Abstract
Weight loss and muscle wasting are of critical importance to cancer patients because of their negative effects on survival, functional status, and tolerability of chemotherapy. Because previous data suggest vascular endothelial growth factor receptor inhibitors disrupt skeletal muscle pathways, such as PI3K and AKT, the current study explored weight loss and muscle wasting in colorectal cancer patients treated with bevacizumab. Patients were assessed for serial weight and radiographic changes in skeletal muscle at baseline and again within 3 months of starting cancer therapy. Computed tomography scans were used to assess muscle. Fifty-seven patients are the focus on this report. These patients manifested a decline in mean weight from 85 to 83 kilograms (P = 0.002). Mean skeletal muscle area at the L3 vertebral level dropped from 148 cm2 to 145 cm2 (P = 0.02). This drop in weight and skeletal muscle occurred independently of cancer progression. No statistically significant differences in survival were observed based on loss of weight or skeletal muscle. Colorectal cancer patients prescribed bevacizumab appear to lose weight and muscle over a few months even in the absence of cancer progression.
Keywords: Wasting, Skeletal muscle, Cancer, Bevacizumab
Introduction
Muscle wasting is of critical importance to cancer patients. First, it predicts a shortened survival. Examining 2115 cancer patients, Prado and others observed that regardless of weight, attrition of skeletal muscle was associated with early death [1]. Secondly, and perhaps intuitively, muscle wasting leads to a decline in functional status. In the same study referred to above, 47% of patients had notable muscle wasting and poor functional scores compared to 26% with less muscle wasting and better scores (P = 0.009). Thirdly, muscle wasting in cancer patients is associated with higher rates of severe toxicity from chemotherapy [2]. A study that examined muscle mass and capecitabine toxicity observed that 50% of patients with muscle wasting experienced severe side effects in contrast to only 20% of patients with no muscle wasting [1, 2]. Thus, muscle and the factors that drive cancer-associated muscle wasting have important implications for cancer patients.
What effect does cancer therapy have on muscle wasting? Although few studies have specifically sought to measure muscle under such circumstances, a robust literature demonstrates weight gain with conventional chemotherapy [3–7]. This weight gain can be dramatic, leading to a several-pound increase in the setting of tumor response or with adjuvant chemotherapy. In fact, weight gain occurs in over 50% of breast cancer patients who receive the latter [3]. Far fewer studies have examined weight changes and body composition in patients who receive some of the newer, better tolerated cancer agents. Recently, however, Antoun and others examined the effects of sorafenib in patients with renal cell carcinoma, reporting a dramatic decline in weight and attrition of lean tissue [8]. Patients lost as much as 2.1 kilograms over 6 months whereas placebo-exposed patients manifested stable weight. Assessment of body composition also revealed a decline in muscle with sorafenib. These findings raise the question of how other, newer antineoplastic agents might detrimentally affect weight and muscle.
Bevacizumab is currently prescribed for the treatment of colorectal, non-small cell lung cancer, as well as a host of other malignancies [9–14]. This vascular endothelial growth factor receptor inhibitor disrupts many of the skeletal muscle pathways, such as PI3K and AKT, in the same manner as sorafenib [15]. To our knowledge, however, no previous studies have examined the effects of bevacizumab on weight and skeletal muscle. The purpose of the present study was to explore how these endpoints change over time in cancer patients treated with this commonly prescribed drug.
Methods
Overview
This study was approved by the Mayo Clinic Institutional Review Board. Its primary goal was to explore serial weight and muscle changes in colorectal cancer patients treated with bevacizumab.
Patient eligibility
Only metastatic colorectal cancer patients who began treatment with bevacizumab in March of 2004 or subsequently at the Mayo Clinic in Rochester, Minnesota were included. This starting point was chosen because the Food and Drug Administration approved bevacizumab that year for the treatment of metastatic colorectal cancer [16]. Bevacizumab-treated patients were included if they had had a computerized tomographic scan of the abdomen less than one month prior to starting bevacizumab and within 3 months of initiation of this drug. Patients who met these criteria were examined consecutively.
Data extraction
The following information was extracted from each medical record: the patient’s date of birth, date of death or last follow-up, weight loss at cancer diagnosis, chemotherapy prior to bevacizumab, dose of bevacizumab, chemotherapy administered in conjunction with bevacizumab, and other cancer treatment such as surgery or radiation administered between scans. In addition, patients’ height and serial weights were gleaned from the medical record. The latter was acquired within two days of each scan. Tumor response between scans was also acquired from the medical record and classified as tumor progression versus other based on real-time clinical assessment. Finally, as described below, muscle area from the L3 level was measured and calculated from each computerized tomographic scan.
Muscle measurements
Computerized tomographic scans were utilized in all patients to assess the area of muscle at the L3 level. QREADS®, an electronic clinical application used purely for viewing medical images and developed at the Mayo Clinic in Rochester, Minnesota, was used to identify this image level and to acquire the measurements described below. A single L3 image was copied to the National Institute of Health’s ImageJ Program, a validated tool for calculating area measurements on radiographic images [17–19]. Skeletal muscle and adipose tissue were distinguished by both gross appearance and by the technique from Lemieux and others [17, 20] (Figs. 1, 2). All portions of the image except the muscle itself were then cropped and removed (Fig. 3). A grayscale pixel histogram of the remaining muscle in the image was then constructed and summed. The number of pixels was then converted to an area measurement in squared centimeters. Whenever possible, measurements and calculations were undertaken in such a manner that the investigator was unaware of the patient’s previously calculated muscle measurement.
Fig. 1.
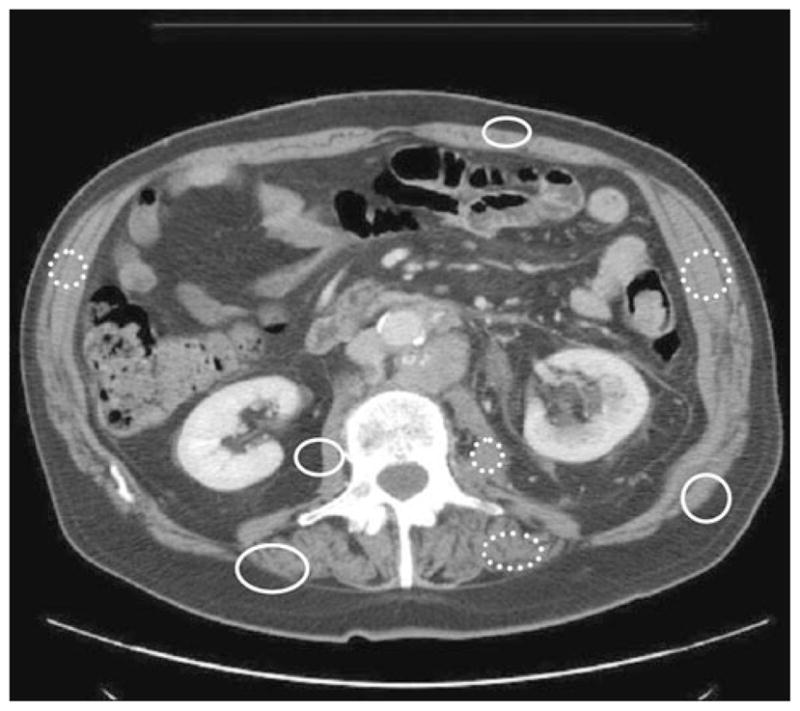
Skeletal muscle gray scale values were defined based on areas that included one-half adipose tissue and one-half skeletal muscle (solid circles) and areas that included only muscle (dotted circles). Such scaling helped define areas of cropping that showed residual muscle
Fig. 2.
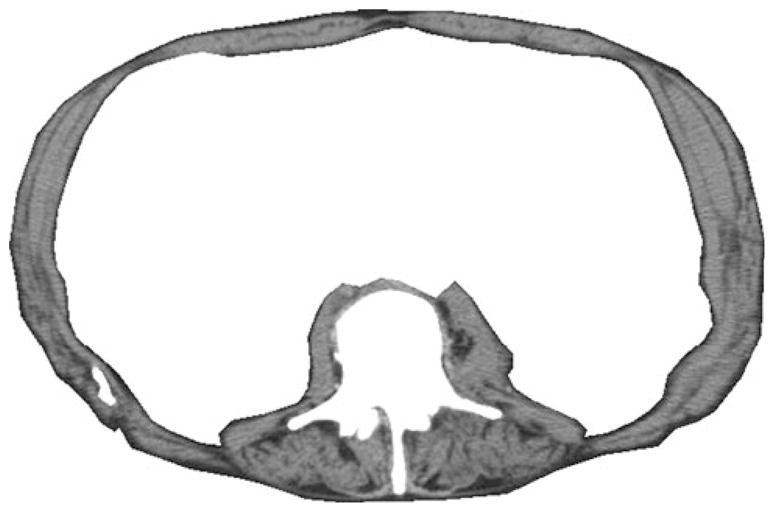
After cropping at the L3 level, only muscle was left. Muscle measurements were based on summing the area of the remaining muscle
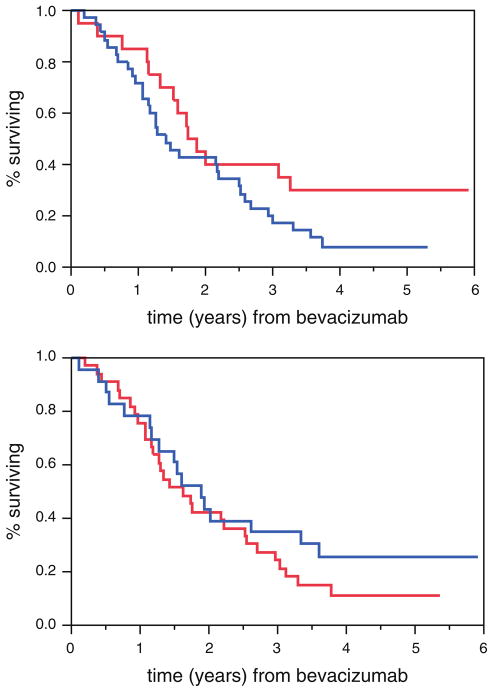
Fig. 3.
There was no statistically significant difference in survival between patients who had gained (1.81 years, top curve on tail) or lost (1.34 years) weight (P = 0.10, log-rank test), as shown in the top graph. Similarly, there was no statistically significant difference between those who had gained (1.73 years, top curve on tail) or lost (1.51 years) muscle (P = 0.33), as shown in the bottom graph
Statistical analyses
Descriptive data include mean values and standard deviations. A paired t-test was used to assess serial differences in continuous outcome measures, such as muscle area and weight. The differences between such measurements were then compared with an independent t-test based on whether or not patients had manifested progressive cancer or not. A log-rank test was used to compare survival based on dichotomized weight and muscle measurements that defined groups. A P-value of <0.05 was considered statistically significant.
A sample size of 57 patients enabled detection of an estimated mean paired difference in muscle area of 4 cm2 (and a standard deviation difference in muscle area of 8 cm2) with greater than 80% power and a 5% type I error rate. These anticipated differences were extrapolated from expected tumor response rates, body composition data from others, and this study’s relatively short interval between serial radiographic assessments [1, 6, 8, 21, 22].
Results
Demographics
A total of 107 patients were consecutively treated between March of 2004 through 2007, and of these, 57 had serial scans. It should be noted that our study aims, which were established a priori, and the sheer lack of study material in patients with no serial scans necessitated that the study focus only on these 57 patients. Patient demographics appear in Table 1. Forty patients had received prior chemotherapy.
Table 1.
Baseline demographics (n = 57)
| Mean age at treatment, in years (range) | 59 (26–84)a |
| Sex | |
| Male | 30 (53) |
| Female | 27 (47) |
| Had chemotherapy been administered prior to bevacizumab? | |
| Yes | 40 (70) |
| No | 17 (30) |
| Weight loss at time of cancer diagnosis? | |
| Yes | 14 (25) |
| No | 15 (26) |
| Unknown | 28 (49) |
| Diabetes? | |
| Yes | 10 (18) |
| No | 47 (82) |
Numbers in parentheses refer to the percentage of the cohort unless otherwise specified
Treatment and tumor response
Most patients received bevacizumab 5 mg/kg; 3 received a higher dose of 7.5 mg/kg. All patients received concurrent conventional chemotherapy along with bevacizumab. This conventional chemotherapy consisted of a combination of fluoropyrimidine, oxaliplatin, and leucovorin (39 patients), an irinotecan-based regimen (14), single agent capecitabine (3), or 5-fluorouracial and leucovorin (1). One patient underwent surgery for a strangulated hernia and 2 required radiation between scans.
In terms of radiographic tumor response, 7 patients manifested progressive cancer. The other 50 manifested stable or responsive cancer.
Serial weight and muscle measurements
The mean weight (standard deviation) of patients at the time of the first and second scans were 85 kilograms (19) and 83 kilograms (19), respectively (P = 0.002). Twenty-two patients had gained weight between scans. Cancer progression did not yield a statistically significant difference in weight loss between groups.
The mean muscle area (standard deviation) on the first and second set of L3 scans was 148 cm2 (38) and 145 cm2 (39), respectively (P = 0.02). Twenty-four patients gained muscle between scans. The mean difference (standard deviation) of muscle area between patients with cancer progression and those with stable or responsive disease was −6.2 (9.3) and −2.0 (7.5), respectively (P = 0.29), thus indicating that tumor progression was not a major determinant of muscle loss.
Follow-up
At the time of this report, 47 of 57 patients had died. Patients who had gained weight between scans had a slightly longer survival compared to those who lost weight, but this difference did not reach statistical significance: 1.81 and 1.34 years, respectively; (P = 0.10). Similarly, patients who had gained muscle between scans also lived longer, but, again, this difference did not reach statistical significance: 1.73 and 1.51 years, respectively; (P = 0.33) (Fig. 3).
Discussion
This study found that the administration of bevacizumab, as described in this group of patients, is associated with loss of weight and attrition of skeletal muscle. Importantly, cancer progression did not predict the decline of either of these endpoints, thus suggesting that perhaps the cancer therapy itself, which included bevacizumab, might account for these findings. Even patients who did not manifest cancer progression manifested a loss of weight and muscle. Our findings parallel similar observations from Antoun and others with sorafenib, but the results from the current study are particularly notable insofar as the weight loss and muscle wasting occurred over a shorter time span of only a few weeks [8]. These observations suggest that patients who receive long-term chemotherapy with bevacizumab or perhaps bevacizumab alone should be monitored carefully for evidence of muscle weakness and other related morbidity.
Do such observations seem plausible? Not all prospectively conducted clinical trials consistently report on the symptoms of fatigue and depression, both of which are thought to be potentially related to generalized weakness [23]. Yet, previous prospective studies with bevacizumab have found that the rates of these symptoms are, in fact, higher with this agent. Miller and others reported that severe fatigue occurred in 9.1% of conventional chemotherapy-treated patients who also received bevacizumab in contrast to only 4.9% who received conventional chemotherapy alone (P = 0.04) [24]. Similarly, Yang and others observed that malaise occurred in 30% of patients treated with bevacizumab 10 mg/kg in contrast to only 16% treated at a dose of bevacizumab 3 mg/kg and 15% exposed only to placebo [25]. Finally, Allegra and others observed that 2.9% of patients treated with bevacizumab in conjunction with conventional chemotherapy suffered severe depression in contrast to only 1.3% who received conventional chemotherapy alone (P<0.01) [26]. The current study begins to suggest an underlying mechanism for these higher rates of fatigue and depression with bevacizumab, pointing to an attrition of lean tissue as a potential culprit.
Finally, the study reported here has limitations. In contrast to the study from Antoun and others that explored body composition in sorafenib-treated patients [8], the current study did not include a placebo group to provide benchmark, comparative muscle attrition rates. Moreover, the current study focused on colorectal cancer patients who had received conventional chemotherapy in conjunction with bevacizumab. The serial loss of muscle tissue reported here might in fact be attributable to the combination of conventional chemotherapy and bevacizumab, not bevacizumab alone. Nonetheless, the rapid weight loss and muscle wasting observed in this study is remarkable. These observations suggest that newer agents—such as bevacizumab—should be further investigated for their contribution to fatigue and to other related adverse events from an epidemiologic and a mechanistic standpoint.
Acknowledgments
This work was funded by 5K24CA131099.
Contributor Information
Timothy Poterucha, Mayo Medical School, Mayo Clinic, Rochester, MN 55905, USA.
Brian Burnette, Department of Oncology, Mayo Clinic, Rochester, MN 55905, USA.
Aminah Jatoi, Email: jatoi.aminah@mayo.edu, Department of Oncology, Mayo Clinic, Rochester, MN 55905, USA. 200 First Street SW, Rochester, MN 55905, USA.
References
- 1.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 2.Prado CM, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13(11):3264–8. doi: 10.1158/1078-0432.CCR-06-3067. [DOI] [PubMed] [Google Scholar]
- 3.Demark-Wahnefried W, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19(9):2381–9. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 4.Camoriano JK, et al. Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. J Clin Oncol. 1990;8(8):1327–34. doi: 10.1200/JCO.1990.8.8.1327. [DOI] [PubMed] [Google Scholar]
- 5.Francini G, et al. Exemestane after tamoxifen as adjuvant hormonal therapy in postmenopausal women with breast cancer: effects on body composition and lipids. Br J Cancer. 2006;95(2):153–8. doi: 10.1038/sj.bjc.6603258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman RJ, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89(5):2248–53. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- 7.Steyn RS, et al. Weight gain as an indicator of response to chemotherapy for oesophageal carcinoma. Clin Oncol (R Coll Radiol) 1995;7(6):382–4. doi: 10.1016/s0936-6555(05)80010-x. [DOI] [PubMed] [Google Scholar]
- 8.Antoun S, et al. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol. 2010;28(6):1054–60. doi: 10.1200/JCO.2009.24.9730. [DOI] [PubMed] [Google Scholar]
- 9.National-Cancer-Institute. Bevacizumab: first-line treatment of metastatic colorectal cancer. 2009 (cited 8/20/2010);Available from: http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab.
- 10.Lynch T, Jr, Kim E. Optimizing chemotherapy and targeted agent combinations in NSCLC. Lung Cancer. 2005;50(S2):S25–32. [PubMed] [Google Scholar]
- 11.Moen MD. Bevacizumab: in previously treated glioblastoma. Drugs. 2010;70(2):181–9. doi: 10.2165/11203890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Escudier B, Cosaert J, Pisa P. Bevacizumab: direct anti-VEGF therapy in renal cell carcinoma. Expert Rev Anticancer Ther. 2008;8(10):1545–57. doi: 10.1586/14737140.8.10.1545. [DOI] [PubMed] [Google Scholar]
- 13.Micha JP, et al. A phase II study of outpatient first-line paclitaxel, carboplatin, and bevacizumab for advanced-stage epithelial ovarian, peritoneal, and fallopian tube cancer. Int J Gynecol Cancer. 2007;17(4):771–6. doi: 10.1111/j.1525-1438.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 14.Tripathy D. Capecitabine in combination with novel targeted agents in the management of metastatic breast cancer: underlying rationale and results of clinical trials. Oncologist. 2007;12(4):375–89. doi: 10.1634/theoncologist.12-4-375. [DOI] [PubMed] [Google Scholar]
- 15.Tsavachidou-Fenner D, et al. Gene and protein expression markers of response to combined antiangiogenic and epidermal growth factor targeted therapy in renal cell carcinoma. Ann Oncol. 2009;21(8):1599–606. doi: 10.1093/annonc/mdp600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 17.Lemieux S, et al. Comparison of two techniques for measurement of visceral adipose tissue cross-sectional areas by computed tomography. Am J Hum Biol. 1999;11(1):61–8. doi: 10.1002/(SICI)1520-6300(1999)11:1<61::AID-AJHB6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Mitsiopoulos N, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85(1):115–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 19.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophoton Int. 2004;11(7):36–42. [Google Scholar]
- 20.Irving BA, et al. NIH ImageJ and Slice-O-Matic computed tomography imaging software to quantify soft tissue. Obesity. 2007;15(2):370–6. doi: 10.1038/oby.2007.573. [DOI] [PubMed] [Google Scholar]
- 21.Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):269–75. doi: 10.1097/SPC.0b013e328331124a. [DOI] [PubMed] [Google Scholar]
- 22.Tol J, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563–72. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 23.Sadler IJ, et al. Preliminary evaluation of a clinical syndrome approach to assessing cancer-related fatigue. J Pain Symptom Manage. 2002;23(5):406–16. doi: 10.1016/s0885-3924(02)00388-3. [DOI] [PubMed] [Google Scholar]
- 24.Miller K, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 25.Yang JC, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allegra CJ, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27(20):3385–90. doi: 10.1200/JCO.2009.21.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]