Abstract
Background
Among some alcohol-dependent individuals, early alcohol abstinence is marked by alcohol withdrawal (AW), a phenomenon mediated by GABA and glutamate signaling. We previously reported that a combination of two medications that affect GABA and glutamate tone, gabapentin and flumazenil, more effectively reduced drinking among individuals with higher pre-treatment AW (Anton et al., 2009). This study evaluated whether this finding is related to changes in neurocognitive performance, which is also affected by cortical GABA and glutamate tone.
Methods
Neurocognitive performance was assessed at baseline and twice during the first week of treatment among 60 alcohol-dependent participants in the previously published clinical trial.
Results
AW was associated with poorer baseline performance on 4 of 8 measures, and individuals with higher baseline AW who received the gabapentin and flumazenil combination demonstrated greater improvement on a measure of response inhibition than those with lower AW or those who received a combination of placebos. Improvement in response inhibition during the first week and medication group interacted in their effect on subsequent drinking, such that improvement predicted greater abstinence only among individuals who received gabapentin and flumazenil. Improvement on other neurocognitive measures was neither differentially impacted by medication or baseline AW nor related to subsequent drinking.
Conclusions
Taken together, these data suggest that acute AW accounts for a small proportion of variance in neurocognitive performance, that gabapentin and flumazenil slightly improve response inhibition during early abstinence, and that such improvement may somewhat reduce later drinking. However, these medications may not affect other neurocognitive domains.
Keywords: alcoholism, anticonvulsant, GABA, neurocognitive, withdrawal
Introduction
Unlike other psychiatric disorders, the process of behavior change in alcohol dependence often occurs rapidly after treatment instigation, with the initiation of alcohol abstinence (Miller et al., 2001). During early abstinence, some treatment-seeking alcoholics experience alcohol withdrawal (AW), the symptoms of which appear 6 to 48 hours after cessation of heavy alcohol use, are initially characterized by sympathetic hyperexcitability (e.g., anxiety, perspiration, tachycardia, nausea, insomnia), and, if untreated, can progress to hallucinations, delirium tremens (DTs), and seizure (Myrick & Anton, 1998). These symptoms result, in part, from the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems. Alcohol acutely enhances presynaptic GABA release (Criswell et al., 2008; Roberto et al., 2003) through allosteric modulation at GABAA receptors (Grobin et al., 1998) and inhibits glutamate function through antagonism of N-methyl-D-aspartate (NMDA) receptors (Woodward, 2000). Chronic alcohol exposure elicits compensatory down-regulated GABAA and up-regulated NMDA expression (Krystal et al., 2003; Kumari & Ticku, 2000), and causes GABAA receptors to shift their composition to contain more α4 subunits, a formation that is less responsive to GABA signaling (Biggio et al., 2007; Sanna et al., 2003) and is associated with increased withdrawal-induced anxiety (Smith et al., 1998). When alcohol intake abruptly ceases and its acute effects dissipate, the sudden reduction in GABAergic and increase in glutamatergic tone engender AW symptoms (Cagetti et al., 2003; Tsai et al., 1998).
Benzodiazepines (BZ), which bind at the BZ site on the GABAA receptor and, like alcohol, acutely enhance GABA and inhibit glutamate signaling, are the current standard of care for AW, as they reduce anxiety and prevent DTs and seizure (Mayo-Smith, 1997). However, concern has increased about these medications’ abuse potential, interactions with alcohol, and cognitive side effects (Myrick et al., 2009). Further, the development of BZ tolerance after high-dose administration may simply forestall withdrawal, as BZ withdrawal pharmacologically mimics AW. Hence, interest has grown in other medications that increase GABA or reduce glutamate signaling.
We previously reported that gabapentin, a GABA analogue that impedes excitatory calcium currents (Hendrich et al., 2008) and may reduce glial glutamate production (Bonnett et. al., 1999), effectively reduces AW symptoms (Myrick et. al., 2009). More recently, we evaluated a proprietary combination (the Prometa protocol; Hythiam, Inc, Los Angeles, CA) of gabapentin and flumazenil, an antagonist at the GABAA BZ site (Anton et al., 2009). This medication combination may affect GABA tone by shifting GABAA receptors to a subunit composition more amenable to GABA effects (i.e., one with fewer α4 subunits) while avoiding the development of BZ tolerance and subsequent withdrawal (Anton et al., 2009; Urschel et al., 2007). We found that gabapentin and flumazenil reduced AW symptoms and drinking, though the latter effect was present, as hypothesized, only in patients who had higher pre-treatment AW.
This finding suggested that gabapentin and flumazenil might differentially affect some aspect of alcoholism that is more dysregulated among individuals who experience AW. One such aspect is neurocognitive functioning. Chronic heavy drinkers display neurocognitive impairment while actively drinking, and impairment remains pronounced during early alcohol abstinence (Bates et al., 2002). Further, neurocognitive deficits seem greater among individuals who experience AW, though most studies have reported an association between current impairment and past detoxifications, rather than acute AW severity (Duka et al., 2003; Loeber et al., 2009). Alcoholics in early abstinence display particular impairment in neurocognitive domains critical to the ability to maintain abstinence, including executive functioning (complex, goal-oriented cognitive processing that requires the coordination of multiple cognitive sub-processes; Nöel et al., 2001; Zinn et al., 2004), response inhibition (the ability to restrain learned behaviors; Cordovil et al., 2010), and impulsivity (the tendency to choose immediate rewards over delayed ones; Mitchell et al., 2005). Those with more severe deficits in these domains are more likely to return to harmful drinking during and after treatment (Wicks et al., 2001; Guardia et al., 2007; Charney et al., 2010) and drop out of treatment (O’Leary et al., 1979; Teichner et al., 2002) and less likely to attain treatment goals (e.g., to learn relapse prevention skills; Teichner et al., 2001), perhaps because such deficits render them less able to conceptualize the complex process of behavior change (McCrady & Smith, 1986), inhibit conditioned associations with alcohol (Guardia et al., 2007), or resist impulsive decision-making when confronted with opportunities to drink (Feldstein Ewing et al., 2009).
Like AW, neurocognitive functioning is affected by GABA and glutamate signaling. GABAA and NMDA receptors are widely expressed throughout the brain, including on pyramidal cells in the prefrontal cortex (PFC) (Mehta & Ticku, 1999), the brain area broadly associated with executive functioning, response inhibition, and impulsivity (Robbins, 2007). PFC activity underlying cognition is driven by excitatory dopaminergic and glutamatergic activity that is regulated by GABA signaling (Tanaka, 2008); deficient GABAergic regulation of this activity is associated with the “hyperactive” neurocognitive deficits characteristic of patients with schizophrenia (Guidotti et al., 2005). Neuroimaging studies suggest that abstinent alcoholics display reduced GABAA receptor expression (Lingford-Hughes et al., 1998) and cortical GABA levels (Behar et al., 1999; Ke et al., 2004) and increased glutamate levels (Lee et al., 2007). Thus, medications that normalize GABA and glutamate tone could resolve alcohol-induced neurocognitive deficits associated with deficient cortical GABA and glutamate.
This study analyzed neurocognitive data collected during a previously published clinical trial of gabapentin and flumazenil (Anton et al., 2009). In light of our finding that this medication combination reduced drinking only among individuals with higher baseline AW, as well as previous reports of association between AW and neurocognitive deficits and the relationship between such deficits and treatment outcome, we hypothesized that 1) higher baseline AW would be associated with greater baseline neurocognitive impairment; 2) gabapentin and flumazenil, as compared to placebos, would improve executive functioning, response inhibition, and impulsivity relative to baseline, and would do so to a greater extent among individuals with higher baseline AW; and 3) individuals who demonstrated greater improvement in these domains would subsequently display improved drinking outcomes.
Materials and Methods
Participants
Participant characteristics and inclusion and exclusion criteria are fully described in Anton et al., 2009. Briefly, participants were 60 individuals recruited through media advertisements who met DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders, revised 4th edition) diagnostic criteria for Alcohol Dependence, as assessed by the Structured Clinical Interview for DSM-IV-TR (SCID; First et al., 2002). Significant inclusion criteria were 1) age 18–70; 2) a minimum of 5 drinks per day on 70% of days in the month before screening; and 3) last drink no more than 72 hours before drug randomization. Significant exclusion criteria were: 1) diagnosis, as assessed by SCID, of any other Axis I psychiatric disorder; 2) recent use of any substance except marijuana or nicotine; 3) history of seizures or DTs during AW (a safety precaution); and 4) use of any psychoactive medication in the previous two weeks.
Procedure
All procedures were approved by the Institutional Review Board for Human Research at the Medical University of South Carolina. Inclusion and exclusion criteria were initially assessed by phone, after which prospective participants were invited to the laboratory for in-person screening, where, before screening began, they provided informed consent. Eligible participants were scheduled for their first treatment appointment (day 1), and instructed to abstain from alcohol the night before the appointment. On day 1, participants were administered the Clinical Institute Withdrawal Assessment for Alcohol-Revised (CIWA-Ar; Sullivan et al., 1989), a clinician-administered rating scale for AW symptoms (range of measure: 0–67), and also completed the Alcohol Dependence Scale (ADS; Skinner & Horn, 1984) and a short (30-item) form of the Profile of Mood States (POMS; McNair et al., 1971). To ensure equal distribution of individuals with higher AW between medication groups, CIWA-Ar scores were subsequently used as an urn variable for medication randomization. Urn randomization assigns participants to treatment groups randomly, with the exception that the assignment is biased toward balance in the various covariate (urn variable) combinations (Stout et al., 1994). Participants with CIWA-Ar scores ≥ 7 were considered to have clinically significant AW (higher AW group), and were approximately evenly randomized to active medications vs. placebos, as were participants with CIWA-Ar scores < 7 (low AW group). As the medication combination assessed here is designed for outpatient administration (Urschel et al., 2007), the cut-off of 7 was chosen to reflect the level of AW severity typically seen in outpatient alcohol treatment settings, rather than the cut-off of 10 originally proposed (Sullivan et al., 1989).
Neurocognitive assessment
Neurocognitive assessment was first conducted before any medication was administered on day 1, and was repeated on days 3 and 7. Participants were required to have a breath alcohol concentration (BrAC) below 0.08% before assessment began. Nine participants had a BrAC between 0 and 0.08% on day 1 (mean = 0.04%); none had a positive BrAC on either subsequent assessment day. To control potential influences on neurocognitive performance, day 1 BrAC was covaried in the analyses of Hypotheses 1 and 2; it did not affect any of the results reported.
On days 1, 3, and 7, participants were administered a brief battery intended to assess executive functioning, response inhibition, and impulsivity, consisting of Parts A and B of the Trail Making Test (TMT; Reitan & Wolfson, 1993), the Stroop Color and Word Test (Stroop, 1935), and Conners’ Continuous Performance Test-II (CPT-II; Conners, 2000). For TMT Part A, which measures psychomotor processing speed, participants must draw, as quickly as possible, a continuous line in ascending order between randomly arranged numerical targets, while for Part B, which measures executive functioning, they must draw such a line in ascending numerical and alphabetical order while alternating between numbers and letters. The Stroop task requires participants to first name, as quickly as possible, the color of ink in which blocks of color are printed (color-naming), then to read the names of color words printed in black ink (word-reading), and finally, to name the color of ink in which an inconsistent color word is printed (interference trial; e.g., the word “RED” printed in blue ink). The color-naming and word-reading trials also assess processing speed, while the interference trial measures the ability to inhibit a prepotent response (i.e., naming the inconsistent color word). Finally, the CPT-II requires participants to respond as quickly as possible to target stimuli (letters of the alphabet except “X”), while refraining from responding to less frequent non-target stimuli (the letter “X”). High numbers of omission errors and long reaction times indicate inattention or slow psychomotor processing, while high numbers of commission errors and short reaction times indicate impulsivity. The TMT and Stroop were administered by a trained research assistant, and participants completed the CPT-II on a laptop computer. For each measure, T-scores were obtained by comparing raw scores to normative tables, which are adjusted, for the TMT, for age, gender, ethnicity, and education (Heaton et al., 2004); for the Stroop, for age and education (Golden & Freshwater, 2002); and for the CPT-II, for age (Conners, 2000).
Medications
The treatment procedure, including detailed time-courses of the flumazenil infusions and gabapentin titration and taper, is fully described in Anton et al., 2009. Briefly, participants received either a combination of flumazenil infusions on days 1 and 2 and oral gabapentin for 39 days, or two matched placebos. All participants also received six weekly manualized therapy sessions (combined behavioral intervention; Miller, 2004). On day 1, after neurocognitive assessment, participants were administered either flumazenil (2 mg) or sterile saline intravenously. Before leaving the laboratory, participants were provided with gabapentin (300 mg) or identically packaged placebo, and were instructed to take the medication orally at bedtime. Participants were also given seven 50 mg hydroxyzine (a mildly sedating antihistamine) pills to be taken for sleep as needed during the first week. Hydroxyzine intake was recorded, and was not significantly different between groups. Covarying the number of hydroxyzine pills taken did not affect the relationships between medication and AW groups and neurocognitive change (Hypothesis 2). Participants returned to the laboratory on day 2, and the flumazenil infusion procedure was repeated. Gabapentin was titrated to 1200 mg by day 4 (day 1: 300 mg; day 2: 600 mg; day 3: 900 mg), and was tapered from day 31 to day 39.
Urine drug screens were obtained on days 1 and 7 and during week 6; cut-offs were 50 ng/mL for marijuana, 300 ng/mL for cocaine, opiates, and benzodiazepines, and 1000 ng/mL for amphetamines. Quantitative metabolite levels were obtained for each sample that was above the cut-off for any substance. No participant was positive for cocaine, opiates, benzodiazepines, or amphetamines on day 1 or 7. Eight participants were positive for marijuana on day 1 and seven were positive on day 7; there were no significant differences between groups on this variable. Because individual clearance rates for Δ9-tetrahydrocannibinol (Δ9-THC) vary, it is difficult to ascertain when participants used marijuana relative to the testing days. To control for the potential influence of recent marijuana use, day 1 quantitative Δ9-THC levels were covaried for the analysis of Hypothesis 1, and day 1 and 7 levels for covaried for the analyses of Hypotheses 2 and 3; neither affected the results described below.
Statistical analysis
All analyses were performed with SPSS 17.0 (SPSS Institute, Chicago, IL); an alpha level of p = 0.05 was considered statistically significant. The relationship between baseline AW and baseline neurocognitive performance (Hypothesis 1) was analyzed using partial correlation. To increase statistical power, baseline CIWA-Ar score was used as a continuous variable for this analysis, rather than the categorical variable used for medication randomization. One-tailed partial correlation coefficients were calculated for the relationships between baseline CIWA-Ar score and baseline T-scores on TMT Parts A and B, the Stroop word-reading, color-naming, and interference trials, and the CPT-II Omissions, Commissions, and Hit Reaction Time (Hit RT) subscales, with other variables that might influence performance (years of alcohol dependence, history of other substance dependence, day 1 BrAc and Δ9-THC levels, and depression symptoms) covaried. The demographic variables not accounted for in the normative adjustments for each measure were also covaried: gender for the Stroop, and gender and years of education for the CPT-II. Years of alcohol dependence were calculated as the difference between each participant’s current age and the age at which he or she first met three DSM-IV-TR alcohol dependence criteria.
Effects of medication and baseline AW on changes in neurocognitive performance during the first week of treatment (Hypothesis 2) were analyzed in a series of linear mixed models (SPSS MIXED), with day of assessment as a repeated measure in each model, using a first-order autoregressive variance-covariance matrix. TMT Part B, Stroop interference, and CPT-II Commissions and Hit RT were the primary measures of interest for these analyses. A full factorial model that included between-participants factors for medication (active or placebo) and AW (low or higher) groups, a within-participants factor for day of assessment (day 3 or 7), and all interactions was used. Day 1 (i.e., baseline) performance on the measure of interest was included as a covariate in each model. To account for the potential effect of continued drinking during week 1 (i.e., slips or relapses), the total number of drinks consumed during this time was also used as a covariate in each model, as were the covariates used in the analysis of Hypothesis 1; none of these variables significantly affected the main effects or interactions reported.
Finally, the relationship between change in neurocognitive performance over the first week of treatment and subsequent drinking (Hypothesis 3) was analyzed in a series of linear mixed models in which percent days abstinent (PDA) during the treatment trial was the outcome variable and week in the study (week 1–6) was a repeated within participants factor. For these analyses, a first-order autoregressive variance/covariance matrix was again used. To best approximate the effect analyzed for Hypothesis 2 (i.e., neurocognitive change between days 3 and 7), change scores (i.e., day 7 T-score – day 3 T-score) for TMT Part B, Stroop interference, and CPT-II Commissions and Hit RT were created. For each of these measures, a factor that indicated whether participants were above or below the mean change score (TMT Part B mean change = 4.3; Stroop interference = 2.7; Commissions = 1.7; Hit RT = 0.6) was then entered as a predictor of PDA in a full factorial model that also included between-participants factors for AW and medication groups, a within-participants factor for week in the trial, and all interactions. PDA during the month before treatment (i.e., baseline abstinence) was used as a covariate in all analyses. Again, each model was run with the covariates previously used for the analyses of Hypotheses 1 and 2; none significantly affected the results reported below.
Results
Medication groups and pre-treatment demographics
Of the 60 participants, 16 had CIWA-Ar scores ≥ 7 (higher AW group; mean CIWA-Ar = 8.2, SD = 2.0), and 44 had scores < 7 (low AW group; mean CIWA-Ar = 2.3, SD = 1.9). Table 1 lists demographic characteristics, baseline drinking measures, years and severity of alcohol dependence (ADS), past other substance dependence, and POMS Depression subscale scores for each group. The only significant differences between groups were on the ADS, for which the higher AW group had higher scores than the low AW group (t(58) = 2.89, p < .01), and past other substance dependence, for which the low AW/active group had more participants with such dependence than the low AW/placebo group (χ2(1, N = 43) = 3.85, p = .05). It is unremarkable that the higher AW group had higher ADS scores, as this measure includes symptoms of AW (e.g., DTs, hallucinations, tachycardia).
Table 1.
| Low AW group | Higher AW group | ||||
|---|---|---|---|---|---|
| PLA | FMZ/GBP | PLA | FMZ/GBP | Test for difference | |
| N | 18 | 26 | 9 | 7 | |
| Age | 47.2 ± 11.2 | 44.1 ± 11.9 | 50.3 ± 8.5 | 46.7 ± 5.6 | Not significant |
| Gender N | |||||
| -Male | 14 | 20 | 6 | 6 | Not significant |
| -Female | 4 | 6 | 3 | 1 | Not significant |
| Education (years) | 14.9 ± 2.6 | 14.4 ± 2.2 | 15.0 ± 1.9 | 13.4 ± 2.5 | Not significant |
| Baseline drinking | |||||
| -Heavy drinking days, % | 91.1 ± 10.0 | 84.3 ± 16.0 | 90.2 ± 16.0 | 86.2 ± 22.0 | Not significant |
| -Days abstinent, % | 5.5 ± 5.9 | 6.4 ± 8.9 | 9.1 ± 16.0 | 12.9 ± 22.0 | Not significant |
|
Alcohol Dependence Scale (range: 0–47) |
12.4 ± 7.0 | 12.6 ± 5.8 | 21.2 ± 10.6 | 17.6 ± 6.9 | Higher AW > Low |
| Years of alcohol dependence | 11.1 ± 8.9 | 10.3 ± 8.4 | 16.3 ± 13.6 | 7.5 ± 8.4 | Not significant |
|
Past other substance dependence N |
1 | 8 | 2 | 2 | Low AW/FMZ-GBP > Low AW/PLA |
|
POMS Depression subscale (range: 0–20) |
6.3 ± 5.6 | 5.7 ± 5.0 | 10.0 ± 6.7 | 5.3 ± 5.0 | Not significant |
Demographic and baseline variables, by medication group within alcohol withdrawal (AW) group. All figures except Ns are means ± standard deviations.
FMZ/GBP = flumazenil/gabapentin; PLA = placebos; POMS = Profile of Mood States.
Hypothesis 1: Association between AW and neurocognitive performance
Baseline AW was significantly and negatively associated with performance on four measures, such that participants with higher AW had lower scores on TMT Parts A and B, Stroop color-naming, and CPT-II Omissions (positive correlations between AW and CPT-II Omissions and Commissions, on which lower T-scores indicate fewer errors, were predicted) (see Table 2). The mean effect size (Cohen’s d) for the relationship between AW and neurocognitive performance was .20, corresponding to a small to medium effect (Cohen, 1992). Of the covariates included, years of alcohol dependence had a significant bivariate correlation (one-tailed Pearson’s r) with Stroop color-naming, such that participants with more years of dependence had lower scores, and day 1 BrAC had a significant correlation with TMT Part A, such that participants with higher BrAC had lower scores.
Table 2.
| Measure subscale | Function measured | Mean baselineT- score (SD) |
Correlation with baseline AW |
|---|---|---|---|
| Trail Making Test | |||
| Part A | Processing speed | 43.4 (9.0) | r = −.31, p = .009 |
| Part B | Executive functioning | 49.7 (9.8) | r = −.24, p = .04 |
| Stroop Color-Naming Test | |||
| Word-reading | Processing speed | 43.1 (10.6) | r = −.14, p = .14 |
| Color-naming | Processing speed | 43.9 (10.5) | r = −.27, p = .02 |
| Interference | Response inhibition | 45.2 (8.1) | r = −.19, p = .08 |
| Conners’ Continuous Performance Test-II | |||
| Omission | Inattention | 45.9 (7.6) | r = .30, p = .01 |
| Commission | Impulsivity | 44.9 (8.3) | r = .04, ns |
| Hit reaction time | Impulsivity | 50.3 (11.3) | r = .07, ns |
Baseline neurocognitive scores and association (one-tailed partial correlations) with baseline AW severity.
SD = standard deviation.
Hypothesis 2: Effects of time, AW, and medication on neurocognitive performance
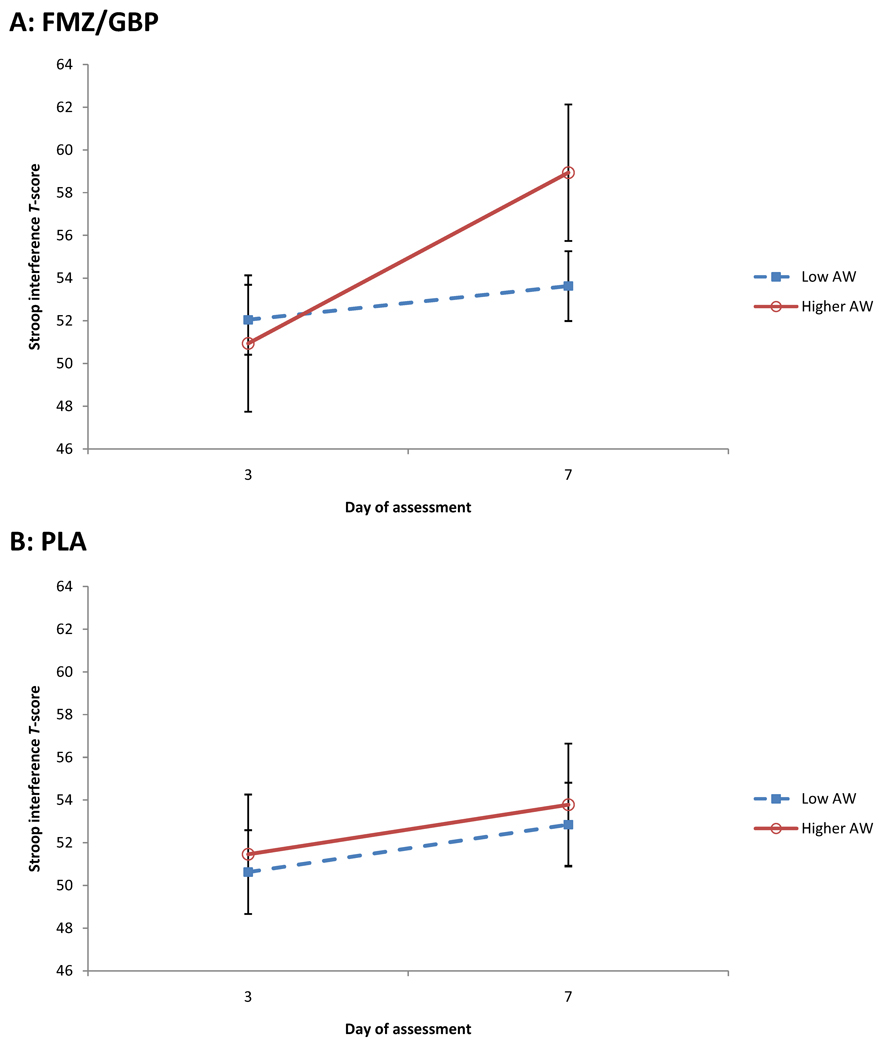
For the primary measures of interest, across all participants, there was a main effect of time on TMT Part B, Stroop interference, and the CPT-II Commissions subscale, such that T-scores improved between days 3 and 7 (see Tables 3 and 4), but no such effect for CPT-II Hit RT. For Stroop interference, there was a significant interaction between AW group and time (F(1, 56.04) = 4.51, p = .04), such that participants with higher AW were more improved between days 3 and 7; however, this effect must be interpreted in the context of the higher-level interaction between medication group, AW group, and time. For this interaction, as predicted, participants who received active medications and who had higher AW were more improved between days 3 and 7 relative to the other three groups (F(1, 56.04) = 3.95, p = .05) (see Figure 1). There were no significant interactions between time and AW or medication group for the other primary measures of interest. When included as a covariate, the number of drinks consumed during week 1 accounted for unique variance for some measures, but did not impact any of the effects reported above.
Table 3.
| Day 3 | Day 7 | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure subscale | PLA-Low | PLA-Higher | FG-Low | FG-Higher | PLA-Low | PLA-Higher | FG-Low | FG-Higher |
| Trail Making Test | ||||||||
| Part A# | 49.0 (1.8) | 48.8 (2.6) | 52.0 (1.5) | 49.5 (2.9) | 51.1 (1.8) | 54.7 (2.7) | 53.7 (1.5) | 57.7 (2.9) |
| Part B | 53.5 (2.1) | 51.5 (2.9) | 54.7 (1.7) | 48.8 (3.3) | 58.6 (2.1) | 55.6 (3.1) | 57.4 (1.7) | 57.3 (3.3) |
| Stroop Color-Naming Test | ||||||||
| Word-reading | 48.1 (1.5) | 49.5 (2.0) | 50.3 (1.2) | 48.5 (2.3) | 52.0 (1.5) | 53.4 (2.1) | 53.5 (1.2) | 52.7 (2.3) |
| Color-naming | 49.2 (1.4) | 50.9 (2.0) | 48.7 (1.2) | 47.5 (2.3) | 53.0 (1.4) | 54.8 (2.1) | 51.5 (1.2) | 52.3 (2.3) |
| Interference* | 49.2 (1.3) | 53.3 (1.8) | 52.4 (1.0) | 50.6 (2.0) | 51.4 (1.3) | 55.8 (1.9) | 53.9 (1.0) | 58.6 (2.0) |
| Conners’ Continuous Performance Test-II | ||||||||
| Omissions | 45.5 (1.6) | 45.1 (2.2) | 45.2 (1.3) | 44.0 (2.7) | 43.2 (1.6) | 44.6 (2.3) | 44.8 (1.3) | 42.2 (2.7) |
| Commissions | 43.1 (1.5) | 43.7 (2.1) | 42.5 (1.2) | 42.5 (2.6) | 40.7 (1.5) | 41.7 (2.1) | 41.8 (1.2) | 38.9 (2.6) |
| Hit RT | 49.3 (2.0) | 48.2 (2.7) | 49.1 (1.6) | 50.0 (3.3) | 49.2 (2.0) | 44.6 (2.8) | 51.7 (1.6) | 51.1 (3.3) |
For each neurocognitive measure, Day 3 and 7 T-scores for the four groups: placebo/low AW (PLA-Low); placebo/higher AW (PLA-Higher); flumazenil/gabapentin/low AW (FG-Low); and flumazenil/gabapentin/higher AW (FG-Higher). Figures are least squares means (standard errors) from the linear mixed models.
p < .05 for interaction between AW group and time.
p < .05 for interaction between AW group, medication group, and time.
Table 4.
| Change between Day 3 and Day 7 | ||||
|---|---|---|---|---|
| Measure subscale | PLA-Low | PLA-Higher | FG-Low | FG-Higher |
| TMT Part B | 5.2 (11.8) | 4.5 (9.3) | 2.7 (10.0) | 8.4 (7.8) |
| Stroop Interference* | 2.2 (4.3) | 2.6 (9.1) | 1.6 (4.7) | 8.0 (3.0) |
| CPT-II Commission | 2.4 (4.2) | 2.2 (4.0) | 0.7 (5.3) | 3.6 (4.0) |
| CPT-II Hit RT | 0.1 (7.2) | 4.1 (5.4) | 2.6 (6.3) | 1.1 (9.7) |
For primary measures of interest, average change (standard deviations) in T-scores between days 3 and 7 for the four groups: placebo/low alcohol AW (PLA-Low); placebo/higher AW (PLA-Higher); flumazenil/gabapentin/low AW (FG-Low); and flumazenil/gabapentin/ higher AW (FG-Higher).
p < .05 for interaction between AW group, medication group, and time.
Figure 1.
T-scores for the Stroop interference trial on Days 3 and 7 of the first week of treatment for participants treated with flumazenil and gabapetin (A: FMZ/GBP) and with placebos (B: PLA). Participants who were treated with active medications and who had higher baseline AW demonstrated greater improvement between Days 3 and 7 than those in the other groups. Figures are least-squares means (± standard errors) from the linear mixed model.
For the other measures, there was a main effect of time for TMT Part A and Stroop word-reading and color-naming, such that T-scores improved between days 3 and 7, but no such effect for CPT-II Omissions. For TMT Part A, there was a significant interaction between AW group and time (F(1, 56.38) = 4.15, p = .05), such that, regardless of medication group, participants with higher AW were more improved between days 3 and 7. There were no significant interactions between time and AW or medication group for the other measures.
Hypothesis 3: Relationship between neurocognitive improvement and subsequent drinking
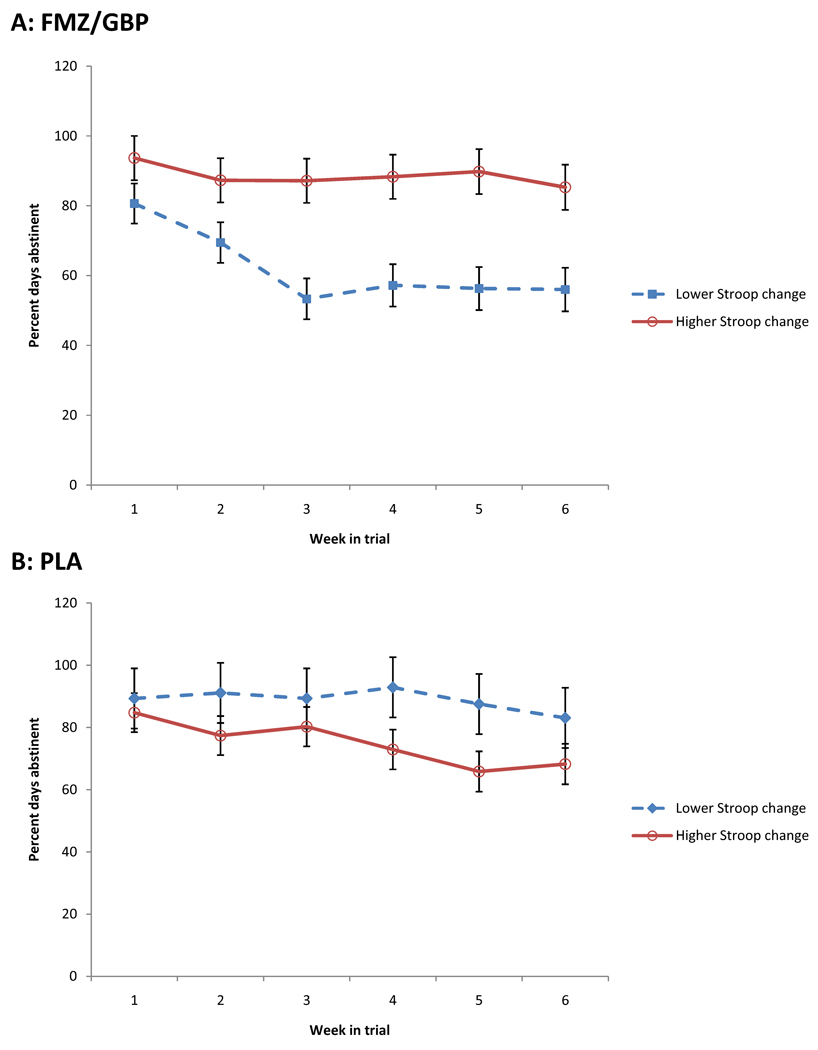
Improvement during week 1 on Stroop interference interacted with medication group in its effect on abstinence (PDA) throughout the trial (F(1, 51.07) = 6.31, p = .02). Follow-up F contrasts revealed that, among participants who received active medications, average PDA across weeks was significantly higher among those who had higher than average interference improvement (F(1, 30.80) = 8.31, p < .01). Among participants who received placebos, average PDA across weeks was significantly higher among those who had lower than average interference improvement (F(1, 21.78) = 4.31, p = .05) (see Figure 2).
Figure 2.
Percent days abstinent during the 6-week treatment trial for participants treated with flumazenil and gabapentin (A: FMZ/GBP) and with placebos (B: PLA). Participants who were treated with active medications who had greater than average improvement on the Stroop interference trial between Days 3 and 7 had more abstinence throughout the trial, while the opposite was true for participants treated with placebos. Figures are least-squares means (± standard errors) from the linear mixed model.
There were no significant effects of TMT Part B, CPT-II Commissions or Hit RT improvement on PDA, nor interactions between such improvement and medication, AW, or time. The only significant effect in the models that included improvement on these measures was the AW by medication interaction observed in the original trial (Anton et. al. 2009), such that participants with higher AW who received active medications had more PDA. Notably, for Stroop interference, this interaction was not significant when improvement on this measure was added to the model (p = .12), suggesting that the interaction between improvement and medication group partially mediated the influence of medication on PDA.
Discussion
Taken together, these data illustrate the effects of a combination of flumazenil and gabapentin on neurocognitive performance during early alcohol abstinence, a period during which some alcoholics experience AW. We previously (Anton et al., 2009) demonstrated that these medications reduced drinking among individuals with higher pre-treatment AW, and this study explored whether neurocognitive improvement accounted for this effect. Most hypotheses were not supported. AW severity was associated with impaired pre-treatment performance on 4/8 neurocognitive measures, but accounted for at most 10% of the variance in performance. Regardless of AW or medication, performance improved on 6/8 measures during the first week of treatment. Response inhibition improved slightly more in the higher AW/active medications group, and participants who received active medications and displayed greater than average improvement in response inhibition subsequently drank less. However, improvements in other domains (i.e., executive functioning, impulsivity) were neither differentially impacted by medication or AW nor related to later drinking.
Few previous studies have examined the relationship between acute AW and neurocognitive performance. The deficits in processing speed, executive functioning, and inattention associated with AW here have also been found among alcoholics during acute intoxication and after short- and long-term abstinence (Bates et al., 2002). Acute AW has been associated with memory impairment (Seifert et al., 2003), but other domains have not been studied. Though impairment in the higher AW group was subtle (i.e., T-scores within one S.D. of normative means), so was AW severity, which was clinically significant, but not medically dangerous. Thus, more severe AW might engender greater neurocognitive impairment.
Though not associated with acute AW severity, response inhibition improved more in the higher AW/active medications group. In alcoholism, response inhibition is especially relevant to cue-elicited craving and relapse. As the disorder progresses, alcoholics become less able to inhibit craving when confronted with alcohol-related cues (Monti et al., 1987). Individuals with higher AW may be particularly vulnerable to cue-elicited craving: according to the conditioned withdrawal theory, alcohol cues induce AW symptoms that motivate drinking, which acts as a negative reinforcer for such symptoms (Niaura et al., 1988). These data suggest that flumazenil and gabapentin improve higher-AW individuals’ ability to inhibit prepotent responses. The magnitude of improvement, about 6 T points, might not be considered clinically significant; however, since improvement in response inhibition also predicted greater abstinence in the active medications group, it may genuinely reflect an increased ability to withstand relapse triggers.
Notably, gabapentin and flumazenil did not impair cognition, consistent with other reports of these medications’ side effects and in contrast to the cognitively impairing effects of BZ, the current standard of care for AW. Among healthy controls, gabapentin marginally slows cortical electroencephalogram frequencies (Salinsky et al., 2002), but produces no significant cognitive side effects (Meador et al., 1999; Salinsky et al., 2002, 2005). Flumazenil, a medication often used to reverse cognitive impairment after BZ sedation, is generally inert among healthy controls (Ghoneim et al., 1993), and may reverse memory impairment among alcoholics (Kapczinski et al., 1995). This study suggests that neither medication produces cognitive side effects among alcoholics.
Limitations
There were several methodological limitations to this study, which was conducted within an outpatient clinical trial. Considerations that facilitated recruitment for this trial (e.g., permitted marijuana and alcohol use) would have been counter-indicated in a study solely of the relationship between acute AW and neurocognitive performance. The higher AW group was relatively small, and contained only four women, of whom one received active medications. Thus, power to detect higher-level interactions was limited, and these interactions, or the lack thereof, should be interpreted with some caution, particularly regarding medication effects among women. Further, the short intervals between assessments may have engendered practice effects, to which the TMT and Stroop are vulnerable (Buck et al., 2003; Davidson et al., 2003), that contributed to neurocognitive improvement (though all groups presumably benefitted equally from such effects). Short-interval testing has been used in other similar studies (Seifert et al., 2003), and was intended to capture short-term changes associated with acute AW.
Finally, it is unclear whether neurocognitive impairment predated the onset of participants’ drinking, alcoholism, or AW. Prospective studies suggest that, while there are no premorbid neurocognitive differences among alcohol-naïve adolescents, those who progress to regular binge drinking subsequently display widespread impairment (Tapert & Brown, 1999). Further, adolescents who more frequently experience AW after binges display more severe impairment at eight-year follow-up (Tapert et al., 2002), suggesting a “kindling” effect, whereby early AW potentiates the severity of future AW (Becker & Hale, 1993). However, neurocognitive impairment may also reflect the effects of recent, rather than lifetime, alcohol use (Horner et al., 1999). The analyses reported here did not covary recent drinking because this variable was quite collinear with baseline AW severity. Thus, it is unclear whether AW is wholly separable from recent heavy drinking.
Conclusions
Despite these limitations, this study evaluated a putative mechanism of action, neurocognitive improvement during early alcohol abstinence, for a combination of GABA- and glutamatergic medications in alcohol dependence. Though most hypotheses were not supported, AW severity was associated with greater impairment in some neurocognitive domains, individuals with higher baseline AW who received gabapentin and flumazenil had slightly greater improvement in response inhibition (but not other domains), and individuals with more improvement in this domain who received active medications subsequently drank less. Thus, flumazenil and gabapentin may differentially improve one aspect of neurocognitive performance during early abstinence, but do not seem to affect other domains.
Acknowledgments
This work was conducted under an unrestricted grant from Hythiam, Inc. Dr. Schacht was supported by an institutional training grant (T32 AA007474) and Dr. Anton by a senior scientist research and mentorship award (K05 AA017435) from the National Institute on Alcohol Abuse and Alcoholism.
References
- Anton RF, Myrick H, Baros AM, Latham PK, Randall PK, Wright TM, Stewart SH, Waid R, Malcolm R. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol. 2009;29:334–342. doi: 10.1097/JCP.0b013e3181aba6a4. [DOI] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Exp Clin Psychopharmacol. 2002;10:193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal "kindling". Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, Shulman GI, Navarro V, Petrakis IL, Charney DS, Krystal JH. Preliminary evidence of low cortical GABA levels in localized 1H–MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- Biggio F, Gorini G, Caria S, Murru L, Sanna E, Follesa P. Flumazenil selectively prevents the increase in alpha(4)-subunit gene expression and an associated change in GABA(A) receptor function induced by ethanol withdrawal. J Neurochem. 2007;102:657–666. doi: 10.1111/j.1471-4159.2007.04512.x. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Banger M, Leweke FM, Maschke M, Kowalski T, Gastpar M. Treatment of alcohol withdrawal syndrome with gabapentin. Pharmacopsychiatry. 1999;32:107–109. doi: 10.1055/s-2007-979203. [DOI] [PubMed] [Google Scholar]
- Buck KK, Atkinson TM, Ryan JP. Evidence of practice effects in variants of the Trail Making Test during serial assessment. J Clin Exp Neuropsychol. 2008;30:312–318. doi: 10.1080/13803390701390483. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol. Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Continuous Performance Test-II. Toronto: Multi-Health Systems; 2000. [Google Scholar]
- Cordovil De Sousa Uva M, Luminet O, Cortesi M, Constant E, Derely M, De Timary P. Distinct effects of protracted withdrawal on affect, craving, selective attention and executive functions among alcohol-dependent patients. Alcohol Alcohol. 2010;45:241–246. doi: 10.1093/alcalc/agq012. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Kelm MK, Breese GR. Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. J Pharmacol Exp Ther. 2008;326:596–603. doi: 10.1124/jpet.107.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson DJ, Zacks RT, Williams CC. Stroop interference, practice, and aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2003;10:85–98. doi: 10.1076/anec.10.2.85.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res. 2003;27:1563–1572. doi: 10.1097/01.ALC.0000090142.11260.D7. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, LaChance HA, Bryan A, Hutchison KE. Do genetic and individual risk factors moderate the efficacy of motivational enhancement therapy? Drinking outcomes with an emerging adult sample. Addict Biol. 2009;14:356–365. doi: 10.1111/j.1369-1600.2009.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB. User’s Guide for the Structured Clinical Interview for DSM-IV-TR Axis I Disorders – Research Version (SCID-I for DSM-IV-TR, November 2002 Revision) 2002 [Google Scholar]
- Ghoneim MM, Block RI, Ping ST, el-Zahaby HM, Hinrichs JV. The interactions of midazolam and flumazenil on human memory and cognition. Anesthesiology. 1993;79:1183–1192. doi: 10.1097/00000542-199312000-00008. [DOI] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM. Stroop Color and Word Test: A Manual for Clinical and Experimental Users. Wood Dale, IL: Stoelting; 2002. [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl.) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Guardia J, Trujols J, Burguete T, Luquero E, Cardús M. Impaired response inhibition scale for alcoholism (IRISA): development and psychometric properties of a new scale for abstinence-oriented treatment of alcoholism. Alcohol Clin Exp Res. 2007;31:269–275. doi: 10.1111/j.1530-0277.2006.00314.x. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl.) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian Adults [manual] 2004 [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc Natl Acad Sci USA. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner MD, Waid LR, Johnson DE, Latham PK, Anton RF. The relationship of cognitive functioning to amount of recent and lifetime alcohol consumption in outpatient alcoholics. Addict Behav. 1999;24:449–453. doi: 10.1016/s0306-4603(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Sherman D, Williams R, Lader M, Curran V. Differential effects of flumazenil in alcoholic and nonalcoholic cirrhotic patients. Psychopharmacology (Berl.) 1995;120:220–226. doi: 10.1007/BF02246197. [DOI] [PubMed] [Google Scholar]
- Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, et al. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 2004;130:283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Kumari M, Ticku MK. Regulation of NMDA receptors by ethanol. Prog Drug Res. 2000;54:152–189. [PubMed] [Google Scholar]
- Lee E, Jang D, Kim J, An SK, Park S, Kim I, Kim S, Yoon K, Namkoong K. Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport. 2007;18:1511–1514. doi: 10.1097/WNR.0b013e3282ef7625. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Acton PD, Gacinovic S, Suckling J, Busatto GF, Boddington SJ, Bullmore E, Woodruff PW, Costa DC, Pilowsky LS, Ell PJ, Marshall EJ, Kerwin RW. Reduced levels of GABA-benzodiazepine receptor in alcohol dependency in the absence of grey matter atrophy. Br J Psychiatry. 1998;173:116–122. doi: 10.1192/bjp.173.2.116. [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, Mann K. Impairment of cognitive abilities and decision making after chronic use of alcohol: The impact of multiple detoxifications. Alcohol Alcohol. 2009;44:372–381. doi: 10.1093/alcalc/agp030. [DOI] [PubMed] [Google Scholar]
- Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278:144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- McCrady BS, Smith DE. Implications of cognitive impairment for the treatment of alcoholism. Alcohol Clin Exp Res. 1986;10:145–149. doi: 10.1111/j.1530-0277.1986.tb05061.x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Meador KJ, Loring DW, Ray PG, Murro AM, King DW, Nichols ME, Deer EM, Goff WT. Differential cognitive effects of carbamazepine and gabapentin. Epilepsia. 1999;40:1279–1285. doi: 10.1111/j.1528-1157.1999.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? Stud Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- Miller WR. Combined Behavioral Intervention Manual. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004. [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29:2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22:38–43. [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Randall PK, Boyle E, Anton RF, Becker HC, Randall CL. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33:1582–1588. doi: 10.1111/j.1530-0277.2009.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Noël X, Van der Linden M, Schmidt N, Sferrazza R, Hanak C, Le Bon O, De Mol J, Kornreich C, Pelc I, Verbanck P. Supervisory attentional system in nonamnesic alcoholic men. Arch Gen Psychiatry. 2001;58:1152–1158. doi: 10.1001/archpsyc.58.12.1152. [DOI] [PubMed] [Google Scholar]
- O'Leary MR, Donovan DM, Chaney EF, Walker RD. Cognitive impairment and treatment outcome with alcoholics: preliminary findings. J Clin Psychiatry. 1979;40:397–398. [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. 2nd ed. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinsky MC, Binder LM, Oken BS, Storzbach D, Aron CR, Dodrill CB. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia. 2002;43:482–490. doi: 10.1046/j.1528-1157.2002.22501.x. [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Storzbach D, Spencer DC, Oken BS, Landry T, Dodrill CB. Effects of topiramate and gabapentin on cognitive abilities in healthy volunteers. Neurology. 2005;64:792–798. doi: 10.1212/01.WNL.0000152877.08088.87. [DOI] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Spiga S, Follesa P, Biggio G. Changes in GABA(A) receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J Neurosci. 2003;23:11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert J, Seeland I, Borsutzky M, Passie T, Rollnik JD, Wiese B, Emrich HM, Schneider U. Effects of acute alcohol withdrawal on memory performance in alcohol-dependent patients: a pilot study. Addict Biol. 2003;8:75–80. doi: 10.1080/1355621031000069918. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Horn JL. Alcohol Dependence Scale: Users Guide. Toronto: Addiction Research Foundation; 1984. [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, French-Mullen JM, Li X. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tanaka S. Dysfunctional GABAergic inhibition in the prefrontal cortex leading to "psychotic" hyperactivation. BMC Neurosci. 2008;9:41. doi: 10.1186/1471-2202-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. J Int Neuropsychol Soc. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Teichner G, Horner MD, Harvey RT. Neuropsychological predictors of the attainment of treatment objectives in substance abuse patients. Int. J. Neurosci. 2001;106:253–263. doi: 10.3109/00207450109149753. [DOI] [PubMed] [Google Scholar]
- Teichner G, Horner MD, Roitzsch JC, Herron J, Thevos A. Substance abuse treatment outcomes for cognitively impaired and intact outpatients. Addict Behav. 2002;27:751–763. doi: 10.1016/s0306-4603(01)00207-6. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Ragan P, Chang R, Chen S, Linnoila VM, Coyle JT. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry. 1998;155:726–732. doi: 10.1176/ajp.155.6.726. [DOI] [PubMed] [Google Scholar]
- Urschel HC, Hanselka LL, Gromov I, White L, Baron M. Open-label study of a proprietary treatment program targeting type A gamma-aminobutyric acid receptor dysregulation in methamphetamine dependence. Mayo Clin Proc. 2007;82:1170–1178. doi: 10.4065/82.10.1170. [DOI] [PubMed] [Google Scholar]
- Wicks S, Hammar J, Heilig M, Wisén O. Factors affecting the short-term prognosis of alcohol dependent patients undergoing inpatient detoxification. Subst Abus. 2001;22:235–245. doi: 10.1080/08897070109511465. [DOI] [PubMed] [Google Scholar]
- Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- Zinn S, Stein R, Swartzwelder HS. Executive functioning early in abstinence from alcohol. Alcohol Clin Exp Res. 2004;28:1338–1346. doi: 10.1097/01.alc.0000139814.81811.62. [DOI] [PubMed] [Google Scholar]