. We believe that this is unlikely based on the large literature on the phenomenology of worry [e.g., Borkovec and Inz, 1990; Breitholtz et al., 1999; Craske et al., 1989; Freeston et al., 1996; Molina et al., 1998; Provencher et al., 2000; Roemer et al., 1997; Szabo and Lovibond, 2002], which shows that worry is predominantly associated with a variety of daily concerns that do not include motor imagery (e.g., finances, interpersonal relationships, school/work performance). Indeed, although the cognitive contents of worry have been well mapped by previous non-physiological paradigms in those studies, motor preparation has never been reported by these methods and thus likely is a more subtle consequence of worry states. TMS uniquely allows the testing of motor preparation in the sense that the motor neural circuitry is recruited even with mental events that are not necessarily predictive of imminent overt behavior. Future studies in depression and anxiety may benefit by continuing to employ a range of central and peripheral physiological measures useful for assessing complex and subtle relationships between mental states and their bodily representations as in this study.
Present findings also contribute to the growing literature using TMS to investigate emotion. Of particular relevance to this report is a recent TMS study highlighting the role of action preparation in theories of emotion, which found greater corticospinal motor excitability (increased MEP amplitude) during the viewing of unpleasant and pleasant pictures compared to neutral pictures [Hajcak et al., 2007]. Present results build on those findings and on other recent TMS studies on emotion [Baumgartner et al., 2007; Lo and Fook-Chong, 2004; Lo et al., 2003; Molnar-Szakacs et al., 2005; Oliveri et al., 2003; Tormos et al., 1997] by implicating heightened corticospinal motor excitability and action preparation in worry.
Given that worry is a central feature of GAD and is also reported widely among patients with other anxiety and mood disorders, present findings contribute to the growing literature using TMS to probe dysregulated emotion. Patients with obsessive-compulsive disorder have been found to have decreased MEP thresholds, another indication of increased corticospinal motor excitability [Greenberg et al., 2000]. With regard to other forms of negative affect, another study found no relationship between neuroticism and MEP threshold [Wassermann et al., 2001]. Our earlier TMS study found that depressed mood was associated with decreased corticospinal motor excitability, as indexed by finger force [Oathes and Ray, 2006]. These latter two studies on neuroticism and depressed mood together with the present results for worry suggest that these different forms of negative affect [Borkovec and Inz, 1990; Metzger et al., 1990; Nitschke et al., 2001] are at least partially independent of one another.
Frequent worry states may contribute to the reported [American Psychiatric Association, 2000] and recorded [Hoehn-Saric et al., 1997] elevations in muscle tension among chronic worriers such as individuals suffering from GAD. Importantly, research on other physiological systems demonstrates that worry is not associated with general physiological hyperactivity. Earlier reports have shown that worry is associated with cardiovascular hyporesponsivity to aversive stimuli [Borkovec and Hu, 1990; Borkovec et al., 1993] and normative potentiation of eyeblink startle in anticipation of aversive stimuli [Nitschke et al., 2002] and while viewing them [Larson et al., 2007]. In addition, the present results indicate that corticospinal motor excitability during worry is not at ceiling, based on the finding of smaller mean MEP amplitude than during motor imagery.
Related to action preparation, corticospinal motor excitability in worry might contribute to speeded response times observed with chronic worriers in behavioral tasks using threatening emotional stimuli [MacLeod et al., 1986; Mogg et al., 1992]. Alternatively, the extensive research on attentional bias in anxiety suggests that fixation on the threat word locations may also facilitate responses to the probes without the need to directly prime the motor system. Further research is needed to determine the relative contributions of corticospinal motor excitability and attentional processes as well as their interaction in responding to threat.
A limitation of this study is the reliance on male research participants. Males were used to avoid complications of hormonal influences on cortical excitability [Smith et al., 1999, 2002, 2003] that may confound the relationship between corticospinal motor response and psychological traits [Wassermann et al., 2001]. TMS research on worry that carefully addresses hormonal variations in women would determine whether the present findings generalize to women. To follow up the consistent pattern of results across the EMG and force metrics employed in this study, a larger sample would provide greater statistical power for detecting whether the hypothesized differences are significant for all metrics used. Future research applying TMS over dominant and non-dominant M1 [Flöel et al., 2003; Lo et al., 2003; Sparing et al., 2007] is needed to assess laterality differences associated with worry [Compton and Mintzer, 2001] and to provide evidence for the assumption that the effects for worry here are not localized to dominant M1 innervation of the hand but rather related to motor facilitation more globally. Paired-pulse TMS techniques would also help to further assess the cortical specificity of our findings. Although similarities between the experiences of worry in clinical and non-clinical populations [Molina et al., 1998; Provencher et al., 2000] support the extrapolation of the present results to chronic worry and GAD, the impact of TMS on worry needs to be investigated in chronic worriers and GAD patients.
This study showcases corticospinal motor activation induced by TMS as a useful method for the study of motor preparation in worry, anxiety, and emotion. Results suggest that worry is accompanied by corticospinal motor excitability that cannot by explained by high cognitive load. These results support theoretical ties between emotion and motor pathways [Ekman and Davidson, 1994; Frijda, 1986; Izard, 1994] as well as specifically between anxiety and motor system recruitment [e.g., Barlow, 2000, 2002].
Acknowledgments
The authors thank Sheng Li for assisting with data acquisition and analysis programing. We also wish to thank Mark Latash for use of his laboratory and equipment as well as for helpful advice on the experimental design.
Appendix
Worry Induction Script
For the next several minutes, we would like you to worry about the topic that you chose earlier in the experiment. Please worry about this topic as intensely as you can, in the way that you usually worry, until you are asked to stop worrying. The stimulations will occur while you are engaged in the worry and may disrupt what you are thinking about. Please do your best to continue your line of thought after each stimulation from where you left off. Make sure to relax your body, including your arms and hands while engaged in the worry. You may begin now.
Mental Math Script
For the next several minutes, we would like you to do a mental subtraction in your head. At the end of this period, we will ask for the number you were last on. The number to start on is 1,320 and we would like you to subtract 7 from this number again and again in your head until we ask you to stop doing the subtraction. The stimulations will occur while you are engaged in the subtraction and may disrupt what you are thinking about. Please do your best to continue your line of thought after each stimulation from where you left off. If you forget the last number you were on, think back to the last number you remember and continue subtracting. Make sure to relax your body, including your arms and hands, while engaged in the subtraction. Remember, start with 1,320 and subtract by 7s. You may begin now.
Motor Imagery Script
For the next several minutes, we would like you to imagine that you are pressing down as hard as you can with your index finger on the button it is now resting on. Please take a moment now to press on the button as hard as you can so that you can imagine later what this feels like.
Now, we want you to imagine pressing as hard as you can but please do not actually press on the button for the remainder of the experiment. In fact, please do your best not to tense any of the muscles in your body. The stimulations will occur while you are engaged in the imagery and may disrupt what you are thinking about. Please do your best to continue your line of thought after each stimulation from where you left off. You may start to imagine pressing now.
Footnotes
Research conducted at The Pennsylvania State University, University Park, PA.
References
- Abbruzzese G, Trompetto C. Clinical and research methods for evaluating cortical excitability. J Clin Neurophysiol. 2002;19:307–321. doi: 10.1097/00004691-200208000-00005. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text revision. [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol. 2000;55:1245–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic. 2. New York: Guilford Press; 2002. [Google Scholar]
- Baumgartner T, Willi M, Jäncke L. Modulation of corticospinal activity by strong emotions evoked by pictures and classical music: a transcranial magnetic stimulation study. Neuroreport. 2007;18:261–265. doi: 10.1097/WNR.0b013e328012272e. [DOI] [PubMed] [Google Scholar]
- Bawa P, Lemon RN. Recruitment of motor units in response to transcranial magnetic stimulation in man. J Physiol (Lond) 1993;471:445–464. doi: 10.1113/jphysiol.1993.sp019909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD, Hu S. The effect of worry on cardiovascular response to phobic imagery. Behav Res Ther. 1990;28:69–73. doi: 10.1016/0005-7967(90)90056-o. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Inz J. The nature of worry in generalized anxiety disorder: a predominance of thought activity. Behav Res Ther. 1990;28:153–158. doi: 10.1016/0005-7967(90)90027-g. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Lyonfields JD, Wiser SL, Diehl L. The role of worrisome thinking in the suppression of cardiovascular response to phobic imagery. Behav Res Ther. 1993;31:321–324. doi: 10.1016/0005-7967(93)90031-o. [DOI] [PubMed] [Google Scholar]
- Breitholtz E, Johansson B, Öst LG. Cognitions in generalized anxiety disorder and panic disorder patients. Behav Res Ther. 1999;37:533–544. doi: 10.1016/s0005-7967(98)00147-8. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analyses for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Compton R, Mintzer DA. Effects of worry and evaluation stress on interhemispheric interaction. Neuropsychology. 2001;15:427–433. [PubMed] [Google Scholar]
- Craske MG, Rapee RM, Jackel L, Barlow DH. Qualitative dimensions of worry in DSM-III-R generalized anxiety disorder subjects and non-anxious controls. Behav Res Ther. 1989;27:397–402. doi: 10.1016/0005-7967(89)90010-7. [DOI] [PubMed] [Google Scholar]
- Ekman P, Davidson RJ, editors. The nature of emotion: fundamental questions. New York: Oxford University Press; 1994. [Google Scholar]
- Eysenck MW, Van Berkum JJA. Trait anxiety, defensiveness, and the structure of worry. Pers Individ Diff. 1992;13:1285–1290. [Google Scholar]
- Fadiga L, Buccino G, Craighero L, Fogassi L, Callese V, Pavesi G. Corticospinal excitability is specifically modulated by motor imagery: a magnetic stimulation study. Neuropsychologia. 1999;37:147–158. doi: 10.1016/s0028-3932(98)00089-x. [DOI] [PubMed] [Google Scholar]
- Flöel A, Elger T, Breitenstein C, Knecht S. Language perception activates the hand motor cortex: implications for motor theories of speech perception. Eur J Neurosci. 2003;18:704–708. doi: 10.1046/j.1460-9568.2003.02774.x. [DOI] [PubMed] [Google Scholar]
- Freeston MH, Dugas MJ, Ladouceur R. Thoughts, images, worry, and anxiety. Cogn Ther Res. 1996;20:265–273. [Google Scholar]
- Frijda NH. The emotions. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- Greenberg BD, Ziemann U, Cora-Locatelli G, Harmon A, Murphy DL, Wassermann E. Altered cortical excitability in obsessive-compulsive disorder. Neurology. 2000;54:142–147. doi: 10.1212/wnl.54.1.142. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Molnar C, George MS, Bolger K, Koola J, Nahas Z. Emotion facilitates action: a transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology. 2007;44:91–97. doi: 10.1111/j.1469-8986.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Rothwell JC. Dynamic changes in corticospinal excitability during motor imagery. Exp Brain Res. 1999;125:75–81. doi: 10.1007/s002210050660. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observation on an amputee. Neurosci Lett. 1986;71:235–240. doi: 10.1016/0304-3940(86)90565-3. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Responses in small hand muscles from magnetic stimulation of the human brain. J Physiology. 1987;388:397–419. doi: 10.1113/jphysiol.1987.sp016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn-Saric R, Hazlett RL, McLeod DR, Pourmotabbed T. Does muscle tension reflect arousal? Relationship between electromyographic and electroencephalographic recordings. Psychiatry Res. 1997;71:49–55. doi: 10.1016/s0165-1781(97)00037-1. [DOI] [PubMed] [Google Scholar]
- Izard CE. Innate and universal facial expressions: evidence from developmental and cross-cultural research. Psychol Bull. 1994;115:288–299. doi: 10.1037/0033-2909.115.2.288. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14:S103–S109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Keselman HJ. Testing treatment effects in repeated measures designs: an update for psychophysiological researchers. Psychophysiology. 1998;35:470–478. [PubMed] [Google Scholar]
- Kischka U, Fajfr R, Fellenberg T, Hess CW. Facilitation of motor evoked potentials from magnetic brain stimulation in man: a comparative study of different target muscles. J Clin Neurophysiol. 1993;10:505–512. doi: 10.1097/00004691-199310000-00008. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2:635–642. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- Larson CL, Nitschke JB, Davidson RJ. Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion. 2007;7:182–191. doi: 10.1037/1528-3542.7.1.182. [DOI] [PubMed] [Google Scholar]
- Latash ML, Yarrow K, Rothwell JC. Changes in finger coordination and response to single pulse TMS of motor cortex during practice of a multifinger force production task. Exp Brain Res. 2003;151:60–71. doi: 10.1007/s00221-003-1480-y. [DOI] [PubMed] [Google Scholar]
- Li S, Latash ML, Zatsiorsky VM. Effects of motor imagery on finger force responses to transcranial magnetic stimulation. Brain Res Cogn Brain Res. 2004;20:273–280. doi: 10.1016/j.cogbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Lo YL, Fook-Chong S. Ipsilateral and contralateral motor inhibitory control in musical and vocalization tasks. Exp Brain Res. 2004;159:258–262. doi: 10.1007/s00221-004-2032-9. [DOI] [PubMed] [Google Scholar]
- Lo YL, Fook-Chong S, Lau DPC, Tan EK. Cortical excitability changes associated with musical tasks: a transcranial magnetic stimulation study in humans. Neurosci Lett. 2003;352:85–88. doi: 10.1016/j.neulet.2003.08.031. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonom Bull Rev. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Metzger RL, Miller M, Cohen M, Sofka M, Borkovec TD. Worry changes decision making: the effect of negative thoughts on cognitive processing. J Clin Psychol. 1990;46:78–88. doi: 10.1002/1097-4679(199001)46:1<78::aid-jclp2270460113>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Eysenck M. Attentional bias to threat in clinical anxiety states. Cogn Emot. 1992;6:149–159. [Google Scholar]
- Molina S, Borkovec TD, Peasley C, Person D. Content analysis of worrisome streams of consciousness in anxious and dysphoric participants. Cogn Ther Res. 1998;22:109–123. [Google Scholar]
- Molnar-Szakacs I, Uddin LQ, Iacobini M. Right-hemisphere motor facilitation by self-descriptive personality-trait words. Eur J Neurosci. 2005;21:2000–2006. doi: 10.1111/j.1460-9568.2005.04019.x. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cogn Ther Res. 2001;25:1–22. [Google Scholar]
- Nitschke JB, Larson CL, Smoller MJ, Navin SD, Pederson AJC, Ruffalo D, Mackiewicz KL, Gray SM, Victor E, Davidson RJ. Startle potentiation in aversive anticipation: evidence for state but not trait effects. Psychophysiology. 2002;39:254–258. doi: 10.1017/S0048577202010156. [DOI] [PubMed] [Google Scholar]
- Oathes DJ, Ray WJ. Depressed mood, index finger force and motor cortex stimulation: a transcranial magnetic stimulation (TMS) study. Biol Psychol. 2006;72:271–277. doi: 10.1016/j.biopsycho.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Babiloni C, Filippi MM, Caltagirone C, Babiloni F, Cicinelli P, Traversa R, Palmieri MG, Rossini PM. Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Exp Brain Res. 2003;149:214–221. doi: 10.1007/s00221-002-1346-8. [DOI] [PubMed] [Google Scholar]
- Provencher MD, Freeston MH, Dugas MJ, Ladouceur R. Catastrophizing assessment of worry and threat schemata among worriers. Behav Cogn Psychother. 2000;28:211–224. [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary motor contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- Riskind JH, Williams NL. The looming cognitive style and generalized anxiety disorder: distinctive danger schemas and cognitive phenomenology. Cogn Ther Res. 2005;29:7–27. [Google Scholar]
- Roemer L, Molina S, Borkovec TD. An investigation of worry content among generally anxious individuals. J Nerv Ment Dis. 1997;185:314–319. doi: 10.1097/00005053-199705000-00005. [DOI] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Tecchio F, Pauri F, Rossini PM. Corticospinal excitability modulation during mental simulation of wrist movements in human subjects. Neurosci Lett. 1998;243:147–151. doi: 10.1016/s0304-3940(98)00088-3. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray N, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Pasqualetti P, Tecchio F. Corticospinal excitability modulation to hand muscles during movement imagery. Cereb Cortex. 1999;9:161–167. doi: 10.1093/cercor/9.2.161. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Ovarian effects on human cortical excitability. Ann Neurol. 2002;51:599–603. doi: 10.1002/ana.10180. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiat. 2003;54:757–762. doi: 10.1016/s0006-3223(02)01924-8. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Keel JC, Greenburg BD, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM. Menstrual cycle effects on cortical excitability. Neurology. 1999;53:2069–2072. doi: 10.1212/wnl.53.9.2069. [DOI] [PubMed] [Google Scholar]
- Sparing R, Meister IG, Wienemann M, Buelte D, Staedtgaen M, Boroojerdi B. Task-dependent modulation of functional connectivity between hand motor cortices and neuronal networks underlying language and music: a transcranial magnetic stimulation study in humans. Eur J Neurosci. 2007;25:319–323. doi: 10.1111/j.1460-9568.2006.05252.x. [DOI] [PubMed] [Google Scholar]
- Szabo M, Lovibond PF. The cognitive content of naturally occurring worry episodes. Cogn Ther Res. 2002;26:167–177. [Google Scholar]
- Todorov E. Direct cortical control of muscle activation in voluntary arm movements: a model. Nat Neurosci. 2000;3:391–398. doi: 10.1038/73964. [DOI] [PubMed] [Google Scholar]
- Tormos JM, Caòete C, Tarazona F, Catalá D, Pascual APL, Pascual-Leone A. Lateralized effects of self-induced sadness and happiness on corticospinal excitability. Neurology. 1997;49:487–491. doi: 10.1212/wnl.49.2.487. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Borkovec TD. A catastrophizing assessment of worrisome thoughts. Cogn Ther Res. 1992;16:505–520. [Google Scholar]
- Wassermann EM, Greenberg BD, Nguyen MB, Murphy DL. Motor cortex excitability correlates with an anxiety-related personality trait. Biol Psychiatry. 2001;50:377–382. doi: 10.1016/s0006-3223(01)01210-0. [DOI] [PubMed] [Google Scholar]