Abstract
Rationale
Intermittent exposure to social defeat stress can induce long-term neural plasticity that may influence escalated cocaine-taking behavior. Stressful encounters can lead to activation of dopamine neurons in the ventral tegmental area (VTA), which are modulated by corticotropin releasing factor (CRF) neurons.
Objective
The study aims to prevent the effects of intermittently scheduled, brief social defeat stress on subsequent intravenous (IV) cocaine self-administration by pretreatment with a CRF receptor subtype 1 (CRF-R1) antagonist.
Materials and methods
Long–Evans rats were submitted to four intermittent social defeat experiences separated by 72 h over 10 days. Two experiments examined systemic or intra-VTA antagonism of CRF-R1 subtype during stress on the later expression of locomotor sensitization and cocaine self-administration during fixed (0.75 mg/kg/infusion) and progressive ratio schedules of reinforcement (0.3 mg/kg/infusion), including a continuous 24-h “binge” (0.3 mg/kg/infusion).
Results
Pretreatment with a CRF-R1 antagonist, CP 154,526, (20 mg/kg i.p.) prior to each social defeat episode prevented the development of stress-induced locomotor sensitization to a cocaine challenge and prevented escalated cocaine self-administration during a 24-h “binge” In addition, pretreatment with a CRF-R1 antagonist (0.3 μg/0.5 μl/side) into the VTA prior to each social defeat episode prevented stress-induced locomotor sensitization to a cocaine challenge and prevented escalated cocaine self-administration during a 24-h “binge”.
Conclusions
The current results suggest that CRF-R1 subtype in the VTA is critically involved in the development of stress-induced locomotor sensitization which may contribute to escalated cocaine self-administration during continuous access in a 24-h “binge”.
Keywords: Stress; Locomotor sensitization; Escalated cocaine self-administration; CRF-R1; CP 154, 526; VTA
Introduction
The 41-amino acid peptide corticotropin releasing factor (CRF) has been implicated as a key component in the mechanisms by which stress promotes cocaine self-administration (Shaham et al. 1998). In particular, the action on CRF receptor subtype 1 (CRF-R1) rather than CRF-R2 may be critical for escalated cocaine, alcohol, and nicotine self-administration in dependent individuals (Goeders and Guerin 2000). In addition, many types of stressors potently activate the CRF system both in the CNS and in the hypothalamic–pituitary–adrenal (HPA) axis (Bhatnagar and Vining 2003; Dunn and Berridge 1990; Iwasaki-Sekino et al. 2009).
The current study focuses on the CRF-R1 subtype in the ventral tegmental area (VTA). Immunohistochemical investigations have demonstrated that CRF-R1 are localized throughout the VTA on a proportion of neurons that synthesize dopamine (DA) and project to forebrain regions such as the nucleus accumbens (Acb) and medial prefrontal cortex (mPFC; Sauvage and Steckler 2001). The VTA receives many synaptic inputs from limbic regions and from the paraventricular nucleus of the hypothalamus (Rodaros et al. 2007). CRF is co-localized in a number of excitatory or inhibitory afferents in the VTA that synapse with dopaminergic and nondopaminergic neurons. Synaptic connections between CRF terminals and dopamine neurons are (83%) predominately asymmetric, glutamatergic, and presumably excitatory (Tagliaferro and Morales 2008). Injections of CRF dose-dependently increased the firing rate of dopamine neurons in the VTA, and this effect was prevented by CRF-R1 antagonism (Wanat et al. 2008). In addition, many neurochemical and behavioral effects have been observed after the injection of a CRF-R1 antagonist into the VTA (Kalivas et al. 1987; Specio et al. 2008; Wang et al. 2005). Our rationale for the current experiments was to study whether it is possible to block the effects of intermittently scheduled, brief social defeat stress on subsequent intravenous cocaine self-administration by pretreatment with a CRF-R1 antagonist into the VTA.
Social defeat stress was chosen due to its translational value to that of human social stress (Björkqvist 2001). Episodic activation of neural and hormonal stress mechanisms can produce behavioral and neural chemical changes that may lead to a sensitized individual who may be more prone to drug-seeking and drug-taking behaviors (“behavioral and neurochemical sensitization” (Goeders 2002; Marinelli and Piazza 2002)). Activation of ascending projections from the mesolimbic pathway is critical for the induction of behavioral sensitization to psychomotor stimulants (Wolf et al. 1994). In addition, repeated social defeat stress increased the cellular activity of Fos-Like immunoreactive labeling in the VTA upon D-amphetamine sensitization (Nikulina et al. 2004). Thus, the VTA represents a critical site for neural and behavioral sensitization to social stress and to psychomotor stimulants (Cador et al. 1995; Miczek et al. 2008). Considerable evidence points to different kinds of social stress as risk factors for initiating, escalating, and resuming drug abuse (Brady et al. 1998; Sinha et al. 2006). Intermittent social defeat stress promotes and intensifies cocaine self-administration assessed by performance under the control of a progressive ratio schedule and by conditions of extended binge-like access (Covington and Miczek 2001; Miczek et al. 2011, under review).
Intra-cerebral ventricular (i.c.v.) injections of CRF induced reinstatement of heroin, alcohol, and cocaine seeking behavior, suggesting a critical role of CRF in stress-induced reinstatement (Brown et al. 2009; Le et al. 2000; Shaham et al. 1997). An initial study reported that i.c.v. injections of alpha-helical CRF9–41 (a nonselective peptide CRF receptor antagonist/partial agonist) decreased footshock-induced reinstatement of heroin seeking (Shaham et al. 1997). Subsequent studies reported that i.c.v. injections of a newer nonselective CRF receptor antagonist, D-Phe CRF12–41 also decreased footshock-induced reinstatement of nicotine, alcohol and cocaine seeking (Erb et al. 1998; Le et al. 2000; Liu and Weiss 2002; Zislis et al. 2007). Most recently, CRF in the VTA has been reported to be a critical site for footshock-induced reinstatement of cocaine seeking (Wang et al. 2005). In addition, local VTA perfusions (via a microdialysis probe) of alpha-helical CRF9–41 decreased footshock-induced reinstatement of cocaine seeking (Wang et al. 2005). Therefore, hyperactivation of CRF-R1 in the VTA could potentially be a target for neural sensitization that may induce escalated cocaine intake as a result of prior experiences with intermittent social stress. Most studies link CRF-R1 to the regulation of emotional behaviors, including anxiety, stress, and drug-taking. Our current study used a selective nonpeptide CRF-R1 antagonist (CP 154,526), to specifically block the release of dopamine by CRF-R1 activation (Schulz et al. 1996). The choice of CP 154,526 is based on its ability to penetrate the blood–brain barrier (Schulz et al. 1996).
We aimed to test the hypothesis that brief, intermittently scheduled social defeat activates CRF-R1 in the VTA, and that this activation is necessary for the development of increased behavioral responses to cocaine. The first aim attempts to characterize whether pretreatment with a CRF-R1 antagonist, CP 154,526, (via systemic administration 20 mg/kg) can block and prevent the stress-induced locomotor sensitization, and subsequently prevent escalated cocaine self-administration. The second aim specifically targets the VTA as a potential site of intervention to examine if microinjections of a CRF-R1 antagonist at this site prior to social stress can attenuate or prevent an escalated response to cocaine.
Materials and methods
Subjects
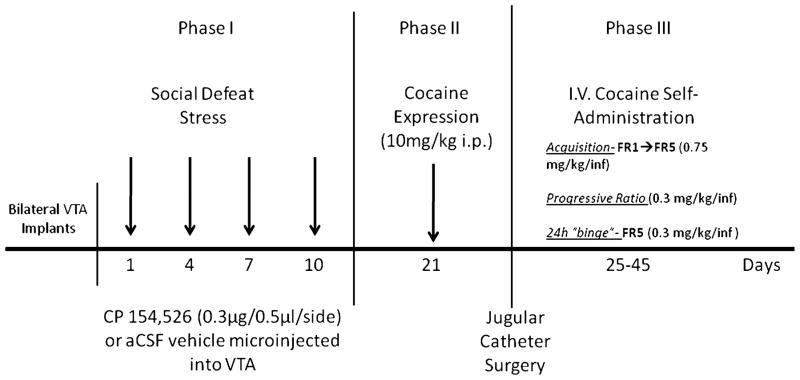
Male Long–Evans rats (Charles River Laboratories, Wilmington, MA) weighing 225–250g were individually housed in custom-built acrylic chambers (30×30.5× 24.5 cm) on arrival (n=105). The floor of each cage was lined with Cellu-Dri™ pellet bedding (Shepherd Specialty Papers, Kalamazoo, MI). All rats were on a reversed light/dark cycle (lights on 2000–0800 h), with controlled temperature (21°C ±10). Rats were given unlimited access to food (Purina laboratory rodent chow) and tap water. The housing conditions were maintained consistently throughout the experiment (see below, social defeat phase, cocaine challenge, and cocaine intravenous (IV) self-administration Fig. 1). All experimental procedures were approved by the Tufts Institutional Animal Care and Use Committee, following the principles of Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Fig. 1.
Experimental design, timeline, and drugs used in phases I–III
Social defeat stress
Upon arrival, rats were randomly assigned to two groups, defeat stressed or non-stressed controls. Subsequently, separate groups of rats were assigned to pharmacological treatment conditions. Experimentally stressed rats were socially defeated as described previously (Covington et al. 2008; Tornatzky and Miczek 1995), on days 1, 4, 7, and 10. Each social defeat episode took place in a room adjacent to the vivarium where the rats were normally housed.
Residents
A separate group of 20 male Long Evans rats (Charles River Laboratories, Wilmington, MA) were housed with a female in large stainless steel cages (45.7× 71.1×45.7 cm) and served as aggressive stimulus rats (residents). Each resident was chosen on the basis of displaying consistent aggressive behavior during confrontations with an intruder rat (Miczek 1979).
Systemic experiment
Before each defeat, rats in the defeat stress and control groups were weighed and then injected with the appropriate agent, either vehicle (saline), or the CRF-R1 antagonist CP 154,526 dissolved in 0.1% methylcellulose with an injection volume of 1.0 ml/kg. Twenty minutes later, experimentally stressed rats were placed in a steel mesh protective cage inside the home cage of a larger aggressive “resident” rat. After 10 min of social threat in the protective cage, experimentally stressed rats were then removed from the protective unit, and exposed to attacks by the resident until they showed behavioral evidence of defeat, defined as the display of 4 s of supine position. Typically, the defeat episode included four attack flurries, within a maximum of 5 min. Immediately after the defeat, the experimentally stressed rats were then returned to the protective cage, which was placed back in the aggressive rat’s home cage for an additional 10 min. Rats were then returned to their home cages in the vivarium until their next defeat, 72 h later. Each experimentally defeated rat was exposed to four different resident aggressors over the course of 10 days.
Intra-VTA experiment
Intra-VTA cannula surgery
The CRF-R1 antagonist CP 154,526 was directly infused into the VTA before each social defeat. Rats were surgically implanted with two guide cannulae for the delivery of CP 154,526 or artificial cerebrospinal fluid (aCSF) vehicle microinjections. While under ketamine (100 mg/kg) and xylazine (6 mg/kg) anesthesia, rats were surgically implanted with bilateral cannulae (23 gauge stainless steel, 11 mm) aimed 1 mm above the VTA (AP, −5.2; DV, −7.5; ML, +1.8 mm) at a 10° angle relative to bregma (Paxinos and Watson 1997). Cannulae were affixed with dental acrylic and three stainless steel screws, #0, anchored into the skull. Patency of cannulae was maintained by inserting obdurators (0.010/.25 mm fit 11 mm with 1 mm projection) except during microinjections. Each infusion of CP 154,526 (0.3 μg/side) or vehicle (aCSF) was delivered over 3 min in a volume of 0.5 μl using a CMA/100 microinfusion pump (CMA Microdialysis, Sweden). After each infusion, the injector was left in place for an additional 1 min to allow for diffusion and to prevent backflow. Infusions were administered once every 72 h, 20 min before each of the four stress episodes, or once every 72 h in the absence of social stress (control). After the completion of the self-administration experiments, rats were killed and cannula placement was verified by mounting 50-μm sections on gelatin coated slides and then staining them with cresyl violet (Fig. 2).
Fig. 2.
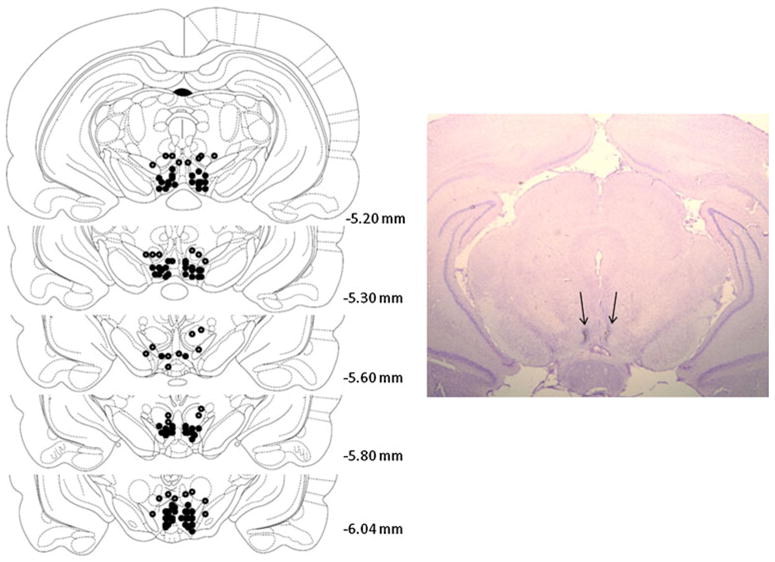
Left. Schematic portrayal of accurately placed intra-VTA sites. Each figure corresponds to coronal sections of the rat brain from −5.2 to −6.04 mm from bregma. Filled circles represent the average location of each pair of bilateral cannulae. The injection sites from 17 rats were inaccurate placements and are shown as open circles. Right Photomicrograph of an intra-VTA injection site
IV catheter surgery
All rats were permanently implanted with indwelling catheter (Siltastic® silicon tubing, ID 0.63 mm, OD 1.17 mm) into the right jugular vein (Covington and Miczek 2001; Remie et al. 1990) under a combination of ketamine (100 mg/kg) and xylazine (6 mg/kg) anesthesia. The catheter was passed subcutaneously through the rat’s back where it exited through a small incision and was affixed to a small plastic pedestal (Plastics One, Roanoke, VA) mounted inside a harness (Instech Laboratories Inc., Plymouth Meeting, PA). After catheter surgery, rats were allowed 5 days to recover, and were handled and weighed daily.
Locomotor sensitization
Cocaine challenge
Eleven days after the fourth social defeat episode (day 21), stressed and non-stressed rats were given a challenge injection of cocaine (10 mg/kg i.p.), to asses locomotor sensitization (Covington and Miczek 2001). Each rat was moved to an adjacent room and was briefly removed from its cage to be weighed and injected with saline, after which it was immediately returned to the home cage. Five minutes after the saline injection, rats were videotaped for 5 min. All rats were then injected with cocaine (10 mg/kg i.p.) and additional behavioral recordings took place 5–10 and 25–30 min later. A trained observer analyzed each video recording, using a custom keyboard and commercial software (The Observer Video-Pro© version 8.0, Noldus Information Technology, Wageningen, The Netherlands) to record the frequency and duration of rearing, walking, grooming, and inactivity.
Cocaine self-administration
After 5 days of recovery, rats were moved from their home cage and housed in the IV self-administration test chambers (Miczek and Mutschler 1996). Catheters were flushed with 0.2 ml of saline and 0.2 ml of heparinized saline (20 IU/ml) each morning, and 0.17 ml pulses of saline were delivered every 30 min except during the daily cocaine self-administration sessions.
Acquisition and maintenance
Initially, rats were allowed to self-administer cocaine (0.75 mg/kg/infusion), without a priming injection, and each lever press resulted in an IV infusion (fixed ratio; FR 1 schedule of reinforcement) followed by a 30-s timeout. Each daily session terminated after the delivery of 15 infusions or after 5 h of access. After reliable self-administration behavior was verified (two consecutive days of 15 infusions), the FR schedule was progressively increased over three to five additional days until every fifth lever press resulted in an IV infusion (FR 5). Rats were maintained on a limited access FR 5 schedule for at least five consecutive days. Rats that did not meet the criterion of two consecutive days of 15 infusions underwent behavioral shaping to facilitate lever pressing for IV cocaine self-administration.
Progressive ratio schedule
Once rats showed reliable, stable self-administration on FR5 for 5 days, they were tested using a progressive ratio (PR) schedule (Richardson and Roberts 1996). The progressive response requirement incremented as follows: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178. Sessions terminated once no cocaine infusion was delivered within 60 min. The average number of completed infusions for each rat was the dependent variable. Three daily PR sessions alternated with FR 5 sessions of 15 infusions, 0.75 mg/kg/infusion.
Twenty-four-hour binge
After their final PR session, rats were given one more day of limited access to cocaine (FR5, 0.75 mg/kg/infusion) and the following day rats were given access to a continuous 24-h “binge” (FR5, 0.3 mg/kg/infusion). The total number of infusions of cocaine was the dependent variable for analysis. Upon completion of the 24-h binge, catheter patency was verified by IV administration of methohexital sodium (Brevital™).
Statistics
Two-way analyses of variance (ANOVA) were used to analyze walking frequency in response to a cocaine injection (10 mg/kg i.p.), average number of infusions received over 3 PR self-administration sessions, total number of infusions received during the 24-h “binge”, and in the cumulative cocaine infusions at hour 24 across the treatment groups (stress or control, CP 154,526 or vehicle). When the F ratios were statistically significant Holm–Sidak post hoc analyses of all pair-wise comparisons were made.
Systemic experiment (n=58)
Cocaine challenge: non-stressed vehicle n=17; non-stressed CP 154–526 n=10; episodic defeat vehicle n=20; episodic defeat CP 154–526 n=11.
Cocaine self-administration: Non-stressed vehicle n=9; non-stressed CP 154–526 n=8; episodic defeat vehicle n= 9; episodic defeat CP 154–526 n=9.
Intra-VTA experiment (n=47)
Cocaine challenge: non-stressed vehicle n=11; non-stressed CP 154–526 n=9; episodic defeat vehicle n=14; episodic defeat CP 154–526 n=13.
Cocaine self-administration: non-stressed vehicle n=8; non-stressed CP 154–526 n=7; episodic defeat vehicle n= 7; episodic defeat CP 154–526 n=8.
Animals dropped from analysis during the cocaine self-administration phase were due to illness, or failure of catheter patency. Seventeen microinjected animals were excluded from the cocaine challenge analysis due to missed placements.
Drug solutions
Cocaine hydrochloride was obtained from the Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD) and was dissolved in sterile 0.9% saline. CP 154,526 was obtained from Pfizer as a gift or was purchased from Tocris Bioscience (St Louis, MO). The dosing for CP 154,526 was based on previous reports (Schulz et al. 1996; Shaham et al. 1998; Mansbach et al. 1997).
Results
Locomotor sensitization to cocaine
Systemic experiment
Cocaine challenge
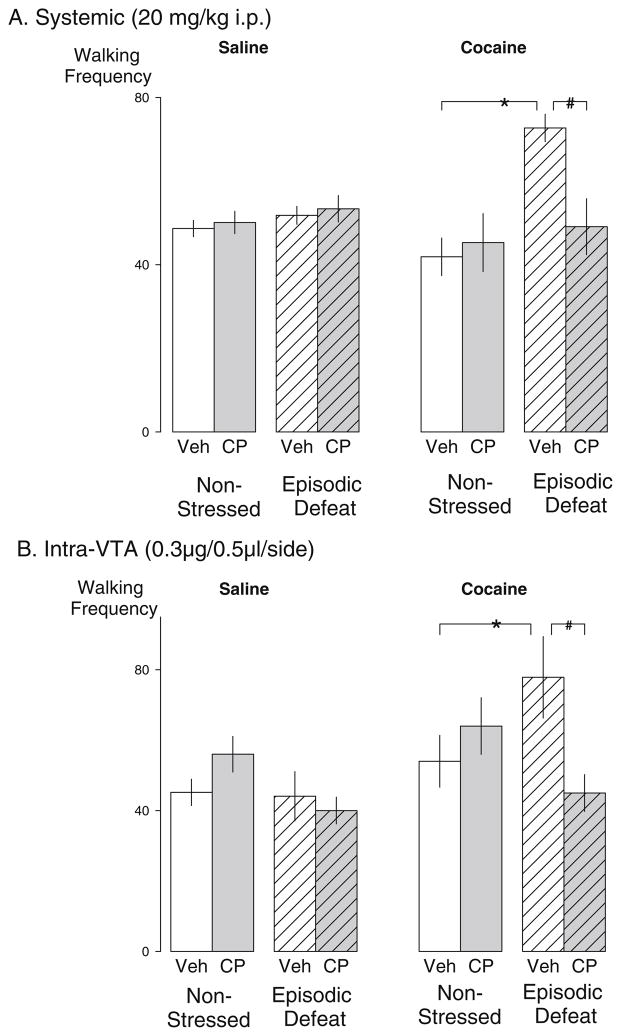
Eleven days after the last social defeat (i.e., day 21) no significant differences were found between groups in terms of the frequency of any behavior after an initial saline challenge. However, the cocaine (10 mg/kg i.p.) challenge produced a significant increase in the frequency of walking behavior in those rats that were episodically stressed and pretreated with vehicle injections prior to each defeat (Fig. 3a). During the cocaine challenge, a significant main effect of stress was detected on the frequency of walking behavior in episodically stressed animals compared to non-stressed (F1,54 =11.502, p=0.001), and a significant interaction between stress and drug treatment was also observed (F1,54 =7.014, p =0.011).
Fig. 3.
Systemic or intra-VTA injections of a CRF-R1 receptor antagonist blocked and prevented stress-induced locomotor sensitization in rats. a The mean (±SEM) walking frequency during a saline challenge (left) and a cocaine challenge (right; 10 mg/kg i.p.) 11 days after the social defeat phase with or without prior defeat stress. Non-stressed vehicle n=17; Non-stressed CP 154–526 n=10; episodic defeat vehicle n=20; episodic defeat CP 154–526 n=11. b Mean (±SEM) walking frequency after 10 mg/kg i.p. cocaine challenge 11 days after episodic defeat stress modified by microinjection of CRF-R1 antagonist CP-154526 (0.3 μg/0.5 μL/side) into the VTA of non-defeated and episodically defeated rats. Non-stressed vehicle n=11; non-stressed CP 154–526 n=9; episodic defeat vehicle n=14; episodic defeat CP 154–526 n=13. The asterisk indicates groups that were significantly different from the non-defeated vehicle condition at the p<0.05 level. The pound sign indicates the groups that were significantly different from the stressed-vehicle condition compared to the stressed drug condition at the p<0.05 level
Post hoc analysis revealed a significant increase in the walking frequency in those rats exposed to intermittent stress and pretreated with vehicle compared to the vehicle-treated non-stress condition (p<0.001). A significant reduction was detected in the walking frequency during the cocaine challenge in stressed rats pretreated with CP 154,526 (20 mg/kg i.p.), as compared to the vehicle-treated stress condition (p=0.001), showing that systemic injections of CP 154,526 before each exposure to stress prevented the induction of locomotor sensitization.
Intra-VTA experiment
Cocaine challenge
Microinfusions of CP 154,526 (0.3 μg/0.5 μl/side) into the VTA prior to each episode of social defeat significantly reduced the walking frequency in response to a cocaine challenge as compared to the vehicle-treated stress condition (Fig. 3b). Overall, a significant interaction between stress and drug treatment was observed (F1,43=6.317 p =0.016).
Walking frequency was significantly higher in rats that were stressed and microinfused with vehicle compared to non-stressed vehicle-treated controls (p=0.049), while walking frequency was significantly reduced in stressed rats pretreated with CP 154,526 compared to the vehicle-treated stress condition (p=0.006). Of the 17 missed placements, 4 were part of the group that received both stress and microinjections of CP 154,526. The average walking frequency for rats with misplacements outside the VTA was 75±10.6, roughly 1.7 times higher than those rats receiving CP 154,526 directly into the VTA.
Cocaine self-administration
Systemic experiment
Acquisition and maintenance
The statistical power was not great enough to run accurate statistics for the first two daily sessions of cocaine self-administration, rather the percentage of rats that did meet the criterion within the first two daily sessions were reported. Maintenance (resp/min) for self-administering cocaine was significantly more in those rats that received systemic injections of vehicle compared to those rats that received systemic injections of CP 154,526 (F1,36 =5.288 p=.027). In addition, rats that were stressed compared to non-stressed controls showed a significant increase in rate for self-administering cocaine (F1,36 =8.417 p=0.006). (see Table 1)
Table 1.
Effects of CP 154,526 on acquisition of cocaine self-administration, and maintenance of cocaine taking according to either a fixed ratio (FR) or progressive ratio (PR) schedule
| Condition | Treatment | Acquisition within two daily sessions | Maintenance | PR break point (infusions obtained) | |||
|---|---|---|---|---|---|---|---|
| Systemic (20 mg/kg) CP154,526 | |||||||
| Control | Vehicle | n=10 | 30% | n=10 | 0.840±0.085* | n=9 | 10.7±0.627* |
| Control | CP 154,526 | n=10 | 10% | n=10 | 0.548±0.081 | n=8 | 9.3±0.665 |
| Stress | Vehicle | n=13 | 7% | n=10 | 0.990±0.085*** | n=9 | 11.9±0.627* |
| Stress | CP 154,526 | n=11 | 27% | n=9 | 0.891±0.089** | n=9 | 10.2±0.627 |
| Intra-VTA (0.3 μg/0.5 μl/side) CP154,526 | |||||||
| Control | Vehicle | n=12 | 8% | n=12 | 0.630±0.086 | n=8 | 9.7±0.590 |
| Control | CP 154,526 | n=10 | 10% | n=8 | 0.639±0.109 | n=7 | 9.9±0.631 |
| Stress | Vehicle | n=10 | 30% | n=10 | 0.870±0.098 | n=7 | 10.1±0.631 |
| Stress | CP 154,526 | n=10 | 10% | n=10 | 0.630±0.058 | n=8 | 10.6±0.590 |
p<0.05; values rendered in italics indicate a significant drug effect between vehicle and CP 154,526 groups
p<0.05; values rendered in italics indicate a significant stress effect between stress and control groups
Progressive ratio of cocaine reinforcement
The “break point” for self-administering cocaine was significantly less in those rats that received systemic injections of CP 154,526 compared to those rats that received systemic injections of vehicle. Specifically, there was an overall drug effect between conditions (F1,31 =6.451, p=0.016; see Table 1).
Twenty-four-hour cocaine binge
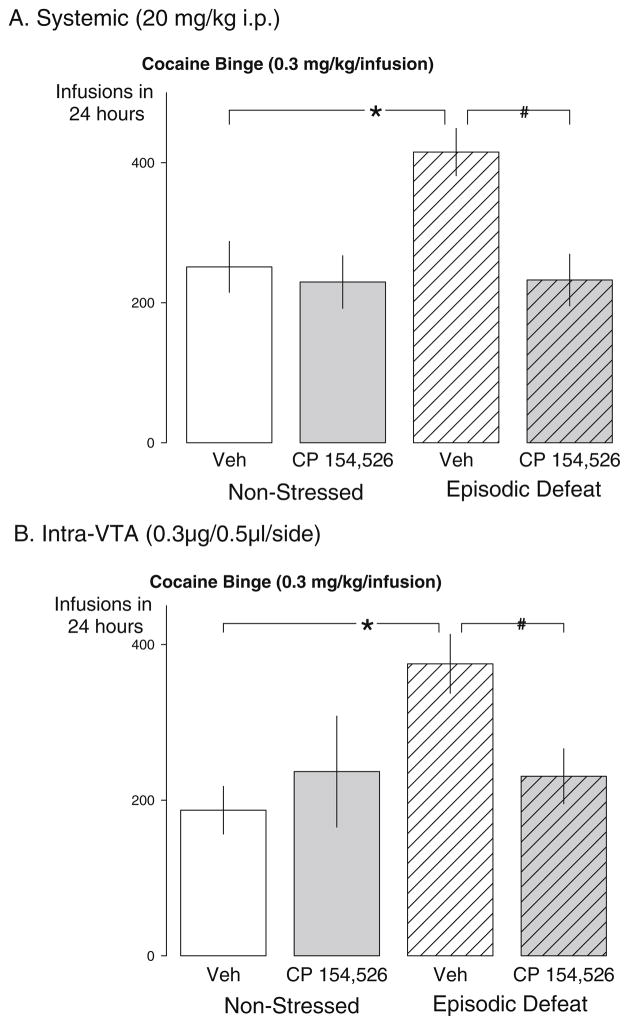
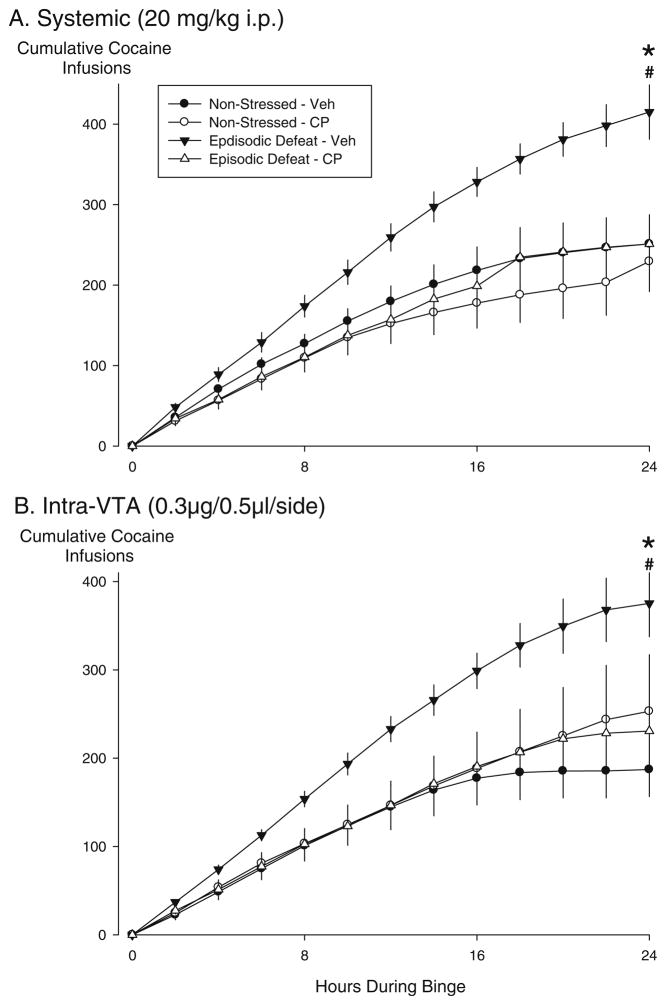
The total number of cocaine infusions during the 24-h “binge” was highest in the vehicle-treated stress sensitized rats, which contributed to a significant main effect of stress (F1,31=8.0, p =0.008), a significant main effect of drug (F1,31=5.330, p =0.028), and a significant interaction between stress and drug treatment (F1,31=4.975, p=0.033; Fig. 4a). Stressed, vehicle-treated rats achieved significantly more cocaine infusions during the 24-h “binge” than non-stressed vehicle-treated controls (p=0.001). Rats given systemic injections of CP 154,526 prior to each defeat showed a significant reduction in the total number of cocaine infusions during the 24-h “binge” compared to the stressed vehicle-treated group (p=0.003). In addition, cumulative cocaine infusions represent the same statistics described above since we only did statistics at hour 24 during the cocaine binge (Fig. 5a).
Fig. 4.
Effects of systemically administered or intra-VTA infusions of CRF-R1 antagonists prior to each episode of social defeat on subsequent cocaine taking behavior during a 24-h continuous access “binge”. The mean (±SEM) total number of cocaine infusions self-administered by non-stressed control rats (left bars) and episodically stressed rats (right bars). a Data from rats that received systemic injections of saline (open bars) or CP 154,526 (gray bars) during the social defeat phase of the experiment. Non-stressed vehicle n=9; non-stressed CP 154–526 n=8; episodic defeat vehicle n=9; episodic defeat CP 154–526 n=9. b Data from rats that received aCSF (open bars) prior to each episode of social defeat, and rats that received CP 154,526 (gray bars).
Non-stressed vehicle n=8; non-stressed CP 154–526 n=7; episodic defeat vehicle n=7; episodic defeat CP 154–526 n=8. The asterisk indicates groups that were significantly different from the non-defeated vehicle condition at the p<0.05 level. The pound sign indicates the groups that were significantly different from the stressed-vehicle condition compared to the stressed drug condition at the p<0.05 level
Fig. 5.
Effects of systemically administered or intra-VTA infusions of CRF-R1 antagonists during episodic social defeat on subsequent cocaine taking behavior during a 24-h continuous access “binge”. The mean (±SEM) cumulative number of cocaine infusions self-administered over 24 h by non-stressed control rats (circles) and episodically stressed rats (triangles). a The data from rats that received systemic injections of saline (black) or CP 154,526 (white) prior to each social defeat. Non-stressed vehicle n=9; non-stressed CP 154–526 n=8; episodic defeat vehicle n=9; episodic defeat CP 154–526 n=9. b The data from rats that received aCSF (black) prior to each social defeat, and rats that received CP 154,526 (white). Non-stressed vehicle n=8; non-stressed CP 154–526 n=7; episodic defeat vehicle n=7; episodic defeat CP 154–526 n=8. The asterisk indicates groups that were significantly different from the non-defeated vehicle condition at the p<0.05 level. The pound sign indicates the groups that were significantly different from the stressed-vehicle condition compared to the stressed drug condition at the p<0.05 level
Intra-VTA experiment
Acquisition and maintenance
The statistical power was not great enough to run accurate statistics for the first two daily sessions of cocaine self-administration, rather the percentage of rats that did meet the criterion within the first two daily sessions were reported. There were no significant differences observed for the maintenance phase of cocaine self-administration (see Table 1)
Progressive ratio of cocaine reinforcement
There were no significant differences in the progressive ratio phase of cocaine self-administration (see Table 1).
Twenty-four-hour cocaine binge
Furthermore, a significant role for VTA activation during social stress was revealed by the effects of CP 154,526 microinfusion prior to each defeat on subsequent cocaine taking during the 24-h “binge”. The total number of cocaine infusions during the “binge” was significantly reduced in those rats receiving microinjections of CP 154,526 prior to each exposure to defeat compared to vehicle-injected stressed rats, which contributed to an overall significant interaction between stress and drug treatment (F1,26=4.628,p =0.041; Fig. 4b).
The number of cocaine infusions during a 24-h “binge” in vehicle-treated stressed rats was significantly higher compared to vehicle-treated controls (p=0.007). Intra-VTA infusions of CP 154,526 prior to intermittent social stress significantly reduced the total number of cocaine infusions over the 24-h period as compared to the stressed vehicle-treated condition (p=0.032). In addition, cumulative cocaine infusions represent the same statistics described above since we only did statistics at hour 24 during the cocaine binge (Fig. 5b). Of the 17 missed placements, 1 rat received both stress and microinjections of CP 154,526 that completed the “binge”. This rat accumulated 181 infusions of cocaine which is below the group’s average.
Discussion
The current results suggest that CRF-R1 subtype in the VTA is critically involved in the development of stress-induced sensitization which can lead to escalated cocaine self-administration during continuous access in a 24-h “binge”. These results significantly extend previous demonstrations that the CRF-R1 subtype in the VTA may be an important site for the prevention of behavioral and neural sensitization to repeated exposure to cocaine and in cocaine self-administration (Goeders 2002; Lodge and Grace 2005; Phillips et al. 2003; Specio et al. 2008). These data report that CRF-R1 antagonism in the VTA prevented locomotor sensitization to a cocaine challenge and prevented escalation of cocaine “binges” after experiencing brief intermittent episodes of social defeat stress. The current findings suggest that CRF may be activated by intermittent social stress episodes and, after repeated intermittent exposures, CRF may exert a significant role in inducing neuroplastic changes in the mesolimbic DA system (Miczek et al. 2008).
CRF-immunoreactive fibers densely innervate many intra-hypothalamic and extra-hypothalamic brain areas, including the VTA. Afferent projections of neurons containing high concentrations of CRF are primarily located within the paraventricular nucleus of the hypothalamus (Sawchenko et al. 1993), an intra-hypothalamic structure that is part of the HPA axis and is a major contributor in regulating the behavioral and physiological responses to stress. In addition, extra-hypothalamic CRF-containing neurons primarily exist in the bed nucleus of the stria terminalis, the central nucleus of the amygdala (Gray 1993; Sakanaka et al. 1986), and the prefrontal cortex (PFC; Lewis et al. 1989; Millan et al. 1986; Rodaros et al. 2007; Swanson et al. 1983). CRF acts on two G-protein linked receptors, CRF-R1 and CRF-R2, and can interact with the CRF-binding protein (CRF-BP) (Higelin et al. 2001). More specifically, in the VTA these receptor subtypes are expressed in a topographically intricate manner and are proposed to be involved in brain reward and stress circuits (Sauvage and Steckler 2001; Van Pett et al. 2000). Efferent projections of CRF activation in the VTA is part of an interconnected network involving structures such as, the PFC, the shell of the nucleus accumbens, and the basolateral amygdala (Abercrombie et al. 1989; Kalivas and Duffy 1995; Moghaddam 2002).
The cellular distribution in the VTA has recently revealed CRF and glutamate (GLU) axon terminals establishing asymmetric synaptic connections with VTA DA neurons which are part of the mesolimbic pathways mediating brain reward and stress (Tagliaferro and Morales 2008). Long-lasting alterations in the mesolimbic DA pathway have been proposed to increase the risk of stress-induced relapse to cocaine seeking (Wang et al. 2005). In a previous study, rats were trained to self-administer cocaine, extinguish the cocaine seeking and taking habit, and then reinstate the seeking response by footshock stress. Twenty-minute microdialysis samples from the VTA were collected during the footshock-induced reinstatement phase of the experiment. Results indicate that footshock stress can increase the CRF levels in the VTA in cocaine-treated and cocaine-naïve animals. Along with CRF increases, GLU and DA levels were increased in the cocaine-trained but not in the cocaine-naïve animals suggesting specific cellular signaling mediated by CRF release. Furthermore, micro-injections of a non-specific CRF receptor antagonist prevented the increase in GLU and DA levels in the VTA, suggesting that CRF may acquire control over VTA GLU and DA release projecting to other brain regions. Footshock-induced reinstatement of extinguished cocaine-seeking behavior is reestablished by central CRF injections (Erb et al. 2006) and is prevented by CRF antagonism (Erb et al. 1998; Shaham et al. 1998). The VTA seems to be a critical site for footshock-induced reinstatement as CRF application appears to elevate extracellular glutamate levels (Wang et al. 2007). Glutamatergic regulation of DA neurons in the VTA is potentiated by NMDA receptor mediated neurotransmission which is also facilitated by CRF (Ungless et al. 2003). These activational effects within the VTA most likely involve downstream elevation of DA and activation of DA receptors in the mPFC and Acb (Capriles et al. 2003; McFarland et al. 2004; Kalivas et al. 1987).
Peptidergic modulation of monoaminergic nuclei continue to be investigated as targets for therapeutic intervention in stress disorders. These include serotonin cells in the dorsal raphe (Bubar and Cunningham 2008), and norepinephrine cells in the locus coeruleus (Reyes et al. 2008; Valentino and Van 2008). The current results suggest an additional target for intervention by focusing on the CRF-R1 subtype in the VTA. Brief intermittently scheduled social stress can cause long-term alterations that sensitize the mesolimbic DA pathway (Tidey and Miczek 1996; Miczek et al. 2011, under review). More specifically, intermittent brief social defeat experiences appear to augment c-Fos expression in the VTA and PFC after being challenged with a moderate dose of amphetamine (1.0 mg/kg; Covington, III et al. 2005; Nikulina et al. 2004). This sensitized cellular activity might reflect a dysregulated cascade of intracellular processes that may promote the escalation of stress-induced drug-taking behavior. Electrophysiological studies demonstrated that application of CRF increased VTA dopamine neuronal firing in a concentration dependent manner. In addition, experiments using CRF agonists, CRF antagonists, and CRF-receptor deficient mice all led to the same conclusion that CRF increased VTA dopamine neurons through CRF-R1 activation (Wanat et al. 2008). The previous studies identify a link between dopamine and CRF in the VTA and this link may be relevant to a specific role in stress-induced escalation of cocaine self-administration. Our results demonstrate that stress-escalated cocaine taking can be prevented by pretreatment with a CRF-R1 antagonist into the VTA prior to each social defeat experience. Thus, a CRF-R1 antagonist may be a promising therapeutic intervention for stress-escalated drug dependence. Once the side-effect profile of CRF-R1 antagonist is significantly improved, particularly at the human level, it is feasible to envision therapeutic interventions that rely primarily on sites of action at monoaminergic cells in the brain stem. Future studies will have to define the dopaminergic cells and their targets that are selectively modulated by CRF-R1 antagonist.
In addition to the CRF-R1 subtype in the VTA, CRF-R2 and CRF binding protein have been identified in a subpopulation of VTA neurons (Ungless et al. 2003; Wang et al. 2007). Pharmacological and electrophysiological data suggest participation of CRF-R2 and CRF-BP in VTA interactions with glutamate signaling to dopamine neurons (Wang and Morales 2008). Mixed results using RT-PCR and in-situ hybridization reveal detectible evidence for CRF-R2 mRNA in the VTA of cocaine-naïve mice (Ungless et al. 2003), but other studies did not replicate this finding and could not detect CRF-R2 mRNA in cocaine-naïve mice. Our ongoing research aims to characterize the CRF-R2 subtype in the VTA by microinfusion of a selective CRF-R2 antagonist, Astressin 2B. Our preliminary results regarding the CRF-R2 subtype seem to reveal an amplified response to cocaine after brief intermittent exposure to social stress. To date, the current literature has at least two hypotheses regarding the CRF-R2 subtype. One hypothesis supports CRF-R2 as an additive receptor that can exert similar cellular signaling to that of CRF-R1 (Risbrough et al. 2003; Risbrough et al. 2004; Risbrough et al. 2009). The second hypothesis is that the CRF-R2 subtype exerts opposite functions to those of the CRF-R1 (Makino et al. 2002; Müller and Wurst 2004; Wang et al. 2007). Our ongoing research involving the CRF-R2 subtype seems to agree with the latter hypothesis and may amplify the effects caused by intermittent social stress, and subsequently amplify the reinforcing effects of cocaine.
Epidemiological evidence and neurobiological data converge to link social stress and drug use (Sinha et al. 2006; Sinha 2009; Substance Abuse and Mental Health Services Administration 2010). Some specific types of social stress can promote drug abuse and trigger relapse, whereas others do not, each stressor activating discrete neurobiological mechanisms (Miczek et al. 2008). Social stress, depending on intensity and duration, may increase the susceptibility to anxiety, post traumatic stress syndrome, panic disorders or depression. In spite of several promising leads, no effective pharmacological intervention has been approved for the treatment of stress-induced escalated cocaine self-administration.
In conclusion, the transition from initial drug use to escalated drug-taking behavior is a complex issue that likely involves neural sensitization in specific mesolimbic DA neurons (Covington et al. 2008; Miczek et al. 2008; Wang et al. 2009). Based on the current results, we have replicated our previous finding that brief intermittent social stress can induce locomotor sensitization which in turn can escalate the reinforcing effects of cocaine self-administration during a 24-h “binge” (Covington et al. 2008; Yap et al. 2005). Overall, we prevented stress-induced locomotor sensitization to cocaine by administering a CRF-R1 antagonist into the VTA prior to each episode of social defeat. These results indicate that brief intermittent episodes of social stress can activate the mesolimbic DA circuit via CRF-R1 subtype, which contribute to long-lasting neural adaptations that may influence the reinforcing effects of cocaine.
Acknowledgments
This paper was funded by the National Institute on Drug Abuse grant DA-002632.
Contributor Information
Christopher O. Boyson, Email: christopher.boyson@tufts.edu, Department of Psychology, Tufts University, Medford, MA, USA
Tarciso T. Miguel, UFSCar/UNESP-Araraquara, Araraquara, SP, Brazil
Isabel M. Quadros, Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil
Joseph F. DeBold, Department of Psychology, Tufts University, Medford, MA, USA
Klaus A. Miczek, Department of Psychology, Tufts University, Medford, MA, USA
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic–pituitary–adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–165. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Björkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Brady KT, Dansky BS, Sonne SC, Saladin ME. Posttraumatic stress disorder and cocaine dependence. Order of onset. Am J Addict. 1998;7:128–135. [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Stinus L. Evidence of a complete independence of the neurobiological substrates for the induction and expression of behavioral sensitization to amphetamine. Neuroscience. 1995;65:385–395. doi: 10.1016/0306-4522(94)00524-9. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology (Berl) 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin- releasing factor administration—is CRF a mediator of anxiety or stress responses? Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Petrovic A, Yi D, Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology (Berl) 2006;187:112–120. doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2000;23:577–586. doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann NY Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Higelin J, Py-Lang G, Paternoster C, Ellis GJ, Patel A, Dautzenberg FM. 125I-Antisauvagine-30: a novel and specific high-affinity radioligand for the characterization of corticotropin-releasing factor type 2 receptors. Neuropharmacology. 2001;40:114–122. doi: 10.1016/s0028-3908(00)00105-2. [DOI] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Latimer LG. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp Ther. 1987;242:757–763. [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotropin-releasing factor instress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Foote SL, Cha CI. Corticotropin-releasing factor immunoreactivity in monkey neocortex: an immunohistochemical analysis. J Comp Neurol. 1989;290:599–613. doi: 10.1002/cne.902900412. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Ther. 2005;314:201–206. doi: 10.1124/jpet.105.084913. [DOI] [PubMed] [Google Scholar]
- Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav. 2002;73:147–158. doi: 10.1016/s0091-3057(02)00791-8. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Brooks EN, Chen YL. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur J Pharmacol. 1997;323:21–26. doi: 10.1016/s0014-2999(97)00025-3. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology (Berl) 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MA, Jacobowitz DM, Hauger RL, Catt KJ, Aguilera G. Distribution of corticotropin-releasing factor receptors in primate brain. Proc Natl Acad Sci USA. 1986;83:1921–1925. doi: 10.1073/pnas.83.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Müller MB, Wurst W. Getting closer to affective disorders: the role of CRH receptor systems. Trends Mol Med. 2004;10:409–415. doi: 10.1016/j.molmed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. Academic; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Remie R, van Dongen JJ, Rensema JW. Permanent cannulation of the jugular vein (acc. to Steffens) In: van Dongen JJ, editor. Manual of microsurgery on the laboratory rat. Elsevier; Amsterdam: 1990. pp. 159–169. [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology (Berl) 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, Holsboer F. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;34:1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and para-ventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei-potential implication for arousal and attention. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, III, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci USA. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker C, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:0–4. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Sinha R. Stress and addiction: a dynamic interplay of genes, environment, and drug intake. Biol Psychiatry. 2009;66:100–101. doi: 10.1016/j.biopsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic–pituitary–adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of national findings. US Department of Health and Human Services; Rockville: 2010. [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Alcohol, anxiolytics and social stress in rats. Psychopharmacology (Berl) 1995;121:135–144. doi: 10.1007/BF02245600. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van BE. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J Comp Neurol. 2008;509:302–318. doi: 10.1002/cne.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Xue CJ, White FJ, Dahlin SL. MK-801 does not prevent acute stimulatory effects of amphetamine or cocaine on locomotor activity or extracellular dopamine levels in rat nucleus accumbens. Brain Res. 1994;666:223–231. doi: 10.1016/0006-8993(94)90776-5. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Covington HE, III, Gale MC, Datta R, Miczek KA. Behavioral sensitization due to social defeat stress in mice: antagonism at mGluR5 and NMDA receptors. Psychopharmacology (Berl) 2005;179:230–239. doi: 10.1007/s00213-004-2023-3. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]