Abstract
Numerous peptides released from endocrine cells in the intestinal mucosa were established early on to be involved in the physiological regulation of food intake with a prominent role in termination of food ingestion when nutrients pass along the intestinal tract. Recently, peptides released from X/A-like endocrine cells of the gastric oxyntic mucosa were recognized as additional key players in the regulation of feeding and energy expenditure. Gastric X/A-like cells release the octanoylated peptide, ghrelin, the only known peripherally produced hormone stimulating food intake through interaction with growth hormone secretagogue 1a receptor (GHS-R1a). Additionally, non-octanoylated (des-acyl) ghrelin present in the circulation at higher levels than ghrelin is currently discussed as potential modulator of food intake by opposing ghrelin’s action independent from GHS-R1a although the functional significance remains to be established. Obestatin, a ghrelin-associated peptide was initially reported as anorexigenic modulator of ghrelin’s orexigenic action. However, subsequent reports did not support this contention. Interesting is the recent identification of nesfatin-1, a peptide derived from the nucleobindin2 gene prominently expressed in the stomach in gastric X/A-like cells in different vesicles than ghrelin. Circulating nesfatin-1 levels vary with metabolic state and peripheral or central injection inhibits dark phase feeding in rodents. Overall, these data point to an important role of X/A-like cells of the stomach in food intake regulation through the expression of the orexigenic peptide ghrelin along with des-acyl ghrelin and nesfatin-1 capable of reducing food intake upon exogenous injection although their mechanisms of action and functional significance remain to be established.
1. Introduction
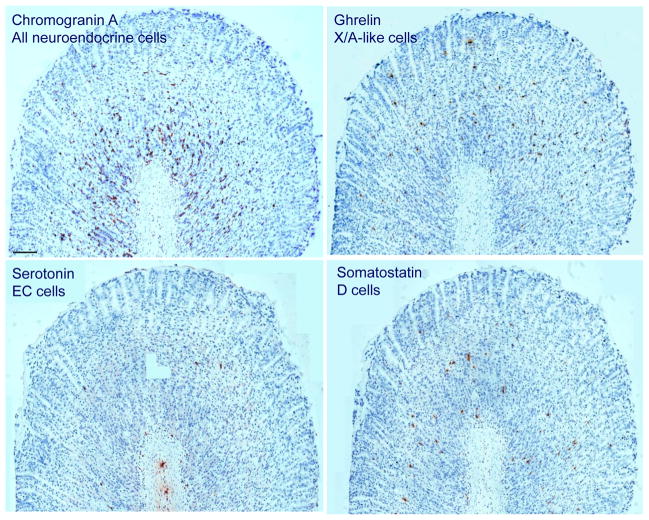
Several enteroendocrine cells scattered within the intestinal mucosa [114] have been recognized early on to influence food intake by releasing peptide hormones in response to changes in nutritional status [57, 77, 101] while endocrine cells in the gastric mucosa were mainly implicated in the regulation of acid secretion [34]. The gastric endocrine cells encompass enterochromaffin-like cells releasing histamine (ECL, 30% in human and 65% in the rat), gastrin-producing cells (G cells), somatostatin-containing cells (D cells >20% of gastric oxyntic endocrine cells in humans and 5–10% in rats), and the less abundant serotonin-containing enterochromaffin (EC) cells (Fig. 1) [113, 114]. In addition, there is a distinct 5th endocrine cell type without connection to the lumen (closed-type) distributed throughout the gastric oxyntic glands that was labeled P/D1 cell in humans and X/A-like cell in rats due to similarity with the rat pancreatic A-cell [21]. This cell type accounts for up to 20–30% of the oxyntic endocrine cell population and represents the second most frequent type among the gastric endocrine cells [21, 114]. Their content and function remained largely unknown until ghrelin was identified in rat X/A-like and human P/D1 cells as the only peripherally produced and centrally acting peptide hormone known so far to increase food intake [84, 115]. This seminal discovery revealed that the gastric mucosa contains endocrine cells able to influence food consumption by the release of a specific peptide as previously identified in the intestinal mucosa. Of functional relevance was the subsequent demonstration of interaction between ghrelin and anorexigenic peptides produced by intestinal endocrine cells such as cholecystokinin (CCK), peptide YY (PYY), and glucagon-like peptide 1 (GLP-1) (for review see [77, 101]).
Fig. 1.
Immunohistochemical picture of neuroendocrine cells in the rat gastric oxyntic mucosa of ad libitum fed male rats. While the majority of neuroendocrine cells reside in the lower part of gastric glands, ghrelin positive X/A-like cells are evenly distributed throughout the entire length of the glands. Somatostatin-positive D cells are mostly localized in the lower half of the oxyntic glands. Serotonin-positive EC cells are rare. Scale bar represents 100 μm.
Furthermore, the last years witnessed the identification and characterization of additional gene products within the X/A-like cells also able to influence food intake. These include peptides resulting from differential post-translational modifications of pro-ghrelin (non-octanoylated ghrelin or des-acyl ghrelin and n-decanoyl ghrelin) [59, 63], distinct potential processing of the ghrelin gene (obestatin) [164] or the processing of a distinct gene such as nucleobindin2 (nesfatin-1) [137]. Recent functional reports indicate the reduction of food intake induced by exogenous administration of des-acyl ghrelin [9, 31] and nesfatin-1 [132] whereas the action of obestatin remains largely equivocal [49]. In the present review we will highlight gastric X/A-like cells and the actions of peptides processed in these cells on food intake and modalities pursued targeting these peptides for potential novel therapeutic venues in the treatment of obesity with emphasis on recent developments.
2. Ghrelin
Ghrelin (also known as acyl ghrelin or octanoylated ghrelin) was discovered in 1999 by Kojima and colleagues as the endogenous ligand of the long known growth hormone secretagogue receptor 1a isoform (GHS-R1a, Fig. 2) [84]. The 28-amino acid peptide ghrelin displays a unique feature among peptides which is an acyl group at the serine-3 residue providing increased lipophilicity essential for the binding to the GHS-R1a receptor [19]. While an n-octanoyl group (8-carbon chain with no double bond) is the primary acyl chain in human and rodent ghrelin [85], an additional acyl modification namely n-decanoyl (10-carbon chain without double bond) ghrelin is also produced in X/A-like cells contributing up to 40–60% of the circulating esterified ghrelin in mice [59]. In the human stomach, the ratio of octanoylated to decanoylated ghrelin is around 3:1 [64]. Additional studies in mice established that orally ingested medium chain fatty acids are directly incorporated to form the acyl group of ghrelin resulting in stomach concentrations of ghrelin with an acyl group that bears the signature of the corresponding carbon chain lengths (n-hexanoyl ghrelin, n-octanoyl ghrelin and n-decanoyl ghrelin) of medium-chain fatty acids added to the diet [105]. The enzyme responsible for acylation of ghrelin was unknown for almost 10 years and has recently been identified in mice and humans as a member of the superfamily of membrane-bound O-acyltransferases (MBOATs) and was termed ghrelin-O-acyltransferase (GOAT) [55, 160]. GOAT is highly co-expressed with ghrelin in cells of the mouse gastric mucosa as shown by in situ hybridization [120]. The enzyme octanoylates pro-ghrelin before its transport to the Golgi apparatus where it is cleaved by prohormone convertase (PC) to form the mature ghrelin [160]. Among seven mammalian PCs, PC1/3 has been reported to be primarily involved in the processing of the proghrelin precursor protein [167]. However, in vitro studies further indicate that not only PC1/3 but also both PC2 and furin could process proghrelin to the 28-amino acid ghrelin in presence of GOAT expression in the cells as well as n-octanoic acid in the culture medium which are both required to produce n-octanoyl ghrelin [142].
Fig. 2.
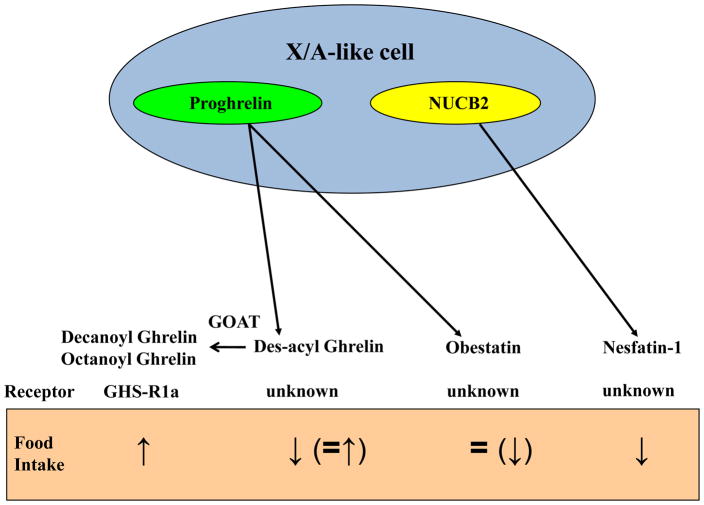
Peptide products of the rat gastric X/A-like cell and their effects on food intake in rodents. ↑, stimulation; ↓, inhibition; =, no effect; GHS-R1a, growth hormone secretagogue receptor 1a; GOAT, ghrelin-O-acyltransferase; NUCB2, nucleobindin2
2.1 Ghrelin release and receptor interaction
Ghrelin positive X/A-like cells distributed throughout the gastric oxyntic mucosa [38, 98] are the main source of circulating ghrelin [7] as demonstrated by the sharp decline of ghrelin levels following gastrectomy [69]. Moreover, ghrelin is produced, although in much lower amounts, in the small and large bowel [38], pancreas [40] as well as other peripheral viscera such as kidney, liver, heart, testis, adipose tissue and skin [18, 48]. These production sites could contribute to the circulating ghrelin but are also thought to respond to ghrelin through autocrine or paracrine mechanisms. Moreover, ghrelin has been detected in the central nervous system in the arcuate nucleus of the hypothalamus [95] as well as in neurons adjacent to the third ventricle [33]. Circulating ghrelin levels increase before and decline after a meal in experimental animals and humans (Fig. 3) [36]. Likewise, n-decanoyl ghrelin immunoreactivity is increased by fasting in the murine stomach and plasma [59]. Furthermore, total ghrelin levels inversely correlate with body mass index with increased levels in anorexic and cachectic patients and decreased levels under conditions of obesity (Fig. 3) [37, 148]. However, in the Prader-Willi syndrome, the most common hereditary cause for obesity, plasma ghrelin levels are elevated despite the increased body weight [35], likely contributing to the sustained appetite in those patients.
Fig. 3.
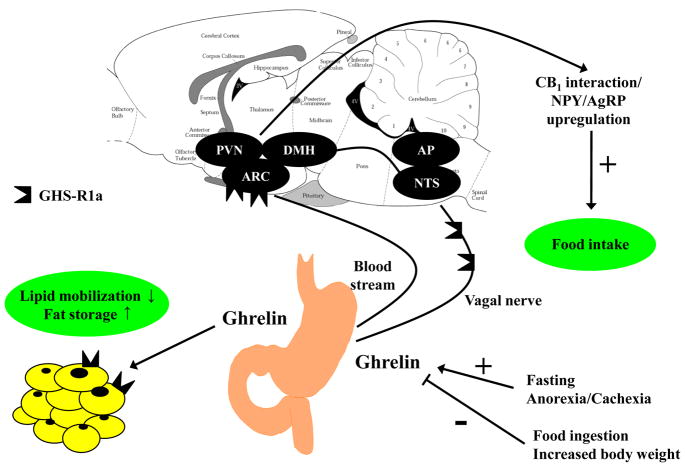
Schematic representation of factors influencing ghrelin release and mediating ghrelin’s effects on food intake and fat storage. ↑, increase; ↓, decrease; +, stimulation; −, inhibition, AgRP, agouti-related peptide; AP, area postrema; ARC, arcuate nucleus; DMH, dorsomedial nucleus of the hypothalamus; GHS-R1a, growth hormone secretagogue receptor 1a; NPY, neuropeptide Y; NTS, nucleus of the solitary tract; PVN, paraventricular nucleus of the hypothalamus.
Ghrelin secretion is influenced by a number of hormones, neurotransmitters and cytokines. In particular, circulating ghrelin levels are decreased by peripheral administration of bombesin/gastrin releasing peptide [42], somatostatin [42, 129], CCK [22], GLP-1 [56, 94, 110], insulin [117], interleukin-1 [152] and prostacyclin [97] and increased by adrenaline, noradrenaline [42], secretin [42], central vagal stimulation [5], sham feeding [134], endothelin 1 and 3 [42, 144], and the stable analog of the endogenous cannabinoid, anandamide (Fig. 4) [162]. However, it is not known whether most of these substances act directly on ghrelin cells expressing the respective receptors or indirectly by releasing downstream signals that then influence X/A-like cells. Isolation of purified X/A-like cells will help to address the mechanisms regulating ghrelin secretion. Genetic labeling of X/A-like cells with markers such as green fluorescent protein has recently been successfully used to enrich ghrelin cells from the murine gastric mucosa [118, 120]. Further studies on enriched ghrelin cell populations will help to delineate receptor expression and underlying signaling mechanisms regulating gastric ghrelin release.
Fig. 4.
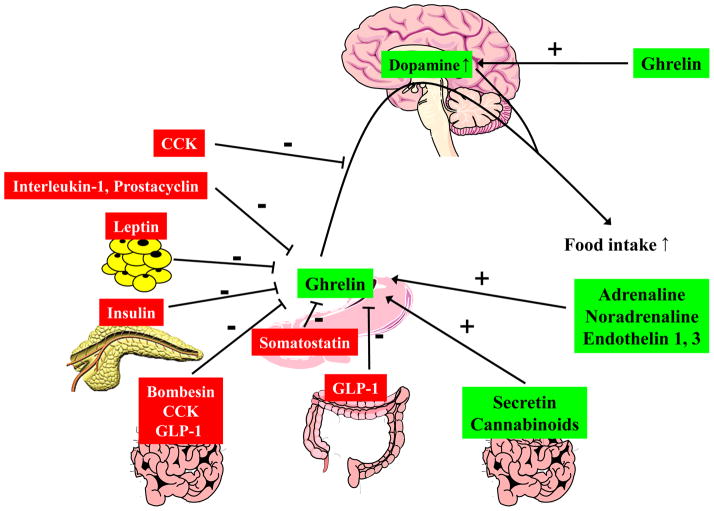
Schematic illustration of the interaction of ghrelin with other gut peptides and transmitters. ↑, increase; +, stimulation; −, inhibition.
Ghrelin binds to the GHS-R1a, which is expressed in the brain as well as in the periphery at sites not only involved in food intake regulation and reward but also memory and a variety of other functions [48, 54, 65, 103]. In the brain, ghrelin receptor mRNA is expressed in the arcuate nucleus, the ventromedial nucleus, the hippocampus in areas CA2 and CA3, the dentate gyrus, substantia nigra, ventral tegmental area, dorsal and median raphe nuclei and in the pituitary [54, 65, 103, 168]. Compared to the pituitary, ghrelin receptor mRNA has been detected in lower amounts in the thyroid, pancreas, spleen, myocardium and adrenal gland [48]. Additionally, ghrelin receptor mRNA expression was described in adipose tissue, immune cells and on vagal afferents [58, 125].
The octanoyl group on the serine in the third position is essential for binding to and activating the receptor [84, 85]. Moreover, structure function studies established that the active core of the ghrelin receptor-ligand interaction encompasses the first five N-terminal amino acids coupled to the hydrophobic residue [19]. Recent studies show a modulation of ghrelin signaling linked with homo- and heterodimerization of the GHS-R1a [125]. Homodimerization of the GHS-R1a seems to contribute to the synergistic interaction between growth hormone-releasing hormone and ghrelin on growth hormone release [125]. In contrast, heterodimerization of GHS-R1a and GHS-R1b decreases ghrelin responsiveness whereas heterodimerization between GHSR-1a and cannabinoid 1 (CB1) receptors is likely to play a role in the food intake stimulatory interaction between ghrelin and endocannabinoids [125]. It is noteworthy to mention that GHS-R1a is among the few G-coupled protein receptors which are constitutively active, resulting in signaling even in the absence of the ligand [62].
2.2 Stimulatory effect of ghrelin on food intake
Ghrelin is well established to stimulate food consumption in both lean and obese humans [44] and food intake upon peripheral and brain injection in various naïve animal species (Fig. 3) [157]. In addition, exogenous injection of ghrelin is able to restore food intake suppressed by endotoxin [152] or in experimental conditions mimicking chronic renal failure by subtotal nephrectomy in rats [3]. Recent studies to assess the biological action of other acylated forms of ghrelin produced in the stomach show that n-decanoyl ghrelin displays similar orexigenic effects as ghrelin in mice [59]. Ghrelin’s orexigenic action is GHS-R1a-mediated as characterized by the suppression of exogenous and endogenous (fasting) ghrelin-induced food intake and activation of hypothalamic ghrelin responsive cells by several developed GHS-R1a antagonists (e.g. JMV 3002, 2959 and 2810) [121]. In addition, GHS-R1a knockout mice do not increase food intake after a single ghrelin injection [141, 169].
Ghrelin can reach hypothalamic food intake regulatory centers directly after passage of the blood-brain barrier (Fig. 3) [17, 109]. In addition, the presence of ghrelin receptors on vagal afferents and neurons in the rat nodose ganglion provides anatomical support for ghrelin signaling to the brain via the vagus nerve (Fig. 3) [39, 119]. This is further supported by the demonstration that vagotomy abolishes the ghrelin-induced food intake [39]. Moreover, exogenous ghrelin failed to stimulate food intake in patients after surgical procedures involving vagotomy [92]. However there is also a report showing that intraperitoneal injection of ghrelin can stimulate food intake in rats with selective subdiaphragmatic vagal deafferentation [8] indicating that peripheral ghrelin-induced stimulation of food intake may be mediated via the afferent vagus nerve along with direct access to the brain.
2.3 Effects of ghrelin on fat mass, energy expenditure and body weight
Besides regulating food consumption, ghrelin is also involved in body weight modulation. Chronic delivery of the peptide leads to body weight gain in rodents not only through increasing appetite but more prominently by promoting fat storage in white adipose tissue [41, 148]. Particularly, adipose tissues such as retroperitoneal and inguinal fat depots are enlarged whereas superficial adipose fat in subcutaneous depots is not altered and such lipogenic effect is growth hormone-independent [41, 123]. Other studies indicate that reduced lipid mobilization reflected by an increased respiratory exchange ratio may underlie ghrelin’s adipose tissue promoting effect [41] independently of its orexigenic properties (Fig. 3) [145]. Genetic models directed to knockdown or over-express the ghrelin signaling pathways result in a phenotype that is not as clear as that induced by pharmacological approaches. Ghrelin over-expressing mice resulting in increased circulating levels of ghrelin and des-acyl ghrelin have no alterations of food intake or body weight, however, they have an impaired glucose tolerance most likely due to suppressed insulin secretion [68]. Mice lacking both ghrelin and the ghrelin GHS-R1a however show increased energy expenditure and consecutively decreased body weight when fed a normal diet whereas single knockout (KO) mice (for either ghrelin or its receptor) do not show this phenotype [112]. When fed a high fat diet, ghrelin null mice exhibited a reduced respiratory quotient indicating increased fat utilization [156]. Moreover, GHS-Ra knockout (KO) mice were shown to be resistant to diet-induced obesity, which further supports the role of ghrelin in adipogenesis [169]. In contrast, another research group showed in GHS-Ra KO mice that ghrelin is involved in insulin sensitivity and glucose regulation while these mice were not resistant to diet-induced obesity [140]. This discrepancy has been suggested to be related to difference in the genetic background of the KO mice colonies. Taken together, these studies indicate that chronic alterations of ghrelin signaling pathways more prominently impact on energy expenditure than food intake although adaptive and compensatory regulatory mechanisms may also take place under conditions of chronically altered ghrelin signaling by genetic modifications.
Recently the role of GOAT and ghrelin in the control of energy balance and adiposity was investigated in mice lacking the GOAT gene (Mboat4) or over-expressing human ghrelin and GOAT [79]. GOAT knockout mice did not show alterations in body weight when fed a standard rodent diet but displayed a decreased body weight when kept on a high fat diet compared to their wildtype littermates [79]. Moreover, when mice are fed with a diet enriched in medium-chain triglycerides, GOAT deficient mice have a lower body weight and reduced fat mass compared to wildtype [79]. These findings provide new insight to the ghrelin-GOAT system as a lipid sensor not only in the absence of food but also when high caloric food is available [79, 135]. Moreover, a recent study described that GOAT prefers n-hexanoyl-CoA over n-octanoyl-CoA as the acyl donor in vitro [108]. However, the concentration of n-hexanoyl ghrelin in the murine stomach is markedly lower than n-octanoyl ghrelin [108] probably due to the lower amount of this acyl donor in vivo.
2.4 Mediation of ghrelin’s orexigenic effect by appetite-regulating neuropeptides in the hypothalamus
As described above ghrelin is also produced centrally in the arcuate nucleus of the hypothalamus [95] and in neurons adjacent to the third ventricle [33]. The arcuate nucleus is strongly implicated in the regulation of food intake [126]. Ghrelin-containing neurons in the in the arcuate nucleus send projections to neuropeptide Y (NPY) and agouti-related peptide (AgRP) positive neurons [33, 53]. NPY and AgRP are orexigenic neuropeptides [1] and regulated by ghrelin. Peripheral injection of ghrelin selectively activates NPY-containing neurons of the arcuate nucleus in mice [153]. Likewise, intracerebroventricular (icv) administration of ghrelin activates NPY/AgRP-expressing neurons and stimulates the expression of NPY and AgRP mRNA in the arcuate nucleus (Fig. 3) [75]. Conversely, the orexigenic effect of icv ghrelin is blocked by the co-injection of an anti-NPY or anti-AgRP antibody [103]. Studies using double NPY and AgRP KO mice corroborated these findings with peripherally injected ghrelin. Whereas single deletion of NPY or AgRP was not effective in blocking the food intake stimulatory action of intraperitoneally administered ghrelin suggesting compensatory actions, deletion of both, NPY and AgRP genes resulted in a complete suppression of ghrelin’s orexigenic action [32]. Thus, there is convincing evidence that the central mediation of ghrelin’s orexigenic effect involves hypothalamic NPY and AgRP.
2.5 Interaction between ghrelin and gut peptides or brain transmitters
The interplay between gastric and intestinal peptides is of importance since they are often released simultaneously in response to changes in metabolic conditions and nutritional status. The orexigenic action of ghrelin and related Fos expression in specific hypothalamic nuclei regulating food intake are completely abolished by intraperitoneal co-injection with CCK-8 in rats (Fig. 4) [82]. Likewise, peripheral injection of bombesin, unlike amylin, abolishes ghrelin’s orexigenic effect in rats (Fig. 4) [80]. Moreover, GLP-17–36 amide inhibits ghrelin secretion in the isolated rat stomach [94]. Likewise, exendin-4, a GLP-1 receptor agonist, reduces circulating ghrelin levels by up to 70% after peripheral as well as central injection in rats [110]. Therefore, GLP-1 may contribute to the postprandial decline in ghrelin levels. Conversely, the anorexigenic effect of PYY3–36 or GLP-1 is attenuated by ghrelin by up to 64% [30].
The anorexigenic hormone leptin is a 16 kDa protein mainly derived from the white adipose tissue [46] but also expressed in the gastric mucosa [14]. Gastric ghrelin mRNA levels are decreased by peripheral administration of leptin (Fig. 4) and increased in leptin-deficient ob/ob mice [11] indicative of a negative regulation of gastric ghrelin gene expression by leptin. In the brain, central injection of ghrelin reverses the central leptin-induced inhibition of food intake in rats [103] possibly due to restoring the leptin-induced decrease in hypothalamic NPY mRNA levels [133]. These data indicate an interaction between leptin and ghrelin in the hypothalamus on neuronal circuits regulating food intake. Whether endogenous ghrelin released into the circulation and crossing the blood-brain barrier [17, 109] or locally produced ghrelin in the brain [33, 84, 95] interact with leptin remains to be investigated.
The mesocorticolimbic dopamine system is involved in the hedonic aspects of food [124]. Several lines of evidence highlight the impact of ghrelin on reward mechanisms [73]. Central as well as peripheral administration of ghrelin induces dopamine overflow in the nucleus accumbens of mice (Fig. 4) [70, 71]. Furthermore, microinjection of ghrelin into the ventral tegmental area and latero-dorsal tegmental area increased the extracellular concentration of accumbal dopamine [72]. A single intra-tegmental infusion of ghrelin significantly increases food intake [2]. Moreover, ghrelin directly influences the electrophysiological responses in most dopamine-positive neurons in mice and rats, increases dopamine turnover and thus enhances the activity of dopaminergic cells in the nucleus accumbens of the ventral tegmental area as shown by whole-cell patch clamp recordings [2]. At the cellular level, activation of GHS-R1a by ghrelin also amplifies dopamine/dopamine1 receptor-induced cAMP accumulation with a mechanism consistent with agonist-dependent formation of GHS-R1a/dopamine1 receptor heterodimers [74]. These direct actions of ghrelin on dopamine-producing cells could underlie the effects of ghrelin on reward.
Cannabinoids (CB) are lipids that stimulate food intake and body weight gain similarly to ghrelin and could therefore share common pathways [125]. This is supported by the demonstration that central injection of ghrelin is unable to increase food intake in CB1 receptor knockout mice [86] indicating that the endocannabinoid signaling system is necessary for ghrelin’s orexigenic effect. Anandamide, an endocannabinoid, injected peripherally increases circulating ghrelin levels [162]. Conversely, rimonabant, a CB1 receptor antagonist, reduces systemic ghrelin levels (Fig. 4) [25]. The crosstalk between ghrelin and endocannabinoids could take place at the receptor level based on the observation that the GHS-R1a and CB1 are expressed in the hypothalamus with high expression levels in the arcuate nucleus and the assumption of the formation of heterodimers which warrants further characterization [125]. Evidence so far indicates a complex interplay between ghrelin and other peripheral and central mechanisms modulating food intake.
2.6 Ghrelin in the clinical setting
Much attention has been given to ghrelin due to its unique orexigenic action in animals and humans. Ghrelin is able to stimulate food intake under conditions of impaired appetite as shown in two randomized placebo-controlled trials with patients suffering from end-stage kidney disease (Table 1) [12, 158]. Moreover, daily oral application of a formula rich in octanoic acids over a period of two weeks increases plasma ghrelin levels as well as nutritional status in cachectic patients with chronic respiratory disease (Table 1) [13].
Table 1.
Effects of targeting ghrelin in pre-clinical versus clinical studies to modulate food intake and/or body weight.
| Goal | Compound | Species and conditions | Route | Duration | Effect | Reference |
|---|---|---|---|---|---|---|
| Stimulate food intake and/or body weight |
Pre-clinical
|
|||||
| ghrelin | rats with chronic renal failure | ip | 7 days | food intake ↑, body weight = | [3] | |
|
| ||||||
| ghrelin | rats after lipopolysaccharide injection | iv | acute | food intake↑ | [152] | |
|
| ||||||
|
Clinical
| ||||||
| ghrelin | peritoneal dialysis patients with malnutrition | sc | acute | food intake↑ | [158] | |
|
| ||||||
| ghrelin | dialysis patients with malnutrition | sc | 7 days | appetite ↑, energy intake ↑ | [12] | |
|
| ||||||
| formula rich in octanoic acids | cachectic patients with chronic respiratory disease | po | 14 days | body weight ↑ | [13] | |
|
| ||||||
| Inhibit food intake and/or body weight |
Pre-clinical
|
|||||
| anti-ghrelin vaccine | rats | ip | 13 weeks | body weight gain ↓ | [171] | |
|
| ||||||
| anti-ghrelin vaccine | pigs | sc | 40 days | food intake ↓, body weight ↓ | [151] | |
|
| ||||||
| anti-ghrelin vaccine | mice on high fat diet | sc | 22 weeks | body weight gain = | [78] | |
|
| ||||||
| anti-ghrelin antibody | mice with diet-induced obesity | ip | 4 weeks | acute: food intake ↓, chronic: food intake =, body weight = | [96] | |
|
| ||||||
| anti-ghrelin oligonucleotide | rats | ip | acute | food intake ↓, neuronal activation ↓ | [81] | |
|
| ||||||
| anti-ghrelin oligonucleotide | mice with diet-induced obesity | sc | 13 days | food intake ↓, body weight ↓ | [128] | |
|
| ||||||
| GHS-R antagonist | lean and diet-induced obese mice | ip | 5 days | food intake ↓, body weight gain ↓ | [10] | |
|
| ||||||
|
Clinical
| ||||||
| anti-ghrelin vaccine | obese patients | ns | 24 weeks | body weight = | [20] | |
↑, increase; ↓, decrease; =, no effect; ip, intraperitoneal; iv, intravenous; ns, not specified; po, per os; sc, subcutaneous
The blocking of endogenous or exogenous ghrelin was actively investigated using various approaches in order to reduce food intake and body weight gain for potential new therapeutic venues. Anti-ghrelin vaccination gave very promising results by curtailing body weight gain in pre-clinical studies in pigs and rats (Table 1) [151, 171]. However, in mice fed a high fat diet anti-ghrelin vaccination failed to decrease body weight (Table 1) although acutely reducing food intake [78]. One difference between those studies, possibly contributing to the divergent outcome, is the adjuvant used. In pigs, the ghrelin peptide was linked to bovine serum albumin [151] and in rats to the carrier protein, keyhole limpet hemocyanin [171], while in mice Pan-DR epitope peptides were used [78]. However, the exact underlying mechanism remains to be determined. Likewise, another study using a ghrelin-neutralizing antibody failed to demonstrate a reduction in body weight in mice kept on a high fat diet (Table 1) [96]. Both studies in mice showed an increase in circulating ghrelin levels [78, 96] that may account for the lack of effect under conditions of already reduced ghrelin sensitivity [111]. Furthermore, species differences could also contribute to the different outcomes. Likewise, in obese human subjects ghrelin vaccination failed to induce body weight loss (Table 1) [20] suggesting compensatory mechanisms. Another experimental approach was the use of synthetic L-isomer oligonucleotides which are able to specifically bind to ghrelin with high affinity and to be resistant to endogenous nucleases [81]. This anti-ghrelin Spiegelmer (German Spiegel = mirror) NOX-B11 acutely blocks the exogenous ghrelin-induced food intake and neuronal activation [81]. A more advanced version (NOX-B11-2) also reduced body weight gain in high fat diet-induced obese mice upon chronic subcutaneous administration (Table 1) [128]. Interfering with ghrelin receptors, GHS-R1a antagonists that can cross the blood-brain barrier also reduce energy intake in lean and obese rodents (Table 1) [10].
Until now, no anti-obesity drug targeting ghrelin or the GHS-R1a has reached clinical efficacy as shown by the lack of sustained body weight loss in one clinical study [20]. This is most likely related to associated compensatory mechanisms [125] which may occur after chronic treatment design in human studies (e.g. 24 weeks) [20] when compared to animal studies (e.g. 12 weeks) [171], as like in humans, ghrelin KO mice also displayed normal growth and a similar food intake pattern as wildtype [156]. The existence of additional ghrelin receptor subtypes that have yet to be identified [125] may also contribute to the lack of efficacy of the current drugs, thereby bypassing the effect of selective antagonists.
Bariatric surgery is the most effective treatment for long-term weight loss in morbidly obese patients as shown by reports with the commonly performed Roux-en-Y gastric bypass surgery (RYGB) [143]. In those patients circulating ghrelin levels are low postoperatively and no meal-related ghrelin changes can be observed which has been proposed to underlie, or at least contribute, to the efficacy of bariatric surgery (Table 2) [37]. Likewise, sleeve gastrectomy, a technique that involves removal of the gastric fundus, reduces body weight in animal models [154] as well as obese patients (Table 2) [76]. Ghrelin levels decrease after this procedure [90], consistent with the stomach being the major production site of circulating ghrelin (Table 2). This long-lasting decrease is thought to play a role in the beneficial effect of sleeve gastrectomy on body weight [45] and also the observed improvement of glucose control [93]. This decrease contrasts with the increased levels of circulating ghrelin seen after diet-induced weight loss in obese patients (Table 2) [87]. Similarly, after gastric banding that maintains the normal food passage as well as keeps the gastric fundus intact but decreases the volume of the proximal gastric reservoir, circulating ghrelin levels increase (Table 2) [87, 90]. One proposed interpretation relates to the ghrelin response as reflecting a state of starvation to restore the previous obese “normal” state since the homeostatic set-point is changed in obese patients and consecutively the obese state is perceived as “normal” [155].
Table 2.
Effects of three common surgical procedures compared to a dietary approach on body mass index (BMI) and circulating ghrelin levels.
| Intervention/Technique | Duration | Effects on BMI | Effects on circulating ghrelin | Reference |
|---|---|---|---|---|
| Roux-en-Y gastric bypass | 9 – 31 months | 36% reduction of BMI | 77% reduction of the 24 h ghrelin profile (area under the curve) | [37] |
| Sleeve gastrectomy | 12 months | 36% reduction of BMI | 34% reduction of fasting total ghrelin plasma levels | [76] |
| Gastric banding | 18 months | 38% reduction of BMI | 65% increase of fasting total ghrelin plasma levels | [87] |
| Diet | 6 months | 9% reduction of BMI | 110% increase of fasting total ghrelin plasma levels | [87] |
3. Des-acyl ghrelin
Initial reports indicated that the vast majority of the circulating ghrelin form is represented by des-acyl ghrelin with an acyl/total ghrelin ratio of 1:55 [63]. However, we have recently shown that higher yields of the labile acylated ghrelin form can be obtained when a novel method is applied to blood processing [139] compared with the commonly used standard method using mainly EDTA blood on ice [15]. We found in ad libitum fed rats that the acyl/total ghrelin ratio is 1:5 with the new blood processing method compared to 1:19 with the standard method. In fasted rats the acyl/total ghrelin ratio remains 1:5 with the new method and increased to 1:41 with the standard method of blood processing [139]. The novel RAPID method encompasses Reduced temperatures, Acidification, Protease inhibition, Isotopic exogenous controls and Dilution as recently detailed [139]. Such a procedure of blood treatment immediately after collection results in an 80% higher recovery of circulating acyl ghrelin levels and therefore represents a valuable advance to detect circulating concentrations of orexigenic ghrelin form [139]. Recent studies in the vascularly perfused rat stomach indicate that des-acyl ghrelin release is enhanced under conditions of low pH while that of acyl ghrelin is not [98]. Moreover, while des-acyl ghrelin co-localized with acyl ghrelin in round closed-type cells of the oxyntic mucosa, open-type cells were positive for des-acyl but not acyl ghrelin [98]. Furthermore a portion of des-acyl ghrelin-positive open-type cells contained somatostatin [98]. Taken together, these recent findings indicate a differential regulation in the post-translational processing of proghrelin leading to ghrelin and des-acyl ghrelin.
3.1 Des-acyl ghrelin and receptor interaction
In contrast to ghrelin, des-acyl ghrelin does not bind to the GHS-R1a due to the lack of the fatty acid group and therefore has been initially categorized as a non-active peptide (Fig. 2) [84]. However, since both ghrelin and des-acyl ghrelin affect cells that do not bear the GHS-R1a, the existence of yet unknown additional ghrelin receptors is strongly assumed [125, 136]. This is supported by the observations that des-acyl ghrelin stimulates insulin release from INS-1E cells independently of the GHS-R1a [47] and inhibits cell proliferation in human breast cancer [28] and prostate cancer [27] cell lines which do not express the GHS-R1a.
3.2 Des-acyl ghrelin: effects on food intake
Compared to ghrelin the effects of des-acyl ghrelin on food intake are less well characterized [67]. However, it is currently discussed as a possible anorexigenic hormone and modulator of ghrelin-induced food intake although some findings are still equivocal [67] (Table 3). Initial studies showed a reduction of food intake following intraperitoneal injection of des-acyl ghrelin in fasted rats during the light phase or ad libitum fed rats during the dark phase [31] and intracerebroventricular or intraperitoneal injection in fasted mice during the light phase [9]. Underlying mechanisms of the des-acyl ghrelin-induced anorexigenic effect following peripheral injection have been reported to be capsaicin-insensitive and associated with the activation of neurons in the paraventricular nucleus of the hypothalamus along with the disruption of motor activity in the stomach [31]. Whether the peptide action is secondary to induction of food aversion or altered behavior including the experience of sickness has not yet been investigated. However, subsequent studies failed to reproduce the anorexigenic effect following peripheral injection of des-acyl ghrelin in fasted rats [66] or mice [104]. In addition, one report shows a stimulation of food intake after intracerebroventricular injection of a low dose (1.9 μg/kg) of the peptide in rats [146]. Whether this reflects a direct mechanism of the peptide action or an effect after acylation remains to be elucidated. Recently, we demonstrated that des-acyl ghrelin, which does not influence food intake during the light as well as dark phase when injected alone, blocked the ghrelin-induced increase in food intake following simultaneous intraperitoneal injection in rats [66] (Table 3) suggesting a possible interaction between the two peptide forms depending upon the ratio released. The identification of des-acyl ghrelin receptors mediating these effects will increase our knowledge on mechanisms and site of action.
Table 3.
Effect of des-acyl ghrelin on food intake in rodents.
| Site of administration | Dose (μg/kg) | Feeding condition | Circadian phase | Species | Effect on food intake | Reference |
|---|---|---|---|---|---|---|
| icv | 1.9 | ad lib | light, dark | rats | ↑ | [146] |
| icv | 9.1–91 | ad lib | light | mice | = | [9] |
| icv | 91 | fasted | light | mice | ↓ | [9] |
|
| ||||||
| iv | 14.4–48 | ad lib | light | rats | = | [146] |
|
| ||||||
| ip | 128 | ad lib | light | mice | = | [146] |
| ip | 274 | fasted | light | mice | ↓ | [9] |
| ip | 64 | ad lib | light | rats | = | [31] |
| ip | 64 | fasted | light | rats | ↓ | [31] |
| ip | 64 | ad lib | dark | rats | ↓ | [31] |
| ip | 960 | ad lib, fasted | ns | mice | = | [104] |
| ip | 64–125 | ad lib, fasted | light | rats | = | [66] |
icv: intracerebroventricular, iv: intravenous, ip: intraperitoneal, ad lib: ad libitum fed, fas: fasted, ns: not specified
3.3 Effect of des-acyl ghrelin on fat mass and energy expenditure
Transgenic mice that over-express des-acyl ghrelin display a reduced body size accompanied by a decreased body weight compared to their wildtype littermates [6]. These changes were observed in the absence of altered food intake suggesting that undernutrition does not contribute to the observed effects on body weight. However, it has to be noted that these transgenic mice had 10 to 50-fold higher, and therefore supraphysiological, des-acyl ghrelin levels than their wildtype littermates. When des-acyl ghrelin expression was increased by the fatty acid-binding protein-4 (FABP4) promoter, circulating des-acyl ghrelin levels were markedly increased whereas ghrelin or obestatin levels were not altered [165]. Epididymal and perirenal fat depots were reduced in those transgenic mice compared to their wildtype littermates whereas brown adipose tissue was not changed [165]. Furthermore, these mice also display increased insulin sensitivity [165] suggesting that des-acyl ghrelin may play a role not only to reduce food intake and control body weight but also in glucose homeostasis. However, des-acyl ghrelin stimulates intracellular lipid accumulation in human adipocytes in vitro that were obtained from obese subjects [116] which may contribute to the fat accumulation in those patients. Whether this does reflect species differences or is attributable to divergent responses under in vivo and in vitro conditions or obesity status requires further investigations.
3.4 Possible use of targeting the ghrelin acylation process in the treatment of obesity
The recently identified enzyme that acetylates ghrelin, GOAT, attracted much attention as a potential target in the drug treatment of obesity. One study reported the first pentapeptide representing the octanylated N-terminus of ghrelin able to inhibit GOAT [161]. However, further studies will be required to develop new inhibitors without receptor-signaling activity. This strategy may provide a mean to selectively reduce circulating ghrelin and concomitantly increase des-acyl ghrelin.
4. The ghrelin-associated peptide, obestatin
Obestatin or proghrelin(53–75) is a proposed 23 amino acid residue amidated peptide resulting from alternative splicing and posttranslational modification from a computer-based predicted cleavage site of C-terminal proghrelin that was initially reported to have anorexigenic properties (Fig. 2) [136, 164]. Obestatin immunoreactivity can be detected together with ghrelin in the vesicles of the same cells in normal human gastric mucosa as well as gastric endocrine tumors [52, 149]. The co-localization between obestatin and preproghrelin was also shown in the rat gastric oxyntic mucosa, however less co-localization (60%) was shown with mature ghrelin pointing towards differential post-translational expression [166].
4.1 Release of obestatin and receptor interaction
In contrast to the fasting-induced increased expression of ghrelin both at a cellular and a systemic level, obestatin immunoreactivity in the gastric mucosa does not change during food deprivation [166]. Furthermore, circulating obestatin levels are below detection threshold [16, 100] and not affected by fasting or feeding [164] or in presence of a gastric neuroendocrine tumor [149]. However, one study showed a significant reduction of circulating obestatin after fasting [170]. Taken together, these data suggest differential mechanisms regulating post-translational modifications/cleavage leading to obestatin and ghrelin in X/A-like cells under conditions of changes in metabolic status.
When obestatin was described in 2005 it was thought to be the endogenous ligand of the seven transmembrane domain G protein-coupled receptor, GPR39 [164]. This receptor, which displays high constitutive activity, was an orphan member of the ghrelin receptor family [61]. However, all subsequent studies performed by several independent groups [29, 60, 91, 147] and also the original investigators [163] could reproduce neither the binding of obestatin to tissue homogenates and to cells transiently transfected with recombinant GPR39 nor the activation of the receptor by obestatin. Therefore, the endogenous ligand of GPR39 is still unknown as well as the cognate receptor for obestatin.
4.2 Controversial effects of obestatin on food intake
The original report on obestatin in 2005 suggested that the peptide suppresses food intake, decreases body weight gain and is able to counteract ghrelin’s orexigenic action [164]. However, only a few studies were able to partially reproduce these data when using very specific conditions [23, 26, 88, 102] and some of those have been recently retracted [89]. So far the vast majority of studies conducted by several independent groups have not been able to confirm the inhibitory effect of obestatin on food intake, body weight gain or its interaction with ghrelin [4, 24, 43, 50, 60, 83, 88, 99, 100, 106, 122, 127, 147, 150, 159, 170] as recently exhaustively reviewed [49]. Taken together, these data do not support the assumption of obestatin as a physiologically relevant peptide in the regulation of food intake. Taken together, obestatin does not appear to be a “statin” for obesity and is proposed to be renamed ghrelin-associated peptide (GAP) [51].
5. Nesfatin-1
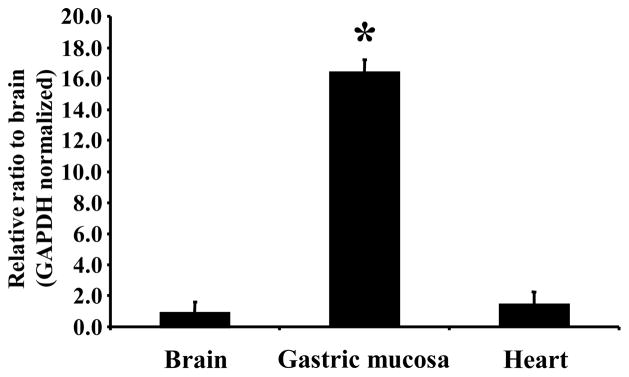
Nesfatin-1 was identified by Oh-I et al. in the rat hypothalamus and reported to decrease food intake upon 3rd ventricle injection and named nesfatin for NUCB2 encoded satiety and fat-influencing protein [107]. Posttranslational processing results in nesfatin-1, nesfatin-2 and nesfatin-3 (Table 4), however, only nesfatin-1 exhibits a food intake-reducing effect [107]. We could recently establish that the nesfatin-1 precursor NUCB2 is more prominently expressed at the mRNA and protein level in the rat gastric oxyntic mucosa as shown by microarray analysis substantiated by RT-qPCR and Western Blot (Fig. 2) than in other viscera such as the heart and even the brain (Fig. 5) [137]. Moreover, NUCB2 mRNA expression is significantly enriched in a population of gastric oxyntic small endocrine cells [137]. Co-localization of ghrelin and nesfatin-1 immunoreactivity in the X/A-like cells could be demonstrated by confocal microscopy where both peptides reside in different pools of vesicles (Fig. 6) [137]. This co-localization is further supported by the occurrence of PC1/3 in X/A-like cells [160] as PC1/3 is involved in the processing of NUCB2 to nesfatin-1 in the brain [131]. Moreover, nesfatin-1 immunoreactivity was also detected in the pancreas, testis and pituitary gland [137]. However, the function at these production sites remains unclear but may point towards an action in an autocrine or paracrine fashion.
Table 4.
Amino acid sequences of rat nesfatin-1, 2 and 3.
| Nesfatin-1 (aa 1–82) | VPIDV DKTKV HNVEP VESAR IEPPD TGLYY DEYLK QVIEV |
| LETDP HFREK LQKAD IEEIR SGRLS QELDL VSHKV RTRLD EL | |
| Nesfatin-2 (aa 85–163) | QEVGR LRMLI KAKLD ALQDT GMNHH LLLKQ FEHLN HQNPD |
| TFESK DLDML IKAAT ADLEQ YDRTR HEEFK KYEMM KEHE | |
| YLKTL SEEKR KEEEA KFAEM KRKHE DHPKV NHPGS KDQLK | |
| EVWEE TDGLD PNDFD PKTFF KLHDV NNDGF LDEQE LEALF | |
| Nesfatin-3 (aa 166–396) | TKELD KVYNP QNAED DMIEM EEERL RMREH VMNEI DNNKD |
| RLVTL EEFLR ATEKK EFLEP DSWET LDQQQ LFTEE ELKEY | |
| ESIIA IQESE LKKKA DELQK QKEEL QRQHD HLEAQ KQEYQ | |
| QAVQQ LEQKK FQQGI APSGP AGELK FEPHT |
Fig. 5.
NUCB2 mRNA is significantly higher expressed in the gastric oxyntic mucosa than in brain and heart. Data are expressed as mean ± SD, * p < 0.001 vs. brain and heart. (Reproduced with permission from reference [137]; Copyright 2009, The Endocrine Society).
Fig. 6.
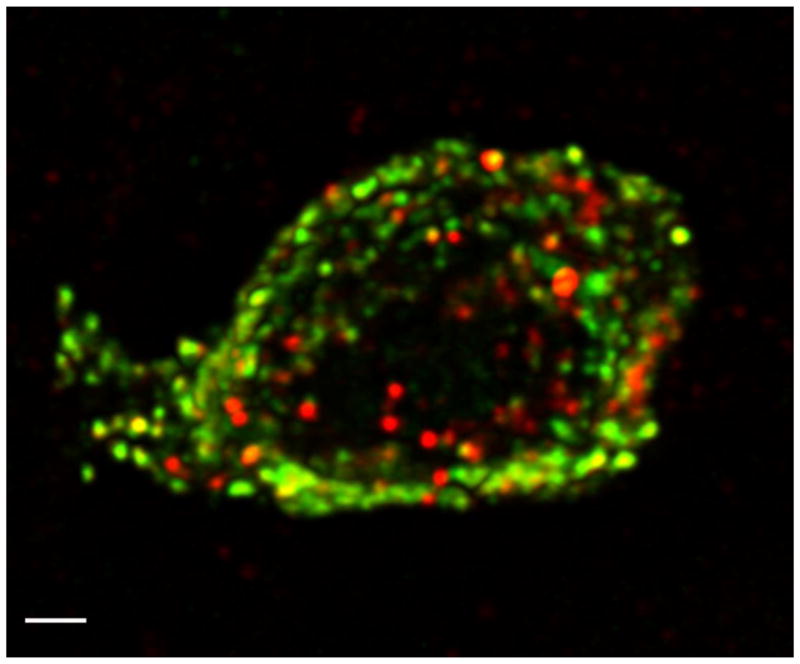
High resolution confocal image of a single gastric mucosal endocrine cell co-expressing ghrelin (green) and nesfatin-1 (red) immunoreactivity. Confocal microscopy was performed using a 100 × objective and digital zoom, 8 images in z-axis (stack size 24 × 24 × 7 μm) with dual laser excitation at 488 and 543 nm. Images were collected in two optical channels and photomultiplier tubes using FITC and TRITC filter sets, de-convoluted and reconstructed into a 3-dimensional image. The scale bar represents 1 μm. (Reproduced with permission from reference [137]; Copyright 2009, The Endocrine Society).
5.1 Release of nesfatin-1
We recently reported that fasting for 24 h decreases NUCB2 mRNA expression in a pool of enriched small gastric endocrine cells [137] and significantly reduces nesfatin-1 plasma levels in rats [138]. These data indicate that NUCB2/nesfatin-1 expression and release are regulated by nutritional status. Nesfatin-1 has also been detected in human serum by Western blot analysis and possible changes in circulating levels in various nutritional conditions and relationship to body mass index are currently under investigation [131]. Further studies are warranted to elucidate the differential regulation of nesfatin-1 and ghrelin within the same X/A-like cells along with the receptor for nesfatin-1 to characterize its action sites.
5.2 Inhibitory effect of nesfatin-1 on food intake
Recently, Shimizu and colleagues showed that the intraperitoneal injection of nesfatin-1 reduces the dark phase food intake in mice without causing taste aversion [130]. Furthermore, repeated intraperitoneal injections over a period of 6 days inhibit body weight gain [131]. The mid segment (aa 24–53) of the 82 aa nesfatin-1 is also biologically active to reduce food intake after peripheral injection, whereas the N-23 or C-29 amino acid terminal fragments are inactive [130]. The anorexigenic action of nesfatin-1 was observed in lean, ob/ob and high fat diet-induced obese mice providing evidence for a leptin-independent mechanism of action [130]. Furthermore, preliminary data suggest that nesfatin-1 also induces a long lasting inhibition of food intake in mice when injected subcutaneously (over 14 h) as well as after intranasal administration in rats (6 h) [131]. The observation that a nesfatin-1 mid fragment is not able to suppress food intake in capsaicin pretreated mice [132] points towards a vagal transmission of peripheral nesfatin-1 signals to the brain.
Injection of nesfatin-1 in pmol amounts into the lateral, or 4th ventricle and cisterna magna also decreases dark phase food intake for 6 h whereas the 24 h cumulative food intake is not altered [138]. However, the body weight of rats was significantly reduced 24 h following an acute intracerebroventricular injection of nesfatin-1 [138] suggesting increased energy expenditure. Further studies investigating respiratory quotient and uncoupling protein levels in brown adipose tissue are needed to corroborate this assumption.
5.3 Possible use of nesfatin-1 in the drug treatment of obesity
Peripheral nesfatin-1 exerts an anorexigenic action that is still retained in diet-induced obese and ob/ob mice [130] pointing towards a leptin-independent action. Since obese subjects commonly display leptin resistance or at least decreased leptin sensitivity this finding could make nesfatin-1 a promising target in the drug treatment of obesity as recently suggested [131].
6. Conclusions
Until now, the gastric endocrine X/A-like cells were thought to be restricted to the stimulation of food intake and adiposity linked with the synthesis and release of the orexigenic peptide, ghrelin. However, recent developments including the characterization of the possible modulatory effect of des-acyl ghrelin as well as the identification of nesfatin-1 in X/A-like cells highlight the importance of this cell type as regulator of food intake able to stimulate and also inhibit food consumption and influence body weight. Ghrelin’s orexigenic action can be modulated by various other food intake regulatory gut peptides (e.g. CCK, bombesin, GLP-1 and PYY), proteins (e.g. leptin) and lipids (e.g. endocannabinoids) originating from the stomach and the intestine. The receptors involved in des-acyl ghrelin’s and nesfatin-1’s biological actions are still unknown and their identification along with the classification of possible additional ghrelin receptor subtypes will markedly enhance our understanding of these peptides’ and the X/A-like cells’ physiology.
The incidence and prevalence of overweight, obesity and associated diseases are rising in the developed countries throughout the world which represents an important socioeconomic burden. Drug treatment options are very limited and peptide products of the X/Alike cell have been tackled as potential promising candidates. Ghrelin signaling pathways especially have been in the spotlight not only as a mean to curtail food consumption and body weight gain by blocking GHS-R1a mediated actions but also to stimulate these pathways to promote food consumption in pathological states linked with reduced appetite. While preclinical as well as clinical data look promising for the stimulation of food intake and consecutive improvement of the nutritional status using ghrelin analogs, the preclinical data showing a decrease in food intake and body weight by applying various strategies to block ghrelin signaling have not been successfully translated into the clinical setting. This suggests the existence of other factors compensating ghrelin’s effect as well as additional ghrelin receptor subtypes yet to be identified which may be subject to species differences. A deeper understanding how different peptides are individually regulated and secreted from the same cell will require detailed characterization at the cellular and subcellular level in order to unravel the regulation and physiological function of the gastric X/A-like endocrine cell.
Acknowledgments
Y.T. is in receipt of VA Research Career Scientist Award and NIH R01grants DK-33061, DK-57238 and VA Merit Award. A.S. and M. G. are supported by the German Research Foundation Grants STE 1765/1-1 and GO 1718/1-1 respectively. We thank Ms. Eugenia Hu for careful reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abizaid A, Horvath TL. Brain circuits regulating energy homeostasis. Regul Pept. 2008;149:3–10. doi: 10.1016/j.regpep.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Garcia O, Garcia-Lopez E, Rodriguez J, Gil-Pena H, Molinos I, Carbajo-Perez E, et al. Administration of ghrelin to young uraemic rats increases food intake transiently, stimulates growth hormone secretion and does not improve longitudinal growth. Nephrol Dial Transplant. 2007;22:1309–13. doi: 10.1093/ndt/gfl645. [DOI] [PubMed] [Google Scholar]
- 4.Annemie VD, Debby VD, Valentijn V, Bart de S, Walter L, Liliane S, et al. Central administration of obestatin fails to show inhibitory effects on food and water intake in mice. Regul Pept. 2009;156:77–82. doi: 10.1016/j.regpep.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Ao Y, Go VL, Toy N, Li T, Wang Y, Song MK, et al. Brainstem thyrotropin-releasing hormone regulates food intake through vagal-dependent cholinergic stimulation of ghrelin secretion. Endocrinology. 2006;147:6004–10. doi: 10.1210/en.2006-0820. [DOI] [PubMed] [Google Scholar]
- 6.Ariyasu H, Takaya K, Iwakura H, Hosoda H, Akamizu T, Arai Y, et al. Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology. 2005;146:355–64. doi: 10.1210/en.2004-0629. [DOI] [PubMed] [Google Scholar]
- 7.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–8. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 8.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–60. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947–52. doi: 10.1136/gut.52.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–45. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 12.Ashby DR, Ford HE, Wynne KJ, Wren AM, Murphy KG, Busbridge M, et al. Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney Int. 2009;76:199–206. doi: 10.1038/ki.2009.114. [DOI] [PubMed] [Google Scholar]
- 13.Ashitani J, Matsumoto N, Nakazato M. Effect of octanoic acid-rich formula on plasma ghrelin levels in cachectic patients with chronic respiratory disease. Nutr J. 2009;8:25. doi: 10.1186/1475-2891-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature. 1998;394:790–3. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 15.Banfi G, Salvagno GL, Lippi G. The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin Chem Lab Med. 2007;45:565–76. doi: 10.1515/CCLM.2007.110. [DOI] [PubMed] [Google Scholar]
- 16.Bang AS, Soule SG, Yandle TG, Richards AM, Pemberton CJ. Characterisation of proghrelin peptides in mammalian tissue and plasma. J Endocrinol. 2007;192:313–23. doi: 10.1677/JOE-06-0021. [DOI] [PubMed] [Google Scholar]
- 17.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–7. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 18.Barreiro ML, Gaytan F, Caminos JE, Pinilla L, Casanueva FF, Aguilar E, et al. Cellular location and hormonal regulation of ghrelin expression in rat testis. Biol Reprod. 2002;67:1768–76. doi: 10.1095/biolreprod.102.006965. [DOI] [PubMed] [Google Scholar]
- 19.Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem. 2000;43:4370–6. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- 20.Biotechnology C. Cytos Biotechnology: Press Release November 7. 2006. Phase I/IIa clinical trial with obese individuals shows no effect of CYT009-GhrQb on weight loss. [Google Scholar]
- 21.Bordi C, D’Adda T, Azzoni C, Ferraro G. Classification of gastric endocrine cells at the light and electron microscopical levels. Microsc Res Tech. 2000;48:258–71. doi: 10.1002/(SICI)1097-0029(20000301)48:5<258::AID-JEMT3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 22.Brennan IM, Otto B, Feltrin KL, Meyer JH, Horowitz M, Feinle-Bisset C. Intravenous CCK-8, but not GLP-1, suppresses ghrelin and stimulates PYY release in healthy men. Peptides. 2007;28:607–11. doi: 10.1016/j.peptides.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Bresciani E, Rapetti D, Dona F, Bulgarelli I, Tamiazzo L, Locatelli V, et al. Obestatin inhibits feeding but does not modulate GH and corticosterone secretion in the rat. J Endocrinol Invest. 2006;29:RC16–8. doi: 10.1007/BF03344175. [DOI] [PubMed] [Google Scholar]
- 24.Brunetti L, Leone S, Orlando G, Recinella L, Ferrante C, Chiavaroli A, et al. Effects of obestatin on feeding and body weight after standard or cafeteria diet in the rat. Peptides. 2009;30:1323–7. doi: 10.1016/j.peptides.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, Montoya ML, Neyrinck AM, Delzenne NM, Lambert DM. Potential modulation of plasma ghrelin and glucagon-like peptide-1 by anorexigenic cannabinoid compounds, SR141716A (rimonabant) and oleoylethanolamide. Br J Nutr. 2004;92:757–61. doi: 10.1079/bjn20041256. [DOI] [PubMed] [Google Scholar]
- 26.Carlini VP, Schioth HB, Debarioglio SR. Obestatin improves memory performance and causes anxiolytic effects in rats. Biochem Biophys Res Commun. 2007;352:907–12. doi: 10.1016/j.bbrc.2006.11.112. [DOI] [PubMed] [Google Scholar]
- 27.Cassoni P, Ghe C, Marrocco T, Tarabra E, Allia E, Catapano F, et al. Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur J Endocrinol. 2004;150:173–84. doi: 10.1530/eje.0.1500173. [DOI] [PubMed] [Google Scholar]
- 28.Cassoni P, Papotti M, Ghe C, Catapano F, Sapino A, Graziani A, et al. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab. 2001;86:1738–45. doi: 10.1210/jcem.86.4.7402. [DOI] [PubMed] [Google Scholar]
- 29.Chartrel N, Alvear-Perez R, Leprince J, Iturrioz X, Reaux-Le Goazigo A, Audinot V, et al. Comment on “Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake”. Science. 2007;315:766. doi: 10.1126/science.1135047. author reply. [DOI] [PubMed] [Google Scholar]
- 30.Chelikani PK, Haver AC, Reidelberger RD. Ghrelin attenuates the inhibitory effects of glucagon-like peptide-1 and peptide YY(3–36) on food intake and gastric emptying in rats. Diabetes. 2006;55:3038–46. doi: 10.2337/db06-0730. [DOI] [PubMed] [Google Scholar]
- 31.Chen CY, Inui A, Asakawa A, Fujino K, Kato I, Chen CC, et al. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129:8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–12. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 33.Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 34.Cui G, Waldum HL. Physiological and clinical significance of enterochromaffin-like cell activation in the regulation of gastric acid secretion. World J Gastroenterol. 2007;13:493–6. doi: 10.3748/wjg.v13.i4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8:643–4. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 36.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 37.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 38.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–61. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 39.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 40.Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124–9. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 41.Davies JS, Kotokorpi P, Eccles SR, Barnes SK, Tokarczuk PF, Allen SK, et al. Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol Endocrinol. 2009;23:914–24. doi: 10.1210/me.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de la Cour CD, Norlen P, Hakanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regul Pept. 2007;143:118–26. doi: 10.1016/j.regpep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Depoortere I, Thijs T, Moechars D, De Smet B, Ver Donck L, Peeters TL. Effect of peripheral obestatin on food intake and gastric emptying in ghrelin-knockout mice. Br J Pharmacol. 2008;153:1550–7. doi: 10.1038/sj.bjp.0707683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes (Lond) 2005;29:1130–6. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 45.Frezza EE, Chiriva-Internati M, Wachtel MS. Analysis of the results of sleeve gastrectomy for morbid obesity and the role of ghrelin. Surg Today. 2008;38:481–3. doi: 10.1007/s00595-007-3648-8. [DOI] [PubMed] [Google Scholar]
- 46.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 47.Gauna C, Delhanty PJ, van Aken MO, Janssen JA, Themmen AP, Hofland LJ, et al. Unacylated ghrelin is active on the INS-1E rat insulinoma cell line independently of the growth hormone secretagogue receptor type 1a and the corticotropin releasing factor 2 receptor. Mol Cell Endocrinol. 2006;251:103–11. doi: 10.1016/j.mce.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 48.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 49.Goebel M, Stengel A, Taché Y. Continued controversy on obestatin as a gut hormone influencing food intake and gastrointestinal motility. Obes Metab. 2008;4:143–8. [Google Scholar]
- 50.Gourcerol G, Million M, Adelson DW, Wang Y, Wang L, Rivier J, et al. Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides. 2006;27:2811–9. doi: 10.1016/j.peptides.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Gourcerol G, St-Pierre DH, Taché Y. Lack of obestatin effects on food intake: should obestatin be renamed ghrelin-associated peptide (GAP)? Regul Pept. 2007;141:1–7. doi: 10.1016/j.regpep.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 52.Gronberg M, Tsolakis AV, Magnusson L, Janson ET, Saras J. Distribution of Obestatin and Ghrelin in Human Tissues: Immunoreactive Cells in the Gastrointestinal Tract, Pancreas, and Mammary Glands. J Histochem Cytochem. 2008;56:793–801. doi: 10.1369/jhc.2008.951145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guan JL, Wang QP, Kageyama H, Takenoya F, Kita T, Matsuoka T, et al. Synaptic interactions between ghrelin- and neuropeptide Y-containing neurons in the rat arcuate nucleus. Peptides. 2003;24:1921–8. doi: 10.1016/j.peptides.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 54.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–9. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 55.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105:6320–5. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hagemann D, Holst JJ, Gethmann A, Banasch M, Schmidt WE, Meier JJ. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regul Pept. 2007;143:64–8. doi: 10.1016/j.regpep.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Hameed S, Dhillo WS, Bloom SR. Gut hormones and appetite control. Oral Dis. 2009;15:18–26. doi: 10.1111/j.1601-0825.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- 58.Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. 2001;86:4284–91. doi: 10.1210/jcem.86.9.7866. [DOI] [PubMed] [Google Scholar]
- 59.Hiejima H, Nishi Y, Hosoda H, Yoh J, Mifune H, Satou M, et al. Regional distribution and the dynamics of n-decanoyl ghrelin, another acyl-form of ghrelin, upon fasting in rodents. Regul Pept. 2009;156:47–56. doi: 10.1016/j.regpep.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2007;148:13–20. doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- 61.Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem. 2004;279:53806–17. doi: 10.1074/jbc.M407676200. [DOI] [PubMed] [Google Scholar]
- 62.Holst B, Schwartz TW. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol Sci. 2004;25:113–7. doi: 10.1016/j.tips.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–13. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 64.Hosoda H, Kojima M, Mizushima T, Shimizu S, Kangawa K. Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing. J Biol Chem. 2003;278:64–70. doi: 10.1074/jbc.M205366200. [DOI] [PubMed] [Google Scholar]
- 65.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–7. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 66.Inhoff T, Mönnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29:2159–68. doi: 10.1016/j.peptides.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inhoff T, Wiedenmann B, Klapp BF, Mönnikes H, Kobelt P. Is desacyl ghrelin a modulator of food intake? Peptides. 2009;30:991–4. doi: 10.1016/j.peptides.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 68.Iwakura H, Ariyasu H, Li Y, Kanamoto N, Bando M, Yamada G, et al. A mouse model of ghrelinoma exhibited activated growth hormone-insulin-like growth factor I axis and glucose intolerance. Am J Physiol Endocrinol Metab. 2009;297:E802–11. doi: 10.1152/ajpendo.00205.2009. [DOI] [PubMed] [Google Scholar]
- 69.Jeon TY, Lee S, Kim HH, Kim YJ, Son HC, Kim DH, et al. Changes in plasma ghrelin concentration immediately after gastrectomy in patients with early gastric cancer. J Clin Endocrinol Metab. 2004;89:5392–6. doi: 10.1210/jc.2004-0872. [DOI] [PubMed] [Google Scholar]
- 70.Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–63. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- 71.Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 72.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 73.Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106:11318–23. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol. 2006;20:1772–85. doi: 10.1210/me.2005-0084. [DOI] [PubMed] [Google Scholar]
- 75.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–43. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 76.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–7. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 77.Karra E, Batterham RL. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol Cell Endocrinol. 2009 doi: 10.1016/j.mce.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 78.Kellokoski E, Kummu O, Serpi R, Lehenkari P, Ukkola O, Kesaniemi YA, et al. Ghrelin vaccination decreases plasma MCP-1 level in LDLR(−/−)-mice. Peptides. 2009 doi: 10.1016/j.peptides.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 79.Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–5. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kobelt P, Goebel M, Stengel A, Schmidtmann M, van der Voort IR, Tebbe JJ, et al. Bombesin, but not amylin, blocks the orexigenic effect of peripheral ghrelin. Am J Physiol Regul Integr Comp Physiol. 2006;291:R903–13. doi: 10.1152/ajpregu.00681.2005. [DOI] [PubMed] [Google Scholar]
- 81.Kobelt P, Helmling S, Stengel A, Wlotzka B, Andresen V, Klapp BF, et al. Anti-ghrelin Spiegelmer NOX-B11 inhibits neurostimulatory and orexigenic effects of peripheral ghrelin in rats. Gut. 2006;55:788–92. doi: 10.1136/gut.2004.061010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobelt P, Tebbe JJ, Tjandra I, Stengel A, Bae HG, Andresen V, et al. CCK inhibits the orexigenic effect of peripheral ghrelin. Am J Physiol Regul Integr Comp Physiol. 2005;288:R751–8. doi: 10.1152/ajpregu.00094.2004. [DOI] [PubMed] [Google Scholar]
- 83.Kobelt P, Wisser AS, Stengel A, Goebel M, Bannert N, Gourcerol G, et al. Peripheral obestatin has no effect on feeding behavior and brain Fos expression in rodents. Peptides. 2008;29:1018–27. doi: 10.1016/j.peptides.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 85.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 86.Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kotidis EV, Koliakos GG, Baltzopoulos VG, Ioannidis KN, Yovos JG, Papavramidis ST. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment--a prospective study. Obes Surg. 2006;16:1425–32. doi: 10.1381/096089206778870058. [DOI] [PubMed] [Google Scholar]
- 88.Lagaud GJ, Young A, Acena A, Morton MF, Barrett TD, Shankley NP. Obestatin reduces food intake and suppresses body weight gain in rodents. Biochem Biophys Res Commun. 2007;357:264–9. doi: 10.1016/j.bbrc.2007.03.138. [DOI] [PubMed] [Google Scholar]
- 89.Lagaud GJ, Young A, Acena A, Morton MF, Barrett TD, Shankley NP. Retracted notice to: Obestatin reduces food intake and suppresses body 4 weight gain in rodents” [Biochem. Biophys. Res. Commun 357(1) (2007) 5 264–269] Biochem Biophys Res Commun. 2009;388:619. doi: 10.1016/j.bbrc.2007.03.138. [DOI] [PubMed] [Google Scholar]
- 90.Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–9. doi: 10.1381/0960892054621125. [DOI] [PubMed] [Google Scholar]
- 91.Lauwers E, Landuyt B, Arckens L, Schoofs L, Luyten W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem Biophys Res Commun. 2006;351:21–5. doi: 10.1016/j.bbrc.2006.09.141. [DOI] [PubMed] [Google Scholar]
- 92.le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab. 2005;90:4521–4. doi: 10.1210/jc.2004-2537. [DOI] [PubMed] [Google Scholar]
- 93.Li F, Zhang G, Liang J, Ding X, Cheng Z, Hu S. Sleeve Gastrectomy Provides a Better Control of Diabetes by Decreasing Ghrelin in the Diabetic Goto-Kakizaki Rats. J Gastrointest Surg. 2009 doi: 10.1007/s11605-009-0997-1. [DOI] [PubMed] [Google Scholar]
- 94.Lippl F, Kircher F, Erdmann J, Allescher HD, Schusdziarra V. Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regul Pept. 2004;119:93–8. doi: 10.1016/j.regpep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 95.Lu S, Guan JL, Wang QP, Uehara K, Yamada S, Goto N, et al. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neurosci Lett. 2002;321:157–60. doi: 10.1016/s0304-3940(01)02544-7. [DOI] [PubMed] [Google Scholar]
- 96.Lu SC, Xu J, Chinookoswong N, Liu S, Steavenson S, Gegg C, et al. An acyl-ghrelin-specific neutralizing antibody inhibits the acute ghrelin-mediated orexigenic effects in mice. Mol Pharmacol. 2009;75:901–7. doi: 10.1124/mol.108.052852. [DOI] [PubMed] [Google Scholar]
- 97.Madison LD, Scarlett JM, Levasseur P, Zhu X, Newcomb K, Batra A, et al. Prostacyclin signaling regulates circulating ghrelin during acute inflammation. J Endocrinol. 2008;196:263–73. doi: 10.1677/JOE-07-0478. [DOI] [PubMed] [Google Scholar]
- 98.Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, et al. Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00147.2009. [DOI] [PubMed] [Google Scholar]
- 99.Moechars D, Depoortere I, Moreaux B, de Smet B, Goris I, Hoskens L, et al. Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology. 2006;131:1131–41. doi: 10.1053/j.gastro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 100.Mondal M, Toshinai K, Ueno H, Koshinaka K, Nakazato M. Characterization of obestatin in rat and human stomach and plasma, and its lack of acute effect on feeding behavior in rodents. J Endocrinol. 2008;198:339–46. doi: 10.1677/JOE-08-0082. [DOI] [PubMed] [Google Scholar]
- 101.Moran TH, Dailey MJ. Minireview: Gut peptides: targets for antiobesity drug development? Endocrinology. 2009;150:2526–30. doi: 10.1210/en.2009-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagaraj S, Peddha MS, Manjappara UV. Fragments of obestatin as modulators of feed intake, circulating lipids, and stored fat. Biochem Biophys Res Commun. 2008;366:731–7. doi: 10.1016/j.bbrc.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 103.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 104.Neary NM, Druce MR, Small CJ, Bloom SR. Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect. Gut. 2006;55:135. [PMC free article] [PubMed] [Google Scholar]
- 105.Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, et al. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146:2255–64. doi: 10.1210/en.2004-0695. [DOI] [PubMed] [Google Scholar]
- 106.Nogueiras R, Pfluger P, Tovar S, Arnold M, Mitchell S, Morris A, et al. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology. 2007;148:21–6. doi: 10.1210/en.2006-0915. [DOI] [PubMed] [Google Scholar]
- 107.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–12. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 108.Ohgusu H, Shirouzu K, Nakamura Y, Nakashima Y, Ida T, Sato T, et al. Ghrelin O-acyltransferase (GOAT) has a preference for n-hexanoyl-CoA over n-octanoyl-CoA as an acyl donor. Biochem Biophys Res Commun. 2009;386:153–8. doi: 10.1016/j.bbrc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 109.Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27:911–6. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 110.Perez-Tilve D, Gonzalez-Matias L, Alvarez-Crespo M, Leiras R, Tovar S, Dieguez C, et al. Exendin-4 potently decreases ghrelin levels in fasting rats. Diabetes. 2007;56:143–51. doi: 10.2337/db05-0996. [DOI] [PubMed] [Google Scholar]
- 111.Perreault M, Istrate N, Wang L, Nichols AJ, Tozzo E, Stricker-Krongrad A. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int J Obes Relat Metab Disord. 2004;28:879–85. doi: 10.1038/sj.ijo.0802640. [DOI] [PubMed] [Google Scholar]
- 112.Pfluger PT, Kirchner H, Gunnel S, Schrott B, Perez-Tilve D, Fu S, et al. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol Gastrointest Liver Physiol. 2008;294:G610–8. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- 113.Prinz C, Kajimura M, Scott DR, Mercier F, Helander HF, Sachs G. Histamine secretion from rat enterochromaffinlike cells. Gastroenterology. 1993;105:449–61. doi: 10.1016/0016-5085(93)90719-s. [DOI] [PubMed] [Google Scholar]
- 114.Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. [DOI] [PubMed] [Google Scholar]
- 115.Rindi G, Necchi V, Savio A, Torsello A, Zoli M, Locatelli V, et al. Characterisation of gastric ghrelin cells in man and other mammals: studies in adult and fetal tissues. Histochem Cell Biol. 2002;117:511–9. doi: 10.1007/s00418-002-0415-1. [DOI] [PubMed] [Google Scholar]
- 116.Rodriguez A, Gomez-Ambrosi J, Catalan V, Gil MJ, Becerril S, Sainz N, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 2009;33:541–52. doi: 10.1038/ijo.2009.40. [DOI] [PubMed] [Google Scholar]
- 117.Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, et al. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab. 2002;87:3997–4000. doi: 10.1210/jcem.87.8.8879. [DOI] [PubMed] [Google Scholar]
- 118.Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, Perello M, et al. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155:91–8. doi: 10.1016/j.regpep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sakata I, Yamazaki M, Inoue K, Hayashi Y, Kangawa K, Sakai T. Growth hormone secretagogue receptor expression in the cells of the stomach-projected afferent nerve in the rat nodose ganglion. Neurosci Lett. 2003;342:183–6. doi: 10.1016/s0304-3940(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 120.Sakata I, Yang J, Lee CE, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, et al. Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. Am J Physiol Endocrinol Metab. 2009;297:E134–41. doi: 10.1152/ajpendo.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salome N, Haage D, Perrissoud D, Moulin A, Demange L, Egecioglu E, et al. Anorexigenic and electrophysiological actions of novel ghrelin receptor (GHS-R1A) antagonists in rats. Eur J Pharmacol. 2009;612:167–73. doi: 10.1016/j.ejphar.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 122.Samson WK, White MM, Price C, Ferguson AV. Obestatin acts in brain to inhibit thirst. Am J Physiol Regul Integr Comp Physiol. 2007;292:R637–43. doi: 10.1152/ajpregu.00395.2006. [DOI] [PubMed] [Google Scholar]
- 123.Sangiao-Alvarellos S, Vazquez MJ, Varela L, Nogueiras R, Saha AK, Cordido F, et al. Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology. 2009;150:4562–74. doi: 10.1210/en.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 125.Schellekens H, Dinan TG, Cryan JF. Lean Mean Fat Reducing “Ghrelin” Machine: Hypothalamic Ghrelin and Ghrelin Receptors as Therapeutic Targets in Obesity. Neuropharmacology. 2010;58:2–16. doi: 10.1016/j.neuropharm.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 126.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 127.Seoane LM, Al-Massadi O, Pazos Y, Pagotto U, Casanueva FF. Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats. J Endocrinol Invest. 2006;29:RC13–5. doi: 10.1007/BF03344174. [DOI] [PubMed] [Google Scholar]
- 128.Shearman LP, Wang SP, Helmling S, Stribling DS, Mazur P, Ge L, et al. Ghrelin neutralization by a ribonucleic acid-SPM ameliorates obesity in diet-induced obese mice. Endocrinology. 2006;147:1517–26. doi: 10.1210/en.2005-0993. [DOI] [PubMed] [Google Scholar]
- 129.Shimada M, Date Y, Mondal MS, Toshinai K, Shimbara T, Fukunaga K, et al. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochem Biophys Res Commun. 2003;302:520–5. doi: 10.1016/s0006-291x(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 130.Shimizu H, Oh-I S, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, et al. Peripheral Administration of Nesfatin-1 Reduces Food Intake in Mice: The leptin-independent mechanism. Endocrinology. 2009;150:662–71. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 131.Shimizu H, Oh IS, Okada S, Mori M. Nesfatin-1: An Overview and Future Clinical Application. Endocr J. 2009;56:537–43. doi: 10.1507/endocrj.k09e-117. [DOI] [PubMed] [Google Scholar]
- 132.Shimizu H, Ohsaki A, Oh IS, Okada S, Mori M. A new anorexigenic protein, nesfatin-1. Peptides. 2009;30:995–8. doi: 10.1016/j.peptides.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 133.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–32. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 134.Simonian HP, Kresge KM, Boden GH, Parkman HP. Differential effects of sham feeding and meal ingestion on ghrelin and pancreatic polypeptide levels: evidence for vagal efferent stimulation mediating ghrelin release. Neurogastroenterol Motil. 2005;17:348–54. doi: 10.1111/j.1365-2982.2004.00634.x. [DOI] [PubMed] [Google Scholar]
- 135.Smith RG. From GH to Billy Ghrelin. Cell Metab. 2009;10:82–3. doi: 10.1016/j.cmet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 136.Soares JB, Leite-Moreira AF. Ghrelin, des-acyl ghrelin and obestatin: Three pieces of the same puzzle. Peptides. 2008;29:1255–70. doi: 10.1016/j.peptides.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 137.Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, et al. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232–8. doi: 10.1210/en.2008-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, et al. Central Nesfatin-1 Reduces Dark Phase Food Intake and Gastric Emptying in Rats: Differential Role of Corticotropin-Releasing Factor2 Receptor. Endocrinology. 2009;150:4911–9. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Taché Y, et al. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–8. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149:843–50. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 2004;101:4679–84. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Takahashi T, Ida T, Sato T, Nakashima Y, Nakamura Y, Tsuji A, et al. Production of n-octanoyl-modified ghrelin in cultured cells requires prohormone processing protease and ghrelin O-acyltransferase, as well as n-octanoic acid. J Biochem. 2009;146:675–82. doi: 10.1093/jb/mvp112. [DOI] [PubMed] [Google Scholar]
- 143.Tanner BD, Allen JW. Complications of bariatric surgery: implications for the covering physician. Am Surg. 2009;75:103–12. doi: 10.1177/000313480907500201. [DOI] [PubMed] [Google Scholar]
- 144.Thanthan S, Mekaru C, Seki N, Hidaka K, Ueno A, Thidarmyint H, et al. Endogenous ghrelin released in response to endothelin stimulates growth hormone secretion in cattle. Domest Anim Endocrinol. 2009 doi: 10.1016/j.domaniend.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 145.Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116:1983–93. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, et al. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147:2306–14. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- 147.Tremblay F, Perreault M, Klaman LD, Tobin JF, Smith E, Gimeno RE. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology. 2007;148:501–6. doi: 10.1210/en.2006-1275. [DOI] [PubMed] [Google Scholar]
- 148.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 149.Tsolakis AV, Grimelius L, Stridsberg M, Falkmer SE, Waldum HL, Saras J, et al. Obestatin/ghrelin cells in normal mucosa and endocrine tumours of the stomach. Eur J Endocrinol. 2009;160:941–9. doi: 10.1530/EJE-09-0001. [DOI] [PubMed] [Google Scholar]
- 150.Unniappan S, Speck M, Kieffer TJ. Metabolic effects of chronic obestatin infusion in rats. Peptides. 2008;29:1354–61. doi: 10.1016/j.peptides.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 151.Vizcarra JA, Kirby JD, Kim SK, Galyean ML. Active immunization against ghrelin decreases weight gain and alters plasma concentrations of growth hormone in growing pigs. Domest Anim Endocrinol. 2007;33:176–89. doi: 10.1016/j.domaniend.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 152.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St-Pierre DH, et al. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol. 2006;291:G611–20. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 153.Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- 154.Wang Y, Liu J. Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. Obes Surg. 2009;19:357–62. doi: 10.1007/s11695-008-9688-3. [DOI] [PubMed] [Google Scholar]
- 155.Wells T. Ghrelin - Defender of fat. Prog Lipid Res. 2009;48:257–74. doi: 10.1016/j.plipres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 156.Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci U S A. 2004;101:8227–32. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–8. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 158.Wynne K, Giannitsopoulou K, Small CJ, Patterson M, Frost G, Ghatei MA, et al. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J Am Soc Nephrol. 2005;16:2111–8. doi: 10.1681/ASN.2005010039. [DOI] [PubMed] [Google Scholar]
- 159.Yamamoto D, Ikeshita N, Daito R, Herningtyas EH, Toda K, Takahashi K, et al. Neither intravenous nor intracerebroventricular administration of obestatin affects the secretion of GH, PRL, TSH and ACTH in rats. Regul Pept. 2007;138:141–4. doi: 10.1016/j.regpep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 160.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–96. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 161.Yang J, Zhao TJ, Goldstein JL, Brown MS. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci U S A. 2008;105:10750–5. doi: 10.1073/pnas.0805353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zbucki RL, Sawicki B, Hryniewicz A, Winnicka MM. Cannabinoids enhance gastric X/A-like cells activity. Folia Histochem Cytobiol. 2008;46:219–24. doi: 10.2478/v10042-008-0033-4. [DOI] [PubMed] [Google Scholar]
- 163.Zhang JV, Klein C, Ren P-G, Kass S, Donck LV, Moechars D, Hsueh AJW. Response to Comment on “Obestatin, a Peptide Encoded by the Ghrelin Gene, Opposes Ghrelin’s Effects on Food Intake”. Science. 2007;315:766d. doi: 10.1126/science.1135047. [DOI] [PubMed] [Google Scholar]
- 164.Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996–9. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 165.Zhang W, Chai B, Li JY, Wang H, Mulholland MW. Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology. 2008;149:4710–6. doi: 10.1210/en.2008-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao CM, Furnes MW, Stenstrom B, Kulseng B, Chen D. Characterization of obestatin- and ghrelin-producing cells in the gastrointestinal tract and pancreas of rats: an immunohistochemical and electron-microscopic study. Cell Tissue Res. 2008;331:575–87. doi: 10.1007/s00441-007-0514-3. [DOI] [PubMed] [Google Scholar]
- 167.Zhu X, Cao Y, Voogd K, Steiner DF. On the processing of proghrelin to ghrelin. J Biol Chem. 2006;281:38867–70. doi: 10.1074/jbc.M607955200. [DOI] [PubMed] [Google Scholar]
- 168.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–72. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zizzari P, Longchamps R, Epelbaum J, Bluet-Pajot MT. Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology. 2007;148:1648–53. doi: 10.1210/en.2006-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zorrilla EP, Iwasaki S, Moss JA, Chang J, Otsuji J, Inoue K, et al. Vaccination against weight gain. Proc Natl Acad Sci U S A. 2006;103:13226–31. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]