Abstract
Glutamatergic synapse formation has been rigorously investigated for the past two decadesat both the molecular and cell biological level yet a comparable intensity of investigation into the cellular and molecular mechanisms of GABAergic synapses has been lacking until relatively recently. This review will provide a detailed overview of the current understanding of GABAergic synapse formation with a particular emphasis on assembly of synaptic components, molecular mechanisms of synaptic development, and a subset of human disorders which manifest when GABAergic synapse development is disrupted. An unexpected and emerging theme from these studies is that glutamatergicand GABAergic synapse formation share a number of overlapping molecular and cell biologicalmechanisms that will be emphasized in this review.
Keywords: synapse development, GABAergic, glutamatergic
Introduction
The complex circuitry of the mammalian central nervous system (CNS) enables the execution of fundamental cognitive processes such as learning, speech, and memory. A given neuron, through its synaptic connections, either excites or inhibits other neurons in the circuit. If the synaptic connections of this circuit are disrupted, there are devastating consequences for nervous system function, as evidenced by the variety of neurological disorders that are thought to be the manifestation of inappropriate circuit formation. In fact, aberrant development of either excitatory or inhibitory synapses is now widely accepted as a contributing factor to many neurological impairments such as mental retardation, autism spectrum disorders, and epilepsy (Bear et al., 2004; Fernandez and Garner, 2007; Hong et al., 2005; Hong et al., 2009; Rubenstein and Merzenich, 2003; Tabuchi et al., 2007; Zoghbi, 2003)
In the central nervous system, the majority of synapses are chemical: they release neurotransmitter into the synaptic cleft, the space between the presynaptic neuron and the postsynaptic neuron. Neurotransmitters then bind to receptors in the postsynaptic membrane, and ion flow through these receptors can depolarize or hyperpolarize the postsynaptic neuron. Chemical synapses are defined by the neurotransmitter that they release. Here, we will focus our discussion on synapses in the hippocampus that release GABA (γ-aminobutyric acid), and compare and contrast the development of these synapses to those that release glutamate. Generally, mature GABAergic synapses are inhibitory, as they act to hyperpolarize the postsynaptic neuron, while glutamatergic synapses are excitatory and depolarize the postsynaptic neuron (Ben-Ari et al., 2007). While glutamatergic neurotransmission is the primary mode of excitatory neurotransmission in the hippocampus and is essential for fast neuronal communication, inhibitory synapses are thought provide a brake to neural firing and are important for a number of purposes which include, but are not limited to, preventing postsynaptic neurons from reaching threshold (Andersen et al., 1963; Buhl et al., 1994; Kandel et al., 1961; Miles and Wong, 1987), modulating the pattern of action potential firing (Andersen et al., 1964; Cobb et al., 1995; Miles and Wong, 1984), and modifying synaptic strength (Davies et al., 1991).
The vast majority of research on synapse development to date has focused on glutamatergic synapses. The deficit in knowledge with respect to GABAergic synapse development is based in large part on the difficulties inherent in a biochemical purification of material from sparse GABAergic synapses, and only relatively recently has there been significant interest in this topic. Therefore, in this review we will aim to discuss the current understanding of how GABAergic synapse development proceeds in the hippocampus, and compare and contrast to glutamatergic synapse development when it is relevant or informative.
1. The Mature GABAergic Synapse
In this section we will discuss the properties of a mature GABAergic synapse, to help guide later discussion of GABAergic synaptogenesis and maturation.
1.1 Presynaptic Terminal
Ultrastructural studies have revealed that the presynaptic terminal of GABAergic synapses contains synaptic vesicles poised at the active zone, a presynaptic structure that is the region where synaptic vesicles cluster, dock, and release into the synaptic cleft (Figure 1). Studies using aldehyde fixation techniques demonstrate that GABAergic synapses contain synaptic vesicles that are pleiomorphic in shape, whereas glutamatergic synapses contain vesicles that are larger and more uniformly spherically structured (Peters and Harriman, 1990; Peters and Palay, 1996). However, recent structural analysis of glutamatergic synapses indicates that classic aldehyde fixation of tissue samples leads to structural artifacts that may not accurately depict living tissue (Frotscher et al., 2007; Rostaing et al., 2006; Siksou et al., 2007; Siksou et al., 2009). An alternative method to aldehyde fixation, high-pressure freezing, has recently been used to study the structure of glutamatergic synapses, and shows that the synaptic terminals are larger than previously thought (Rostaing et al., 2006; Siksou et al., 2007). In addition, high-pressure freezing has revealed that synaptic vesicles are less tightly packed and small filaments linking vesicles into subgroups within the presynaptic terminal are present (Rostaing et al., 2006; Siksou et al., 2009). These results suggest that there may be structural differences in GABAergic synapses after aldehyde fixation compared to methods that maintain structural integrity more accurately. Thus far, a comprehensive analysis of GABAergic synapse ultrastructure using high-pressure freezing techniques has not been performed.
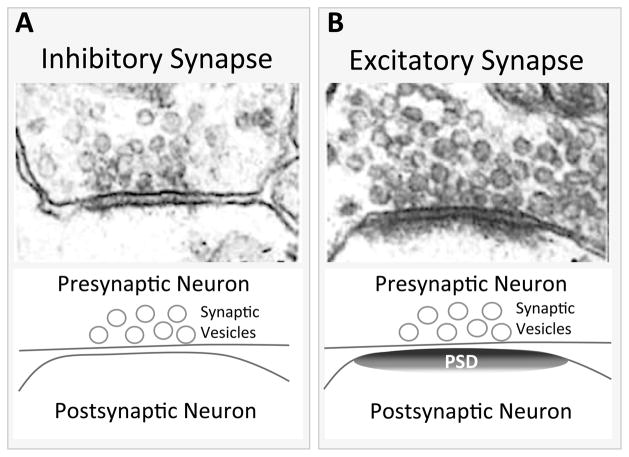
Figure 1.
(modified from Collonnier et al 1968) Top A) An electron micrograph (EM) of a symmetric, inhibitory synapse in cortex. Here, both the presynaptic membrane and the postsynaptic membrane have approximately equal electron densities. Top B) An EM image of an asymmetric, excitatory synapse in cortex. Here, you can see the presence of a postsynaptic density (PSD) and synaptic vesicles.
Bottom A and B) Schematic diagram of the EM images above, depicting the major structural entities observed.
Glutamatergic synapses contain two different functional pools of synaptic vesicles, the readily-releasable pool and the reserve pool. However, studies using the pH sensitive fluorophore synaptopHluorin have found that the readily-releasable pool is less variable in size at GABAergic synapses compared to glutamatergic synapses (Moulder et al., 2007). This was determined by measuring the fluorescence intensity of synaptopHluorin (i.e. VAMP2 tagged with a pH sensitive GFP (Burrone et al., 2006) puncta after a train of stimulation was applied to cultured rat hippocampal neurons (Moulder et al., 2007). Although this experiment allows a glimpse of the size of vesicle pools at GABAergic synapses, it is not clear if there are multiple pools as in glutamatergic synapses, and if so, how these pools are utilized in GABAergic synapses.
There are a number of different proteins that are present at both GABAergic and glutamatergic synapses that aid in the organization and release of synaptic vesicles from the presynaptic terminal. One such molecule is Synapsin I, which binds presynaptic molecules such as F-actin (Ceccaldi et al., 1993) and also binds to synaptic vesicles (Cheetham et al., 2003). Synapsins are important in regulating synaptic vesicle clustering at glutamatergic synapses (Cesca et al., 2010). The Synapsin I knockout mouse displays a decrease in the amplitude of evoked inhibitory postsynaptic currents (IPSCs) in cultured hippocampal neurons (Chiappalone et al., 2009). Using multiple probability fluctuation analysis (MPFA) of evoked IPSCs, the authors conclude that this phenotype is due to a reduction in the size of the readily-releasable pool at GABAergic synapses (Baldelli et al., 2007; Baldelli et al., 2005; Chiappalone et al., 2009). By contrast, the lack of Synapsin I leads to an increase in the amplitude of evoked excitatory postsynaptic currents (EPSCs) as a result of an increase in the size of the readily releasable pool (Chiappalone et al., 2009). GABAergic synapses also contain other active zone associated proteins, such as Bassoon, which is important in regulating the loading of synaptic vesicles to the active zone in glutamatergic synapses (Richter et al., 1999; Sanmarti-Vila et al., 2000). However, the precise role of Bassoon at GABAergic synapses remains to be determined. Presynaptic adhesion molecules, such as neurexins, play a role in the formation and stabilization of GABAergic synapses and will be discussed in detail in a later section of this review (Section 3.3).
1.2 Postsynaptic terminal
GABAergic synapses are most frequently found on the dendritic shaft or soma of the postsynaptic neuron (Gray, 1959; Peters and Palay, 1996). In contrast to a glutamatergic synapse, which has a thick, electron-dense postsynaptic density, GABAergic synapses have a much thinner postsynaptic density as visualized by electron microscopy (EM) (Colonnier, 1968; Peters and Palay, 1996), and are thus frequently referred to as “symmetric synapses” (Figure 1). Pre-embedding immunogold labeling of GABA receptors (GABARs) using high pressure freezing in the monkey subthalamic nucleus revealed a postsynaptic structure of GABAergic synapses that contains an electron dense band along the plasma membrane with a less dense filamentous region beneath (Galvan et al., 2004).
While a large number of proteins have been identified at glutamatergic synapses, relatively few have been identified at GABAergic synapses. This is in part due to the lack of a postsynaptic density (PSD) in GABAergic synapses, as synaptic proteins localized to glutamatergic synapses were largely identified by isolation of the PSD and subsequent biochemical characterization (Kim and Sheng, 2004). This, combined with the fact that GABAergic synapses are less abundant than glutamatergic synapses in cortex and hippocampus, has made it prohibitively difficult to isolate and characterize GABAergic synapses using a biochemical approach.
Despite these technical obstacles, a variety of proteins present and functioning at GABAergic synapses continue to be discovered and described. These include, but are not limited to, scaffolding proteins, cell adhesion molecules, and signal transduction proteins, all of which will be discussed in detail below (see Section 3). Of course, neurotransmitter receptors are the hallmark of the postsynaptic terminal in both GABAergic and glutamatergic synapses. In the hippocampus, ionotropic GABAA receptors (GABAARs) predominate in the GABAergic postsynaptic density (Figure 2A). GABAARs are ligand-gated ion channels that are permeable to chloride and in mature neurons, activation of these receptors leads to hyperpolarization of the cell (Ben-Ari et al., 2007).
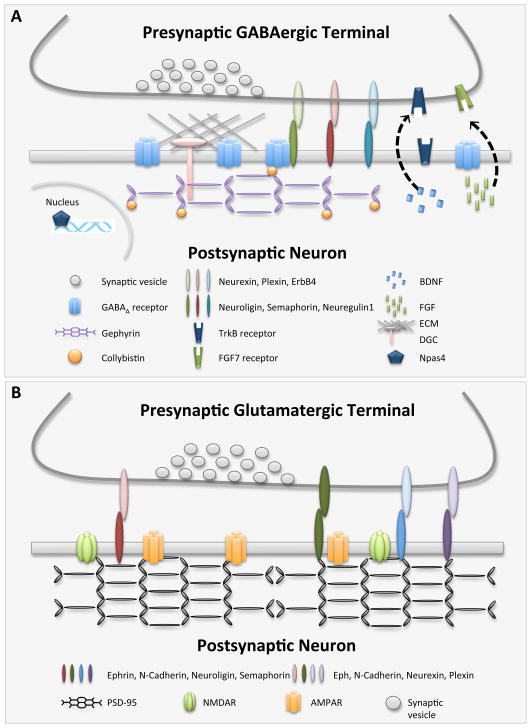
Figure 2.
A) Schematic of molecules that have been implicated in GABAergic synapse development. See Section 3 for details. B) Schematic of cell adhesion molecules that have been implicated in glutamatergic synapse development. See Section 3 and Dalva et al., 2007 for details. Note that a subset of molecules (e.g. Semaphorins, Neuroligins) are implicated in both glutamatergic and GABAergic synapse development.
GABAARs are heteromeric pentamers and the molecular makeup of each GABAR determines its functional properties. To date, 19 GABAAR subunits have been identified in the mammalian CNS. However, each of these 19 subunits can be alternatively spliced, thereby further increasing the heterogeneity of GABAARs (Cossart et al., 2006; Dumitriu et al., 2007; Freund and Buzsaki, 1996; Parra et al., 1998). A functional GABAAR requires the presence of both α and β subunits (Pritchett et al., 1989). The inter- and intracellular distribution of different GABAAR subunits helps to confer different functions and specificity to a GABAergic synapse. For example, the α1 subunit is found in the postsynaptic membrane of almost all GABAergic synapses throughout the soma and dendrites, while the α2 subunit is only located at a subset of synapses, primarily those in the axon initial segment (Baumann et al., 2001; Chang et al., 1996; Farrar et al., 1999; Nusser et al., 1996). The γ2 subunit of the GABAAR is synaptically localized (Essrich et al., 1998). Functional GABAARs form in the absence of the γ2 subunit, however these synapses are primarily localized to extrasynaptic sites, indicating that the γ2 subunit is required for the proper localization of GABAARs at synapses (Essrich et al., 1998) Mice genetically devoid of the γ2 subunit of GABAARs proceed normally through embryonic development. However, postnatal development of γ2 −/− mice is marked by sensorimotor dysfunction and retarded growth, and most only live for a few hours after birth (Essrich et al., 1998; Gunther et al., 1995).
Another type of GABAR, the metabotropic GABAB receptor (GABABR), plays an important role in the postsynaptic terminal of GABAergic synapses. Metabotropic GABABRs function through Go and Gi proteins that can lead to a number of different downstream consequences, such as activation of potassium-permeable ion channels (Gahwiler and Brown, 1985) or inhibition of adenylyl cyclase, leading to a decrease in protein kinase A (PKA) activity (Chalifoux and Carter, 2011; Xu and Wojcik, 1986). Unlike GABAARs, where 19 different subunits have been identified, only two subunits have been discovered for the GABABRs, GABAB1/B2 (Bowery and Brown, 1997; Isomoto et al., 1998; Kaupmann et al., 1997; Pfaff et al., 1999; Schwarz et al., 2000). GABABRs elicit a number of different downstream signals, presumably in part by signaling through auxiliary binding proteins such as GISP and Mupp1 (Balasubramanian et al., 2007; Chalifoux and Carter, 2011; Kantamneni et al., 2007).
Postsynaptic GABABRs are located extrasynaptically and are activated by GABA spillover (Kulik et al., 2003; Scanziani, 2000). In hippocampal pyramidal neurons, the activation of GABABRs can lead to hyperpolarization of the postsynaptic cell (Dutar and Nicoll, 1988; Nicoll et al., 1990; Scanziani, 2000). This hyperpolarization is distinct from the hyperpolarization elicited by GABAARs, as it is slower and prolonged (Dutar and Nicoll, 1988), and is necessary for rhythmic hippocampal activity (Scanziani, 2000). GABABRs are also found in the presynaptic terminal of glutamatergic synapses and in GABAergic axons (Chalifoux and Carter, 2011; Kulik et al., 2003; Vigot et al., 2006). There are a number of different roles of presynaptic GABABRs; they can activate K+ channels and indirectly limit calcium release (Thompson and Gahwiler, 1992), as well as suppress multivesicular release (Chalifoux and Carter, 2010).
Despite our limited knowledge of the ultrastructural and molecular composition of GABAergic synapses at the present time, thus far it seems that the overall organization of GABAergic postsynaptic terminals is similar to glutamatergic postsynaptic sites, but with different molecular players. Currently, there is renewed interest in GABAergic synapse composition and development and it will be interesting to see how this vision changes as more focus is directed toward GABAergic synapses.
2. Assembly of GABAergic synapses
Although identifying the major events in glutamatergic synapse assembly has been a focus in the field of synapse development for many years (Ahmari et al., 2000; Dai and Peng, 1996; Gerrow et al., 2006; Sabo et al., 2006; Shapira et al., 2003; Zakharenko et al., 1999; Zhai et al., 2001), assembly of GABAergic synapses is considerably less well- understood. Here, we will discuss what is known about the order of events that comprise GABAergic synapse assembly.
2.1 First Contact
An initial step in synapse development is contact between the presynaptic axon and the postsynaptic dendrite and subsequent stabilization of this contact. The Bonhoeffer group has provided an intriguing look at this issue using two-photon laser scanning microscopy in hippocampal slice cultures derived from GAD65-GFP expressing mice, which express GFP in a subset of interneurons in the hippocampus (Wierenga et al., 2008). By visualizing GABAergic presynaptic boutons in this manner, and postsynaptic CA1 pyramidal cell dendrites by Alexa Fluor 594 injection, the authors observed dendritic and axonal protrusions that came in contact with each other; however, these contacts were always transient (Wierenga et al., 2008). Instead, mature GABAergic synapses form at pre- existing axo-dendritic crossings and are marked by the appearance of new, stable GABAergic boutons (Wierenga et al., 2008). In addition, retrospective immunostaining after time-lapse imaging indicates that new synapses form on the order of a few hours (Wierenga et al., 2008), similar to the time reported for glutamatergic synapse formation (Friedman et al., 2000). This study represents the first report that visualizes the initial contact between a GABAergic axon and its postsynaptic partner, and provides exciting evidence that initiation of contact in GABAergic synapse development has both marked similarities and differences when compared to glutamatergic synaptogenesis. Future studies likely will aim to elucidate which proteins are recruited to young GABAergic synapses, and how these proteins arrive at the synapse.
2.2 Arrival of Synaptic Components
Unlike glutamatergic synapses which have been extensively studied by time-lapse imaging of fluorescently-tagged proteins such as the AMPA receptor (ionotrophic glutamate receptor α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptor; AMPAR) subunits and PSD-95 (Barrow et al., 2009; Bresler et al., 2001; Correia et al., 2008; Friedman et al., 2000; Gerrow et al., 2006; Washbourne et al., 2002; Washbourne et al., 2004), the order of assembly of the components of the pre- and postsynaptic specializations of GABAergic synapses has not been elucidated. However, it is instructive to summarize what has been learned from such studies of glutamatergic synapse development to provide a framework with which to think about GABAergic synapse development.
Significant evidence supports vesicular transport of both pre-and postsynaptic proteins (McAllister, 2007). For example, an emerging view of postsynaptic assembly reveals that mobile transport packets deliver preformed scaffold complexes containing PSD-95, GKAP, and Shank to nascent synapses (Gerrow et al., 2006; Prange and Murphy, 2001). Scaffolding proteins accumulate in the postsynaptic terminal concurrently with ionotrophic glutamate receptors, AMPARs and NMDARs (N-methyl D-aspartate receptor) (Barrow et al., 2009; Bresler et al., 2001; Friedman et al., 2000; Gerrow et al., 2006; Washbourne et al., 2002). Presynaptic assembly is also characterized by the accumulation of discrete transport vesicles that carry a characteristic set of presynaptic molecules. These transport vesicles can accumulate at a specific site to begin formation of the presynaptic active zone (Ahmari et al., 2000; Shapira et al., 2003; Zhai et al., 2001). This pre-fabrication and organization of synaptic components into modular packets could facilitate the rapid rate of synaptogenesis, which can occur in 30–60 minutes (Friedman et al., 2000).
To date, it is unclear if GABAergic synapses use similar transport vesicles to deliver components to the synapse. However, on the postsynaptic side, time-lapse imaging has been used to investigate the trafficking of GABAARs in and out of mature synapses (for comprehensive reviews see (Jacob et al., 2008; Luscher et al., 2011). To summarize a large and ongoing body of work, GABAARs assemble in the endoplasmic reticulum, are transported through the secretory pathway, and are then inserted into the plasma membrane. Some mechanisms by which GABAARs are trafficked to and retained at synaptic sites will be discussed below (Section 3). For example, binding of GABAARs to components of the GABAergic postsynaptic scaffold such as gephyrin and collybistin could serve to recruit or retain GABAARs at synaptic sites (Bannai et al., 2009; Bogdanov et al., 2006).
Kinesin family motor proteins have been implicated in the transport of GABAARs from internal compartments to extrasynaptic and synaptic sites (Twelvetrees et al., 2010), as demonstrated by immunostaining, live imaging, and electrophysiological recordings of synaptic currents from cultured neurons. For example, when the function of the kinesin KIF5 is blocked with a kinesin-specific antibody, the amplitude of miniature inhibitory postsynaptic currents (mIPSCs) decreases, while the frequency of mIPSC events remains the same as control conditions (Twelvetrees et al., 2010). In addition, coimmunoprecipitation using GABAAR β subunits pulls down KIF5 as well as the adaptor protein Huntingtin-associated protein HAP1 (Twelvetrees et al., 2010). These and other experiments from this study suggest that disruption of GABAAR trafficking may underlie some of the etiology of Huntington’s Disease.
2.3 Localization of Synaptic Contacts
During development, synapses form within specific regions on the postsynaptic neuron. For example, the excitatory synapses on pyramidal neurons and cerebellar Purkinje neurons form primarily onto dendritic spines, while GABAergic synapses are localized to the dendritic shaft or cell soma (Lardi-Studler and Fritschy, 2007; Tretter and Moss, 2008; Wierenga et al., 2008). Recent studies have suggested that a “molecular code” regulates the subcellular organization of GABAergic synapses. For example, in the cerebellar cortex, synapses from inhibitory stellate cells form onto the dendrites of Purkinje cells, while inhibitory basket cells localize their synapses onto the Purkinje cell soma and axon initial segment (Ango et al., 2004; Lardi-Studler and Fritschy, 2007). Neurofascin186, a cell adhesion molecule, is expressed as a gradient along the axon initial segment of Purkinje cells (Ango et al., 2004). This gradient is necessary for proper localization of basket cell innervation and is demarcated by the scaffolding protein ankyrin- G (Ango et al., 2004).
In addition to cell adhesion molecules, proper synapse localization can be dictated through postsynaptic receptors, such as the GABAAR α1 subunit. Mice lacking the GABAAR subunit α1 are not able to maintain GABAergic synapses from stellate cells onto Purkinje cells in the cerebellum. However, basket cells are unaffected by the absence of this postsynaptic receptor in the innervated Purkinje cell soma (Fritschy et al., 2006). It is likely that the differential expression of receptor subunits throughout the neuron helps to orchestrate the complex pattern of connections between different types of neurons. This may be accomplished by receptor subunits selectively binding to specific cell adhesion molecules within the postsynaptic site, which are in turn recognized by their binding partners expressed at presynaptic terminals in axons of specific subtypes of neurons. Examples of such molecules that are known to mediate GABAergic synapse formation are discussed below (Section 3).
2.4 Relative order of Glutamatergic and GABAergic Synapse Development in Hippocampus
The weight of the evidence suggests that GABAergic synapse development is initiated prior to glutamatergic synapse development in the hippocampus (Hennou et al., 2002; Tyzio et al., 1999). Although nowhere near as well-studied as in the hippocampus, this order seems to be recapitulated during development of other areas of the CNS such as the neocortex and spinal cord (for a comprehensive discussion of this issue see (Ben-Ari et al., 2007)). Electrophysiological recordings from CA1 neurons in acute hippocampal slices taken from postnatal day 0 (P0) rats demonstrate that the vast majority of pyramidal cells are synaptically silent (Tyzio et al., 1999). Of the non-silent neurons, approximately equal numbers of neurons contain GABAergic synapses only or GABAergic and glutamatergic synapses, and synapse number increases as the neurons mature (Tyzio et al., 1999). In addition, electrophysiological recordings from interneurons in acute hippocampal slices taken from rats at P0 reveals that approximately 20% of interneurons have only GABAergic synapses while approximately 75% of interneurons have both glutamatergic and GABAergic synapses (the remaining 5% are silent) (Hennou et al., 2002). Thus, the order of synaptogenesis is similar between pyramidal and interneurons. Further, on the basis of this study and others, the presence of an interneuron circuit has been proposed, where interneurons first innervate other interneurons before they innervate pyramidal cells (Ben-Ari et al., 2004; Gozlan and Ben-Ari, 2003; Gulyas et al., 1996). This is perhaps not surprising, as interneurons are more mature than pyramidal cells as they migrate into the hippocampus.
In contrast, the first glutamatergic synapses are established around birth to postnatal day two in hippocampal CA1 pyramidal neurons as assayed by whole-cell voltage clamp recordings (Durand et al., 1996; Tyzio et al., 1999). However, most of these synapses are ‘silent,’ meaning that they contain only NMDARs and not AMPARs, and therefore are not able to participate in rapid synaptic communication (Durand et al., 1996; Wu et al., 1996). The percentage of silent synapses steadily decreases around postnatal day five until they represent only about 20% of the synapse population after postnatal week 1 (Ben-Ari et al., 1997).
However, even though GABAergic synapse development begins a few days before glutamatergic synapse development, robust synapse development continues throughout the first few postnatal weeks of life, and of course proceeds throughout the life of the animal. Therefore, much of GABAergic and glutamatergic synapse development occurs concurrently (Chen et al., 1995; Hennou et al., 2002; Koller et al., 1990; Tyzio et al., 1999; Walton et al., 1993). This leads to an interesting set of problems for say, a developing CA1 pyramidal neuron onto which glutamatergic and GABAergic synapses are simultaneously forming. For example, what is the signaling mechanism by which glutamate receptors get trafficked to glutamatergic postsynaptic terminals and not GABAergic terminals?
Some insight into these issues might come from studies performed using retrospective immunostaining of dissociated cultures of hippocampal neurons isolated from embryonic day (E18) rat pups. These results of these studies indicate that glutamatergic synapse development precedes GABAergic synapse development (Danglot et al., 2006). This finding is also recapitulated by the work of Deanna Bensen and colleagues discussed below in Section 2.5 (Anderson et al., 2004). It is not clear why these neuronal culture studies do not recapitulate the studies of acute neonatal hippocampal slice discussed above. However, dissociation of neurons before plating involves the shearing of the neuronal processes, which must regrow in culture. Therefore, any synaptic contacts that had already been established in vivo must be re-established in vitro, and this appears to take a few days. Thus, it could be that this “re-setting” of synapse development overlaps with the period of time when robust glutamatergic and GABAergic synapse development is occurring simultaneously, and thus the relative order is lost. Alternatively, it could be that the molecular mechanisms that govern glutamatergic synapse development are more promiscuous or inappropriately activated in the context of dissociated cultures of neurons.
2.5 Fidelity of Synapse Assembly
Using time-lapse confocal imaging of glutamatergic synapses in the CA3 region of hippocampus in organotypic slice culture, the Bonhoeffer group demonstrated that dendritic filopodia differentiate between a glutamatergic axon and GABAergic axon (Lohmann and Bonhoeffer, 2008). They show that a local calcium transient occurs in a dendritic filipodium within seconds after making initial contact with a presynaptic axon, and is therefore unlikely to be synaptic in origin (Lohmann and Bonhoeffer, 2008). Interestingly, these dendritic filopodia selectively establish contact with glutamatergic axons and not with GABAergic axons, and these calcium transients occur at higher frequencies when contacting glutamatergic axons (Lohmann and Bonhoeffer, 2008). This elegant study demonstrates not only that dendritic filopodia are capable of selectively choosing their targets, but that they do so via a contact-dependent calcium transient.
Despite this intriguing study, there is an accumulating body of evidence that demonstrates “mismatching” of glutamatergic and GABAergic synaptic elements during the early stages of synapse development. The group of Deanna Benson performed a comprehensive study, using immunostaining for various glutamatergic and GABAergic synaptic proteins, of the protein composition of synapses while they were forming in cultured hippocampal neurons over more than two weeks in vitro (Anderson et al., 2004). They found that early in the development of the cultures, for example at 7 days in vitro (DIV), a significant percentage of GAD65 positive presynaptic terminals co-stain for both gephyrin and PSD-95, postsynaptic GABAergic and glutamatergic synaptic markers, respectively (Anderson et al., 2004). By 17 DIV however, the percentage of mismatching significantly decreases. Additionally, the authors made similar observations at glutamatergic presynaptic terminals: a subset of PSD-95 clusters are observed apposed to GABAergic presynaptic markers such as GAD65 in young cultures (Anderson et al., 2004). Interestingly, blockade of GABAAR or NMDAR activity from 7 to 17 DIV leads to a decrease in fidelity of matching at GABAergic terminals, indicating that activity contributes to matching of proper components at GABAergic synapses (Anderson et al., 2004). In contrast, NMDAR blockade leads to an increase in fidelity of matching of glutamatergic synapses (Anderson et al., 2004).
Other groups have made similar observations in traditional hippocampal cultures (Brunig et al., 2002), autaptic cultures of hippocampal neurons (Rao et al., 2000), cultured cerebellar granule cells (Studler et al., 2002) and Purkinje cells (Fritschy et al., 2006). Importantly, mismatched synapses have also been observed in vivo in adult rodents, where immuno-EM of mossy fiber to granule cell synapses in cerebellum revealed the presence of both GABAAR and GluA subunit of AMPARs opposed to these glutamatergic terminals (Nusser et al., 1998). Also, double-innervation of dendritic spines in cortex by glutamatergic and GABAergic inputs has been reported (Micheva and Beaulieu, 1995; Micheva et al., 2010).
Taken together, these studies suggest that glutamatergic and GABAergic synapse development are neither spatially nor temporally separable processes. It seems reasonable to postulate that as transient contacts between axons and dendrites are formed, components of the postsynaptic specializations of both glutamatergic and GABAergic synapses, such as scaffolding proteins and neurotransmitter receptors, are shuttled to these nascent synaptic sites in a stochastic manner. As synapse development proceeds, the identity of the synapse as either glutamatergic or GABAergic is established by retention of the appropriate synaptic proteins, perhaps in an activity-dependent manner. It could be that examples of “mismatched” synapses from intact tissue preparations are rare simply because researchers have not yet examined this question in a systematic manner. Along those lines, recent work has provided evidence for co-release of GABA and glutamate at synapses in hippocampus, cortex, and cerebellum (Boulland et al., 2004; Noh et al., 2010; Somogyi et al., 2004; Zander et al., 2010). Thus, it could be that “mismatched” synapses are actually synapses that are designed to respond to co-release of two different neurotransmitters from the same presynaptic terminal.
3. Molecular Mechanisms of GABAergic Synapse Development
Biochemical and candidate gene approaches by a large number of labs over the past thirty years have led to the identification of many molecules that function at excitatory, glutamatergic synapses in processes such as neurotransmitter release and reception. However, despite this knowledge, we have only just begun to define the molecules that mediate the actual assembly of the glutamatergic synapse. And, in contrast to glutamatergic synapse formation, even less is known about inhibitory, GABAergic synapse formation in mammals. Notably, the only directed screen to identify genes that are specifically required for GABAergic synapse formation and transmission published to date was performed in C. elegans (Vashlishan et al., 2008). This study implicated a number of molecules in regulating GABAergic synaptic transmission, a subset of which may be involved in assembling the appropriate pre- and postsynaptic specializations.
In this section, we will focus our discussion on molecules that have been shown to play a role specifically in inhibitory synapse formation, with a focus on GABAergic synapses (Figure 2A). Interestingly, a number of these molecules/families of molecules have been shown to play a role in both glutamatergic and GABAergic synapse formation. We will discuss the role of these proteins in GABAergic synapse formation specifically. As a number of recent reviews have comprehensively detailed the identity and mechanism of action of molecules that are thought to be involved in mediating glutamatergic synapse development (Dalva et al., 2007; McAllister, 2007; Scheiffele, 2003), we will not discuss these molecules further.
3.1 Gephyrin
Gephyrin was originally identified as a polypeptide that co-purifies with glycine receptor (GlyR) subunits (Pfeiffer et al., 1982). Gephyrin is expressed throughout the nervous system and in non-neuronal tissues (Fritschy et al., 2008), is a prominent component of the postsynaptic specialization of glycinergic and GABAergic synapses, and is exclusively localized to these synapses (Sassoe-Pognetto et al., 1995).
For many years, gephyrin has been hypothesized to function analogously to the MAGUK scaffolding proteins such as PSD-95 at excitatory synapses that bind to and localize neurotransmitter receptors at the postsynaptic density (Kim and Sheng, 2004). Support for this model arises in part from the fact that gephyrin can form both dimers and trimers and further, these interactions can lead to a hexagonal lattice of gephyrin molecules (Fritschy et al., 2008). Surprisingly, cloning and characterization of gephyrin revealed that it is a mediator of molybdenum cofactor (Moco) biosynthesis (Feng et al., 1998), which is required for oxidation of nitrate and nitrite and sulfate to sulfite (Fritschy et al., 2008). This family of enzymes is well-conserved from plants to bacteria to animals, and perhaps gephyrin the result of an ancient gene fusion event, which resulted in a multimeric protein with a novel function (Feng et al., 1998). Interestingly, it remains to be determined if the two known functions of gephyrin (i.e. clustering of neurotransmitter receptors, and Moco biosynthesis) are related (Fritschy et al., 2008). An analysis of the spinal cord and hypothalamus of mutant mice in which the entire gephyrin gene was constitutively deleted revealed an absolute requirement for gephyrin in clustering of GlyRs at synapses (Feng et al., 1998).
In contrast, a requirement for gephyrin to localize GABAARs to the synapse is less clear. The gephyrin knockout mice exhibit a decrease in the number of synaptically- localized GABAR puncta in retina, spinal cord, and hippocampus (Fischer et al., 2000; Kneussel et al., 2001; Kneussel et al., 1999; Levi et al., 2004). Whole-cell voltage clamp recordings of mIPSCs from cultured hippocampal neurons obtained from gephyrin knockout mice reveal a modest decrease in the amplitude of these events and no change in the frequency (Levi et al., 2004). In addition, demonstration of a direct interaction between gephyrin and GABAARs has been elusive (Meyer et al., 1995), although recently, an interaction between the α2 subunit of the GABAAR and gephyrin has been reported (Saiepour et al., 2010; Tretter et al., 2008). Additional work from the laboratory of Stephen Moss has also demonstrated that cell-surface GABAARs are more mobile and less likely to form synaptic clusters in the absence of gephyrin (Jacob et al., 2005).
The fact that at least a subset of GABAAR subunits are able to localize at synaptic sites and form functional synapses in the absence of gephyrin suggests that other, non- gephyrin dependent mechanisms exist to localize GABAARs to synapses. Interestingly, post- synaptic clustering of gephyrin is disrupted in the absence of certain GABAARs subunits (Essrich et al., 1998; Kralic et al., 2006; Studer et al., 2006) suggesting a dynamic relationship between gephyrin and GABAARs. Further, an immuno-EM study of gephyrin knockout mice examining synapses in the brainstem of P0 mice demonstrated that GABA and VIAAT-positive synapses are morphologically normal in the absence of gephyrin (O’Sullivan et al., 2009). Thus, although gephyrin clearly plays an important role in modulating GABAAR subunit abundance at symmetric synapses, the precise nature of this modulation and the molecular mechanism by which it is achieved are not well understood.
3.2 Collybistin
Collybistin is a brain-specific Rho-family guanine nucleotide exchange factor (GEF) that was originally identified in a yeast two-hybrid screen as protein that interacts with gephyrin (Kins et al., 2000). Work from a number of labs has implicated collybistin as a factor required for clustering gephyrin and GABAARs, and mouse models in which the collybistin gene has been deleted support this hypothesis (Harvey et al., 2004; Kins et al., 2000; Papadopoulos et al., 2008; Papadopoulos et al., 2007). In support of the crucial role of collybistin in mediating GABAergic synapse development, a human patient presenting with a variety of symptoms including mental retardation, seizures, and increased anxiety was found to have a mutation in the collybistin gene (Kalscheuer et al., 2009).
Interestingly, the presence of the collybistin N-terminal SH3 domain interferes with the ability of collybistin to form gephyrin aggregates in non-neuronal cells (Harvey et al., 2004; Kins et al., 2000), presumably through an auto-inhibitory mechanism, and a recent study has uncovered a possible mechanistic explanation for this observation (Poulopoulos et al., 2009). Binding of the collybistin SH3 domain to the intracellular domain of the Neuroligin 2 protein allows a collybistin/gephyrin complex to nucleate assembly of a gephyrin scaffold specifically at the GABAergic postsynaptic membrane and recruit GABAARs (Poulopoulos et al., 2009) (see further discussion of Neuroligin 2 below). However, another study demonstrated that overexpression of an isoform of collybistin which lacks the SH3 domain in cultured hippocampal neurons is able to induce synaptic clustering of overexpressed gephyrin without a concomitant increase in Neuroligin 2 clustering (Chiou et al., 2011). Thus, the precise relationship and timing of interactions between Neuroligin 2, collybisitin, and gephyrin clustering remains to be determined. Along those lines, a group investigating the relationship between gephyrin, collybistin, and various subunits of the GABAAR demonstrated that collybistin itself binds directly to the α2 subunit of the GABAAR in yeast and HEK293 cells (Saiepour et al., 2010), suggesting a more direct role for collybistin in GABAAR clustering than was previously assumed. In addition, the authors of this study provide evidence that GABAARα2, gephyrin, and collybistin are able to simultaneously co-associate (Saiepour et al., 2010), suggesting a complex interaction between these proteins to regulate GABAAR synaptic clustering.
3.3 Neurexin-Neuroligin Signaling
The presynaptic, transmembrane neurexin proteins and postsynaptic, transmembrane neuroligin proteins comprise a large family of trans-synaptic, calcium- dependent cell adhesion molecules that function to regulate synapse development (Craig and Kang, 2007; Dalva et al., 2007; Dean and Dresbach, 2006; Huang and Scheiffele, 2008). In mammals, the neurexins are encoded by three genes, and transcription from two different promoters produces an α or β neurexin transcript from each gene (Dalva et al., 2007). Additional alternative splicing generates more than 1000 neurexin isoforms (Dalva et al., 2007). The neuroligins are encoded by at least 4 genes in rodents (Nlgn1-4); Neuroligin 1 is localized primarily to excitatory synapses (Song et al., 1999) while Neuroligin 2 is localized predominantly to GABAergic synapses (Varoqueaux et al., 2004)(Figure 2). Interestingly, alternative splicing in the extracellular domain of both neurexins and neuroligins regulates binding selectivity and influences function at the synapse (Boucard et al., 2005; Chih et al., 2006; Graf et al., 2006). Both neuroligins and neurexins are transmembrane proteins, contain PDZ domains in their intracellular, C- terminal regions, and have been shown to interact with putative scaffolding proteins such as PSD-95 (see references within (Craig and Kang, 2007; Dalva et al., 2007)).
The current interest in these proteins as synaptogenic molecules stems from the work of Peter Scheiffele and colleagues who discovered that expression of neuroligins in heterologous cells drives assembly of presynaptic structures in co-cultured neurons (Scheiffele et al., 2000). Using a similar heterologous cell/neuron co-culture system (Craig et al., 2006), a subsequent study demonstrated that expression of β-neurexins is sufficient to drive assembly of both glutamatergic and GABAergic postsynaptic specializations in neurons (Graf et al., 2004). Since that time, results from a large number of in vitro experiments suggested a model where an interaction between a postsynaptic neuroligin and a presynaptic β-neurexin induces the formation of glutamatergic and GABAergic synapses (Chih et al., 2005; Dean et al., 2003; Graf et al., 2004; Scheiffele et al., 2000).
Interestingly, in vivo work has both supported and extended this relatively straightforward model. Analysis of mice in which the Nlgn1,2,3 genes have been constitutively deleted reveals that the predominant function of these proteins is to mediate GABAergic synaptic transmission and development (Varoqueaux et al., 2006). These mice die at birth from respiratory failure, and an analysis of brainstem circuitry reveals a significant deficit in GABAergic, glycinergic, and glutamatergic synaptic transmission, but only modest changes in synapse number ((Varoqueaux et al., 2006) see particularly Figs 4– 6). Similarly, a mouse in which all three α-neurexin isoforms are deleted displays a similar phenotype and also has a decrease in GABAergic synapse density in brainstem (Missler et al., 2003). A careful analysis of the phenotype of single Nlgn1−/− or Nlgn2−/− mice revealed that neuroligin 1 acts specifically to promote the functional maturation of glutamatergic synapses while neuroligin 2 acts specifically to promote the functional maturation of GABAergic synapses (Chubykin et al., 2007). Taken together, the in vitro and in vivo data suggest a model where the neuroligin/neurexin adhesion complex acts at multiple steps in synapse development, perhaps stabilizing nascent contacts and recruiting molecules such as neurotransmitter receptors to synapses as they are developing. In addition, the rather modest reductions in both synapse number and synaptic transmission in the various neurexin and neuroligin knockout mouse models suggest that these molecules certainly act redundantly with other proteins to promote synapse development.
Although the neurexin/neuroligin proteins were initially studied as molecules which promote excitatory synapse development, the specificity of Neuroligin 2 localization to GABAergic synapses and the prominent deficit in GABAergic synapse development in mice in which neurexin/neuroligin signaling is perturbed has led to an increased interest in the role of these proteins in GABAergic synapse development. To this end, two relatively recent studies deserve specific discussion. As mentioned above, the cytoplasmic domain of Neuroligin 2 binds both gephyrin and collybistin, resulting in nucleation of a gephyrin/collybistin scaffold at GABAergic synapses (Poulopoulos et al., 2009). Further, the neuroligin 2/gephyrin/collybistin complex mediates clustering of GABAARs at synapses (Poulopoulos et al., 2009). Intriguingly, neurexins directly interact with and impair the function of synaptic GABAARs, presumably through a trans-synaptic mechanism (Zhang et al., 2010a). Thus, it seems as though neuroscientists are only beginning to uncover the numerous and varied functions of these cell adhesion complexes in synapse development.
3.4 Dystrophin
Dystrophin was initially cloned and characterized in 1987; mutations in this gene are the underlying cause of Duchenne muscular dystrophy, a disorder that is characterized by muscle wasting and mental retardation (Hoffman et al., 1987). Dystrophin is expressed in the muscle and brain and is now known to be a major component of a molecularly heterogenous complex called the Dystrophin-associated glycoprotein complex (DGC) (Pilgram et al., 2010). Despite decades of intensive investigation, the function of the DGC is not well understood, although it is largely believed to form a scaffold which spans the plasma membrane and is thought to connect the extracellular matrix to the intracellular actin cytoskeleton (Pilgram et al., 2010). The DGC has been shown to play a role in neurotransmitter receptor clustering and synaptic transmission at both neuromuscular junction (NMJ) and CNS synapses from worms to mammals (Pilgram et al., 2010). Specifically, immunohistochemistry experiments reveal that dystrophin clusters co-localize with clusters of both α1 and α2 subunits of GABAAR in hippocampus and cerebellum (Brunig et al., 2002) (Knuesel et al., 1999). Further, GABAARα1 and α2 clusters are decreased in the hippocampus and cerebellum of mice lacking the full-length dystrophin isoform (Knuesel et al., 1999). In addition, these same mice also show a reduction in the number of GABAAR α2 clusters and the frequency of norepinephrine-induced GABAergic IPSCs in amygdala (Sekiguchi et al., 2009). Taken together, these results, in conjunction with similar analyses of other components of the DGC (see refs in (Pilgram et al., 2010)) point to an important role for the DGC in clustering GABAARs at synapses.
3.5 Neuregulin 1/ErbB4 Signaling
Neuregulin 1 (NRG1) and its receptor ErbB4 have been in the spotlight recently due to the identification of both genes as schizophrenia susceptibility loci (Mei and Xiong, 2008). The NRG1 locus generates at least 31 different isoforms which are either cleaved or membrane-bound; all isoforms contain an epidermal growth factor (EGF)-like domain (Mei and Xiong, 2008). The ErbB4 receptor tyrosine kinase is one of four potential NRG1 receptors (ErbB1-4) and is the only one in which NRG1 binding activates ErbB tyrosine kinase activity (Mei and Xiong, 2008). NRG1/ErbB4 signaling has been implicated in a variety of developmental processes in the nervous system including the migration of both pyramidal and interneurons, axon guidance, axon myelination, and synapse formation at the NMJ (Mei and Xiong, 2008).
Previous work from a number of groups suggests a role for NRG1 and ErbB4 in mediating glutamatergic synapse development. For example, ErbB signaling regulates glutamatergic synaptic transmission and dendritic spine number and size in pyramidal neurons in vitro and in vivo (Barros et al., 2009; Li et al., 2007). However, it is entirely unclear if this is due to a cell automonous effect of ErbB4 function in pyramidal neurons, or is perhaps due to a local circuit effect, a hypothesis supported by new experimental evidence outlined below (Wen et al., 2010). In fact, although the expression pattern of ErbB4 in the rodent brain remains controversial (Garcia et al., 2000; Huang et al., 2000; Woo et al., 2007), the weight of the evidence now points in favor of ErbB4 expression in a subset of GABAergic interneurons in cortex and not in glutamatergic, pyramidal cells (Fazzari et al., 2010; Vullhorst et al., 2009). Specifically, both immunostaining and electron microscopy indicate that ErbB4 is predominantly expressed by parvalbumin-positive cortical interneurons, where it can be found both in the axon and in the postsynaptic density of asymmetric synapses formed onto these interneurons (Fazzari et al., 2010).
After making this discovery, the Marin and Rico groups went on to perform a genetic analysis to demonstrate that ErbB4 is cell-autonomously required in interneurons to mediate GABAergic synapse formation. To reach this conclusion, the authors performed both a morphological and functional analysis of parvalbumin-positive chandelier cells in which ErbB4 expression was eliminated using Cre-mediated recombination in two independent strains of mice and found that the number of GABAergic synapses made by these cells was reduced (Fazzari et al., 2010). Performing similar experiments, the authors did not observe a requirement for ErbB4 in pyramidal neurons to form glutamatergic synapses onto other pyramidal cells (Fazzari et al., 2010). In addition, these effects are presumably mediated by a Nrg1/ErbB4 ligand-receptor interaction, as overexpression of Nrg1 by pyramidal neurons increases the number of GABAergic synapses formed onto these cells (Fazzari et al., 2010). Thus, the loss-of-function and gain-of-function data agree and support a requirement for ErbB4 signaling in interneurons to mediate GABAergic synapse formation.
3.6 FGF7
Fibroblast Growth Factors (FGFs) are secreted glycoproteins which mediate their diverse biological functions in large part through binding to and activating FGF receptor tyrosine kinases (Turner and Grose, 2010). The range of processes in which FGF signaling has been implicated includes patterning the early embryo, organogenesis, and cell proliferation and survival (Turner and Grose, 2010). FGFs were initially shown to regulate presynaptic differentiation of motoneurons and mossy fibers from the cerebellum both in vitro and in vivo (Umemori et al., 2004). To follow up on this finding, the Umemori group chose to examine two particular family members, FGF7 and FGF22, in detail in the hippocampus. This group examined both the intact brain of mice in which either FGF7 or FGF22 was deleted, in combination with studies of neuronal cultures obtained from these animals, to define a role for FGF signaling in the pyramidal neurons of the CA3 region of the hippocampus. Specifically, it was shown that FGF7 is localized to the postsynaptic specialization of GABAergic synapses, and is required to promote the clustering of synaptic vesicles at the presynaptic termini of GABAergic synapses while having no effect on glutamatergic synapses (Terauchi et al., 2010). Interestingly, FGF22 is localized to the postsynaptic density of glutamatergic synapses, and is required in the same cells as FGF7 to promote clustering of synaptic vesicles at the presynaptic termini of glutamatergic synapses. Thus, FGF7 represents the only molecule thus far implicated in the presynaptic differentiation of GABAergic synapses specifically.
3.7 Npas4
Npas4 is a transcription factor whose expression is strongly upregulated in neurons by calcium influx in response to neuronal depolarization (Lin et al., 2008). Knockdown of Npas4 expression by RNAi causes a decrease in the density of GABAergic synapses formed onto pyramidal neurons in the hippocampus while overexpression of Npas4 increases GABAergic synapse density (Lin et al., 2008). Among many identified Npas4 targets, it was shown that Npas4 regulates BDNF transcription in response to neuronal depolarization, and that the effect of Npas4 on GABAergic synapse density is partially dependent on BDNF (Lin et al., 2008). Further studies of Npas4 targets should help to elucidate the molecular mechanisms by which neuronal activity regulates the density of GABAergic synapses and in turn, the balance of excitation and inhibition in the nervous system.
3.8 Semaphorins
The Semaphorin family of proteins consists of over 25 members grouped into eight different classes based largely on their sequence homology and protein domain structures. The hallmark of a Semaphorin family member is the extracellular Semaphorin (Sema) domain: a conserved, cysteine-rich region of ~500 amino acids at the N-terminus of the protein (Yazdani and Terman, 2006). Semaphorins are perhaps best known by the function of the secreted, Class 3 Semaphorins as axon guidance molecules in the developing nervous system, although the majority of Semaphorins, including Class 4 Semaphorins, are in fact membrane-associated proteins. Semaphorin function has been implicated in numerous developmental processes outside of the nervous system, including lymphocyte specification and signaling, angiogenesis, cell migration, and vascular and heart morphogenesis (Kruger et al., 2005; Yazdani and Terman, 2006). Currently, there are numerous examples of Semaphorin family members mediating synapse development in both vertebrate and invertebrate systems (Bouzioukh et al., 2006; Godenschwege et al., 2002; Lin et al., 2007; Morita et al., 2006; Murphey et al., 2003; O’Connor et al., 2009; Paradis et al., 2007; Pecho-Vrieseling et al., 2009; Tran et al., 2009).
Our work has functionally implicated two members of the Class 4 Semaphorins in glutamatergic and/or GABAergic synapse formation: Sema4B and Sema4D. Importantly, our work has identified Class 4 Semaphorins as novel regulators of GABAergic synapse formation. Specifically, RNAi-mediated knockdown of Sema4B in the postsynaptic neuron leads to a decrease in the density of both glutamatergic and GABAergic synapses in cultured hippocampal neurons, as assessed by immunostaining and electrophysiological measurements (Paradis et al., 2007) (Figure 2). In addition, RNAi-mediated knockdown of Sema4D in the postsynaptic neuron leads to a decrease in the density of GABAergic synapses without an apparent effect on glutamatergic synapses (Paradis et al., 2007). Interestingly, knockdown of Sema4B and Sema4D in the postsynaptic neuron leads to a decrease in the density of postsynaptic specializations of GABAergic synapses while having no effect on the density of presynaptic specializations (Paradis et al., 2007). This result implies that Sema4B and Sema4D might be acting in the postsynaptic neuron to recruit neurotransmitter receptors or in other ways organize the postsynaptic density.
To date, the focus of most studies of Semaphorin signaling has been on the function of Semaphorins as ligands for various receptors (i.e. “forward signaling”). However, as Class 4 Semaphorins are membrane-associated proteins with a C-terminal intracellular domain, the possibility of a role for Semaphorins as bidirectional signaling proteins exists (i.e. both “forward” and “reverse signaling”). Currently, the best candidate for a Sema4D receptor to mediate its effects on synapse development is the transmembrane receptor PlexinB1 (PlxnB1). Sema4D has been shown to bind to PlxnB1 in heterologous cells with high affinity (Tamagnone et al., 1999). In addition, PlxnB1 has been reported to be expressed in hippocampal neurons by antibody staining (Swiercz et al., 2002), and by in situ hybridization (Brain Atlas of Gene Expression, (Magdaleno et al., 2006)). Thus, in the case of Sema4D “reverse signaling,” Sema4D might be activated by binding to PlxnB1. Alternatively, Sema4D could “forward signal” either as a soluble factor or membrane- bound factor to activate PlxnB1. As our work demonstrates that loss of Sema4D in the postsynaptic neuron leads to a decrease in GABAergic synapse density in this same neuron (Paradis et al., 2007), the most parsimonious model suggests that Sema4D might act in an autocrine manner by binding to PlxnB1 expressed on the same cell which releases Sema4D. Current work is focused on testing these models.
3.9 Brain-Derived Neurotrophic Factor (BDNF)
BDNF is a member of the neurotrophin family of peptide growth factors that were originally isolated and described based on their ability to promote survival and differentiation of peripheral nervous system (PNS) neurons. Since that time, a multitude of functions for BDNF in the developing and mature PNS and CNS have been described (Ernfors et al., 1994; Gottmann et al., 2009; Huang and Reichardt, 2001; Jones et al., 1994; Riddle et al., 1996; Russo et al., 2008; Yoshii and Constantine-Paton, 2010). BDNF is a secreted protein that exerts its signaling effects through binding to its high-affinity receptor TrkB, a receptor tyrosine kinase, and a low affinity receptor p75NTR (Huang and Reichardt, 2001). Interestingly, BDNF is thought to be predominantly secreted from excitatory, pyramidal neurons in cortex and hippocampus (Kokaia et al., 1993; Miranda et al., 1993) while it’s receptor, TrkB, is expressed by both pyramidal cells and GABAergic interneurons (Cabelli et al., 1996) (Cellerino et al., 1996). Thus, secreted BDNF is poised to act in both an autocrine and paracrine manner.
BDNF has an unusual gene structure: transcription is initiated at eight distinct promoters resulting in, via alternative splicing and polyadenylation, at least eighteen different transcripts that remarkably all code for an identical BDNF protein. One of the interesting aspects of this gene structure is that BDNF transcription from promoter IV is dependent on neuronal activity, mediated in part by the calcium-responsive CREB transcription factor (Cohen and Greenberg, 2008). Investigation into the function of BDNF in the CNS has focused on modulatory effects of secreted BDNF on glutamatergic and GABAergic synaptic transmission, synaptic and structural plasticity, and synapse development (Gottmann et al., 2009; Greenberg et al., 2009; Yoshii and Constantine-Paton, 2010).
A role for BDNF in GABAergic synapse formation has been elucidated by a number of beautiful studies (see references contained in (Gottmann et al., 2009)), a subset of which will be highlighted here. To circumvent the gross morphological defects and lethality associated with deletion of the BDNF gene, Huang and colleagues took a gain-of–function approach to studying BDNF in the CNS. Specifically, they expressed BDNF in forebrain under the control of the α-CaMKII promoter and found that maturation of GABAergic interneurons and presynaptic terminals is accelerated in visual cortex (Huang et al., 1999). Using a related approach, Aguado et al. overexpressed BDNF in CNS progenitors and assayed GABAergic maturation both by in situ hybridization and electron microscopy. They found that GAD65/67 mRNA is dramatically upregulated in hippocampi in which BDNF is overexpressed and further, that the density of GABA-positive terminals is increased (Aguado et al., 2003). Similarly, exogenous treatment of cultured hippocampal neurons with BDNF increases both the number of GABAAR clusters and the percentage of synaptically localized GABAA R clusters (Elmariah et al., 2004).
The Reichardt group took a loss-of-function approach to studying BDNF signaling in synaptogenesis. To circumvent the lethality issues associated with constitutive knockout of genes in the BDNF signaling pathway, Rico and colleagues generated a conditional allele of the trkB gene to produce mice in which trkB was deleted in the cerebellum (Rico et al., 2002a). The cerebellum in these mice is normal with no change in cell number and morphology, and the overall cerebellar architecture is grossly normal as well. (Rico et al., 2002a). Both immunohistochemistry and electron microscopy revealed a deficit in the number of GABAergic synapses formed onto granule cells, with no change in the number of glutamatergic synapses formed onto these cells (Chen et al., 2011; Rico et al., 2002b). The Reichardt group went on to demonstrate a cell-autonomous requirement for TrkB in both the presynaptic Golgi cell to cluster presynaptic GABAergic proteins such as GAD67, and the postsynaptic granule cell to cluster postsynaptic GABAergic proteins such as gephyrin (Chen et al., 2011). Further, use of a TrkB “knock in” in which the kinase activity of TrkB can be inhibited by feeding the animals 1NMPP1 (Chen et al., 2005) revealed that TrkB is required both in the intial stages of GABAergic synapse assembly (P0–28) and also later in development for synapse maintenance (P30–50) (Chen et al., 2011).
Similarly, Kohara and colleagues injected virus expressing Cre recombinase into the cortex of mice containing a floxed allele of the BDNF gene. Immunohistochemical analysis of neurons in which the BDNF gene had been deleted by Cre-mediated recombination revealed fewer GAD65-positive boutons onto the soma of excitatory neurons and a decrease in the frequency of mIPSCs (Kohara et al., 2007), consistent with a decrease in GABAergic synapse number. Again, BDNF is expressed by the excitatory neurons and not the interneurons, suggesting that local secretion of BDNF by the target cell influences whether a GABAergic synapse gets made onto that cell. Taken together, both overexpression and loss-of-function analyses of BDNF signaling indicate a requirement for BDNF signaling to mediate formation of GABAergic synapses in multiple brain regions.
Until recently, it has been difficult to tease apart the activity and non-activity- dependent functions of BDNF using straight loss of function or overexpression approaches. However, an elegant set of experiments from the laboratory of Michael Greenberg directly and definitively addressed this issue. Hong and colleagues generated a “knock in” mouse in which the CREB binding site in the activity-dependent promoter IV of the BDNF gene was mutated; they went on to show that transcription from promoter IV is no longer responsive to activity in this animal (Hong et al., 2008). Importantly, the authors found that GABAergic synapse development is perturbed in the cortex of mice that are unable to express BDNF in response to neuronal activity. Specifically, both immunohistochemistry and an analysis of mIPSC frequency revealed a decrease in the density of GABAergic synapses formed onto layer II/III pyramidal cells in primary visual cortex (Hong et al., 2008). Thus, the in vivo function of BDNF that is produced in response to activity is to promote GABAergic synapse development. This finding has far-reaching implications for cortical development in general as it suggests that circuits respond to sensory experience by dampening excitation through increased inhibition.
3.10 Astrocyte-derived factors
Astrocytes were long thought to function as merely “support” cells in the CNS, playing an important role in maintaining a healthy metabolic state of neurons (Coles and Abbott, 1996; Tsacopoulos and Magistretti, 1996). However, they are now recognized as having crucial cell signaling roles in a structure that has been coined a “tripartite” synapse. The tripartite synapse contains astrocytic projections that closely appose the presynaptic and postsynaptic neuronal termini at classical chemical synapses (Araque et al., 1999; Haydon and Carmignoto, 2006).
With respect to synapse development, astrocyte-derived factors play an important role in both glutamatergic and GABAergic synapse formation and development (Boehler et al., 2007; Elmariah et al., 2005; Hughes et al., 2010; Nagler et al., 2001; Pfrieger and Barres, 1997; Ullian et al., 2004; Ullian et al., 2001). Co-culture of neurons and glia, in conjunction with neuronal growth in astrocyte-conditioned media (ACM), demonstrates that astrocytes release molecules that are necessary to maintain and promote the structure and function of early synapses (Beattie et al., 2002; Faissner et al., 2010; Nagler et al., 2001; Pfrieger and Barres, 1997; Slezak and Pfrieger, 2003; Ullian et al., 2004; Ullian et al., 2001). In the past decade, much effort has been devoted to biochemical purifications of astrocyte-conditioned media in order to identify the factors that mediate these effects with great success (Christopherson et al., 2005; Dowell et al., 2009; Hughes et al., 2010; Jeon et al., 2010). For example, the extracellular matrix proteins Thrombospondins (TSPs) are both necessary and sufficient for glutamatergic synapse formation in cultured retinal ganglion cells (Christopherson et al., 2005).
Research on GABAergic synapse formation and astrocytes is still in its infancy. Early work suggested that astrocytes play an important role at GABAergic synapses because hippocampal neurons grown either on astrocytes or with ACM had larger GABA-induced Cl− currents relative to neurons that were grown in the absence of astrocytes (Elmariah et al., 2005; Liu et al., 1997; Liu et al., 1996). In addition, ACM increases the number of GABAergic presynaptic terminals, the frequency of mIPSCs, and the number GABAAR clusters during the first 10 DIV of hippocampal neuron cultures (Elmariah et al., 2005). This increase in GABAergic synapse development is due to a secreted protein(s) from astrocytes, as conditioned media from fibroblasts was unable to alter synapse density in neuron cultures, and trypsizination of ACM blocks increases in GABAergic synapse density (Hughes et al., 2010). The authors ruled out TSPs because ACM that is immuno-depleted of endogenous TSPs using antisera against TSP-1 and TSP-2 is still able to induce GABAergic synapse formation when compared to unaltered ACM (Hughes et al., 2010). Thus, the identity of this secreted factor or factors remains elusive.
In summary, astrocytes play an important role in both GABAergic and glutamatergic synapse development, but so far at least, they exert their effects on these two types of synapses through distinct molecular mechanisms.
4. Role of GABAergic Synaptic Transmission in Synapse Development
There is no doubt that neuronal activity is an important modulator of synapse development (Anderson et al., 2004; Chattopadhyaya et al., 2004; Fritschy and Brunig, 2003; Katz and Shatz, 1996; Rao et al., 2000). However, activity is not absolutely required as synapses still form in the absence of activity (Craig, Blackstone et al. 1994; Verhage, Maia et al. 2000; Studler, Fritschy et al. 2002; Varoqueaux, Sigler et al. 2002; Harms and Craig 2005). For example, deletion of the protein Munc-18, which is required for synaptic vesicle fusion, leads to a complete loss of neurotransmitter release from the presynaptic terminal (Verhage et al., 2000). Despite this profound deficit in synaptic transmission, synapses form proper morphological structures as determined by electron microscopy and immunohistochemistry (Verhage, Maia et al 2000). Similarly, simultaneous deletion of the Munc13-1 and Munc13-2 proteins, which are essential for vesicle priming, causes the complete absence of neurotransmitter release (Varoqueaux et al., 2002). However, morphologically normal synapses form in neuronal cultures derived from the hippocampi of these animals (Varoqueaux et al., 2002).
Despite the finding that activity is not an absolute requirement for synapse development, the fact is that synapses do develop in the presence of activity in the intact nervous system. Furthermore, there exists a growing body of literature that supports the idea that GABAergic synaptic transmission contributes to both glutamatergic and GABAergic synapse development, as outlined below.
4.1 Depolarizing actions of GABA: relevance to glutamatergic synapse development
Unlike GABA’s action in mature neurons, where the cell is hyperpolarized after GABAAR channel opening, binding of GABA to GABAARs in immature neurons leads to depolarization (Figure 3)(Ben-Ari, 2002; Ben-Ari et al., 2007; Khazipov et al., 2004; Tyzio et al., 2008). This is the result of a change in the GABAA reversal potential due to changes in the Cl− gradient across the cell membrane, which is established by K+-Cl− transporters. The expression of these transporters is dynamically regulated during development (Figure 3C)(Inoue et al., 1991; Takebayashi et al., 1996). For example, during embryonic and early postnatal development the Na+-K+-Cl− transporter NKCC1 is preferentially expressed in neurons. This protein actively pumps Na+, K+ and Cl− across the membrane into the cell, causing an increase in intracellular Cl− concentration (Figure 3B). Postnatally, the expression of another K+-Cl− transporter, KCC2, dramatically increases between P0 and P9 in the rat hippocampus (Rivera et al., 1999). KCC2 pumps Cl− across the membrane and out of the cell, thereby reversing the Cl− equilibrium potential, thus rendering GABA as hyperpolarizing (Figure 3B). Although the expression of NKCC1 has not been shown to decrease with development in the hippocampus, in the neocortex, the expression of NKCC1 peaks at P7 and decreases substantially by P20 (Dzhala et al., 2005). Likewise, the expression of KCC2 in the neocortex begins to increase in expression around P10 and peaks in the adult rat (Dzhala et al., 2005). This change in expression from NKCC1 to KCC2 marks the developmental switch from GABAergic depolarization to hyperpolarization. Interestingly, GABA-mediated depolarization and subsequent Ca2+ influx into the neuron drives this developmental switch (Ganguly et al., 2001).
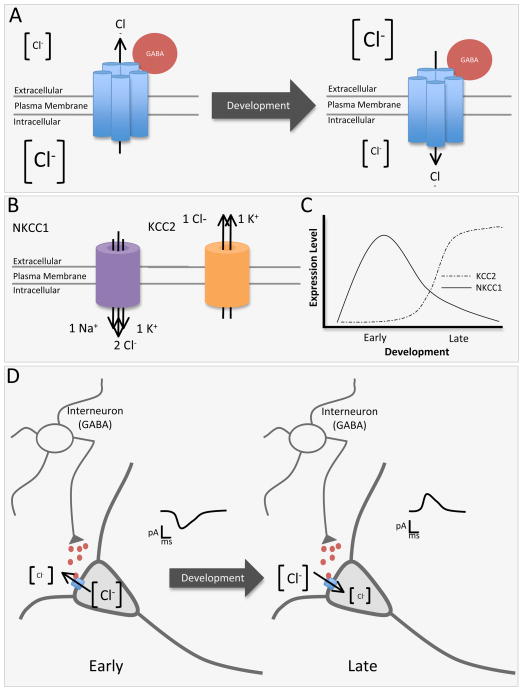
Figure 3.
A) GABAA receptors are permeable to Cl− ions. The direction of Cl− ion flow depends on the electrochemical driving force (larger Cl− indicates higher Cl− concentration). B) The Cl− concentration gradient is established by the expression of the chloride co-transporters NKCC1 and KCC2. C) Expression of KCC2 and NKCC1 is developmentally regulated, with NKCC1 predominating early in development and KCC2 later in development. D) GABAA receptor activation after release of GABA from interneurons causes an outward flow of Cl− ions and subsequent membrane depolarization during early development. This efflux of negative ions across the membrane is equivalent to an influx of positive ions and thus is depicted as an inward current. In later development, a hyperpolarization of the GABAA reversal potential occurs due to a change in the Cl− gradient across the cell membrane, where the extracellular Cl− concentration is now higher than the intracellular Cl− concentration. The change in the Cl− gradient causes an inward flow of Cl− ions through GABAA receptors which is depicted as an outward current.
The importance of the timing of this shift from depolarization to hyperpolarization and its relationship to glutamatergic synapse development is exemplified by perturbations in the expression of either KCC2 or NKCC1. In one study, premature KCC2 expression prompted an increase in GABAergic synapse density, without altering the density of glutamatergic synapses, thus tipping the delicate equilibrium between excitation and inhibition (Chudotvorova et al., 2005; Hubner et al., 2001). Similarly, an electrophysiological study from Xenopus laevus tectal neurons demonstrated that manipulating EGABA in vivo by prematurely expressing KCC2 leads to a strengthening of GABAergic inputs onto tectal neurons (Akerman and Cline, 2006). However. in contrast to the previous work, the KCC2 pertubation in this study prevented the maturation of functional glutamatergic synapses (Akerman and Cline, 2006). In addition, RNAi-mediated knockdown of NKCC1 expression in early development inhibited glutamatergic synaptogenesis, without perturbing GABAergic synaptogenesis (Wang and Kriegstein, 2008). The authors propose that this NKCC1-dependent increase in glutamatergic synaptogenesis acts through NMDAR activation to recruit AMPARs to silent synapses (Wang and Kriegstein, 2008).
However, it should be noted that relatively recent work (Holmgren et al., 2010; Rheims et al., 2009; Tyzio et al., 2011; Zilberter et al., 2010) but see (Ben-Ari et al., 2007; Tyzio et al., 2011)) has called into question the depolarizing effects of GABA during early postnatal development. The controversy, summarized nicely in (Khakhalin, 2011), stems from the use of glucose as the sole energy source in artificial cerebral spinal fluid (ACSF) for in vitro neuronal preparations and electrophysiological recordings. Supplementing ACSF with other energy sources such as pyruvate caused a shift in the reversal potential of GABA towards more hyperpolarized potentials and further, an amelioration of GDPs (see next section for description of GDPs). This suggests that the depolarizing effects of GABA are an experimental artifact and therefore not biologically relevant. The resolution of this controversy will not come quickly for sure, but hopefully will be aided by an analysis of transgenic mouse models in which intracellular Cl− concentration is changed by deletion or overexpression of Cl− transporters and further, by supplementation of ACSF with alternative energy sources for future experiments (Khakhalin, 2011).
4.2 Giant Depolarizing Potentials (GDPs)
The depolarizing capability of GABA release in early postnatal development is important for regulating Ca2+ currents, and could be used to facilitate circuit formation and synaptogenesis through synchronous activity (Ben-Ari et al., 2007). Giant Depolarizing Potentials (GDPs) are polysynaptic, slowly-propagating network oscillations that have been observed both in vivo and in vitro in the developing hippocampus in rodents and in primates (Ben-Ari et al., 2007). GDPs are the first synaptically generated oscillations in the hippocampus (Allene and Cossart, 2010) and are present during the first postnatal week, during a time when the action of GABA is excitatory (Ben-Ari et al., 1989; Ben-Ari et al., 2007). They subside by the second postnatal week (Ben-Ari et al., 2007); correlating with the shift from predominantly GABAergic synapse development to the maturation of glutamatergic synapses (Durand and Konnerth, 1996; Hennou et al., 2002; Tyzio et al., 1999).
4.3 A model for the synchronized development of GABAergic and glutamatergic synapses in hippocampus
The above data, combined with the fact that GABAergic synapse development initiates before glutamatergic synapse development (see Section 2.4, above) has led to the following model for synapse development in the hippocampus, largely based upon work by Dr. Ben-Ari and colleagues (see (Ben-Ari, 2002; Ben-Ari et al., 2007) and references therein). To begin, the development of depolarizing, GABAergic synapses drive glutamatergic synapse development. In particular, the depolarizing nature of early GABAergic synaptic transmission is sufficient to relieve the NMDAR Mg2+ block, thus allowing for Ca2+ entry through NMDARs in response to glutamate release. This in turn drives AMPAR insertion into NMDAR-only containing “silent” synapses. In addition, the depolarizing nature of early GABA release drives the GDPs and subsequent Ca2+ influx into neurons, which is required for the formation of proper hippocampal networks.
4.4 Role of activity in GABAergic synapse development
Not only does GABAergic activity initiate glutamatergic synaptogenesis as discussed above, it may strengthen existing GABAergic synapses. The 65kDa GAD65 and 67kDa GAD67 proteins are isoforms of the enzyme that produces GABA, glutamic acid decarboxylase (GAD) (Soghomonian and Martin, 1998). The Z. J. Huang group created a conditional allele of the gene encoding GAD67 (i.e. Gad1). Next, they simultaneously knocked out expression of GAD67 in interneurons while labeling them with GFP by transfection of an interneuron-specific GFP-IRES-Cre recombinase construct in organotypic cultures derived from cortex of these mice (an analysis of the knockout animal is complicated by lethality) (Chattopadhyaya et al., 2007). Using immunostaining and imaging, the group found that knockdown of GAD67 in basket cells leads to a profound defect in axon branching and perisomatic synapse formation onto pyramidal neurons in cortex, and these defects can be rescued by increasing GABA lifetime by pharmacological blockade of GABA transporters (Chattopadhyaya et al., 2004; Chattopadhyaya et al., 2007). A previous study from the same group demonstrated that either intraocular injection of tetrodotoxin (TTX) or activity blockade by TTX application to cortical organotypic cultures at discrete time points caused decreased perisomatic synapse density of pyramidal neurons (Chattopadhyaya et al., 2004). However, in this study it is unclear whether the TTX-dependent effects on synapse development reflect a change in GABAergic or glutamatergic synaptic transmission or both. While these intriguing results suggest that GABA signaling is required for GABAergic innervation of pyramidal cells, it is difficult to separate the effects of decreased axon branching from synapse development per se in these studies. Further, a study using organotypic hippocampal culture found that treatment with the GABAAR antagonist bicuculline increased the density of GABAergic presynaptic terminals (Marty et al., 2000). While it is difficult to explain the disparate results from these two studies, possibilities include the area of the brain studied and the timing and duration of pharmacological manipulations.
Evidence from Purkinje cells of the cerebellum suggests that different synapses (i.e. axo-dendritic vs. axo-somatic) are differentially affected by loss of GABAergic transmission. Electrophysiology and immunoelectron microscopy analysis of a mouse in which the α1 subunit of the GABAAR is deleted and therefore lacks GABAergic synaptic transmission reveals that GABAergic synapses from basket cells form normally onto the soma but not the dendrites of Purkinje cells (Fritschy et al., 2006). In addition, GABAergic axons from basket cells go on to form inappropriate synapes onto Purkinje cell dendritic spines (Fritschy et al., 2006). Another study engineered a mutation into the β3 subunit of GABAARs, which prevents endocytosis at the synapse from occurring, and found an increase in both the size and the number of GABAergic synapses (Jacob et al., 2009). Neurons with increased number of synaptic GABAARs exhibit an increase in the ratio of immature filopodial-like spines to mature, mushroom-like spines as well as a decrease in PSD-95 expression. Interestingly, the immature spine morphology and decrease in PSD-95 expression is rescued by pharmacological blockade of GABAARs (Jacob et al., 2009), indicating that increased GABAergic transmission is responsible for the delay in excitatory synapse development.
5. GABAergic Synapse Development and Neurological Disorders
It is now widely believed that aberrant development of excitatory and/or inhibitory synapses contributes to neurological impairments such as mental retardation, autism spectrum disorders (ASDs) and epilepsy (Bear et al., 2004; Fernandez and Garner, 2007; Hong et al., 2005; Rubenstein and Merzenich, 2003; Tabuchi et al., 2007; Zoghbi, 2003). Numerous genetic studies on human populations with ASDs have found linkage to genes that regulate synapse development, axon pathfinding, and neuronal morphology (Gilman et al., 2011; Jamain et al., 2003; Levy et al., 2011; Sanders et al., 2011; Szatmari et al., 2007). Thus, one hypothesis to explain ASDs posits that changes in circuit connectivity alter information processing in affected individuals. Below, we have chosen to discuss in depth Rett syndrome and ASDs in which changes in inhibitory drive contribute to the disorder, as an emerging model for a connection between synaptogenic proteins and these disease states.
5.1 Rett Syndrome
Rett syndrome is an X-linked disease that results from mutations in a single gene, Mecp2 (Amir et al., 1999). Mecp2 encodes for methyl-CpG binding protein-2, which binds methylated CpG sequences in vertebrate genomes (Tate et al., 1996). It is believed that methylated CpG islands recruit other proteins that can lead to downstream transcriptional regulation. Loss of MeCP2 can lead to both activation and repression of transcription across the genome. However, ~85% of genes assayed in Mecp2 null mice by Zoghbi and colleagues are downregulated, suggesting a bias towards transcriptional activation for MeCP2 function (Chahrour et al., 2008).
Rett syndrome affects approximately 1:10,000 girls worldwide (Percy and Lane, 2005). Females with Rett syndrome develop normally for the first 6–18 months of life at which point there is a rapid regression in development. Interestingly, this age is after neurogenesis and migration have occurred, and corresponds with the period of synaptic development that includes later stages of synaptogenesis and synaptic pruning (Zoghbi, 2003). In humans, onset of symptoms typically involves a deceleration in head and body growth along with a regression in motor and speech abilities and abnormal breathing patterns. Cognitive impairments are also present and manifest themselves similarly to ASDs (Percy and Lane, 2005).
There are a number of mouse models of Rett syndrome, many of which have a loss- of-function of the Mecp2 gene. Two of these models are deletions in the Mecp2 gene: the Jaenisch mouse lacks exon 3 of Mecp2 (Chen et al., 2001), and the Bird mouse lacks both exons 3 and 4 (Guy et al., 2001). Mecp2 null mice (Jaenisch and Bird models) appear normal until about 5 weeks postnatally. At this point, mice begin to demonstrate abnormal behaviors resembling Rett syndrome, such as hindlimb clasping and irregular breathing (please see reviews for further discussion of symptoms (Cobb et al., 2010; Zoghbi, 2003).
Classically, Rett syndrome has been thought of as a disorder of neural development. However recent work has extended this intepretation, as mice in which Mecp2 is deleted in adulthood display phenotypes similar to mice in which Mecp2 is constitutively deleted (McGraw et al., 2011). This suggests that Mecp2 expression is required throughout the life of the animal for normal nervous system function. Interestingly, a recent study from the C. Chen lab examining development of the retinogeniculate synapse in the Bird Mecp2 null mouse found aberrant remodeling of this synapse, specifically during the phase of development which requires visual experience (Noutel et al., 2011). The implication of this finding is that defects in activity-dependent synapse development occur in the absence of MeCP2.
Although the behavioral symptoms of Rett syndrome are prominent in human patients as well as in the mouse models, the neuropathological phenotypes are relatively subtle. CA2 pyramidal neurons in Mecp2 null mice are ~15–25% smaller than the corresponding neurons in wild type littermate control mice (Chen et al., 2001). In addition, Mecp2 null mice have simplified dendritic arbors in their neocortical projections (Kishi and Macklis, 2004). In layer5 of the somatosensory cortex of the Jaenisch Mecp2 null mice, spontaneous activity of pyramidal neurons is reduced (Dani et al., 2005). While there is no change in the intrinsic properties of the cortical neurons studied, there is a shift in the balance between excitation and inhibition, which favors inhibition (Dani et al., 2005). The increase in inhibition is due to a reduced connectivity and weaker excitatory synapses in the Mecp2 null mice compared to wild-type littermates (Chao et al., 2007; Dani and Nelson, 2009). Similarly to the somatosensory cortex, a decrease in the excitatory drive and an increase of inhibitory drive was detected in the thalamus of the Bird Mecp2 null mice (Zhang et al., 2010b).
A recent study again points to an important role for MeCP2 in regulating inhibition in the nervous system (Chao et al., 2010), although the conclusions from this study differ from those discussed above. In this study, Zoghbi and colleagues generate a knockout of Mecp2 that is confined to GABA-expressing neurons and demonstrate that in this mouse, many of the same aspects of nervous system function are impaired as has been observed with the Mecp2 null animals, such as motor control, breathing, and weight regulation (Chao et al., 2010). The authors also find a broad spectrum of behavioral deficits in this mouse and conclude from this analysis that loss of Mecp2 from GABAergic neurons recapitulates features of Rett syndrome (Chao et al., 2010). In addition, the authors go on to show that deletion of MeCP2 in GABAergic neurons decreases the amount of GABA by altering Gad1/2 expression, leading to a decrease in mIPSC quantal size (Chao et al., 2010). This observation differs from studies using mice in which Mecp2 was deleted in all neurons that show a slight increase in mIPSC quantal size (Dani et al., 2005).
Clearly, much work remains to sort out the differences between the various mouse models in terms of behavior, cellular physiology, and circuit development to uncover the key sites of action of MeCP2 function. In addition, as MeCP2 functions as a global regulator of gene expression, further studies are needed to elucidate the specific molecular mechanisms which lead to alterations in excitatory/inhibitory ratio in mouse models of Rett syndrome. Taken together, these findings, coupled with the fact that the appearance of Rett symptoms in humans occurs during a period of rapid synaptogenesis, suggest that a defect in synapse development or maintentnace may be the underlying cause of Rett syndrome. In addition, these studies point to the importance of maintaining the appropriate balance of excitation and inhibition for proper cortical circuit function.
5.2 Autism Spectrum Disorders (ASDs)
Autism Spectrum Disorders (ASDs) are diagnosed based on the presence of three behavioral features: a deficit in social communication, stereotypies, and a delay or absence of language skills (van Spronsen and Hoogenraad, 2010). ASDs are typically detected and diagnosed before the second or third year of life. Again, this time frame is potentially important for understanding the underlying pathology of the disorder because, as with Rett syndrome, the onset and detection of the disorder corresponds with a period of robust synapse formation and maturation in humans (Lord et al., 2000). There is a strong genetic basis to these disorders as they are highly heritable (~80–90%: (Bailey et al., 1995; van Spronsen and Hoogenraad, 2010) with a large degree of genetic heterogeneity. Interestingly, linkage analyses on families with ASDs have revealed mutations or single nucleotide polymorphisms (SNPs) in many synaptic proteins. These synaptic proteins include the cell adhesion molecules Neurexin and Neuroligin (Abrahams and Geschwind, 2008; Jamain et al., 2003; Marshall et al., 2008; Tabuchi et al., 2007)), Semaphorins (Weiss et al., 2009), and Shank family members (Moessner et al., 2007; Pinto et al., 2010) as well as many GTPases, which play a role in regulating cytoskeletal dynamics in dendrites and dendritic spines (Pinto et al., 2010).
A small percentage of ASD patients have mutations in Neuroligin 3. One of these is a change from an arginine to cysteine at amino acid 451 of the coding sequence (R451C) (Jamain, Quach, et al 2003). To ask if this mutation could mimic ASDs in a rodent model, a genetic knock-in strategy was employed to introduce the R451C mutation into the Nlgn3 gene in mice. Employing this experimental approach, one group found that the Neuroligin 3 R451C knock-in mouse displays increases in both GABAergic synaptic number and strength and autism-like behavioral deficits such as impaired social interactions (Tabuchi et al., 2007), suggesting a role for GABAergic synaptic function and development in ASDs. However, another group using this same strategy did not find any behavioral deficits in their Neuroligin 3 R451C mouse model (Chadman et al., 2008); electrophysiological recordings were not reported in this study. Thus, a conclusion with respect to the role of the R451C mutation in synapse development and ultimately, ASD-like phenotypes, awaits further investigation.
As mentioned previously, the Semaphorins have recently emerged as important mediators of both glutamatergic and GABAergic synapse development (Bouzioukh et al., 2006; Godenschwege et al., 2002; Lin et al., 2007; Morita et al., 2006; Murphey et al., 2003; O’Connor et al., 2009; Paradis et al., 2007; Pecho-Vrieseling et al., 2009; Tran et al., 2009). Interestingly, in the largest genome-wide association study of ASDs to date, a study of 1,553 patients led by the Daly and Chakravarti labs, the strongest genome-wide-significant association involved markers adjacent to SEMA5A on chromosome 5p15 (Weiss et al., 2009). In addition, two studies have reported altered regulation of SEMA5A mRNA in patients with ASDs (Melin et al., 2006; Weiss et al., 2009). Interestingly, Sema5a is strongly expressed in the dentate gyrus of the hippocampus and in layers 5 and 6 of the cortex in rodents (Lein et al., 2007), two areas where morphological changes correlate with ASDs (Hutsler and Zhang, 2010; Schumann et al., 2004). The prevalence of SEMA5A SNPs in autistic patients, coupled with studies that demonstrate that Semaphorin family members mediate both glutamatergic and GABAergic synapse development, suggests the intriguing possibility that the underlying cause of ASD symptoms in patients with Sema5A mutations may be defects in synapse development.
Scaffolding proteins and cytoskeletal regulation are important in synapse development. Genetic mapping has implicated the scaffolding proteins of the Shank family in ASDs (Durand et al., 2007; Marshall et al., 2008; Moessner et al., 2007; Pinto et al., 2010). Shank proteins bind many synaptic proteins and together with Homer can synergistically increase dendritic spine size through formation of a scaffolding network in the dendritic spine (Hayashi et al., 2009; Sala et al., 2001). It will be interesting to determine if the mutations in Shank family members that are linked to ASDs cause an increase or a decrease in the amount of Shank expression and likewise, how this change in expression affects synaptic development. In addition, small, Ras-like GTPases are important regulators of the actin cytoskeleton and as such, control both dendritic spine development and dendritic branching (Ghiretti and Paradis, 2010, 2011; McNair et al., 2010; Paradis et al., 2007; Tashiro et al., 2000). Interestingly, a recent linkage analysis demonstrated that copy number variants (CNVs) of Rho family GTPases are found with increased frequency in individuals with ASDs compared to non-ASD individuals (Pinto et al., 2010).
For obvious reasons, alterations in synapses are difficult to assess in autistic patients and further, the causal relationship between any morphological changes and the ASD symptoms is unclear. Changes in the shape of dendritic spines may affect synaptic connections by modulating the chemical microenvironment of the synapse (Kasai et al., 2003; Robinson and Kolb, 2004; Tsay and Yuste, 2004). A recent study demonstrates that post-mortem brains of autistic patients have significant increases in spine density in the superficial layers of cortical pyramidal neurons, and this change was found in every brain from an autisitic individual surveyed in the study (Hutsler and Zhang, 2010). This study is the first of its kind to present a clear correlation between morphological synapses and ASDs. Because spines are easier experimentally to investigate in post-mortem tissue samples from humans than other types of synapses, it may be more challenging to uncover the role of inhibitory synapse development in ASDs. However, one inroad into this problem lies in further linkage analysis to identify autism susceptibility loci, coupled with increased understanding of the molecular mechanisms of GABAergic synapse development.
Conclusions
The past decade has brought astounding progress to the field of GABAergic synapse development. No longer the forgotten younger sibling, only appreciated for its ability to rein-in glutamatergic synaptic transmission, GABAergic synapses are now being studied and appreciated by numerous researchers as signal transducers in their own right. However, much remains to be discovered. For example, live-imaging studies that observe components of GABAergic synapses assembling at nascent synaptic sites are needed to inform our understanding of the steps in GABAergic synapse development. More gene discovery efforts, in any organism, geared at identifying molecules that regulate all steps in GABAergic synapse development (contact stabilization, recruiting pre- and post-synaptic components to contact sites) must be a significant area of focus in the near future.
Much of the data presented in this review points to the idea that similar mechanisms regulate glutamatergic and GABAergic synapse development, as observed at the molecular level. No longer can we be content to think of these synaptic subtypes developing via independent mechanisms. It is abundantly clear that glutamatergic and GABAergic synapses co-develop in time and in space, utilize the same or homologous signal transduction pathways, and have a complicated, activity-based relationship which influences their co-development. In addition, the field of synapse development must confront two interesting observations which promise to further influence how we think about shared mechanisms of synapse development: co-release of glutamate and GABA from central synapses, and co-innervation of dendritic spines by both glutamatergic and GABAergic synapses.
Research Highlights.
Glutamatergic and GABAergic synapses differ at the ultrastructural and molecular level.
Thus far, little is known about the order of assembly of components at GABAergic synapses.
Many molecules mediate both glutamatergic and GABAergic synapse development.
The depolarizing action of GABA-mediated currents drives glutamatergic synapse development.
An underlying cause of neurological disease is aberrant GABAergic synapse development.
Acknowledgments
Work in the Paradis laboratory is supported by the Richard and Susan Smith Family Foundation, Chestnut Hill, MA, an Alfred P. Sloan Research Fellowship, and NIH grants R01NS065856 and R21DA026977. We thank Amy Ghiretti, Dr. Sonia Cohen, Dr. Elizabeth Hong, Dr. Anna Moore and anonymous reviewers for helpful comments on this manuscript.
Abbreviation List
- GABA
γ-aminobutyric acid
- VAMP2
vesicle-associated membrane protein 2
- GFP
green fluorescent protein
- GABAR
GABA receptor
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptor
- NMDARs
N -methyl D -aspartate receptor
- PSD
postsynaptic density
- GlyR
glycine receptor
- GISP
G protein-coupled receptor interacting scaffold protein
- Mupp1
multi-PDZ domain protein 1
- GAD65/67
glutamic acid decarboxylase 65kDa/67 kDa
- CA1/CA3
cornu ammonis 1/3
- GKAP
guanylate kinase-associated protein
- mIPSC
miniature inhibitory postsynaptic current
- HAP1
Huntingtin-associated protein
- P#
postnatal day #
- E#
embryonic day #
- DIV
day in vitro
- EM
electron microscopy
- VIAAT
vescicular inhibitory amino acid transporter
- GEF
Guanine nucleotide exchange factor
- DGC
Dystrophin-associated glycoprotein complex
- NMJ
neuromuscular junction
- CNS
central nervous system
- NRG1
Neuregulin 1
- EGF
epidermal growth factor
- FGF
Fibroblast Growth Factors
- Sema
Semaphorin
- Plxn
Plexin
- BDNF
Brain-Derived Neurotrophic Factor
- PNS
peripheral nervous system
- CREB
cAMP response element-binding
- CaMKII
calcium calmodulin kinase II
- ACM
astrocyte-conditioned media
- TSP
Thrombospondin
- NKCC1
Na+-K+-Cl− transporter NKCC1
- KCC2
K+-Cl− transporter
- RNAi
RNA interference
- ACSF
artificial cerebral spinal fluid
- GDP
Giant Depolarizing Potential
- TTX
tetrodotoxin
- ASD
autism spectrum disorder
- MECP2
methyl-CpG binding protein-2
- SNP
single nucleotide polymorphisms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci. 2006;26:5117–5130. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Loyning Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963;198:540–542. doi: 10.1038/198540a0. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Loyning Y. Pathway of Postsynaptic Inhibition in the Hippocampus. J Neurophysiol. 1964;27:608–619. doi: 10.1152/jn.1964.27.4.608. [DOI] [PubMed] [Google Scholar]
- Anderson TR, Shah PA, Benson DL. Maturation of glutamatergic and GABAergic synapse composition in hippocampal neurons. Neuropharmacology. 2004;47:694– 705. doi: 10.1016/j.neuropharm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin- based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation atpurkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends in neurosciences. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Fam SR, Hall RA. GABAB receptor association with the PDZ scaffold Mupp1 alters receptor stability and function. The Journal of biological chemistry. 2007;282:4162–4171. doi: 10.1074/jbc.M607695200. [DOI] [PubMed] [Google Scholar]
- Baldelli P, Fassio A, Valtorta F, Benfenati F. Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J Neurosci. 2007;27:13520–13531. doi: 10.1523/JNEUROSCI.3151-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli P, Hernandez-Guijo JM, Carabelli V, Carbone E. Brain-derived neurotrophic factor enhances GABA release probability and nonuniform distribution of N- and P/Q-type channels on release sites of hippocampal inhibitory synapses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:3358–3368. doi: 10.1523/JNEUROSCI.4227-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H, Levi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Muller U. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow SL, Constable JR, Clark E, El-Sabeawy F, McAllister AK, Washbourne P. Neuroligin1: a cell adhesion molecule that recruits PSD-95 and NMDA receptors by distinct mechanisms during synaptogenesis. Neural Dev. 2009;4:17. doi: 10.1186/1749-8104-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Subunit arrangement of gamma-aminobutyric acid type A receptors. J Biol Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215– 1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks. Trends Neurosci. 2004;27:422–427. doi: 10.1016/j.tins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Boehler MD, Wheeler BC, Brewer GJ. Added astroglia promote greater synapse density and higher activity in neuronal networks. Neuron Glia Biol. 2007;3:127–140. doi: 10.1017/S1740925X07000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta- neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gundersen V, Bergersen LH, Fremeau RT, Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. doi: 10.1002/cne.20354. [DOI] [PubMed] [Google Scholar]
- Bouzioukh F, Daoudal G, Falk J, Debanne D, Rougon G, Castellani V. Semaphorin3A regulates synaptic function of differentiated hippocampal neurons. Eur J Neurosci. 2006;23:2247–2254. doi: 10.1111/j.1460-9568.2006.04783.x. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Brown DA. The cloning of GABA(B) receptors. Nature. 1997;386:223– 224. doi: 10.1038/386223a0. [DOI] [PubMed] [Google Scholar]
- Bresler T, Ramati Y, Zamorano PL, Zhai R, Garner CC, Ziv NE. The dynamics of SAP90/PSD-95 recruitment to new synaptic junctions. Mol Cell Neurosci. 2001;18:149–167. doi: 10.1006/mcne.2001.1012. [DOI] [PubMed] [Google Scholar]
- Brunig I, Suter A, Knuesel I, Luscher B, Fritschy JM. GABAergic terminals are required for postsynaptic clustering of dystrophin but not of GABA(A) receptors and gephyrin. J Neurosci. 2002;22:4805–4813. doi: 10.1523/JNEUROSCI.22-12-04805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Burrone J, Li Z, Murthy VN. Studying vesicle cycling in presynaptic terminals using the genetically encoded probe synaptopHluorin. Nature protocols. 2006;1:2970–2978. doi: 10.1038/nprot.2006.449. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Allendoerfer KL, Radeke MJ, Welcher AA, Feinstein SC, Shatz CJ. Changing patterns of expression and subcellular localization of TrkB in the developing visual system. J Neurosci. 1996;16:7965–7980. doi: 10.1523/JNEUROSCI.16-24-07965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi PE, Benfenati F, Chieregatti E, Greengard P, Valtorta F. Rapid binding of synapsin I to F- and G-actin. A study using fluorescence resonance energy transfer. FEBS Lett. 1993;329:301–305. doi: 10.1016/0014-5793(93)80242-m. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Maffei L, Domenici L. The distribution of brain-derived neurotrophic factor and its receptor trkB in parvalbumin-containing neurons of the rat visual cortex. Eur J Neurosci. 1996;8:1190–1197. doi: 10.1111/j.1460-9568.1996.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91:313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron. 2010;66:101–113. doi: 10.1016/j.neuron.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABA(B) receptor modulation of synaptic function. Current opinion in neurobiology. 2011;21:339–344. doi: 10.1016/j.conb.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham JJ, Murray J, Ruhkalova M, Cuccia L, McAloney R, Ingold KU, Johnston LJ. Interaction of synapsin I with membranes. Biochem Biophys Res Commun. 2003;309:823–829. doi: 10.1016/j.bbrc.2003.08.082. [DOI] [PubMed] [Google Scholar]
- Chen AI, Nguyen CN, Copenhagen DR, Badurek S, Minichiello L, Ranscht B, Reichardt LF. TrkB (tropomyosin-related kinase B) controls the assembly and maintenance of GABAergic synapses in the cerebellar cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:2769–2780. doi: 10.1523/JNEUROSCI.4991-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. GABA receptors precede glutamate receptors in hypothalamic development; differential regulation by astrocytes. J Neurophysiol. 1995;74:1473–1484. doi: 10.1152/jn.1995.74.4.1473. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Chiappalone M, Casagrande S, Tedesco M, Valtorta F, Baldelli P, Martinoia S, Benfenati F. Opposite changes in glutamatergic and GABAergic transmission underlie the diffuse hyperexcitability of synapsin I-deficient cortical networks. Cereb Cortex. 2009;19:1422–1439. doi: 10.1093/cercor/bhn182. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans- synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Chiou TT, Bonhomme B, Jin H, Miralles CP, Xiao H, Fu Z, Harvey RJ, Harvey K, Vicini S, De Blas AL. Differential Regulation of the Postsynaptic Clustering of {gamma}-Aminobutyric Acid Type A (GABAA) Receptors by Collybistin Isoforms. The Journal of biological chemistry. 2011;286:22456–22468. doi: 10.1074/jbc.M111.236190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte- secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudotvorova I, Ivanov A, Rama S, Hubner CA, Pellegrino C, Ben-Ari Y, Medina I. Early expression of KCC2 in rat hippocampal cultures augments expression of functional GABA synapses. J Physiol. 2005;566:671–679. doi: 10.1113/jphysiol.2005.089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb S, Guy J, Bird A. Reversibility of functional deficits in experimental models of Rett syndrome. Biochem Soc Trans. 2010;38:498–506. doi: 10.1042/BST0380498. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75– 78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183– 209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JA, Abbott NJ. Signalling from neurones to glial cells in invertebrates. Trends in neurosciences. 1996;19:358–362. doi: 10.1016/0166-2236(96)10042-4. [DOI] [PubMed] [Google Scholar]
- Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968;9:268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- Cossart R, Petanjek Z, Dumitriu D, Hirsch JC, Ben-Ari Y, Esclapez M, Bernard C. Interneurons targeting similar layers receive synaptic inputs with similar kinetics. Hippocampus. 2006;16:408–420. doi: 10.1002/hipo.20169. [DOI] [PubMed] [Google Scholar]
- Craig AM, Graf ER, Linhoff MW. How to build a central synapse: clues from cell culture. Trends Neurosci. 2006;29:8–20. doi: 10.1016/j.tins.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Peng HB. Dynamics of synaptic vesicles in cultured spinal cord neurons in relationship to synaptogenesis. Mol Cell Neurosci. 1996;7:443–452. doi: 10.1006/mcne.1996.0032. [DOI] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglot L, Triller A, Marty S. The development of hippocampal interneurons in rodents. Hippocampus. 2006;16:1032–1060. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J Neurosci. 2009;29:11263–11270. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell JA, Johnson JA, Li L. Identification of astrocyte secreted proteins with a combination of shotgun proteomics and bioinformatics. J Proteome Res. 2009;8:4135– 4143. doi: 10.1021/pr900248y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Cossart R, Huang J, Yuste R. Correlation between axonal morphologies and synaptic input kinetics of interneurons from mouse visual cortex. Cereb Cortex. 2007;17:81–91. doi: 10.1093/cercor/bhj126. [DOI] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand GM, Konnerth A. Long-term potentiation as a mechanism of functional synapse induction in the developing hippocampus. J Physiol Paris. 1996;90:313–315. doi: 10.1016/s0928-4257(97)87905-3. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. A physiological role for GABAB receptors in the central nervous system. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Elmariah SB, Crumling MA, Parsons TD, Balice-Gordon RJ. Postsynaptic TrkB-mediated signaling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J Neurosci. 2004;24:2380–2393. doi: 10.1523/JNEUROSCI.4112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmariah SB, Oh EJ, Hughes EG, Balice-Gordon RJ. Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J Neurosci. 2005;25:3638–3650. doi: 10.1523/JNEUROSCI.3980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, Seidenbecher C. Contributions of astrocytes to synapse formation and maturation - Potential functions of the perisynaptic extracellular matrix. Brain Res Rev. 2010;63:26–38. doi: 10.1016/j.brainresrev.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J Biol Chem. 1999;274:10100–10104. doi: 10.1074/jbc.274.15.10100. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Garner CC. Over-inhibition: a model for developmental intellectual disability. Trends Neurosci. 2007;30:497–503. doi: 10.1016/j.tins.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Fischer F, Kneussel M, Tintrup H, Haverkamp S, Rauen T, Betz H, Wassle H. Reduced synaptic clustering of GABA and glycine receptors in the retina of the gephyrin null mutant mouse. The Journal of comparative neurology. 2000;427:634–648. doi: 10.1002/1096-9861(20001127)427:4<634::aid-cne10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299– 323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P, Kralic JE, Vogt KE, Sassoe-Pognetto M. Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAA receptors containing the alpha1 subunit in Purkinje cells. J Neurosci. 2006;26:3245–3255. doi: 10.1523/JNEUROSCI.5118-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Zhao S, Graber W, Drakew A, Studer D. New ways of looking at synapses. Histochem Cell Biol. 2007;128:91–96. doi: 10.1007/s00418-007-0305-7. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Brown DA. GABAB-receptor-activated K+ current in voltage- clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985;82:1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Charara A, Pare JF, Levey AI, Smith Y. Differential subcellular and subsynaptic distribution of GABA(A) and GABA(B) receptors in the monkey subthalamic nucleus. Neuroscience. 2004;127:709–721. doi: 10.1016/j.neuroscience.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Ghiretti AE, Paradis S. The GTPase Rem2 regulates synapse development and dendritic morphology. Dev Neurobiol. 2010 doi: 10.1002/dneu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiretti AE, Paradis S. The GTPase Rem2 regulates synapse development and dendritic morphology. Developmental neurobiology. 2011;71:374–389. doi: 10.1002/dneu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70:898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphey RK. Bi- directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat Neurosci. 2002;5:1294–1301. doi: 10.1038/nn976. [DOI] [PubMed] [Google Scholar]
- Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res. 2009;199:203–234. doi: 10.1007/s00221-009-1994-z. [DOI] [PubMed] [Google Scholar]
- Gozlan H, Ben-Ari Y. Interneurons are the source and the targets of the first synapses formed in the rat developing hippocampal circuit. Cereb Cortex. 2003;13:684–692. doi: 10.1093/cercor/13.6.684. [DOI] [PubMed] [Google Scholar]
- Graf ER, Kang Y, Hauner AM, Craig AM. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J Neurosci. 2006;26:4256–4265. doi: 10.1523/JNEUROSCI.1253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16:3397– 3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, et al. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Harvey K, Duguid IC, Alldred MJ, Beatty SE, Ward H, Keep NH, Lingenfelter SE, Pearce BR, Lundgren J, Owen MJ, et al. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J Neurosci. 2004;24:5816– 5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM, Li H, Sala C, Hayashi Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–171. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hennou S, Khalilov I, Diabira D, Ben-Ari Y, Gozlan H. Early sequential formation of functional GABA(A) and glutamatergic synapses on CA1 interneurons of the rat foetal hippocampus. Eur J Neurosci. 2002;16:197–208. doi: 10.1046/j.1460-9568.2002.02073.x. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Holmgren CD, Mukhtarov M, Malkov AE, Popova IY, Bregestovski P, Zilberter Y. Energy substrate availability as a determinant of neuronal resting potential, GABA signaling and spontaneous network activity in the neonatal cortex in vitro. Journal of neurochemistry. 2010;112:900–912. doi: 10.1111/j.1471-4159.2009.06506.x. [DOI] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, West AE, Greenberg ME. Transcriptional control of cognitive development. Curr Opin Neurobiol. 2005;15:21–28. doi: 10.1016/j.conb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hong Q, Zhang M, Pan XQ, Guo M, Li F, Tong ML, Chen RH, Guo XR, Chi X. Prefrontal cortex Homer expression in an animal model of attention- deficit/hyperactivity disorder. J Neurol Sci. 2009;287:205–211. doi: 10.1016/j.jns.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Scheiffele P. GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development. Curr Opin Neurobiol. 2008;18:77–83. doi: 10.1016/j.conb.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Elmariah SB, Balice-Gordon RJ. Astrocyte secreted proteins selectively increase hippocampal GABAergic axon length, branching, and synaptogenesis. Mol Cell Neurosci. 2010;43:136–145. doi: 10.1016/j.mcn.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- Inoue M, Hara M, Zeng XT, Hirose T, Ohnishi S, Yasukura T, Uriu T, Omori K, Minato A, Inagaki C. An ATP-driven Cl− pump regulates Cl− concentrations in rat hippocampal neurons. Neurosci Lett. 1991;134:75–78. doi: 10.1016/0304-3940(91)90512-r. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kaibara M, Sakurai-Yamashita Y, Nagayama Y, Uezono Y, Yano K, Taniyama K. Cloning and tissue distribution of novel splice variants of the rat GABAB receptor. Biochem Biophys Res Commun. 1998;253:10–15. doi: 10.1006/bbrc.1998.9706. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Wan Q, Vithlani M, Saliba RS, Succol F, Pangalos MN, Moss SJ. GABA(A) receptor membrane trafficking regulates spine maturity. Proc Natl Acad Sci U S A. 2009;106:12500–12505. doi: 10.1073/pnas.0903943106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H, Lee S, Lee WH, Suk K. Analysis of glial secretome: the long pentraxin PTX3 modulates phagocytic activity of microglia. J Neuroimmunol. 2010;229:63–72. doi: 10.1016/j.jneuroim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalscheuer VM, Musante L, Fang C, Hoffmann K, Fuchs C, Carta E, Deas E, Venkateswarlu K, Menzel C, Ullmann R, et al. A balanced chromosomal translocation disrupting ARHGEF9 is associated with epilepsy, anxiety, aggression, and mental retardation. Hum Mutat. 2009;30:61–68. doi: 10.1002/humu.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Spencer WA, Brinley FJ., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Kantamneni S, Correa SA, Hodgkinson GK, Meyer G, Vinh NN, Henley JM, Nishimune A. GISP: a novel brain-specific protein that promotes surface expression and function of GABA(B) receptors. Journal of neurochemistry. 2007;100:1003–1017. doi: 10.1111/j.1471-4159.2006.04271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure- stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, et al. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Khakhalin AS. Questioning the depolarizing effects of GABA during early brain development. Journal of neurophysiology. 2011 doi: 10.1152/jn.00293.2011. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. The European journal of neuroscience. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771– 781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kins S, Betz H, Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat Neurosci. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Gasnier B, Feng G, Sanes JR, Betz H. Gephyrin-independent clustering of postsynaptic GABA(A) receptor subtypes. Mol Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289– 9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Mastrocola M, Zuellig RA, Bornhauser B, Schaub MC, Fritschy JM. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice) Eur J Neurosci. 1999;11:4457–4462. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27:7234– 7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia Z, Bengzon J, Metsis M, Kokaia M, Persson H, Lindvall O. Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc Natl Acad Sci U S A. 1993;90:6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller H, Siebler M, Schmalenbach C, Muller HW. GABA and glutamate receptor development of cultured neurons from rat hippocampus, septal region, and neocortex. Synapse. 1990;5:59–64. doi: 10.1002/syn.890050105. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J Comp Neurol. 2006;495:408–421. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Lujan R, Haas CA, Lopez-Bendito G, Shigemoto R, Frotscher M. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardi-Studler B, Fritschy JM. Matching of pre- and postsynaptic specializations during synaptogenesis. Neuroscientist. 2007;13:115–126. doi: 10.1177/1073858406296803. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, Kendall J, Marks S, Lakshmi B, Pai D, Ye K, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Ogiya M, Takahara M, Yamaguchi W, Furuyama T, Tanaka H, Tohyama M, Inagaki S. Sema4D-plexin-B1 implicated in regulation of dendritic spine density through RhoA/ROCK pathway. Neurosci Lett. 2007;428:1–6. doi: 10.1016/j.neulet.2007.09.045. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QY, Schaffner AE, Chang YH, Vaszil K, Barker JL. Astrocytes regulate amino acid receptor current densities in embryonic rat hippocampal neurons. J Neurobiol. 1997;33:848–864. [PubMed] [Google Scholar]
- Liu QY, Schaffner AE, Li YX, Dunlap V, Barker JL. Upregulation of GABAA current by astrocytes in cultured embryonic rat hippocampal neurons. J Neurosci. 1996;16:2912– 2923. doi: 10.1523/JNEUROSCI.16-09-02912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–260. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdaleno S, Jensen P, Brumwell CL, Seal A, Lehman K, Asbury A, Cheung T, Cornelius T, Batten DM, Eden C, et al. BGEM: an in situ hybridization database of gene expression in the embryonic and adult mouse nervous system. PLoS Biol. 2006;4:e86. doi: 10.1371/journal.pbio.0040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, Shago M, Moessner R, Pinto D, Ren Y, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S, Wehrle R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw CM, Samaco RC, Zoghbi HY. Adult Neural Function Requires MeCP2. Science. 2011 doi: 10.1126/science.1206593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair K, Spike R, Guilding C, Prendergast GC, Stone TW, Cobb SR, Morris BJ. A role for RhoB in synaptic plasticity and the regulation of neuronal morphology. J Neurosci. 2010;30:3508–3517. doi: 10.1523/JNEUROSCI.5386-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin M, Carlsson B, Anckarsater H, Rastam M, Betancur C, Isaksson A, Gillberg C, Dahl N. Constitutional downregulation of SEMA5A expression in autism. Neuropsychobiology. 2006;54:64–69. doi: 10.1159/000096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. An anatomical substrate for experience-dependent plasticity of the rat barrel field cortex. Proc Natl Acad Sci U S A. 1995;92:11834–11838. doi: 10.1073/pnas.92.25.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Busse B, Weiler NC, O’Rourke N, Smith SJ. Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron. 2010;68:639–653. doi: 10.1016/j.neuron.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Wong RK. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. J Physiol. 1984;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Wong RK. Inhibitory control of local excitatory circuits in the guinea- pig hippocampus. J Physiol. 1987;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda RC, Sohrabji F, Toran-Allerand CD. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci U S A. 1993;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, et al. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita A, Yamashita N, Sasaki Y, Uchida Y, Nakajima O, Nakamura F, Yagi T, Taniguchi M, Usui H, Katoh-Semba R, et al. Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling. J Neurosci. 2006;26:2971–2980. doi: 10.1523/JNEUROSCI.5453-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, Jiang X, Taylor AA, Shin W, Gillis KD, Mennerick S. Vesicle pool heterogeneity at hippocampal glutamate and GABA synapses. J Neurosci. 2007;27:9846– 9854. doi: 10.1523/JNEUROSCI.2803-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey RK, Froggett SJ, Caruccio P, Shan-Crofts X, Kitamoto T, Godenschwege TA. Targeted expression of shibire ts and semaphorin 1a reveals critical periods for synapse formation in the giant fiber of Drosophila. Development. 2003;130:3671–3682. doi: 10.1242/dev.00598. [DOI] [PubMed] [Google Scholar]
- Nagler K, Mauch DH, Pfrieger FW. Glia-derived signals induce synapse formation in neurones of the rat central nervous system. J Physiol. 2001;533:665–679. doi: 10.1111/j.1469-7793.2001.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci. 2010;13:232–238. doi: 10.1038/nn.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutel J, Hong YK, Leu B, Kang E, Chen C. Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice. Neuron. 2011;70:35– 42. doi: 10.1016/j.neuron.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693– 1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TP, Cockburn K, Wang W, Tapia L, Currie E, Bamji SX. Semaphorin 5B mediates synapse elimination in hippocampal neurons. Neural Dev. 2009;4:18. doi: 10.1186/1749-8104-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan GA, Hofer W, Betz H. Inhibitory postsynaptic membrane specializations are formed in gephyrin-deficient mice. Neurosci Lett. 2009;458:106–110. doi: 10.1016/j.neulet.2009.04.036. [DOI] [PubMed] [Google Scholar]
- Papadopoulos T, Eulenburg V, Reddy-Alla S, Mansuy IM, Li Y, Betz H. Collybistin is required for both the formation and maintenance of GABAergic postsynapses in the hippocampus. Mol Cell Neurosci. 2008;39:161–169. doi: 10.1016/j.mcn.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Papadopoulos T, Korte M, Eulenburg V, Kubota H, Retiounskaia M, Harvey RJ, Harvey K, O’Sullivan GA, Laube B, Hulsmann S, et al. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 2007;26:3888– 3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, Zhu L, Brass LF, Chen C, Greenberg ME. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra P, Gulyas AI, Miles R. How many subtypes of inhibitory cells in the hippocampus? Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy AK, Lane JB. Rett syndrome: model of neurodevelopmental disorders. J Child Neurol. 2005;20:718–721. doi: 10.1177/08830738050200090301. [DOI] [PubMed] [Google Scholar]
- Peters A, Harriman KM. Different kinds of axon terminals forming symmetric synapses with the cell bodies and initial axon segments of layer II/III pyramidal cells. I. Morphometric analysis. J Neurocytol. 1990;19:154–174. doi: 10.1007/BF01217295. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL. The morphology of synapses. J Neurocytol. 1996;25:687–700. doi: 10.1007/BF02284835. [DOI] [PubMed] [Google Scholar]
- Pfaff T, Malitschek B, Kaupmann K, Prezeau L, Pin JP, Bettler B, Karschin A. Alternative splicing generates a novel isoform of the rat metabotropic GABA(B)R1 receptor. Eur J Neurosci. 1999;11:2874–2882. doi: 10.1046/j.1460-9568.1999.00704.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F, Graham D, Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982;257:9389–9393. [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Pilgram GS, Potikanond S, Baines RA, Fradkin LG, Noordermeer JN. The roles of the dystrophin-associated glycoprotein complex at the synapse. Mol Neurobiol. 2010;41:1–21. doi: 10.1007/s12035-009-8089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Prange O, Murphy TH. Modular transport of postsynaptic density-95 clusters and association with stable spine precursors during early development of cortical neurons. J Neurosci. 2001;21:9325–9333. doi: 10.1523/JNEUROSCI.21-23-09325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Rao A, Cha EM, Craig AM. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J Neurosci. 2000;20:8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheims S, Holmgren CD, Chazal G, Mulder J, Harkany T, Zilberter T, Zilberter Y. GABA action in immature neocortical neurons directly depends on the availability of ketone bodies. Journal of neurochemistry. 2009;110:1330–1338. doi: 10.1111/j.1471-4159.2009.06230.x. [DOI] [PubMed] [Google Scholar]
- Richter K, Langnaese K, Kreutz MR, Olias G, Zhai R, Scheich H, Garner CC, Gundelfinger ED. Presynaptic cytomatrix protein bassoon is localized at both excitatory and inhibitory synapses of rat brain. J Comp Neurol. 1999;408:437–448. doi: 10.1002/(sici)1096-9861(19990607)408:3<437::aid-cne9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002a;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nature neuroscience. 2002b;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DR, McAllister AK, Lo DC, Katz LC. Neurotrophins in cortical development. Cold Spring Harb Symp Quant Biol. 1996;61:85–93. [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rostaing P, Real E, Siksou L, Lechaire JP, Boudier T, Boeckers TM, Gertler F, Gundelfinger ED, Triller A, Marty S. Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur J Neurosci. 2006;24:3463– 3474. doi: 10.1111/j.1460-9568.2006.05234.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo SL, Gomes RA, McAllister AK. Formation of presynaptic terminals at predefined sites along axons. J Neurosci. 2006;26:10813–10825. doi: 10.1523/JNEUROSCI.2052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiepour L, Fuchs C, Patrizi A, Sassoe-Pognetto M, Harvey RJ, Harvey K. Complex role of collybistin and gephyrin in GABAA receptor clustering. J Biol Chem. 2010;285:29623–29631. doi: 10.1074/jbc.M110.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115– 130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, Chu SH, Moreau MP, Gupta AR, Thomson SA, et al. Multiple Recurrent De Novo CNVs, Including Duplications of the 7q11.23 Williams Syndrome Region, Are Strongly Associated with Autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmarti-Vila L, tom Dieck S, Richter K, Altrock W, Zhang L, Volknandt W, Zimmermann H, Garner CC, Gundelfinger ED, Dresbach T. Membrane association of presynaptic cytomatrix protein bassoon. Biochem Biophys Res Commun. 2000;275:43–46. doi: 10.1006/bbrc.2000.3256. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Kirsch J, Grunert U, Greferath U, Fritschy JM, Mohler H, Betz H, Wassle H. Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. J Comp Neurol. 1995;357:1–14. doi: 10.1002/cne.903570102. [DOI] [PubMed] [Google Scholar]
- Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz DA, Barry G, Eliasof SD, Petroski RE, Conlon PJ, Maki RA. Characterization of gamma-aminobutyric acid receptor GABAB(1e), a GABAB(1) splice variant encoding a truncated receptor. J Biol Chem. 2000;275:32174–32181. doi: 10.1074/jbc.M005333200. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Zushida K, Yoshida M, Maekawa M, Kamichi S, Sahara Y, Yuasa S, Takeda S, Wada K. A deficit of brain dystrophin impairs specific amygdala GABAergic transmission and enhances defensive behaviour in mice. Brain. 2009;132:124–135. doi: 10.1093/brain/awn253. [DOI] [PubMed] [Google Scholar]
- Shapira M, Zhai RG, Dresbach T, Bresler T, Torres VI, Gundelfinger ED, Ziv NE, Garner CC. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38:237–252. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohtsuka T, Fejtova A, Kao HT, Greengard P, Gundelfinger ED, Triller A, et al. Three-dimensional architecture of presynaptic terminal cytomatrix. J Neurosci. 2007;27:6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksou L, Triller A, Marty S. An emerging view of presynaptic structure from electron microscopic studies. J Neurochem. 2009;108:1336–1342. doi: 10.1111/j.1471-4159.2009.05888.x. [DOI] [PubMed] [Google Scholar]
- Slezak M, Pfrieger FW. New roles for astrocytes: regulation of CNS synaptogenesis. Trends Neurosci. 2003;26:531–535. doi: 10.1016/j.tins.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, Shigemoto R, Watanabe M, Somogyi P. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19:552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer R, von Boehmer L, Haenggi T, Schweizer C, Benke D, Rudolph U, Fritschy JM. Alteration of GABAergic synapses and gephyrin clusters in the thalamic reticular nucleus of GABAA receptor alpha3 subunit-null mice. Eur J Neurosci. 2006;24:1307– 1315. doi: 10.1111/j.1460-9568.2006.05006.x. [DOI] [PubMed] [Google Scholar]
- Studler B, Fritschy JM, Brunig I. GABAergic and glutamatergic terminals differentially influence the organization of GABAergic synapses in rat cerebellar granule cells in vitro. Neuroscience. 2002;114:123–133. doi: 10.1016/s0306-4522(02)00206-3. [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi M, Kagaya A, Hayashi T, Motohashi N, Yamawaki S. gamma- Aminobutyric acid increases intracellular Ca2+ concentration in cultured cortical neurons: role of Cl− transport. Eur J Pharmacol. 1996;297:137–143. doi: 10.1016/0014-2999(95)00734-2. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927– 938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- Tate P, Skarnes W, Bird A. The methyl-CpG binding protein MeCP2 is essential for embryonic development in the mouse. Nat Genet. 1996;12:205–208. doi: 10.1038/ng0296-205. [DOI] [PubMed] [Google Scholar]
- Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783–787. doi: 10.1038/nature09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. The Journal of physiology. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD, Kolodkin AL. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–1069. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Moss SJ. GABA(A) Receptor Dynamics and Constructing GABAergic Synapses. Front Mol Neurosci. 2008;1:7. doi: 10.3389/neuro.02.007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:877– 885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay D, Yuste R. On the electrical function of dendritic spines. Trends Neurosci. 2004;27:77–83. doi: 10.1016/j.tins.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, et al. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Allene C, Nardou R, Picardo MA, Yamamoto S, Sivakumaran S, Caiati MD, Rheims S, Minlebaev M, Milh M, et al. Depolarizing actions of GABA in immature neurons depend neither on ketone bodies nor on pyruvate. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:34–45. doi: 10.1523/JNEUROSCI.3314-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Minlebaev M, Rheims S, Ivanov A, Jorquera I, Holmes GL, Zilberter Y, Ben-Ari Y, Khazipov R. Postnatal changes in somatic gamma-aminobutyric acid signalling in the rat hippocampus. Eur J Neurosci. 2008;27:2515–2528. doi: 10.1111/j.1460-9568.2008.06234.x. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010;10:207–214. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Vashlishan AB, Madison JM, Dybbs M, Bai J, Sieburth D, Ch’ng Q, Tavazoie M, Kaplan JM. An RNAi screen identifies genes that regulate GABA synapses. Neuron. 2008;58:346–361. doi: 10.1016/j.neuron.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, Buonanno A. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MK, Schaffner AE, Barker JL. Sodium channels, GABAA receptors, and glutamate receptors develop sequentially on embryonic rat spinal cord cells. J Neurosci. 1993;13:2068–2084. doi: 10.1523/JNEUROSCI.13-05-02068.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci. 2008;28:5547–5558. doi: 10.1523/JNEUROSCI.5599-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004;24:8253– 8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Arking DE, Daly MJ, Chakravarti A. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461:802–808. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, Yin DM, Lai C, Terry AV, Jr, Vazdarjanova A, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Becker N, Bonhoeffer T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat Neurosci. 2008;11:1044–1052. doi: 10.1038/nn.2180. [DOI] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, et al. Neuregulin-1 enhances depolarization- induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Xu J, Wojcik WJ. Gamma aminobutyric acid B receptor-mediated inhibition of adenylate cyclase in cultured cerebellar granule cells: blockade by islet-activating protein. J Pharmacol Exp Ther. 1986;239:568–573. [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko S, Chang S, O’Donoghue M, Popov SV. Neurotransmitter secretion along growing nerve processes: comparison with synaptic vesicle exocytosis. J Cell Biol. 1999;144:507–518. doi: 10.1083/jcb.144.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander JF, Munster-Wandowski A, Brunk I, Pahner I, Gomez-Lira G, Heinemann U, Gutierrez R, Laube G, Ahnert-Hilger G. Synaptic and vesicular coexistence of VGLUT and VGAT in selected excitatory and inhibitory synapses. J Neurosci. 2010;30:7634–7645. doi: 10.1523/JNEUROSCI.0141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Zhang C, Atasoy D, Arac D, Yang X, Fucillo MV, Robison AJ, Ko J, Brunger AT, Sudhof TC. Neurexins physically and functionally interact with GABA(A) receptors. Neuron. 2010a;66:403–416. doi: 10.1016/j.neuron.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Zak JD, Liu H. MeCP2 is required for normal development of GABAergic circuits in the thalamus. J Neurophysiol. 2010b;103:2470–2481. doi: 10.1152/jn.00601.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y, Zilberter T, Bregestovski P. Neuronal activity in vitro and the in vivo reality: the role of energy homeostasis. Trends Pharmacol Sci. 2010;31:394–401. doi: 10.1016/j.tips.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]