Abstract
Complex cognitive functions, such as learning and memory, arise from the interaction of multiple brain regions that comprise functional circuits and different components of these circuits make unique contributions to learning. The hippocampus and the retrosplenial cortex (RSC) are anatomically interconnected and both regions are involved in learning and memory. Previous studies indicate that the hippocampus exhibits unique firing patterns for different contexts and that RSC neurons selectively respond to cues that predict reinforcement or the need for a behavioral response, suggesting a hippocampal role in encoding contexts and an RSC role in encoding behaviorally significant cues. To test this, we simultaneously recorded hippocampal and RSC neuronal activity as rats learned to discriminate two behavioral contexts. The rats learned to approach the east arm of a plus maze for reward during the first half of each session and to approach the west arm during the second half. The ‘go east’ and ‘go west’ conditions constitute distinct behavioral contexts, which were cued by the reward location. Neurons in both regions developed highly context-specific responses as subjects learned to discriminate the contexts, but the response patterns differed in the two brain regions. Consistent with a context processing role, hippocampal neurons developed context specific responses to a variety of task stimuli and events. In contrast, RSC neurons only developed context specific responses to the reward location, which served as the context identifying cue. These results suggest that the hippocampus and RSC play distinct, but complimentary roles in mediating context appropriate memories and behaviors.
Keywords: hippocampus, retrosplenial cortex, cingulate cortex, context, place cell
Introduction
There is broad consensus that complex cognitive functions, such as learning and memory, arise from the interaction of multiple brain regions that comprise functional circuits (Aggleton and Brown, 1999; Gabriel, 1993; Mizumori et al., 2000) and that different components of these circuits make unique contributions to learning (Kesner and Rogers, 2004; White and McDonald, 2002). The hippocampus and the retrosplenial cortex (RSC) are anatomically interconnected (Amaral and Witter, 1995; van Groen and Wyss, 1990; van Groen and Wyss, 1992) and both regions are involved in learning and memory. Damage to either region results in amnesia (Scoville and Milner, 2000; Valenstein et al., 1987) and both regions have been implicated in spatial cognition (Cho and Sharp, 2001; O’Keefe and Dostrovsky, 1971; Olton et al., 1979; Sutherland et al., 1988) and contextual learning (Keene and Bucci, 2008b; Kim and Fanselow, 1992).
Despite these similarities, the functional roles of the hippocampus and RSC are not identical. For example, the hippocampus is critically involved in contextual learning (Maren, 2001; Smith, 2008) and hippocampal neurons exhibit context unique firing patterns that could serve as a neural code for the context (Smith and Mizumori, 2006b). The RSC, in addition to being involved in contextual learning (Keene and Bucci, 2008b), is known to be involved in discrimination tasks that do not depend on the hippocampus. For example, RSC lesions severely impair instrumental discrimination learning and RSC neurons preferentially respond to predictive cues (Gabriel, 1993; Smith et al., 2002), suggesting that the RSC plays a general role in encoding behaviorally significant stimuli (i.e. stimuli that predict reinforcement or the need for a particular behavioral response).
These findings suggested the hypothesis that the hippocampus encodes contexts and the RSC encodes behaviorally significant cues and that these two structures function together to produce context-appropriate memories and behaviors. Supporting this hypothesis, fornix lesions disrupted context specific firing patterns in the RSC and impaired contextual learning (Smith et al., 2004). In the present study, we investigated this hypothesis by simultaneously recording neuronal activity in the hippocampus and RSC while rats learned to discriminate two behavioral contexts.
Contexts typically involve a complex array of static cues, making it difficult to measure neuronal responses to specific context identifying cues. In the present study, the contexts were defined only by the reward location which allowed us to record RSC neuronal responses to a single context identifying cue (the reward location). In this task, rats learned to approach the east arm of a plus maze for reward during the first half of each training session and to approach the west arm during the second half. The ‘go east’ and ‘go west’ conditions constitute two distinct behavioral contexts with different task demands. We have used this task to examine the hippocampal role in behavioral context discrimination and found that after learning, hippocampal neurons exhibit highly distinctive firing patterns in the go east and go west contexts, including context specific spatial firing, reward responses and responses during the intertrial interval (Gill et al., 2010; Smith and Mizumori, 2006a; Smith and Mizumori, 2006b). Here, we compared hippocampal neuronal responses with those in the RSC and we examined the development of the task relevant neuronal responses in each region by recording neuronal responses before learning, in a control condition that involved searching for rewards in unpredictable locations, and during each of the daily training sessions as the rats learned to discriminate the go east and go west behavioral contexts. If the neurons in these regions play a role in learning to discriminate behavioral contexts, then firing should be similar across the two halves of the control session, which did not involve a context manipulation, but the firing should become distinct in the two behavioral contexts as the rats learn the task.
Materials and Methods
Subjects and Surgical Procedures
The subjects were 16 food restricted (80–85% of free feeding weight) adult male Long-Evans rats (Simonsen, CA). Movable stereotrode recording electrodes, fabricated by twisting together 2 25μm lacquer coated tungsten wires (McNaughton et al., 1983), were stereotaxically positioned just above the CA1 field of the hippocampus (2.5–4.5mm posterior to bregma, 2.5mm lateral, and 1.7mm ventral to the brain surface) and the RSC (3.5–4.5mm posterior to bregma, 0.5mm lateral and 0.3mm ventral). RSC electrodes were implanted in the granular retrosplenial area b (Rgb), also referred to as the posterior cingulate cortex or Brodman’s Area 29c. The rats were anesthetized with sodium pentobarbital (40 mg/kg). They were also given atropine sulphate (0.2 mg/kg) to prevent respiratory congestion, an antibiotic (5 mg/kg Baytril) and an analgesic (5 mg/kg ketoprofen). All procedures complied with guidelines established by the University of Washington Animal Care and Use Committee.
Behavioral Training
We reasoned that if the rats were required to perform different responses in two contexts that contained no distinguishing features, they would be forced to rely on internal representations to differentiate the contexts. Therefore, the rats were trained to retrieve rewards from one location on a plus maze during the first half of each training session and from a different location in the same environment during the second half of the sessions. The two session halves constituted separate behavioral contexts, which were defined by the task demands rather than the background stimuli. The environment and the specific motor behaviors (e.g. locomotion, right and left turns) were equivalent across contexts. Thus, any differential firing patterns in the two contexts cannot be attributed to these factors.
The maze was enclosed by curtains with objects placed around the perimeter to serve as extramaze cues. Trials began when the rats were placed on the maze facing the curtain at the end of an arm and ended when the rat arrived at the reward. During a 30 sec intertrial interval (ITI), the rats were placed on a platform adjacent to the maze. The position of the ITI platform was constant throughout training.
Before beginning regular training sessions, the rats were given a random foraging control session during which baseline neuronal and behavioral data were collected for comparison with later training sessions. During this session, the rats started each trial on a randomly designated arm and foraged for rewards on a (different) randomly designated arm. The rats were given two blocks of 10 training trials, separated by 30 seconds of darkness. The training procedures and behavioral requirements did not differ between the two blocks of trials. Since the random foraging sessions did not have a context manipulation, firing patterns were expected to be similar across the two blocks of this session and any differences in firing would be due to changes in satiety, fatigue or other factors unrelated to learning.
After the random foraging session, the rats were given daily training sessions consisting of 2 blocks of 15 trials each. During the first block of every training session, the reward was placed at the end of the east arm. During the second block, the reward was placed at the end of the west arm. The start positions for each trial were randomly designated from among the 3 non-reward arms. The two blocks were separated by 30 seconds of darkness to cue the rats that the second block was about to begin. Training continued with the same 2 reward locations until the rats attained a behavioral criterion of at least 75% correct choices on two consecutive sessions. After achieving this criterion, the rats were given 2–10 post-criterial training sessions for the collection of additional neuronal data during asymptotic performance.
Data Collection
Neuronal spike data and video data were collected throughout learning with the Cheetah Data Acquisition System (Neuralynx, Inc., Bozeman, MT). Signals from the electrodes were amplified 2000–10000 times, filtered at 600 Hz and 6 kHz, and digitized. All waveforms exceeding a user-defined threshold were stored to disk along with their time of occurrence for offline analysis. Standard spike sorting techniques were used to separate the multi-unit records into component single units (MClust, A. D. Redish). Waveform features used for sorting included spike amplitude, spike width, waveform principle components, and waveform area. Additional custom template matching algorithms were used to facilitate sorting. The rat’s position and head direction were monitored by digitized video (sampled at 30 Hz) of an LED array attached to the rats head. Video data were also used to establish the time of the trial start, arrival at the reward and return to the ITI platform after each trial.
General Analysis Strategy for the Neuronal Data
This study is part of a series of experiments designed to identify the neural mechanisms of behavioral context discrimination (Gill et al., 2010; Smith and Mizumori, 2006a; Smith and Mizumori, 2006b). The goal of the present study is to compare the development of various types of neuronal responses in the hippocampus and RSC during learning. Previously, we reported that hippocampal neurons exhibit context specific responses after learning. Thus, the data of the random foraging session and asymptotic performance have been reported elsewhere (Smith and Mizumori, 2006b). Some of those data are reproduced here for comparison with RSC neuronal data. In order to facilitate these comparisons, all the neuronal data were analyzed using the same methods as previous reports in this series.
Before beginning training, the recording probes were advanced until isolatable neuronal spikes were obtained. The same neurons were sometimes recorded for several training sessions (e.g. Fig. 2). However, it was typically not possible to reliably maintain records throughout training. Therefore, a strategy was adopted in which various population measures of neural responses were examined at different stages of learning. In keeping with this strategy, electrodes were advanced to obtain new units whenever records were lost.
Fig. 2.
The development of context-specific neuronal firing is shown in an individual hippocampal neuron which was recorded for 5 consecutive days (labels as in Fig. 1). Plots are shown for the first and second halves (Block 1 and Block 2) of the random foraging session (RF) and for the first, second and fourth regular acquisition sessions (ACQ1, ACQ2 and ACQ4). Spatial correlation coefficients (r) of the firing rate maps are given for each pair of plots. Behavioral performance during each half session is indicated by the percentage of trials with correct responses. The firing patterns were similar during the random foraging session and the first acquisition session. However, they became more distinct as the rat learned the context appropriate responses.
Neurons were first classified as to whether they exhibited spatial or event responses. Neurons that had responses were then analyzed to determine whether context-specific responses developed as the rats learned to distinguish the two contexts. If the neuronal responses were sensitive to the behavioral context, firing should be similar across the two blocks of the random foraging session, which did not involve a context manipulation, but the firing across the two blocks (contexts) of regular training sessions should become differentiated with learning. The rats took varying numbers of sessions to reach the criterion. Therefore, the learning related development of the neuronal responses were assessed by analyzing the data from a set of training sessions that were common to all rats, including the random foraging session, the first acquisition session, the middle training session and asymptotic performance sessions. The middle training session was simply the session that was half way through the duration of training for each rat. For example, for a rat that required seven sessions to reach the criterion the fourth training session served as the middle session. For those rats that took an even number of sessions, the session after the half way mark served as the middle session. The session in which the rats reached the behavioral criterion was included in the asymptotic performance data.
Analysis of Spatial Firing Patterns
Hippocampal pyramidal neurons and RSC neurons were classified as having a place field if they a) fired in at least 4 adjacent pixels (2.8 × 2.8 cm/pixel) but less than half of the maze area b) had a within field firing rate at least twice that of the firing rate outside the field and c) fired during more than 50% of the passes through the field (Smith and Mizumori, 2006b). To examine learning related changes in spatial firing patterns, separate firing rate maps were constructed for the first and second blocks of each training session and a pixel by pixel correlation coefficient (Pearson’s r) of the firing rates was computed. Only the pixels visited in both blocks were used. The correlation coefficients served as a measure of the similarity of the spatial firing patterns across the two blocks of trials. To assess learning related changes in the spatial firing patterns, the correlation coefficients of neurons recorded during different stages of training were compared using ANOVA.
Analysis of Event-Related Firing
This analyses examined neuronal responses to the reward and the return of the subjects to the ITI platform after training trials. ITI responses were examined because they could be involved in the maintenance of memories for past or future reward locations during the delay in between trials (Pastalkova et al., 2008). Peri-event time histograms were constructed with the data centered on the arrival at the reward location and the ITI platform. Separate histograms were constructed for the two blocks of trials within each session. The firing frequencies of the 10 pre-event bins (100 msec bins) were compared to the 10 post-event bins using the Wilcoxon signed rank test. Neurons with a significant (p<.05) difference between pre-and post-event firing rates were classified as having an event response.
The data of neurons that exhibited event responses were further analyzed to detect differences in event related neuronal firing across the two blocks of each training session. For these analyses, the values in the histograms were normalized (z-transformation) using the mean and standard deviation of the firing rate recorded for 5 seconds prior to the event. Thus, the firing rate data were expressed in standard units of change from the pre-event baseline. The 10 post-event bins of the first block were compared to 10 post-event bins of the second block. Neurons with an event response that also exhibited a significant (p<.05) difference between the two blocks were classified as having a context-specific event response. Learning related development of event responses was assessed by submitting the percentage of neurons with context-specific responses at different stages of training to Chi Square analysis.
The spatial firing and the firing around the time of the rewards was examined for each neuron in order to ensure that place fields near the goal locations were not mistakenly classified as reward responses, and vice versa. Responses were classified as reward responses if they were time-locked to the reward and if they did not occur in the same location and facing the same direction when there was no reward, such as when the rat was placed at the end of that arm for the start of trials. Otherwise, they were classified as spatial responses.
The numbers of neurons recorded in each brain region during each stage of training are given in Table 1. The variation in the numbers of neurons recorded during the different stages of training was largely due to our recording procedures, rather than some training-related change in the physiological properties of the neurons (e.g. silent neurons becoming active with training). The electrodes were advanced until isolatable units were located before the critical training session, such as the first session or the criterial session. However, it was not possible to anticipate which session would fall in the middle of acquisition so it was less likely that electrodes were advanced prior to those sessions. The largest number of neurons in each area was recorded during asymptotic performance, because we gave subjects up to 10 post-criterial (asymptotic) training sessions and advanced the electrodes between each session in order to improve the yield.
Table 1.
The numbers and percentages of neurons that exhibited various responses are shown for each brain region and stage of training. The table shows the total number of neurons (N) recorded in each brain region. The number and percentage of neurons that exhibited place fields which met our criteria are shown, along with the average spatial correlation (r) and SEM for those neurons. Also shown are the number of neurons that exhibited reward and ITI responses that did not differ in the two contexts (Non-Specific) and the number that exhibited significantly different responses in the two contexts (Context-Specific).
| Hippocampus | N | Place | r (SEM) | Non-Specific Reward | Context-Specific Reward | Non-Specific ITI | Context-Specific ITI |
|---|---|---|---|---|---|---|---|
| Random Foraging | 105 | 34 (32.4%) | 0.35 (.043) | 4 (3.8%) | 7 (6.7%) | 2 (1.9%) | 8 (7.6%) |
| First Session | 72 | 28 (38.9%) | 0.27 (.048) | 2 (2.8%) | 14 (19.4%)* | 4 (5.6%) | 10 (13.9%) |
| Middle Session | 48 | 23 (47.9%) | 0.20 (.065)† | 0 (0.0%) | 15 (31.3%)* | 0 (0.0%) | 7 (14.6%) |
| Asymptote | 310 | 120 (38.7%) | 0.22 (.028)* | 10 (3.2%) | 68 (21.9%)* | 7 (2.3%) | 59 (19.0%)* |
| Total | 535 | 205 (38.3%) | 16 (3.0%) | 104 (19.4%) | 13 (2.4%) | 84 (15.7%) | |
| Retrosplenial Cortex | N | Place | r (SEM) | Non-Specific Reward | Context-Specific Reward | Non-Specific ITI | Context-Specific ITI |
| Random Foraging | 56 | 5 (8.9%) | 0.12 (.039) | 6 (10.7%) | 4 (7.1%) | 6 (10.7%) | 8 (14.3%) |
| First Session | 45 | 7 (15.6%) | 0.16 (.039) | 1 (2.2%) | 19 (42.2%)* | 5 (11.1%) | 8 (17.8%) |
| Middle Session | 40 | 9 (22.5%) | 0.22 (.072) | 3 (7.5%) | 12 (30.0%)* | 1 (2.5%) | 7 (17.5%) |
| Asymptote | 225 | 63 (28.0%) | 0.21 (029) | 11 (4.9%) | 73 (32.4%)* | 10 (4.4%) | 60 (26.7%) |
| Total | 366 | 84 (23.0%) | 21 (5.7%) | 108 (29.5%) | 22 (6.0%) | 83 (22.7%) | |
Values that differed significantly (p<.05) from the random foraging control session are indicated with a (*) while values that approached significance (.05<p<.10) are indicated with a (†).
Results
Behavior
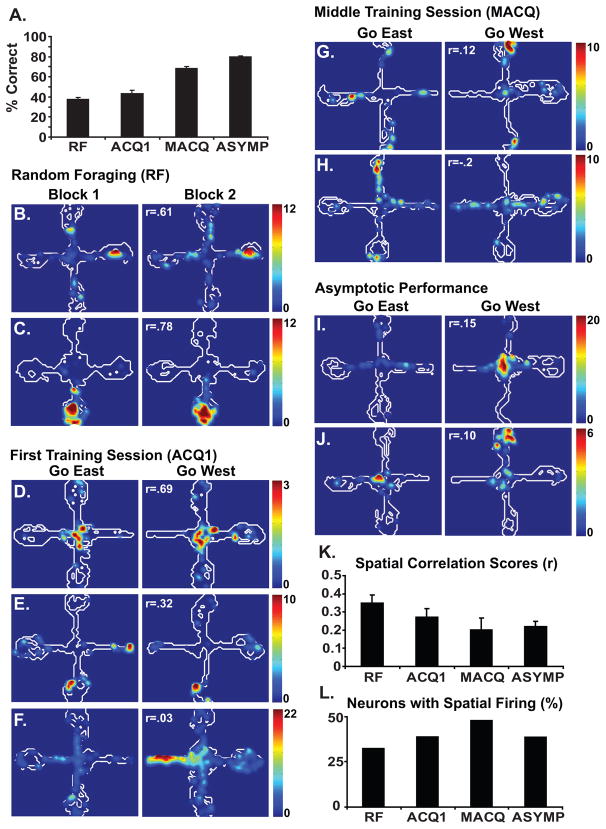
The rats achieved the performance criterion in an average of 6.7 sessions. As expected, the rats chose the rewarded arm at chance levels during the random foraging control session (35.71% correct, compared to chance performance of 33.3%, one-sample t-test, t(13)=1.01, p=.33). Choice accuracy improved significantly with training (F[3,39]=72.47, p<.001) and, although post hoc comparisons indicated that choice accuracy did not improve during the first training session, performance did improve significantly at each stage of training thereafter until the rats reached an average of 83.63% correct at asymptote (p<.05, Fig. 1A).
Fig. 1.
Behavioral data are shown in A. The average percentage of trials in which the rats made a correct arm entry (with no erroneous entries into non-rewarded arms) are shown for the random foraging (RF) session, the first training session (ACQ1), the session half way through training (MACQ) and asymptotic performance (ASYMP). The contour plots (B–J) show examples of the spatial firing patterns of individual hippocampal neurons recorded during each of stage of training. The regions of the maze visited by the rat are outlined in white. The firing rates are illustrated by the colored contour peaks, with the scale (in Hz) indicated for each neuron. Each pair of plots shows the data of a single neuron during a single session. Spatial correlation scores (r) indicating the similarity of the spatial firing patterns are given for each pair of plots. Plots B and C illustrate the firing patterns of two neurons recorded during the first and second halves of the random foraging session (Block 1 and Block 2). For each trial, rewards were placed at the end of randomly designated arms and the rat started on one of the three non-rewarded arms. Plots D–F illustrate the firing patterns of neurons recorded during the first training session. Each pair of plots illustrates neuronal firing during the first half of the session (Go East) when the reward was always placed on the east arm, and during the second half (Go West) when the reward was always placed on the west arm. Similarly, plots G–H and plots I–J illustrate the firing patterns of neurons recorded during the middle acquisition session and during asymptotic performance, respectively. Prior to training, the neuronal firing patterns were highly similar across the two blocks of training (e.g. B and C). Early in training, the firing patterns of some neurons were similar in the two contexts (e.g. D) while others were more distinct in the two contexts (e.g. F). As learning progressed, the firing patterns of the neurons became more distinct (e.g. G–J). This can be seen in the bar plots, which summarize the spatial firing of the hippocampal neuronal population during each stage of training. The average spatial correlation scores declined as the rats learned to discriminate the two contexts (K). The percentage of neurons that exhibited a place field did not change with training (L). Plots B, I and J were adapted from Smith and Mizumori, 2006b.
After completing the first block of trials, the maze room lights were extinguished for 30 sec to cue the rats that the second block of trials was about to begin. However, the rats apparently did not use this cue. Instead, on the first trial of the second block, they incorrectly approached the previously rewarded (east) location 97% of the time, on average, even after their overall performance had reached asymptote. There were no context-identifying cues available at the start of the training trials. However, the rats were always allowed to search until they found the reward. Finding the reward on the east arm confirmed that the go east context was still in effect. When the reward was no longer found on the east arm, the rats began to go to the west arm. Thus, the reward was not only the reinforcing stimulus. The reward and its location also served as a discriminative cue that allowed the rats to differentiate the east and west contexts.
Spatial Firing Patterns of Hippocampal and Retrosplenial Cortical Neurons
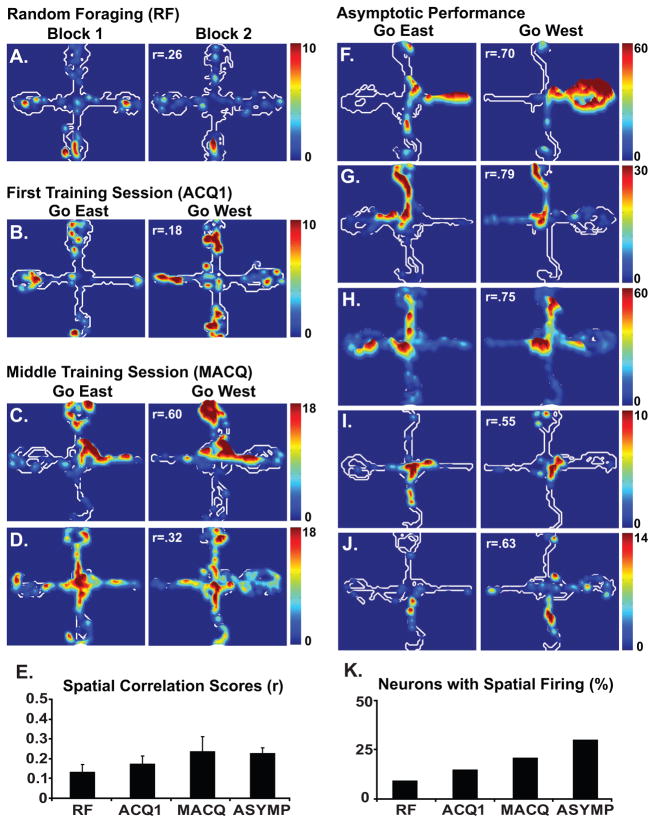
Hippocampal neurons have long been known to exhibit spatially localized firing (i.e place fields, O’Keefe and Dostrovsky, 1971). Previous studies have shown that RSC neurons also exhibit spatially localized firing patterns (Cho and Sharp, 2001). In the present study, neurons in both structures exhibited place fields although they differed with respect to how the fields developed (Figs. 1 and 3). Consistent with previous reports (e.g. Frank et al., 2004), hippocampal neurons exhibited place fields during the first recording session and they continued to exhibit place fields throughout learning. The proportion of neurons that exhibited place fields did not change significantly with training (X2(3)=3.47, p=.33, Fig. 1L). In contrast, RSC neurons acquired place fields as the rats learned the task. The proportion of RSC neurons that exhibited place fields increased significantly with learning (X2(3)=13.70, p<.01, Fig. 3K).
Fig. 3.
The contour plots show examples of the spatial firing patterns of individual RSC neurons recorded during each of stage of training (labels as in Fig 1). Data are shown for the random foraging session, the first training session, the middle training session and during asymptotic performance. The neuronal firing patterns were generally similar in the two contexts and this did not change with training. This can be seen in the bar plots, which summarize the spatial firing of the RSC neuronal population during each stage of training. In contrast to the hippocampus, the average spatial correlation scores of RSC neurons did not change with training (E). However, the percentage of neurons that exhibited a place field increased with training (K).
Previously, we reported that hippocampal neurons exhibited different spatial firing patterns after the rats had learned to discriminate the go east and go west contexts (Smith and Mizumori, 2006b). Importantly, the differential firing patterns were not due to differences in the direction of travel or differences in the specific trajectories taken to the reward. For example, rats typically passed through the place fields in the same direction during the east and west contexts, either because the rat made errors or because the place field was located on a part of the maze that was traversed similarly in both contexts. Even when the analysis was limited to passes through the fields in the same direction, neuronal firing was significantly different in the two contexts. Additionally, the place fields did not rotate 180 degrees when the reward location changed from the east arm to the west arm. Each of these possibilities was examined and eliminated previously for this data set (for details see Smith and Mizumori, 2006b).
In the present study, we examined the development of these responses over the course of several training sessions. Spatial correlation scores reflecting the similarity of the spatial firing patterns were computed for each neuron that exhibited a place field. The average spatial correlation scores exhibited a marginally significant decline with training (F[3,170]=2.25, p<.085), suggesting that the spatial firing patterns became more distinct as the rats learned to distinguish the two contexts (Fig. 1K). Post hoc comparisons indicated that the spatial correlation scores were significantly reduced during asymptotic performance (p<.05) and marginally significantly reduced during the middle training session (p=.065). Thus, the spatial correlation scores did not begin to decline, on average, until the middle of acquisition (marginally) or until asymptote.
Although the spatial correlation scores declined gradually over the course of several sessions, our observations suggested that hippocampal neurons developed context-specific firing patterns at different times. Some hippocampal neurons exhibited context-specific firing patterns on the first day of training (e.g. Fig. 1F). Consistent with this idea, the percentage of the spatial correlation scores that fell in the bottom quartile of the distribution increased from 23.5% during the random foraging session to 36.7% during the first training session. This result suggests that, although the average correlation scores were not significantly reduced during the first session, some neurons began to differentiate the two contexts during the first training session. Most neurons started out with similar responses during the random foraging session and, at some point in training, the responses became differentiated in the two contexts (Fig. 2). Context specific firing patterns may have emerged at different times for different neurons.
Like hippocampal neurons, RSC neurons exhibited spatial firing. However, unlike hippocampal neurons, the proportion of RSC neurons that exhibited place fields increased significantly with learning (described above) and the spatial correlation scores did not change with training (F[3,80]=0.38, p=.77, Fig. 3E). Thus, the learning related changes in the spatial firing patterns of hippocampal and RSC neurons differed in two ways. First, hippocampal place fields were present from the outset of recording, whereas RSC place fields developed with training. Second, the spatial firing patterns of hippocampal neurons clearly differentiated the go east and go west behavioral contexts whereas the RSC firing patterns did not. The fact that only one of the two regions exhibited differential firing patterns in the east and west contexts (the hippocampus) indicates that such differential firing patterns were not simply an artifact of the training procedures or analysis methods.
Previously, RSC neurons have been shown to exhibit directionally selective firing (i.e. head direction neurons) and place fields (i.e. place fields, Cho and Sharp, 2001). Additionally, many of the RSC place cells exhibited directionality or were responsive to complex combinations of location, directional heading and various kinds of movements. In the present study, we did not find head directional firing and RSC neurons exhibited very similar spatial firing patterns in the east and west contexts, suggesting that they were not modulated by directional and movement variables. This may have been due to regional differences in the RSC. The recordings of the previous study were 1.2–2.2 mm caudal to those of the present study. Although both recording sites are part of the regions commonly referred to as the RSC in the rat, there may be important differences in the cytoarchitecture and connectivity along the rostral-caudal extent of the RSC (for reviews see van Groen et al., 1993; Vogt, 1993).
Hippocampal and Retrosplenial Cortical Event Related Neuronal Responses
Hippocampal and retrosplenial cortical neurons responded to task relevant events, such as the reward and the return of the rat to the intertrial interval (ITI) platform after training trials. However, the two regions differed in the way these responses developed over the course of learning.
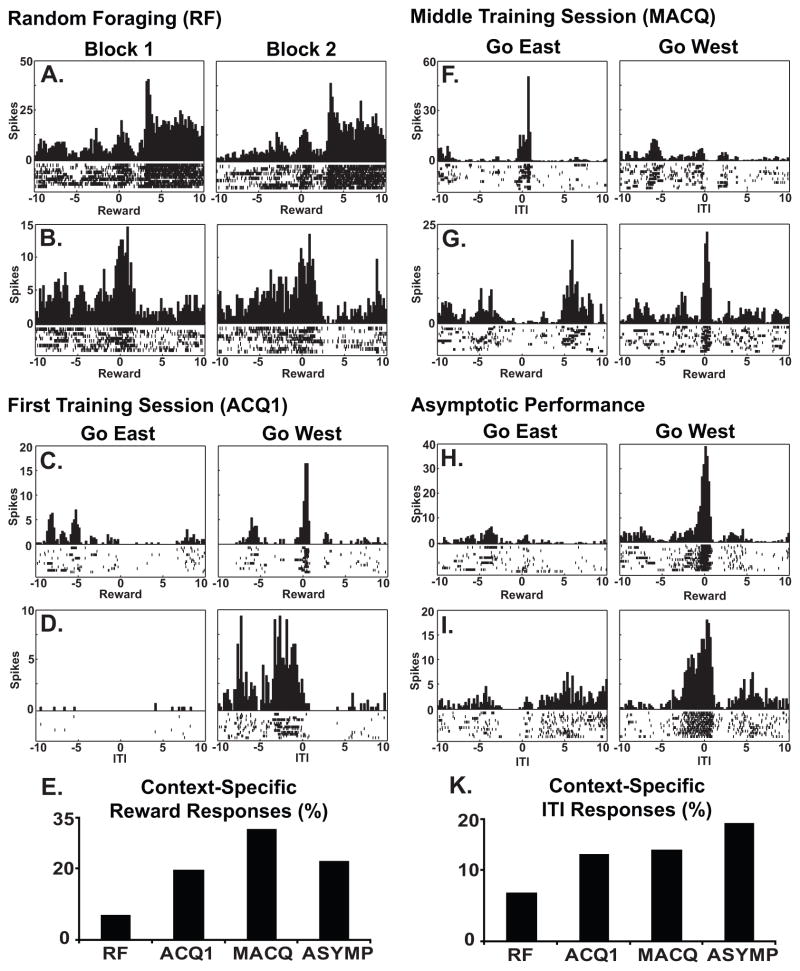
Hippocampal neurons developed highly context-specific responses to the reward (Fig. 4 and Table 1). The percentage of neurons exhibiting a context-specific reward response increased significantly across the four stages of training (Fig. 4E, X2(3)=16.45, p<.001) and this percentage increased significantly during the first training session, relative to the random foraging session (X2(1)=6.67, p<.05).
Fig. 4.
Peri-event time histograms illustrate event related firing of individual hippocampal neurons. Each pair of plots shows the data of a single neuron during a single session. The histograms show neuronal firing, summed across the 15 trials of each block, while the raster plots show trial by trial firing with one row of tick marks for each trial. The plots are centered on the event (time zero) and twenty seconds of data are shown, from 10 seconds before to 10 seconds after the event. Examples of event responses are shown for the random foraging session (A and B), the first training session (C and D), the middle training session (F and G) and during asymptotic performance (H and I). The responses recorded during the random foraging session were similar across the two blocks of training. The neurons in A and B both fired in response to the reward. Interestingly, the neuron in A also fired about 2.5 sec after the reward when the rat was returned to the ITI platform. Additional examples of reward responses are shown in C, G and H. Additional examples of ITI responses are shown in D, F and I. As shown in the bar plots (E and K), the percentage of hippocampal neurons that exhibited context specific reward and ITI responses increased over the course of training.
Hippocampal neurons also developed context-specific responses at the end of training trials when the rat was returned to the ITI platform (X2(3)= 8.01, p<.05, Fig. 4K). These responses developed more gradually than the context-specific reward responses. The percentage of neurons with an ITI response did not increase significantly during the first training session (X2(1)=1.84, p=.18), but it was increased during asymptotic performance (X2(1)=7.55, p<.01), relative to the random foraging session.
RSC neurons developed robust context-specific responses to the reward during the first training session. Prior to training, during the random foraging session, 17.86% (10/56) of the neurons exhibited significant responses to the reward. That percentage increased to 44.44% (20/45) on the first day of training and the responses became markedly different in the two contexts for all but one of these neurons (Fig. 5 and Table 1). Overall, the percentage of RSC neurons with a context-specific reward response increased significantly with training (X2(3)=17.48, p<.001, Fig 5H). Moreover, this percentage increased significantly during the first training session, relative to random foraging (X2(1)=10.60, p<.001). Interestingly, the dramatic increase in context-specific reward responses occurred during the first session, before behavioral performance had improved significantly from chance levels.
Fig. 5.
Peri-event time histograms illustrate event related firing of individual RSC neurons recorded during each of stage of training (labels as in Fig. 4). Examples of reward responses recorded during the first training session are shown in plots A–C. Insets in A and B illustrate the firing of the same neurons on the previous day’s random foraging session when no reward response was evident. Examples of reward responses recorded during the middle training session (D) and asymptotic performance (E-G) are also shown. The percentage of RSC neurons that exhibited context specific reward responses increased during the first training session and remained high throughout training (plot H).
Previous studies showed that RSC neurons respond to behaviorally significant cues (i.e. those cues that predict reinforcement or the need for a particular behavioral response, Smith et al., 2002). The behavioral results described above indicate that, in the present experiment, the reward and its location was a critically important cue that the rats used to identify the current context (go east or go west). Thus, the rapid development of highly selective RSC neuronal responses to the reward and its location may have been critical for the rats to be able to discriminate the two contexts.
RSC neurons also responded to other task relevant events, such as the end of training trials when the rat was returned to the ITI platform after the completion of training trials, and some of these responses differed in the go east and go west contexts (Table 1). However, unlike hippocampal ITI responses, these responses did not develop significantly with training (X2(3)=5.43, p<.15). Since the percentage of the neuronal population responding to these events was the same during the random foraging control condition and during learning, these responses cannot be clearly associated with contextual learning.
Differential Responses Did Not Develop During Control Sessions
Context-specific spatial and event related responses developed only when the rats were trained to discriminate the two behavioral contexts. As discussed above, neuronal populations exhibited context-specific responses during training, but not during random foraging. As an additional control, 3 rats were given 2–5 additional random foraging sessions before beginning context training. These sessions allowed for neuronal recording during repeated training sessions without the context manipulation. Significant block differences in the place fields and event responses did not develop during these sessions. As reported previously (Smith and Mizumori, 2006b), the average spatial correlation coefficients of hippocampal neurons recorded after repeated random foraging sessions did not differ from those of neurons recorded during the initial random foraging session (F[1,28]=2.06, p=.17). The percentage of neurons with block specific reward responses also did not change with repeated random foraging sessions (6.9% after repeated sessions compared to 6.1% during the initial session, X2(1)=0.03, p=.34). Similarly, the reward responses of RSC neurons did not develop block specificity with repeated random foraging sessions (22.2% during the initial session and 22.2% after repeated sessions, X2(1)=0.0, p=1.0). These results indicated that the development of context-specific firing patterns could not be attributed to repeated exposure to the training environment or to changes in arousal or motivation over the course of training or during a given training session.
Comparison of HPC and RSC Neuronal Responses
Both hippocampal and RSC neurons exhibited spatial and event related firing. However, there were some regional differences in the characteristics of these responses (see Table 2). Overall, 38.3% of hippocampal pyramidal neurons exhibited place fields compared to 23.0% of RSC neurons. The average firing rate of RSC neurons was much higher than hippocampal neurons (average overall firing rate: 17.5 Hz compared to 2.2 Hz in the hippocampus), and this was apparent in the place fields and the event responses. RSC neurons also had larger place fields, which often encompassed an entire arm of the maze. Although RSC neurons fired at a higher rate within their place fields, the contrast between in field and out of field firing was much greater in hippocampal neurons. On average, the within field firing rate of hippocampal neurons was more than 50 times the out of field firing rate, compared to 9 times in RSC neurons.
Table 2.
Place field statistics for hippocampal and RSC neurons. The average area, the within field firing rate, the ratio of within field firing rate to out of field firing and the reliability (the proportion of passes through the field during which the neuron fired) are given.
| Area (cm2) | Rate (Hz) | Ratio | Reliability | |
|---|---|---|---|---|
| Hippocampus | 368.16 | 16.34 | 50.34 | 0.75 |
| RSC | 1031.44 | 45.70 | 8.90 | 0.86 |
Event responses also differed in the two regions. Due to the low baseline firing rate, hippocampal event responses typically consisted of increased firing at the time of the event. In contrast, RSC neurons exhibited a variety of response types. Some RSC neurons exhibit relatively brief increases in firing which were closely time-locked to the reward (e.g. Fig. 5G). Others exhibited a more gradual buildup in firing as the rat approached the reward, typically with a peak in firing during reward consumption. Many of these neurons also exhibited sustained firing at the reward until the rat was removed from the maze and placed on the ITI platform (e.g. Fig. 5C and F). Interestingly, context specificity often appeared as increased firing in response to the reward in one context, but decreased firing in the other context (e.g. Fig. 5A, E and F). This pattern of responses could serve to amplify the differential processing of the two reward locations.
Discussion
Hippocampal and RSC neurons developed highly context-specific responses as subjects learned to discriminate the two behavioral contexts. These results, in combination with contextual learning impairments resulting from lesions of either region (e.g. Keene and Bucci, 2008b; Kim and Fanselow, 1992), indicate that the hippocampus and RSC are part of a circuit that mediates contextual learning and memory, including those contexts that are defined by their behavioral demands. However, hippocampal and RSC neurons exhibited different kinds of responses and the responses developed differently in the two brain regions, suggesting that they make different contributions to the contextual learning process. Importantly, hippocampal and RSC neuronal responses developed as a function of learning and they did not develop in a control condition that did not involve contextual learning.
Not surprisingly, hippocampal neurons exhibited place fields during the first session and throughout training. However, the spatial representations became progressively more distinct in the go east and go west contexts as the rats learned. At the population level, the average spatial correlation scores changed gradually until the firing patterns became context specific after learning. However, some individual neurons developed context specific firing patterns during the early stages of learning, suggesting that context specific firing patterns emerged at different times for different neurons. Previously, we have suggested that these context specific firing patterns could serve as a neural representation of the context (Smith and Mizumori, 2006b). The present results suggest that individual context specific neuronal responses accumulate as the rats learn the task, eventually resulting in a unique population code for each context. Consistent with this idea, differential firing develops rapidly in tasks that are learned quickly (Lee et al., 2006) and more slowly in tasks that require more training (the present study and the ‘skipped reward’ task of Bower et al., 2005).
Hippocampal neurons also responded to task relevant events, including the reward and the ITI, and these responses also became highly context specific as the rats learned to discriminate the east and west contexts. The context specific reward responses developed on the first day of training and they continued to develop with training. The context specific ITI responses developed more slowly, reaching maximum prevalence during asymptotic performance. These ITI responses are consistent with recent reports of differential firing during the delay period of spatial alternation tasks and they may play an important role in maintaining memory during the delay (Ainge et al., 2007; Gill et al., 2010; Pastalkova et al., 2008).
The pattern of results in the RSC was quite different from the hippocampus. RSC neurons also exhibited place fields but they differed from hippocampal neurons in terms of their development and context sensitivity. Whereas hippocampal place fields were present from the outset and they became context specific as the rats learned, RSC place fields developed over the course of learning and they were not context specific. RSC neurons also responded to the reward and the ITI. Like the spatial responses, ITI responses in the RSC were not context specific. Only the reward responses differentiated the east and west contexts.
In the present study, the reward and its location played a critical role as the cue that subjects used to identify the current behavioral context (see behavioral results). Although RSC neurons responded to many of the same task stimuli as hippocampal neurons, they only developed context specific responses to this important cue. Studies of instrumental discrimination learning have shown that RSC neurons preferentially respond to cues that predict reinforcement or the need for a behavioral response and lesions of the RSC impair learning (Gabriel, 1993; Keene and Bucci, 2008a; Smith et al., 2002). Interestingly, RSC neurons respond to any predictive cue, regardless of whether the reinforcement is aversive or appetitive and regardless of the specific behavioral response (e.g. avoidance or approach responses, Smith et al., 2004). Our results support the idea that RSC neurons encode behaviorally significant cues and expand this idea to include context-identifying cues.
Findings from studies of spatial navigation are also consistent with a RSC role in processing behaviorally significant cues. For example, rats with RSC lesions failed to use extra-maze cues for navigation in a radial maze task (Vann and Aggleton, 2005) and showed impaired navigation to a visible platform in the Morris water maze even though swimming behavior was normal (Cain et al., 2006). In the present study, the hippocampus and RSC both generated spatial representations (place fields). However, the hippocampal representation was highly sensitive to changes in the behavioral context while the RSC was not, perhaps because the spatial geometry was the same in the two contexts and spatial cues were not useful for context discrimination. That is, unlike hippocampal place neurons, RSC neurons may encode spatial geometry as a potentially significant cue with differential responses only when the spatial cues have important discriminative value. This account suggests that if the contexts were defined by the spatial cues, with different behavioral responses required in different environments, RSC neurons would develop different place fields in the two contexts. The RSC has been proposed as an important component of the brain’s navigation system (e.g. Mizumori et al., 2000) and the present results are consistent with this idea. However, the RSC role in navigation may reflect a more general role in processing behaviorally significant cues, including spatial cues.
The overall pattern of results from this study indicates that hippocampal neurons develop context specific responses to a wide variety of task stimuli and events, but RSC neurons only developed context specific responses to the cue that subjects used to identify the current behavioral context. The result is that, regardless of the rat’s location or the stimuli being encountered, hippocampal output is unique to the current context and could therefore serve as a continuous neural code for the context. In contrast, context specific firing in the RSC was focused exclusively on the reward. This differential RSC signal was generated rapidly, during the first day of training, before the rats exhibited significant behavioral evidence of learning, and was very robust, with more than 40% of RSC neurons exhibiting the differential response. Thus, these results are consistent with the hypothesis that the hippocampus encodes contexts and the RSC encodes behaviorally significant cues, including those cues that identify the current context.
If so, then the hippocampus and RSC must work in concert to allow subjects to produce context-appropriate memories and behaviors. In our task, RSC input may be needed for the development of hippocampal context representations. Because the environment was the same for the east and west trials, most of the input to the hippocampus was similar in the two contexts. However, the early developing context specific RSC reward responses may have prompted the development of context specific firing patterns in the hippocampus. This account suggests that RSC lesions would impair the use of specific cues to identify the context and disrupt hippocampal context representations. Although this has not been tested directly, the results of lesion studies are consistent with this idea. Inactivation of the RSC causes remapping of hippocampal place fields in a familiar environment (Cooper and Mizumori, 2001). This disruption of hippocampal firing patterns may have occurred because, without RSC input, the subjects failed to identify the familiar context, leading the hippocampus to generate a new context representation. Interestingly, RSC inactivation also impaired performance when rats were tested in a novel context (in the dark, Cooper and Mizumori, 1999). Thus, the RSC may provide cue-related information to update hippocampal spatial representations.
Information flow in the opposite direction, from the hippocampus to the RSC, is also important, particularly when the significance of a cue depends upon the context. For example, RSC neurons exhibit different responses to discriminative auditory cues depending on the context (Freeman et al., 1996). Fornix lesions, which partially disconnect the hippocampus and RSC, disrupt the context specific firing patterns in the RSC and impair the ability to learn different behaviors in different contexts (Smith et al., 2004). Thus, the hippocampal-RSC interactions that mediate contextual learning processes are bi-directional.
A growing literature from human fMRI studies highlights the shared memory role of the hippocampus and RSC. For example, both regions are active during spatial navigation (Maguire, 2001) and episodic memory (Ranganath et al., 2005; Steinvorth et al., 2006). In a result that is remarkably consistent our findings, Bar and Aminoff (2003) reported that cues with strong contextual associations evoked activity in the RSC. Several recent studies have examined the neural substrates of imagining future episodes (e.g. imagining your next birthday party). This process probably involves mentally constructing a context and placing yourself within that context (Szpunar and McDermott, 2008). The hippocampus and RSC have both been repeatedly implicated in this task (Addis et al., 2007; Schacter et al., 2008; Szpunar et al., 2007). Thus, considerable evidence indicates that the hippocampus and RSC function together in contextual memory tasks. The present results highlight this interaction while pointing out the distinct contributions each structure makes to contextual memory.
Acknowledgments
This work was supported by Grant sponsor: NIH, Grant number: MH83809 to D. Smith and Grant sponsor: NIH, Grant number: MH58755 to S. Mizumori.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–77. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-thalamic axis. Behavioral & Brain Sciences. 1999;22(3):425–444. discussion 444–489. [PubMed] [Google Scholar]
- Ainge JA, van der Meer MA, Langston RF, Wood ER. Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus. 2007 doi: 10.1002/hipo.20301. [DOI] [PubMed] [Google Scholar]
- Amaral D, Witter M. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. 2. New York: Academic Press; 1995. pp. 443–493. [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38(2):347–58. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bower MR, Euston DR, McNaughton BL. Sequential-context-dependent hippocampal activity is not necessary to learn sequences with repeated elements. J Neurosci. 2005;25(6):1313–23. doi: 10.1523/JNEUROSCI.2901-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DP, Humpartzoomian R, Boon F. Retrosplenial cortex lesions impair water maze strategies learning or spatial place learning depending on prior experience of the rat. Behav Brain Res. 2006;170(2):316–25. doi: 10.1016/j.bbr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Cho J, Sharp PE. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behavioral Neuroscience. 2001;115(1):3–25. doi: 10.1037/0735-7044.115.1.3. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. Retrosplenial cortex inactivation selectively impairs navigation in darkness. Neuroreport. 1999;10(3):625–30. doi: 10.1097/00001756-199902250-00033. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus. Journal of Neuroscience. 2001;21(11):3986–4001. doi: 10.1523/JNEUROSCI.21-11-03986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Stanley GB, Brown EN. Hippocampal plasticity across multiple days of exposure to novel environments. J Neurosci. 2004;24(35):7681–9. doi: 10.1523/JNEUROSCI.1958-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Cuppernell C, Flannery K, Gabriel M. Context-specific multi-site cingulate cortical, limbic thalamic, and hippocampal neuronal activity during concurrent discriminative approach and avoidance training in rabbits. Journal of Neuroscience. 1996;16(4):1538–49. doi: 10.1523/JNEUROSCI.16-04-01538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M. Discriminative avoidance learning: A model system. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser; 1993. pp. 478–523. [Google Scholar]
- Gill PR, Mizumori SJY, Smith DM. Hippocampal Episode Fields Develop With Learning. Hippocampus. 2010 doi: 10.1002/hipo.20832. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Involvement of the retrosplenial cortex in processing multiple conditioned stimuli. Behav Neurosci. 2008a;122(3):651–8. doi: 10.1037/0735-7044.122.3.651. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behav Neurosci. 2008b;122(5):1070–7. doi: 10.1037/a0012895. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rogers J. An analysis of independence and interactions of brain substrates that subserve multiple attributes, memory systems, and underlying processes. Neurobiol Learn Mem. 2004;82(3):199–215. doi: 10.1016/j.nlm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lee I, Griffin AL, Zilli EA, Eichenbaum H, Hasselmo ME. Gradual translocation of spatial correlates of neuronal firing in the hippocampus toward prospective reward locations. Neuron. 2006;51(5):639–50. doi: 10.1016/j.neuron.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol. 2001;42(3):225–38. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, O’Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. Journal of Neuroscience Methods. 1983;8(4):391–7. doi: 10.1016/0165-0270(83)90097-3. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Cooper BG, Leutgeb S, Pratt WE. A neural systems analysis of adaptive navigation. Mol Neurobiol. 2000;21(1–2):57–82. doi: 10.1385/MN:21:1-2:057. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handelman GE. Hippocampus, space, and memory. Brain and behavioral sciences. 1979;2:313–365. [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321(5894):1322–7. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15(8):997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957 [classical article] Journal of Neuropsychiatry & Clinical Neurosciences. 2000;12(1):103–13. doi: 10.1176/jnp.12.1.103. [DOI] [PubMed] [Google Scholar]
- Smith DM. The hippocampus, context processing and episodic memory. In: Huston JP, editor. Handbook of Behavioral Neuroscience, Vol 18, Handbook of Episodic Memory, Ekrem Dere, Alexander Easton, Lynn Nadel and Joseph P. Huston. The Netherlands: Elsevier; 2008. pp. 465–481. [Google Scholar]
- Smith DM, Freeman JH, Jr, Nicholson D, Gabriel M. Limbic Thalamic Lesions, Appetitively-Motivated Discrimination Learning and Training-Induced Neuronal Activity In Rabbits. Journal of Neuroscience. 2002;22(18):8212–21. doi: 10.1523/JNEUROSCI.22-18-08212.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJY. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006a;16(9):716–29. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJY. Learning-Related Development of Context-Specific Neuronal Responses to Places and Events: The Hippocampal Role in Context Processing. Journal of Neuroscience. 2006b;26(12):3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Wakeman D, Patel J, Gabriel M. Fornix Lesions Impair Context-Related Cingulothalamic Neuronal Patterns and Concurrent Discrimination Learning. Behavioral Neuroscience. 2004;118(6):1225–1239. doi: 10.1037/0735-7044.118.6.1225. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: an fMRI study of recent and remote memory retrieval. Neuroimage. 2006;30(1):285–98. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. Contributions of cingulate cortex to two forms of spatial learning and memory. Journal of Neuroscience. 1988;8(6):1863–72. doi: 10.1523/JNEUROSCI.08-06-01863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, McDermott KB. Episodic future thought and its relation to remembering: evidence from ratings of subjective experience. Conscious Cogn. 2008;17(1):330–4. doi: 10.1016/j.concog.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci U S A. 2007;104(2):642–7. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–46. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- van Groen T, Vogt BA, Wyss JM. Interconnections between the thalamus and retrosplenial cortex in the rodent brain. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser; 1993. pp. 478–523. [Google Scholar]
- van Groen T, Wyss JM. The connections of presubiculum and parasubiculum in the rat. Brain Res. 1990;518(1–2):227–43. doi: 10.1016/0006-8993(90)90976-i. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol. 1992;315(2):200–16. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behav Neurosci. 2005;119(6):1682–6. doi: 10.1037/0735-7044.119.6.1682. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Structural organization of cingulate cortex: Areas, neurons, and somatodendritic transmitter receptors. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser; 1993. pp. 19–70. [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77(2):125–84. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]