Abstract
Background
Intermittent access to drugs of abuse, as opposed to continuous access, is hypothesized to induce a kindling-type transition from moderate to escalated use, leading to dependence. Intermittent 24-hour cycles of ethanol access and deprivation can generate high levels of voluntary ethanol drinking in rats.
Methods
The current study uses C57BL/6J mice (B6) in an intermittent access to 20% ethanol protocol to escalate ethanol drinking levels. Adult male and female B6 mice were given intermittent access to 20% ethanol on alternating days of the week with water available ad libitum. Ethanol consumption during the initial 2 hours of access was compared to a short term, limited access “binge” drinking procedure, similar to drinking-in-the-dark (DID). B6 mice were also assessed for ethanol dependence with handling-induced convulsion (HIC), a reliable measure of withdrawal severity.
Results
After 3 weeks, male mice given intermittent access to ethanol achieved high stable levels of ethanol drinking in excess of 20 g/kg/24h, reaching above 100 mg/dl BEC, and showed a significantly higher ethanol preference than mice given continuous access to ethanol. Also, mice given intermittent access drank about twice as much as DID mice in the initial 2-hour access period. B6 mice that underwent the intermittent access protocol for longer periods of time displayed more severe signs of alcohol withdrawal. Additionally, female B6 mice were given intermittent access to ethanol and drank significantly more than males (ca. 30 g/kg/24h).
Discussion
The intermittent access method in B6 mice is advantageous because it induces escalated, voluntary, and preferential per os ethanol intake, behavior that may mimic a cardinal feature of human alcohol dependence, though the exact nature and site of ethanol acting in the brain and blood as a result of intermittent access has yet to be determined.
Keywords: alcoholism, intermittent access, C57BL/6J, animal models, dependence
Introduction
Over several decades, preclinical models for excessive, binge-like alcohol consumption have been developed, yet it remains challenging to fully represent some of the essential features of elevated alcohol drinking that characterize human alcohol use disorders. Ethanol exposure via vapor inhalation has been the current prototypical method for rendering rodents dependent on alcohol (O’Dell et al., 2004). Rodents reliably demonstrate alcohol dependence after ethanol vapor exposure (Becker and Lopez, 2004; Roberts et al., 2000; Schulteis et al., 1995), whereas dependence induced per os would be more applicable for a translation of pharmacologically motivated drinking behavior (Dole et al., 1985). Ethanol liquid diet or one-bottle forced drinking protocols also engender escalated oral ethanol consumption (Lieber et al., 1963; Lieber and DeCarli, 1982), though incorporating free-choice preferential alcohol drinking would enhance external validity. Developing a paradigm that induces animals to voluntarily consume high amounts of alcohol would be a valuable tool for approximating human alcoholic-like drinking.
Intermittent access appears to be a cardinal feature of many conditions that promote drinking large amounts of alcohol (Le Magnen, 1960; Rodd-Henricks et al., 2000; Sinclair and Senter, 1968). It has been thought that the progression from high alcohol intake to alcohol dependence may be driven by repeated cycles of heavy drinking followed by deprivation (Breese et al., 2005; Meisch, 1983). According to the kindling hypothesis (Ballenger and Post, 1978; Becker, 1998), increasing amounts of ethanol ingested over repeated episodes may intensify ensuing symptoms of withdrawal when alcohol is absent (Begleiter and Porjesz, 1979; Overstreet et al., 2002; Overstreet et al., 2004b), thus generating a compensatory mechanism for relapse (Becker and Hale, 1993; Holter et al., 1998).
C57BL/6J (B6) mice have been studied for more than five decades for their high alcohol intake (Belknap et al., 1993; Rodgers, 1966). Compared to other inbred strains, B6 mice voluntarily consume the most alcohol per unit of body weight and exhibit the highest ethanol preference (Elmer et al., 1987; McClearn and Rodgers, 1959; Yoneyama et al., 2008). Within the DID protocol, adult male B6 mice show pharmacologically relevant blood ethanol concentrations (BEC) ranging from 80–100 mg per dl over a 2–4h period (Lyons et al., 2008; Rhodes et al., 2005; Rhodes et al., 2007). DID represents a replicable model for binge-like drinking up to intoxication (Lowery et al., 2010; Sparta et al., 2008), but the duration of ethanol intoxication does not seem to be sustained sufficiently to induce dependence.
The present study explored escalated alcohol drinking behavior in adult B6 mice given intermittent access to 20% ethanol in a 2-bottle choice paradigm, as previously implemented in rats (Simms et al., 2008). We examined the time course of drinking, when given intermittent access versus continuous access to increasing concentrations of ethanol, until stable 20% ethanol drinking was observed. To assess if the procedure produced a shift from heavy alcohol drinking to dependence, signs of withdrawal severity were assessed, as measured by responses to handling-induced convulsion (HIC). B6 male drinking behavior in the intermittent access paradigm was compared to a short term, limited access “binge” drinking procedure, similar to DID. Additionally, since females of the B6 strain are known to self-administer higher amounts of ethanol than males under most conditions (Finn et al., 2005; Lopez and Becker, 2003), we compared female ethanol drinking behavior to male drinking behavior in the intermittent access paradigm in an initial exploration of sex differences. We propose a pharmacologically motivated model of alcoholic-like drinking in mice (Dole et al., 1985), as a novel approximation of episodic and excessive drinking in humans.
Materials and Methods
Animals and Housing
Adult male and female C57BL/6J mice (Jackson Laboratories, Bar Harbour, ME) were 8 weeks old upon arrival and were initially housed as male/female pairs in polycarbonate cages (28 × 17 × 12 cm) with stainless steel wire mesh lids and pine shavings covering the floor. Ethanol-naïve animals were given 1 week to habituate to the vivarium conditions on a 12-hour reversed light/dark cycle (lights off at 7 AM) and constant temperature (21 ± 2°C) and humidity (25%). Standard rodent chow (LabDiet 5001 Rodent Diet; PMI Nutrition International, Brentwood, MO) and tap water were unrestricted. All procedures were approved by the Tufts University Institutional Animal Care and Use Committee and were in accordance with the NIH Guide for Care and use of Laboratory Animals.
Ethanol Intake Procedures
Ethanol solutions (w/v) were prepared in tap water from 95% ethyl alcohol (Pharmaco-AAPER, Brookfield, CT). Males and females were changed to individual housing at least 24h before the presentation of two centrifuge tubes of water on the cage lid for three days for acclimation to drinking from sipper tubes. Fluids were presented in 50-ml plastic centrifuge tubes (Nalgene) with no. 5 rubber stoppers (Fisher Scientific, Agawam, MA 01001) containing stainless steel ball-bearing sippers (Ancare Corp., Bellmore, NY). Centrifuge tubes were securely held through the wire mesh cage lid and presented to mice 3 hours into the dark cycle. Bottles were weighed to the nearest hundredth of a gram 24 hours after the fluids were given. To control for spillage due to experimenter handling or evaporation, weekly “drip” averages (loss of fluid in a cage with no animal present) were subtracted from individual fluid intakes. Mice were weighed to the nearest tenth of a gram before every ethanol drinking session to calculate the grams of ethanol intake per kilogram of body weight.
Experiment 1: Intermittent-Access 20% Ethanol 2-Bottle Choice Drinking
Male (n = 15) and female (n = 12) mice were given intermittent access to 20% ethanol without sweeteners in a recently revived drinking paradigm for outbred rats (Simms et al., 2008), originally established by Wise (1973). A second cohort of male (n = 12) mice were given intermittent access to be contemporaneously matched with the female cohort. On the first Monday, Wednesday, and Friday, mice were given 3%, 6%, and 10% (w/v) ethanol solutions in one centrifuge tube and water in a second centrifuge tube to fade in increasing ethanol concentrations. For the remainder of the experiments, mice received one bottle of 20% ethanol and one bottle of water every Monday, Wednesday, and Friday for 24 hours. At the same hour the following day, bottles were removed, weighed, and the ethanol solution in one bottle was thoroughly washed and replaced by water. The two water bottles remained in place until the next alcohol drinking session. The placement of bottles was alternated before each ethanol drinking session to avoid side preferences.
Another group of male mice (n = 12) were given continuous access to 20% ethanol without sweeteners. The first week of alcohol drinking, mice were given 3% ethanol solution and water Monday and Tuesday, 6% ethanol solution and water Wednesday and Thursday, and 10% ethanol solution and water Friday, Saturday, and Sunday. For the duration of the experiment, mice received one bottle of 20% ethanol and one bottle of water 24-hours, 7 days a week. Bottles were weighed at the same time as they were for mice with intermittent access, and the placement of bottles were switched daily.
Ethanol Withdrawal Assessments
Handling-Induced Convulsion (HIC)
After 4 weeks, or 16 weeks, of intermittent access or 4 weeks of continuous access to ethanol, male mice were tested for alcohol dependence by assessing physical reactions to ethanol withdrawal (Goldstein and Pal, 1971). The scoring system for convulsions elicited by lifting a mouse by the tail, or HIC, are described by Goldstein (1972) on a 0–4 scale [0 = no withdrawal signs; 1 = tonic convulsion when the mouse is lifted and given a gentle 180° turn; 2 = tonic-clonic convulsion elicited by the gentle spin, or tonic convulsion when lifted without turning; 3 = tonic-clonic convulsion not requiring any spin; 4 = violent tonic-clonic convulsion, often continuing after release of the mouse]. Ethanol bottles were removed at the end of the 24-hr exposure, which was 3 hours into the dark cycle (10 AM). Observations by two experimenters were made every 2 hours after the ethanol bottle had been withdrawn for 10 hours. Withdrawal severity was assessed throughout the remainder of the dark cycle and one hour into the light cycle (8 PM).
Experiment 2: Limited Access “binge” drinking vs. Intermittent Access
Prior to the first binge drinking test all animals received exposure to ethanol drinking in a 24 hour 2 bottle choice test (Belknap et al., 1993; Yoneyama et al., 2008). Male mice were offered two bottles with either water or ethanol (3, 6, 10, 20% w/v) in ascending order on consecutive days for 24 hours, 3 hours in to the dark phase (10 AM). Once mice consumed stably (less than 15% variability across 3 ethanol sessions) 20% ethanol, male mice (n = 12) were given 1 bottle of water or ethanol (20% w/v) for 2 hours, 3 hours into the dark phase (10 AM) of the light cycle, based on previous research (Rhodes et al., 2005). On test days 1 and 3, mice were given 1 bottle of water for 2 hours. On test days 2 and 4, mice were given 1 bottle of 20% ethanol for 2 hours. Water (ml) or ethanol (ml) consumption after the 2-hour test session was averaged for each individual over the 2 test days. Drinking bottles were removed at the same time and weighed daily. Fresh 20% ethanol solution was prepared daily, and all mice were weighed before replacing drinking bottles. Placement of water and ethanol bottles (left or right side of cage lid) was counterbalanced across test days. No blood samples were taken.
In order to make comparisons to short term “binge” drinking, male mice that had undergone the dependence-inducing intermittent access procedure for 4 weeks were tested for their initial 2-hr and 4-hr drinking behavior. Although mice have 24-hr total access to 2-bottle choice ethanol and water, we sought to explore the initial drinking during the first few hours of alcohol access after a 24-hr deprivation. It would also be useful to establish 2-hr and 4-hr fluid intake measurements for comparisons against intake behavior influenced by short-acting pharmacological manipulations. For these additional measurements, male mice given intermittent access (n = 15) and continuous access (n = 12) for 4 weeks were used from Experiment 1. Fluid intake (ml), ethanol drinking (g/kg), and ethanol preference were measured after the initial 2-hour access period (12 PM) and 4-hour access period (2 PM) to 20% ethanol and water during an alcohol session. Mice given continuous access to alcohol had intake measurements occur 2 hours and 4 hours after bottle weighing, which were contemporaneous with the intermittent access mice, at 12 PM and 2 PM.
Blood Ethanol Concentration Analysis
When male mice from the previous experiments had maintained a stable baseline in the intermittent access or continuous access procedure, blood samples were collected from the submandibular vein 2 hours after ethanol fluid presentation. For all animals, blood samples were collected and then centrifuged at 4°C for 10 min at 3000 r.p.m. Blood ethanol concentrations (mg/dl) were assessed with an ethanol assay kit using NAD-ADH reagent (Genzyme Diagnostics, Charlottetown, Canada) and absorbance was analyzed with a SmartSpec 3000 spectrophotometer (Bio-Rad, Hercules, CA).
Statistical Analysis
Statistical analyses were performed using SigmaStat version 3.1 (Systat Software, San Jose, CA). Ethanol intake (g/kg), body weight (g), volume of ethanol intake (ml), water intake (ml), total fluid intake (ml), and ethanol preference (%) during the increasing ethanol concentrations between intermittent and continuous access groups were analyzed with multiple two-way analyses of variance (ANOVA), followed by Bonferroni post hoc analysis when significant group effects were found (p < 0.05). Single daily values from the intermittent group were compared to the mean of the 2 or 3 days of intake from the continuous group. For the maintenance phase at 20% ethanol, ethanol intake (g/kg), body weight (g), volume of ethanol intake (ml), water intake (ml), total fluid intake (ml), and ethanol preference (%) were analyzed between intermittent and continuous access groups with multiple one-way ANOVAs. 3-week average intakes for individual mice in the intermittent group (9 ethanol sessions) were compared to 3-week average intakes for individual mice in the continuous group (21 ethanol sessions). For comparing male versus female consumption, additional two-way ANOVAs were performed for the acquisition phase on similar dependent variables previously mentioned, and also one-way ANOVAs were used to analyze sex differences in the 20% ethanol maintenance phase in intermittent access procedure.
Group differences for HIC scores were analyzed with a Kruskall-Wallis One-Way ANOVA by Ranks. The Kruskall-Wallis test was followed by Mann-Whitney rank sum tests when significant group differences for HIC scores were found. Graded HIC scores were interpreted as ordinal data, so data are reported in median values and interquartile ranges for each 2-hour assessment. The trapezoidal method was used to calculate area-under-the-curve for withdrawal severity over time.
For 2-hour and 4-hour ethanol consumption, a one-way ANOVA was performed to compare limited access binge ethanol intake (g/kg/2h) to continuous access ethanol intake (g/kg) and intermittent access ethanol intake (g/kg) during the same time period, followed by post hoc Bonferroni t-tests. Volume of ethanol intake (ml), water intake (ml), total fluid intake (ml), and ethanol preference (%) between groups during the 2-hour and 4-hour access period were also analyzed with additional one-way ANOVAs. Descriptive statistics for all measurements, except for HIC scores, are reported as mean plus or minus standard error of the mean (M ± SEM).
Results
Experiment 1: Intermittent Access to 20% ethanol
Acquisition and Maintenance
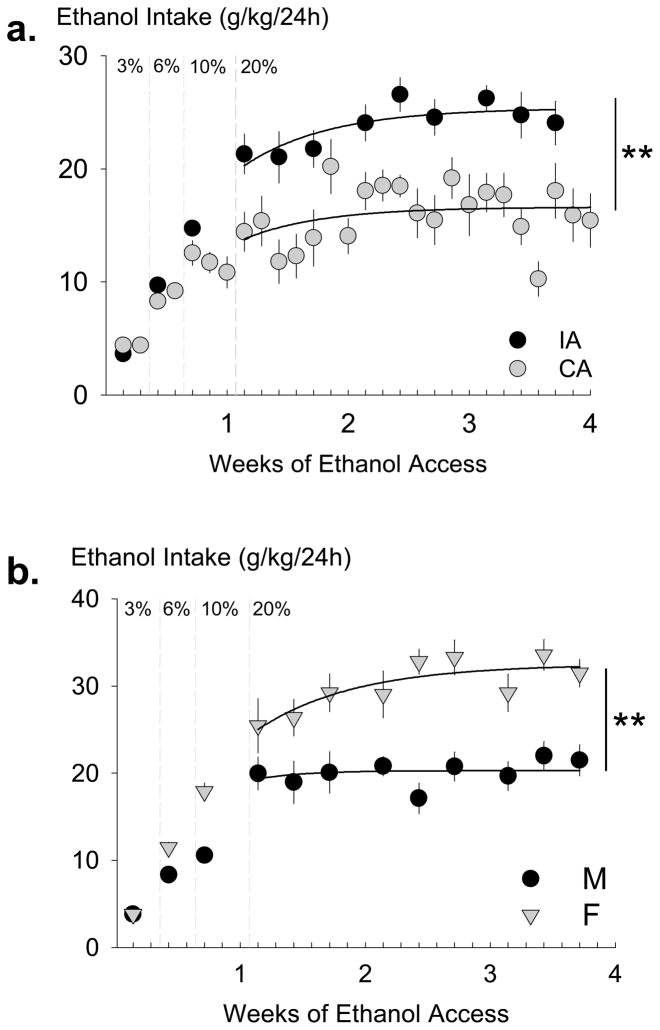
Adult male B6 mice were given either intermittent (n = 15) or continuous (n = 12) 2-bottle choice to increasing concentrations of ethanol and water, which induced high ethanol drinking behavior (Fig. 1A). Initially, a two-way ANOVA revealed no group differences to ethanol intake (g/kg) while animals had intermittent or continuous 24-hour access to 3%, 6%, and 10% ethanol. There were significant increases in ethanol intake between ethanol concentrations [F(1,2) = 23.91, p < 0.05] during the 3%, 6%, 10% acclimation period. After one week of 3%, 6%, 10% (w/v) ethanol and water 2-bottle choice, mice were given access to 20% ethanol which was maintained for 3 additional weeks. Mice with 24-hour intermittent access to 20% ethanol intake stably consumed an average 23.82 ± 0.68 g/kg [mean (M) ± standard error of the mean (SEM)] across the 3-week period. In contrast, mean 24-hour ethanol intake was 15.95 ± 0.56 g/kg for the continuous access group. A one-way ANOVA revealed a significant group difference [F(1,25) = 8.65, p < 0.01] for the 20% ethanol maintenance period.
Fig. 1.
Ethanol intake (g/kg) over 24 hours for male C57BL/6J mice given continuous access (CA, gray circles, n = 12) or intermittent access (IA, black circles, n = 15) to alcohol (Panel A). Ethanol concentrations were faded from 3%, 6%, 10% ethanol to be maintained on 20% ethanol for the remainder of the experiment. Mice receiving alcohol intermittently had significantly higher alcohol consumption during 3-week maintenance phase on 20% ethanol. Data are mean intake ± SEM. ** p < 0.01 difference between groups. Ethanol intake (g/kg) over 24 hours for male (M, black circles, n = 12) and female (F, gray triangles, n = 12) C57BL/6J mice with intermittent access to alcohol (Panel B). Ethanol concentrations were faded from 3%, 6%, 10% ethanol to be maintained on 20% ethanol for the remainder of the experiment. Female mice had significantly higher alcohol consumption during 3-week maintenance phase on 20% ethanol. Data are mean intake ± SEM. ** p < 0.01 difference between groups.
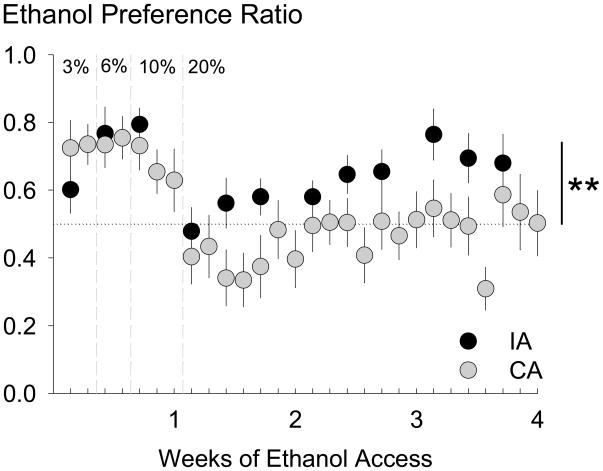
The acquisition and maintenance of ethanol preference is shown in Fig. 2. Preference for alcohol was calculated for ethanol compared to water, with the formula being volume of ethanol intake (ml) divided by total volume fluid intake (ml). A two-way ANOVA (alcohol concentration × alcohol access group) was performed to analyze initial acclimation to alcohol with increasing concentrations. There were no preference differences between 3%, 6%, or 10% ethanol concentrations or group differences between mice in different alcohol access groups. After the initial acclimation to alcohol, a one-way ANOVA found significant group differences for male B6 mice given 20% ethanol versus water [F(1,25) = 4.84, p < 0.05), where mice given intermittent access showed higher alcohol preference [0.63 ± 0.04] than mice given continuous access [0.46 ± 0.07].
Fig. 2.
Preference ratios were calculated for male C57BL/6J mice by dividing the amount of ethanol solution consumed by the total volume of fluid consumed over 24 hours on alcohol-drinking days. Mice given intermittent access (IA, black circles, n = 15) had a significantly higher preference for 20% ethanol than mice given continuous access (CA, gray circles, n = 12). Data are mean ethanol preference ± SEM. ** p < 0.01 difference between groups.
Body weight, volume of ethanol intake (ml), water intake (ml), and total fluid intake (ml) were monitored during the acquisition phase through the 3%, 6%, 10% ethanol days and during the maintenance phase with the 20% concentration (Table 1). Continuous access males (n = 12; Table 1) were compared to the later described group of intermittent males (n = 12) contemporaneous with the group of females (n = 12). Again, for the 3-week maintenance phase with 20% ethanol, individual averages across the ethanol sessions (9 sessions for intermittent mice, 21 sessions for continuous mice) were compared between groups. Reflecting differences in ethanol consumption in grams per kilogram, 24-hour ethanol intake volume (ml) was also significantly different between continuous access males and intermittent access males for the 10% concentration [F(1, 22) = 10.23, p < 0.01] and the 20% concentration [F(1,22) = 20.91, p < 0.001]. During the 20% ethanol maintenance phase, body weight (g), water intake (ml), and total fluid intake (ml) did not show any significant group differences.
Table 1.
C57BL/6J fluid consumption when given differential access to alcohol
| Sessions (days) | BW (g) | EtOH (mL) | H2O (mL) | Total (mL) | |
|---|---|---|---|---|---|
| 3%
|
|||||
| Continuous Male | 2 | 24.2±0.2 | 3.55±0.23 | 1.45±0.29 | 4.99±0.16 |
| Intermittent Male | 1 | 24.6±0.4 | 2.97±0.36 | 1.98±0.36 | 4.95±0.21 |
| Intermittent Female | 1 | 22.0±0.8* | 2.76±0.33 | 1.92±0.48 | 4.68±0.24 |
| 6%
|
|||||
| Continuous Male | 2 | 24.5±0.2 | 3.52±0.20 | 1.35±0.28 | 4.87±0.16 |
| Intermittent Male | 1 | 24.6±0.3 | 3.63±0.37 | 1.15±0.39 | 4.77±0.18 |
| Intermittent Female | 1 | 21.8±0.8* | 4.17±0.27 | 0.90±0.31 | 5.07±0.21 |
| 10%
|
|||||
| Continuous Male | 3 | 24.6±0.2 | 2.86±0.22 | 1.63±0.24 | 4.48±0.13 |
| Intermittent Male | 1 | 24.8±0.3 | 3.65±0.12† | 1.08±0.29 | 4.74±0.22 |
| Intermittent Female | 1 | 22.1±0.8* | 3.91±0.22 | 1.11±0.22 | 5.02±0.30 |
| 20%
|
|||||
| Continuous Male | 21 | 25.7±0.4 | 1.99±0.16 | 2.95±0.46 | 4.93±0.23 |
| Intermittent Male | 9 | 26.0±0.3 | 3.03±0.16† | 2.22±0.31 | 5.20±0.32 |
| Intermittent Female | 9 | 22.7±0.7* | 3.37±0.10* | 1.90±0.10* | 5.25±0.10 |
Several elements of 24-hour C57BL/6J drinking behavior were assessed during the one-week acclimation phase using 3%, 6%, and 10% ethanol vs. water and the 3 weeks of maintenance with 20% ethanol vs. water for males given continuous access to alcohol (n=12), males given intermittent access to alcohol (n=12), and females (n=12) given intermittent access to alcohol. Measurements are mean ± standard error of the mean. Body weight [BW] in grams (g), volume of ethanol consumption [EtOH] in milliliters (mL), volume of water consumption [H2O] in milliliters (mL), and total volume of fluid intake [Total] in milliliters (mL) were recorded.
p <.01 compared to continuous access males.
p <.01 compared to intermittent access males.
A group of female B6 mice (n = 12) were given the same schedule of intermittent access as another contemporaneous group of male B6 mice (n = 12) (Fig. 1B). There were no significant differences between intermittent access male (n = 15) groups tested at different times (n = 12). Two-way ANOVAs (ethanol concentration × sex) were used to compare sex differences between ethanol intake (g/kg) and preference during the acclimation phase to rising concentrations of ethanol. During the acclimation phase, there was a significant effect of sex on ethanol intake [F(1,65) = 16.44; p < 0.001] and a significant effect of concentration on ethanol intake [F(2,65) = 81.50; p < 0.001]. There was no significant interaction. Post hoc tests revealed that female mice given intermittent access consumed more ethanol at each rising concentration than male mice given intermittent access, and both males and females consumed more ethanol as the ethanol concentration increased. During the 3-week maintenance phase with intermittent access to 20% ethanol and water, the contemporaneous group of male B6 mice (n = 12) consumed 20.10 ± 0.48 g/kg/24h 20% ethanol and the group of female B6 mice (n = 12) consumed 32.13 ± 1.45 g/kg/24h, roughly fifty percent more (Fig. 1B). A one-way ANOVA showed that this sex difference in 24-hour ethanol consumption (g/kg) was statistically significant [F(1,22) = 62.03, p < 0.001].
An additional two-way ANOVA was performed to analyze ethanol preference across rising concentrations, 3%, 6%, 10% ethanol, and sex. Male and female mice given intermittent access showed no significant differences for preference across the acclimation phase. Also, female mice did not significantly differ from male mice in ethanol preference across the 20% ethanol maintenance period.
Also shown in Table 1, significant group differences in body weight (g) between intermittent access males and intermittent access females existed at all ethanol concentrations [3% ethanol F(1,22) = 9.60, p < 0.01; 6% ethanol F(1,22) = 11.47, p < 0.01; 10% ethanol F(1,22) = 11.27, p < 0.01; 20% ethanol F(1,22) = 23.13, p < 0.001]. On average, females also consumed higher volumes of 20% ethanol (ml) [F(1,22) = 4.89, p < 0.05] and showed significantly lower water intake than the intermittent access males during the 20% ethanol maintenance phase [F(1,22) = 4.87, p < 0.05]. All other measurements between intermittent access males and intermittent access females were not statistically different.
Ethanol Withdrawal Assessments
Handling-induced Convulsion (HIC)
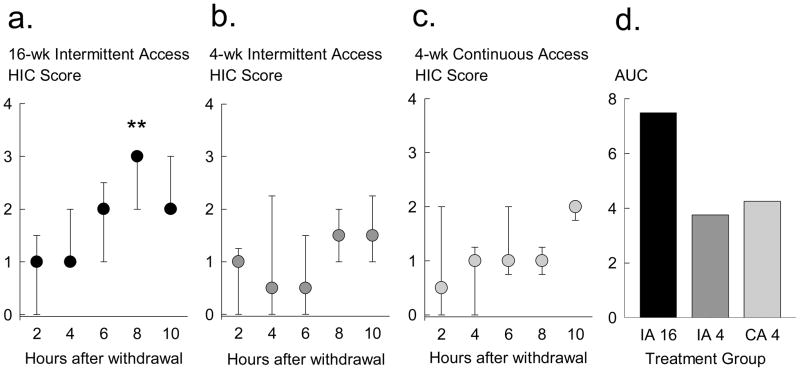
Severity of ethanol withdrawal was judged every 2 hours using the HIC rating scale described by Goldstein (1972). Of the total sample size for intermittent access mice (n = 15), nine mice were randomly selected to continue with intermittent alcohol access for an additional 12 weeks before HIC ratings, while six mice were assessed for HIC ratings after 4 weeks. Of the total sample size for continuous access mice (n = 12), we were only able to perform HIC scores for 6 mice. The other six continuous access mice were used for other experiments. All mice that underwent HIC assessments showed stable ethanol intake over 3 ethanol sessions before they were deprived of ethanol. Median HIC scores (ordinal scale 0–4) increased over time (Fig. 3A, B, C), with 8–10 hours into ethanol withdrawal having the highest HIC scores from the three experimental groups: intermittent access for 16 weeks (IA 16; n = 9; Fig. 3A), intermittent access for 4 weeks (IA 4; n = 6; Fig. 3B), and continuous access for 4 weeks (CA 4; n = 6; Fig. 3C). A Kruskall-Wallis One-Way ANOVA by Ranks indicated significant differences between median 8-hour HIC scores of the groups [H = 13.64, p < 0.01]. Mann-Whitney rank sum t-tests revealed that the intermittent access for 16 weeks group exhibited greater 8-hour HIC reactions than both the intermittent access for 4 weeks group [T = 25.5, p < 0.01] and the continuous access for 4 weeks group [T = 22.5, p < 0.01]. Mice that were given intermittent access for 16 weeks displayed the highest median of graded withdrawal reactions; a score of 3 indicates a tonic-clonic convulsion not requiring any spin (Goldstein, 1972). As indicated by the interquartile range (Fig. 3A), most mice in this group displayed a HIC score of 3. Median HIC scores over time were also expressed as area-under-the-curve (AUC) between treatment groups (Fig. 3D), which additionally revealed marked group differences.
Fig. 3.
Handling-induced convulsion (HIC) ratings of withdrawal severity (Goldstein, 1972) were measured every two hours after ethanol withdrawal for male C57BL/6J mice given intermittent access for 16 weeks (black, n = 9; Panel A), mice given intermittent access for four weeks (dark gray, n = 6; Panel B), and mice given continuous access for four weeks (light gray, n = 6; Panel C). In Panels A, B, and C, median HIC scores are shown with interquartile ranges for each 2-hour measurement after ethanol was removed. ** p < 0.01 difference between groups.
Panel D compares the area-under-the-curve (AUC) of HIC scores over time between 16-week intermittent access (IA 16, black), 4-week intermittent access (IA 4, dark gray), and 4-week continuous access (CA 4, light gray) groups.
Experiment 2: Limited Access “binge” drinking vs. Intermittent Access
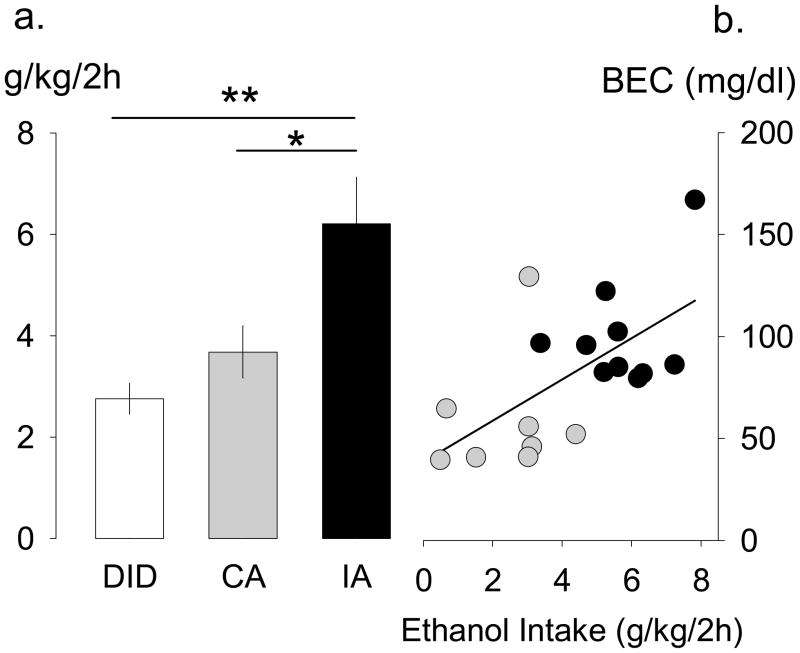
A separate group of adult male B6 mice (n = 12) underwent the 2-hour limited access binge drinking procedure, adapted from DID. B6 males given intermittent access (n = 15) and continuous access (n = 11) for 4 weeks were used from the previous experiment, and we recorded drinking during the initial 2 hour access period to 20% ethanol and water during an alcohol session. One male in the continuous access group was excluded from analyses due to significant spillage on test day. Ethanol consumption (g/kg/2h) is displayed in Fig. 4A, but volume of ethanol intake (ml), water intake (ml), total fluid intake (ml), and ethanol preference (%) were also measured. Since one bottle was presented to limited access mice during test sessions, no preference measures are available. A one-way ANOVA indicated significant group differences for 2-hour ethanol intake (g/kg) [F(2,35)=7.01, p < 0.01], and post hoc Bonferroni t-tests revealed that mice given intermittent access drank more ethanol in 2 hours than both the mice given continuous access [t = 2.55, p < 0.05] and mice in the limited access binge drinking procedure [t = 3.58, p < 0.01]. Average 2-hour ethanol intakes for B6 mice given different schedules were 6.21 ± 0.92 g/kg/2h given intermittent access, 3.68 ± 0.51 g/kg/2h given continuous access, and 2.75 ± 0.31 g/kg/2h given the limited access binge drinking procedure.
Fig. 4.
Panel A compares 2-hour 20% ethanol intake (g/kg) for male C57BL/6J mice in the limited access “binge” drinking procedure (DID, white, n = 12), continuous access (CA, light gray, n = 11), and intermittent access (IA, black, n = 15) procedures. IA intake (g/kg) was significantly higher than both DID and CA intake. Data are mean ± SEM. * p < 0.05, ** p < 0.01 difference between groups. Panel B shows the correlation between ethanol intake (g/kg/2h) and blood ethanol concentrations (BEC) (mg/dl) after 2-hour access period for a subset of individuals in the IA (black circles, n = 10) and CA (light gray, n = 8) schedules of ethanol presentation.
Further one-way ANOVAs were performed for the other measures during the 2-hour consumption period (not shown). Similar to ethanol intake (g/kg), volume of 20% ethanol (ml) also was significantly different between groups [F(2,35) = 10.13, p < .001], where mice given intermittent access consumed greater volume of ethanol (ml) than mice given continuous access [t = 2.99, p < .05] and mice in the binge drinking paradigm [t = 4.33, p < .001]. Group differences in ethanol preference during the 2-hour access period showed a non-significant trend for higher ethanol preference in mice given intermittent access [0.68 ± 0.08] than for mice given continuous access [0.54 ± 0.10]. Since one bottle was offered at a time to limited access binge drinking mice during test sessions, neither total fluid nor preference ratio were analyzed. Unlike the significant difference in ethanol drinking, water consumption (ml) and total fluid consumption (ml) were not significantly different between groups.
BEC was analyzed for a set of individuals in the intermittent access (n = 10) and continuous access (n = 8) procedures after 2 hours access to alcohol (Fig. 4B). Mice that did not show reliable ethanol intake on the test day (n = 2, continuous group) or did not have clear plasma samples after centrifugation (n = 5, intermittent group; n =2, continuous group) were excluded from BEC assessment. Mice that consumed increasing quantities of alcohol showed matching BECs (mg/dl). BECs for continuous access mice ranged from 39.49–129.24 mg/dl, and intermittent access mice ranged from 79.58–166.93 mg/dl.
Four-hour cumulative drinking was also assessed for intermittent access (n = 15) and continuous access (n = 11) mice after 4 weeks of stable ethanol drinking (not shown). During the 4-hour access period, ethanol consumption (g/kg) was not statistically different between the two groups, although volume of ethanol consumed (ml) was significantly different [F(1,24) = 9.70, p < 0.01]. Although ethanol preference values in the 2-hour access period for intermittent [0.68 ± 0.08] and continuous access animals [0.54 ± 0.10] seemed to be conserved in the 4-hour access period for intermittent [0.70 ± 0.10] and continuous [0.50 ± 0.08] access groups, statistical analysis revealed no significant group differences in ethanol preference for the 4-hour access period. Additional one-way ANOVAs showed that neither water consumption (ml) nor total fluid consumption (ml) were different between ethanol access groups during the 4-hour period.
Discussion
Male B6 mice given intermittent access to 20% ethanol and water will voluntarily and preferentially consume significantly higher quantities of alcohol than male B6 mice given continuous access to alcohol. In less than 4 weeks, this procedure with alternating daily limited access and deprivation can induce a reliable and steady escalation of per os alcohol intake; male B6 drink more than 20 g/kg/24h and female B6 drink ca. 30 g/kg/24h. Under these conditions, significant signs of dependence begin to emerge. Also, mice that drink escalated amounts of alcohol in the intermittent access procedure consumed more alcohol in the first two hours than mice in the limited access binge drinking procedure, plus showing pharmacologically relevant BECs greater than 100 mg/dl.
The results of the present experiment complement previous studies that generated high ethanol consumption with the same intermittent access 20% ethanol paradigm in rats (Simms et al., 2008; Wise, 1973). Since the 1970s, intermittent presentations of alcohol have also been shown to substantially elevate 2-bottle choice alcohol drinking in rats on a 24-hour basis (Pinel and Huang, 1976; Wayner and Greenberg, 1972; Wise, 1973). The current study, like Simms and colleagues (Simms et al., 2008; Simms et al., 2010), revives a protocol that demonstrated that intermittent access to 20% ethanol induced high ethanol intake without the need of sucrose fading (Samson, 1986) or water deprivation (Meisch and Thompson, 1972). The present results also complement, and exceed, the escalated drinking seen in a protocol using every other day (EOD) 15% ethanol drinking in B6 mice recently reported by Melendez (2011).
The basic principle of intermittent access has been implemented in various protocols that ultimately lead to elevated ethanol drinking (Tomie et al., 2005; Tomie et al., 2006; Melendez, 2011). From this perspective, one can also consider the alcohol deprivation effect (ADE), where periods of ethanol access alternate with periods of deprivation in weekly cycles (Melendez et al., 2006; Rodd-Henricks et al., 2000; Sinclair, 1972). Relapse-like drinking has been studied in the expression of ADE (Breese et al., 2005; Heyser et al., 1997; Sanchis-Segura and Spanagel, 2006), which is measured by a pronounced increase in ethanol preference and consumption after imposed abstinence. So far, methods investigating the ADE with 2-bottle choice testing have not consistently shown to produce dependence, perhaps due to the increased deprivation length (Melendez et al., 2006). Though the ethanol drinking after the ADE and other intermittent protocols (O’Dell et al., 2006) is substantially escalated, the current procedure has the potential to lead to ethanol dependence without forced vapor exposure (Becker and Lopez, 2004; Schulteis et al., 1995) that incorporates free-choice drinking.
In the present experiment, some animals given intermittent access to alcohol exhibited high withdrawal scores, indicative of dependence. Similar to alcoholic patients who show withdrawal symptoms as severe as insomnia, nausea, autonomic hyperactivity, and even tonic-clonic seizures (Cornish et al., 2001; Hillbom et al., 2003), ethanol-dependent animals display enhanced anxiety-like behaviors, handling-induced convulsions (Goldstein and Pal, 1971) and heightened startle responses (Macey et al., 1996) during acute and protracted withdrawal (Baldwin et al., 1991; Kliethermes et al., 2004; Overstreet et al., 2002; Valdez et al., 2002). Chronic intermittent ethanol (CIE) treatment via gavage has also resulted in increased seizure severity that has been attributed to a kindling-like process (Gonzalez et al., 2001). Altogether, the precise nature of whether excessive drinking observed in the current intermittent access procedure induces dependence has yet to be characterized more comprehensively.
We hypothesize that the repeated cycling of high ethanol consumption followed by deprivation induces persistent neuroadaptations. Previous research implicates neuropeptides corticotropin-releasing factor (CRF) and neuropeptide Y as prominent candidates for the modulation of dependent, intermittent drinking behavior (Heilig and Koob, 2007; Koob et al., 1993; Rasmussen et al., 2000; Rivier et al., 1984). In our ongoing investigations of central CRF during intermittent access drinking in B6 mice and Long-Evans rats, we have found that antagonism of the CRF type-1 receptor leads to selective suppression of alcohol drinking behavior (Hwa et al., 2010). Since CRF receptor antagonists also alleviate anxiogenic withdrawal symptoms in dependent rats (Breese et al., 2004; Knapp et al., 2004; Overstreet et al., 2004a; Rassnick et al., 1993), CRF may be a likely contributor to the emergence of excessive alcohol consumption seen after intermittent access and consequently interact with dopamine and serotonin in the ventral tegmental area (Gatto et al., 1994; Rodd et al., 2004) and also the dorsal raphé nucleus (Kirby et al., 2000; Valentino et al., 2010).
The present experiment demonstrated that female C57BL/6J mice achieve a significantly higher average ethanol intake value (g/kg) of 20% ethanol than male mice. These findings confirm previously reported high ethanol intake in female B6 mice using a one-bottle paradigm or food or water deprivation (Middaugh et al., 1992; Middaugh et al., 1999) as well as continuous access in a two-bottle choice procedure (Yoneyama et al., 2008). Since the females showed similar volumes of ethanol consumption (ml) as the males, their higher alcohol intake (g/kg) appears to be due to their lower body weights. Additionally, there may be underlying physiological differences such as the higher water content in females and differences in metabolism (Crippens et al., 1999), or neurological differences in brain architecture and endocrine systems (Devaud et al., 2003; Witt, 2007). The variability among females remained low despite potential fluctuations in their estrous cycle. This suggests that the estrous cycle and associated hormones may play a lesser role in alcohol drinking in females, and the role of gonadal hormones in escalated ethanol drinking will be pursued in future experiments.
The current study extends the key principles of intermittent ethanol availability combined with the use of C57BL/6J mice that have been long-known for their high alcohol intake (Belknap et al., 1993; Elmer et al., 1987; McClearn and Rodgers, 1959; Rodgers, 1966; Yoneyama et al., 2008). The pairing of B6 mice and intermittent availability of high concentrations of ethanol for 24 hours has generated ethanol intake in the initial 2 hours that may exceed current short-term, limited access models for binge-like drinking leading to intoxication (Lowery et al., 2010; Rhodes et al., 2005; Sparta et al., 2008). We sought to adapt the DID procedure described by Rhodes et al., (2005) to include an acclimation phase to increasing concentrations of ethanol before the 4-day limited access procedure. Despite this change to the history of the limited access binge drinking mice, 2-hour ethanol consumption was similar to levels previously described, at around 2–3 g/kg/2h. BECs from the intermittent access and continuous access individuals remain lower than those seen in previous binge-drinking reports (Becker and Lopez, 2004; Finn et al., 2007), so ongoing projects are trying to clarify the time course of initial peak binge drinking during intermittent access.
Procedures mimicking binge-like drinking such as DID demonstrate the principle of intermittency on an hourly basis. However, we draw a crucial distinction in that the DID procedure, which is most commonly assessed over 4 days, represents non-dependent binge-like drinking. It is expected that chronic intermittent access should produce higher levels of binge-like drinking, due to potential mechanisms driven by dependence. In line with the kindling hypothesis of escalated alcohol intake (Ballenger and Post, 1978; Becker, 1998; Breese et al., 2010), the intermittent access protocol allows for longer periods of deprivation paired with longer episodes of high drinking, repeated for several weeks, which may contribute to the higher intake values during the initial 2-hour access period. The current dependence-producing intermittent access protocol extends the timeline of the short-term, limited access procedures, like DID, significantly. We show the intermittent access procedure induced mice to drink more ethanol in a 2-hour access period, in the presence of freely available water. These features constitute a strong binge-like drinking model that may be a step closer to approximating the human condition.
In conclusion, we have described a procedure that incorporates ethanol-preferring B6 mice within a schedule that allows frequent limited access to alcohol during the week, thereby generating excessive, preferential voluntary alcohol drinking. This procedure complements the intermittent access 20% ethanol drinking protocol using outbred rats, though the observed levels of alcohol drinking can be up to three times higher in the inbred mice. We submit this straightforward protocol for mimicking the escalation to alcoholic-like drinking.
Acknowledgments
This project was supported by an NIH research grant R01 AA013983 awarded to KAM. We would like to thank Monita Wong for her excellent methodological assistance and Sally McIver, Ph.D. from the Tufts Sackler School for Biological Sciences for her technical expertise in assaying blood ethanol concentrations.
Support received from NIH R01 AA013983 awarded to KAM.
References
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology. 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barros H, Miczek KA. Withdrawal from oral cocaine in rats:ultrasonic vocalizations and tactile startle. Psychopharmacology. 1996;125:379–384. doi: 10.1007/BF02246021. [DOI] [PubMed] [Google Scholar]
- Becker HC. Kindling in alcohol withdrawal. Alcohol Health Res World. 1998;22:25–33. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Persistence of a “subacute withdrawal syndrome” following chronic ethanol intake. Drug Alcohol Depend. 1979;4:353–357. doi: 10.1016/0376-8716(79)90019-x. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O’Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: A risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2010 doi: 10.016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JW, McNicholas LF, O’Brien CP. Treatment of substance-related disorders. In: Schatzberg AF, Nemeroff CB, editors. Essentials of clinical psychopharmacology. American Psychiatric Press; Washington (DC): 2001. pp. 519–538. [Google Scholar]
- Crippens D, White ML, George MA, Jaworski JN, Brunner LJ, Lancaster FE, Gonzales RA. Gender differences in blood levels, but not brain levels, of ethanol in rats. Alcohol Clin Exp Res. 1999;23:414–420. [PubMed] [Google Scholar]
- Davis M. Neurochemical modulation of sensory-motor reactivity: acoustic and tactile startle reflexes. Neurosci Biobehav Rev. 1980;4:241–263. doi: 10.1016/0149-7634(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Alele P, Ritu C. Sex differences in the central nervous system actions of ethanol. Crit Rev Neurobiol. 2003;15:41–59. doi: 10.1615/critrevneurobiol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- Dole VP, Ho A, Gentry RT. Toward an analogue of alcoholism in mice: criteria for recognition of pharmacologically motivated drinking. Proc Natl Acad Sci U S A. 1985;82:3469–3471. doi: 10.1073/pnas.82.10.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Meisch RA, George FR. Mouse strain differences in operant self-administration of ethanol. Behav Genet. 1987;17:439–451. doi: 10.1007/BF01073111. [DOI] [PubMed] [Google Scholar]
- Falk JL. Production of polydipsia in normal rats by an intermittent food schedule. Science. 1961;133:195–196. doi: 10.1126/science.133.3447.195. [DOI] [PubMed] [Google Scholar]
- Falk JL, Tang M. What schedule-induced polydipsia can tell us about alcoholism. Alcohol Clin Exp Res. 1988;12:577–585. doi: 10.1111/j.1530-0277.1988.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC. A procedure to produce high alcohol intake in mice. Psychopharmacology (Berl) 2005;178:471–480. doi: 10.1007/s00213-004-2039-8. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Alcohol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Goldstein DB. Relationship of alcohol dose to intensity of withdrawal signs in mice. J Pharmacol Exp Ther. 1972;180:203–215. [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Gonzalez LP, Veatch LM, Ticku MK, Becker HC. Alcohol withdrawal kindling: mechanisms and implications for treatment. Alcohol Clin Exp Res. 2001;25:197S–201S. doi: 10.1097/00000374-200105051-00032. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased ethanol self administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res. 1997;21:784–791. [PubMed] [Google Scholar]
- Hillbom M, Pieninkeroinen I, Leone M. Seizures in alcohol-dependent patients: epidemiology, pathophysiology and management. CNS Drugs. 2003;17:1013–1030. doi: 10.2165/00023210-200317140-00002. [DOI] [PubMed] [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9:41–48. [PubMed] [Google Scholar]
- Hwa LS, Quadros IM, DeBold JF, Miczek KA. Selective suppression of escalated alcohol intake in rats and mice with a CRF-R1 antagonist in VTA but not DRN. San Diego: Society for Neuroscience Online Meeting PlannerPoster; 2010. #365.19/EE11. [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- Le Magnen J. Study of some factors associated with modifications of spontaneous ingestion of ethyl alcohol by the rat. J Physiol (Paris) 1960;52:873–884. [PubMed] [Google Scholar]
- Lester D. Self-maintenance of intoxication in the rat. Q J Stud Alcohol. 1961;22:223–231. [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Jones DP, Mendelson J, DeCarli LM. Fatty liver, hyperlipemia and hyperuricemia produced by prolonged alcohol consumption, despite adequate dietary intake. T Assoc Am Physicians. 1963;76:289–300. [Google Scholar]
- Lopez MF, Becker HC. Gender differences in ethanol intake prior to and following repeated chronic ethanol exposure and withdrawal in C57BL/6J mice. Alcohol Clin Exp Res. 2003;27:15A. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AM, Lowery EG, Sparta DR, Thiele TE. Effects of food availability and administration of orexigenic and anorectic agents on elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:1962–1968. doi: 10.1111/j.1530-0277.2008.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: Effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Rodgers DA. Differences in alcohol preference among inbred strains of mice. Q J Stud Alcohol. 1959;20:691–695. [Google Scholar]
- Meisch RA. Relationship between physical dependence on ethanol and reinforcing properties of ethanol in animals. In: Cicero TJ, editor. Ethanol Tolerance and Dependence: Endocrinological Aspects. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1983. pp. 27–33. [Google Scholar]
- Meisch RA, Thompson T. Ethanol intake during schedule-induced polydipsia. Physiol Behav. 1972;8:471–475. doi: 10.1016/0031-9384(72)90331-9. [DOI] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (Every-Other-Day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:1–7. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Merlo-Pich E, Lorang M, Yeganeh M, Rodriguez DF, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh LD, Frackelton WF, Boggan WO, Onofrio A, Shepherd CL. Gender differences in the effects of ethanol on C57BL/6 mice. Alcohol. 1992;9:257–260. doi: 10.1016/0741-8329(92)90062-f. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy ALE, McGroarty KK. Ethanol consumption by C57BL/6 mice: Influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004a;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004b;78:459–464. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel JP, Huang E. Effects of periodic withdrawal on ethanol and saccharin selection in rats. Physiol Behav. 1976;16:693–698. doi: 10.1016/0031-9384(76)90238-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology. 1992;106:351–358. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of alcohol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000;24:747–753. [PubMed] [Google Scholar]
- Rodgers DA. Factors Underlying Differences in Alcohol Preference Among Inbred Strains of Mice. Psychosom Med. 1966;28:498–513. [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–1463. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD. The alcohol-deprivation effect. Influence of various factors. Q J Stud Alcohol. 1972;33:769–782. [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Gittleman J, Dranoff E, Pohorecky LA. Social interaction opportunity and intermittent presentations of ethanol sipper tube induce ethanol drinking in rats. Alcohol. 2005;35:43–55. doi: 10.1016/j.alcohol.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Tomie A, Miller WC, Dranoff E, Pohorecky LA. Intermittent presentations of ethanol sipper tube induce ethanol drinking in rats. Alcohol. 2006;41:225–230. doi: 10.1093/alcalc/agl002. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Br Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I. Effects of hypothalamic stimulation, acclimation and periodic withdrawal on ethanol consumption. Physiol Behav. 1972;9:737–740. doi: 10.1016/0031-9384(72)90043-1. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]